Abstract
Pseudomonas aeruginosa secretes a wide range of hydrolytic enzymes into the external medium by the Xcp secretion machinery. To better understand the role played by envelope constituents in the functioning of this type II secretory system, we have studied the influence of lipopolysaccharide (LPS) on the secretion of two extracellular enzymes, the elastase LasB and the lipase LipA. Strains with defective LPS decreased production of LasB and altered the secretion processes of both LasB and LipA without any apparent effect on the composition of the Xcp machinery. The PAO1algC strain, defective in the outer core of LPS, was leaky, as shown by the extracellular release of the periplasmic β-lactamase. Generation of an xcpR mutation in this mutant led only to a partial accumulation of LasB within the cells, indicating that in strain PAO1algC with a functional xcpR gene, LasB was released in the extracellular medium partly by leakage and partly by secretion. The pool of LasB released into the medium by leakage was not recovered in an active form, while extracellular LasB was active when secreted via the secretory machinery. Further analysis revealed that the presence of a functional Xcp machinery is strictly required for the activation process of LasB. Our results provide evidence that the Xcp system is not fully functional when the LPS structure of P. aeruginosa is altered.
Gram-negative bacteria secrete a wide range of hydrolytic enzymes, toxins, and virulence factors in the extracellular medium by using at least three major secretory pathways. The type I pathway is a one-step process (bypassing the periplasm) that involves a machinery of three components, including an ABC protein (20). The type III, or contact site-dependent, pathway is a one-step process widely used by animal pathogens and involves a complex machinery with about 20 envelope proteins (32). Proteins using the type II, or general secretion pathway (GSP), system are secreted by a two-step process involving a transient periplasmic intermediate. They first cross the inner membrane via the Sec machinery (12), before interacting in the periplasm with the secretion system, which carries out their translocation through the outer membrane (14, 39, 42). In Pseudomonas aeruginosa, this system consists of at least 12 proteins defined as XcpA and XcpP to -Z. Most of these proteins are localized in the inner membrane (XcpA, XcpP, and XcpSXYZ) or associated with it (XcpR). XcpR is thought to play an important role in the energization of the secretory process, since it possesses an ATP binding domain (14). XcpT to -W are recovered in both inner and outer membranes (4). Only one protein, the secretin XcpQ, is localized in the outer membrane under a multimeric organization and might function as a specialized pore (5, 14). Some of the Xcp proteins are homologous to proteins involved in pilin biogenesis. XcpT to -X have been shown to present homologies with PilA, the subunit of the type IV pilin in P. aeruginosa, and have been defined as pseudopilins. Maturation of these pseudopilins is catalyzed by the peptidase XcpA (or PilD), which is also involved in the processing of the precursor form of PilA (4, 35). Other proteins of the Xcp machinery, XcpR and XcpQ, have also been found to present homologies with proteins involved in the Pil system, respectively, PilB and PilQ. These homologies suggest that the Xcp machinery could be organized as a complex structure comparable to that of the type IV pilus necessary for pilus assembly (14). However, Xcp protein complexes spanning the periplasm and the outer membrane have not yet been demonstrated.
In P. aeruginosa and other organisms possessing type II secretion machineries, much attention has been paid to the mechanisms involved in the assembly of the GSP system, the interaction between secreted proteins and the secretory apparatus, and the function of the GSP components (14). In contrast, little attention has been devoted to the influence of envelope constituents on the functioning efficiency of the GSP secretory systems. Due to the localization of its components and its postulated structure, it seems likely that the Xcp machinery interacts with other envelope components, such as peptidoglycan or lipopolysaccharide (LPS). This proposed influence of compounds unrelated to the Xcp machinery on the secretion process is suggested by the results obtained by Kagami et al. (23), who reported xcpT(Ts) suppressors localized outside the xcp operons.
LPS is one of the virulence factors produced by P. aeruginosa. This bacterium is able to coexpress two LPS types, which are known as the A and B bands (30, 33, 40). Type A LPS is an antigenically conserved molecule with an O polysaccharide region composed of trisaccharide repeating units of d-rhamnose (2). Type B LPS is the serotype-specific LPS that divides P. aeruginosa into 20 distinct serotypes that are dependent on the composition of the O antigen (33, 34). It is likely that functioning of the Xcp machinery is tightly related to envelope stability, namely the stability of the outer membrane. It is known that LPS plays an important role in outer membrane stabilization. Indeed, enhanced sensitivity to antibiotics, detergents, and EDTA and leakage of periplasmic enzymes have been observed in deep rough mutants of Escherichia coli and Salmonella enterica serovar Typhimurium (19). In P. aeruginosa, the permeation of steroids has been shown to be increased up to 5.5-fold when the outer membrane contained defective LPS (38). Furthermore, both in vivo and in vitro studies have demonstrated that LPS plays a role in protein assembly (10, 28, 45). More recently, the stability of OmpF trimers has also been shown to be affected by the ratio of phospholipids to LPS in E. coli (25). In other studies, Wandersman and Létoffé showed that secretion of E. coli hemolysin and proteases from Erwinia chrysanthemi by the type I pathway is defective in E. coli mutants affected in LPS biosynthesis (47). These defects are caused by outer membrane alterations that interfere with the formation of functional secretion machinery complexes. Moreover, translocation of TcpA, the pilin subunit of Vibrio cholerae, has been shown to be affected in LPS mutants from V. cholerae defective in O antigen (21). These observations that emphasize the role of LPS in envelope organization and stability suggest that protein secretion in gram-negative bacteria could be affected by LPS alterations. In this study, we have investigated the influence of this outer membrane component on the functioning of the type II secretion system of P. aeruginosa. Our results provide evidence that alteration of the LPS structure causes an important perturbation of the envelope organization. These modifications lead to leakage of the periplasm-located β-lactamase and alteration of the secretion process of the elastase (LasB) and the lipase (LipA), two extracellular proteins of P. aeruginosa secreted by the GSP secretory system. We also report that the LasB activation process is strictly dependent on the presence of a functional Xcp machinery, thereby giving new insights into the interaction between secreted proteins and the GSP apparatus.
MATERIALS AND METHODS
Bacterial strains, plasmids, and plasmid transfer.
The bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa and E. coli strains were grown respectively in tryptone soy broth (TSB) medium (Difco laboratories) or in Luria-Bertani medium at 37°C under agitation. Absorbance was measured at an optical density of 600 nm (OD600). Plasmids were transferred to P. aeruginosa strains by electroporation essentially as described by Smith and Iglewski (46) with minor modifications. TSB medium was used instead of SOC buffer immediately after electric shock, because SOC caused lysis of electroporated LPS mutant cells. Transformants were isolated on Pseudomonas isolation agar (PIA) medium containing glycerol and the required antibiotic (300 μg of carbenicillin per ml or 1,000 μg of kanamycin per ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAO1 | Prototroph, chl-2 | |
| AK1012 | Rough LPS derivative of PAO1, core deficient, A− B− | 21 |
| PAO1algC | algC mutant derivative of PAO1 obtained by Tc cartridge insertion, A− B− | 8 |
| PAO1/::xcpR′ | Obtained by insertion of pGMR′ suicide vector in xcpR | This study |
| PAO1algC/::xcpR′ | idem as PAO1/::xcpR′ | This study |
| E. coli TG1 | supE hsd Δ thi Δ(lac-proAB) F′ (traD36 proAB+ lacIqlacZΔM15) | |
| Plasmids | ||
| pAX24 | Tcr, IncP1 oriT pLAFR3 with xcpP to −Z on 20-kb DNA insert | 13 |
| pUC19 | Apr, ColE1, lacI, φ80dlacZ | 48 |
| pUCR | xcpR gene cloned into the SmaI site of pUC19 | This study |
| pMMB67EH | Apr, RSF 1010 (IncQ), tac promoter | 15 |
| pUCBM20 | Apr, ColE1 | Boehringer |
| pGMR′ | Apr, 0.5-kb SalI internal fragment of xcpR from pUCR cloned into the SalI site of pUCBM20 | This study |
| pUCP18 | Apr, ColE1, broad-host-range plasmid | 44 |
| pML27 | Apr, lasB in pMMB67EH | 11 |
| pLPS 188 | Apr, algC gene in pUCP18 | 17 |
| pJRD215 | Kmr, RSF 1010, wide-host-range cosmid-cloning plasmid | 9 |
| p24 | Kmr, P. alcaligenes lipAB genes cloned into pJRD215 | Similar to pJRDlipAB (16) |
| pTS400 | Apr, translational lasB-lacZ fusion in pSW205 | 37 |
Generation of xcpR mutation in P. aeruginosa.
xcpR mutants were generated by insertion in the host chromosome of a suicide vector (pGMR′) containing an internal fragment from xcpR according to the strategy defined by Akrim et al. (1). A 0.5-kb SalI fragment of xcpR corresponding to the central region of the gene was subcloned into the SalI site of pUCBM20, giving pGMR′. The suicide plasmid was introduced into parental and LPS mutant cells by electroporation, and recombinants were isolated on PIA plates containing carbenicillin. Carbenicillin-resistant colonies were then tested for proteolytic activity on protease assay plates (tryptic soy agar [TSA]) containing skim milk (Difco Laboratories), and those giving no hydrolytic halo were selected for further studies. A secretion-defective clone (PAO1/::xcpR′) was isolated and complemented by expression in trans of the entire xcp gene cluster cloned into pLAFR3 (giving plasmid pAX24) (13). Insertion of the suicide vector pGMR′ in the xcpR gene was checked by PCR analysis with the ORG5 (5′-ATGATGACAGCCCATTACCC-3′) and ORG6 (5′-AAGGCGGCCATTATTCTTCCC-3′) primers (corresponding, respectively, to the 5′ and 3′ ends of the xcpR gene), M13 universal (−40) and reverse primers, and genomic DNA from strain PAO1/::xcpR′ as a template. DNA from the wild-type strain PAO1 was used as a control.
Immunoblotting analysis.
Cells were harvested at the transition between the late-exponential and early-stationary growth phases (OD600 of between 2 and 4). Supernatants were precipitated with 10% (wt/vol) trichloroacetic acid (final concentration), and precipitates were washed twice with 90% (vol/vol) acetone. Cellular and extracellular proteins were solubilized in the same volume of sodium dodecyl sulfate-sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described by Laemmli (27). Each lane containing cellular extracts was loaded with 0.1 OD600 equivalent unit (approximately 100 μg of proteins), and the same volume of the corresponding extracellular extracts was loaded on the gel in order to compare the amounts of cellular and extracellular LasB and LipA protein. Proteins were transferred to nitrocellulose membrane (BA85; Schleicher & Schuell) by using a semidry blotter apparatus (Bio-Rad) and reacted with appropriately diluted polyclonal or monoclonal antiserum. Either peroxidase-conjugated goat anti-rabbit, peroxidase-conjugated goat anti-mouse, or alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Jackson) was used as the secondary antibody. Proteins were revealed by chemiluminescence with peroxidase-conjugated secondary antibodies (Pierce) or by colorimetric reaction with a 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP-NBT) kit from Eurogentec when alkaline phosphatase-conjugated secondary antibodies were used. LasB antibodies were raised in rabbit against purified P. aeruginosa elastase obtained from Nagase Company (Osaka, Japan). β-lactamase antibodies were obtained from 5 Prime-3 Prime, Inc. XcpR antibodies were obtained as described by Ball et al. (3) by using a cro′-lacI′-lacZ′-′xcpR fusion. XcpY antibodies were raised in rabbit against a purified glutathione S-transferase (GST)–XcpY fusion protein (6). XcpT antibodies (raised in rabbit) were a gift from D. Nunn (36), and XcpQ antibodies (raised in rabbit) were obtained from W. Bitter (5). Monoclonal LipA antibodies raised in mouse against Pseudomonas alcaligenes LipA were obtained from G. Gerritse.
Protein quantification.
Protein concentration was estimated by the method described by Schaecterle and Pollach (43) or with the Bio-Rad protein assay.
lasB expression.
Expression of lasB was studied during growth by measuring the β-galactosidase activity of a translational lasB-lacZ fusion according to the method described by Latifi et al. (31). One enzymatic unit is defined as the amount of enzyme which liberated 1 μmol of p-nitrophenol per min. Results were standardized to 1 OD600 unit.
Cellular and extracellular extracts.
Cells (4-ml culture) were harvested (7,000 × g, 10 min, 4°C) at the transition between the late-exponential and early-stationary growth phases (OD600 of 2 to 4). Culture supernatants containing extracellular proteins were stored at 4°C for further analysis. Cell pellets were resuspended in the same volume of 10 mM Tris-HCl (pH 8), and cells were disrupted by sonication in ice with a Branson sonifier (three periods of 15 s at 45-s intervals). Unbroken cells and cellular debris were removed by centrifugation (3,000 × g, 10 min, 4°C), and the supernatant containing cellular proteins was used for activity tests. In each experiment, the different cellular extracts were adjusted to an equivalent protein concentration. The same dilution factor was applied to extracellular extracts in order to compare cellular and extracellular activities.
Lipase activity assays.
Lipase activity from cellular extracts and from culture medium was assayed according to the method of Kordel et al. (26) with p-nitrophenylpalmitate (Sigma) as the substrate. One unit of lipase activity was defined as the amount of enzyme that liberated 1 μmol of p-nitrophenol per min. Results were standardized to one OD600 unit.
Proteolytic activity assay on plates.
Cellular and extracellular extracts obtained as described above were loaded (100 μl) into wells prepared in TSA plates containing skim milk and 200 μg of tetracycline per ml (in order to stop growth of residual cells), and plates were incubated overnight at 37°C. Elastase LasB is the most abundant protease produced by P. aeruginosa. Since other proteolytic activities produced by P. aeruginosa were barely detected on skim milk agar plates under the experimental conditions used, this test was taken as indicative of elastase activity. The elastase assay with elastin Congo red as a substrate (41) was not used in this study, because it was found to cause an artifactual activation of LasB under the experimental conditions used.
RESULTS AND DISCUSSION
Effect of defective LPS structure on LasB secretion.
The elastase LasB is one of the exoenzymes produced in large quantities by P. aeruginosa and efficiently secreted into the extracellular medium. Secretion of this enzyme was studied by immunoblotting in the parental strain, PAO1, and two derived LPS mutants, AK1012 and PAO1algC (Table 1). These two mutants are characterized by the same LPS alteration due to the loss of phosphoglucomutase activity (8, 22). As shown in Fig. 1A, LasB was recovered in the extracellular medium of strain PAO1 and was barely detected in cells. In the two mutants, LasB was also found to be extracellular, but it was produced in smaller amounts than in the parental strain (Fig. 1A). Interestingly, the low level of extracellular LasB secreted by the LPS mutants was not associated with a corresponding intracellular accumulation of the exoprotein. When LasB was overexpressed, the secretory machinery was saturated and the enzyme accumulated within the wild-type cells. Such an intracellular accumulation was not observed in the PAO1algC mutant (Fig. 1B).
FIG. 1.
Elastase secretion in LPS mutants of P. aeruginosa. (A) LasB was revealed on an immunoblot by chemiluminescence as indicated in Materials and Methods. Only the relevant part of the immunoblot is shown. C, cellular fraction; E, extracellular fraction. Samples were withdrawn at OD600s of 2.7 (PAO1), 2.9 (AK1012), and 2.7 (PAO1algC). (B) Effect of saturation of the Xcp machinery on LasB secretion. The lasB gene expressed from pML27 was overproduced after induction with 1 mM isopropyl-β-d-thiogalactopyranoside. Samples were withdrawn at OD600s of 2.6, 2.6, 2.6, and 2.8, respectively, for the PAO1(pMMB), PAO1(pML27), PAO1algC(pMMB), and PAO1algC(pML27) strains. Immunoblots were revealed by detection of activity of alkaline phosphatase conjugated to secondary antibody. The position of LasB (33 kDa) is indicated by an arrow. pMMB = pMMB67EH.
Effect of alteration of the LPS structure on lasB expression.
The lack of intracellular accumulation of LasB in LPS mutants when LasB was overexpressed suggested that this protein could not be intracellularly observed, either because it was unstable and degraded inside the cells, or because its expression was lowered as a result of a feedback control resulting from a defect in elastase secretion. Both possibilities could also explain the small amount of external LasB observed in LPS mutants (Fig. 1). In order to test the last hypothesis, expression of the lasB gene was studied by assaying β-galactosidase activity from a translational lasB-lacZ fusion expressed in the wild-type strain PAO1 and the LPS mutant PAO1algC. As shown in Fig. 2, expression of the fusion was cell density dependent in strain PAO1 and was increased at the transition between the exponential and stationary growth phases, after about 5 h of growth (Fig. 2A). This result was expected, since it was previously shown that lasB expression is under the control of quorum sensing (37). In contrast, lasB was expressed at a lower level in the algC mutant (Fig. 2B). Therefore, our results explain (at least in part) the decreased amount of LasB detected in LPS mutants and show that alteration of the LPS structure caused a decreased expression of lasB in P. aeruginosa.
FIG. 2.
Effect of alteration of the LPS structure on elastase expression in P. aeruginosa. LasB expression was studied by assaying the β-galactosidase activity of a translational lasB-lacZ fusion. Samples corresponding to equivalent amounts of proteins were withdrawn at intervals and treated as described by Latifi et al. (31). β-Galactosidase activity in PAO1(pTS400) (□) and PAO1algC(pTS400) (▵) strains is expressed in units per milliliter of culture and standardized to 1 OD600 unit.
Lam et al. (29) reported that the thickness of the outer leaflet of the outer membrane of the rough strain, AK1012, is decreased compared to that of the parent strain, PAO1. Such a modification of the envelope architecture could also occur in strain PAO1algC, decreasing membrane stability and weakening interactions between Xcp components, which are viewed as a multiprotein complex spanning the periplasm and outer membrane. These changes might lead to a decreased efficiency of the secretion process. The same structural modifications could also alter interactions between the propeptide-LasB protein complex and elements of the secretory apparatus. It could be speculated that a decreased efficiency of the machinery slows down the secretion process, with, as a consequence, a feedback inhibition effect on LasB synthesis. In other respects, it could also be proposed that alteration of lasB expression is the consequence of a deficient quorum-sensing response in LPS mutants. However, we do not yet have any result supporting such a hypothesis. Further studies are now required to demonstrate precisely the cause of the alteration of lasB expression in strain PAO1algC.
Lesion of the LPS also affects lipase secretion.
Secretion of another exoenzyme, lipase LipA, by the type II secretory system in P. aeruginosa was also investigated with the LPS mutant. As a first approach, the secretion of LipA was determined by assaying lipase activity in extracellular and cellular fractions obtained from strain PAO1 and the rough mutant PAO1algC. As shown in Table 2, although only slight differences were observed between the wild-type and mutant strains, lipase was found associated with cells at a higher level in strain PAO1algC than in strain PAO1. Since no reactive antibody directed against P. aeruginosa LipA was available, the presence of inactive enzyme in the fractions tested could not be excluded. Therefore, a different experimental approach was used. The lipase gene of P. alcaligenes, an organism phylogenetically close to P. aeruginosa, was expressed from plasmid p24 in strain PAO1 and in the algC mutant. Analysis by immunoblotting with monoclonal antibodies directed to P. alcaligenes lipase showed that this heterologous enzyme is efficiently produced and secreted into the extracellular medium by strain PAO1 (Fig. 3). In contrast, larger amounts of lipase were found to be cell associated in strain PAO1algC. These results are in good agreement with those obtained by assay of lipase activity (Table 2). Because the total amounts of lipase produced by both strains were similar, it was concluded that the lipase secretion process was affected by LPS mutation. Thus, the decreased efficiency of the secretion process in an LPS-defective mutant is not restricted to LasB but could be a more general phenomenon.
TABLE 2.
Influence of LPS alteration on lipase secretion in P. aeruginosaa
| Sample type | Activity in mU/ml (%)b
|
|||
|---|---|---|---|---|
| PAO1 | PAO1algC | PAO1(p24) | PAO1algC(p24) | |
| Cells | 9.5 ± 3.3 (53.4) | 18.5 ± 4.2 (71.9) | 99.2 (21.5) | 293.1 (73.9) |
| Supernatant | 8.3 ± 2.7 (46.6) | 7.2 ± 2.5 (28.1) | 362.6 (78.5) | 103.4 (26.1) |
| Total | 17.8 ± 5.4 (100) | 25.7 ± 2 (100) | 461.8 (100) | 396.5 (100) |
Cells were collected at the transition between the late-exponential and early-stationary growth phases, and lipase activity was assayed both in the supernatant of the growth culture and in the cell extracts obtained from sonicated cells as described in Materials and Methods.
Results are mean values (± standard error) of three independent experiments, respectively, for PAO1 and PAO1algC strains. PAO1(p24) and PAO1algC(p24) extracts were obtained from the same cultures as those used to analyze P. alcaligenes LipA on the immunoblot (Fig. 3).
FIG. 3.
Lipase secretion in strain PAO1algC. P. alcaligenes lipase was expressed in PAO1 and PAO1algC strains and samples withdrawn at OD600 = 3.5 and 3.1, respectively, for the PAO1(p24) and PAO1algC(p24) strains. Cell and extracellular extracts were analyzed by Western blotting, and immunodetection was carried out by chemiluminescence with a monoclonal antiserum directed to P. alcaligenes lipase, as described in Materials and Methods. Only the relevant part of the immunoblot is shown. C, cellular fraction; E, extracellular fraction. The position of LasB (33 kDa) is indicated by an arrow.
Outer membrane integrity of PAO1algC.
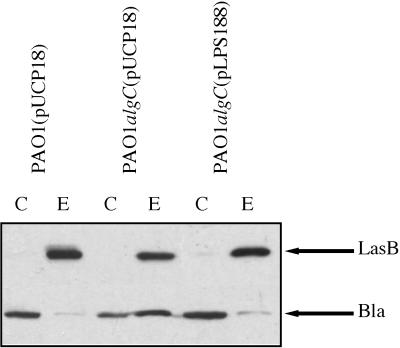
LPS mutations are known to cause perturbations of the outer membrane in gram-negative bacteria (19). Outer membrane integrity was tested by studying the localization of a periplasmic enzyme, β-lactamase, expressed from the plasmid pUCP18 introduced into the PAO1 and PAO1algC strains. As expected, the β-lactamase was mainly intracellular in strain PAO1(pUCP18). However, it was released in large amounts into the extracellular medium of the LPS mutant (Fig. 4). Furthermore, the decrease in extracellular LasB and the leakage of β-lactamase in the LPS mutant appear to be directly or at least indirectly related to the LPS lesion, since complementation of the mutation by expression of the cloned algC gene (pLPS188) led to the recovery of increased levels of extracellular elastase and to decreased leakage of β-lactamase (Fig. 4). Thus, under these experimental conditions, it seems likely that the outer membrane integrity of P. aeruginosa is affected by LPS alteration and that the severe disturbance of the outer membrane function observed in the LPS mutant is related to the algC mutation. Similar alterations of outer membrane integrity were also observed in P. aeruginosa mutants devoid of outer membrane protein F and showing leakage of periplasmic enzymes (18). However, although LPS defective, strain PAO1algC was not found to be affected in OprF (data not shown).
FIG. 4.
Effect of algC complementation on the localization of β-lactamase (Bla) and the production of elastase in strain PAO1algC. LasB and β-lactamase were immunodetected by chemiluminescence as indicated in Materials and Methods. Samples were withdrawn at OD600s of 3.9, 3.7, and 3.6, respectively, for the PAO1(pUCP18), PAO1algC(pUCP18), and PAO1algC(pLPS188) strains. Only the relevant parts of the immunoblots are shown. C, cellular fraction; E, extracellular fraction. The positions of LasB (33 kDa) and β-lactamase (30 kDa) are indicated by arrows.
Influence of LPS alteration on the organization of the Xcp machinery.
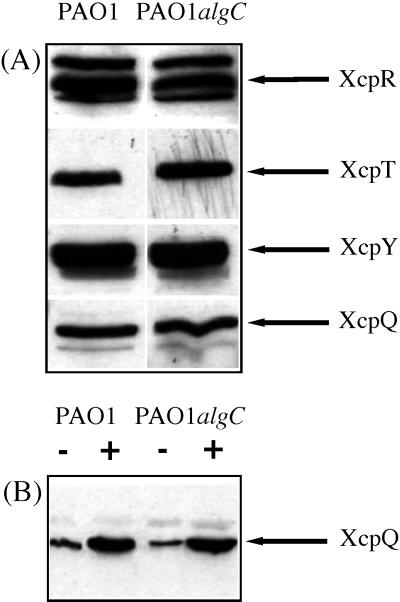
The decreased amounts of extracellular LasB and LipA observed as a consequence of LPS modification could also reflect some alterations in the composition of the Xcp machinery. As shown by immunoblotting experiments, the level of four Xcp proteins tested, XcpR, XcpT, XcpY, and XcpQ, did not seem to be affected by the algC mutation (Fig. 5A). Recently, it has been reported that XcpQ, the only outer membrane protein of the Xcp machinery, is organized as a multimer and that the functionality of this protein strictly depends on multimerization (5). XcpQ multimers are resistant to 2% sodium dodecyl sulfate at 20°C but can be dissociated to monomers upon heating (5). Thus, samples from PAO1 and PAO1algC strains were solubilized at either 20 or 95°C, and the amount of XcpQ monomers was revealed by immunodetection. As shown in Fig. 5B, LPS alteration did not affect XcpQ multimerization, since the amounts of XcpQ monomers increased similarly in the two protein extracts studied upon heating. Therefore, under these experimental conditions, the levels of the various Xcp proteins do not seem to be significantly altered by the LPS deficiency in strain PAO1algC, and it seems unlikely that secretion defects observed in the algC mutant could be directly related to an obvious alteration in the composition of the Xcp machinery. Our results rather suggest that LPS deficiency causes modifications of the envelope organization. Such modifications could weaken interactions between secreted proteins and the Xcp machinery or between the Xcp proteins themselves and thus decrease the efficiency of the secretion process.
FIG. 5.
Effect of LPS alteration on the organization of the Xcp machinery (A) and on the multimerization of XcpQ (B). (A) Cellular proteins from the PAO1 and PAO1algC strains were analyzed by Western blotting and immunodetection of XcpR (55 kDa), XcpT (15 kDa), XcpY (41 kDa), and XcpQ (70 kDa) carried out by chemiluminescence with their respective antisera. (B) Cellular proteins from the PAO1 and PAO1algC strains were solubilized either at 20°C (−) or at 95°C (+) and analyzed by Western blotting. Immunodetection was performed with XcpQ-directed antiserum, and proteins were detected by chemiluminescence. Only the relevant parts of the immunoblots are shown. Samples were withdrawn at OD600s of 2.2 (strain PAO1) and 2.1 (strain PAO1algC).
Mechanism of LasB translocation into the extracellular medium by strain PAO1algC: leakage or secretion?
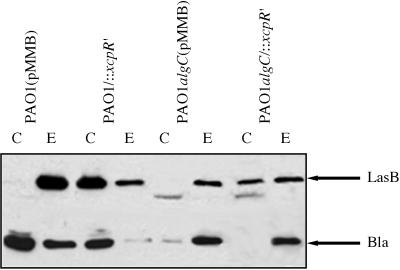
The periplasmic leakage of β-lactamase observed in strain PAO1algC begs the question of the mechanism by which elastase crosses the outer membrane of this mutant: is it a passive mechanism (e.g., diffusion through the outer membrane [leakage]) or an active one (secretion)? In order to answer this question, we generated an xcpR mutation in PAO1 and PAO1algC strains (see Materials and Methods and reference 1). No xcpR product was immunodetected in these mutants. In contrast, XcpT and XcpY were normally produced, showing no polar effect of the mutation on downstream genes of the operon xcpR to -Z (14) and suggesting that promoter activity from the suicide plasmid could drive expression of xcpS to -Z genes. Moreover, expression of genes expressed from the divergent operon xcpPQ did not appear to be affected by the xcpR mutation, since XcpQ was found in normal amounts in both mutants (data not shown). As expected, alteration of the Xcp machinery caused the massive intracellular accumulation of LasB in strain PAO1/::xcpR′, whereas this protein was only detected in the extracellular medium of strain PAO1(pMMB) (Fig. 6). Furthermore, in these two strains, β-lactamase was found to weakly leak into the medium, suggesting that extracellular LasB could also be partially released outside by a xcp-independent process, e.g., a leakage process through the outer membrane. Therefore, in wild-type LPS strains, given the slight β-lactamase leakage in strain PAO1(pMMB) and the ratio of cellular LasB to extracellular LasB in strain PAO1/::xcpR′, it can be deduced that only a minor part of LasB could leak from the cells. In the double mutant PAO1algC/::xcpR′, LasB was also intracellularly accumulated, but to a lesser extent than in the single mutant PAO1/::xcpR′. Indeed, the ratio of cellular LasB to extracellular LasB was clearly lower in the double mutant than in the xcpR single mutant, and elastase was recovered in significant amounts in the extracellular medium of strain PAO1algC/::xcpR′. Moreover, in this double mutant, β-lactamase was found to be almost entirely localized in the extracellular medium (Fig. 6). These results indicate that in secretion-defective mutants, the LasB fraction which is extracellular is released into the medium by a leakage process and that this process is enhanced when the LPS structure is affected. On the other hand, in the xcpR mutants, the LasB fraction retained in the cells represents the pool of elastase normally secreted by the Xcp machinery when the xcpR gene is functional. From these observations, it was concluded that in the LPS mutant, elastase was recovered in the extracellular medium partly by leakage and partly by secretion, while in the wild-type strain, LasB was mostly secreted. Thus, the functioning efficiency of the secretion mechanism is decreased when the LPS structure of P. aeruginosa is altered.
FIG. 6.
Localization of LasB in strain PAO1algC defective in functional Xcp machinery. Elastase and β-lactamase (Bla) were analyzed by Western blotting with extracts from PAO1 and PAO1algC strains deficient or not in xcpR and antibodies directed to LasB and β-lactamase. Only the relevant parts of the immunoblots are shown. C, cellular fraction; E, extracellular fraction. Samples were withdrawn at OD600s of 2.2, 2.25, 2.3, and 2.4, respectively, for the PAO1(pMMB), PAO1/::xcpR′, PAO1algC(pMMB), and PAO1algC/::xcpR′ strains.
Influence of the functionality of the Xcp machinery on the activation process of elastase.
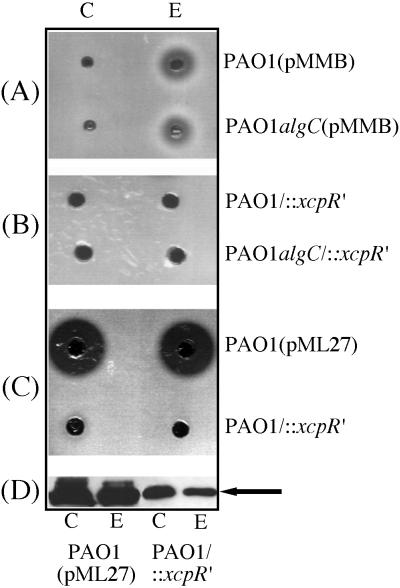
The process of LasB activation is a complex mechanism involving several steps. As previously described by Braun et al. (7), LasB is synthesized as a protein complex with a propeptide which is required for targeting the mature enzyme to the secretion apparatus. Moreover, binding of the propeptide to the mature enzyme inhibits its enzymatic activity (24) and LasB activation is concomitant with dissociation of the complex. In order to study the influence of the functionality of the Xcp machinery on this process, we assayed cellular and extracellular elastase activity in P. aeruginosa strains either altered in LPS structure, secretion defective, or both. As shown in Fig. 7A, elastase activity was only detected in the extracellular medium of the PAO1(pMMB) and PAO1 algC(pMMB) strains. This result was expected, since in these strains, only extracellular LasB was observed on immunoblots (Fig. 6). When the Xcp machinery was affected by mutation, although LasB was accumulated within PAO1/::xcpR′ and PAO1algC/::xcpR′ cells (Fig. 6), no intracellular elastase activity could be detected (Fig. 7B). Moreover, in the double mutant, LasB released into the extracellular medium by leakage was not active, suggesting that secretion of an active form of elastase requires prior interaction of the protein with a fully functional Xcp machinery. As a control, extracts from strain PAO1/::xcpR′ were concentrated fivefold, and their activity was compared to that of unconcentrated extracts from strain PAO1(pML27) which overexpressed LasB. LasB intracellularly accumulated within PAO1(pML27) cells (Fig. 7D) was found to be active (Fig. 7C). However, although LasB was accumulated in large amounts in PAO1/::xcpR′ cells (Fig. 7D), no proteolytic activity could be detected even after concentration of fresh extracts (Fig. 7C). Nevertheless, it should be noted that proteolytic activity of cellular extracts can be detected on protease assay plates after storage for at least 2 days at 4°C before use (data not shown). Immunoblotting experiments showed that activation was due to the disappearance of the propeptide from the propeptide-mature elastase complex, which is an essential and ultimate step for activity (reference 24 and data not shown). The same results were obtained with an xcpQ deletion mutant derivative of strain PAO1 (data not shown). These observations lend support to the idea that activation of LasB strictly depends on interaction of the propeptide-mature enzyme complex with a functional Xcp machinery and, therefore, that LasB released into the medium by leakage does not undergo an activation process, probably because it remains associated with the propeptide.
FIG. 7.
Influence of the state of functionality of the Xcp system on the cellular activity of LasB. (A) Cellular (C) and extracellular (E) extracts obtained from strains PAO1(pMMB) and PAO1algC(pMMB) were assayed for proteolytic activity on plates as described in Materials and Methods. Samples were withdrawn at OD600s of 2.65 for strain PAO1(pMMB) and 2.6 for strain PAO1algC(pMMB). (B) Proteolytic activity of cellular and extracellular extracts from PAO1/::xcpR′ and PAO1algC/::xcpR′ strains. Samples were withdrawn at OD600s of 3.4 and 2.1, respectively. (C) Cellular and extracellular extracts from strain PAO1/::xcpR′ were concentrated fivefold by concentration on a Centricon 10 (Amicon). Unconcentrated extracts from strain PAO1(pML27) and concentrated extracts of strain PAO1/::xcpR′ were used for proteolytic activity tests on plates. (D) Western blot analysis of extracts from strains PAO1(pML27) and PAO1/::xcpR′. Only the relevant part of the immunoblot is shown. Protein analysis was carried out with unconcentrated extracts from strain PAO1/::xcpR′. Samples were withdrawn at OD600s of 3.9 for strain PAO1(pML27) and 4.2 for strain PAO1/::xcpR′. The position of LasB (33 kDa) is indicated by an arrow.
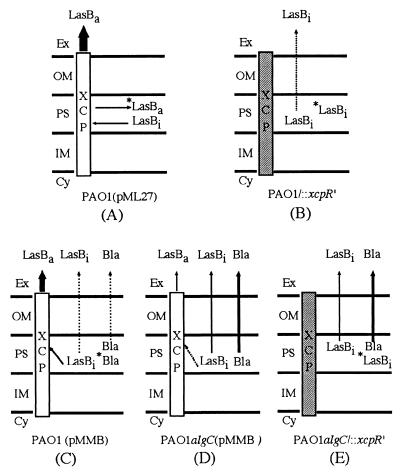
In summary, different models of LasB secretion can be proposed which take into account the results obtained in this study. When the secretory system is saturated by elastase overproduction [strain PAO1(pML27)], LasB is partly secreted by the Xcp machinery and partly accumulated within the cells in an active form (Fig. 8A). In a secretion-defective strain derived from strain PAO1, inactive LasB is slightly released into the medium by leakage and mostly accumulated within the cells, showing that activation of elastase requires prior interaction of the LasB-propeptide complex with a functional Xcp machinery. This result suggests that intracellular LasB represents the fraction of the protein that is secreted when the Xcp machinery is functional (Fig. 8B). As expected, in strain PAO1(pMMB), LasB is mainly secreted by the Xcp secretory machinery in an active form and slightly released into the medium by leakage, as indicated by minor amounts of β-lactamase recovered outside the cells (Fig. 8C). Alteration of the LPS structure affects the functioning of the secretory machinery, probably by decreasing interaction of secreted proteins with the Xcp apparatus (Fig. 8D, dashed arrow). β-Lactamase almost entirely leaks from the cells, while LasB is partly secreted in an active form and partly released into the medium by leakage in an inactive state (Fig. 8D). Generation of an xcpR mutation in an LPS-defective strain (PAO1algC/::xcpR′) causes only a partial accumulation of LasB within the cells (Fig. 8E). As in strain PAO1/::xcpR′, intracellular LasB found in the double mutant represents the protein fraction which is secreted in the presence of a functional Xcp system. In strain PAO1algC/::xcpR′, LasB is mostly released outside by leakage, whereas this process is minor in a mutant only affected in the secretory system (Fig. 8B and E). Therefore, it seems likely that a lesion of the LPS directly or indirectly alters the functioning of the secretory system in P. aeruginosa and lowers its efficiency.
FIG. 8.
Elastase secretion models in P. aeruginosa strains derived from PAO1 and PAO1algC. Active Xcp machinery is represented as an empty rectangle, while a shaded rectangle corresponds to a defective secretory system. Relative levels of elastase (LasB) and β-lactamase (Bla) released into the medium are indicated by arrow width. Ex, extracellular medium; OM, outer membrane; PS, periplasmic space; IM, inner membrane; Cy, cytoplasm; *LasBa, active intracellular elastase; LasBa, active extracellular elastase; *LasBi, inactive intracellular elastase; LasBi, inactive elastase; *Bla, intracellular β-lactamase (see text for commentaries).
Another interesting observation is that although a periplasmic leakage occurred in strain PAO1algC/::xcpR′, as shown by the high release of the periplasmic enzyme β-lactamase in the culture medium, LasB remained at least partly cell associated. Moreover, in this double mutant, lipase activity was not detected in the external medium, but was localized in the cells (data not shown). Therefore, it appears that although β-lactamase leaked from rough mutant cells, two exoenzymes, LasB and LipA, could at least partly remain cell associated and weakly interact with the secretory machinery. These observations suggest that LPS could play a role in the stabilization of the Xcp apparatus and that this machinery is not fully functional in the LPS-deficient strain PAO1algC, probably because secreted enzymes interact less efficiently with components of the secretory system. Our results showing that LasB activation depends on a functional Xcp system are consistent with such a hypothesis.
ACKNOWLEDGMENTS
We are indebted to Barbara Iglewski for plasmid pTS400, David Nunn for XcpT antiserum, W. Bitter for XcpQ antiserum, Gijs Gerritse for plasmid p24 and LipA antibodies, and Terry Beveridge for strain AK1012 and for his interest in this work. We also thank Alain Filloux and Claude Lazdunski for critical reading of the manuscript.
This work was supported by the French cystic fibrosis foundation AFLM (Association pour la Lutte contre La Mucoviscidose) and by Biotech Framework IV grant BI04 CT960119 from the European Union to Cell Factories Network.
REFERENCES
- 1.Akrim M, Bally M, Ball G, Tommassen J, Teerink H, Filloux A, Lazdunski A. Xcp-mediated protein secretion in Pseudomonas aeruginosa: identification of two additional genes and evidence for regulation of xcp gene expression. Mol Microbiol. 1993;10:431–443. doi: 10.1111/j.1365-2958.1993.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 2.Arseneault T L, Hughes D W, McLean D B, Szarck W A, Kropinski A M, Lam J S. Structural studies on the polyacrylamide portion of ′A-band′ lipopolysaccharide from a mutant AK1401 of Pseudomonas aeruginosa strain PAO1. Can J Chem. 1991;69:1273–1280. [Google Scholar]
- 3.Ball G, Chapon-Hervé V, Bleves S, Michel G, Bally M. Assembly of XcpR in the cytoplasmic membrane is required for extracellular protein secretion in Pseudomonas aeruginosa. J Bacteriol. 1999;181:382–388. doi: 10.1128/jb.181.2.382-388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bally M, Filloux A, Akrim M, Ball G, Lazdunski A, Tommassen J. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol Microbiol. 1992;6:1121–1131. doi: 10.1111/j.1365-2958.1992.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 5.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 6.Bleves S, Voulhoux R, Michel G, Lazdunski A, Tommassen J, Filloux A. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family) Mol Microbiol. 1998;27:31–40. doi: 10.1046/j.1365-2958.1998.00653.x. [DOI] [PubMed] [Google Scholar]
- 7.Braun P, Tommassen J, Filloux A. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol. 1996;19:297–306. doi: 10.1046/j.1365-2958.1996.381908.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyne M J, Jr, Russell K S, Coyle C L, Goldberg J B. The Pseudomonas aeruginosa algC gene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J Bacteriol. 1994;176:3500–3507. doi: 10.1128/jb.176.12.3500-3507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 10.deCock H, Van Blokland S, Tommassen J. In vivo insertion and assembly of outer membrane protein PhoE of Escherichia coli K12 into the outer membrane. J Biol Chem. 1996;271:12885–12890. doi: 10.1074/jbc.271.22.12885. [DOI] [PubMed] [Google Scholar]
- 11.de Groot A, Filloux A, Tommassen J. Conservation of xcp genes involved in the two-step secretion process in different Pseudomonas species and other Gram-negative bacteria. Mol Gen Genet. 1991;229:278–284. doi: 10.1007/BF00272167. [DOI] [PubMed] [Google Scholar]
- 12.Duong F, Eichler J, Price A, Rice-Leinard M, Wickner W. Biogenesis of the gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- 13.Filloux A, Bally M, Murgier M, Wretlind B, Lazdunski A. Cloning of xcp genes located at the 55 min region of the chromosome and involved in protein secretion in Pseudomonas aeruginosa. Mol Microbiol. 1989;3:261–265. doi: 10.1111/j.1365-2958.1989.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 14.Filloux A, Michel G, Bally M. The Xcp machinery of Pseudomonas aeruginosa: a model system for studying GSP-dependent protein secretion. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 15.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 16.Gerritse G, Hommes R W J, Quax W J. Development of a lipase fermentation process that uses a recombinant Pseudomonas alcaligenes strain. Appl Environ Microbiol. 1998;64:2644–2651. doi: 10.1128/aem.64.7.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg J B, Hatano K, Pier G B. Synthesis of lipopolysaccharide O side chains by Pseudomonas aeruginosa PAO1 requires the enzyme phosphomannomutase. J Bacteriol. 1993;175:1605–1611. doi: 10.1128/jb.175.6.1605-1611.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh N, Wakebe H, Yoshihara E, Nakae T, Nishino T. Role of protein F in maintaining structural integrity of the Pseudomonas aeruginosa outer membrane. J Bacteriol. 1989;171:983–990. doi: 10.1128/jb.171.2.983-990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock R E W. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- 20.Holland I B, Kenny B, Blight M. Haemolysin secretion from E. coli. Biochimie. 1990;72:131–141. doi: 10.1016/0300-9084(90)90138-7. [DOI] [PubMed] [Google Scholar]
- 21.Iredell J R, Manning P A. Outer membrane translocation arrest of the TcpA pilin subunit in rfb mutants of Vibrio cholerae O1 strain 569B. J Bacteriol. 1997;179:2038–2046. doi: 10.1128/jb.179.6.2038-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarrell K F, Kropinski A M B. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981;38:529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagami Y, Ratliff M, Surber M, Martinez A, Nunn D. Type II secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol Microbiol. 1998;27:221–233. doi: 10.1046/j.1365-2958.1998.00679.x. [DOI] [PubMed] [Google Scholar]
- 24.Kessler E, Safrin M. The propeptide of Pseudomonas aeruginosa elastase acts as an elastase inhibitor. J Biol Chem. 1994;269:22726–22731. [PubMed] [Google Scholar]
- 25.Kloser A, Laird M, Deng M, Misra R. Modulations in lipid A and phospholipid biosynthesis pathways influence outer membrane protein assembly in Escherichia coli K-12. Mol Microbiol. 1998;27:1003–1008. doi: 10.1046/j.1365-2958.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- 26.Kordel M, Hofmann B, Schomburg D, Schmid R D. Extracellular lipase of Pseudomonas sp strain ATCC 21808: purification, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol. 1991;173:4836–4841. doi: 10.1128/jb.173.15.4836-4841.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Laird M W, Kloser A W, Misra R. Assembly of LamB and OmpF in deep rough and lipopolysaccharide mutants of Escherichia coli K-12. J Bacteriol. 1994;176:2259–2264. doi: 10.1128/jb.176.8.2259-2264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam J S, Graham L L, Lightfoot J, Dasgupta T, Beveridge T J. Ultrastructural examination of the lipopolysaccharides of Pseudomonas aeruginosa strains and their isogenic rough mutants by freeze-substitution. J Bacteriol. 1992;174:7159–7167. doi: 10.1128/jb.174.22.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam M Y C, McGroarty E J, Kropinski A M, MacDonald L A, Pederson S S, Øiby N H, Lam J S. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J Clin Microbiol. 1989;27:962–967. doi: 10.1128/jcm.27.5.962-967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eucaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 33.Liu P V, Matsumoto H, Kusama H, Bergman T. Survey of heat-stable, major somatic antigens of Pseudomonas aeruginosa. Int J Syst Bacteriol. 1983;33:256–264. [Google Scholar]
- 34.Liu P V, Wang S. Three new major somatic antigens of Pseudomonas aeruginosa. J Clin Microbiol. 1990;28:922–925. doi: 10.1128/jcm.28.5.922-925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunn D N, Lory S. Product of the Pseudomonas aeruginosa gene pilD is a prepilin peptidase. Proc Natl Acad Sci USA. 1991;88:3281–3285. doi: 10.1073/pnas.88.8.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunn D N, Lory S. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J Bacteriol. 1993;175:4375–4382. doi: 10.1128/jb.175.14.4375-4382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 38.Plesiat P, Ramos Aires J, Godard C, Köhler T. Use of steroids to monitor alterations in the outer membrane of Pseudomonas aeruginosa. J Bacteriol. 1997;179:7006–7010. doi: 10.1128/jb.179.22.7004-7010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivera M, McGroarty E J. Analysis of a common antigen lipopolysaccharide from Pseudomonas aeruginosa. J Bacteriol. 1989;171:2244–2248. doi: 10.1128/jb.171.4.2244-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rust L, Messing C R, Iglewski B. Elastase assays. Methods Enzymol. 1994;235:554–562. doi: 10.1016/0076-6879(94)35170-8. [DOI] [PubMed] [Google Scholar]
- 42.Salmond G, Reeves P J. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 43.Schaecterle G R, Pollach R L. A simplified method for the quantitative assay of protein in biological material. Anal Biochem. 1975;51:654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- 44.Schweizer H P. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene. 1991;97:109–112. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 45.Sen K, Nikaido H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J Bacteriol. 1991;173:926–928. doi: 10.1128/jb.173.2.926-928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith A W, Iglewski B H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989;17:10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wandersman C, Létoffé S. Involvement of lipopolysaccharide in the secretion of Escherichia coli α-haemolysin and Erwinia chrysanthemi proteases. Mol Microbiol. 1993;7:141–150. doi: 10.1111/j.1365-2958.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 48.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]