Abstract
Introduction
The COVID-19 pandemic resulted in a significant disruption of colorectal cancer (CRC) care pathways. This study evaluates the management and outcomes of patients with primary locally advanced or recurrent CRC during the pandemic in a single tertiary referral centre.
Methods
Patients undergoing elective surgery for advanced or recurrent CRC with curative intent between March 2020 and March 2021 were identified. Following first multidisciplinary team discussion patients were broadly classified into two groups: straight to surgery (n=22, 45%) or neoadjuvant therapy followed by surgery (n=27, 55%). Primary outcome was COVID-19-related complication rate.
Results
Forty-nine patients with a median age of 66 years (interquartile range: 54–73) were included. No patients developed a COVID-19 infection or related complication during hospital admission. Significant delays were identified in the treatment pathway of patients in the straight to surgery group, mostly due to delays in referral from external centres. Nine of 22 patients in the straight to surgery group had evidence of tumour progression compared with 3 of 27 in the neoadjuvant group (p=0.015839). Seven of 27 patients in the neoadjuvant group showed evidence of tumour regression. During the study, surgical waiting times were reduced, and more operations were performed during the second wave of COVID-19.
Conclusion
This study suggests that it is possible to mitigate the risks of COVID-19-related complications in patients undergoing complex surgery for locally advanced and recurrent CRC. Delay in surgical intervention is associated with tumour progression, particularly in patients who may not have neoadjuvant therapy. Efforts should be made to prioritise resources for patients requiring time-sensitive surgery for advanced and recurrent CRC.
Keywords: Surgery, Advanced colorectal cancer, Recurrent colorectal cancer, COVID-19
Introduction
The COVID-19 pandemic that evolved in the spring of 2020 led to unprecedented pressures on the UK National Health Service (NHS) due to an exponential increase in demand for hospital and intensive care unit beds, staff redeployment/absence, revised standard operating procedures to prevent nosocomial transmission, limited primary care access, reduced diagnostics availability and lockdown of the population. These factors resulted in a drastic disruption of the UK cancer care pathways.1
Patients with locally advanced and recurrent colorectal cancer (CRC) require a beyond total mesorectal excision (bTME) surgical approach,2 including advanced pelvic resections3 which are resource-intensive procedures. The need to repurpose wards to isolation facilities and reallocate intensive care unit bed capacity as well as theatre staff for the management of COVID-19 patients poses a logistical and ethical challenge for the surgical management of patients with locally advanced primary or recurrent rectal cancers requiring complex surgical resections planes.2 Without surgical intervention, the survival of this sub-group of patients with CRC is poor.4 Patients with advanced or recurrent cancer are therefore at risk of collateral damage of the COVID-19 pandemic due to treatment delays contributing to disease progression beyond resectability.5
Many surgeons advocated the continuation of cancer services during the COVID-19 pandemic to prevent delays in diagnosis and treatment.6 However, the resources required to manage these complex patients with locally advanced primary or recurrent CRC, along with a perception of generally poorer outcomes may have contributed to initial COVID-19-related guidance indicating that such extended surgery may be de-prioritised.7 This study evaluates the safety and outcomes of elective colorectal surgery for patients with advanced primary or recurrent CRC during the COVID-19 pandemic, and focuses on the causes and sequalae of delays in the standard cancer pathways for advanced and recurrent CRC.
Methods
Study design
This was a retrospective observational study of adult patients undergoing elective resections for locally advanced or recurrent CRC requiring surgery beyond conventional surgical planes and/or synchronous multivisceral resections at a single tertiary referral centre. Relevant approvals were obtained and data on eligible consecutive patients, between March 2020 and March 2021, were collected and stored in a secured departmental database.
Patients and procedures
All patients over 18 years of age listed for an elective surgical resection for locally advanced, recurrent CRC or multivisceral resection with curative intent were eligible for inclusion. Patients were identified using a prospectively maintained electronic database of all cases discussed at the advanced and recurrent CRC multidisciplinary team (MDT) meeting. Those individuals who had progressed while waiting for surgical intervention, but remained candidates for an R0 resection, based on the last MDT recommendation, were included. Patients who progressed during work-up and had surgery with palliative intent, based on the last MDT recommendation, were excluded.
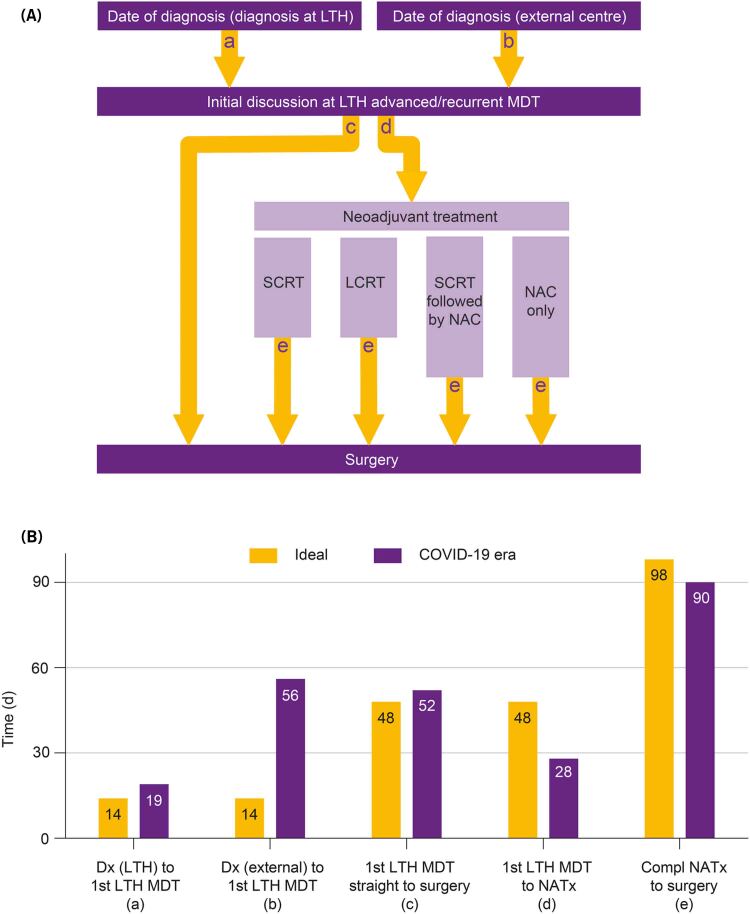
After the first tertiary MDT discussion at Leeds Teaching Hospitals NHS Trust (LTHT), patients were either offered surgery or neoadjuvant treatment. For this study, patients were categorised into one of five groups (Figure 1a): (i) straight to surgery, (ii) short course radiotherapy (SCRT) followed by delayed total mesorectal excision (TME), (iii) long course chemoradiotherapy (LCRT) followed by TME, (iv) SCRT followed by chemotherapy before TME and (v) neoadjuvant chemotherapy (NAC) followed by surgery.
Figure 1 .
Treatment pathways and breakdown of time intervals in the treatment pathway. (A) Schematic representation of different patient pathways depending on nature of neoadjuvant treatment. (a) Time from diagnosis to first Leeds Teaching Hospitals NHS Trust (LTHT) MDT if diagnosis in our centre, (b) time from diagnosis to first LTHT MDT if patient was referred from an external centre, (c) time from LTHT MDT to surgery for straight to surgery, (d) time from LTHT MDT to neoadjuvant treatment and (e) time from end of neoadjuvant treatment to surgery. (B) Comparison of ideal (light grey) and median actual time intervals (dark grey) during COVID-19 pandemic. The ‘62-day target’ was split to 14 days from diagnosis to MDT and 48 days from MDT to first definitive treatment. The 98-day interval includes 10 weeks of delay after CRT and 4 weeks for imaging, repeat MDT discussion and organisation of surgery. LCRT = long course chemoradiotherapy; LTH = Leeds Teaching Hospitals; NAC = neoadjuvant chemotherapy; NATx = neoadjuvant treatment; SCRT = short course radiotherapy.
Preoperative work-up included up-to-date radiological imaging and cardiopulmonary exercise testing when indicated. Once an operation date was confirmed, patients were asked to isolate for a period of 10–14 days (guidance varied during the pandemic) and were required to have a negative COVID-19 polymerase chain reaction (PCR) test, at least three days prior to surgery.
All patients underwent surgery in and were admitted to a ‘cold site’8 with regular postoperative COVID-19 PCR swabs, in addition to routine postoperative management. The surgical team comprised three advanced CRC surgeons, two post CCT fellows and two advanced nurse practitioners in addition to a dedicated theatre team. Back-up from other specialties including critical care, urology, vascular surgery, neurosurgery and plastic surgery was available. A collaborative decision was made by the advanced colorectal malignancy surgical team alongside the institutional management to have a ring-fenced critical care facility in the ‘cold site’, shared with other tertiary surgical services. Exenteration cases were prioritised following a vetting process in weekly MDT meetings attended by surgeons, anaesthetists/critical care physicians and hospital management.
Data variables
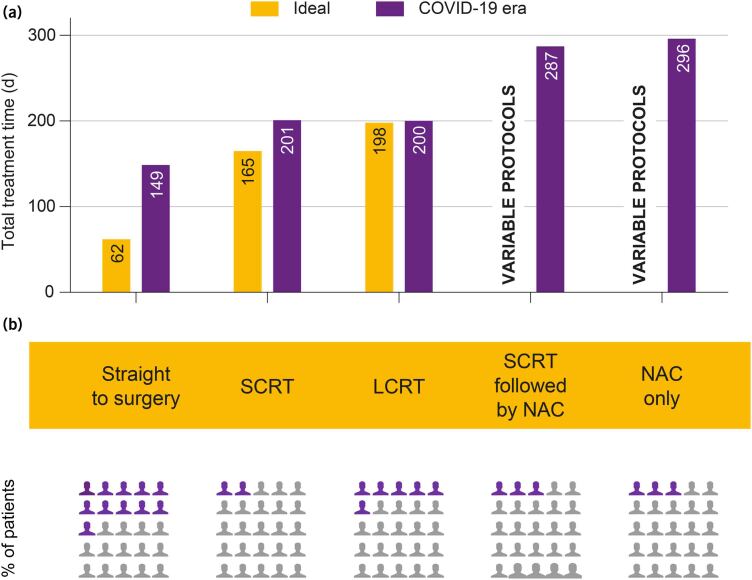
Baseline patient characteristics included age, sex, date of diagnosis, date and route of referral to MDT (internal vs external), and tumour characteristics. Details of the first LTHT MDT decision, ideal treatment time and actual treatment time were calculated for all patients in each group (Figure 2a). The rationale for ideal treatment duration was: (i) straight to surgery=62-day target; (ii) SCRT=165 days, 62 days to first definitive treatment+5 days of radiotherapy (RT)+70 days of delay+28 days to surgery including re-imaging and repeat MDT discussion;9 and (iii) LCRT=198 days, 62 days to first definitive treatment+38 days of RT+70 days of delay+28 days to surgery including re-imaging and repeat MDT discussion.10 The ideal duration of treatment pathways for patients having NAC followed by surgery, and SCRT followed by chemotherapy before TME were not calculated as these were non-standardised protocols.
Figure 2 .
Total treatment time and proportions of patients undergoing each treatment strategy. (a) Ideal total treatment time (light grey) and median values of the actual total treatment time during the COVID-19 pandemic (dark grey), calculated from date of diagnosis to date of surgery, for the different treatment pathways. Rationale for ideal time limits: straight to surgery=62-day target. SCRT: 165 days=62 days to treatment+5 days of RT+70 days of delay+28 days to surgery (reimaging, repeat MDT). LCRT: 198 days=62 days to treatment+38 days of RT+70 days of delay+28 days to surgery (reimaging, repeat MDT). No limits are stated for the latter two categories because of variations in applied protocols. (b) Proportion of patients undergoing each treatment strategy. LCRT = long course chemoradiotherapy; NAC = neoadjuvant chemotherapy; SCRT = short course radiotherapy.
Based on serial preoperative imaging, intraoperative findings of other organ involvement and postoperative histopathology, tumour growth was broadly classified as remained stable, regressed or progressed. Operative variables and postoperative outcomes collected included details of procedure (Supplementary Table S1), change in COVID-19 PCR status while an inpatient, COVID-19-related complications, Clavien–Dindo complications11 and in-hospital mortality.
Outcomes
The primary outcome measure was COVID-19-related complication rate following surgery for locally advanced and recurrent CRC. Secondary outcome measures were postoperative complications, 30-day mortality, delays in standard treatment protocols and tumour growth (stable, progression, regression) while waiting for surgical intervention.
Statistical analysis
The study was conducted according to guidelines set by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies.12 Discrete variables were expressed as counts and percentages, continuous variables as median with interquartile range (IQR). A chi-square test was used to calculate the difference in proportion of patients who developed disease progression in straight to surgery versus neoadjuvant treatment followed by surgery groups. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 24.0 for Windows (SPSS, Chicago, IL, USA).
Results
Patients and disease characteristics
Forty-nine patients were included, with median age of 66 years (IQR 54–73), the majority were male (67%) and most were external tertiary referrals (69%). Four patients were excluded; one patient was listed but died due to COVID-19 infection prior to admission while waiting for surgery, one declined surgery due to fears of hospital acquired COVID-19-related complications and two patients developed disease progression based on radiological imaging rendering them unresectable while being worked up for surgery. Table 1 summarises patient demographics, tumour characteristics, type of surgery and postoperative course of the study population. Approximately half of the patients had locally advanced CRC; of the remainder, 17 patients had surgery for recurrences and six for re-recurrences. Median time to recurrence was 24 months (IQR 12–56). There was one case each of locally advanced sigmoid cancer and recurrent anal cancer, three cases of advanced caecal malignancies (one involving bladder, one invading external iliac artery and one with synchronous liver metastasis) and two cases of abdominal recurrences from colon cancer. All others were primary, locally advanced, rectal cancers or pelvic recurrences from rectal cancer. Median maximum dimension of primary tumours was 6cm (IQR 5–9.75) and there was evidence of extramural venous invasion in 72.2% of histopathology specimens. Surgical procedures were classified as bTME or grade A–D as defined in Table 1. A full list of operative procedures is included in Supplementary Table S1. Eighty per cent (39/49) of patients had a planned admission to critical care postoperatively.
Table 1 .
Patient demographics, tumour characteristics, type of surgery and postoperative course
| Variable | Data |
|---|---|
| Patient characteristics | |
| Age, median (IQR), years | 66 (54–73) |
| Sex, n (%) | |
| Male | 33 (67) |
| Female | 16 (33) |
| Anaerobic threshold, median (IQR), ml/kg/min | 12.8 (10.4–13.7) |
| Referral pathways | |
| Internal, n (%) | 15 (31) |
| External, n (%) | 34 (69) |
| Tumour characteristics | |
| Maximum diameter, median (IQR), cm | 6 (5–9.75) |
| Primary, n (%) | 26 (53) |
| Recurrence, n (%) | 17 (35) |
| Re-recurrence, n (%) | 6 (12) |
| Operation category, n (%) | |
| Beyond TME | 6 (12) |
| Grade A exenteration | 12 (24) |
| Grade B exenteration | 19 (39) |
| Grade C exenteration | 2 (4) |
| Other curative | 7 (14) |
| Other palliative | 3 (6) |
| Postoperative course | |
| COVID-19-related complications | 0 |
| Change in COVID-19 status as inpatient | 0 |
| Claviden–Dindo complications, n (%) | |
| 0 | 11 (22) |
| 1 | 10 (2) |
| 2 | 14 (28) |
| 3 | 9 (18) |
| 4 | 2 (4) |
| 5 | 3 (6) |
Beyond TME=any extra-anatomical resection beyond the TME plane up to what constitutes an exenteration; exenteration=multivisceral resection of the rectum or recurrence with en bloc resection of >50% of two or more unrelated organs and/or major pelvic neurovascular sidewall structures or bone; Grade A=total pelvic exenteration (complete resection of all soft tissue remaining in the pelvis excluding side wall); Grade B=any visceral resection with distal sacrectomy (distal to S2/3 junction) and/or pelvic side wall including lymphadenectomy; Grade C=any visceral resection with high sacrectomy (at or above S2/3 junction) and/or major vascular reconstruction; IQR=interquartile range.
Outcomes after surgery
No patient had a positive COVID-19 PCR test or thorax computed tomography scan showing radiological features of COVID-19 pneumonia while an inpatient at LTHT and hence there were no COVID-19 related complications during the study period (March 2020 to March 2021). There was one 30-day mortality; this patient had undiagnosed preoperative peripheral arterial disease and developed critical bilateral lower limb ischaemia in the postoperative period that was not salvageable. Three patients died during hospital admission (postoperative days 3, 34 and 104). The other two patients had a protracted postoperative course due to chest sepsis, multiorgan failure and were ultimately palliated following repeated critical care admissions. Table 1 summarises all postoperative complications.
Treatment pathways and tumour progression
Following first discussion at the advanced CRC MDT at LTHT, patients were either recommended to have surgery n=22 (45%) or neoadjuvant treatment prior to surgery in the form of SCRT n=4 (8%), LCRT n=13 (27%), SCRT followed by chemotherapy n=5 (10%) or NAC=5 (10%). Figure 1a describes the different patient pathways and Figure 1b compares ideal and actual median time intervals for defined processes in patient management during COVID-19 pandemic, highlighting that the maximum delay was in the time to referral from external centres. Figure 2a compares the ideal and actual median time intervals from diagnosis to surgery for patients in the straight to surgery or each neoadjuvant treatment group. The maximum delay was noted in the straight to surgery group during the COVID-19 pandemic.
In the straight to surgery group (n=22), there was evidence of disease progression in 9 patients and stable disease in 13. In the SCRT group, one of four patients progressed. There were no cases of regression in straight to surgery or SCRT groups. Tumour characteristics in the LCRT group (n=13) were progression in two, stable disease in eight and regression in three. We identified no cases of disease progression in the SCRT followed by NAC and NAC only groups, with proportion of tumour regression identified in 1/5 and 3/5 patients, respectively. There was a statistically significant difference (p=0.015839) in cases of tumour progression between straight to surgery (9/22) vs neoadjuvant treatment followed by surgery (3/27). Seven of the 27 patients in the neoadjuvant treatment group (26%) showed evidence of regression, with three cases of complete pathological response based on post-operative histology.
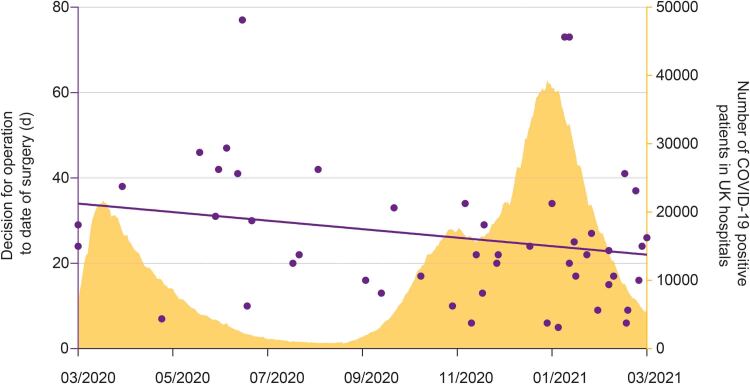
Unit’s productivity
Figure 3 shows the waiting times for surgery once a decision to operate was made (each dot representing one patient) in the context of the two waves of COVID-19 pandemic in the UK. Waiting times were reduced and more patients underwent surgery during the second wave despite the higher numbers of hospitalisations due to COVID-19, nationally.
Figure 3 .
Trends of waiting time for surgery during the COVID-19 pandemic. Each dot represents one patient. x axis=date of surgery; left y axis=time interval from decision for operation to date of surgery; right y axis=evolution of the COVID-19 pandemic, indicated by number of COVID-19-positive patients in UK hospitals. The waiting time was reduced, and more patients were operated during the second wave despite the higher numbers of hospitalisations due to COVID-19.
Discussion
To the best of our knowledge, this is the first study reporting on the postoperative outcomes of patients undergoing complex abdomino/pelvic surgery for locally advanced and recurrent CRC during the COVID-19 pandemic. Following the initial surge of COVID-19 cases worldwide in March 2020, several opinion pieces were published highlighting the logistical challenges, ethical dilemmas and practical guidelines for the continuation of surgery for CRC.13,14 Subsequent publications have looked at the outcomes from elective CRC surgery during the COVID-19 pandemic15–17 but none of these have focused on the specific group of patients with advanced or recurrent CRC whose management is more demanding from a technical perspective as well as resource utilisation.
Our data suggest that it is safe and feasible to continue with complex surgical resections for patients with locally advanced or recurrent CRC without significant COVID-19-related complications. These findings are in keeping with reported literature for conventional CRC surgery.17,18 Untreated, CRC progresses over time, and delay in care has been associated with poorer outcomes. Guidelines recommend a short interval from symptoms to diagnosis with a benchmark of initiation of the first treatment within six weeks from diagnosis for 90% of patients.19 We had to balance the risk of poorer cancer-specific survival associated with any prolonged delay in treatment, against the risk of deterioration and death from contracting COVID-19.20 With meticulous preoperative planning, strict adherence to standard operating procedures to prevent nosocomial infections and the availability of a dedicated ‘cold’ site,21 including critical care, we have been able to avoid the detrimental effects of postoperative COVID-19 infections. These have been reported up to 51.2% for pulmonary complications and a 30-day mortality rate of up to 23.8% in published series.22
CRCs are considered to have intermediate rates of proliferation with an estimated median doubling time of 211 days.23 In our cohort, a significant proportion (41%) of patients who could only have surgery as definitive treatment and had delays in surgical intervention were noted to have disease progression, three of whom became inoperable. This is an important observation based on objective data highlighting the importance of access to surgery for patients with advanced and recurrent CRC. We found that in our series, a delay in referral from external centres (likely COVID-related) accounted for the interruption in standard pathway in most cases, underscoring the importance of more efficient and streamlined processes for referrals, especially during a pandemic. Because of COVID-19-related uncertainties encountered by healthcare systems worldwide, such delays have been a universal phenomenon. Authors of the DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19), an international survey, found that CRC surgery was delayed in 58.3% (434/745) of departments. For 90% of respondents, the delay was five to eight weeks beyond normal waiting time, exceeding eight weeks for the remaining 10%.24 Similar delays have been reported in a UK-based national evaluation25 as well as the PelvEx Collaborative Group who reported on 50 exenterative units worldwide in May 2020 and identified that fewer than half (42%) units operated at usual capacity, 44% reported varying reductions in referrals and caseload and 14% stopped operating.14 We encountered delays, particularly in the pathway of patients who required surgery as their first definitive treatment. However, the main reason for delayed treatment was disruption in referral pathway from external referral centres. In cases in which neoadjuvant treatment was required prior to surgery, we were able to adhere to treatment targets in most cases as there was sufficient time to organise theatre access from a logistical perspective.
The ability of preoperative radiotherapy to prevent locoregional recurrence for locally advanced rectal cancer has been well established for SCRT26 as well as LCCRT.27 A longer interval from radiation to surgery results in greater tumour downstaging for both SCRT9 and LCCRT.10 The recently published results of the RAPIDO study, have shown that SCRT followed by chemotherapy before TME in locally advanced primary rectal cancer also has favourable oncological outcomes.28 In our series, few patients receiving neoadjuvant therapy prior to surgery progressed. Interestingly, approximately a quarter of patients receiving neoadjuvant therapy prior to surgery showed evidence of tumour regression. Based on these findings it may be reasonable to consider giving chemotherapy to patients who have received SCRT and encounter delays in definitive surgery due to lack of theatre capacity.
This study has several limitations. It is a retrospective, single-institution analysis with the potential for selection bias in patients in different treatment groups. The study does not account for the disease progression in all patients referred to our MDT because there was a proportion who were deemed inoperable at the time of first MDT discussion at LTHT. It is also worth noting that the preoperative isolation protocols and inpatient COVID-19 testing regimens have been variable during the study period because of changes in institutional and national guidance. Having long-term oncological data and a control group with time scales of treatment pathways in the pre-COVID era would have been more informative.
Our study highlights the importance of accessibility to ‘cold sites’, regional coordination, triage of patients based on clinical urgency and proactive surgical leadership, which is pivotal in these uncertain times to meet the ethical, clinical and logistical challenges of oncological surgery. It is clear that due to diagnostic delays during the first peak of pandemic, there will be a higher proportion of locally advanced CRC diagnoses in the future. Based on population data sets in England, Morris et al predict that due to the disruption in CRC referral pathways and reduction in diagnostics between April and October 2020, some 3,500 fewer patients were diagnosed and treated for CRC than in 2019.25 These are likely to present as emergencies or advanced malignancies. It is therefore imperative that even in such unprecedented times, we undertake workforce planning so that any current or future pandemic does not delay access by advanced CRC patients to appropriate care. Cancer treatments are time sensitive and disruption to treatment pathways leading to delays results in collateral mortality. These factors need to be considered in the broader administrative plan.
This study suggests that it is possible to mitigate the risks of COVID-19-related complications in patients undergoing complex surgery for locally advanced and recurrent CRC. Delay in surgical intervention is associated with tumour progression, particularly in patients who may not have neoadjuvant therapy. Efforts should be made to prioritise resources for patients requiring time-sensitive surgery for advanced and recurrent CRC.
References
- 1.Kutikov A, Weinberg DS, Edelman MJet al. A War on two fronts: cancer care in the time of COVID-19. Ann Intern Med 2020; 172: 756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyond TMEC. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 2013; 100: E1–E33. [DOI] [PubMed] [Google Scholar]
- 3.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007; 14: 447–454. [DOI] [PubMed] [Google Scholar]
- 4.PelvEx C. Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg 2019; 269: 315–321. [DOI] [PubMed] [Google Scholar]
- 5.Tan KK, Moran BJ, Solomon MJ. Avoiding collateral mortality in a pandemic - time to change our mindset in surgical oncology. Nat Rev Clin Oncol 2020; 17: 383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downs JS, Wilkinson MJ, Gyorki DE, Speakman D. Providing cancer surgery in the COVID-19 crisis. Br J Surg 2020; 107: e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Leary MP, Choong KC, Thornblade LWet al. Management considerations for the surgical treatment of colorectal cancer during the global Covid-19 pandemic. Ann Surg 2020; 272: e98–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eardley I. A New normal?: The COVID-19 pandemic has heralded different ways of working, triage of workload, collaborative research and cold-site surgery. BJU Int 2020; 126: 215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaytan-Saglam E, Balik E, Saglam Set al. Delayed versus immediate surgery following short-course neoadjuvant radiotherapy in resectable (T3N0/N+) rectal cancer. J Cancer Res Clin Oncol 2017; 143: 1597–1603. [DOI] [PubMed] [Google Scholar]
- 10.Hoendervangers S, Burbach JPM, Lacle MMet al. Pathological complete response following different neoadjuvant treatment strategies for locally advanced rectal cancer: A systematic review and meta-analysis. Ann Surg Oncol 2020; 27: 4319–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger Met al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 13.Wexner SD, Cortes-Guiral D, Gilshtein Het al. COVID-19: impact on colorectal surgery. Colorectal Dis 2020; 22: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PelvEx C. The impact of the COVID-19 pandemic on the management of locally advanced primary/recurrent rectal cancer. Br J Surg 2020; 107: e547–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborative CO. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis 2020; 23: 732–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K, Sheng Y, Huang Cet al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020; 21: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrano FM, Foppa C, Carvello M, Spinelli A. With adequate precautions colorectal cancer surgery can be safely continued during COVID-19 pandemic. Br J Surg 2020; 107: e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobrado LF, Nahas CSR, Marques CFSet al. Is it safe to perform elective colorectal surgical procedures during the COVID-19 pandemic? A single institution experience with 103 patients. Clinics (Sao Paulo) 2021; 76: e2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemming JA, Nanji S, Wei Xet al. Association between the time to surgery and survival among patients with colon cancer: A population-based study. Eur J Surg Oncol 2017; 43: 1447–1455. [DOI] [PubMed] [Google Scholar]
- 20.Lei S, Jiang F, Su Wet al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine 2020; 21: 100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyle JM, Kuryba A, Blake HAet al. The impact of the first peak of the COVID-19 pandemic on colorectal cancer services in England and Wales: A national survey. Colorectal Dis 2021; 23: 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collaborative CO. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020; 396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke JR, Brown P, Quyn Aet al. Tumour growth rate of carcinoma of the colon and rectum: retrospective cohort study. BJS Open 2020; 4: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santoro GA, Grossi U, Murad-Regadas Set al. DElayed COloRectal cancer care during COVID-19 pandemic (DECOR-19): global perspective from an international survey. Surgery 2021; 169: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris EJA, Goldacre R, Spata Eet al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol 2021; 6: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebag-Montefiore D, Stephens RJ, Steele Ret al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009; 373: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer R, Becker H, Hohenberger Wet al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 28.Bahadoer RR, Dijkstra EA, van Etten Bet al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22: 29–42. [DOI] [PubMed] [Google Scholar]