Abstract
Aims
The optimal timing of an invasive strategy (IS) in non-ST-elevation acute coronary syndrome (NSTE-ACS) is controversial. Recent randomized controlled trials (RCTs) and long-term follow-up data have yet to be included in a contemporary meta-analysis.
Methods and results
A systematic review of RCTs that compared an early IS vs. delayed IS for NSTE-ACS was conducted by searching MEDLINE, Embase, and Cochrane Central Register of Controlled Trials. A meta-analysis was performed by pooling relative risks (RRs) using a random-effects model. The primary outcome was all-cause mortality. Secondary outcomes included myocardial infarction (MI), recurrent ischaemia, admission for heart failure (HF), repeat re-vascularization, major bleeding, stroke, and length of hospital stay. This study was registered with PROSPERO (CRD42021246131). Seventeen RCTs with outcome data from 10 209 patients were included. No significant differences in risk for all-cause mortality [RR: 0.90, 95% confidence interval (CI): 0.78–1.04], MI (RR: 0.86, 95% CI: 0.63–1.16), admission for HF (RR: 0.66, 95% CI: 0.43–1.03), repeat re-vascularization (RR: 1.04, 95% CI: 0.88–1.23), major bleeding (RR: 0.86, 95% CI: 0.68–1.09), or stroke (RR: 0.95, 95% CI: 0.59–1.54) were observed. Recurrent ischaemia (RR: 0.57, 95% CI: 0.40–0.81) and length of stay (median difference: −22 h, 95% CI: −36.7 to −7.5 h) were reduced with an early IS.
Conclusion
In all-comers with NSTE-ACS, an early IS does not reduce all-cause mortality, MI, admission for HF, repeat re-vascularization, or increase major bleeding or stroke when compared with a delayed IS. Risk of recurrent ischaemia and length of stay are significantly reduced with an early IS.
Keywords: Non-ST-elevation acute coronary syndrome, Invasive, Timing, Percutaneous coronary intervention, Mortality
Structured Graphical Abstract
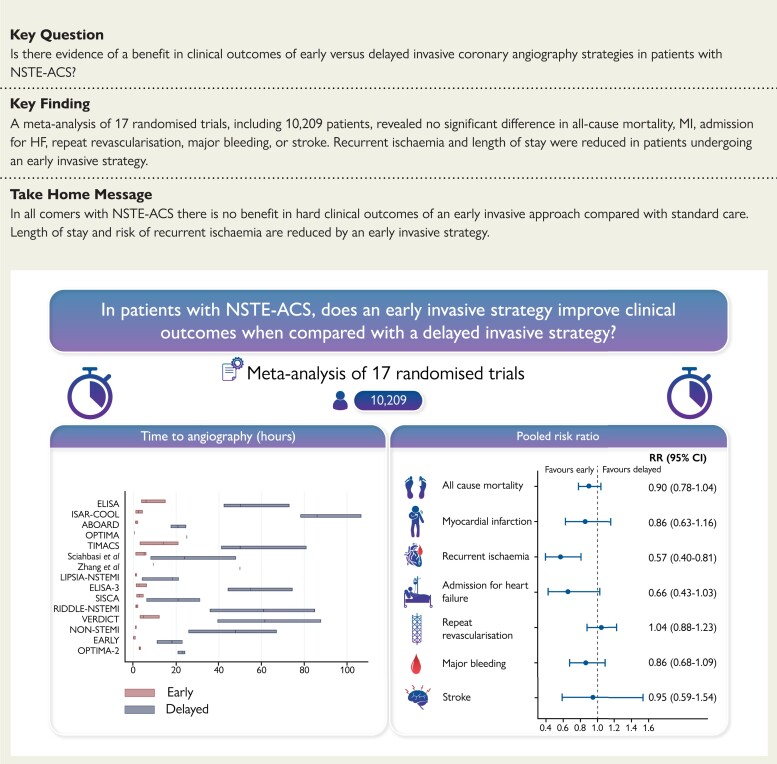
Structured Graphical Abstract.
Left: Time to invasive coronary angiography in the included randomized controlled trials. The bars represent median time and interquartile ranges in the early invasive strategy group (red) and the delayed invasive strategy group (blue). The Tekin et al.17 and Liu et al.18 studies are not displayed as medians were not reported. Interquartile ranges were not reported in the OPTIMA and Zhang et al.14 trials. Right: Summary relative risks for all-cause mortality, myocardial infarction, recurrent ischaemia, admission for heart failure, repeat revascularization, major bleeding, and stroke.
See the editorial comment for this article ‘Timing of invasive management of NSTE-ACS: is the time up for early management?’, by Paul Guedeney et al., https://doi.org/10.1093/eurheartj/ehac212.
Introduction
International guidelines recommend a routine invasive strategy (IS) for most patients with non-ST-elevation acute coronary syndrome (NSTE-ACS),1,2 supported by evidence of improved composite ischaemic outcomes when compared with a selective IS.3 However, the optimal timing of a routine IS is unclear.
Plaque passivation with anti-thrombotic agents and statins was initially proposed as a therapeutic approach to permit optimal conditions for deferred percutaneous coronary intervention (PCI).4 In contrast, an early IS with re-vascularization may attenuate ongoing or subclinical ischaemia and reduce the risk of abrupt vessel occlusion. International guidelines recommend that the timing of an IS for NSTE-ACS is determined by patient characteristics that include factors such as the risk of future ischaemic events.1
Prior meta-analyses include an aggregate study-level investigation of 6397 patients from 10 randomized controlled trials (RCTs), and a patient-level data analysis of 8 RCTs totalling 5324 patients. Both studies found no difference in hard clinical endpoints when an early IS was compared with a delayed IS in all-comers with NSTE-ACS.5,6 However, a further four RCTs that investigated the optimal timing of IS in NSTE-ACS have since reported, while long-term outcomes of patients enrolled in earlier studies have been published. The aim of the present study was to produce an updated meta-analysis to best inform contemporary clinical practice.
Methods
We performed a systematic review and updated meta-analysis of RCTs that compared the efficacy and safety of early vs. delayed invasive coronary angiography strategies in patients with NSTE-ACS. The study was reported in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-analyses statement and registered with the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42021246131).
Search strategy
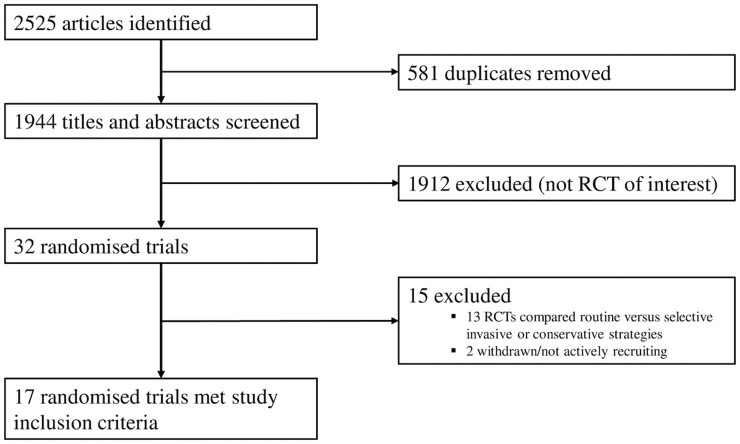
Literature search strategies were developed using medical subject headings and keywords related to NSTE-ACS. Keywords used included ‘myocardial infarction’, ‘non-ST-elevation acute coronary syndrome’, ‘NSTE-ACS’, ‘non-ST-elevation myocardial infarction’, ‘NSTEMI’, ‘early’, ‘immediate’, ‘delayed’, ‘deferred’, ‘invasive’, ‘timing’, ‘strategy’, ‘intervention’, ‘angiography’, ‘angioplasty’, ‘approach’, and ‘treatment’. The RCTs were identified using recognized search strategies with support from a research librarian (STL) with expertise in systematic review methodology.7 We searched MEDLINE (Ovid interface, 1948 onwards), Embase (Ovid interface, 1980 onwards), and the Cochrane Central Register for Clinical Trials without language restrictions up to 20 April 2021. The electronic database search was supplemented by using clinical trial registries (ClinicalTrials.gov and metaRegister of Controlled Trials) to identify any other relevant studies. Furthermore, references of included trials were assessed for other appropriate trials. Once duplicates had been removed, full study titles and abstracts were independently screened by two authors (T.A.K. and S.A.K.) according to the study inclusion criteria. In instances of uncertainty, full text articles were also independently screened (T.A.K. and S.A.K.). Any disagreement regarding inclusion or exclusion was resolved through discussion and final adjudication by a third independent author (A.L.). The MEDLINE search strategy used is described in see Supplementary material online, Appendix S1. A flow chart detailing the literature search and screening process is provided in Figure 1.
Figure 1.
Flow diagram outlining the process of article screening and trial inclusion for the present meta-analysis.
Inclusion criteria
To be eligible for inclusion, studies were required to be RCTs that compared an early vs. delayed IS in patients with NSTE-ACS and reported all-cause mortality for a minimum follow-up period of 30 days following randomization (Table 1).8–24 The RCTs that compared a routine invasive vs. selective IS or conservative management were excluded. Accordingly, for the three-arm LIPSIA-NSTEMI trial that compared immediate, early, and selective invasive strategies, we excluded the selective invasive group.15 That is, data from the immediate arm (median 1.1 h) were included in the early IS group and data from the early arm (median 18.3 h) were included in the delayed IS group of the meta-analysis. The timings used were from randomization to receipt of invasive coronary angiography, except in the OPTIMA trial that randomized patients at the time of invasive coronary angiography (the reported time interval was from randomization to receipt of PCI),11 and the Sciahbasi et al.13 and OPTIMA-2 trials in which the reported interval was from admission to angiography.24 The Tekin et al.17 and Liu et al.18 studies did not provide median timing to angiography data, rather target thresholds for each group.
Table 1.
Randomized controlled trials that investigated early vs. delayed invasive strategies in patients with non-ST-elevation acute coronary syndrome and met inclusion criteria for the present meta-analysis
| Study/author | Year | Patients, n | Point of randomization | Timing of ICA, median (h) | Mode of re-vascularization, n (%) | Primary endpoint | Longest clinical outcome follow-up available | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Early | Delayed | Early | Delayed | Early | Delayed | |||||
| ELISA van’t Hof et al.8 |
2003 | 109 | 111 | In-hospital | 6 | 50 | Medical: 27 (25) | Medical: 26 (23) | Enzymatic infarct size | 12 months |
| PCI: 66 (61) | PCI: 64 (58) | |||||||||
| CABG: 15 (14) | CABG: 21 (19) | |||||||||
| ISAR-COOL Neumann et al.9 |
2003 | 203 | 207 | In-hospital | 2.4 | 86 | Medical: 44 (22) | Medical: 58 (28) | Death or MI | 12 months |
| PCI: 143 (70) | PCI: 133 (64) | |||||||||
| CABG: 16 (8) | CABG: 16 (8) | |||||||||
| ABOARD Montalescot et al.10 |
2009 | 175 | 177 | In-hospital | 1.1 | 20.5 | Medical: 42 (24) | Medical: 55 (31) | Enzymatic infarct size | 30 days |
| PCI: 117 (67) | PCI: 105 (59) | |||||||||
| CABG: 16 (9) | CABG: 17 (10) | |||||||||
| OPTIMA Riezebos et al.11 |
2009 | 73 | 69 | Initial coronary angiography if suitable for PCI | 0.5a | 25a | Medical: 0 (0) | Medical: 0 (0) | Death, MI, or unplanned re-vascularization | 5 years |
| PCI: 73 (100) | PCI: 73 (100) | |||||||||
| CABG: 0 (0) | CABG: 0 (0) | |||||||||
| TIMACS Mehta et al.12 |
2009 | 1593 | 1438 | In-hospital | 14 | 50 | Medical: 384 (24) | Medical: 423 (29) | Death, MI, or stroke | 6 months |
| PCI: 954 (60) | PCI: 796 (55) | |||||||||
| CABG: 225 (16) | CABG: 219 (15) | |||||||||
| Sciahbasi et al.13 | 2010 | 27 | 27 | In-hospital | 5b | 24b | Medical: 0 (0) | Medical: 0 (0) | Myocardial blush grade on contrast enhanced TTE | 12 months |
| PCI: 27 (100) | PCI: 27 (100) | |||||||||
| CABG: 0 (0) | CABG: 0 (0) | |||||||||
| Zhang et al.14 | 2010 | 446 | 369 | In-hospital | 9.3 | 49.9 | Medical: 91 (20) | Medical: 20 (22) | Death, MI, major bleeding, re-PCI, RI | 6 months |
| PCI: 314 (70) | PCI: 252 (68) | |||||||||
| CABG: 41 (9) | CABG: 37 (10) | |||||||||
| LIPSIA-NSTEMI Thiele et al.15 |
2012 | 200 | 200 | In-hospital | 1.1 | 18.3 | Medical: 33 (17) | Medical: 34 (17) | Enzymatic infarct size | 6 months |
| PCI: 151 (76) | PCI: 141 (71) | |||||||||
| CABG: 16 (8) | CABG: 25 (13) | |||||||||
| ELISA-3 Badings et al.16 |
2013 | 269 | 265 | In-hospital | 2.6 | 54.9 | Medical: 27 (10) | Medical: 33 (12) | Death, MI, or RI | 2 years |
| PCI: 180 (67) | PCI: 164 (62) | |||||||||
| CABG: 62 (23) | CABG: 68 (26) | |||||||||
| Tekin et al.17 | 2013 | 69 | 62 | In-hospital | <24 hc | 24–72 hc | Medical: 0 (0) | Medical: 0 (0) | Death, MI, re-hospitalization for cardiac cause | 3 months |
| PCI: 69 (100) | PCI: 62 (100) | |||||||||
| CABG: 0 (0) | CABG: 0 (0) | |||||||||
| Liu et al.18 | 2015 | 22 | 20 | In-hospital | <12 hc | 12–24 hc | Medical: 0 (0) | Medical: 0 (0) | Not specified | 6 months |
| PCI: 22 (100) | PCI: 20 (100) | |||||||||
| CABG: 0 (0) | CABG: 0 (0) | |||||||||
| SISCA Reuter et al.19 |
2015 | 83 | 87 | Pre-hospital | 2.8 | 20.9 | Medical: 25 (32) | Medical: 23 (30) | Death, MI, urgent re-vascularization | Median 4.1 yearsd |
| PCI: 45 (58) | PCI: 45 (59) | |||||||||
| CABG: 8 (10) | CABG: 8 (11) | |||||||||
| RIDDLE-NSTEMI Milosevic et al.20 |
2016 | 162 | 161 | In-hospital | 1.4 | 61 | Medical: 15 (9) | Medical: 18 (11) | Death or MI | 3 years |
| PCI: 127 (78) | PCI: 104 (65) | |||||||||
| CABG: 20 (12) | CABG: 38 (24) | |||||||||
| VERDICT Kofoed et al.21 |
2018 | 1075 | 1072 | In-hospital | 4.7 | 61.6 | Medical: 445 (41) | Medical: 498 (46) | Death, MI, admission for heart failure, or admission for refractory ischaemia | Median 4.3 years |
| PCI: 498 (46) | PCI: 442 (41) | |||||||||
| CABG: 132 (12) | CABG: 132 (12) | |||||||||
| Non-STEMI Rasmussen et al.22 |
2019 | 245 | 251 | Pre-hospital | 1.0 | 47.8 | Medical: 14 (8) | Medical: 13 (7) | Death, MI admission for heart failure | 12 months |
| PCI: 124 (73) | PCI: 122 (68) | |||||||||
| CABG: 21 (12) | CABG: 36 (20) | |||||||||
| Hybrid: 10 (6) | Hybrid: 8 (5) | |||||||||
| EARLY Lemesle et al.23 |
2020 | 346 | 363 | In-hospital | 0 | 18 | Medical: 82 (25) | Medical: 64 (19) | CV death or RI | 30 days |
| PCI: 230 (72) | PCI: 262 (78) | |||||||||
| CABG: 9 (3) | CABG: 10 (3) | |||||||||
| OPTIMA-2 Fagel et al.24 |
2021 | 125 | 124 | In-hospital | 2.9b | 22.8b | Medical: 41 (33) | Medical: 33 (26) | Enzymatic infarct size | 12 months |
| PCI: 59 (47) | PCI: 75 (61) | |||||||||
| CABG: 24 (19) | CABG: 15 (12) | |||||||||
CABG, coronary artery bypass grafting; ICA, invasive coronary angiography; MI, myocardial infarction; PCI, percutaneous coronary intervention; RI, recurrent ischaemia; TTE, transthoracic echocardiography.
In the OPTIMA trial, timing of ICA was the interval from randomization (performed at initial angiography when PCI was deemed to be the most appropriate re-vascularization strategy) to receipt of PCI.
In the Sciahbasi et al.13 and OPTIMA-2 trials, timing of ICA was reported as the interval from admission to angiography.
In the trials by Liu et al.18 and Tekin et al.,17 median timing of ICA for each group was not provided. Timing targets specified in the study methodology are listed.
In the SISCA trial only all-cause mortality was reported at a median 4.1 year follow-up. The remaining endpoints were reported at 30 days.
Data extraction
Baseline demographic and clinical outcome data were extracted from the main study reports (see Supplementary material online, Appendix S2). Any Supplementary material or appendices were also reviewed. Long-term follow-up data in the case of the OPTIMA, ELISA-3, and RIDDLE-NSTEMI trials were obtained from subsequent publications.25–27 Since both ELISA and ISAR-COOL trials provided only 30-day outcomes in their primary reports, 12-month outcomes for these studies were extracted from the Katritsis et al.28 study-level data meta-analysis published in 2011. Data available from the Zhang et al.14 study were limited.
We systematically recorded study baseline characteristics as mean and standard deviation, or median and interquartile range (IQR) if these were not normally distributed. Frequencies and percentages were used to summarize categorical variables. Clinical outcome data were extracted on an intention-to-treat basis. For studies with multiple follow-up periods, we included data from the longest follow-up period reported for each individual endpoint.
Two independent authors (T.A.K. and S.A.K.) assessed the methodological quality of the included trials according to the Cochrane tool for assessing risk of bias in RCTs.29 Any disagreement was resolved through discussion and final adjudication by a third independent author (A.L.). These assessments are provided in see Supplementary material online, Appendix S3.
Study endpoints
The primary outcome of the study was all-cause mortality. Secondary outcomes included myocardial infarction (MI), recurrent ischaemia, admission for heart failure (HF), repeat re-vascularization, major bleeding, stroke, and length of hospital stay. Individual study endpoint definitions are detailed in see Supplementary material online, Appendix S4.
Statistical analysis
For binary outcomes, we extracted the number of events and total number of patients for both the early and delayed IS groups in the included RCTs. The number of patients without events was calculated by subtracting the number of events from the total number of patients. The number of patients with, and without, events in each group was then used to calculate the individual and the pooled relative risks (RRs) with corresponding 95% confidence intervals (CIs) using the DerSimonian and Laird random-effects model.30 For the continuous outcome of length of hospital stay, the median length of hospitalization (in hours) from each study, alongside the first and third quartiles, was extracted and the pooled effect calculated using the quantile estimation method which also employed a DerSimonian and Laird random-effects model.31
Following Cochrane recommendations, between study heterogeneity was assessed using Cochran’s Q statistic (with the significance level set at 0.10) and quantified using the I2 statistic.7 Heterogeneity was classified as no important, moderate, substantial, and considerable if the I2 percentage was <25, 25–50, 51–75, and >75%, respectively.32 Publication bias was estimated, when meta-analyses included 10 or more studies,33 through Egger’s linear regression test,34 while funnel plots were inspected to evaluate the presence or absence of asymmetry. To evaluate stability of results, sensitivity analyses were performed by removing one study at a time (i.e. leave-one-out meta-analysis) and re-calculating the pooled effect size.
Statistical analyses for the binary outcomes were performed using STATA (Version 17.0; StataCorp, College Station, TX, USA). Statistical analysis for the length of hospital stay was performed in R (version 4.0.3, https://www.R-project.org/) using the package ‘metamedian’.35 All tests were two-sided and the significance level was set at 0.05.
Results
The literature searches returned 2525 studies, of which 581 were duplicates. After independent screening of titles and abstracts, 32 RCTs were scrutinized in detail. Of these, 17 met the inclusion criteria and were included in this meta-analysis (Figure 1). For the primary outcome, the study by Sciahbasi et al.13 was excluded because no mortality events in either the early IS or delayed IS groups were reported. Therefore, the present meta-analysis includes a further six RCTs and a total of 10 155 patients with all-cause mortality data, almost 4000 additional patients when compared with the last major aggregate data meta-analysis published in 2016.5
Trial characteristics are displayed in Table 1. Additional tables that summarize patient demographics, inclusion criteria, and endpoint definitions across studies are included in see Supplementary material online, Appendices S2 and S4. These data were available for all trials except Zhang et al.14
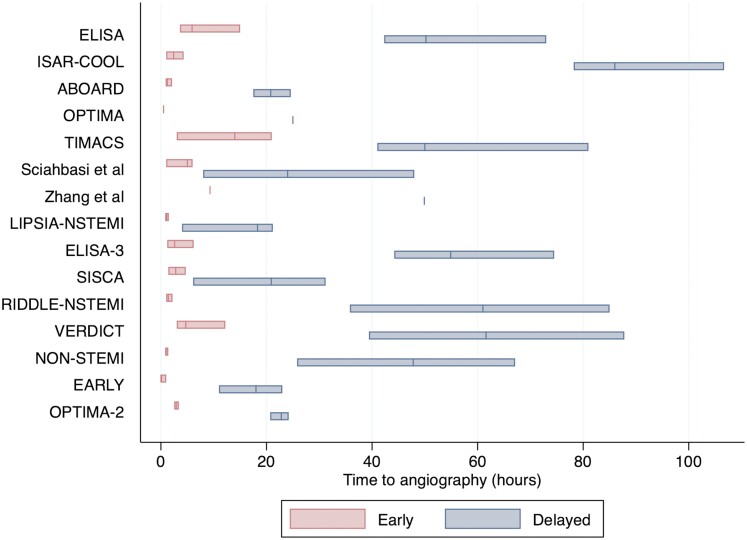
Of the 17 included articles, in total 5215 patients received an early IS, while 4994 received a delayed IS. The pooled medians timings to angiography across the included trials were 3.43 h (1.47–5.40 h) in the early IS group and 41.3 h (29.3–53.2 h) in the delayed IS group. Four studies totalling 1130 patients were excluded from this analysis because IQR data were not reported (Figure 2).11,14,17,18 Baseline demographics across trials were well balanced (see Supplementary material online, Appendix S2). There was heterogeneity of inclusion criteria and endpoint definitions across the included trials, in particular with respect to MI and recurrent ischaemia (see Supplementary material online, Appendix S4). All patients received either PCI, coronary artery bypass grafting, or optimal medical therapy except four studies in which all patients underwent PCI.11,13,17,18 All patients were treated with dual anti-platelet therapy prior to invasive coronary angiography, except in the EARLY trial where this was only given if re-vascularization was undertaken.23 The median follow-up period across all trials was 12 months (IQR 6–24 months).
Figure 2.
Time to invasive coronary angiography in the included randomized controlled trials. The bars represent median time and interquartile ranges in the early invasive strategy group (left) and the delayed invasive strategy group (right). The Tekin et al. and Liu et al. studies are not displayed as medians were not reported. Interquartile ranges were not reported in the OPTIMA and Zhang et al.14 trials.
Risk of bias
Risk of bias assessments are displayed in see Supplementary material online, Appendix S3. In general, there was a low risk of bias across the included trials. However, several studies did not provide sufficient information regarding their process of randomization, allocation concealment, and blinded adjudication of outcomes, thus they were graded as ‘unclear risk of bias’. Three studies, Zhang et al.14, Tekin et al.17, and Liu et al.,18 were considered to be at high risk of bias due to concerns regarding the method of randomization and the blinding of outcome assessment. Very few patients (<5%) were lost to follow-up across the included trials, with any such cases reported appropriately. Study participants and personnel were not blinded to their treatment allocation and timing of the IS, as is convention in pragmatic strategy trials that investigate timing of IS in NSTE-ACS.
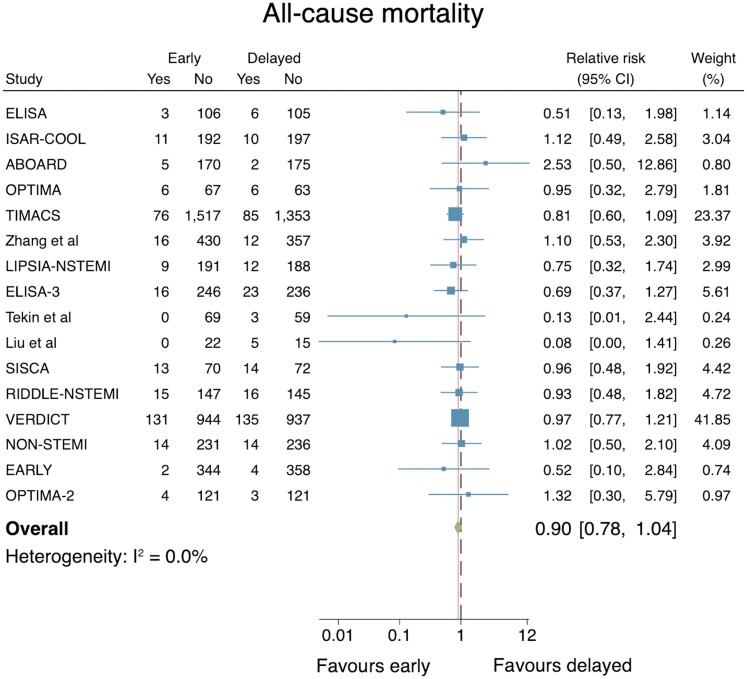
All-cause mortality
All 17 studies reported the effect of timing of an IS on all-cause mortality. Excluding the Sciahbasi et al.13 trial (as no mortality events were reported), data from 10 155 patients in 16 RCTs were included in the primary analysis. No difference was demonstrated when an early IS was compared with a delayed IS (RR: 0.90, 95% CI: 0.78–1.04; Figure 3). No important heterogeneity across the trials was identified (I2 = 0.0%). The associated funnel plot was relatively symmetrical and did not suggest evidence of significant publication bias, supported by Egger’s test for small-study effects (P = 0.37, see Supplementary material online, Appendix S5). A leave-one-out meta-analysis did not change the statistical significance of the results. Sensitivity analyses that excluded six trials in which the median time to angiography in the delayed IS arm was <24 h did not alter the point estimate or significance of the results (RR: 0.90, 95% CI 0.77–1.05). Subgroup analyses stratified by length of follow-up did not alter the results significantly: short term (30 days) including three RCTs, RR: 1.17 (95% CI: 0.25–5.48); medium term (>30 days to 12 months) including 11 RCTs, RR: 0.85 (95% CI: 0.68–1.06); long-term (>12 months) including 5 RCTs, RR: 0.93 (95% CI: 0.77–1.13).
Figure 3.
Individual and summary relative risks for all-cause mortality in randomized controlled trials that compared early vs. delayed invasive strategies.
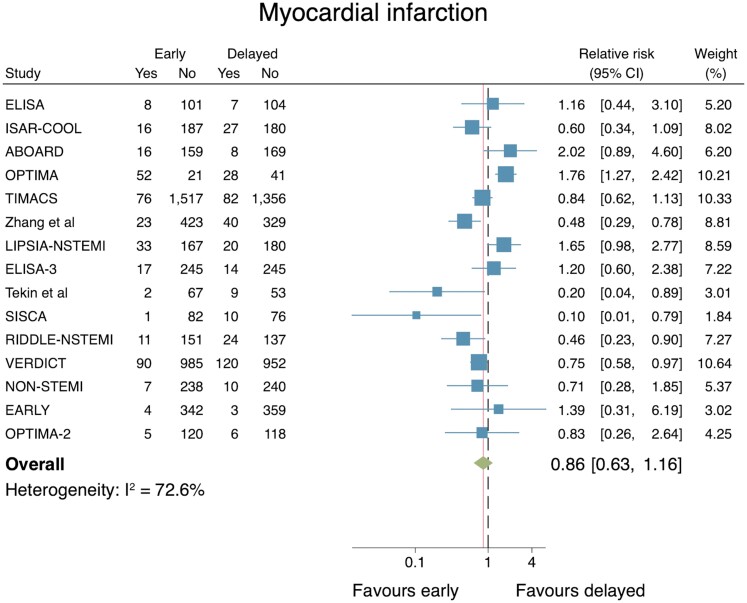
Myocardial infarction
The effect of an early IS vs. a delayed IS on MI was reported in 16 trials. Again, the Sciahbasi et al.’s study reported no events in either group. The total number of patients included in the analysis of 15 RCTs was 10 113. An early IS did not reduce the risk for MI (RR: 0.86, 95% CI: 0.63–1.16; Figure 4). Although several studies lay outside the 95% CIs, the associated funnel plot appears symmetrical. Moreover, there was no evidence of publication bias when excluding estimates for smaller studies compared with larger studies (Egger’s test, P = 0.16, see Supplementary material online, Appendix S5). Substantial evidence of heterogeneity between the studies was observed (I2 = 72.6%), yet a leave-one-out meta-analysis did not change the statistical significance of the results.
Figure 4.
Individual and summary relative risks for myocardial infarction in randomized controlled trials that compared early vs. delayed invasive strategies.
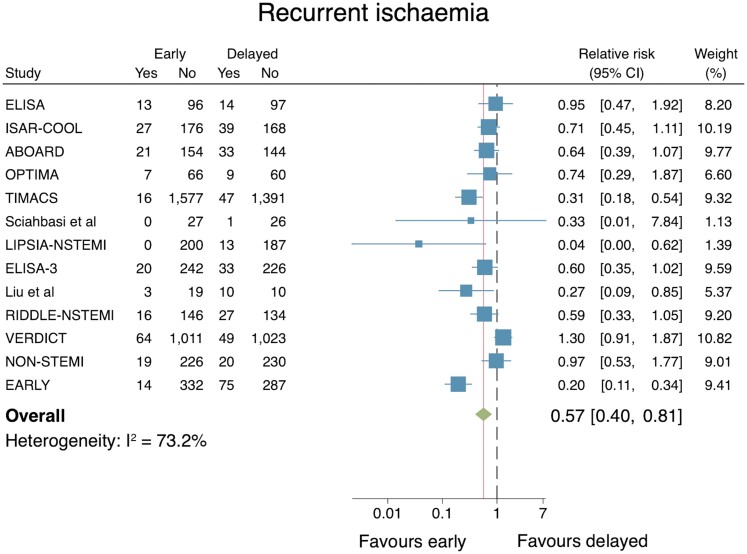
Recurrent ischaemia
The effect of an early IS vs. a delayed IS on recurrent ischaemia was reported in 13 trials (n = 8845). An early IS was associated with a reduced risk for recurrent ischaemia (RR: 0.57, 95% CI: 0.40–0.81; Figure 5). However, substantial heterogeneity across the trials was noted (I2 = 73.2%), while there are some evidence of small-study effects (Egger’s test P = 0.08; see Supplementary material online, Appendix S5). A leave-one-out meta-analysis did not markedly change the pooled point estimate nor alter the statistical significance of the results.
Figure 5.
Individual and summary relative risks for recurrent ischaemia in randomized controlled trials that compared early vs. delayed invasive strategies.
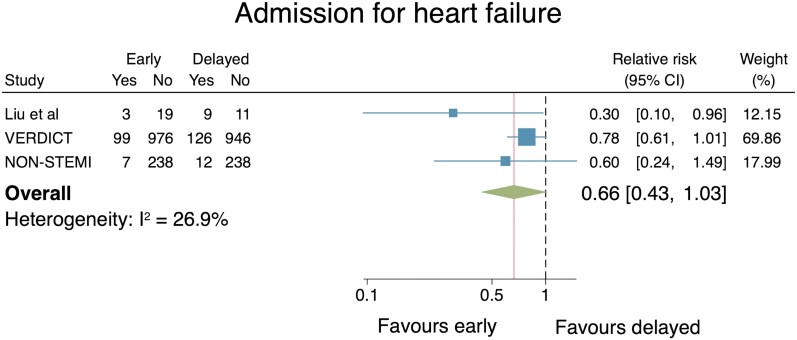
Admission for heart failure
Only three RCTs reported the effect of an early IS vs. a delayed IS on admission with HF (n = 2684). No difference was demonstrated when the two strategies were compared (RR: 0.66, 95% CI: 0.43–1.03; Figure 6). Heterogeneity across the studies was classified as no important (I2 = 26.9%). Publication bias testing was inappropriate due to the small (<10) number of included studies.33 When a leave-one-out meta-analysis was performed the pooled estimates differed and met significance, indicating that some studies were more influential than others. Individual removal of the Liu et al., VERDICT, and non-STEMI trials from the analysis resulted in pooled RR estimates of 0.77 (95% CI: 0.60–0.98), 0.46 (95% CI: 0.22–0.94), and 0.58 (95% CI: 0.24–1.38), respectively.
Figure 6.
Individual and summary relative risks for admission for heart failure in randomized controlled trials that compared early vs. delayed invasive strategies.
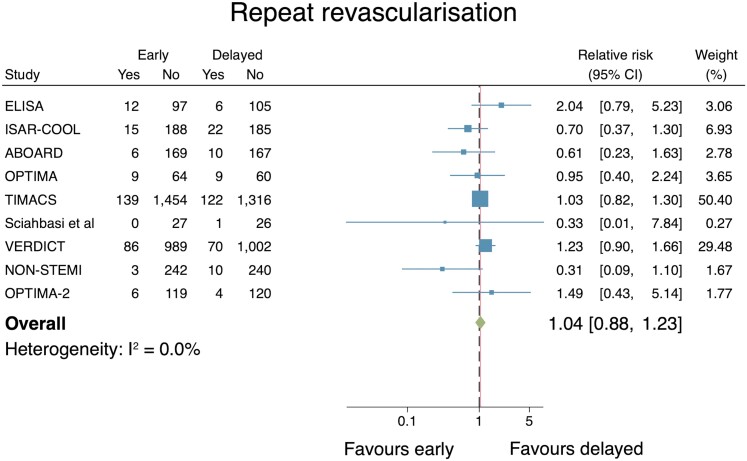
Repeat re-vascularization
The effect of an early IS vs. a delayed IS on repeat re-vascularization was reported in 9 studies with n = 7100 in the final analysis. There was no significant difference between the two groups (RR: 1.04, 95% CI: 0.88–1.23; Figure 7). No important heterogeneity of the included trials was identified according to the I2 statistic (0.0%). Publication bias testing was inappropriate due to the small (<10) number of included studies.33 Sensitivity analyses conducted via a leave-one-out meta-analysis did not change the statistical significance of the results.
Figure 7.
Individual and summary relative risks for repeat re-vascularization in randomized controlled trials that compared early vs. delayed invasive strategies.
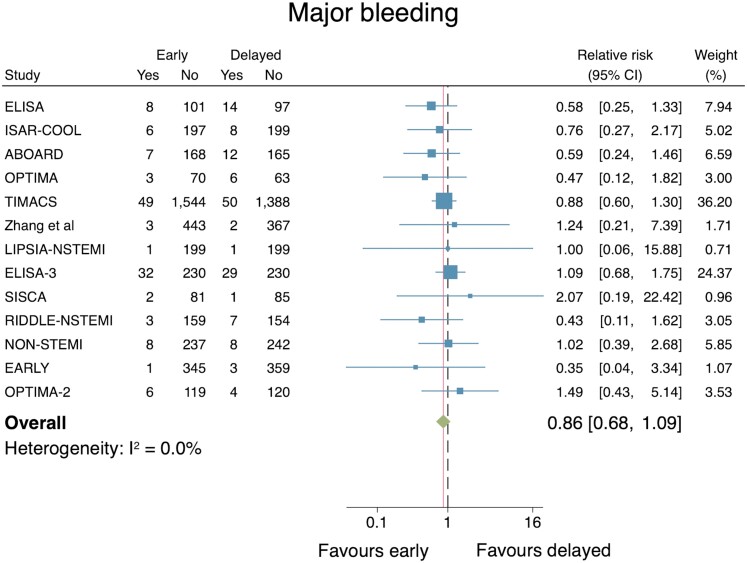
Major bleeding
The effect of an early IS vs. a delayed IS on major bleeding was reported in 13 studies (n = 7835). Moreover, the VERDICT trial was excluded as the investigators reported all bleeding events and did not categorize these according to accepted major or minor bleeding criteria. No significant difference between the two groups was observed (RR: 0.86, 95% CI: 0.68–1.09; Figure 8) and no important heterogeneity was identified (I2 = 0.0%). The associated funnel plot did not suggest evidence of significant publication bias, while Egger’s test for small-study effects was non-significant at the 5% level (P = 0.55, see Supplementary material online, Appendix S5). A leave-one-out meta-analysis did not markedly change the pooled RR estimate nor alter the statistical significance of the results.
Figure 8.
Individual and summary relative risks for major bleeding in randomized controlled trials that compared early vs. delayed invasive strategies.
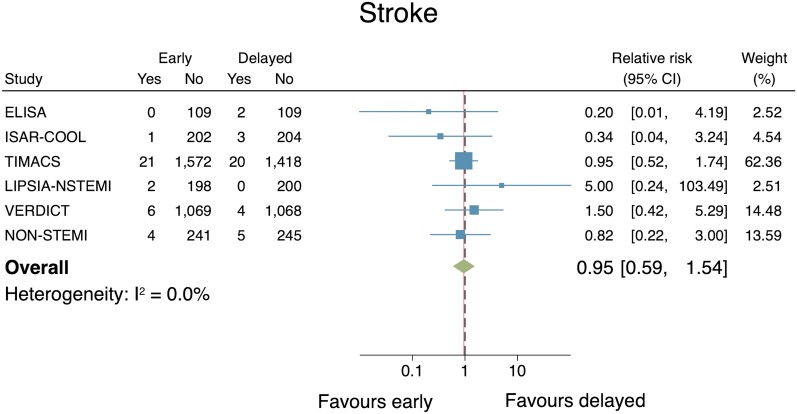
Stroke
Six studies reported the effect of an early IS vs. a delayed IS on stroke (n = 6703). There was no significant difference between groups (RR: 0.95, 95% CI: 0.59–1.54; Figure 9). No evidence of important heterogeneity across the included studies was found (I2 = 0.0%). Publication bias testing was inappropriate due to the small (<10) number of included studies.33 Sensitivity analyses conducted via a leave-one-out meta-analysis did not change the statistical significance of the results.
Figure 9.
Individual and summary relative risks for stroke in randomized controlled trials that compared early vs. delayed invasive strategies.
Length of stay
Eight studies reported the effect of an early IS vs. a delayed IS on length of hospital stay (n = 3029). An early IS was associated with a reduction in length of stay (median difference: −22 h, 95% CI: −37 h to −8 h; P = 0.003). Pooled medians for the early IS and delayed IS groups were 86 h (95% CI: 60–111 h) and 111 h (95% CI: 74–148 h), respectively.
Discussion
This study, to our knowledge the largest and most contemporary meta-analysis in this field, found that an early IS does not reduce all-cause mortality, MI, admission for HF, or repeat re-vascularization when compared with a delayed IS. However, an early IS reduces risk of recurrent ischaemia, albeit there is potential of publication bias regarding this outcome, and length of hospital stay. Safety outcomes of major bleeding and stroke were no different between strategies (Structured Graphical abstract).
Prior meta-analyses have demonstrated a reduction in death and MI when a routine IS was compared with a selective invasive or conservative strategy in patients with NSTE-ACS.36,37 Nevertheless, the optimal timing of an invasive approach and follow-on re-vascularization is uncertain. International guidelines recommend that decision processes concerning timing of an IS are informed by risk stratification.1,2 Unstable or very high-risk patients with a clinical indication to undergo an immediate IS (<2 h) have largely been excluded from prior RCTs. Thereafter, The European Society of Cardiology (ESC) guidelines recommend, as a Class IA recommendation, an early IS (<24 h) is recommended in clinically stabilized high-risk NSTE-ACS patients which exhibits either (i) temporal change in troponin or (ii) Global Registry of Acute Coronary Events (GRACE) score ≥140.1,2 The guidelines additionally recommend that such an approach be undertaken in patients with dynamic ST-T segment electrocardiogram changes or transient ST-segment elevation.1 A selective invasive or ischaemia-guided strategy is reserved for the remaining cohort who do not meet the above criteria and are therefore deemed to be of low baseline risk.
Previous RCTs and meta-analyses of those trials have sequentially failed to demonstrate a significant difference in death or MI between an early IS and delayed IS in patients with NSTE-ACS.5,6,28,38 In the present study, these findings have been replicated in a larger data set and provide firmer evidence that there is no survival benefit with an early IS in NSTE-ACS all-comers. Moreover, the study demonstrates that no significant risk reduction for MI is associated with either strategy, although heterogeneity in endpoint definitions across the included RCTs must be acknowledged. The present analysis does show an approximate 50% reduction in risk of recurrent ischaemia in those patients who received an early IS, yet this does not translate into a lower rate of either MI or repeat re-vascularization in this population. This counterintuitive result may be due to the inconsistent definitions of recurrent ischaemia which were used across the trials, with often a single episode of ischaemic chest pain meeting such endpoint criteria. Of note, the non-STEMI and SISCA trials found that 17% and 21% of patients randomized to a delayed IS required accelerated angiography due to clinical deterioration, respectively.19,20 As presented, however, these results in totality imply that recurrent ischaemia as defined in these studies is not a direct risk factor for, or predictor of, subsequent spontaneous MI or greater need for re-vascularization.
Importantly, to the best of our knowledge this is the first meta-analysis to evaluate timing of IS on admission for HF in patient with NSTE-ACS. Although the initial findings demonstrated a non-statistically significant trend to reduced HF hospitalization, it is noteworthy that a sensitivity analysis excluding the 42 patients from the Liu et al. trial, which is of poor methodological quality with very high risk of bias, narrowed the CIs, and resulted in a significant reduction in admission for HF with use of an early IS. Therefore, pooling of RRs from the VERDICT and non-STEMI RCTs suggests that an early IS reduces future risk of HF hospitalization. However, given that only three RCTs reported hospitalization with HF as an endpoint with a total of 256 events, and that this finding was not linked to a reduction in MI in the early IS group, this finding may only be considered hypothesis generating. Admission with HF as a reported outcome is recognized to be of growing significance because of a documented association with morbidity and as a predictor of poor outcomes.39 Future ACS RCTs should aim to capture HF hospitalization as a key secondary outcome.
Guideline recommendations for an early IS in high-risk NSTE-ACS are predominantly based on a priori subgroup analyses of GRACE score ≥140 patients from the TIMACS and VERDICT trials.12,21 These demonstrated a reduction in composite ischaemic outcomes in patients that underwent an early IS, but the findings have yet to be confirmed in a RCT that specifically investigates this high-risk subgroup. The Jobs et al.6 individual patient data meta-analysis also suggested an early IS may benefit patient subgroups with elevated biomarkers at baseline, diabetes mellitus, and age >75 years, although statistical test for interaction were inconclusive. The RAPID NSTEMI trial set out to answer this question but lower than expected event rates and slow recruitment due to the coronavirus disease 2019 pandemic resulted in early termination of the study.40 This question may only be definitively answered by a future patient-level meta-analysis of this subgroup.
If current ESC guidelines definitions are applied in a real-world setting,1,2 of 137 000 NSTE-ACS patients who underwent an IS in England and Wales between 2010 and 2015, 94% would have met the ‘high-risk’ NSTE-ACS criteria. However, in this report only 16% of the high-risk cohort received an IS within the guideline recommended 24 h of hospital admission.41 Delivery of an early IS will likely be unachievable for many healthcare systems without significant and potentially costly restructuring of ACS pathways. It could be argued that the most recent ESC guidelines have moved ahead of the currently available evidence—and the current Class IA recommendations questioned. An early IS for all patients with NSTE-ACS and a temporal change in troponin may be unnecessary, since there are no randomized trial data that support that such an approach reduces death, MI, or repeat re-vascularization in this specific patient group. Perhaps more in line with current evidence, the recently published ACC/AHA guidelines on myocardial re-vascularization recommend that in initially stabilized patients considered to be of high risk of clinical events (defined as those with a GRACE score of ≥140), it is reasonable to choose an early IS (within 24 h) over a delayed IS to improve outcomes (strength of recommendation IIa, level of evidence B-R). They do not recommend this approach for all patients with NSTE-ACS and a temporal change in troponin.42 This meta-analysis has shown that in all-comers with NSTE-ACS, an early IS results in shorter length of stay and less recurrent ischaemia, which is not associated with a higher rate of death or MI. Our results themselves do not directly challenge the ESC recommendation for an early IS for all patients with NSTE-ACS and a temporal change in troponin. However, aside from those with a GRACE score of ≥140, the benefit from randomized trials of an early IS is limited to a shorter length of stay and less recurrent ischaemia. It is questionable whether these benefits would be sufficient for a major restructuring of care pathways for all patients with NSTE-ACS and a temporal change in troponin.
Furthermore, safety and cost-efficacy must be rigorously considered for the routine use of an early IS to be widely recommended in clinical guidelines. This meta-analysis found that no excess risk for major bleeding or stroke was associated with an early IS. In addition, patients who underwent an early IS experienced significantly shorter length of hospital stay, with these differences likely to be exaggerated in countries where the wait for standard of care angiography is longer than the delayed IS group timings of the included RCTs. In a post hoc analysis of the TIMACS trial participants, healthcare cost savings were indeed associated with an early IS,43 yet there are few additional data that provide robust insights as to the economic benefits of this approach in patients with NSTE-ACS.
Despite advances in NSTE-ACS care over recent years, timing of an IS remains a contentious issue to be resolved for higher risk patients in particular. Future clinical research studies that focus on identifying those higher risk NSTE-ACS patients who may benefit most from an early IS are required. Moreover, means of better selecting appropriate individuals with obstructive coronary disease that require re-vascularization is also necessary. Research initiatives directed at investigating these strategies may yield results that relieve pressure on catheter laboratories, and thus obviate the need for widespread NSTE-ACS pathway and service re-configuration.
This study has limitations. First, as this is an aggregate study-level data meta-analysis, our results are limited by a lack of individual patient-level data that affords closer examination of subgroups and specific treatment effects. However, it is considered that a robustly conducted aggregate data meta-analysis produces comparable results to individual patient data studies, and that similar conclusions are often drawn.44 Second, substantial heterogeneity across the included RCTs with respect to inclusion criteria, timing of IS, endpoint definitions, and follow-up periods may have impacted on the validity of our results. Perhaps the most important difference is the timing of IS across trials. In 30% of studies, the delayed arm was in fact quite early (median <24 h), with any potential treatment effect of an early IS possibly diluted by the limited time separation between groups. Third, the included trials span a 20-year time period during which diagnostic, pharmacological, and invasive strategies for NSTE-ACS have evolved significantly. For example, many trials used highly variable diagnostic criteria for MI and enrolled patients prior to the widespread use of high-sensitivity troponin, meaning ascertainment of early re-MI (spontaneous or peri-procedural) was not robust. Importantly, approximately 25% of patients across the included trials were biomarker negative and thus could be classified as unstable angina. This could dilute any potential treatment effect from an early IS in higher risk patients with myocardial injury and limits the conclusions that can be drawn. However, it should also be noted that all but one of the included trials were conducted using conventional troponin assays. The reduced sensitivity of these assays when compared with contemporary high-sensitivity troponin assay is important to acknowledge since a proportion of the patients labelled as ‘unstable angina’ may have in fact have had smaller degrees of myocardial injury and infarction and met current diagnostic criteria for NSTEMI.45
Conclusion
In conclusion, an early IS was not associated with a reduction in risk of all-cause mortality, MI, admission for HF, and repeat re-vascularization compared with a delayed IS in an all-comer NSTE-ACS population. An early IS resulted in risk reduction for recurrent ischaemia and length of hospital stay. Safety outcomes consisting of major bleeding and stroke were no different between strategies. International guideline recommendations require greater scrutiny since data that support an early IS in NSTE-ACS patients are limited. Future RCTs ought to focus on identification of pre-defined high-risk subgroups that may benefit from an early IS.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgement
The authors acknowledge the support of the National Institute of Health Research Biomedical Research Centre in Leicester.
Contributor Information
Thomas A Kite, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Sameer A Kurmani, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Vasiliki Bountziouka, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Nicola J Cooper, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Selina T Lock, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Chris P Gale, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Marcus Flather, Norwich Medical School, University of East Anglia and Norfolk and Norwich University Hospital, Norwich, UK.
Nick Curzen, University Hospital Southampton NHS Foundation Trust and School of Medicine, University of Southampton, Southampton, UK.
Adrian P Banning, Department of Cardiology, Oxford Heart Centre, John Radcliffe Hospital, Oxford, UK.
Gerry P McCann, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Andrew Ladwiniec, Department of Cardiovascular Sciences and the NIHR Leicester Biomedical Research Centre, Glenfield Hospital, University of Leicester and University Hospitals of Leicester NHS Trust, Leicester, UK.
Funding
This work received no specific grant from any funding agency.
Data Availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Collet JP, Thiele H, Barbato E, Bauersachs J, Dendale P, Edvardsen T, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 2. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: Executive Summary. Circulation 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 3. Mehta S, Cannon CP, Fox KAA, Wallentin L, Boden WE, Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA 2005;293:2908–2917. [DOI] [PubMed] [Google Scholar]
- 4. Monroe VS, Kerensky RA, Rivera E, Smith KM, Pepine CJ. Pharmacologic plaque passivation for the reduction of recurrent cardiac events in acute coronary syndromes. J Am Coll Cardiol 2003;41:S23–S30. [DOI] [PubMed] [Google Scholar]
- 5. Bonello L, Laine M, Puymirat E, Lemesle G, Thuny F, Paganelli F, et al. Timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes and clinical outcomes: an updated meta-analysis. JACC Cardiovasc Interv 2016;9:2267–2276. [DOI] [PubMed] [Google Scholar]
- 6. Jobs A, Mehta SR, Montalescot G, Vicaut E, van’t Hof AWJ, Badings EA, et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: a meta-analysis of randomised trials. Lancet 2017;390:737–746. [DOI] [PubMed] [Google Scholar]
- 7. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., eds. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). In: Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021. www.training.cochrane.org/handbook [Google Scholar]
- 8. van’t Hof AWJ, De Vries ST, Dambrink JHE, Miedema K, Suryapranata H, Hoorntje JCA, et al. A comparison of two invasive strategies in patients with non-ST elevation acute coronary syndromes: results of the early or late intervention in unstable angina (ELISA) pilot study: 2b/3a upstream therapy and acute coronary syndromes. Eur Heart J 2003;24:1401–1405. [DOI] [PubMed] [Google Scholar]
- 9. Neumann F-J, Kastrati A, Pogatsa-Murray G, Mehilli J, Bollwein H, Bestehorn HP, et al. Evaluation of prolonged antithrombotic pretreatment (“Cooling-Off” Strategy) before intervention in patients with unstable coronary syndromes a randomized controlled trial. JAMA 2003;290:1593–1599. [DOI] [PubMed] [Google Scholar]
- 10. Montalescot G, Cayla G, Collet JP, Elhadad S, Beygui F, Le BH, et al. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 2009;302:947–954. [DOI] [PubMed] [Google Scholar]
- 11. Riezebos RK, Ronner E, Ter Bals E, Slagboom T, Smits PC, Ten Berg JM, et al. Immediate versus deferred coronary angioplasty in non-ST-segment elevation acute coronary syndromes. Heart 2009;95:807–812. [DOI] [PubMed] [Google Scholar]
- 12. Mehta S, Granger C, Boden W, Steg P, Bassand J, Faxon D, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009;360:2165–2175. [DOI] [PubMed] [Google Scholar]
- 13. Sciahbasi A, Madonna M, De Vita M, Agati L, Scioli R, Summaria F, et al. Comparison of immediate vs early invasive strategy in patients with first acute non-ST-elevation myocardial infarction. Clin Cardiol 2010;33:650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Qiao S, Zhu J, Chinese Cooperative Group of the Timing of Intervention in Acute Coronary Syndromes . Outcome of patients with non-ST segment elevation acute coronary syndrome undergoing early or delayed intervention. Zhonghua Xin Xue Guan Bing Za Zhi 2010;38:865–869. [PubMed] [Google Scholar]
- 15. Thiele H, Rach J, Klein N, Pfeiffer D, Hartmann A, Hambrecht R, et al. Optimal timing of invasive angiography in stable non-ST-elevation myocardial infarction: the leipzig immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA-NSTEMI Trial). Eur Heart J 2012;33:2035–2043. [DOI] [PubMed] [Google Scholar]
- 16. Badings EA, The SHK, Dambrink JHE, Van Wijngaarden J, Tjeerdsma G, Rasoul S, et al. Early or late intervention in high-risk non-ST-elevation acute coronary syndromes: results of the ELISA-3 trial. EuroIntervention 2013;9:54–61. [DOI] [PubMed] [Google Scholar]
- 17. Tekin K, Cagliyan C IHT, Balli M, Uysal O BO, Arik O, Cayli M. Influence of the timing of percutaneous coronary intervention on clinical outcomes in non-ST-elevation myocardial infarction. Korean Circ J 2013;43:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Zhao L, Li Y, Wang Z, Liu L, Zhang F. Evaluation of early interventional treatment opportunity of the elderly & high-risk patients with non-ST segment elevation acute myocardial infarction. Pakistan J Med Sci 2015;31:1053–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reuter PG, Rouchy C, Cattan S, Benamer H, Jullien T, Beruben A, et al. Early invasive strategy in high-risk acute coronary syndrome without ST-segment elevation. The Sisca randomized trial. Int J Cardiol 2015;182:414–418. [DOI] [PubMed] [Google Scholar]
- 20. Milosevic A, Vasiljevic-Pokrajcic Z, Milasinovic D, Marinkovic J, Vukcevic V, Stefanovic B, et al. Immediate versus delayed invasive intervention for non-STEMI patients: the RIDDLE-NSTEMI study. JACC Cardiovasc Interv 2016;9:541–549. [DOI] [PubMed] [Google Scholar]
- 21. Kofoed KF, Kelbæk H, Riis Hansen P, Torp-Pedersen C, Høfsten D, Kløvgaard L, et al. Early versus standard care invasive examination and treatment of patients with non-ST-segment elevation acute coronary syndrome verdict randomized controlled trial. Circulation 2018;138:2741–2750. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen MB, Stengaard C, Sørensen JT, Riddervold IS, Søndergaard HM, Niemann T, et al. Comparison of acute versus subacute coronary angiography in patients with non-ST-elevation myocardial infarction (from the NONSTEMI Trial). Am J Cardiol 2019;124:825–832. [DOI] [PubMed] [Google Scholar]
- 23. Lemesle G, Laine M, Pankert M, Boueri Z, Motreff P, Paganelli F, et al. Optimal timing of intervention in NSTE-ACS without pre-treatment: the EARLY randomized trial. JACC Cardiovasc Interv 2020;13:907–917. [DOI] [PubMed] [Google Scholar]
- 24. Fagel ND, Amoroso G, Vink MA, Slagboom T, van der Schaaf RJ, Herrman JP, et al. An immediate or early invasive strategy in non-ST-elevation acute coronary syndrome: the OPTIMA-2 randomized controlled trial. Am Heart J 2021;234:42–50. [DOI] [PubMed] [Google Scholar]
- 25. Oosterwerff EFJ, Fagel ND, Slagboom T, Tijssen JGP, Herrman JP, Smits PC, et al. Impact of percutaneous coronary intervention timing on 5-year outcome in patients with non-ST-segment elevation acute coronary syndromes. The ‘wait a day’ approach might be safer. Neth Heart J 2016;24:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Badings EA, Remkes WS, The SH, Dambrink JHE, Tjeerdsma G, Rasoul S, et al. Two-year outcome after early or late Intervention in non-ST elevation acute coronary syndrome. Open Heart 2017;4:e000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Milasinovic D, Milosevic A, Vasiljevic-Pokrajcic Z, Marinkovic J, Vukcevic V, Stefanovic B, et al. Three-year impact of immediate invasive strategy in patients with non-ST-segment elevation myocardial infarction (from the RIDDLE-NSTEMI Study). Am J Cardiol 2018;122:54–60. [DOI] [PubMed] [Google Scholar]
- 28. Katritsis DG, Siontis GCM, Kastrati A, Vant Hof AWJ, Neumann FJ, Siontis KCM, et al. Optimal timing of coronary angiography and potential intervention in non-ST-elevation acute coronary syndromes. Eur Heart J 2011;32:32–40. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 31. McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J 2020;62:69–98. [DOI] [PubMed] [Google Scholar]
- 32. Deeks J, Higgins J, Altman D, eds. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021. www.training.cochrane.org/handbook [Google Scholar]
- 33. Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGrath S, Zhao X, Steele R, Benedetti A. Metamedian: meta-analysis of medians. R package version 0.1.5. https://cran.r-project.org/package=metamedian.
- 36. O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, et al. Early invasive vs conservative treatment unstable angina and non-ST-segment. JAMA 2008;300:71–80. [DOI] [PubMed] [Google Scholar]
- 37. Fox KAA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JGP, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome. A meta-analysis of individual patient data. J Am Coll Cardiol 2010;55:2435–2445. [DOI] [PubMed] [Google Scholar]
- 38. Navarese E, Gurbel P, Andreotti F, Tantry U, Jeong Y, Kozinski M, et al. Optimal timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes: a systematic review and meta-analysis. Ann Intern Med 2013;158:261–270. [DOI] [PubMed] [Google Scholar]
- 39. Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJV, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006;27:65–75. [DOI] [PubMed] [Google Scholar]
- 40. Kite TA, Gershlick AH. High-risk NSTE-ACS: high time for robust data. Eur Heart J 2021;42:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rashid M, Curzen N, Kinnaird T, Lawson CA, Myint PK, Kontopantelis E, et al. Baseline risk, timing of invasive strategy and guideline compliance in NSTEMI: Nationwide analysis from MINAP. Int J Cardiol 2020;301:7–13. [DOI] [PubMed] [Google Scholar]
- 42. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e21–e129. [DOI] [PubMed] [Google Scholar]
- 43. Bainey KR, Gafni A, Rao-Melacini P, Tong W, Steg PG, Faxon DP, et al. The cost implications of an early versus delayed invasive strategy in acute coronary syndromes: the TIMACS study. J Med Econ 2014;17:415–422. [DOI] [PubMed] [Google Scholar]
- 44. Smith CT, Marcucci M, Nolan S, Iorio A, Sudell M, Riley R, et al. Analyses based on aggregate data. Cochrane Database Syst Rev 2016;9:MR000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lipinski MJ, Baker NC, Escárcega RO, Torguson R, Chen F, Aldous SJ, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J 2015;169:6–16.e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.