Abstract
Aims
Oxygen-pulse morphology and gas exchange analysis measured during cardiopulmonary exercise testing (CPET) has been associated with myocardial ischaemia. The aim of this analysis was to examine the relationship between CPET parameters, myocardial ischaemia and anginal symptoms in patients with chronic coronary syndrome and to determine the ability of these parameters to predict the placebo-controlled response to percutaneous coronary intervention (PCI).
Methods and results
Patients with severe single-vessel coronary artery disease (CAD) were randomized 1:1 to PCI or placebo in the ORBITA trial. Subjects underwent pre-randomization treadmill CPET, dobutamine stress echocardiography (DSE) and symptom assessment. These assessments were repeated at the end of a 6-week blinded follow-up period.
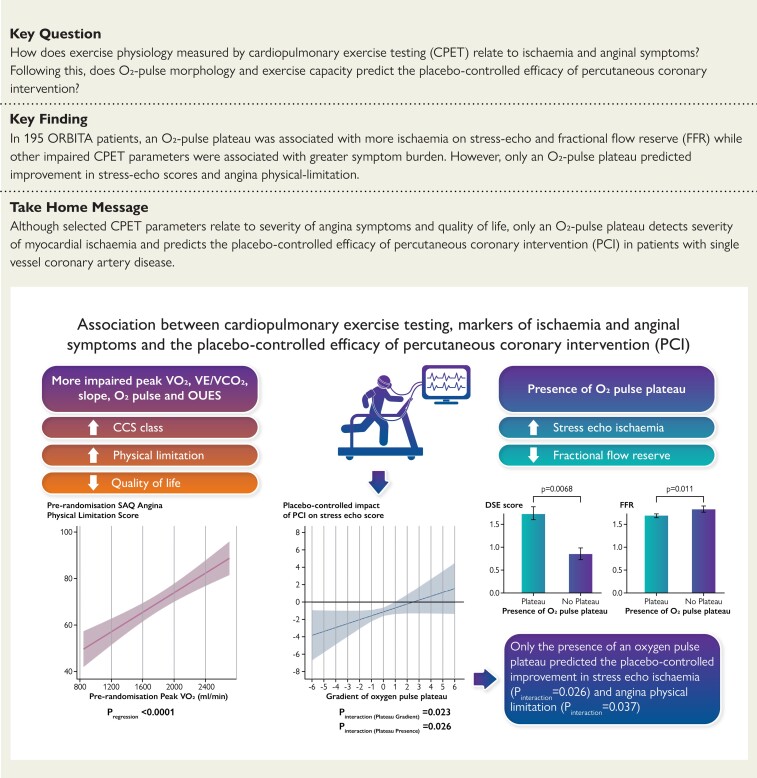
A total of 195 patients with CPET data were randomized (102 PCI, 93 placebo). Patients in whom an oxygen-pulse plateau was observed during CPET had higher (more ischaemic) DSE score [+0.82 segments; 95% confidence interval (CI): 0.40 to 1.25, P = 0.0068] and lower fractional flow reserve (−0.07; 95% CI: −0.12 to −0.02, P = 0.011) compared with those without. At lower (more abnormal) oxygen-pulse slopes, there was a larger improvement of the placebo-controlled effect of PCI on DSE score [oxygen-pulse plateau presence (Pinteraction = 0.026) and oxygen-pulse gradient (Pinteraction = 0.023)] and Seattle angina physical-limitation score [oxygen-pulse plateau presence (Pinteraction = 0.037)]. Impaired peak VO2, VE/VCO2 slope, peak oxygen-pulse, and oxygen uptake efficacy slope was significantly associated with higher symptom burden but did not relate to severity of ischaemia or predict response to PCI.
Conclusion
Although selected CPET parameters relate to severity of angina symptoms and quality of life, only an oxygen-pulse plateau detects the severity of myocardial ischaemia and predicts the placebo-controlled efficacy of PCI in patients with single-vessel CAD.
Keywords: Cardiopulmonary exercise testing, Oxygen pulse, Stable coronary artery disease, Chronic coronary syndrome, Ischaemia, Angina, Peak oxygen uptake
Structured Graphical Abstract
Structured Graphical Abstract.
Only an oxygen pulse plateau relates to markers of ischaemia and predicts the placebo-controlled efficacy of percutaneous coronary intervention in patients with chronic coronary syndrome.
See the editorial comment for this article ‘Subjective angina or myocardial ischaemia to justify PCI? Never mistake the finger for the moon’, by Alessandro Spirito et al., https://doi.org/10.1093/eurheartj/ehac353.
Introduction
Cardiopulmonary exercise testing (CPET) provides a non-invasive assessment of integrated exercise physiology through evaluation of ventilatory gas exchange (VGE). In chronic coronary syndrome, CPET permits reproducible quantification of cardio-respiratory fitness,1 discriminates between exercise-limiting pathophysiological adaptations2 and provides prognostic stratification.3,4
Several CPET parameters have been associated with inducible myocardial ischaemia in symptomatic patients with stable coronary artery disease (CAD).5,6 Compared WITH exercise electrocardiography alone, assessments of work-rate trajectory (VO2/WR), oxygen-pulse (O2-pulse), and ventilatory efficiency during CPET have been shown to offer enhanced sensitivity and specificity for the detection of inducible myocardial ischaemia and perfusion defects.7–9
A linear increase of O2-pulse has been described, which reflects the progressive stroke volume response during incremental exercise.10 An O2-pulse plateau may indicate the inability to augment stroke volume, a feature of exercise-induced cardiac dysfunction which precedes angina and has been reported as valuable in quantifying myocardial ischaemia.7,11 Thus, this suggests that CPET, which provides an integrated assessment of cardiac function and exercise capacity, will be closely associated with indices of myocardial ischaemia and severity of anginal symptoms. However, the relationship between the O2-pulse trajectory and other CPET parameters with gold-standard invasive and non-invasive measures of ischaemia (or even anginal symptoms), has never been assessed in patients with chronic coronary syndrome.
Previous unblinded studies have evaluated CPET as an effective technique to assess functional outcomes following revascularization. Significant improvements have been observed following percutaneous coronary intervention (PCI), in peak oxygen consumption (VO2), oxygen uptake kinetic responses, ventilatory anaerobic threshold and O2-pulse response following PCI.12–17 However, these observed beneficial effects of revascularization have never been tested against placebo control. Furthermore, it may be hypothesized that patients with more impaired functional capacity at baseline (i.e. those who were more symptomatic) or have features suggestive of ischaemia (such as an O2-pulse plateau) on CPET may derive a greater placebo-controlled benefit from PCI.
Hence, in this analysis, we utilized data from the ORBITA (Objective Randomized Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina) trial to assess how the O2-pulse morphology, measured through an automated quantitative analysis, and other CPET parameters, relate to severity of CAD scored by indices of myocardial ischaemia and symptom questionnaires. We assessed whether these parameters can predict the placebo-controlled efficacy of PCI. We also evaluated the effect of placebo-controlled PCI on CPET endpoints in patients with stable single-vessel CAD.
Methods
This study was approved by the London Central Research Ethics Committee (reference 13/LO/1340) and all trial participants provided written consent before enrolment.
Study design
The design of ORBITA has been reported previously.18 ORBITA recruited patients with stable angina and angiographically severe single-vessel CAD (≥70% stenosis), who were referred for elective PCI.
Following enrolment, patients underwent a 6-week medical optimization phase during which anti-anginal therapy was initiated and uptitrated. Patients then underwent pre-randomization assessments including CPET, dobutamine stress echocardiography (DSE) and symptom assessments including the physician-assessed Canadian Cardiovascular Society (CCS) class, patient-reported symptoms using the Seattle Angina Questionnaire (SAQ) and quality of life using the EuroQOL-5 (EQ-5D-5L) questionnaire. Invasive assessments of inducible myocardial ischaemia, including fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR), were made immediately prior to randomization.
Patient blinding was achieved with conscious sedation and auditory isolation using over-the-ear headphones playing music. Once sedated, patients were randomly assigned 1:1 to PCI or a placebo procedure (randomization performed with SRUB Version 8.4.20). Patients and all subsequent caregivers remained blinded to treatment allocation for a 6-week follow-up period.
At the end of this period, all pre-randomization assessments including CPET, DSE, symptom, and quality of life questionnaires were repeated, prior to unblinding.
The full CPET assessment methodology and exercise testing protocol is described in the Supplementary Appendix.
Cardiopulmonary exercise testing reporting
CPET endpoints were reported twice by two cardiologists who were blinded to treatment allocation, time point of the test (pre- or post-randomization) and the other reporter’s opinion.18 Resting haemodynamics and VGE indices were measured as average values for 30 s prior to initiation of exercise. Anaerobic threshold (AT) time was estimated from breath-by-breath data using the V-slope method and validated with other plots, using the ventilatory-equivalent method and the respiratory exchange ratio (RER) method.19 VO2 at AT was taken at this time point. The VE/VCO2 slope was calculated from exercise onset to peak exercise by linear regression.20 The oxygen uptake efficiency slope (OUES) was determined by the slope of the regression-line between the log10VE (x-axis) and VO2 (y-axis) during the whole exercise period (VO2 = a logVE + b, where a = OUES, b = intercept).21
At peak exercise, VO2 (ml/min/kg) was stated as the highest 30 s average within the last minute of exercise until the first 15 s of recovery.5 Other peak values were calculated at identical timepoints. Peak O2-pulse was calculated as the ratio of peak oxygen consumption and heart rate (VO2/HR). Breathing reserve (%) was calculated as: (MVV-VEpeak)/MVV x 100%, where MVV is the maximum voluntary ventilation. Maximum voluntary ventilation was estimated as 40 x FEV122
The analysis of the O2-pulse morphology (presence of a plateau and gradient of slope) was determined by an automated quantitative technique, implemented using the Python Programming Language. Initially, two expert cardiologists identified the presence of an O2-pulse plateau if there was a horizontal plateau of the O2-pulse curve (an inflection in the slope) during exercise, where there was no increase in the VO2/HR on visual assessment. A subset of these cases was then randomly selected to determine optimal cut-offs for the O2-pulse morphology quantitative analysis to reproduce these expert opinions. We generated an automated algorithm from this subset of cases and applied it to the ORBITA data set. This began with applying a Savitzky–Golay filter with window length corresponding to 30 s of data using a second-order polynomial, to prove smoothed estimates of the VO2, heart rate, stroke volume, and RER. Where subjects had reached a sufficient degree of exercise intensity on the treadmill (deemed as exercise times of at least 300 s), we compared the slope of the O2-pulse in the last 2 min of exercise (considered as the late slope), to that of the remaining exercise slope until the last 2 min of exercise (considered as the early slope). These slopes were calculated using Huber regression, which is more resistant to outlier than simple linear regression. If the ratio of these two slopes (late slope over early slope) was lower than 0.4, this was deemed as a plateau in the O2-pulse morphology. If the patient exercised for less than 5 min, we only analysed the morphology of the late slope (last 2 min of exercise), where a late slope of less than 0.3 ml/beat/min was deemed as a plateau. If there was less than 2 min of exercise data, this was considered inappropriate for O2-pulse morphology analysis. In addition to the presence of an O2-pulse plateau (dichotomous analysis), we also treated the O2-pulse morphology as a continuous variable using either the ratio or late slope value based on the time exerted on the treadmill. Example cases for morphology analysis are presented in the Supplementary Appendix. We also provide the Python Programming Language code and example data sheet for data formatting and analysis purposes as Supplementary material.
Ischaemia assessment and reporting
Invasive physiology assessment (FFR and iFR) was performed with the clinical operator blinded to the result. This enabled patients with a range of clinically representative FFR and iFR values to be randomized within single trial and allowed investigation of the ability of these parameters to predict the placebo-controlled efficacy of PCI.
Dobutamine stress echocardiography was performed according to a standardized protocol by a sonographer and a physician, blinded to other assessments and has been previously described.23 Each scan was examined twice by six imaging cardiologists, blinded to time point, treatment allocation and their first opinion and reported as previously described.23 This was conducted to reduce inter- and intra-observer variability of stress echocardiography assessment.
In our analysis, ischaemia was assessed using mean DSE scores, iFR and FFR; these parameters were treated as continuous variables.
Statistical analysis
In this CPET-stratified analysis of ORBITA, data were obtained from all patients who underwent pre-randomization CPET. Summary statistics for baseline characteristics were presented with normality assessed using the Kolmogorov–Smirnov test.
The ORBITA primary analysis utilized two-sample t-tests to determine change scores of continuous variables, as had been prespecified in the statistical plan. In this and previous ORBITA stratified analysis,23,24 regression modelling (a generalized form of analysis of covariance) was used, to assess the relationship between CPET parameters (e.g. O2-pulse plateau morphology, peak oxygen uptake, etc.) and DSE, invasive coronary physiology, patient-reported or physician-assessed symptoms and to determine the interaction between pre-randomization CPET parameters on the treatment effect of PCI on each endpoint. Regression modelling provides increased statistical power and allows incorporation of baseline values and clinical characteristics (such as age, weight, height, and sex) to determine treatment effect and associations.
Regression models were fitted for each endpoint. For continuous endpoints such as the VGE parameters, SAQ physical-limitation score, SAQ quality of life (QOL) score, EQ-5D-5L Visual Analogue Score (VAS) and exercise time, ordinary least squares models were used. For ordinal variables including the SAQ angina-frequency score, Duke treadmill score, freedom from angina (calculated from SAQ) and the CCS angina class, proportional odds logistics models were used.25 For each component of the SAQ, EQ-5D-5L VAS and freedom from angina, a higher score indicatesa better health status. Hence, an odds ratio (OR) of more than one (natural logarithm of OR = 0) indicates that PCI achieved a better health status than placebo.26
For both continuous and ordinal study endpoints, we modelled the follow-up (post-randomization) endpoint values conditioned on the pre-randomization endpoint values. Each covariate in the model was tested for linearity (where a P-value of >0.05 for non-linearity suggested that the covariate had a linear relationship with the endpoint).27 A model was then fitted for each study endpoint with pre-randomization CPET variable interacting with the randomization arm. Age, weight, height, and sex were also adjusted in the model (and also tested for non-linearity) to account for the effects of these baseline characteristics on pre-randomization CPET parameter.28 For covariates that were non-linear, knots were positioned at the 25th, 50th, and 75th percentile of the covariate distribution. The graphs are shown of endpoints against pre-randomization CPET variables. The difference in study endpoint values between the two arms, conditioned on the pre-randomization value, was represented on the vertical axis. We report the interaction as the P-value (Pinteraction) from the combined main effect and interaction effect.
All statistical analyses were performed using open-source statistical environment R.29 Regression models were built using the rms package 27 and graphs were created with the tidyverse package.30
Results
Of the 200 patients randomized in ORBITA, pre-randomization CPET data were available for 195 patients (102 PCI and 93 placebo). CPET data were unreliable in three patients due to persistent mask leaks, and in two patients, who declined to wear the mask required for CPET.
Patient and procedural characteristics
Patient demographic data are shown in Table 1. Most patients had physician-assessed CCS Class II or III angina severity at enrolment (98% in the PCI arm and 96.8% in the placebo arm). A total of 187 (95.9%) patients had at least one positive ischaemia test prior to randomization. This included any pre-enrolment clinical positive functional test (Table 1), pre-randomization stress echocardiography score ≥1, FFR ≤0.80 and iFR ≤0.89 (see Supplementary material online, Table S1).
Table 1.
Patient demographics at enrolment
| Demographics | PCI (n = 102) | Placebo (n = 93) | Total (n = 195) |
|---|---|---|---|
| Age, years | 65.9 ± 9.6 | 66.2 ± 8.5 | 66.1 ± 9.1 |
| Male sex | 71 (69.6) | 72 (77.4) | 143 (73.3) |
| Height, cm | 168.5 ± 9.9 | 169.2 ± 8.6 | 168.9 ± 9.3 |
| Weight, kg | 79.6 ± 15.0 | 83.3 ± 16.0 | 81.4 ± 15.6 |
| Smoking status | |||
| Current | 11 (10.8) | 14 (15.1) | 25 (12.8) |
| Previous | 38 (37.3) | 36 (38.7) | 74 (38.0) |
| Never | 53 (51.9) | 43 (46.2) | 96 (49.2) |
| Hypertension | 70 (68.6) | 65 (69.9) | 135 (69.2) |
| Hypercholesterolaemia | 80 (78.4) | 61 (65.6) | 141 (72.3) |
| Diabetes mellitus | 15 (14.7) | 20 (21.5) | 35 (17.9) |
| Previous MI | 5 (4.9) | 5 (5.4) | 10 (5.1) |
| Previous PCI | 10 (9.8) | 13 (14.0) | 23 (11.8) |
| CCS Class | |||
| I | 2 (2.0) | 3 (3.2) | 5 (2.6) |
| II | 64 (62.7) | 53 (57.0) | 117 (60.0) |
| III | 36 (35.3) | 37 (39.8) | 73 (37.4) |
| Left ventricular systolic function | |||
| Normal | 95 (93.1) | 84 (90.3) | 179 (91.8) |
| Mild impairment | 3 (2.9) | 7 (7.5) | 10 (5.1) |
| Moderate impairment | 4 (4.0) | 2 (2.2) | 6 (3.1) |
| Severe impairment | 0 (0) | 0 (0) | 0 (0) |
| Angina duration, months | 5 (4–10) | 6 (4–9) | 6 (4–9) |
| Pre-enrolment clinical positive functional test | 54 (52.9) | 42 (45.2) | 96 (49.2) |
| ETT | 26 (25.5) | 17 (18.3) | 43 (22.1) |
| MIBI | 9 (8.8) | 11 (11.8) | 20 (10.3) |
| DSE | 19 (18.6) | 13 (14.0) | 32 (16.3) |
Values are given as mean ± standard deviation, n (%), or median (interquartile range).
CCS, Canadian Cardiovascular Society angina class; DSE, dobutamine stress echocardiography; ETT, exercise tolerance test; MI, myocardial infarction; MIBI, nuclear medicine myocardial perfusion scan; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention.
Pre-randomization lung function and cardiopulmonary exercise testing assessment
Pre-randomization resting lung function and CPET data are shown in Supplementary material online, Table S2. These CPET parameters were well balanced between groups. A total of 152 patients (77.9%) were on beta-blockers and 6 patients (3.1%) were in non-sinus rhythm during exercise testing. Most patients (76.9%) attained a RER of ≥1.00 (a marker of acceptable exercise effort).
The association between cardiopulmonary exercise testing parameters and markers of ischaemia
DSE, FFR, and iFR data were available in 178 (91.3%), 189 (96.9%), and 191 (97.9%) patients, respectively. A plateau of the O2-pulse was detected in 142/192 (74%) patients using the automated analysis (see Supplementary material online, Table S2). There were no clinical (risk factors for cardiovascular disease, Table 1) or demographic factors (age, gender) associated with a greater probability of attaining an O2-pulse plateau.
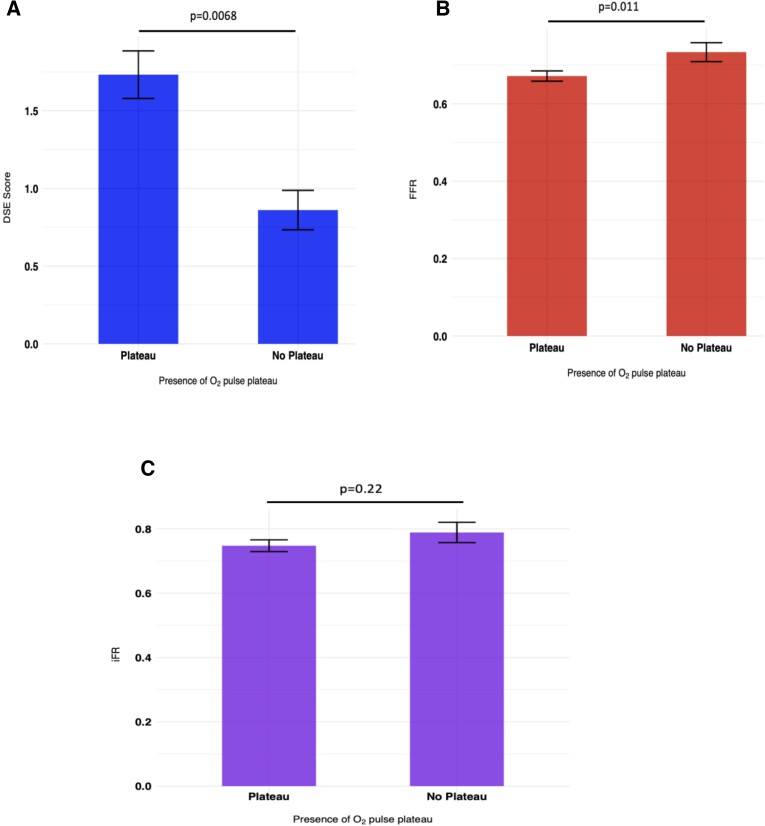
Patients with an O2-pulse plateau (detected via automated analysis) had significantly higher DSE scores [+0.82 segments; 95% confidence interval (CI) 0.40 to 1.25, P = 0.0068; Figure 1A] and lower FFR values (−0.07; 95% CI: −0.12 to −0.02, P = 0.011; Figure 1B) compared with those without. Although there was a trend towards patients with an O2-pulse plateau having a lower iFR, this difference was not statistically significant (−0.04; 95% CI: −0.11 to 0.02, P = 0.22; Figure 1C). Incorporation of peak RER attained by each participant in the regression model also showed similar results; [DSE scores: +0.98 segments (95% CI: 0.30 to 1.66, P = 0.0047), FFR: −0.06 (95% CI: −0.12 to −0.003, P = 0.041) and iFR: −0.05 (95% CI: −0.13 to 0.03, P = 0.22)]. Analysis of the O2-pulse morphology as a continuous variable however did not suggest a significant relationship between these parameters (see Supplementary material online, Figure S1).
Figure 1.
Relationship between the presence of an O2-pulse plateau and severity of ischaemia. Relationship between the presence of an oxygen-pulse plateau and (A) DSE score, (B) FFR, and (C) iFR. Data are presented as mean with error bars indicating standard error values. DSE, Dobutamine stress echocardiography; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; and O2, oxygen. P values were calculated from the regression model which was adjusted for age, weight, height, and sex.
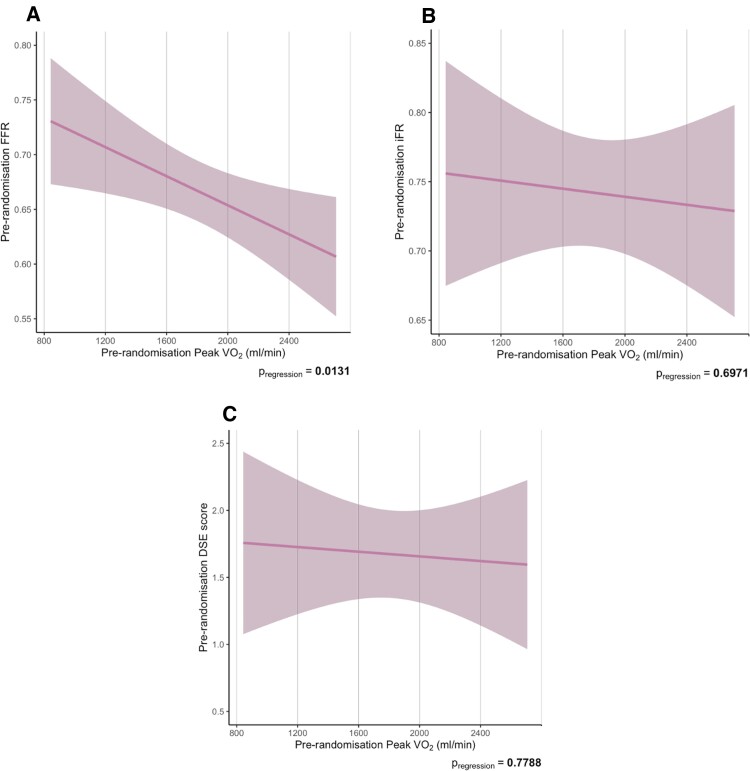
Similarly, greater impairment of other CPET parameters at pre-randomization (peak VO2, VE/VCO2 slope, peak O2-pulse, and OUES) did not suggest more ischaemia as assessed by FFR, iFR and DSE (Figure 2 for peak VO2 and see Supplementary material online, Figure S2 for VE/VCO2, peak O2-pulse, and OUES). Patients with more impaired peak VO2, peak O2-pulse, and OUES had significantly higher FFR.
Figure 2.
Relationship between pre-randomization peak VO2 and pre-randomization FFR (A), iFR (B) and DSE score (C). Data are presented as mean with error bars indicating standard error values. DSE, dobutamine stress echocardiography; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; O2, oxygen; OUES, oxygen uptake efficiency slope; VE/VCO2: Minute ventilation to carbon dioxide VO2: oxygen uptake P values were calculated from the regression model which was adjusted for age, weight, height, and sex.
Relationship between cardiopulmonary exercise testing parameters and symptom severity
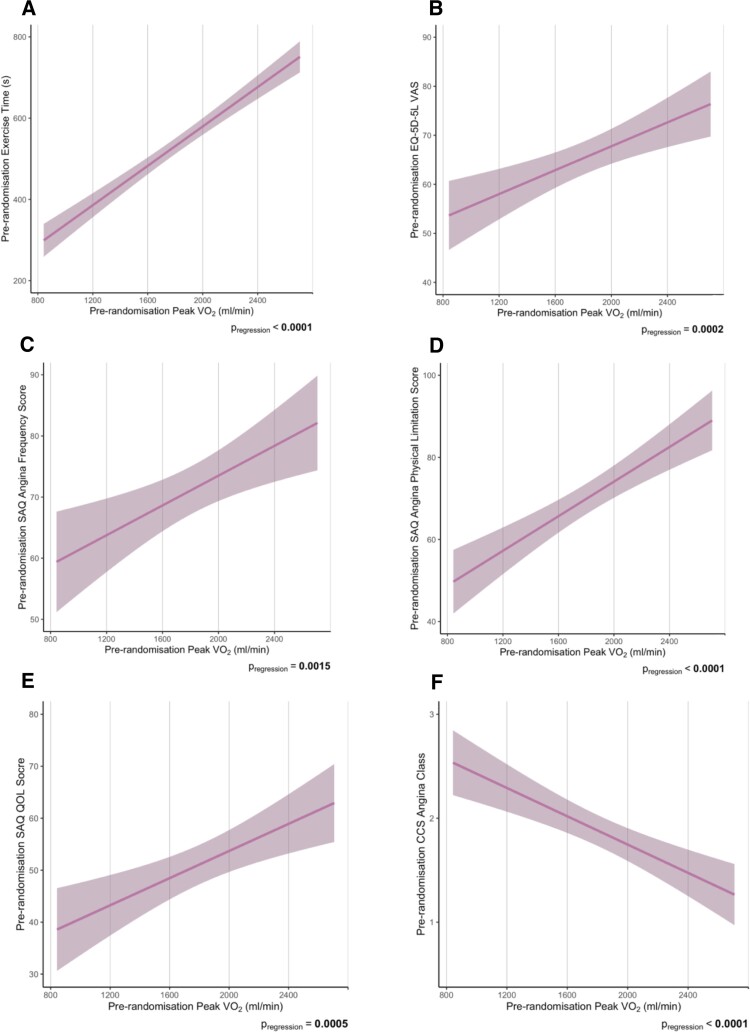
Across the cohort, a significantly positive association was observed between the total duration of treadmill exercise and the peak VO2 achieved (P < 0.0001; Figure 3A). Patients with higher peak VO2 also had higher EQ-5D-5L VAS (P = 0.0002; Figure 3B), higher SAQ domain scores including angina-frequency (P = 0.0015; Figure 3C), physical-limitation (P < 0.0001; Figure 3D), and QOL (P = 0.0005; Figure 3E) and lower (milder) physician-assessed CCS score (P < 0.0001; Figure 3F).
Figure 3.
Relationship between pre-randomization peak VO2 and pre-randomization patient-reported and physician-assessed anginal symptoms including exercise time (A), EQ-5D-5L VAS (B), SAQ angina frequency score (C), SAQ angina physical limitation score (D), SAQ quality of life score (E) and CCS angina class (F). The shaded area represents the 95% CI for the estimate of this mean effect. Higher scores on the questionnaires indicate a better angina health status and quality of life. CCS indicates Canadian Cardiovascular Society; EQ-5D-5L indicates five level version of EuroQol 5 dimensions; QOL, quality of life; SAQ, Seattle Angina Questionnaire; VAS, visual analogue score; VO2, oxygen uptake. P values were calculated from the regression model which was adjusted for age, weight, height, and sex.
Patients with a lower VE/VCO2 (less impaired) slope had significantly better patient-reported and physician-assessed symptoms (see Supplementary material online, Figure S3). Similarly, higher peak O2-pulse and OUES were mostly associated with better physician-assessed CCS and patient-reported symptoms (see Supplementary material online, Figures S4 and S5).
Placebo-controlled effect of percutaneous coronary intervention on Duke treadmill score, ventilatory gas exchange, and exercise haemodynamics
At 6-week follow-up, there was no significant effect of PCI compared with placebo on peak VO2 (−8.03 ml/min, 95% CI: −80.07 to 64.00, P = 0.826), presence of O2-pulse plateau (OR: 1.13, 95% CI: 0.56–2.26, P = 0.735), or any CPET parameters during exercise (Table 2). However, PCI was more likely to result in improvement in Duke treadmill score compared with placebo (OR: 1.73, 95% CI: 1.05–2.85, P = 0.032, Table 2).
Table 2.
Placebo-controlled effect of PCI on CPET parameters, exercise haemodynamics and Duke treadmill score
| CPET endpoint | ANCOVA estimate with the covariate modelled as a restricted cubic spline (PCI over placebo) | ANCOVA P-value |
|---|---|---|
| CPET variable during exercise | ||
| Peak VO2 (mL/min) | −8.03 (95% CI: −80.07 to 64.00) | 0.826 |
| Peak O2-pulse (ml/beat) | 0.04 (95% CI: −0.65 to 0.73) | 0.903 |
| O2-pulse plateau presence | OR: 1.13 (95% CI: 0.56–2.26) | 0.735 |
| O2-pulse plateau gradient | −0.26 (95% CI: −0.60 to 0.087) | 0.142 |
| OUES | −40.72 (95% CI: −131.29 to 49.86) | 0.376 |
| VE/VCO2 slope | 0.22 (95% CI: −9.57 to 22.33) | 0.431 |
| Peak VE (L) | −1.35 (95% CI: −5.24 to 2.54) | 0.495 |
| Peak RER | 0.0076 (95% CI: −0.020 to 0.035) | 0.585 |
| VO2 at AT (mL/min) | 2.22 (95% CI: −57.78 to 62.22) | 0.942 |
| Exercise haemodynamics | ||
| Peak SBP (mmHg) | 4.52 (95% CI: −2.56 to 11.59) | 0.210 |
| Peak HR (bpm) | −0.34 (95% CI: −3.86 to 3.18) | 0.847 |
| RPP (mmHg • bpm) | 740.89 (95% CI: −432.65 to 1914.40) | 0.215 |
| Duke treadmill score | OR: 1.73 (95% CI: 1.05–2.85) | 0.032 |
| Maximal ST-segment depression (mm) | −0.55 (95% CI: −1.10 to −0.02) | 0.041 |
| Treadmill angina index | OR: 1.07 (95% CI: 0.49–2.34) | 0.869 |
Treatment effect estimates were generated using regression modelling. The follow-up values were modelled conditioned to pre-randomization value and treatment.
ANCOVA, analysis of covariance; AT, anaerobic threshold; bpm, beats per minute; CI, confidence interval; CPET, cardiopulmonary exercise testing; DBP, diastolic blood pressure; HR, heart rate; OUES, oxygen uptake efficiency slope; OR, odds ratio, RER, respiratory exchange ratio; RPP, rate pressure product; SBP, systolic blood pressure; VE, minute ventilation; VO2, oxygen uptake.
Estimates are either expressed as absolute values for continuous variables or odds ratios for discrete variables. Duke treadmill score was calculated as follows: Duration of exercise in minutes = (5 × the maximal net ST-segment deviation during or after exercise, in millimetres)−(4 × the treadmill angina index). The angina index has a value of 0 if the patient had no angina during exercise, 1 if the patient had non-limiting angina, and 2 if angina was the reason the patient stopped exercising.
Cardiopulmonary exercise testing as a predictor of the placebo-controlled effect of percutaneous coronary intervention on study endpoints
O2-pulse morphology
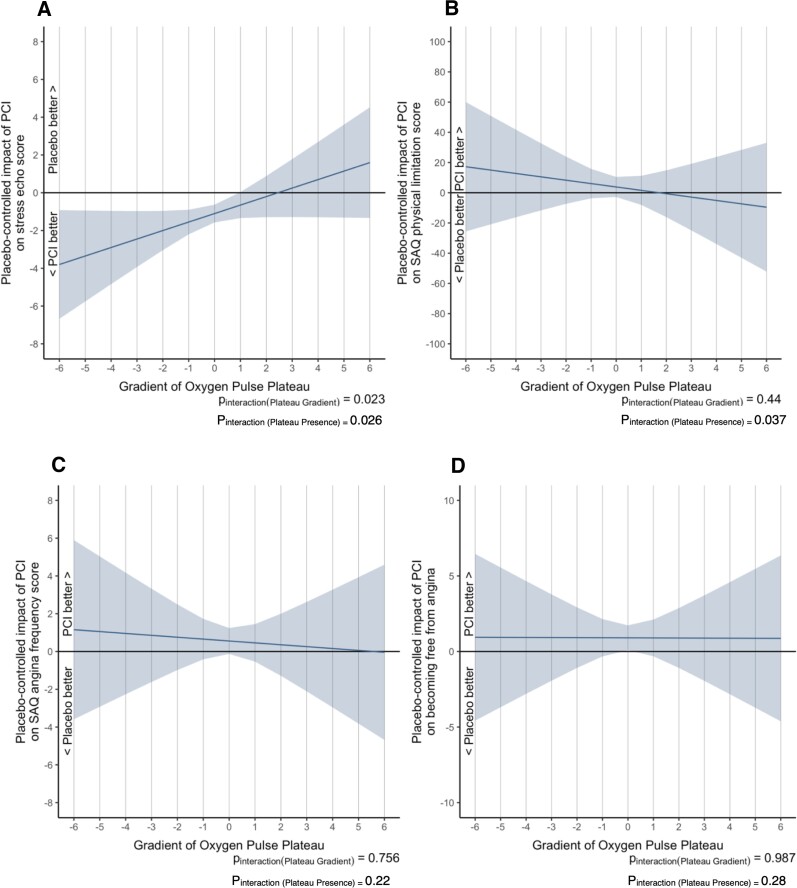
Paired (pre- and post-) DSE scores were available for 155 patients (88 in the PCI arm and 67 in the placebo arm). PCI significantly reduced the DSE score compared with placebo (−1.083, 95% Cl: −1.46 to −0.71, P < 0.0001, see Supplementary material online, Table S3). The presence of an O2-pulse plateau (Pinteraction = 0.026, Figure 4A) and the O2-pulse gradient (Pinteraction = 0.023, Figure 4A) predicted progressively larger improvement of DSE scores at lower (more abnormal) O2-pulse slopes.
Figure 4.
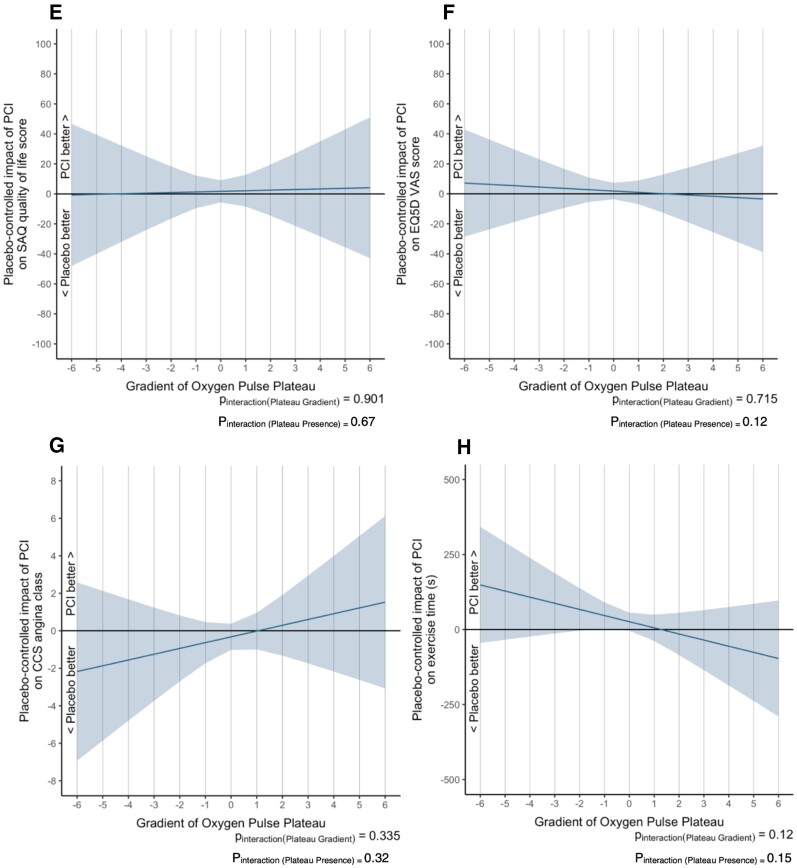
Interaction between pre-randomization O2-pulse plateau gradient and the efficacy of placebo-controlled PCI on ORBITA endpoints. The shaded area represents the 95% CI for the estimate of this mean effect. In panels 4A, 4B, 4E, 4F, and 4H, the vertical axis shows the absolute improvement or worsening with PCI over placebo where an unweighted linear regression model was used to calculate the improvement or worsening in dobutamine stress echocardiography (DSE) scores, SAQ physical-limitation score, SAQ quality of life (QOL) score, EQ-5D-5L visual analogue score (VAS) and exercise time. In panels 4C, 4D, and 4G, the vertical axis shows the natural logarithm of the odds ratio for improvement or worsening with PCI over placebo where an ordinal cumulative probability model was used to calculate the improvement or worsening in SAQ angina frequency, becoming free from angina and CCS angina class. CCS, Canadian Cardiovascular Society; EQ-5D-5L indicates 5 level version of EuroQol 5 dimensions; PCI, percutaneous coronary intervention; SAQ; SAQ, Seattle Angina Questionnaire; VAS, Visual Analogue Score; VO2, oxygen uptake.
Although PCI did not improve SAQ physical-limitation score (4.14, 95% CI: −1.33 to 9.62, P = 0.137, see Supplementary material online, Table S3), the presence of an O2-pulse plateau significantly modified this effect (Pinteraction = 0.037, Figure 4B) with greater benefit of PCI seen in patients with an O2-pulse plateau compared to those without. This was despite there being no interaction between the gradient of the O2-pulse morphology and physical-limitation at baseline (Pinteraction = 0.44, Figure 4B).
Percutaneous coronary intervention was more likely to result in improvement of SAQ angina-frequency score (OR: 1.73, 95% CI: 1.02–2.96, P = 0.0432, see Supplementary material online, Table S3) and lead to freedom from angina (OR 2.58, 95% CI: 1.35–4.92, P = 0.004, see Supplementary material online, Table S3) vs. placebo. However, there was no detectable interaction between O2-pulse morphology (O2-pulse plateau presence Pinteraction = 0.22; Pinteraction = 0.28 and O2-pulse gradient Pinteraction = 0.76, Pinteraction = 0.99, Figure 4C–4D, respectively) and the effect of PCI on these study endpoints.
Similarly, there was no interaction between the pre-randomization O2-pulse morphology and the placebo-controlled efficiency of PCI on SAQ QOL (Figure 4E), EQ-5D-5L VAS (Figure 4F), CCS class (Figure 4G), and exercise time (Figure 4H).
Exercise capacity and other cardiopulmonary exercise testing parameters
There was no interaction between pre-randomization exercise capacity as assessed by exercise time or peak VO2 and the placebo-controlled efficacy of PCI on any of the study endpoints in this stratified analysis. Furthermore, there were no convincing interactions between other CPET parameters such as VE/VCO2 slope, peak O2-pulse, and OUES with the PCI study endpoints (see Supplementary material online, Figures S6–S9). These results were consistent, despite the finding that patients who had more impaired peak VO2, VE/VCO2, O2-pulse, and OUES were more symptomatic and exercised for lesser times at baseline (Figure 3).
Discussion
This is the first placebo-controlled analysis of the effect of revascularization with PCI in chronic coronary syndrome on VGE parameters during exercise. Principally, through an automated quantitative analysis of O2-pulse morphology, we found that the presence of an O2-pulse plateau during CPET was associated with more ischaemia as assessed by DSE or invasive coronary physiology (FFR). The presence of an O2-pulse plateau also predicted greater reduction of DSE ischaemia and greater improvement in angina physical-limitation score at 6 weeks following placebo-controlled PCI. Secondly, we found that although a greater impairment of peak VO2, VE/VCO2 slope, peak O2-pulse and OUES was associated with more severe symptoms and worse QOL, these did not predict the placebo-controlled efficacy of PCI. Finally, we found that in comparison to a placebo procedure, PCI with drug-eluting stents had no significant effect on any conventionally reported measure of VGE during exercise (Graphical abstract).
Association between assessments at pre-randomization
We found that the presence of an O2-pulse plateau selected individuals with more ischaemia as evidenced by more wall motion abnormalities on DSE and lower FFR. This finding supports the theory that an O2-pulse plateau occurs when there is an inability to adequately increase the stroke volume response during exercise. While previous studies solely utilized visual assessment and applied scoring scales to grade individual O2-pulse curves in relation to the normal appearance of the slope,12,15,31 we provide a reproducible method which reduces bias from highly subjective categorization. Our sensitivity analysis for varying cut-offs of ratio and late slope gradient (shown in Supplementary Appendix) further emphasizes O2-pulse morphology as a physiological manifestation of ischaemia.
However, greater impairment of other CPET parameters (including peak VO2, VE/VCO2 slope, peak O2-pulse, and OUES) was not associated with more ischaemia. This finding contrasts with previously reported smaller, unblinded studies.32–34 Although these selected parameters are not necessarily specific for cause of exercise limitation, the OUES, which was found to discriminate between circulatory and respiratory limitation, was equally not related to ischaemia severity.2 Our blinded analysis is hence robust to these conclusions.
Despite this lack of association with severity of ischaemia, these CPET parameters did correlate with severity of symptoms and QOL in patients with single-vessel CAD, suggesting that a patient’s exercise capacity is linked to the symptoms they report. Patients with the lowest exercise capacity (and consequently lowest peak VO2), consistently reported the greatest angina symptom burden. The same relationship was observed for physician-assessed and patient-reported symptoms despite previous reported discrepancies between these metrics.35,36 Similar associations were also observed with our VE/VCO2 slope and the OUES, other indicators of functional capacity which incorporate volitional effort. Much like patients with chronic heart failure, potential mechanisms implicated in these exercise ventilatory efficiency abnormalities in patients with CAD include early lactic acidosis and greater sympathetic and neurohormonal activation.37–39 The symptom of angina may draw parallels with these impairments, leading to elevation of the VE/VCO2 slope or decrease in OUES.
Effect of percutaneous coronary intervention on ventilatory gas exchange
In ORBITA, PCI was found to be highly effective in normalizing the anatomical and physiological features of a coronary stenosis, and in doing so, ischaemia was essentially eradicated in the active treatment arm. However, we found no effect of ischaemia eradication on exercise physiology (VGE and haemodynamics), contrasting with previous unblinded CPET and exercise haemodynamic studies.33,40 Importantly, the lack of placebo control in unblinded studies increases the likelihood of misinterpretation of the placebo effect as a treatment effect.
Peak exercise measures are strongly influenced by volition and are limited by symptoms. Hence, they are endpoints highly vulnerable to bias in an unblinded trial. Indeed, patients who are aware of their treatment arm are more likely to be influenced by a therapy that is believed to improve symptoms, especially during the anticipation of these symptoms during exertion. Submaximal VGE measures such as the O2 uptake efficiency slope and anaerobic/ventilatory threshold aim to reduce the influence of subject motivation by consideration of a range of data points or by identification of frequently attained thresholds.41 However, these parameters are determined by the operator using methods which are subject to considerable interobserver variability. The subjective decisions of the physician to terminate CPET based on symptoms,42,43 further emphasizes a point in the assessment chain that is, again, vulnerable to bias with an unblinded design.
Interestingly, we did not find a change in the O2-pulse morphology following placebo-controlled PCI, again contrasting with previous studies.12,15 Since this morphology incorporates volitional effort (also adjusted into our algorithm based on their total exercise time) and hence limited by symptom perception, there is considerable bias in the attainment of a plateau/slope of the morphology in unblinded trials. Furthermore, O2-pulse morphology is based on other factors such as peripheral O2 extraction, a training effect and deconditioning.44,45 It is therefore a global functional assessment as opposed to a simple measure of ischaemia. A follow-up of more than 6 weeks may have possibly been required to manifest a change in this metabolic endpoint of exercise along with other CPET parameters.
We found an improvement in the Duke treadmill score, driven by a decrease in ST-segment deviations in the PCI group compared with the placebo group. This improvement in an ischaemic endpoint is concordant with our analysis of placebo-controlled improvement in DSE scores,23 suggesting a further point on the ischaemic cascade amenable to PCI. Whether this translates into improvement in long-term prognosis among our patients with single-vessel CAD remains uncertain.
Baseline cardiopulmonary exercise testing as a predictor of placebo-controlled percutaneous coronary intervention
In patients enrolled in ORBITA, the average pre-randomization peak VO2 was 76% of the predicted value, whereas using anthropometric and resting VO2 measurements in our patient cohort, the average metabolic equivalent of task was between 4.85 and 6.10. These values are well below age predicted normal ranges.46 This suggests that the disparity of our results with previous studies and the unexpected primary result of ORBITA on exercise time, was not due to enrolment of patients with a ‘well-preserved’ exercise capacity at baseline and hence less likely to benefit from PCI.
However, for the prediction of patients most able to benefit symptomatically from PCI, it has previously been reported that patients with a lower baseline peak VO2 (≤15 ml/kg/min) derived more benefit following revascularization, with greater improvements in exercise capacity compared with patients who were less impaired at baseline.13,47 In direct contrast, however, we found no association between pre-randomization exercise capacity assessed by peak VO2 or exercise time (or other CPET parameters) and the placebo-controlled effect of PCI on nearly all symptom endpoints. We treated exercise capacity as a continuum using high quality VGE data, rather than estimates solely from treadmill time and speed, hence permitting a more sensitive interpretation. However, despite this, baseline CPET parameters (peak VO2, VE/VCO2 slope, peak O2-pulse, and OUES) which significantly relate to symptoms burden, QOL, and exercise capacity, were also not able to predict which patients would likely benefit from PCI on most endpoints.
Only the O2-pulse morphology at baseline predicted the placebo-controlled efficacy of PCI on DSE scores (both gradient and presence of plateau) and angina physical limitation (only presence of plateau). The O2-pulse morphology was associated with greater ischaemia on DSE and FFR (ischaemic potential) at pre-randomization. Hence, this finding establishes the O2-pulse morphology as an important physiological parameter. Although the O2-pulse morphology is not linearly related to the severity of ischaemia,11 its presence indicates more severe ischaemia which is amenable to intervention. This finding also suggests that eradication of ischaemia on DSE is related to patient-reported angina, concordant with our previous DSE stratified analysis.23
Clinical implications
Primary analysis of the ISCHAEMIA trial48 and previously from COURAGE49 have suggested that coronary revascularization, in addition to medical therapy, does not lead to reduction in all-cause mortality or risk of ischaemic cardiovascular events. Subsequent hypothesis-generating secondary analyses of ISCHAEMIA have suggested that the results may be more nuanced than at first considered. For example, greater ischaemia burden at baseline or presence of lesions such as proximal left anterior descending artery stenosis of ≥ 70%, were not associated with greater risk50; Type 1 myocardial infarction rates were more frequent with the conservative strategy51 and that the invasive strategy lowered cardiovascular death and myocardial infarction rates in those with the most extensive anatomic disease.50 More data are required to understand which patients are most likely to benefit from PCI but it does seem that in the majority, the primary remit of PCI is symptom relief.
Given that ORBITA demonstrated a smaller than expected effect size of PCI vs. placebo for angina relief in patients with single-vessel disease, it is key to determine which patients would benefit symptomatically from this invasive procedure.18 Intracoronary pressures measured invasively (FFR/iFR) did not show a detectable ability to predict symptom relief from PCI.24 However, non-invasive ischaemia testing using DSE 23 and in this analysis, O2-pulse morphology from CPET, suggested that the presence of a greater burden of ischaemia at pre-randomization predicted a greater placebo-controlled effect of PCI.
Although our results, and those of previous studies, suggest that CPET may provide improved granularity in the evaluation of patients with chronic coronary syndrome compared with standard exercise testing, there may be multiple reasons that have meant that CPET has not been integrated into routine clinical practice in this condition. Expert staff are required for calibration, administration, and interpretation of test results, and facial masks or mouthpiece use may cause discomfort for some patients. Furthermore, exercise testing may not be suitable in all patient groups.5
However, once these challenges are met, incorporation of CPET in clinical practice is relevant, practical and potentially cost-effective. In patients presenting with symptoms attributed to stable CAD and ischaemia manifested as an O2-pulse plateau, CPET may select patients who are most likely to derive symptomatic benefit from PCI thereby allowing intervention to be targeted to those patients who are most likely to benefit from the procedure.
Limitations
This CPET-stratified analysis of ORBITA incorporated 195 patients (97.5%) of the original cohort and is therefore the largest analysis to date examining the relationship between baseline exercise capacity and symptomatic improvement following PCI. Despite this, the sample size may limit the power of this analysis since the effect of PCI on the primary endpoint of exercise times (and symptoms) was smaller than expected. Matched pre-post data for study endpoints were also not available for all patient in this stratified analysis (see Supplementary material online, Table S3); 79.4% of participants (155/195) had paired (pre- and post-) DSE scores: 86.3% in the PCI arm (88/102) and 72.0% in the placebo arm (67/93). There is a potential for selection bias if the remaining patients differed in some way. Furthermore, only 30% of participants in this analysis were women and it has been shown that women have more frequent angina and less severe ischaemia than men.52
Our assessments of VGE, exercise capacity and symptom severity are vulnerable to the presence of possible alternative confounding diagnoses, which may be an explanation for the absence of association with myocardial ischaemia in most CPET parameters. However, the patients enrolled into ORBITA were deemed to be symptomatic from their epicardial coronary stenosis and were candidates listed for PCI.18 Indeed, cardiac dysfunction detected by CPET also identifies a global ischaemic burden, including ischaemia from non-obstructive coronary arteries. In ORBITA, we did not quantify coronary flow reserve or microvascular resistance. Similarly, antianginals such beta-blockers (despite a blunted heart rate response) and ranolazine have shown to improve peak VO2 through enhanced microcirculatory and endothelial function.53–55 Hence, the effect of intensive guideline-directed medical therapy at pre-randomization may have also reduced the incremental effect size from PCI. Cardiopulmonary exercise testing was not performed at enrolment, therefore we do not know the effect size of medical therapy alone on VGE in patients with stable CAD.56
Although we used treadmill testing, studies identifying exercise-induced myocardial ischaemia with CPET have previously utilized cycle ergometry.7 Cycle ergometry permits assessment of the HR/WR slope and the O2 uptake work rate (ΔVO2/ΔWR) slope, which allows estimation of the exercise load at which myocardial ischaemia develops.57 Treadmill CPET does not allow for these measurements as work rate is not expressed as Watts and does not increase linearly. However, treadmill testing confers a more physiological and familiar form of daily exercise that reduces localized fatigue and works larger muscle groups. Hence, treadmill testing typically produces a greater peak VO2 and haemodynamic response within an individual compared with cycle ergometry. These are potential reasons why treadmill testing has recently appeared more reliable than cycle ergometry in CAD quantification.9
Finally, ORBITA recruited patients with single-vessel CAD. All patients were taking optimal medical therapy with a mean number of three anti-anginal agents at the time of the pre-randomization tests. ORBITA-2 (NCT03742050) is recruiting patients with single and multi-vessel disease taking real-world anti-anginal therapy and will therefore test the placebo-controlled efficacy of PCI in wider range of patients.
Conclusion
Although CPET parameters relate to severity of angina symptoms and quality of life, only the presence of an O2-pulse plateau detects the severity of myocardial ischaemia and predicts the placebo-controlled efficacy of PCI in patients with stable single-vessel CAD.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors are grateful for infrastructural support from the NIHR Imperial Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Contributor Information
Sashiananthan Ganesananthan, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Christopher A Rajkumar, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Michael Foley, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
David Thompson, University College London, London, UK.
Alexandra N Nowbar, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK.
Henry Seligman, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Ricardo Petraco, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Sayan Sen, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Sukhjinder Nijjer, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Simon A Thom, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK.
Roland Wensel, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; DRK-Kliniken-Berlin and Charité Berlin, Germany.
John Davies, Essex Cardiothoracic Centre, Basildon.
Darrel Francis, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Matthew Shun-Shin, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
James Howard, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Rasha Al-Lamee, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, Du Cane Road W12 0HS, London, UK; Imperial College Healthcare NHS Trust, London, UK.
Funding
C.A.R. (MR/S021108/1) and M.F. (MR/V001620/1) are supported by the Medical Research Council (United Kingdom). A.N.N. was supported by the NIHR Academy. R.P. (FS/11/46/28861), D.F. (FS 04/079), and M.S.-S. (FS/14/27/30752) were supported by the British Heart Foundation. J.H. was supported by the Wellcome Trust (212183/Z/18/Z).
References
- 1. Coeckelberghs E, Buys R, Goetschalckx K, Pattyn N, Vanhees L, Cornelissen V. Test-retest reliability of maximal and submaximal gas exchange variables in patients with coronary artery disease. J Cardiopulm Rehabil Prev 2016;36:263–269. [DOI] [PubMed] [Google Scholar]
- 2. Barron A, Francis DP, Mayet J, Ewert R, Obst A, Mason M, et al. . Oxygen uptake efficiency slope and breathing reserve, not anaerobic threshold, discriminate between patients with cardiovascular disease over chronic obstructive pulmonary disease. JACC Heart Fail 2016;4:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, et al. . Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol 2003;42:2139–2143. [DOI] [PubMed] [Google Scholar]
- 4. Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, et al. . Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002;106:666–671. [DOI] [PubMed] [Google Scholar]
- 5. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. . Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 6. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 7. Belardinelli R, Lacalaprice F, Carle F, Minnucci A, Cianci G, Perna G, et al. . Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J 2003;24:1304–1313. [DOI] [PubMed] [Google Scholar]
- 8. Dominguez-Rodriguez A, Abreu-Gonzalez P, Gomez MA, del Garcia-Baute MC, Arroyo-Ucar E, Avanzas P, et al. . Myocardial perfusion defects detected by cardiopulmonary exercise testing: role of VE/VCO2 slope in patients with chest pain suspected of coronary artery disease. Int J Cardiol 2012;155:470–471. [DOI] [PubMed] [Google Scholar]
- 9. Popovic D, Guazzi M, Jakovljevic DG, Lasica R, Banovic M, Ostojic M, et al. . Quantification of coronary artery disease using different modalities of cardiopulmonary exercise testing. Int J Cardiol 2019;285:11–13. [DOI] [PubMed] [Google Scholar]
- 10. Chaudhry S, Arena R, Wasserman K, Hansen JE, Lewis GD, Myers J, et al. . Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. Am J Cardiol 2009;103:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munhoz EC, Hollanda R, Vargas JP, Silveira CW, Lemos AL, Hollanda RMK, et al. . Flattening of oxygen pulse during exercise may detect extensive myocardial ischemia. Med Sci Sports Exerc 2007;39:1221–1226. [DOI] [PubMed] [Google Scholar]
- 12. Klainman E, Fink G, Lebzelter J, Zafrir N. Assessment of functional results after percutaneous transluminal coronary angioplasty by cardiopulmonary exercise test. Cardiology 1998;89:257–262. [DOI] [PubMed] [Google Scholar]
- 13. Barmeyer A, Meinertz T. Anaerobic threshold and maximal oxygen uptake in patients with coronary artery disease and stable angina before and after percutaneous transluminal coronary angioplasty. Cardiology 2002;98:127–131. [DOI] [PubMed] [Google Scholar]
- 14. Adachi H, Koike A, Niwa A, Sato A, Takamoto T, Marumo F, et al. . Percutaneous transluminal coronary angioplasty improves oxygen uptake kinetics during the onset of exercise in patients with coronary artery disease. Chest 2000;118:329–335. [DOI] [PubMed] [Google Scholar]
- 15. Inbar O, Yamin C, Bar-On I, Nice S, David D. Effects of percutaneous transluminal coronary angioplasty on cardiopulmonary responses during exercise. J Sports Med Phys Fitness 2008 Jun;48:235–245. [PubMed] [Google Scholar]
- 16. Mashayekhi K, Neuser H, Kraus A, Zimmer M, Dalibor J, Akin I, et al. . Successful percutaneous coronary intervention improves cardiopulmonary exercise capacity in patients with chronic total occlusions. J Am Coll Cardiol 2017;69:1095–1096. [DOI] [PubMed] [Google Scholar]
- 17. Ajisaka R, Watanabe S, Yamanouchi T, Masuoka T, Sugishita Y. Effect of percutaneous transluminal coronary angioplasty on exercise ventilation in patients with coronary artery disease and normal left ventricular function. Am Heart J 1996;132:48–53. [DOI] [PubMed] [Google Scholar]
- 18. Al-Lamee R, Thompson D, Dehbi H-M, Sen S, Tang K, Davies J, et al. . Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomized controlled trial. Lancet 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 19. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1985 1986 Jun;60:2020–2027. [DOI] [PubMed] [Google Scholar]
- 20. Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev 2008;13:245–269. [DOI] [PubMed] [Google Scholar]
- 21. Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, et al. . Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 1996;28:1567–1572. [DOI] [PubMed] [Google Scholar]
- 22. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. . Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 23. Al-Lamee RK, Shun-Shin MJ, Howard JP, Nowbar AN, Rajkumar C, Thompson D, et al. . Dobutamine stress echocardiography ischemia as a predictor of the placebo-controlled efficacy of percutaneous coronary intervention in stable coronary artery disease: the stress echocardiography-stratified analysis of ORBITA. Circulation 2019;140:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Lamee R, Howard JP, Shun-Shin MJ, Thompson D, Dehbi H-M, Sen S, et al. . Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation 2018;138:1780–1792. [DOI] [PubMed] [Google Scholar]
- 25. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis [Internet]. 2nd ed. Springer International Publishing; 2015 [cited 2020 May 14]. (Springer Series in Statistics). Available from: https://www.springer.com/gp/book/9783319194240
- 26. Garratt AM, Hutchinson A, Russell I. Network for Evidence-Based Practice in Northern and Yorkshire (NEBPINY). The UK version of the Seattle Angina Questionnaire (SAQ-UK): reliability, validity and responsiveness. J Clin Epidemiol 2001;54:907–915. [DOI] [PubMed] [Google Scholar]
- 27. Jr FEH. rms: Regression Modeling Strategies [Internet]. 2019 [cited 2020 May 14]. Available from: https://CRAN.R-project.org/package=rms
- 28. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016;133:e694–711. [DOI] [PubMed] [Google Scholar]
- 29.R: The R Project for Statistical Computing [Internet]. [cited 2020 May 15]. Available from: https://www.r-project.org/
- 30.Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C, et al. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics [Internet]. 2020 [cited 2020 May 15]. Available from: https://CRAN.R-project.org/package=ggplot2
- 31. De Lorenzo A, Da Silva CL, Castro Souza FC, De Souza Leão Lima R. Value of the oxygen pulse curve for the diagnosis of coronary artery Disease. Physiol Res 2018:679–686. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka S, Noda T, Segawa T, Minagawa T, Watanabe S, Minatoguchi S. Relationship between functional exercise capacity and functional stenosis in patients with stable angina and intermediate coronary stenosis. Circ J 2009;73:2308–2314. [DOI] [PubMed] [Google Scholar]
- 33. Cook CM, Ahmad Y, Howard JP, Shun-Shin MJ, Sethi A, Clesham GJ, et al. . Association between physiological stenosis severity and angina-limited exercise time in patients with stable coronary artery disease. JAMA Cardiol 2019;4:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinkstaff S, Peberdy MA, Kontos MC, Fabiato A, Finucane S, Arena R. Usefulness of decrease in oxygen uptake efficiency slope to identify myocardial perfusion defects in men undergoing myocardial ischemic evaluation. Am J Cardiol 2010;106:1534–1539. [DOI] [PubMed] [Google Scholar]
- 35. Saxon JT, Chan PS, Tran AT, Angraal S, Jones PG, Grantham JA, et al. . Comparison of patient-reported vs physician-estimated angina in patients undergoing elective and urgent percutaneous coronary intervention. JAMA Netw Open 2020;3:e207406–e207406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shafiq A, Arnold SV, Gosch K, Kureshi F, Breeding T, Jones PG, et al. . Patient and physician discordance in reporting symptoms of angina among stable coronary artery disease patients: insights from the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study. Am Heart J 2016;175:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. . Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 2000;21:154–161. [DOI] [PubMed] [Google Scholar]
- 38. Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, et al. . Development of a ventilatory classification system in patients with heart failure. Circulation 2007;115:2410–2417. [DOI] [PubMed] [Google Scholar]
- 39. Wensel R, Georgiadou P, Francis DP, Bayne S, Scott AC, Genth-Zotz S, et al. . Differential contribution of dead space ventilation and low arterial pCO2 to exercise hyperpnea in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2004;93:318–323. [DOI] [PubMed] [Google Scholar]
- 40. Parisi AF, Folland ED, Hartigan P. A Comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. N Engl J Med 1992;326:10–16. [DOI] [PubMed] [Google Scholar]
- 41. Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol 2000;36:194–201. [DOI] [PubMed] [Google Scholar]
- 42. Kaczmarek S, Habedank D, Obst A, Dörr M, Völzke H, Gläser S, et al. . Interobserver variability of ventilatory anaerobic threshold in asymptomatic volunteers. Multidiscip Respir Med 2019;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vainshelboim B, Rao S, Chan K, Lima RM, Ashley EA, Myers J. A comparison of methods for determining the ventilatory threshold: implications for surgical risk stratification. Can J Anaesth J Can Anesth 2017;64:634–642. [DOI] [PubMed] [Google Scholar]
- 44. Warburton DER, McKenzie DC, Haykowsky MJ, Taylor A, Shoemaker P, Ignaszewski AP, et al. . Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol 2005;95:1080–1084. [DOI] [PubMed] [Google Scholar]
- 45. Chuang M-L, Lin I-F, Huang S-F, Hsieh M-J. Patterns of Oxygen pulse curve in response to incremental exercise in patients with chronic obstructive pulmonary disease – An observational study. Sci Rep 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wasserman K, editor. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. 5th ed.Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. p572. [Google Scholar]
- 47. Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. . Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation 2002;105:1291–1297. [DOI] [PubMed] [Google Scholar]
- 48. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. . Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 50. Reynolds HR, Shaw LJ, Min JK, Page CB, Berman DS, Chaitman BR, et al. . Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation 2021;144:1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chaitman BR, Alexander KP, Cyr DD, Berger JS, Reynolds HR, Bangalore S, et al. . Myocardial infarction in the ISCHEMIA trial. Circulation 2021;143:790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, et al. . Association of sex with severity of coronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary analysis of the ISCHEMIA. Randomized clinical trial. JAMA Cardiol 2020;5:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pavia L, Orlando G, Myers J, Maestri M, Rusconi C. The effect of beta-blockade therapy on the response to exercise training in postmyocardial infarction patients. Clin Cardiol 1995;18:716–720. [DOI] [PubMed] [Google Scholar]
- 54. Deshmukh SH, Patel SR, Pinassi E, Mindrescu C, Hermance EV, Infantino MN, et al. . Ranolazine improves endothelial function in patients with stable coronary artery disease. Coron Artery Dis 2009;20:343–347. [DOI] [PubMed] [Google Scholar]
- 55. Willis LH, Slentz CA, Johnson JL, Kelly LS, Craig KP, Hoselton AL, et al. . Effects of exercise training with and without ranolazine on peak oxygen consumption, daily physical activity, and quality of life in patients with chronic stable angina pectoris. Am J Cardiol 2019;124:655–660. [DOI] [PubMed] [Google Scholar]
- 56. Foley M, Rajkumar CA, Shun-Shin M, Ganesananthan S, Seligman H, Howard J, et al. . Achieving optimal medical therapy: insights from the ORBITA trial. J Am Heart Assoc 2021;10:e017381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chaudhry S, Kumar N, Behbahani H, Bagai A, Singh BK, Menasco N, et al. . Abnormal heart-rate response during cardiopulmonary exercise testing identifies cardiac dysfunction in symptomatic patients with non-obstructive coronary artery disease. Int J Cardiol 2017;228:114–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.