Abstract
Aims
The diagnostic performance of non-invasive imaging in patients with prior coronary artery disease (CAD) has not been tested in prospective head-to-head comparative studies. The aim of this study was to compare the diagnostic performance of qualitative single-photon emission computed tomography (SPECT), quantitative positron emission tomography (PET), and qualitative magnetic resonance imaging (MRI) in patients with a prior myocardial infarction (MI) or percutaneous coronary intervention (PCI).
Methods and results
In this prospective clinical study, all patients with prior MI and/or PCI and new symptoms of ischaemic CAD underwent 99mTc-tetrofosmin SPECT, [15O]H2O PET, and MRI, followed by invasive coronary angiography with fractional flow reserve (FFR) in all coronary arteries. All modalities were interpreted by core laboratories. Haemodynamically significant CAD was defined by at least one coronary artery with an FFR ≤0.80. Among the 189 enrolled patients, 63% had significant CAD. Sensitivity was 67% (95% confidence interval 58–76%) for SPECT, 81% (72–87%) for PET, and 66% (56–75%) for MRI. Specificity was 61% (48–72%) for SPECT, 65% (53–76%) for PET, and 62% (49–74%) for MRI. Sensitivity of PET was higher than SPECT (P = 0.016) and MRI (P = 0.014), whereas specificity did not differ among the modalities. Diagnostic accuracy for PET (75%, 68–81%) did not statistically differ from SPECT (65%, 58–72%, P = 0.03) and MRI (64%, 57–72%, P = 0.052). Using FFR < 0.75 as a reference, accuracies increased to 69% (SPECT), 79% (PET), and 71% (MRI).
Conclusion
In this prospective head-to-head comparative study, SPECT, PET, and MRI did not show a significantly different accuracy for diagnosing FFR defined significant CAD in patients with prior PCI and/or MI. Overall diagnostic performances, however, were discouraging and the additive value of non-invasive imaging in this high-risk population is questionable.
Keywords: Single-photon emission computed tomography, Positron emission tomography, Magnetic resonance imaging, Chronic coronary syndrome, Fractional flow reserve
Structured Graphical Abstract
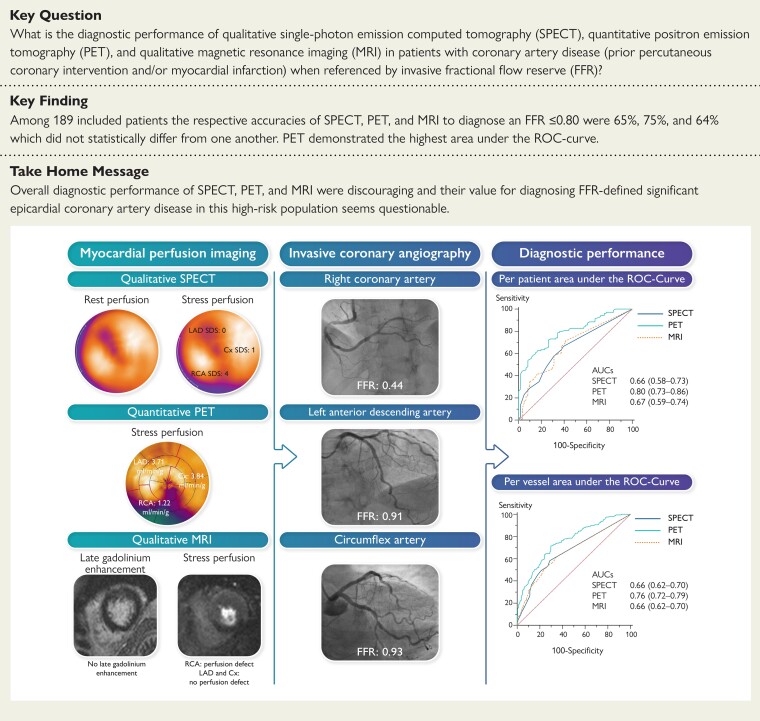
Structured Graphical Abstract.
In this prospectively conducted study, every patient underwent myocardial perfusion imaging with SPECT, PET, and MRI (left panel) with subsequent ICA and FFR measurements in all vessels regardless of imaging results and stenosis severity (middle panel). This resulted in modest diagnostic performances of whom PET revealed the greatest AUC on a per-patient and per-vessel level (right panel). In this case example, qualitative SPECT shows a reversible defect during stress in the inferior wall with an SDS of 5. The quantitative PET polar map depicts abnormal absolute myocardial blood flow during stress in the RCA territory of 1.22 mL/min/g myocardial tissue, indicative for ischaemia. Qualitative MRI showed a stress perfusion defect without myocardial scar, suggestive of ischaemia in the inferior wall. The ICA revealed a haemodynamically significant lesion in the proximal RCA as indicated by an abnormal FFR of 0.44.
AUC, area under the receiver operating characteristic curve; Cx, circumflex artery; FFR, fractional flow reserve; ICA, invasive coronary angiography; LAD, left anterior descending artery; MRI, magnetic resonance imaging; PET, positron emission tomography; RCA, right coronary artery; ROC, receiver operating characteristic curve; SDS, summed difference score; and SPECT, single-photon emission computed tomography.
See the editorial comment for this article ‘Stress imaging versus fractional flow reserve: what comes first—the chicken or the egg?’, by Patrizio Lancellotti et al., https://doi.org/10.1093/eurheartj/ehac287.
Introduction
Current guidelines recommend the use of non-invasive imaging as an initial test for diagnosing obstructive coronary artery disease (CAD) in patients with an intermediate risk of chronic coronary syndromes (CCSs) and to determine the appropriateness of referral for invasive coronary angiography (ICA) and subsequent revascularization.1 At present, several non-invasive imaging modalities are available, including single-photon emission computed tomography (SPECT), positron emission tomography (PET), and magnetic resonance imaging (MRI). With increasing clinical likelihood of obstructive CAD, guidelines favour ischaemia testing over anatomic testing with coronary computed tomography angiography (CCTA). A specific functional imaging technique, however, is not recommended. Interestingly, the existing literature lacks studies about the diagnostic performance in specific patient categories with a known history of CAD such as prior myocardial infarction (MI) or revascularization. Patients with prior CAD or a reduced ejection fraction are often excluded from clinical studies or, at most, they represent a minority of the study population.2–4 Paradoxically, these patients represent a majority of the cardiac outpatient clinic population5 and it is in these high-risk patients that guidelines recommend utilization of non-invasive stress imaging to diagnose and risk stratify patients with equivocal new or worsening symptoms.1 Evaluation with non-invasive imaging in these patients is, however, generally thought to be more challenging and scarcely available data from subgroup analyses have shown ambiguous results.6–8 Furthermore, many previous diagnostic performance studies were hampered by the lack, or inconsistent use, of fractional flow reserve (FFR) as a reference standard. This disregards the prognostic importance of FFR in guiding revascularization when compared with guidance with only angiography.9–11 Therefore, the present prospective study was initiated to compare the diagnostic performance of myocardial perfusion imaging (MPI) with SPECT, PET, and MRI in patients with prior MI and/or percutaneous coronary intervention (PCI), against an FFR reference standard.
Methods
Study design and population
The Prospective Comparison of Cardiac PET, SPECT, and MRI Perfusion Imaging with Invasive Coronary Angiography in Patients with Prior CAD (PACIFIC 2) study was a prospective controlled clinical single-centre, head-to-head comparative study conducted from January 2014 to October 2020, at the Amsterdam UMC, VU University Medical Center, Amsterdam, The Netherlands. The study was pre-maturely ended mainly because of lack of imaging capacity during the COVID-19 pandemic. Patients with suspected obstructive CAD, who were referred for a clinically indicated ICA, had a history of previous MI or PCI (>3 months before), and were suitable for all imaging tests were evaluated for inclusion in the PACIFIC 2 study. Detailed in- and exclusion criteria are presented in Supplementary material online, Information S1. In short, exclusion criteria included contraindications for adenosine; contraindications for iodinated contrast; cardiac devices or non-MRI proof metal implants; prior coronary artery bypass graft (CABG) surgery; claustrophobia; atrial fibrillation; pregnancy; and acute MI. The study complied with the Declaration of Helsinki, the study protocol was approved by the VUmc Medical Ethics Review Committee, and all patients provided written informed consent.
Image acquisition and analysis
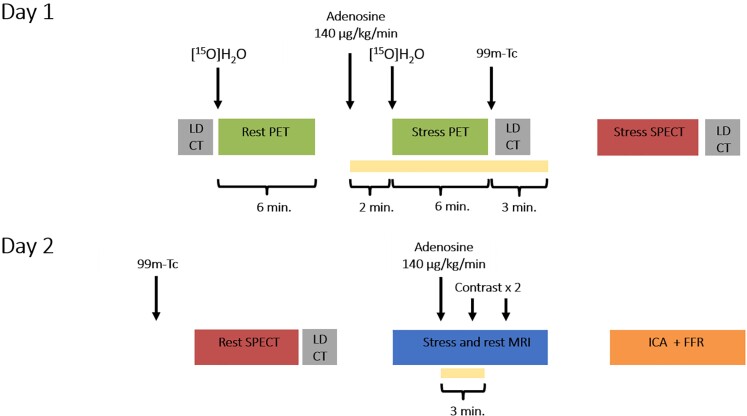
Patients were scheduled to undergo SPECT, PET, and MRI followed by ICA and FFR measurements of all coronary arteries regardless of non-invasive test results, within 2 weeks. A schematic overview of the study protocol is shown in Figure 1. Patients were instructed to avoid the intake of caffeine or xanthine containing products 24 h prior to the scans and to be in a fasting state on the day of the imaging protocol. Medication was neither discontinued nor changed during the execution of the study protocol. Detailed descriptions of imaging acquisition and analyses are provided in Supplementary material online, Information S2. Image data sets were transferred to core laboratories: SPECT (Royal Brompton Hospital, London, England), PET (Turku University Hospital, Turku, Finland), and MRI (University Hospital Frankfurt, Frankfurt am Main, Germany). These dedicated laboratories analysed and interpreted the images blinded for the other imaging and ICA results.
Figure 1.
Schematic illustration of the study protocol. All patients underwent the same 2-day protocol with positron emission tomography, single-photon emission computed tomography, and magnetic resonance imaging, followed by invasive coronary angiography with routine fractional flow reserve measurements. FFR, fractional flow reserve; ICA, invasive coronary angiography; LD-CT, low-dose computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; and SPECT, single-photon emission computed tomography.
Single-photon emission computed tomography/computed tomography
Images were obtained on a dual-head hybrid SPECT/CT scanner (Symbia T2; Siemens Healthineers, Erlangen, Germany). Patients underwent a 2-day stress-rest protocol using intravenous adenosine (140 mg/kg/min) as a hyperaemic agent and a weight-adjusted dose of 370–550 MBq 99mTc-tetrofosmin as radiotracer. Images were acquired using electrocardiographic gating and were followed by a low-dose CT scan for attenuation correction. Images were interpreted based on the standardized 17-segment model.12 Radiotracer uptake of each segment during rest and stress was visually scored using a 5-point scoring system (normal; mildly decreased; moderately decreased; severely decreased; and absence of uptake). Based on these rest and stress scores, segmental difference scores were calculated. A summed difference score (SDS) ≥1 was used to define the presence of ischaemia. Perfusion defect percentage was calculated as: (SDS/maximal achievable SDS) × 100. Finally, perfusion defects were qualified as reversible (ischaemia), fixed (MI), or mixed (both MI and ischaemia).
Positron emission tomography/computed tomography
Patients were scanned on a PET/CT device (Philips Gemini TF 64 or Ingenuity TF 128; Philips Healthcare, Best, The Netherlands). A dynamic PET perfusion scan was performed using 370 MBq of [15O]H2O during resting and adenosine-induced (140 mg/kg/min) hyperaemic conditions. Low-dose CT scans allowed for attenuation correction. Parametric images with quantitative myocardial blood flow (MBF) were generated. Hyperaemic MBF, expressed in mL/min/g of perfusable myocardial tissue, was calculated for all three vascular territories using standardized segmentation.12 A hyperaemic MBF ≤2.3 mL/min/g of perfusable myocardial tissue in ≥2 adjacent segments within a vascular territory was used to define ischaemia.2,13
Magnetic resonance imaging
Images were acquired on a 1.5-T whole body MR scanner (Magnetom Avanto; Siemens Healthineers). Perfusion images were acquired in three short-axis slices at the basal, mid, and apical level, following a 0.075 mmol/kg bolus of a gadolinium-based contrast agent (DOTAREM; Guerbet, Villepinte, France). Perfusion imaging was performed during rest and adenosine-induced (140 μg/kg/min) stress conditions using identical scanning parameters and slice location. In-plane respiratory motion of the heart was corrected using a non-rigid registration. Cardiac function was assessed with steady-state free-precession cine imaging and late gadolinium enhancement (LGE) was performed using a two-dimensional segmented inversion-recovery gradient-echo pulse sequence. Stress and LGE images were analysed according to the 17-segment model excluding the apex.12 Per-segment, the amount of stress perfusion defect and LGE were visually scored using a 5-point scale (0%, 1–25%, 26–50%, 51–75%, >75%). Ischaemia was defined by a stress perfusion defect extending beyond an area with LGE, or in the absence of LGE by a perfusion defect >1 segment circumferential, or extending >1 slice, or with >50% transmurality. Perfusion defect scores were calculated by subtracting LGE scores from stress perfusion defect scores. Perfusion defect percentage was calculated as: (perfusion defect score/maximal achievable perfusion defect score) × 100.
Invasive coronary angiography and fractional flow reserve
The ICA was performed using a standard protocol in at least two orthogonal directions per evaluated coronary artery segment. Prior to contrast injection, 0.2 mL of intracoronary nitroglycerine was administered to induce epicardial coronary vasodilation. All major coronary arteries were routinely interrogated by FFR, regardless of stenosis severity, except for occluded vessels or subtotal lesions with a diameter stenosis (DS) ≥90%. Intracoronary (150 mg) or intravenous (140 mg/kg/min) adenosine infusion was used to induce maximal coronary hyperaemia. The FFR was calculated as the ratio of mean distal intracoronary pressure and mean arterial pressure. Vessels were considered to have haemodynamically significant CAD in case of an FFR ≤0.80, or stenosis with a DS ≥90% if FFR was missing.
Statistical analysis
The study endpoint was the comparison of SPECT, PET, and MRI, in terms of sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), diagnostic accuracy, and area under the receiver operating characteristic curve (AUC), in identifying patients with haemodynamically significant CAD, referenced by invasive FFR. Patients were considered positive for a modality (including the reference standard), if at least one vessel or vascular territory was positive. In patient-based analysis, these diagnostic measures were calculated as simple proportions with 95% confidence intervals. Sensitivity, specificity, and accuracy on a patient level were compared using the McNemar’s test, whereas PPV and NPV were compared using a marginal regression model using an independent working correlation structure. In vessel-based analysis, diagnostic performance measures were calculated and compared with generalized estimating equations that accounted for multiple measurements within patients using an exchangeable correlation structure (sensitivity, specificity, and accuracy) or an independent correlation structure (NPV and PPV). Hyperaemic MBF for PET and perfusion defect percentage for SPECT and MRI were used to generate AUCs. These AUCs were compared using the method of DeLong et al.14 Continuous variables are presented as mean ± standard deviation or median (interquartile range) as appropriate. Categorical variables are expressed as frequencies and percentages. Differences between continuous and categorical variables were compared using the two-sided Student’s t-test and the χ2 test or Fisher’s exact test, respectively. Logistic regression analyses were used to assess the predictive value of traditional risk factors and imaging results. As we applied a Bonferroni correction for three pairwise comparisons between the three modalities, significance was concluded when P < 0.0167. A power calculation based on the included population is presented in Supplementary material online, Information S3. Statistical analyses were performed using SPSS software package (IBM SPSS Statistics 20.0, Chicago, IL, USA) and MedCalc (MedCalc Software 12.7.8.0, Mariakerke, Belgium).
Results
A total of 3489 patients were assessed for eligibility. Of these, 189 patients with prior MI and/or PCI and suspected obstructive CAD were deemed to be eligible and were included in the study (Supplementary material online, Figure S1). Among them, SPECT result was missing for 4 patients due to an uninterpretable stress scan (N = 1) or missing rest scan (N = 3), PET result was missing in 2 patients due to tracer problems, and MRI result was missing in 18 patients because of failed (N = 6) or incomplete (N = 12) MRI procedures due to refusal of adenosine after PET-SPECT scanning, claustrophobia, and technical reasons. The mean time for completing the entire study protocol, including ICA, was 5.4 ± 3.5 days. Baseline patient characteristics are listed in Table 1. In brief, patient age averaged 63.3 ± 9.3 years, 81% were males, and a total of 100 (53%) patients had suffered a prior MI and 171 (90%) a prior PCI.
Table 1.
Baseline patient characteristics
| Characteristics | |
|---|---|
| Demographics | |
| Age, years (mean ± SD) | 63.3 ± 9.3 |
| Male sex | 153 (81%) |
| Body mass index, kg/m2 (mean ± SD) | 27.4 ± 4.0 |
| History | |
| Hypertension | 122 (65%) |
| Hyperlipidaemia | 128 (68%) |
| Diabetes | 39 (21%) |
| Current smoking | 26 (14%) |
| History of smoking | 78 (41%) |
| Family history of CAD | 95 (50%) |
| Previous PCI | 171 (90%) |
| Previous MI | 100 (53%) |
| Medication | |
| Platelet inhibitor | 187 (99%) |
| Beta-blocker | 115 (61%) |
| Statin | 163 (86%) |
| ACE inhibitor/ARB | 110 (58%) |
| Calcium-channel blocker | 65 (34%) |
| Long-acting nitrate | 50 (27%) |
| Symptoms | |
| Typical angina | 78 (41%) |
| Atypical angina | 78 (41%) |
| Non-specific chest discomfort | 33 (17%) |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Imaging results
Image quality was considered at least average or good in 170 of 185 (92%) SPECT studies, 176 of 187 (94%) PET studies, and 149 of 171 (87%) of MRI studies. The MPI was concluded to be ischaemic in 105 (57%) using SPECT, 119 (64%) using PET, and 95 (56%) using MRI. Supplementary material online, Table S1 shows detailed imaging findings according to FFR-based subgroups. The Structured Graphical Abstract demonstrates typical imaging findings for each modality tested. Mean radiation exposure was 6.2 ± 0.9 mSv for SPECT and 3.1 mSv for PET MPI, while MRI is not associated with ionizing radiation.
Invasive coronary angiography and fractional flow reserve results
In total, 119 (63%) patients were found to have haemodynamically significant CAD defined by ICA and FFR. Among the 567 coronary territories investigated, 9 right coronary arteries appeared right ventricular branches only. Among the remaining 558 vessels, 479 (86%) were directly interrogated with FFR. The FFR was not performed because of (sub)total occlusion (n = 74) or tortuosity (n = 5) as illustrated in Supplementary material online, Figure S2. Ischaemia causing lesions was present in 183 (33%) vessels, including 88 (47%) left anterior descending arteries, 48 (27%) right coronary arteries, and 47 (25%) circumflex arteries. This resulted in the identification of 1-, 2-, 3-vessel disease in 68 (36%), 38 (20%), and 13 (7%) patients, respectively. The FFR values ranged from 0.35 to 1.00 with a mean of 0.87 ± 0.12.
Diagnostic performance of single-photon emission computed tomography, positron emission tomography, and magnetic resonance imaging
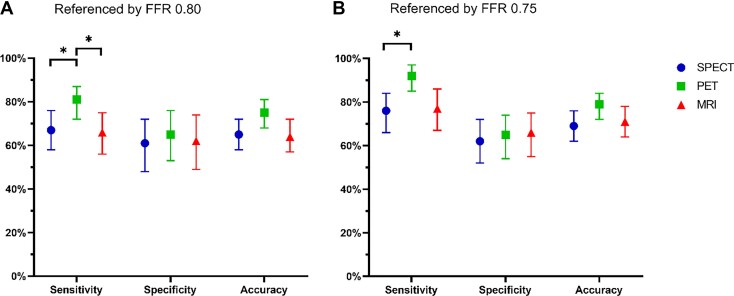
The diagnostic performance of perfusion imaging modalities for the detection of haemodynamically significant CAD are summarized in Table 2 and illustrated in Figures 2 and 3. On a per-patient level, PET showed a higher sensitivity (81%) and NPV (66%) than SPECT (67%, P = 0.016 and 53%, P = 0.03, respectively) and MRI (66%, P = 0.01 and 51%, P = 0.03, respectively). Specificity and PPV did not differ among PET (65% and 80%, respectively), SPECT (61% and 74%, respectively), and MRI (62% and 75%, respectively). Overall, PET had a numerically higher diagnostic accuracy (75%) than SPECT (65%, P = 0.03) and MRI (64%, P = 0.052) and a significantly higher AUC (0.80) than SPECT (0.66, P = 0.001) and MRI (0.67, P = 0.001). The SPECT and MRI showed similar diagnostic performance measures and no significant differences were found between these modalities.
Table 2.
Diagnostic performance of single-photon emission computed tomography, positron emission tomography, and magnetic resonance imaging for the detection of fractional flow reserve-defined significant coronary artery disease
| % (95% CI) | |||
|---|---|---|---|
| SPECT | PET | MRI | |
| Per patient | |||
| Sensitivity | 67 (58–76) | 81 (72–87) | 66 (56–75) |
| Specificity | 61 (48–72) | 65 (53–76) | 62 (49–74) |
| PPV | 74 (68–80) | 80 (74–85) | 75 (68–81) |
| NPV | 53 (45–60) | 66 (57–75) | 51 (43–59) |
| Accuracy | 65 (58–72) | 75 (68–81) | 64 (57–72) |
| AUC | 0.66 (0.58–0.73) | 0.80 (0.73–0.86) | 0.67 (0.59–0.74) |
| Per vessel | |||
| Sensitivity | 60 (52–67) | 73 (66–79) | 44 (35–52) |
| Specificity | 70 (66–75) | 69 (64–73) | 82 (77–86) |
| PPV | 49 (44–54) | 53 (49–58) | 53 (46–60) |
| NPV | 78 (75–81) | 84 (80–87) | 75 (73–78) |
| Accuracy | 67 (63–71) | 70 (66–74) | 70 (65–73) |
| AUC | 0.66 (0.62–0.70) | 0.76 (0.72–0.79) | 0.66 (0.62–0.70) |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; MRI, magnetic resonance imaging; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value; and SPECT, single-photon emission computed tomography.
Figure 2.
Diagnostic performance of qualitative single-photon emission computed tomography, quantitative positron emission tomography, and qualitative magnetic resonance imaging for diagnosing significant coronary artery disease on a patient-based level, as defined by fractional flow reserve 0.80 (A) and 0.75 (B). Diagnostic values are represented as point estimates and 95% confidence intervals. Only the sensitivity was significantly different across the modalities, in favour of positron emission tomography. Using the original fractional flow reserve threshold of 0.75 (B) as a reference standard, instead of the later 0.80 (A), improved both sensitivity and accuracy among single-photon emission computed tomography, positron emission tomography, and magnetic resonance imaging. Abbreviations as in Figure 1. *A significant difference (P < 0.0167) between modalities.
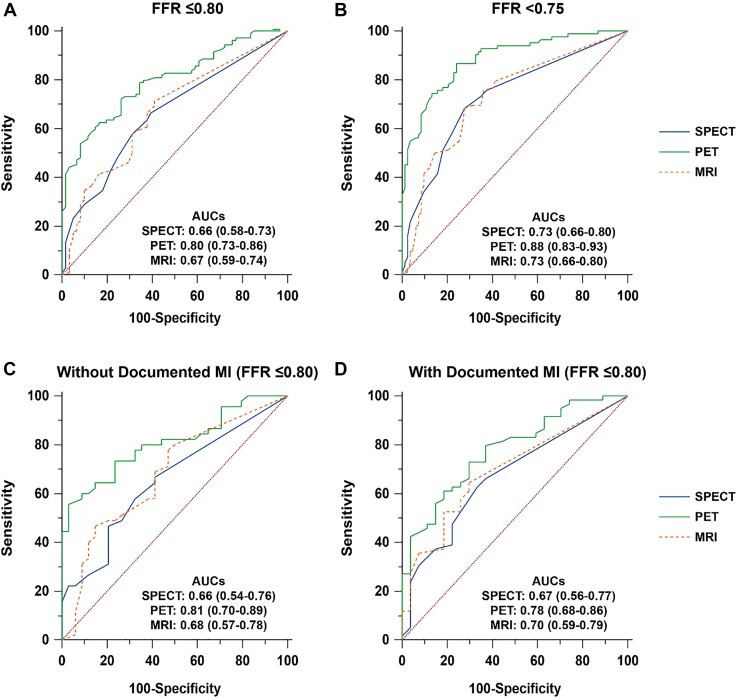
Figure 3.
Discriminative ability of qualitative and quantitative myocardial perfusion imaging modalities for the detection of significant coronary artery disease on a per-patient basis. Receiver operating characteristic curve analysis with corresponding area under the curves and 95% confidence intervals displaying the performance of single-photon emission computed tomography, positron emission tomography, and magnetic resonance imaging as referenced by an (A) fractional flow reserve ≤0.80 and (B) fractional flow reserve <0.75. Similarly, subgroup analyses are shown in patients (C) without a documented myocardial infarction and (D) with a documented myocardial infarction. AUC, area under the receiver operating characteristic curve; other abbreviations are as in Figure 1.
On a per-vessel level, again PET showed the highest sensitivity (73%) and NPV (84%) when compared with SPECT (60%, P = 0.001 and 78%, P = 0.005, respectively) and MRI (44%, P < 0.001 and 75%, P < 0.001, respectively). Specificity was highest for MRI (82%) when compared with SPECT (70%, P < 0.001) and PET (69%, P < 0.001). The PPV was similarly low for all modalities, ranging 49–53%. Accuracy did not significantly differ among modalities, ranging 67–70%. The PET showed the greatest AUC (0.76) when compared with SPECT (0.66, P < 0.001) and MRI (0.66, P = 0.002).
Logistic regression analyses showed that SPECT, PET, and MRI have a modest but significant incremental diagnostic value beyond traditional risk factors and symptoms in predicting haemodynamic significant CAD, as indicated by FFR (Supplementary material online, Table S2).
Diagnostic performance in patients with prior percutaneous coronary intervention, myocardial infarction, and abnormal left ventricular ejection fraction
The overall accuracy and AUC of patients with and without prior MI, as documented in their patient history, did not substantially differ (Figure 3 and Supplementary material online, Table S3). Due to slight changes and smaller subgroups, however, the accuracy and AUC of PET in patients with prior MI did not remain significantly higher than SPECT and MRI. For all modalities, NPV numerically decreased and the PPV numerically increased in patients with prior MI when compared with patients without prior MI. Similar tendencies were found for per-vessel analyses (Supplementary material online, Table S3). A subanalysis of vessels with or without MRI defined MI, revealed a consistent trend for lower accuracy in case of MI (Supplementary material online, Table S4). Conversely, while the AUC numerically increased for SPECT and PET in case of vascular territories with MI, the opposite was found for MRI (Supplementary material online, Figure S3). For all imaging modalities, sensitivity and PPV generally increased with the presence of LGE defined MI, while specificity and NPV generally decreased. In a small subgroup of 25 patients with a left ventricular ejection fraction (LVEF) <50% the AUC was similar to patients with a normal LVEF for SPECT (0.71 vs. 0.66, respectively, P = 0.68), PET (0.84 vs. 0.79, respectively, P = 0.62), and MRI (0.71 vs 0.66, respectively, P = 0.65). Other subanalyses limited to good quality images and stratified by coronary territories did not reveal major changes (Supplementary material online, Tables S5 and S6). A subanalysis limited to obese patients showed similar diagnostic performance of attenuation corrected SPECT and PET imaging, but a lower specificity and accuracy for MRI (Supplementary material online, Table S7). Splenic ‘switch-off’ was seen in 87% of patients. Exclusion of patients without a splenic ‘switch-off’ did not alter the MRI results.
Diagnostic performance of single-photon emission computed tomography, positron emission tomography, and magnetic resonance imaging as referenced by fractional flow reserve 0.75
Figures 2 and 3B show the overall discriminative power of each imaging modality for the detection of significant CAD as defined by an FFR <0.75. Among the 25 patients in the FFR grey-zone (0.75–0.80), a considerable shift was seen from false towards true negatives (16–18 patients depending on the modality). Conversely, only six to nine patients shifted from true to false positive. These alterations resulted in an improved sensitivity and NPV without compromising specificity and PPV and, as such, lead to an enhanced diagnostic accuracy by 4–7% (Table 3).
Table 3.
Diagnostic performance of single-photon emission computed tomography, positron emission tomography, and magnetic resonance imaging for the detection of coronary artery disease when referenced by a fractional flow reserve threshold of <0.75 on a per-patient level
| % (95% CI) | |||
|---|---|---|---|
| SPECT | PET | MRI | |
| Sensitivity | 76 (66–84) | 92 (85–97) | 77 (67–86) |
| Specificity | 62 (52–72) | 65 (54–74) | 66 (55–75) |
| PPV | 67 (60–73) | 73 (66–78) | 68 (61–75) |
| NPV | 73 (64–80) | 90 (81–95) | 75 (66–82) |
| Accuracy | 69 (62–76) | 79 (72–84) | 71 (64–78) |
| AUC | 0.73 (0.66–0.80) | 0.88 (0.83–0.93) | 0.73 (0.66–0.80) |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; MRI, magnetic resonance imaging; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value; and SPECT, single-photon emission computed tomography.
Discussion
This prospective head-to-head comparative study has shown that, in patients with prior PCI and/or MI and suspected ischaemic CAD, quantitative PET outperforms visual SPECT and MRI with regard to the AUC for diagnosing myocardial ischaemia as defined by invasive FFR. The diagnostic accuracy, however, did not significantly differ across the non-invasive imaging modalities. Overall, these diagnostic accuracies were lower than in studies that included patients without prior CAD and were mainly hampered by a low specificity. The present study has several exceptional features: SPECT, PET, and MRI were compared prospectively in a true head-to-head fashion; exclusively patients with a history of PCI or MI were enrolled; coronary arteries were routinely interrogated with invasive FFR as a reference standard, irrespective of imaging results; all scans were analysed by dedicated core laboratories blinded to other imaging findings.
According to current guidelines, symptomatic patients with suspected CAD should be offered diagnostic testing after assessing pre-test probability and clinical likelihood.1 For patients with new or worsening symptom levels and established CCS, such as prior MI or PCI, it is generally recommended to perform functional stress imaging. The choice for a specific stress imaging modality is not advocated and depends predominantly on patient and local preferences. An important reason for the lack of advocating a single imaging modality is probably the paucity of data in this specific patient population. Previous comparative studies have largely focussed on patients without prior CAD.2–4 To our knowledge, no study to date has investigated the diagnostic accuracy of perfusion imaging modalities in a selected population of patients with previous CAD. When compared with patients without a history of previous CAD, perfusion imaging was expected to be more difficult to interpret because of mixed disease (ischaemic and infarction), comorbidities, and an increment of multivessel and microvascular disease. Although even subanalyses are scarce, Arai et al.6 found a numerically lower diagnostic accuracy of MRI in patients with than without prior CAD (65 vs. 69%) and Bernhardt et al.7 described a lower accuracy following PCI or CABG when compared with patients without prior CAD (82 vs. 69 vs. 89%). Conversely, a subanalysis by Perrin et al.8 revealed a decreased accuracy by 4% after excluding all patients with prior CAD (55%) using SPECT. It has to be noted that none of these studies have used FFR as a reference standard. Furthermore, an interesting study by Nakamori et al.15 described a reduced AUC for multivessel disease (0.74) in comparison with single-vessel disease (0.93). The lack of studies with a similar study population hampers an unbiased direct comparison of present diagnostic performance findings. Still, the current diagnostic accuracies of all three modalities are consistently lower than in other recent comparative trials.2–4 For SPECT, a considerably lower accuracy was found in PACIFIC 2 (64%) than in previous studies: Prospective Comparison of Cardiac PET/CT, SPECT/CT Perfusion Imaging and CT Coronary Angiography With Invasive Coronary Angiography (PACIFIC 1, 77%),2 Evaluation of Integrated Cardiac Imaging in Ischemic Heart Disease (EVINCI, 70%),3 and Clinical Evaluation of MAgnetic Resonance imaging in Coronary heart disease (CE-MARC, 76%).4 Similarly, the present accuracy for MRI (65%) was inferior to what was found in CE-MARC (85%). Finally, also PET accuracy (74%) was decreased in comparison with PACIFIC 1 (85%), and EVINCI (85%). Decreased accuracies seem to be hampered predominantly by reduced specificities. This could indeed be explained, at least in part, by the study population characteristics with more microvascular and multivessel disease as well as mixed disease of myocardial ischaemia and infarction. Interestingly, the Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease (MR-IMPACT II) study enrolled a fairly high number of patients with prior MI (28%) and revealed relatively comparable diagnostic accuracies of both SPECT (65%) and MRI (68%).11 In subgroup analyses of the present study, however, we did not find a difference between accuracies or AUCs in patients and vascular territories with vs. without prior MI. In patients with prior MI, a consistent decline was found for NPV and, depending on the modality, an improvement was generally found for PPV and specificity. The largest differences were seen for MRI, suggesting a larger impact of MI on the interpretation of ischaemia. Hypothetically, with MRI it can be more difficult to discriminate scar and residual ischaemia.
An interesting finding of the present study was the resemblance of the performance of visual SPECT and MRI perfusion imaging. This regards the full spectrum of diagnostic performance, including sensitivity, specificity, NPV, PPV, accuracy, and AUC. These similarities could imply some comparable scan features such as the routine visualization of infarcted myocardium and assessment of left ventricular function (both not easily assessed on [15O]H2O PET), suboptimal myocardial tracer and contrast agent uptake characteristics, as well as the lack of routine quantitative evaluation of perfusion.16 Although quantitative myocardial perfusion assessment is an emerging and promising feature for both SPECT and MRI in specialized centres, it is not yet clinical practice in most imaging laboratories. Conversely, absolute MBF is assessed routinely with PET and can lead to improved detection of CAD, especially in case of balanced multivessel disease or discreetly abnormal coronary flow,17 which is reflected by the enhanced sensitivity of quantitative PET, the largest benefit among the diagnostic performance measures. This could be especially important in a patient population like the current one, with a relatively high prevalence of multivessel disease of 27%. More specifically, among patients with discordant PET-SPECT results, patients with abnormal quantitative PET had more cardiovascular risk factors (mean 3.6 vs. 2.9) as predictors of microvascular disease and more multivessel disease in case of significant CAD (31 vs. 25%). Particularly in these patients, especially a pixel-wise MRI-derived MBF quantification may provide better accuracy than the traditional MRI perfusion evaluation utilized in the present study.
In the present study, invasive FFR was used as the reference standard given its ability to favourably guide revascularization strategies in terms of patient outcome.9,10 Nevertheless, FFR also has its limitations as it predominantly evaluates epicardial lesion-specific physiology, while MPI assesses the epicardial and microvasculature bed.16 As such, myocardial territories with diffuse and microvascular disease could result in abnormal myocardial perfusion with a normal FFR. This apparent mismatch does not necessarily represent the failure of either technique, but likely resulted in considerable ‘false positives’ and reduced specificity of MPI in the present study. Conversely, FFR measured distally presumably leads to an overestimation of stenosis severity due to the impact of diffuse CAD. As such, the low sensitivity and NPV of particularly SPECT and MRI may reflect the inability of a visual approach to accurately detect microvascular dysfunction, a homogeneous process which may go undetected with qualitative imaging techniques. Indeed, in the present study, both qualitative techniques, namely SPECT and MRI, demonstrated a significantly lower NPV in comparison with quantitative [15O]H2O PET. However, it has also been suggested that its superior performance is largely owing to its higher spatial resolution that allows for better assessment of endocardial ischaemia. Furthermore, doubts are raised about the impact of infarcted myocardium in discerning ischaemia with FFR. Indeed, intracoronary pressure gradients may vary for a similar stenosis in patients with and without a prior MI. The FFR is expected to be higher in patients with a prior MI for a similar stenosis due to a lower amount of viable myocardium and higher coronary microvascular resistance. However, De Bruyne et al.18 demonstrated that the coronary driving pressure associated with ischaemia remains unchanged in patients with a prior infarction. This is underscored by our findings that the diagnostic accuracy and relationship between perfusion imaging and FFR did not differ between patients with and without prior infarction (Figure 3 and Supplementary material online, Tables S3 and S4). Nevertheless, the agreement with MPI is clearly lower than in studies without patients with prior CAD, which could still be explained by the above mentioned differences between MPI and FFR.2 Notably, applying an FFR reference standard threshold of 0.75 instead of 0.80, as originally determined,19 validated for patients with prior MI18 and perhaps comprising a greater prognostic value,10 considerably improved the diagnostic accuracy of all imaging modalities. Such improvement was partly seen for SPECT and PET in PACIFIC 1,2 but not as pronounced as in the present PACIFIC 2 study. Whether this can be explained by the original derivation of the FFR threshold using non-invasive ischaemia tests including SPECT,19 or by the specific patient population in the present study remains to be elucidated.
The apparently reduced diagnostic accuracies of all perfusion imaging modalities in the currently investigated patient population raise the question whether a limited additional diagnostic value, in patients with a history of CAD and reoccurrence of anginal complaints, outweighs the costs and risks. Although small, risks of non-invasive perfusion imaging should not be neglected. Also in the light of the ever increasing healthcare costs and focus on appropriate use criteria, it should be questioned whether a direct referral for ICA might be preferred. Especially with a high pre-test probability, consistent with our population with a prevalence of CAD being 63%. This is also in line with the current guidelines, stating that in patients with severe angina and a high-risk clinical profile, direct referral for ICA may be reasonable, provided that invasive physiological measures such as FFR are readily available in the catherization laboratory.1 Stress testing followed by ICA, however, could obviate the need for FFR as this technique is underutilized in some clinics. Again, bearing in mind that guiding revascularization with FFR indeed resulted in an event-free survival,9 which was not shown directly for non-invasive ischaemia testing.20 Yet, a recent ISCHEMIA trial subanalysis showed a benefit of an invasive treatment in a small subgroup of patients with non-invasively established ischaemia and either heart failure or reduced LVEF.21 And also the recent multicentre Myocardial Perfusion CMR versus Angiography and FFR to Guide the Management of Patients with Stable Coronary Artery Disease (MR-INFORM) trial showed similar outcomes in patients randomized to either an MRI-based strategy or an FFR-based strategy.22 With regard to symptom relief, it should be noted that an Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina (ORBITA) substudy found a significant interaction between stress echocardiography and patient-reported angina frequency, even though interactions with other symptom responses were not detected.23 Nevertheless, although in our study quantitative PET showed the highest diagnostic accuracy of 75%, this modality is among the least widely available scans and prompts a sophisticated imaging laboratory. Even though logistic regression analysis showed a statistically significant incremental value of each perfusion imaging modality over risk factors and type of chest pain, the odds ratios were discouragingly low and considerably lower than in PACIFIC 1.2 In both studies, PET revealed the highest odds ratio, 2.74 for PACIFIC 2 when compared with 26.59 for PACIFIC 1. It could be debated and further investigated whether the incremental clinical benefit justifies the additional efforts.
Limitations
The present results should be interpreted in the context of the following limitations. Next to the diagnostic accuracy, a physician’s choice of a non-invasive imaging test should depend on multiple other factors including patient characteristics and local availability and experience. Moreover, it is eminent to realize that a binary FFR cut-off value, although practical in comparative studies and necessary to facilitate clinical decision-making, does not reflect the full physiologic spectrum of CAD. In the present study, for instance, additional FFR pullbacks and invasive assessment of microvascular disease and coronary collateral function were not performed. Analyses with the possibly more appropriate threshold of 0.7518 were therefore provided (Table 3 and Figure 2), next to a more detailed evaluation of the interaction between FFR subgroups and perfusion parameters (Supplementary material online, Table S1). Important limitations of this study are the limited sample size, the long recruitment period, and the pre-mature ending so that the target sample size was not reached and a new retrospective sample size calculation with relatively low power was required. This, together with a relatively high dropout rate for MRI, could have underestimated the accuracy of certain imaging modalities. Also other differences between the tested modalities could potentially have altered the diagnostic performance, such as the slightly different image quality (Supplementary material online, Table S5), the visual vs. quantitative perfusion assessment and the order in which the modalities were acquired. The PACIFIC 2 study was designed to reflect clinical practice. Since the start of the PACIFIC 2 study, however, SPECT, PET, and MRI scanners and acquisition protocols have evolved and these advancements may lead to improved diagnostic accuracy. Although the present study MRI acquisition protocol was designed to perform additional quantitative analysis, eventually only a qualitative approach was used because of technical reasons. This acquisition protocol including adjusted gadolinium concentrations, however, could have compromised visual image quality. We recognize that the use of quantitative approaches has gained momentum in parallel with technical advances. While some studies claim its superiority over visual analysis, other studies did not find significant differences regarding the diagnostic accuracy for detecting CAD.15,24 However, new developments in MRI myocardial perfusion assessment techniques will no doubt advance the diagnostic accuracy of ischaemia detection. Future prospective trials are warranted for assessing the surplus value of quantitative imaging over a qualitative MRI approach. Furthermore, it remains uncertain whether the current results may be extrapolated to the more commonly used [13N] ammonia and rubidium 82 tracers.16 Current findings should anyway not be extrapolated to other patient populations such as patients without prior PCI or MI. This present challenging study population might also have caused relatively low performance of SPECT, PET, and MRI perfusion imaging when compared with previous studies. Furthermore, the increasingly used CCTA was not tested in the present study, nor the additional FFR-CT computation, as these are currently not recommended for this specific patient population. With emerging technological improvements, however, it may be an option for patients with prior CAD in the future. Finally, in this diagnostic study, no statement can be made about the prognostic value of the different modalities as outcome data are lacking.
Conclusion
In this prospective head-to-head comparative study, qualitative SPECT and MRI and quantitative PET showed a similar accuracy for diagnosing haemodynamically significant CAD as defined by invasive FFR, in patients with prior PCI or MI. Clinicians should interpret the imaging results in the context of the patient’s history, symptoms, and clinical need. Overall diagnostic performances for all modalities, however, were discouragingly low and the additive value of performing non-invasive imaging in this high-risk population seems questionable.
Supplementary Material
Contributor Information
Roel S Driessen, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Pepijn A van Diemen, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Pieter G Raijmakers, Department of Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Juhani Knuuti, Department of Clinical Physiology, Nuclear Medicine and PET and Turku PET Centre, Turku University Hospital, Kiinamyllynkatu 4-8, FI-20520 Turku, Finland.
Teemu Maaniitty, Department of Clinical Physiology, Nuclear Medicine and PET and Turku PET Centre, Turku University Hospital, Kiinamyllynkatu 4-8, FI-20520 Turku, Finland.
S Richard Underwood, Department of Nuclear Medicine, Royal Brompton Hospital, Sydney St, London SW3 6NP, UK.
Eike Nagel, Institute for Experimental and Translational Cardiovascular Imaging, University Hospital Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, Germany.
Lourens F H J Robbers, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Ahmet Demirkiran, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Martin B von Bartheld, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Peter M van de Ven, Department of Epidemiology and Biostatistics, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Leonard Hofstra, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; Department of Cardiology, Cardiology Centers of the Netherlands, 1073 TB Amsterdam, The Netherlands.
G Aernout Somsen, Department of Cardiology, Cardiology Centers of the Netherlands, 1073 TB Amsterdam, The Netherlands.
Igor I Tulevski, Department of Cardiology, Cardiology Centers of the Netherlands, 1073 TB Amsterdam, The Netherlands.
Ronald Boellaard, Department of Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Albert C van Rossum, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Ibrahim Danad, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Paul Knaapen, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data Availability
The data underlying this article are available on reasonable request to the corresponding author.
References
- 1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 2. Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol 2017;2:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguade-Bruix S, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015;8:e002179. [DOI] [PubMed] [Google Scholar]
- 4. Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al Badarin FJ, Chan PS, Spertus JA, Thompson RC, Patel KK, Kennedy KF, et al. Temporal trends in test utilization and prevalence of ischaemia with positron emission tomography myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging 2020;21:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arai AE, Schulz-Menger J, Berman D, Mahrholdt H, Han Y, Bandettini WP, et al. Gadobutrol-enhanced cardiac magnetic resonance imaging for detection of coronary artery disease. J Am Coll Cardiol 2020;76:1536–1547. [DOI] [PubMed] [Google Scholar]
- 7. Bernhardt P, Spiess J, Levenson B, Pilz G, Hofling B, Hombach V, et al. Combined assessment of myocardial perfusion and late gadolinium enhancement in patients after percutaneous coronary intervention or bypass grafts: a multicenter study of an integrated cardiovascular magnetic resonance protocol. JACC Cardiovasc Imaging 2009;2:1292–1300. [DOI] [PubMed] [Google Scholar]
- 8. Perrin M, Djaballah W, Moulin F, Claudin M, Veran N, Imbert L, et al. Stress-first protocol for myocardial perfusion SPECT imaging with semiconductor cameras: high diagnostic performances with significant reduction in patient radiation doses. Eur J Nucl Med Mol Imaging 2015;42:1004–1011. [DOI] [PubMed] [Google Scholar]
- 9. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 10. Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182–3188. [DOI] [PubMed] [Google Scholar]
- 11. Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al. MR-IMPACT II: magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 2013;34:775–781. [DOI] [PubMed] [Google Scholar]
- 12. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 13. Danad I, Uusitalo V, Kero T, Saraste A, Raijmakers PG, Lammertsma AA, et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J Am Coll Cardiol 2014;64:1464–1475. [DOI] [PubMed] [Google Scholar]
- 14. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 15. Nakamori S, Sakuma H, Dohi K, Ishida M, Tanigawa T, Yamada A, et al. Combined assessment of stress myocardial perfusion cardiovascular magnetic resonance and flow measurement in the coronary sinus improves prediction of functionally significant coronary stenosis determined by fractional flow reserve in multivessel disease. J Am Heart Assoc 2018;7:e007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging 2017;33:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol 2007;14:521–528. [DOI] [PubMed] [Google Scholar]
- 18. De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation 2001;104:157–162. [DOI] [PubMed] [Google Scholar]
- 19. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 20. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopes RD, Alexander KP, Stevens SR, Reynolds HR, Stone GW, Pina IL, et al. Initial invasive versus conservative management of stable ischemic heart disease in patients with a history of heart failure or left ventricular dysfunction: insights from the ISCHEMIA trial. Circulation 2020;142:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagel E, Greenwood JP, McCann GP, Bettencourt N, Shah AM, Hussain ST, et al. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019;380:2418–2428. [DOI] [PubMed] [Google Scholar]
- 23. Al-Lamee RK, Shun-Shin MJ, Howard JP, Nowbar AN, Rajkumar C, Thompson D, et al. Dobutamine stress echocardiography ischemia as a predictor of the placebo-controlled efficacy of percutaneous coronary intervention in stable coronary artery disease: the stress echocardiography-stratified analysis of ORBITA. Circulation 2019;140:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biglands JD, Ibraheem M, Magee DR, Radjenovic A, Plein S, Greenwood JP. Quantitative myocardial perfusion imaging versus visual analysis in diagnosing myocardial ischemia: a CE-MARC substudy. JACC Cardiovasc Imaging 2018;11:711–718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available on reasonable request to the corresponding author.