Abstract
Fermented foods (ffs) and beverages are widely consumed in Southeast Asia (SEA) for their nutritional balance, flavor, and food security. They serve as vehicles for beneficial microorganisms performing a significant role in human health. However, there are still major challenges concerning the safety of ffs and beverages due to the presence of natural toxins. In this review, the common toxins found in traditional ffs in SEA are discussed with special reference to mycotoxins and plant toxins. Also, mitigation measures for preventing risks associated with their consumption are outlined. Ochratoxin, citrinin, aflatoxins were reported to be major mycotoxins present in SEA ffs. In addition, soybean-based ff food products were more vulnerable to mycotoxin contaminations. Common plant toxins recorded in ffs include cyanogenic glycosides, oxalates, phytates and saponins. Combined management strategies such as pre-harvest, harvest and post-harvest control and decontamination, through the integration of different control methods such as the use of clean seeds, biological control methods, fermentation, appropriate packaging systems, and controlled processing conditions are needed for the safe consumption of indigenous ffs in SEA.
Subject terms: Food microbiology, Mass spectrometry
Introduction
The process of fermentation is one of the oldest biotechnologies employed in the production of food products with beneficial properties including prolonged shelf-life and satisfactory organoleptic characteristics1. Finished products from fermented foods (ffs) generally possess enhanced microbial stability and safety and some can be preserved even at room temperature. In addition, the process of food fermentation proffer food products with improved consumers’ palatability. For these reasons, there has been heightened interest in exploring this natural process and more specifically, to link the heterogeneity of the fermenting microbial community and their properties to the quality of the product2. Fermentation is a process used in the production of foods, beverages and other beneficial metabolites either by aerobic or anaerobic microorganisms through the enzymatic conversions of substrates and microbial growth3. By sensing the environment, microbes produce signals to trigger protein synthesis (enzymes), while substrates are converted enzymatically into nutrients, such as amino acids, sugars, nucleotides, fatty acids, and vitamins, which are necessary for the growth of the microorganism. Therefore, the activities of microorganisms are significant in food fermentation as they help to prolong the shelf life of foods, enhance the organoleptic, nutritional and antioxidant properties, and aids in the reduction/removal of toxic ingredients from the raw materials2.
The history of fermentation can be traced back to the Neolithic period (circa 10,000 B.C.) when it was mainly used for extending the shelf-life of perishable foods. The primary aim of food preservation was obtained through the production of inhibitory growth substances namely, organic acid ethanol, and bacteriocins, combined with a decreased water activity4. Therefore, traditional ffs and beverages have been in existence and have been incorporated into the human diet from the onset of civilization. Since then, food fermentation has been widely studied for other purposes including enhancement of food safety by way of suppressing pathogens or elimination of toxigenic compounds; boosting nutritional components, and improving the organoleptic properties of the food5. Fermented foods have the potential to tackle the global problem linked to a having a balanced diet as they are embedded with a distinct group of microflora that improves the nutritional components of foods including proteins, essential amino acids, vitamins, and fatty acids6. Fermenting organisms are also capable of releasing bioactive metabolites such as peptides, conjugated linoleic acids, exopolysaccharides, neurotransmitters (γ-aminobutyric acids also known as GABA), and oligosaccharides7.
In Asia, ffs are important components of human diets and, the international market for fermented ingredients and food products is estimated to rise to $28.4 billion in 20228. In SEA, fermentation of cereal grain to derive different food product varieties have been a tradition for a long time. An example of this is rice wine, a popular alcoholic beverage in Asia. In Malaysia and Indonesia, the fermentation of cassava tubers is carried out to produce the well-known sweet and sour snack known as tapai/tape9. Other ffs consumed in SEA include palm-based products (Sri Lanka), rice/cassava-based (Philippines, Vietnam, Thailand, Indonesia, Malaysia), Soy-based (Thailand, Japan), milk-based (Thailand, Vietnam, Japan, China, Korea), and fish-based products (Thailand, Cambodia, Vietnam, Japan).
The presence of undesirable toxigenic compounds such as mycotoxins and plant toxins especially in amounts in ffs beyond the approved limits may cause serious health problems among consumers or even death. Aspergillus spp., Penicillium spp., Mucor spp., and Rhizopus spp. have been detected in grains, aflatoxins in commercial beverages, and ochratoxin A and zearalenone in some local beverages10. Plant toxins e.g., cyanogenic glycosides have also been reported in cassava and bamboo shoots11. In fermented milk products, the presence of Streptococcus spp. and Clostridium botulinum have led to severe outbreaks12. The quality of ffs becomes doubtful and influences the market especially if this problem occurs concurrently. Hence, there is a need for various precautions in food industries for ensuring high-quality products and safe maximum excess levels of pathogenic microorganisms and toxins in the food products8.
This review summarizes the most found toxins (mycotoxins and plant toxins) in traditional fermented foods in SEA, their sources, and mitigation measures for preventing health risks associated with their consumption.
Fermented foods
As defined by Campbell-Platt13, ffs are foods that are produced by the action of microorganisms or enzymes so that beneficial biochemical changes can generate significant changes in the food. The fermentation process can take place either in a solid-state or liquid state (submerged fermentation). For the solid-state, aerobic microorganisms are responsible for the fermentation process, while the submerged fermentation takes place under anaerobic conditions14. The ffs can be grouped in various ways15; (1) by categories of fermentation process4,16—alcoholic fermentation, acetic acid fermentation, lactic acid fermentation, and alkaline fermentation; (2) by food commodity classes13—dairy-based products, cereal-based products, legume-based products, fish and seafood-based products, meat-based products, root, and tuber-based products and beverages; (3) based on types of microorganism involved17 - yeasts, molds, bacteria (acetic bacteria, lactic acid bacteria (LAB) and Bacillus).
Traditional fermented foods in Southeast Asia
Fermented foods are associated with diverse cultures and civilizations. Climate, history, and the raw materials produced particularly in the regions have led to the exploitation of different fermentation mechanisms to produce indigenous edible foods and beverages which are adapted to the particular environmental conditions18. For instance, in locations where there are animal husbandry and pastoral agricultural practices, there is the general availability of milk and dairy products from cows, goats, sheep, etc. Accordingly, fermented milk, cheese, and other fermented dairy products have evolved across Europe, Middle East, and India. On the contrary, far Eastern regions including Japan, China, and Korea have limited animal husbandry19. Several factors such as economic factors, cultural and religious status also affect the categories of substrates used in producing ffs and alcoholic beverages. In Asia, ffs that have evolved over the years are generally based on rice (and grains), vegetables, soybeans, and fish as main substrates18. In recent years, ffs have received great attention from food industries, and their rising global market was estimated to be about thirty billion USD18. In SEA ffs are still often produced at a family- or community-scale using traditional techniques and as well on an industrial scale. Studies on indigenous ffs were searched for, and relevant works including those carried out by Tamang20; Liu and Tong21; Tamang, et al.22; Swain, et al.23; Beuchat24; Steinkraus17; Soni and Dey25; Owens26 were reviewed and presented in Table 1. From these papers, indigenous ffs and beverages peculiar to the region of SEA were extracted together with their local names, countries of origin, raw materials/substrates, and organoleptic properties. Thus, the indigenous ffs and beverages in SEA have been classified into eight different groups according to their food categories (Table 1a–h). These include fermented dairy products, fermented cereal products, fermented fruits and vegetables, fermented legume products, fermented root crops, fermented meat products, fermented fish products, and fermented alcoholic beverages.
Table 1.
a Indigenous dairy products in SEA, b indigenous fermented cereal products in SEA, c indigenous fermented fruits and vegetable sin SEA, d indigenous legume products in SEA, e indigenous fermented root crop products in SEA, f indigenous fermented meat products in SEA, g indigenous fermented fish products in SEA, h indigenous fermented alcoholic beverages in SEA.
| Local Name | Country | Raw material | Organoleptic properties |
|---|---|---|---|
| a. Fermented dairy products | |||
| Dadih | Indonesia | Buffalo milk | Curd, savory |
| Sua Chua | Vietnam | Dried skim milk, starter, sugar | Acid fermented milk |
| b. Fermented cereal products | |||
| Ang-kak | Thailand, Philippines | Red rice | Colorant |
| Dosa | Malaysia, Singapore | Rice and black gram | Thin crisp pancake, shallow fried |
| Idli | Malaysia, Singapore | Rice and black gram or other dehusked pulses | Soft moist, spongy, mild-acidic |
| Khaomak (Kao-mak) | Thailand | Glutinous rice Look-pang (starter) | Sweet and mild alcoholic Thai desert |
| Puto | Philippines | Rice | Steamed rice cake |
| Tape Ketan | Indonesia | Glutinous rice, Ragi | Sour, sweet, mild alcoholic desert |
| c. Fermented fruits and vegetable products | |||
| Burong mustala | Philippines | Mustard | Wet and acidic |
| Dha muoi | Vietnam | Eggplant, mustard, and beet | Wet and acidic |
| Hom-dong | Thailand | Red onion | Fermented red onion |
| Naw-mai-diong | Thailand | Bamboo shoots | Wet and acidic |
| Pak-gard-dong | Thailand | Leafy vegetable, salt, and boiled rice | Wet, acidic, side dish |
| Pak-sian-dong | Thailand | Gynandropis pentaphylla leaves | Wet, acidic, side dish |
| Dakguadong | Thailand | Mustard leaf | Wet, acidic, sour, salad |
| Sayur asin | Indonesia | Mustard leaves, coconut, salt, and cabbage | Wet, acidic, sour, salad |
| Tempoyak | Malaysia | Durian (Durio zibethinus), salt | Wet, acidic, side dish |
| d. Fermented legume products | |||
| Kecap | Indonesia | Wheat, soybean | Liquid |
| Ketjap | Indonesia | Black soybean | Syrup liquid |
| Oncom Hitam (black) and Oncom Merah (orange) | Indonesia | Tapioca peanut press cake, soybean cord starter | Fermented peanut press cake fried or roasted |
| Indonesia | |||
| Tauco | Indonesia | Soybean | Alkaline, liquid, seasoning |
| Tempeh | Indonesia | Soybean | Paste, flavoring agent, alkaline |
| Thua nao | Thailand | Soybean | Dry, paste, side dish, alkaline |
| e. Fermented root crop products | |||
| Tapé | Indonesia | Cassava | Dessert (sweet) |
| Tapai Ubi | Malaysia | Cassava, Ragi | Dessert (sweet) |
| f. Fermented meat products | |||
| Hham (Musom) | Thailand | Pork meat, pork skin, rice garlic, ginger, salt | Fermented pork |
| Nem-chua | Vietnam | Pork, cooked rice, salt | Fermented sausage |
| Nham or Sai-krok-prieo | Thailand | Pork, rice salt, garlic | Fermented sausage |
| Tocino | Philippines | Pork, potassium nitrate, salt, sugar | Fermented cured pork |
| g. Fermented fish products | |||
| Balao-balao | Philippines | Shrimp, rice, salt | Fermented rice shrimp, condiment |
| Belacan | Malaysia | Shrimp salt | Condiment, paste |
| Bakasang | Indonesia | Shrimp, fish | Condiment, paste |
| Burong Bangus | Philippines | Milkfish, vinegar, rice, salt | Fermented milkfish, sauce |
| Burong Isda | Philippines | Fish, rice, salt | Fermented fish sauce |
| Budu | Malaysia, Thailand | Marine fishes, sugar, salt | Fish sauce, Muslim sauce |
| Hoi-malaeng pu-dong | Thailand | Mussel (Mytilus smaragdinus) salt | Fermented mussel |
| Nam pla | Thailand | Solephorus sp., Ristelliger sp. Cirrhinus sp., water, salt | Fish sauce |
| Nuoc mam | Vietnam | Marine fish | Fish sauce, condiment |
| Patis | Indonesia, Philippines |
Stolephorus sp., Clupea sp., Decapterus sp., Leionathus sp., salt |
Fish sauce |
| Pla-paeng-daeng | Thailand | Marine fish, Ang-kak (red molds rice), salt | Red fermented fish |
| Pla-som (Pla-khao-sug) | Thailand | Marine fish, boiled rice, salt, garlic | Fermented fish, condiment |
| Beverage name | Country | Raw material | Organism/starter | Organoleptic properties |
|---|---|---|---|---|
| h. Fermented alcoholic beverages | ||||
| Basi | Philippines | Sugar cane | Bubod, binubudan | Cloudy or clear liquid |
| Brem | Indonesia | Rice | Ragi | Mild alcoholic, sweet-sour, dry |
| Khao maak | Thailand | Rice | Lookpang | Sweet taste, white colored, juicy, mild alcoholic |
| Krachae | Thailand | Rice | Lookpang | Filtered liquor non-distilled |
| Nam khao | Thailand | Rice | Lookpang | Distilled liquor |
| Ou | Thailand | Rice | Lookpang | Distilled liquor |
| Sato | Thailand | Rice | Lookpang | Distilled liquor |
| Ruou de, Ruou nep | Vietnam | Rice | Men | Clear, distilled liquor |
| Ruou nep than | Vietnam | Purple rice | Men | Viscous thick, non-distilled fermented rice |
| Ruou nep chan | Vietnam | Maize, rice, cassava | Men | Viscous, thick, distilled/non-distilled fermented rice |
| Tapuy | Philippines | Rice | Bubod | Sour, sweet, mild alcoholic |
| Tapai ubi, Tapai pulut | Malaysia | Cassava, rice | Ragi/ jui-piang | Sour, sweet, mild alcoholic |
| Tapé-ketan | Indonesia | Cassava, rice, millet, maize | Ragi | Sweet-sour alcoholic paste |
Fermented dairy products constitute about 20% of the entire economic value of ffs produced globally. Strains of LAB such as Lactobacillus, Lactococcus, and Leuconostoc are generally employed in the fermentation of dairy products27. Yogurt is well known as the major fermented milk worldwide and is prepared mainly from the fermentation of cow milk by two species of LAB (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus)2. Similarly, cheese and other products are made from non-pasteurized milk and may still depend on lactic microbes for fermentation, especially on an industrial scale. Indonesian Dadih, produced from the fermentation of buffalo milk and Vietnamese Sua Chua from dried skimmed milk are examples of common fermented milk found in SEA.
Fermented cereal products play a major role in human nutrition in all regions of the world, including Asia. Among all ffs, fermentation of cereals is the most fermented product, attaining the highest volume28. Generally, cereal-based ffs are majorly produced from rice, maize, sorghum, millet, or wheat. However, most fermented cereal-based products in SEA are mainly derived from rice, which is either liquid (porridge) or stiff gels (solid) in texture. These include Dosa (Malaysia, Singapore), Idli (Malaysia, Singapore), Khaomak (Thailand), Puto (Philippines), and Tapé Ketan (Indonesia).
Fermentation is one of the oldest processing methods for extending the shelf-life quality of perishable foods like fruits and vegetables, especially before storage in the refrigerator. They are various final products of fermented fruits and vegetables produced in SEA based on the kind of substrates used. Most fermented fruits and vegetables in SEA are indigenous to Thailand, the Philippines, Vietnam, Indonesia, and Malaysia. Mustard, beets, eggplant, red onion, bamboo shoots, and durian are mostly fermented into wet and acidic products which are often eaten as side dishes or salads.
Soybean is the major fermented leguminous crop fermented in SEA and products include Kecap, Ketjap, Oncom Hitam (black), Oncom Merah (orange), Tauco, Tempe, and Thua-nao which are mainly produced in Indonesia and Thailand. These fermented products are in the form of syrup liquid, paste, or press cake used as a flavoring agent, seasoning, and side dish.
Processing and consumption of fresh, cured, and fermented meat products have been in practice in the world for many years. Fermented meats such as sausage-like products are not uncommon in European countries and Asia. In Hham (Musom), Nem-chua, Sai-krok-prieo, and Tocino produced basically from pork meat are mostly found in Thailand, Vietnam, and the Philippines, respectively.
The origin of fermented fish products in SEA dates back to around 200 BCE to 200 CE and Thai-Lao, Burmese, and Khmer are concluded as the first ethnic groups to produce fermentation of freshwater fish products20. Marine fishes, shrimps, and mussels are generally fermented into fermented shrimp paste, fermented fish sauce, condiments as shown in Table 2.
Table 2.
Sample preparation and analytical techniques for the determination of mycotoxins in SEA fermented foods and raw materials used for preparing fermented foods.
| Commodity | Country | No of samples | Extraction Solvent | Clean -Up | Analytical method | Mycotoxins | Reference |
|---|---|---|---|---|---|---|---|
| Fermented Wine and Beer | Malaysia | 10 | Acetonitrile | SPE | LC-MS/MS | AF, OTA | Alsharif, et al.140 |
| Thailand | 200 | Chloroform/Acetonitrile | DLME | LC-MS/MS | AF, ZEN, OTA, FB, T-2, DON | Puangkham, et al.141 | |
| Dried Chilli | Thailand | 120 | Methanol | * | TLC | AF, OTA | Chuaysrinule, et al.142 |
| Indonesia | 6 | Methanol-Water | IAC | HPLC-UV | AF, OTA | Wikandari, et al.143 | |
| Malaysia | 10 | Acetonitrile | SPE | LC-MS/MS | AF, OTA | Alsharif, et al.140 | |
| Malaysia | 80 | Methanol-NaHCO3 | IAC | HPLC-FL | AF, OTA | Jalili and Jinap144 | |
| Rice | Thailand | 10 | Methanol | SPE | LC-QTOFMS | AF, ZEN, OTA, FB, T-2, DON | Shiratori, et al.145 |
| Vietnam | 111 | Methanol-Water | * | ELISA | AF, FB | Huong, et al.146 | |
| Thailand | 240 | Methanol | SPE | HPLC-FL | AF, FB | Panrapee, et al.147 | |
| Myanmar | 21 | Acetonitrile | SPE | LC-MS/MS | AF, FB | Lim, et al.148 | |
| Malaysia | 50 | Methanol | IAC | ELISA | AF, OTA | Lim, et al.148 | |
| Fish | Philippines | 31 | Ethanol | SPE | HPLC-UV | AF, OTA | Ebarvia, et al.149 |
| Soybean (Tempeh) | Indonesia | 9 | Acetonitrile-Water | DnS | HPLC-MS/MS | ZEN | Borzekowski, et al.150 |
| Palm Kernel Cake | Indonesia | 20 | Methanol-water | * | ELISA | AF | Reiter, et al.151 |
| Malaysia | 25 | Acetonitrile-Water | * | LC-MS/MS | AF, ZEN, OTA, FB, T-2, DON | Yibadatihan, et al.152 | |
| Corn and Cornmeal | Vietnam | 102 | Methanol-Water | * | ELISA | AF, FB | Huong, et al.146 |
| Thailand | 55 | Methanol | HPLC-UV | OTA | Singkong, et al.153 | ||
| Indonesia | 13 | Methanol | BSA | ELISA | AF | Reiter, et al.151 | |
| Indonesia | 16 | Acetonitrile | IAC | HPLC-UV | AF, ZEN, OTA, FB, T-2, DON | Ali154 | |
| Philippines | 50 | Acetonitrile-Water | SPE | HPLC-FL | AF, ZEN, FB | Yamashita, et al.155 | |
| Thailand | 27 | Acetonitrile-Water | SPE | HPLC-FL | AF, ZEN, FB | Yamashita, et al.155 | |
| Indonesia | 12 | Acetonitrile-Water | SPE | HPLC-FL | AF, FB | Yamashita, et al.155 |
AF aflatoxins, ZEN zearalenone, OTA ochratoxin, FB fumonisins, T-2 T-2 toxin, DON deoxynivalenol, ELISA enzyme linked enzyme linked immunosorbent assay, TLC thin layer chromatography, HPLC high performance liquid chromatography, FL fluorescence, UV ultraviolet, MS mass spectrometry, SPE solid phase extraction, DnS dilute and shoot, IAC immunoaffinity column, BSA bovine serum albumin, ND not detected.
*Not stated or investigated.
Cassava roots are also fermented into popular Indonesian and Malaysian sweet deserts known as Tapé and Tapai Ubi, respectively. Ragi, a dry starter culture consisting of bacteria, yeast, and mold is generally used in this process.
Sugarcane, rice, maize, millet, and cassava serve as common substrates for alcoholic beverages in SEA using Bubod, binubudan, Ragi, Loogpang, Men as starters. Popular alcoholic beverages in this region include Thai Sato produced from rice, Vietnamese Ruou de, Ruou nep, Ruou nep than, Ruou nep chan from rice/purple rice, maize, and cassava, and Indonesian Tapé-ketan from fermented rice, cassava, maize, and millet.
Contamination sources in fermented foods
Contamination in fermented foods can occur either during the primary production of plant/animal-based raw materials or during fermentation itself. Contamination during or after fermentation can also be due to inadequate hygienic conditions or appropriate packaging systems. Several raw materials used in food fermentation naturally contain toxic compounds, e.g., cyanogenic glycosides in cassava, while mycotoxin contamination may take place during pre-and post-harvest of the plant-based raw materials. In addition, toxins such as biogenic amines (Histamine, putrescine, tyramine, cadaverine, and β-phenylethylamine, ethyl carbonate), ethyl carbonate, and bacterial toxins may be released as by-products into the ffs during fermentation.
The fact that ffs are still generally produced at community-scale or family-scale using traditional methods reduces the consumer confidence in the quality and safety of the products, and it is highly important to improve the method of production. For example, consumers do not trust the microbial safety of Nem-chua (Vietnamese fermented sausage) because of the lack of confidence in the microbial safety of the traditionally produced meat29.
Mycotoxin contamination in Southeast Asian fermented foods
All fungal toxins are referred to as mycotoxins which are mainly produced by five species of filamentous fungi, namely Fusarium, Aspergillus, Penicillium, and Alternaria. Recently, more than 400 mycotoxin metabolites have been identified, however, the eight most significant mycotoxin with global relevance regarding public health are aflatoxins, ochratoxin A, zearalenone, deoxynivalenol, fumonisin B1, nivalenol, T-2 toxin and patulin30. Mycotoxin contamination has been discovered in agricultural commodities and specifically in cereals and legumes including rice, wheat, maize, barley, oats, rye, beans, soybeans, peanuts, clover, peas, alfalfa, lentils, chicken peas, and in some other grains that are generally used as raw materials for traditional ffs in SEA4.
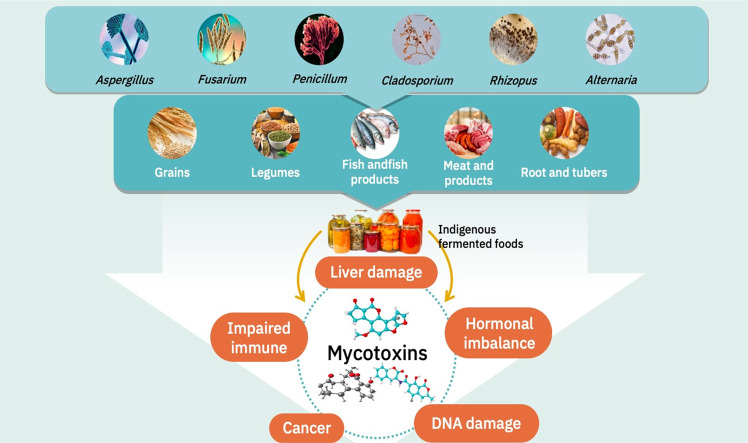
In SEA, diverse mycotoxins including aflatoxins, ochratoxin A, zearalenone, deoxynivalenol, and citrinin have been recorded in ffs (Fig. 1). Studies carried out by Inoue, et al.31 on maize, barley, and other grains used to produce beer traditional and commercial alcoholic beverages, were assessed for mycotoxins. They observed that the grains were contaminated with Aspergillus spp., Penicillium spp., Mucor spp., and Rhizopus spp., and some of the commercial beverages were contaminated with AFs, while a few of the local beverages contained zearalenone and ochratoxin A.
Fig. 1. Mycotoxin contamination in indigenous fermented foods and health implications on human.
Illustrations adapted from Adobe stock images (http://stock.adobe.com/) under Standard Licensing.
In Asian ffs, Rhizopus spp. is generally used in the fermentation process. Some of these fungi species have over time become opportunistic pathogens for consumers with the impaired immune system, while some others have an endosymbiotic interaction with Burkholderia, which has the potential of producing toxic metabolites. Investigation into several strains of Rhizopus showed that about 11% of the strains possess an endosymbiotic association with Burkholderia spp.32. The Burkholderia strains were studied and proven to exhibit the potential of producing rhizoxins.
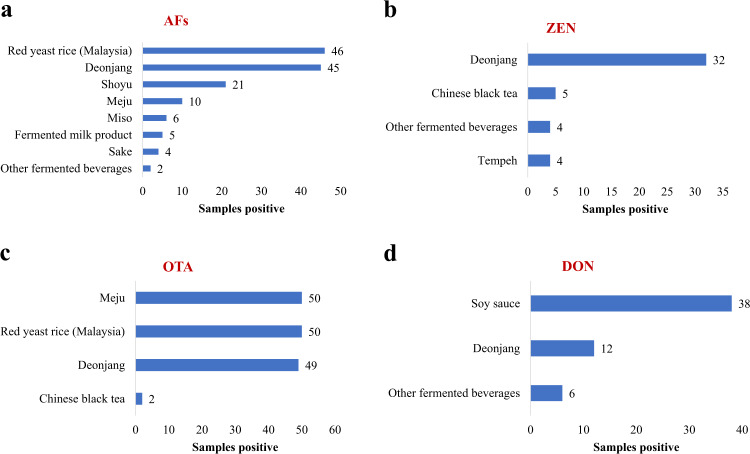
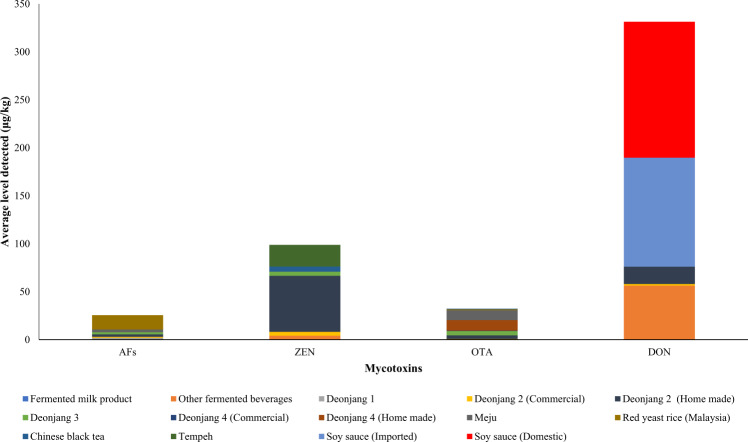
Notwithstanding limited information on the mycotoxin contamination in Southeast Asian ffs, publications relevant to this issue were sourced from the published literature and analyzed. Incidence of mycotoxin contamination in ffs were extracted from fifteen publications and summarized in Fig. 2. The ffs included those from fermented soybean (Deonjang, Meju, Miso, Shoyu, Soy sauce, tempeh); rice (red yeast rice (RYR) and sake); fermented milk products and alcoholic beverages. Most of the AF contaminations were recorded in RYR from Malaysia (n = 46/50)33,34 and Deonjang (n = 45/118)35–38. Zearalenone mostly occurred in Deonjang samples (n = 32/60)35; ochratoxin A mainly in Meju (n = 50/100)37, RYR from Malaysia (n = 50/100)33 and Deonjang (49/109)33–35; deoxynivalenol in Soy sauce (n = 38/40)35 and Deonjang (n = 12/60)35. RYR were the most vulnerable to citrinin contamination as the number of samples from Malaysia and China positive to citrinin were n = 50/50 and n = 43/114, respectively33,34,39. Lastly, fumonisin contamination only occurred in Deonjang, where 30 of 60 samples evaluated were found positive to fumonisin39. Figure 3 shows the maximum mycotoxin concentrations detected in the ffs. RYR from Malaysia was found to contain aflatoxins in average concentrations (2–15 µg/kg of aflatoxins) exceeding the maximum levels (4 µg/kg of aflatoxins) by The European Union Commission40. Also, highest average concentration of zearalenone (58 µg/kg of zearalenone), was detected in homemade Deonjang, which is quite below the maximum level (75 µg/kg of zearalenone) by The European Union Commission41. Average concentrations of samples found to contain ochratoxin A ranged between 1–11 µg/kg of ochratoxin A, with all higher than the regulated limit (3 µg/kg of ochratoxin)42, except for RYR and commercial Doenjang which only contained 1 µg/kg of ochratoxin A. Average deoxynivalenol concentrations detected in both imported and domestic soy sauce were found to be 114 µg/kg and 142 µg/kg of deoxynivalenol, respectively, below the regulated limit (750 µg/kg of deoxynivalenol)41. The average citrinin concentration in RYR from China were found to be 884 mg/kg of citrinin, which may be of serious safety concerns in this ff product when compared to the maximum limit (100 µg/kg of citrinin)43. From this study, a total of 1492 ffs samples consumed in Asia including SEA were evaluated for six different types of mycotoxins and 514 samples (26%) were recorded to be contaminated with at least one type of mycotoxin. Ochratoxin A and aflatoxins were the major mycotoxins identified, representing 29% and 27%, respectively of the total contamination.
Fig. 2. Incidence of mycotoxin contamination in Asian fermented foods.
a Frequency of AFs contamination in Asian fermented foods samples. b Frequency of ZEN contamination in n Asian fermented foods samples. c Frequency of OTA contamination in Asian fermented foods samples. d Frequency of DON contamination in Asian fermented foods samples. AFs Aflatoxins, ZEN Zearalenone, OTA Ochratoxin A, DON Deoxynivalenol.
Fig. 3. Maximum mycotoxin concentrations detected in Asian ffs.
AFs Aflatoxins, ZEN Zearalenone, OTA Ochratoxin A, DON Deoxynivalenol. *Deonjang 1–4 indicated samples analyzed by different authors.
Mycotoxins have also been detected in Thai fermented soy products (Thua-nao) and Tuong as reported in works by44 and45. Tuong is fermented by Aspergillus oryzae, a domesticated type of Aspergillus flavus (AF producer), evidence that explains the presence of AF in brands of Tuong. Aflatoxins are predominantly found in fermented milk products. Different factors influence the free aflatoxins in fermented milk products including storage temperature, storage time, pH, acidity, aflatoxin concentration, and strains used in the fermentation process46. Similarly, aflatoxins have been reported in peanut/soybean press cakes such as Indonesian Oncom Hitam and Oncom Merah that are produced using Neurospora sitophila and Rhizopus oligosporus47. Rhizopus oligosporus can reduce the cyclopentanone moiety resulting in aflatoxicol A, which is about eighteen times less toxic than aflatoxin B1. In all, there is still a major research gap on mycotoxin contaminations, especially in SEA indigenous ffs as there are not much data on this subject.
Phytotoxin contamination in Southeast Asian fermented foods
Cyanogenic glycosides
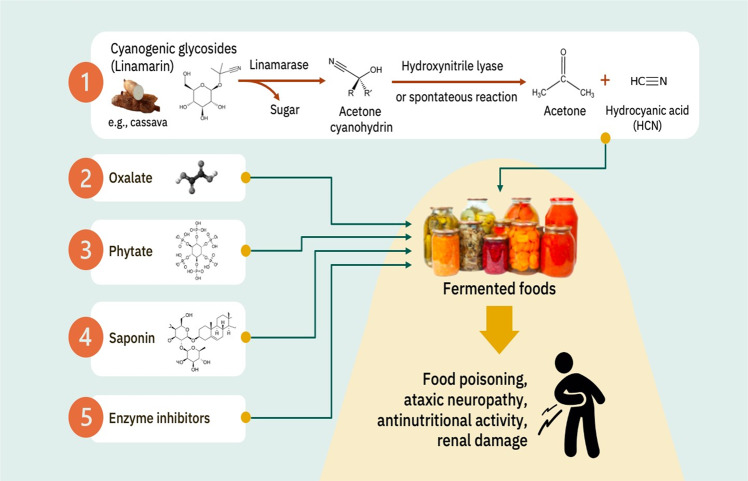
Enzymatic or acid hydrolysis of cyanogenic glycosides produces hydrocyanic acid (HCN), which is strong enough to suppress respiration48. Hydrocyanic acid suppresses cytochrome oxidase, and the enzymatic disintegration of cyanogenic glycosides often takes place in two phases (Fig. 4). In the first phase, beta-glycosidase hydrolyzes the beta-glycosidic bond present between the sugar and aglycone, also known as cyanohydrin. Lyases including hydroxy nitrile lyases catalyze the decomposition of cyanohydrin to HCN and a ketone or an aldehyde, according to the kind of glycoside in the second stage. Cyanogenic glycosides are generally present in tubers of cassava and beans. Cassava tubers consist of two types of cyanogenic glycosides, namely linamarin (95%) and lotaustralin. Cassava tubers contain linamarase (beta-glucosidase), which encounters linamarin (substrate) during the disruption of the cellular structure of the tuber (e.g., grating or grinding process). This may consequently lead to the enzymatic hydrolysis of cyanogenic glycosides and the release of HCN together with unhydrolyzed glycosides. Most human poisonings by cyanogenic glycosides in the tropics including SEA are mainly a result of consumption of cassava products as they still exhibit significant concentrations of HCN regardless of the processing49. Hydrocyanic acid has been reported in tropical ataxic neuropathy, a category of nervous disorders. However, traditional methods of producing cassava-based ffs such as fermentation, soaking, and pressing of soaked pulp have proven effective in eliminating some amounts of HCN.
Fig. 4. Plant toxins from substrates to fermented foods.
Illustrations adapted from Adobe stock images (http://stock.adobe.com/) under Standard Licensing.
Oxalates
Oxalates are scarcely absorbed under non-fasting circumstances in humans. In situations where foods containing excess amounts of oxalates are consumed, they may be excreted as insoluble calcium salts50. There is little knowledge on the oxalate contents present in cereals, legumes, and their products. However, the process of fermentation removes up to 43% of oxalates in some fermented legumes cereals such as soybeans and peanuts and this could be further reduced if subjected to soaking, dehulling, and cooking47. Excess ingestion of oxalates especially from fermented plant foods may inhibit calcium metabolism, resulting in chronic illnesses including renal damage and stone formation.
Phytates
Phytate (myoinositol 1,2,3,4,5,6hexakis dihydrogen phosphate) exists mainly as a result of the monovalent and divalent cations in distinct regions of cereal grains, legumes, and few roots and tubers48,51, where it comprises 85% of the total phosphorus. The presence of phytic acid in ffs causes serious concern as it reduces mineral bioavailability as well as their solubility, functionality, and protein digestibility by forming complexes. This antinutritional substance may also interact with enzymes including alpha-amylase, pepsin, trypsin, and beta-glucosidase, leading to a reduction in their activities. There are two recognized kinds of phytases, namely 3-phytase and 6-phytase. The 6-phytase is commonly found in deeds and grains of higher plants, while the 3-phytase seems to have attributes of microorganisms. During some fermentation and other food processes of tubers, cereals, and grains, phytase contents may be reduced to a certain extent. Phytic acid hydrolysis in Tempeh during fermentation by the action of phytase, which is released during fermentation by Rhizopus oligosporus, thereby reducing the phytate contents in the ffs. Phytic acid contents have been observed to reduce up to 54.5% in Tempeh after fermentation, though it calls for more strategies for safety evaluation52.
Saponins
Saponins are regarded as surface-active sterol or triterpene glycosides to which pentoses or hexoses are linked to sapogenin, a non-polar group that could either be a steroid or a triterpene53. The presence of saponins in foods can easily be identified through their hemolytic activity and their capacity of forming stable foams in aqueous solutions. Although saponins seem to be practically non-toxic to humans, they have been studied to low cause cell inhibition54. Saponins cannot be destroyed by cooking, however, fermentation may reduce the amounts in foods. Studies on decontamination by fermentation carried out by Fenwick and Oakenfull55; Reddy et al.51 revealed a 55.8% decrease in the saponin contents of soybean Tempeh after fermenting with Rhizopus oligosporus.
Enzyme inhibitors
Some naturally existing compounds in plants can inhibit proteolytic and amylolytic enzymes including trypsin, amylase, and chymotrypsin. Trypsin inhibitors can suppress growth by interfering with the digestion of protein, excessive production of pancreatic enzymes, pancreatic hypertrophy, and metabolic disruption in the use of sulfur amino acids in animals fed with plant products containing high concentrations of trypsin inhibitors56. In fermented plant foods, alpha-amylase inhibitor activity has scarcely been reported. The process of fermentation with microorganisms, cooking and controlled heat treatments may reduce the number of enzyme inhibitors in the substrates used in producing ffs. Tannins and some polyphenols are examples of plant-based enzyme inhibitors that form complexes with proteins, leading to the inactivity of the enzyme, decrease in protein solubility and digestibility and reduced ions absorbed57. Enzymes inhibited by these compounds include trypsin, pepsin, chymotrypsin, glucosidase, amylase, and lipases. Fermentation with appropriate fungi or bacteria can help achieve optimal pH conditions for the enzymatic hydrolysis/degradation of tannins and polyphenols58. Fermentation of cooked common beans with Rhizopus oligosporus (Tempe fungi) resulted in a 47% decrease in the activity of trypsin inhibitor due to hydrolysis by the action of Tempe fungi59.
Biogenic amines
Biogenic amines (BA) are low-molecular-weight nitrogenous compounds formed in biological systems by enzymatic decarboxylation of certain amino acids or by amination and transamination of aldehydes and ketones60. BAs are mainly produced in food and beverages by thermal or bacterial decarboxylation of free amino acids during preservative technological processes such as pasteurization and fermentation61. Lactic acid bacteria and some bacteria belonging to family Enterobacteriaceae are the main producers of BA in ffs62,63. Proteolysis and acidification during fermentation process do not only allow these BA-producers to persist and thrive, but also increase the availability of precursor amino acids, resulting in accumulation of BAs63,64.
The most common BAs found in food are histamine (HIS), tryptamine (TRY), putrescine (PUT), spermine (SPM), cadaverine (CAD), agmatine (AGM), spermidine (SPD), β-phenylethylamine (PHE), tyramine (TYM) and trimethylamine (TMA)60,65. Low levels of these compounds in food are not considered as a serious risk. However, when present in high concentrations, they could induce wide range of adverse health effects in consumers, especially in sensitive individuals66–68. HIS and TYR are the most widely studied BA due to their frequent occurrence in ffs and toxic effects including headache, hypotension, and gastroenteritis67. Although, the toxicity of other amines such as PUT, CAD, TRY and PHE remain elusive. However, they have been found to exacerbate the negative effects of HIS and TYR, due to inhibition of HIS and TYR metabolizing enzymes66. Moreover, they can react with nitrites in food, to produce carcinogenic nitrosamines66,68,69. In terms of regulation, HIS is the only BA with regulatory limits. The US Food and Drug Administration (USFDA) set a maximum limit (ML) of 50 mg/kg, while the European Food Safety Authority (EFSA) established a ML of 100 mg/kg for HIS in fish and fish products intended for human consumption65.
It is noteworthy to state here that other plant toxins such as pyrazolidine and tropane alkaloids have been identified in certain cereal crops due to the weeds that grow among the cereals. These toxins may also contribute to toxin contaminations in ffs, although it is an uninvestigated risk.
Analytical techiniques for determination of common natural toxins in sea fermented foods
Mycotoxins
Accurate determination of mycotoxins in ffs can be challenging due to the structure and chemical composition of the matrices. Thus, most sample preparation for mycotoxin analysis involves an extraction and clean-up steps prior to instrumental analysis. Due to the vast variety of raw materials that are fermented, diverse extraction and clean-up procedures can be found in the literature (Table 2)70,71. Also, the choice of solvents has been shown to contribute significantly to the maximal recovery of analytes of interest. Generally, mixtures of methanol–water and acetonitrile-water are the most frequently used extraction solvents during sample preparation72–74. However, for ffs with high fat content and pigments, extraction solvents including ethyl acetate–formic acid, 1-octanol and toluene, dichloromethane, acetone, and chloroform have been found to be more efficient for the recovery of mycotoxins75. The clean-up step following sample extraction helps to improve selectivity and reduce interference compounds (matrix effect), which in turn, contribute to the accurate measurement of mycotoxins in ffs. Clean-up methods commonly employed for food analysis include solid phase extraction (SPE), liquid–liquid extraction (LLE), solid–liquid extraction (SLE), accelerated solvent extraction (ASE), supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), vortex assisted low density solvent–microextraction (VALDS–ME), solid phase extraction (SPE), bovine serum albumins (BSA), aptamer-affinity columns (AACs), molecularly imprinted polymers (MIPs) and immunoaffinity columns (IAC)73–75. The most frequently used clean-up methods for the determination of mycotoxins in ffs are SPE, IAC and BSA.
The SPE technique is fast, requires less solvent, and remove interfering substances present in the food matrix by sorption on a solid adsorbent (usually packed in cartridges), while producing a solution containing mainly the analyte of interest—mycotoxins76. Examples of sorbents used for sample clean-up include C-18 and primary and secondary amine (PSA). In contrast to SPE, IAC isolate and purify mycotoxins through selective binding of antibodies immobilized onto small columns. Analytes are eluted from the column using minimal amounts of organic solvent77. IAC have been developed for both single and multi-mycotoxin, especially the regulated mycotoxins77,78. Despite providing reliable results, IAC still have some important drawbacks such as matrix interference, cross reactivity, column’s short life, column capacity, and are very expensive77. Serum albumins from bovine origin (BSA) that can mimic the recognition properties of antibodies and form a stable binding affinity towards some mycotoxins are currently being used to develop BSA-based sample clean-up columns. They are cost-effective, precise, and accurate as IAC70,79.
Several analytical techniques including immmunoassays, TLC, gas chromatography, and high-performance liquid chromatography coupled with various detectors like FL, diode array, UV, and MS, have been developed and used for the detection and quantification of mycotoxins in ffs (Table 2)80. The most used techniques for determination of mycotoxins in SEA ffs are HPLC-UV, HPLC-FL, and LC-MS/MS (Table 2). For precise and accurate results, and simultaneous determination of multiple mycotoxins at low quantification limits, LC-MS/MS is the method of choice. However, when rapid analysis of mycotoxins is required, immunoassay-based methods including enzyme-linked immunosorbent assay (ELISA), lateral-flow devices (LFDs) and biosensors are very useful despite their numerous drawbacks (such as false-positive results, sensitivity, and reproducibility).
Biogenic amines
Several analytical methods have been developed to detect and quantify BA levels in ffs. These techniques range from the more traditional colorimetric and fluorometric methods; to chromatography methods including gas chromatography (GC) and high-performance liquid chromatography (HPLC) coupled with various detectors such as fluorescence (FL), ultraviolet (UV), photo-diode array (PDA) and mass spectrometry (MS)81,82. HPLC-UV and HPLC-FL are the most frequently reported methods for the detection and quantification of BA in SEA ffs (Table 3). Prior to instrumental analysis of samples, a pre-and post-column derivatization is required to accurately quantify BA. This is due to the lack of chromophore or fluorophore in BA structure83,84. Derivatization agents including dansyl chloride, fluorescamine, fluorenyl-methyl chloroformate, 6-aminoquinolyl-N-hydroxy-succinimidyl carbamate and O-phthaladehydes are suitable agents. However, dansyl chloride is the most preferred, as it can react with both the primary and secondary amino groups to form a more stable and highly sensitive derivatized products84,85.
Table 3.
Sample preparation and analytical techniques for determination of biogenic amines in SEA fermented foods and raw materials used for preparing fermented foods.
| Fermented Food | Local name | Country/Origin | No of samples | Biogenic amines | Extraction solution | Derivatisation agent | Analytical method | Reference |
|---|---|---|---|---|---|---|---|---|
| Wine | Mijiu, Uangjiu, Huangjiu | Malaysia | 18 | TRY, HIS, PUT, TYM, CAD, PHE, SPD | 0.1 M HCl | Dansyl chloride | HPLC-PDA | Yue, et al.156 |
| Fish | Sambal-terasi | Indonesia | 6 | TRY, HIS, PUT, TYM, CAD, PHE | 5% TCA | Dansyl chloride | HPLC-UV | Damanik, et al.157 |
| Fish and vegetables | Teuktrey, Prahok, Trasork chav | Cambodia | 57 | HIS, TYR, PUT, and CAD | 0.4 M PCA | Dansyl chloride | HPLC-UV | Ly, et al.158 |
| Pork | Nham | Thailand | 4 | TRY, HIS, PUT, TYM, CAD, PHE, SPD | 0.4 M PCA | Dansyl chloride | HPLC-PDA | Santiyanont, et al.83 |
| Shrimp paste | Kapi | Philippines | 10 | HIS, PUT, CAD, SPD, SPM | 0.4 M PCA | Dansyl chloride | HPLC-UV | Pilapil, et al.159 |
| Fish | Ikan pekasam | Malaysia | 15 | TRY, HIS, PUT, TYM, CAD, PHE, SPD, SPM | 0.05 M HCl | Benzyl chloride | HPLC-UV | Ezzat, et al.160 |
| Fish and vegetables | — | Malaysia | 67 | TRY, HIS, PUT, TYM, CAD, PHE, SPD, SPM | 6% TCA | Benzyl chloride | HPLC-UV | Zare, et al.161 |
| Cheese | — | Philippines | 10 | CAD, HIS, TYM | 5% TCA | — | TLC | Vallejos, et al.162 |
| Fish, soybean, and vegetables | Teuktrey, Prahok, Trasork chav, Tempe | Malaysia | 62 | TRY, PUT, HIS, TYM, SPD | 5% TCA | Dansyl chloride | HPLC-UV | Saaid, et al.163 |
| Bonito flakes | Katsuobushi | Philippines | 8 | TRY, HIS, PUT, TYM, CAD, PHE, SPD, SPM | 5% TCA | Dansyl chloride | HPLC-UV | Qiao, et al.164 |
| Soybean | Tempe | Indonesia | 10 | TYM, CAD, PHE, SPD, SPM | 5% TCA | Benzyl chloride | HPLC-PDA | Nout, et al.165 |
| Minced fish | Som-fug | Thailand | 7 | TRY, HIS, PUT, TYM, CAD, PHE | 10% TCA | Dansyl chloride | HPLC-UV | Riebroy, et al.166 |
LOD limit of detection, PCA Perchloric acid, TCA Trichloroacetic acid, HCl Hydrochloric acid, HPLC High performance liquid chromatography, TLC Thin layer chromatography, UV Ultraviolet, PDA Photodiode-Array, HIS histamine, TRY Tryptamine, PUT putrescine, SPM spermine, CAD cadaverine, SPD spermidine, PHE β-phenylethylamine, TYM tyramine.
Recent advances in analytical techniques have paved the way for the use of biosensors and flow-injection analysis (FIA), which offer more advantages in comparison to chromatography techniques86,87. They are less-time consuming, much more economical, and do not require sample clean-up or derivatization procedures. Immobilized enzymes (such as diamine oxidase, amine oxidase, peroxidase, and histaminase) that are highly specific for the targeted BA are placed directly on the surface of a working electrode (biosensor) or in a suitable reactor in a FIA arrangement88,89. Most of the available systems also contain a second enzyme such as horseradish peroxidase, for direct detection of BA through chemiluminescence88,89.
In terms of incidences of BA in SEA traditional ffs, various studies have reported the BA content of fermented products as outlined in Table 3. PUT, CAD, TYR, and HIS were the most prevalent BA found in food, particularly in fish and vegetables from Cambodia and Malaysia (Table 3). HIS was detected mostly at levels exceeding the FDA and EFSA maximum permitted limits. The other amines (SPM, PHE and TRY) were detected at low concentrations, with mean total BA levels below 50 mg/kg. The range of total BA found in different types of fermented food as well as the country and analytical methods are summarized in Table 3.
Mitigation strategies
This section discusses the various strategies that have proven effective in the removal or decontamination of mycotoxins and plants in ffs to ensure their safety.
‘Clean substrate’ strategy
Prevention of mycotoxin contamination in raw materials (cereal grains, legumes, fruits and vegetables, roots, and tubers, etc.) (Fig. 3) used in the production of indigenous ffs is of huge importance. The decontamination and detoxification of mycotoxins in agricultural commodities is a problem globally90. It is well known that the drying of crops immediately after harvesting (moisture contents < 14%), the proper handling, and proper storage conditions will aid in the in prevention of mycotoxin accumulation91. Physical processing such as screening and elimination of contaminated material and the peeling, grading, irradiation, segregation, washing, drying, and milling are common treatments applied to decontaminate92. Controlled storage environments such as appropriate ventilation, humidity, managed temperature, and packaging techniques curtail the growth of fungi and accumulation of mycotoxins.93. Essential oils including turmeric oil, clove oil, peppermint and lime oil (single-oil dose and when combined) has been shown to suppress both the production of aflatoxin B1 and the growth of Aspergillus species94,95. In rice grains, the use of the whole clove also inhibited the growth of Aspergillus flavus and Penicillium citrinum and thus reduced mycotoxins levels96.
Fermentation process
The process of fermentation is one of the most economical and easiest ways of preserving foods besides adding organoleptic properties, nutritional quality, and health benefits to the ffs. Thus, fermentation helps in shelf-life extension of the ff product, facilitates transportation by reducing its volume, and eliminates undesirable elements97. In recent years, various microorganisms such as yeasts and molds, bacteria, have shown their ability to reduce or even eliminate mycotoxins98. Many researchers have reported fermentation process as an effective, sustainable, and inexpensive approach to reduce mycotoxins in food99,100. For instance, certain microbes in starter culture can adsorb mycotoxins to their cell wall or use them up as primary source of carbon and nitrogen99,101. Moreover, some enzymes produced by microorganisms have been shown to bio-transform or degrade mycotoxins, leading to mycotoxin reduction44,99. However, following food fermentation, ffs can still contain significant concentration of mycotoxins, particularly when the raw material or substrate is highly contaminated. Moreover, some fermentation processes provide favorable conditions for the growth and production of mycotoxins by toxigenic fungal species of Aspergillus, Fusarium and Penicillium genera102,103. In addition, fermentation conditions such as low pH, facilitate the hydrolysis of conjugated mycotoxins (such as deoxynivalenol-3-glucoside and zearalenone-16-glucoside) back to their precursor mycotoxins, which ultimately, result in the accumulation of mycotoxins. Therefore, there is a need for constant monitoring of mycotoxins in ffs and raw materials used for fermentation, to develop an accurate risk assessment and mitigation measures. More investigations on reducing plant toxins in ffs, specifically by fermentation needs to be carried out. In studies carried out by Shukla, et al.46, plants extracts (Ginkgo biloba, cloves, and Nelumbo nucifera), decreased fungal microflora when added during the fermentation of Meju. Aflatoxins are produced by the fungal isolates in Meju, while the addition of the plant extracts during the fermentation process enhanced the shelf-life quality and lowered the toxic effects from the fermented product. Conventional processes such as washing, decantation, milling, sieving, and fermentation of indigenous ffs have reduced mycotoxin levels by up to 90%104. Some fungi species are also effective in preventing mycotoxin contamination in ffs. For instance, Aspergillus niger, a strain of A. flavus that does not produce aflatoxins, when introduced during fermentation, possesses the ability to decontaminate aflatoxins B1 by converting it into aflatoxicol. In the same vein, Rhizopus oligosporus, when cultured with aflatoxin B1-synthesizing A. flavus, inhibited the production of the aflatoxin B1 and triggered the degradation105.
Fermentation was reported to reduce phytate contents in cereals, legumes, and tubers due to the activity of the endogenous phytase during the process. Fermentation of tempe with Rhizopus oligosporus decreased the phytate content to between 32.9–54.5% of the levels in control samples. As reported by Reddy and Pierson48 a decrease in phytate levels (48.5–96.3%) in fermented peanut press cake was obtained when fermented with either Rhizopus oligosporus or Neurospora sp; fermentation of cooked traditional beans with Rhizopus oligosporus led to a 47% decrease in trypsin inhibitory effects; cassava fermentation (48 h) resulted into reduced HCN amounts to <30% of the initial amounts.
Lactic acid bacteria (LAB) fermentation
Lactic acid bacteria are the most promising bacteria used as fungal antagonists in food fermentation. Application of LAB in indigenous ffs have been in existence from ancient times, and they have gained the status of QPS (Qualified Presumption of Safety) and GRAS (Generally Regarded as Safe) by the European Food Safety Authority (EFSA) and the American Food and Drug Agency, respectively106. So far, many strains of LAB have been considered as “green preservatives” due to their ability to suppress fungal growth in food107. Various antifungal compounds including bacteriocins, organic acids, diacetyl, fatty acids, hydrogen peroxide, lactones, alcohols, bioactive antimycotic oxide, and reuterin have been found to be synthesized by different LABs108. The function of LAB strains is not restricted to fungal growth inhibition, and some are known to bind to fungal mycotoxins, leading to their inactivation.109
Inhibition of mycotoxins by LAB can take place through different mechanisms such as inhibition of productions of mycotoxins (by direct impact on fungal growth110, mycotoxin production inhibition via the modification of external environment111,112, and mycotoxin elimination through adsorption by LAB strains). Adsorption of mycotoxins through their cell wall components is the mechanism mostly common in the removal of mycotoxin by LAB from food matrices. Lactic acid bacteria have been proven to adsorb mycotoxins based upon the functional groups of their cell walls. For example, the cell wall of LAB as with other Gram-positive bacteria is made up of a thick complex peptidoglycan sacculus, enclosed by a cytoplasmic membrane, which is enriched with proteins, polysaccharides, lipoteichoic acids, and teichoic acids. Peptidoglycans and polysaccharides are known to be the major components of LAB for the removal of mycotoxins, and variations in the mycotoxin binding capacity of LAB species is due to the differences that exist in the peptidoglycan structure of their cell wall as well as the amount of available binding sites113. Mechanisms explaining the binding of LAB strains to different mycotoxins that are present in ffs are discussed in the subsequent subsections.
Aflatoxins
Figure 5a outlines all possible mechanisms for the binding of aflatoxin B1 to the cell wall components of LAB. The binding of Lactobacillus rhamnosus to aflatoxin B1 is associated primarily with cell wall peptidoglycans and without the role of cell wall proteins, exopolysaccharides, lipids, and minerals114. In contrast, teichoic acids besides peptidoglycans were also observed to be responsible for the binding of aflatoxin B1 by Lactobacillus reuteri NRRL14171 and Lactobacillus casei Shirota115. Furthermore, it has been studied that aflatoxin B1 can bind to cell β-d-glucans on walls of bacteria involving hydrogen bonds and van der Waals interactions116. Environmental conditions play a key role in the modulation of the number of binding sites available for mycotoxins, bacterial cell wall structure, and physiochemical characteristics112. Acid treatments were reported to increase the hydrophobic interactions because of the denaturing of the cell wall proteins, thereby increasing the number of binding sites to aflatoxin B1117. A better theoretical model was established by Bueno, et al.118 for aflatoxin B1 adsorption to LAB species. According to the authors, the amount of the mycotoxin adsorbed depends on the number of the available binding sites on the cell wall of a bacterium and the equilibrium constant119. However, this could be altered due to chemical, physical, or genetic changes.
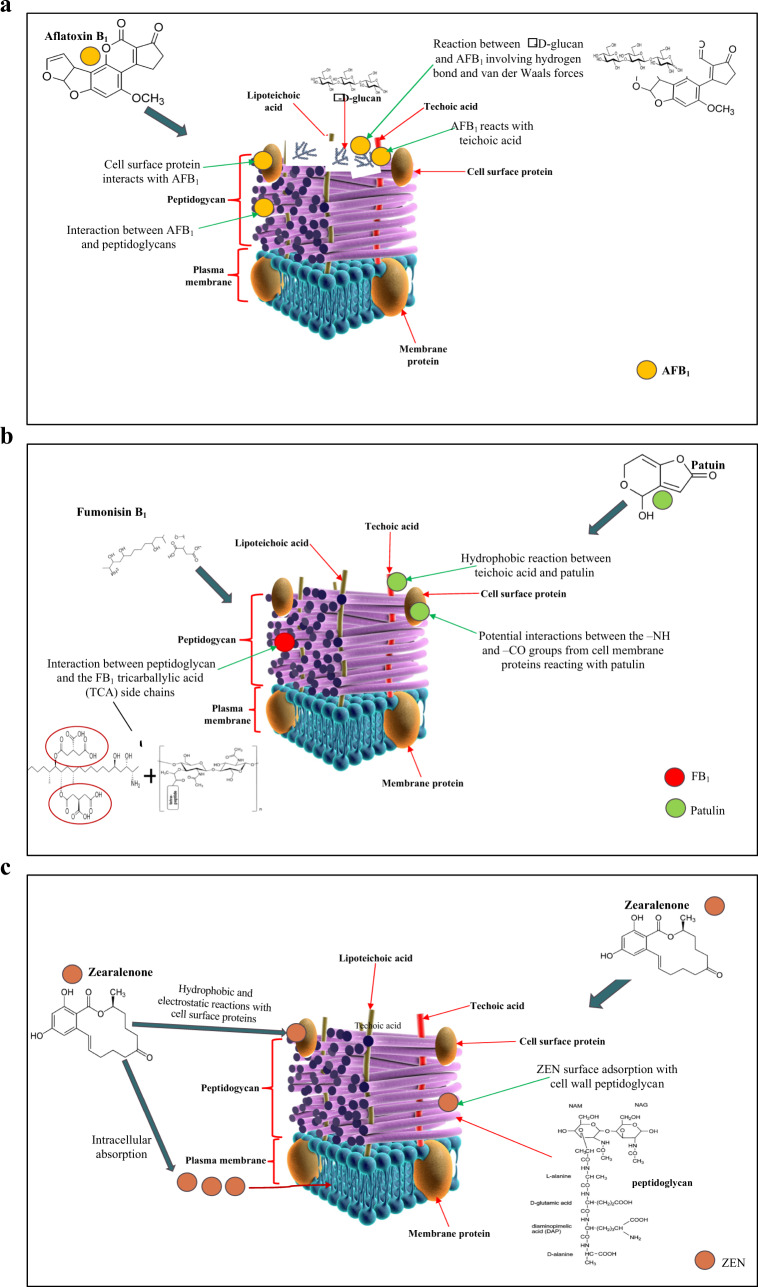
Fig. 5. Diagrams illustrating possible interactions between some selected mycotoxins and bacteria cell wall components.
a The Mechanisms of interactions between aflatoxin B1 with bacterial cell. Wall components such as peptidoglycan, cell wall teichoic acids, β-D-glucan, and cell surface proteins. Illustrations adapted from Adobe stock images, copyright Kateryna_Kon (http://stock.adobe.com/) under Standard Licensing. b The Mechanisms of interactions between fumonisin B1 and patulin with bacterial cell wall components. fumonisin B1 only reacts with cell wall peptidoglycan (interaction between tricarballyllic acid (TCA) of fumonisin B1 and peptidoglycans). The known possible mechanism for bacterial binding to patulin involves interaction with cell surface proteins (-NH or -CO groups). Illustrations adapted from Adobe stock images, copyright Kateryna_Kon (http://stock.adobe.com/) under Standard Licensing. c The Possible mechanisms of interactions between zearalenone and a LAB strain; interaction with intracellular proteins, reaction with cell wall peptidoglycans, or interaction with cell surface proteins (by hydrophobic or electrostatic reaction). Illustrations adapted from Adobe stock images, copyright Kateryna_Kon (http://stock.adobe.com/) under Standard Licensing. AFB Aflatoxin B1, ZEN Zearalenone, OTA Ochratoxin A, DON Deoxynivalenol, FB1 Fumonisin B1.
Ochratoxin A
Ochratoxin A has been found to bind to the cell wall components of L. planetarium, Lactobacillus sanfranciscensis, and L. brevis though the binding was shown to be altered not only by the hydrophobicity of the cell walls but also by the electro donor-acceptor and Lewis’s acid-base reaction120. In addition, the ability of LAB species to enhance mycotoxin binding was reported to be further improved the mutagenesis/genetic control or via the supplementation of some binding-promoting compounds in few cases.
Fumonisins
Figure 5b shows the likely interactions between fumonisin B1 and bacterial cell wall components for mycotoxin removal or decontamination. Cell components and functional groups that are responsible for the interactivity and adsorption of fumonisin B1 and fumonisin B2 by Lactobacillus paraplantarum were studied by Niderkorn, et al.121 and observed that peptidoglycan or some compounds closely linked to it are possibly responsible for fumonisins binding, and without the role of the cell wall lipids, proteins and polysaccharides. Peptidoglycans were also reported to exhibit the maximum capacity of binding to fumonisins by Lactobacillus Pentosus X8 and L. plantarum B7122. The mechanism of the interaction between fumonisins and the cell wall peptidoglycan of LAB stains is yet to be fully understood. Nonetheless, Niderkorn, et al.121 in his study, proposed that tricarballylic acid chains of fumonisins react with peptidoglycans during the process of binding.
Patulin
Zoghi, et al.123 indicated the function of cell surface adhesion proteins as the key structures for patulin binding. Various factors associated with the bio-adsorption of patulin by deactivated LAB species were investigated to further understand the mechanism in a recent study by Wang, et al.124. These authors reported that patulin binding by LAB strains significantly increased by esterification and NaOH pretreatments and reduced by pretreatments using trypsin, iodate, periodate, and lipase. Furthermore, it was revealed that the peptidoglycans of bacterial cell walls do not function in this absorption to any great degree. The authors indicated the possibility of thiol, esters, and alkaline amino acids as the likely compounds responsible for the absorption. Some more novel insights into the mechanisms of the paulin binding by strains of LAB were provided by Wang, et al.125 where they demonstrated the importance of the cell volume and area, and proposed that the higher the cell volume and area, the greater patulin absorption. In addition, the proteins on the cell surface including the carbohydrate components of LAB cells (C–O groups, OH, and NH) were revealed as the likely cell wall components involved in the patulin adsorption. The potential mechanism for patulin adsorption by bacteria strains can be found in Fig. 5b. However, the precise mechanism including the type of reaction between the bacterial cell wall components and patulin is still to be fully understood.
Zearalenone, T-2 toxin, and trichothecene mycotoxins
Król, et al.126 evaluated the kinetics of zearalenone binding by Lactococcus lactis species as a function of time that gave a better understanding of the factors that regulate zearalenone binding. The rate of adsorption of zearalenone by L. lactis reduced from 5.49 μg/mL/min during the stage of absorption (720 min), where about 88% of zearalenone was adsorbed, and to 0.15 μg/mL/min at the second stage of adsorption where equilibrium was attained in the system. The mechanism proposed for the zearalenone removal by strains of LAB involved its interactivity with the cell wall proteins, peptidoglycans, or absorption into the bacterial cell succeeding interaction with the intracellular proteins (Fig. 5c).
Correspondingly, L. plantarum strain 102 was reported to bind T-2 toxin on cell wall components. The binding to cell walls is the only well-known mechanism responsible for trichothecenes removal by strains of LAB127. As studied by Zhou, et al.128, the cell wall of L. lactis CAMT22361 was involved in the removal of T-2 toxins. Non-protein elements of the cell-extracellular section played a major role in the toxin removal followed by the protein components of the extracellular section.
The application of LAB in eliminating mycotoxins in ffs is a general practice in several industries related to foods. Lactobacillus spp., Bifidobacterium spp., Propionibacterium spp., and Streptococcus spp, Lactococcus spp., Leuconostoc spp., Pediococcus spp. strains were reported to be often used to remove mycotoxins in ffs, as they are well known for their resistance and ability to bind to toxins4. Lactobacillus fermentum OYB, L. fermentum RS2, L. plantarum MW, L. plantarum YO, L. brevis WS3, and Lactococcus spp. RS3 has been reported for its anti-AF synthesizing ability when isolated from fermented gruels. Interestingly, L. plantarum YO suppressed the food contaminating aflatoxin B1 including the G-synthesizing Aspergillus spp. in vitro studies. This however suggests the antagonistic ability of the Lactobacillus strain in preventing or reducing mycotoxins in ffs109. In fermented maize meal, Streptococcus lactis and Lactobacillus delbrueckii reduced the amounts of increased zearalenone and fumonisin B1. This result reveals that LAB-mediated fermented maize meal eliminated or reduced the mycotoxins including aflatoxin B1 during the process112. The addition of L. casei during fermentation of kefir greatly about 88.17% of aflatoxin M1. The LAB strain works together with a kefir starter culture to decrease aflatoxin M1 in the food product129. Aspergillus oryzae MAO103, A. oryzae MAO104 isolated from Meju effectively degraded AFB1 to 90% within 2 weeks and further inhibited the growth of aflatoxin B1 produced from Aspergillus flavus130.
Packaging techniques
Food packaging is developed to hold, preserve and protect foods from contamination (environmental and microbial) and other impacts such as shocks, odor, temperature, dust, light, physical damage, and humidity131. In the absence of adequate protection, food will become unappetizing, lose nutritional value, and become unsafe for consumption. For standard food packaging, the required protection is based on the fragility and stability of the food, the target shelf life, and the chain of distribution132. Specific packaging such as effective and smart packaging technologies are essential for fermented foods to preserve the product attributes including flavor, color, and texture, as their fermentation process continues though at a lesser rate133. Fermented foods have the potential of becoming contaminated with degraded products or components due to product-package interactivity. These chemical and physical changes during the package-product interactions may result in the migration of external microbial and chemical contaminants into the food, impacting the organoleptic properties and decreasing the food quality.
Active packaging is one of the innovative packaging used in fermented foods as it makes it convenient to see, feel, read or smell the food characteristics, while intelligent and smart packaging involves the use of sensors/indicators for providing relevant information on the food status or its surrounding system134.
Modified atmosphere packaging135 is a technique that can control gas composition in the environment surrounding the food within a gas-impermeable packaging. For instance, wheat and rye bread artificially inoculated with various fungi species were packaged with 0%, 50%, 75%, or 100% CO2; 1% or 0.03% O2; or with O2 absorber and balanced with N2136. The MAP was more potent against fungal growth on the rye bread, as lesser fungi developed with the increased CO2. But for P. roqueforti, this main contaminant of rye bread was inhibited only in the presence of an O2 absorber. Also, Penicillium roqueforti and A. flavus were unable to grow in both 40% and 60% CO2 environments balanced with N2 and <0.5% O2, but weakly grew (about 30 mm in 30-day incubation) in 20% CO2137.
Fermented products properties, however, are still inadequately understood and some of these products are still packaged using traditional methods. Therefore, there is a need for the development of suitable packaging technology before the commercialization of ffs for enhanced shelf life quality138
Controlled processing conditions
The quality of ff products could be enhanced through the improvement of the process of fermentation and modernization of the automation in food industries. Indeed, several foods manufactures ensure Good Manufacturing Practices (GMP) and Hazard Analysis Critical Control points (HACCP) which greatly reduces potential risks to public health132. On the other hand, indigenous ffs are spontaneously produced at household levels or by small factories usually under hygienically insufficient and unmanaged conditions. Hence, the risks afterward are severe, requiring more rigid hygienic standards139. The process of fermentation itself, if appropriately done, can enhance food safety and public health. This is because fermentation-related microorganisms will generally compete with other microbes, causing competitive exclusion of pathogenic microbes that cause spoilage. For example, inhibitory amounts of ethanol are synthesized during alcohol fermentation; microbes may also produce some inhibitory substances such as bacteriocins, mycosin, and diacetyl, acetaldehydes that may suppress the growth of pathogenic microbes. Lastly, some food fermentation cand involve the addition of salt, hops, sulfites, nitrates, nitrites, and some antimicrobial materials that can suppress the survival or growth of spoilage fungi132.
Indigenous ffs are often consumed as routine diets by many people in SEA. However, pathogenic microbes-producing mycotoxins and plant toxins contribute to the major causes of health issues in humans. This paper has summarized the main mycotoxins and plant toxins present in ffs, and the strategies used for their prevention and/or decontamination. Most substrates used in the production of ffs are a potential source of toxic substances that are detrimental to human health. For instance, cereals and legumes (soybean) are commonly contaminated with mycotoxins (ochratoxin A, aflatoxins, zearalenone, deoxynivalenol and fumonisin), possess plant toxins (cyanogenic glycosides that transforms into HCN during the fermentation process, phytates, saponins, oxalates, and enzyme inhibitors) and accommodate fungal pathogens (Penicillium, Fusarium and Aspergillus spp.).
Studies have demonstrated that the use of substrates that are free from microbial contaminations, specific LAB strains, improved fermentation, and processing conditions (especially washing, prolonged soaking, decanting, and milling), and application of appropriate packaging techniques can effectively mitigate or counteract the toxic substances in ffs. However, it is important to note that, a single method has not been proven 100% effective for ensuring safe food. Hence, more studies into the application of combined management strategies through the integration of numerous potential control methods are required for the safe consumption of indigenous ffs and their sustainability in the global food industry.
Acknowledgements
This study was financially supported by Thailand Science Research and Innovation Fundamental Fund (Project No. 2461863) and Thammasat University Postdoctoral Fellowship (TUPD9/2564).
Author contributions
C.T.E and A.P. conceived the concept, provided guidance; I.O.O. and O.K. and P.J. wrote and edited the various sections of the article; C.T.E and A.P. revised the paper and approved the final version.
Data availability
We declare that data sharing does not apply to our manuscript. This is a review article with no data analyzed during the study.
Competing interests
C.T.E. is an editor for npj Science of Food. C.T.E. was not involved in the journal’s review of, or decisions related to, this manuscript. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Filippis F, Parente E, Ercolini D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017;10:91–102. doi: 10.1111/1751-7915.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray, R. C. & Joshi, V. Fermented Foods: Past, Present and Future (CRC Press,Taylor & Francis Group, 2014).
- 3.Rastogi M, Shrivastava S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017;80:330–340. doi: 10.1016/j.rser.2017.05.225. [DOI] [Google Scholar]
- 4.Anal AK. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: a review. Fermentation. 2019;5:8. doi: 10.3390/fermentation5010008. [DOI] [Google Scholar]
- 5.Bourdichon F, et al. Food fermentations: microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012;154:87–97. doi: 10.1016/j.ijfoodmicro.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Satish Kumar R, et al. Traditional Indian fermented foods: a rich source of lactic acid bacteria. Int. J. Food Sci. Nutr. 2013;64:415–428. doi: 10.3109/09637486.2012.746288. [DOI] [PubMed] [Google Scholar]
- 7.Beermann C, Hartung J. Physiological properties of milk ingredients released by fermentation. Food Funct. 2013;4:185–199. doi: 10.1039/C2FO30153A. [DOI] [PubMed] [Google Scholar]
- 8.Sivamaruthi BS, Kesika P, Chaiyasut C. Toxins in fermented foods: prevalence and preventions—a mini review. Toxins. 2019;11:4. doi: 10.3390/toxins11010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law SV, Abu Bakar F, Mat Hashim D, Abdul Hamid A. Popular fermented foods and beverages in Southeast Asia. Int. Food Res. J. 2011;18:475–484. [Google Scholar]
- 10.Odhav B, Naicker V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002;19:55–61. doi: 10.1080/02652030110053426. [DOI] [PubMed] [Google Scholar]
- 11.Devi YR, Chakma A, Yenkokpam S. Traditional wisdom of Meitei community regarding elimination of cyanogenic glycosides in bamboo shoot food products. Indian J. Traditional Knowl. 2017;16:470–475. [Google Scholar]
- 12.Fernández M, Hudson JA, Korpela R, de los Reyes-Gavilán CG. Impact on human health of microorganisms present in fermented dairy products: an overview. BioMed. Res. Int. 2015;2015:412714. doi: 10.1155/2015/412714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell-Platt G. Fermented foods—a world perspective. Food Res. Int. 1994;27:253–257. doi: 10.1016/0963-9969(94)90093-0. [DOI] [Google Scholar]
- 14.Ray, R. C. & Montet, D. Fermented Foods, Part II: Technological Interventions (CRC Press, 2017).
- 15.Dirar, H. A. The Indigenous Fermented Foods of the Sudan: A Study in African Food and Nutrition (CAB international, 1993).
- 16.Fukushima D. Fermented vegetable protein and related foods of Japan and China. Food Rev. Int. 1985;1:149–209. doi: 10.1080/87559128509540768. [DOI] [Google Scholar]
- 17.Steinkraus KH. Classification of fermented foods: worldwide review of household fermentation techniques. Food Control. 1997;8:311–317. doi: 10.1016/S0956-7135(97)00050-9. [DOI] [Google Scholar]
- 18.Voidarou, C. et al. Fermentative foods: microbiology, biochemistry, potential human health benefits and public health Issues. Foods10.3390/foods10010069 (2021). [DOI] [PMC free article] [PubMed]
- 19.Steinkraus, K. Industrialization of Indigenous Fermented Foods, Revised and Expanded (CRC Press, 2004).
- 20.Tamang, J. P. Ethnic Fermented Foods and Alcoholic Beverages of Asia (Springer, 2016).
- 21.Liu D, Tong C. Bacterial community diversity of traditional fermented vegetables in China. LWT. 2017;86:40–48. doi: 10.1016/j.lwt.2017.07.040. [DOI] [Google Scholar]
- 22.Tamang JP, et al. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020;19:184–217. doi: 10.1111/1541-4337.12520. [DOI] [PubMed] [Google Scholar]
- 23.Swain, M. R., Anandharaj, M., Ray, R. C. & Rani, R. P. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol. Res. Int.2014, 250424 (2014). [DOI] [PMC free article] [PubMed]
- 24.Beuchat, L. R. Biotechnology: Enzymes, Biomass, Food Feed 2nd edn, Vol. 9 (ACS Publications, 1995).
- 25.Soni S, Dey G. Perspectives on global fermented foods. Br. Food J. 2014;116:1767–1787. doi: 10.1108/BFJ-01-2014-0032. [DOI] [Google Scholar]
- 26.Owens, J. D. Indigenous fermented foods of Southeast Asia (CRC Press, 2014).
- 27.Montel M-C, et al. Traditional cheeses: rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014;177:136–154. doi: 10.1016/j.ijfoodmicro.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Brandt MJ. Starter cultures for cereal based foods. Food Microbiol. 2014;37:41–43. doi: 10.1016/j.fm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Sarter, S., Ho, P.-H. & To, K. J. Q. A. Current context of food safety in Vietnam: a glance at food of animal origin. Qual. Assur. Saf. Crop. Foods7, 57–62 (2015).
- 30.FAO F. Agriculture organization of the United Nations. 2004. Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food Nutr. Pap. 2004;81:9–28. [Google Scholar]
- 31.Inoue T, Nagatomi Y, Uyama A, Mochizuki N. Fate of mycotoxins during beer brewing and fermentation. Biosci., Biotechnol., Biochem. 2013;77:1410–1415. doi: 10.1271/bbb.130027. [DOI] [PubMed] [Google Scholar]
- 32.Dolatabadi S, et al. Food preparation with mucoralean fungi: a potential biosafety issue? Fungal Biol. 2016;120:393–401. doi: 10.1016/j.funbio.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Samsudin NIP, Abdullah N. A preliminary survey on the occurrence of mycotoxigenic fungi and mycotoxins contaminating red rice at consumer level in Selangor, Malaysia. Mycotoxin Res. 2013;29:89–96. doi: 10.1007/s12550-012-0154-7. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Zhou Y-C, Yang M-H, Ou-Yang Z. Natural occurrence of citrinin in widely consumed traditional Chinese food red yeast rice, medicinal plants and their related products. Food Chem. 2012;132:1040–1045. doi: 10.1016/j.foodchem.2011.11.051. [DOI] [Google Scholar]
- 35.Woo, S. Y. et al. Simultaneous determination of twenty mycotoxins in the Korean soybean paste doenjang by LC-MS/MS with immunoaffinity cleanup. Toxins10.3390/toxins11100594 (2019). [DOI] [PMC free article] [PubMed]
- 36.Shukla S, et al. Toxicological evaluation of lotus, ginkgo, and garlic tailored fermented Korean soybean paste (Doenjang) for biogenic amines, aflatoxins, and microbial hazards. Food Chem. Toxicol. 2019;133:110729. doi: 10.1016/j.fct.2019.110729. [DOI] [PubMed] [Google Scholar]
- 37.Jeong SE, Chung SH, Hong S-Y. Natural occurrence of aflatoxins and ochratoxin A in meju and soybean paste produced in South Korea. Appl. Biol. Chem. 2019;62:65. doi: 10.1186/s13765-019-0472-y. [DOI] [Google Scholar]
- 38.Ahn S, Lee S, Lee J, Kim B. Accurate determination of ochratoxin A in Korean fermented soybean paste by isotope dilution-liquid chromatography tandem mass spectrometry. Food Chem. 2016;190:368–373. doi: 10.1016/j.foodchem.2015.05.114. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, et al. Comparison of extraction methods for analysis of citrinin in red fermented rice. Food Chem. 2014;157:408–412. doi: 10.1016/j.foodchem.2014.02.060. [DOI] [PubMed] [Google Scholar]
- 40.Regulation EC. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union. 2010;15:1–72. [Google Scholar]
- 41.EC. Commission Regulation (EC) No. 1126/2007 of 28 September 2007 (amending Regulation (EC) No 1881/2006) setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union. 2007;255:14–17. [Google Scholar]
- 42.European Commission Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2006;70:12–34. [Google Scholar]
- 43.EC. Commission Regulation (EU). 2019/1901 of 7 November 2019 (amending Regulation (EC) No 1881/2006) as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Official J. European Union293, 2–4 (2019).
- 44.Petchkongkaew A, Taillandier P, Gasaluck P, Lebrihi A. Isolation of Bacillus spp. from Thai fermented soybean (Thua-nao): screening for aflatoxin B1 and ochratoxin A detoxification. J. Appl. Microbiol. 2008;104:1495–1502. doi: 10.1111/j.1365-2672.2007.03700.x. [DOI] [PubMed] [Google Scholar]
- 45.Thanh, V. N. & Viet Anh, N. T. in Ethnic Fermented Foods and Alcoholic Beverages of Asia (ed Tamang, J. P.) 383–409 (Springer India, 2016).
- 46.Shukla S, et al. Evaluation of fungal microflora for aflatoxin producing possibility in novel quality Meju fermented with single and/or multiple additions of Nelumbo nucifera, Ginkgo biloba, and Allium sativum extracts. J. Food Saf. 2017;37:e12368. doi: 10.1111/jfs.12368. [DOI] [Google Scholar]
- 47.Steinkraus, K. Handbook of Indigenous Fermented Foods, Revised and Expanded (CRC Press, 2018).
- 48.Reddy NR, Pierson MD. Reduction in antinutritional and toxic components in plant foods by fermentation. aaThe term ‘plant foods’ is used in the context of food derived from plant sources. Food Res. Int. 1994;27:281–290. doi: 10.1016/0963-9969(94)90096-5. [DOI] [Google Scholar]
- 49.Bolarinwa, I. F., Oke, M. O., Olaniyan, S. A. & Ajala, A. S. Toxicology (IntechOpen, 2016).
- 50.Franxman, T. J. & Baldwin, J. L. Food Allergy (Mayo Clinic, 2013).
- 51.Reddy, N., Pierson, M. D. & Salunkhe, D. Legume-Based Fermented Foods (CRC Press, 2018). [DOI] [PubMed]
- 52.Azeke MA, Greiner R, Jany K-D. Purification and characterization of two intracellular phytases from the tempeh fungus Rhizopus Oligosporus. J. Food Biochem. 2011;35:213–227. doi: 10.1111/j.1745-4514.2010.00377.x. [DOI] [Google Scholar]
- 53.Lee JH, et al. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Int. J. Food Sci. Technol. 2011;46:2513–2519. doi: 10.1111/j.1365-2621.2011.02775.x. [DOI] [Google Scholar]
- 54.Lorent JH, Quetin-Leclercq J, Mingeot-Leclercq M-P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014;12:8803–8822. doi: 10.1039/C4OB01652A. [DOI] [PubMed] [Google Scholar]
- 55.Fenwick DE, Oakenfull D. Saponin content of food plants and some prepared foods. J. Sci. Food Agric. 1983;34:186–191. doi: 10.1002/jsfa.2740340212. [DOI] [PubMed] [Google Scholar]
- 56.Ruan J-j, et al. Coix lacryma-jobi chymotrypsin inhibitor displays antifungal activity. Pestic. Biochem. Physiol. 2019;160:49–57. doi: 10.1016/j.pestbp.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Nyanzi, R. & Jooste, P. in Probiotics (ed Rigobelo, E.) 161–197 (IntechOpen, 2012).
- 58.Dlamini NR, Taylor JR, Rooney LW. The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chem. 2007;105:1412–1419. doi: 10.1016/j.foodchem.2007.05.017. [DOI] [Google Scholar]
- 59.Starzyńska-Janiszewska A, Stodolak B, Duliński R, Mickowska B. The influence of inoculum composition on selected bioactive and nutritional parameters of grass pea tempeh obtained by mixed-culture fermentation with Rhizopus oligosporus and Aspergillus oryzae strains. Food Sci. Technol. Int. 2012;18:113–122. doi: 10.1177/1082013211414771. [DOI] [PubMed] [Google Scholar]
- 60.Wójcik W, Łukasiewicz M, Puppel K. Biogenic amines: formation, action and toxicity—a review. J. Sci. Food Agric. 2021;101:2634–2640. doi: 10.1002/jsfa.10928. [DOI] [PubMed] [Google Scholar]
- 61.Doeun D, Davaatseren M, Chung M-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017;26:1463–1474. doi: 10.1007/s10068-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li, L. et al. Evaluation of the biogenic amines and microbial contribution in traditional Chinese sausages. Front. Microbiol.10.3389/fmicb.2019.00872 (2019). [DOI] [PMC free article] [PubMed]
- 63.Barbieri, F., Montanari, C., Gardini, F. & Tabanelli, G. Biogenic amine production by lactic acid bacteria: a review. Foods10.3390/foods8010017 (2019). [DOI] [PMC free article] [PubMed]
- 64.Marcobal A, De las Rivas B, Muñoz R. Methods for the detection of bacteria producing biogenic amines on foods: a survey. J. f.ür. Verbraucherschutz und Lebensmittelsicherheit. 2006;1:187–196. doi: 10.1007/s00003-006-0035-0. [DOI] [Google Scholar]
- 65.Hazards EPOB. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:2393. doi: 10.2903/j.efsa.2011.2393. [DOI] [Google Scholar]
- 66.del Rio B, et al. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019;9:120. doi: 10.1038/s41598-018-36239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durak-Dados A, Michalski M, Osek J. Histamine and other biogenic amines in food. J. Vet. Res. 2020;64:281–288. doi: 10.2478/jvetres-2020-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ladero V, Calles-Enríquez M, Fernández M, Alvarez MA. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010;6:145–156. doi: 10.2174/157340110791233256. [DOI] [Google Scholar]
- 69.Warthesen JJ, Scanlan RA, Bills DD, Libbey LM. Formation of heterocyclic N-nitrosamines from the reaction of nitrite and selected primary diamines and amino acids. J. Agric. Food Chem. 1975;23:898–902. doi: 10.1021/jf60201a004. [DOI] [PubMed] [Google Scholar]
- 70.Agriopoulou, S., Stamatelopoulou, E. & Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods10.3390/foods9040518 (2020). [DOI] [PMC free article] [PubMed]
- 71.Zhang K. Evaluation of automated sample preparation for mycotoxin analysis in foods. J. AOAC INTERNATIONAL. 2020;103:1052–1059. doi: 10.1093/jaoacint/qsz044. [DOI] [PubMed] [Google Scholar]
- 72.Arroyo-Manzanares N, Huertas-Pérez JF, García-Campaña AM, Gámiz-Gracia L. Mycotoxin analysis: new proposals for sample treatment. Adv. Chem. 2014;2014:547506. doi: 10.1155/2014/547506. [DOI] [Google Scholar]
- 73.Breidbach, A. A greener, quick and comprehensive extraction approach for LC-MS of multiple mycotoxins. Toxins10.3390/toxins9030091 (2017). [DOI] [PMC free article] [PubMed]
- 74.Njumbe Ediage E, Diana Di Mavungu J, Monbaliu S, Van Peteghem C, De Saeger S. A Validated Multianalyte LC–MS/MS Method for Quantification of 25 Mycotoxins in Cassava Flour, Peanut Cake and Maize Samples. J. Agric. Food Chem. 2011;59:5173–5180. doi: 10.1021/jf2009364. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, L., Dou, X.-W., Zhang, C., Logrieco, A. F. & Yang, M.-H. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins10.3390/toxins10020065 (2018). [DOI] [PMC free article] [PubMed]
- 76.Kong D, et al. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale. 2016;8:5245–5253. doi: 10.1039/C5NR09171C. [DOI] [PubMed] [Google Scholar]
- 77.Ertekin Ö, Kaymak T, Pirinçci ŞŞ, Akçael E, Öztürk S. Aflatoxin-specific monoclonal antibody selection for immunoaffinity column development. BioTechniques. 2019;66:261–268. doi: 10.2144/btn-2018-0143. [DOI] [PubMed] [Google Scholar]
- 78.Hu X, et al. Development of a multiple immunoaffinity column for simultaneous determination of multiple mycotoxins in feeds using UPLC–MS/MS. Anal. Bioanal. Chem. 2016;408:6027–6036. doi: 10.1007/s00216-016-9626-5. [DOI] [PubMed] [Google Scholar]
- 79.Leal T, Abrunhosa L, Domingues L, Venâncio A, Oliveira C. BSA-based sample clean-up columns for ochratoxin A determination in wine: Method development and validation. Food Chem. 2019;300:125204. doi: 10.1016/j.foodchem.2019.125204. [DOI] [PubMed] [Google Scholar]
- 80.Singh J, Mehta A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: a review. Food Sci. Nutr. 2020;8:2183–2204. doi: 10.1002/fsn3.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Önal A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007;103:1475–1486. doi: 10.1016/j.foodchem.2006.08.028. [DOI] [Google Scholar]
- 82.Munzi G, Failla S, Di Bella S. Highly selective and sensitive colorimetric/fluorometric dual mode detection of relevant biogenic amines. Analyst. 2021;146:2144–2151. doi: 10.1039/D0AN02336A. [DOI] [PubMed] [Google Scholar]
- 83.Santiyanont P, et al. Dynamics of biogenic amines and bacterial communities in a Thai fermented pork product Nham. Food Res. Int. 2019;119:110–118. doi: 10.1016/j.foodres.2019.01.060. [DOI] [PubMed] [Google Scholar]
- 84.Liu S-J, Xu J-J, Ma C-L, Guo C-F. A comparative analysis of derivatization strategies for the determination of biogenic amines in sausage and cheese by HPLC. Food Chem. 2018;266:275–283. doi: 10.1016/j.foodchem.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Mantoanelli JOF, Gonçalves LM, Pereira EA. Dansyl chloride as a derivatizing agent for the analysis of biogenic amines by CZE-UV. Chromatographia. 2020;83:767–778. doi: 10.1007/s10337-020-03896-x. [DOI] [Google Scholar]
- 86.Vanegas, D. C. et al. Laser scribed graphene biosensor for detection of biogenic amines in food samples using locally sourced materials. Biosensors10.3390/bios8020042 (2018). [DOI] [PMC free article] [PubMed]
- 87.Vasconcelos, H. et al. Biosensors for biogenic amines: a review. Biosensors10.3390/bios11030082 (2021). [DOI] [PMC free article] [PubMed]
- 88.Kannan SK, et al. A review on chemical and electrochemical methodologies for the sensing of biogenic amines. Anal. Methods. 2020;12:3438–3453. doi: 10.1039/D0AY00358A. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen HTT, Lee S, Lee J, Ha J-H, Kang SH. Ultrasensitive biogenic amine sensor using an enhanced multiple nanoarray chip based on competitive reactions in an evanescent field. Sens. Actuators B: Chem. 2021;345:130354. doi: 10.1016/j.snb.2021.130354. [DOI] [Google Scholar]
- 90.Awuchi, C. G. et al. Mycotoxins affecting animals, foods, humans, and plants: types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—a revisit. Foods10.3390/foods10061279 (2021). [DOI] [PMC free article] [PubMed]
- 91.Sarrocco, S., Mauro, A. & Battilani, P. Use of competitive filamentous fungi as an alternative approach for mycotoxin risk reduction in staple cereals: state of art and future perspectives. Toxins10.3390/toxins11120701 (2019). [DOI] [PMC free article] [PubMed]
- 92.Chilaka, C. A., De Boevre, M., Atanda, O. O. & De Saeger, S. The status of fusarium mycotoxins in sub-Saharan Africa: a review of emerging trends and post-harvest mitigation strategies towards food control. Toxins10.3390/toxins9010019 (2017). [DOI] [PMC free article] [PubMed]
- 93.Gonçalves A, et al. Pre- and postharvest strategies to minimize mycotoxin contamination in the rice food chain. Compr. Rev. Food Sci. Food Saf. 2019;18:441–454. doi: 10.1111/1541-4337.12420. [DOI] [PubMed] [Google Scholar]
- 94.Owolabi IO, Songsamoe S, Matan N. Combined impact of peppermint oil and lime oil on Mangosteen (Garcinia Mangostana) fruit ripening and mold growth using closed system. Postharvest Biol. Technol. 2021;175:111488. doi: 10.1016/j.postharvbio.2021.111488. [DOI] [Google Scholar]
- 95.Hu Y, Zhang J, Kong W, Zhao G, Yang M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017;220:1–8. doi: 10.1016/j.foodchem.2016.09.179. [DOI] [PubMed] [Google Scholar]
- 96.Aiko V, Mehta A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015;40:943–954. doi: 10.1007/s12038-015-9569-6. [DOI] [PubMed] [Google Scholar]
- 97.Ogrodowczyk AM, et al. Crossroad of tradition and innovation-the application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products–a review. Pol. J. Food Nutr. Sci. 2021;71:107–134. [Google Scholar]
- 98.Fabiszewska AU, Zielińska KJ, Wróbel B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: a minireview. World J. Microbiol. Biotechnol. 2019;35:76. doi: 10.1007/s11274-019-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ji C, Fan Y, Zhao L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016;2:127–133. doi: 10.1016/j.aninu.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Byakika S, et al. Potential application of lactic acid starters in the reduction of aflatoxin contamination in fermented sorghum-millet beverages. Int. J. Food Contamination. 2019;6:4. doi: 10.1186/s40550-019-0074-9. [DOI] [Google Scholar]
- 101.Hathout AS, Aly SE. Biological detoxification of mycotoxins: a review. Ann. Microbiol. 2014;64:905–919. doi: 10.1007/s13213-014-0899-7. [DOI] [Google Scholar]
- 102.Atter A, et al. Microbial diversity and metabolite profile of fermenting millet in the production of Hausa koko, a Ghanaian fermented cereal porridge. Front. Microbiol. 2021;12:1752. doi: 10.3389/fmicb.2021.681983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolawole O, Meneely J, Petchkongkaew A, Elliott C. A review of mycotoxin biosynthetic pathways: associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021;37:8–26. doi: 10.1016/j.fbr.2021.04.003. [DOI] [Google Scholar]
- 104.Okeke CA, et al. Traditional processing impacts mycotoxin levels and nutritional value of ogi—A maize-based complementary food. Food Control. 2018;86:224–233. doi: 10.1016/j.foodcont.2017.11.021. [DOI] [Google Scholar]
- 105.Xing F, et al. Aflatoxin B1 inhibition in Aspergillus flavus by Aspergillus niger through down-regulating expression of major biosynthetic genes and AFB1 degradation by atoxigenic A. flavus. Int. J. Food Microbiol. 2017;256:1–10. doi: 10.1016/j.ijfoodmicro.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 106.Leuschner RGK, et al. Qualified presumption of safety (QPS): a generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA) Trends Food Sci. Technol. 2010;21:425–435. doi: 10.1016/j.tifs.2010.07.003. [DOI] [Google Scholar]
- 107.Pawlowska, A. M., Zannini, E., Coffey, A. & Arendt, E. K. in Advances in Food and Nutrition Research Vol. 66 (ed Henry, J.) 217–238 (Academic Press, 2012). [DOI] [PubMed]
- 108.Crowley S, Mahony J, van Sinderen D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013;33:93–109. doi: 10.1016/j.tifs.2013.07.004. [DOI] [Google Scholar]
- 109.Ahlberg SH, Joutsjoki V, Korhonen HJ. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015;207:87–102. doi: 10.1016/j.ijfoodmicro.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 110.Gomaa EZ, Abdelall MF, El-Mahdy OM. Detoxification of aflatoxin B1 by antifungal compounds from lactobacillus brevis and lactobacillus paracasei, isolated from dairy products. Probiotics Antimicrobial Proteins. 2018;10:201–209. doi: 10.1007/s12602-017-9350-2. [DOI] [PubMed] [Google Scholar]
- 111.Guimarães A, Venancio A, Abrunhosa L. Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit. Contam. Part A. 2018;35:1803–1818. doi: 10.1080/19440049.2018.1500718. [DOI] [PubMed] [Google Scholar]
- 112.Sadiq FA, et al. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: a comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019;18:1403–1436. doi: 10.1111/1541-4337.12481. [DOI] [PubMed] [Google Scholar]
- 113.Ambrosini VMDE, Gonzalez S, Perdigon G, Holgado APDER, Oliver G. Chemical composition of the cell wall of lactic acid bacteria and related species. Chem. Pharm. Bull. 1996;44:2263–2267. doi: 10.1248/cpb.44.2263. [DOI] [PubMed] [Google Scholar]
- 114.Assaf JC, Atoui A, Khoury AE, Chokr A, Louka N. A comparative study of procedures for binding of aflatoxin M1 to Lactobacillus rhamnosus GG. Braz. J. Microbiol. 2018;49:120–127. doi: 10.1016/j.bjm.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hernandez-Mendoza A, Garcia HS, Steele JL. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009;47:1064–1068. doi: 10.1016/j.fct.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 116.Yiannikouris A, et al. Chemical and conformational study of the interactions involved in Mycotoxin complexation with β-d-Glucans. Biomacromolecules. 2006;7:1147–1155. doi: 10.1021/bm050968t. [DOI] [PubMed] [Google Scholar]
- 117.Lili Z, Junyan W, Hongfei Z, Baoqing Z, Bolin Z. Detoxification of cancerogenic compounds by lactic acid bacteria strains. Crit. Rev. Food Sci. Nutr. 2018;58:2727–2742. doi: 10.1080/10408398.2017.1339665. [DOI] [PubMed] [Google Scholar]
- 118.Bueno DJ, Casale CH, Pizzolitto RP, Salvano MA, Oliver G. Physical adsorption of aflatoxin B1 by lactic acid bacteria and saccharomyces cerevisiae: a theoretical model. J. Food Prot. 2007;70:2148–2154. doi: 10.4315/0362-028X-70.9.2148. [DOI] [PubMed] [Google Scholar]
- 119.Barroso E, et al. Lactobacillus plantarum IFPL935 impacts colonic metabolism in a simulator of the human gut microbiota during feeding with red wine polyphenols. Appl. Microbiol. Biotechnol. 2014;98:6805–6815. doi: 10.1007/s00253-014-5744-1. [DOI] [PubMed] [Google Scholar]
- 120.Piotrowska, M. The adsorption of ochratoxin A by lactobacillus species. Toxins10.3390/toxins6092826 (2014). [DOI] [PMC free article] [PubMed]
- 121.Niderkorn V, Morgavi DP, Aboab B, Lemaire M, Boudra H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J. Appl. Microbiol. 2009;106:977–985. doi: 10.1111/j.1365-2672.2008.04065.x. [DOI] [PubMed] [Google Scholar]
- 122.Li X, et al. Lactobacillus plantarum X1 with α-glucosidase inhibitory activity ameliorates type 2 diabetes in mice. RSC Adv. 2016;6:63536–63547. doi: 10.1039/C6RA10858J. [DOI] [Google Scholar]
- 123.Zoghi A, Khosravi-Darani K, Sohrabvandi S, Attar H, Alavi SA. Effect of probiotics on patulin removal from synbiotic apple juice. J. Sci. Food Agriculture. 2017;97:2601–2609. doi: 10.1002/jsfa.8082. [DOI] [PubMed] [Google Scholar]
- 124.Wang L, et al. Identification of key factors involved in the biosorption of Patulin by inactivated Lactic Acid Bacteria (LAB) cells. PLoS One. 2015;10:e0143431. doi: 10.1371/journal.pone.0143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang L, et al. A new insight into the adsorption mechanism of patulin by the heat-inactive lactic acid bacteria cells. Food Control. 2015;50:104–110. doi: 10.1016/j.foodcont.2014.08.041. [DOI] [Google Scholar]
- 126.Król A, et al. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018;410:943–952. doi: 10.1007/s00216-017-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zou Z-Y, et al. In vitro removal of deoxynivalenol and T-2 toxin by lactic acid bacteria. Food Sci. Biotechnol. 2012;21:1677–1683. doi: 10.1007/s10068-012-0223-x. [DOI] [Google Scholar]
- 128.Zhou L-H, et al. Analysis of T-2 Toxin removal factors in a lactococcus fermentation system. J. Food Prot. 2017;80:1471–1477. doi: 10.4315/0362-028X.JFP-17-051. [DOI] [PubMed] [Google Scholar]
- 129.Sani AM, Marhamati Z, Marhamatizade M. Bio-detoxification of aflatoxin M1 in kefir using Lactobacillus casei. Biotechnol. Indian J. 2014;9:219–224. [Google Scholar]
- 130.Lee KR, et al. Aflatoxin B 1 detoxification by aspergillus oryzae from Meju, a traditional Korean fermented soybean starter. J. Microbiol. Biotechnol. 2017;27:57–66. doi: 10.4014/jmb.1607.07064. [DOI] [PubMed] [Google Scholar]
- 131.Schaefer D, Cheung WM. Smart packaging: opportunities and challenges. Procedia CIRP. 2018;72:1022–1027. doi: 10.1016/j.procir.2018.03.240. [DOI] [Google Scholar]
- 132.Tamang, J. P. & Kailasapathy, K. Fermented Foods and Beverages of the World (CRC press, 2010).
- 133.Lee B-J, et al. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010;122:271–276. doi: 10.1016/j.foodchem.2010.02.071. [DOI] [Google Scholar]
- 134.Kerry, J. P. in InnovationsinFood Packaging 2nd edn (ed Jung H. Han) 549–584 (Academic Press, 2014).
- 135.Thuwapanichayanan R, Yoosabai U, Jaisut D, Soponronnarit S, Prachayawarakorn S. Enhancement of γ-aminobutyric acid in germinated paddy by soaking in combination with anaerobic and fluidized bed heat treatment. Food Bioprod. Process. 2015;95:55–62. doi: 10.1016/j.fbp.2015.03.010. [DOI] [Google Scholar]
- 136.Liu Y, Galani Yamdeu JH, Gong YY, Orfila C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020;19:1521–1560. doi: 10.1111/1541-4337.12562. [DOI] [PubMed] [Google Scholar]
- 137.Taniwaki MH, Hocking AD, Pitt JI, Fleet GH. Growth and mycotoxin production by food spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int. J. Food Microbiol. 2009;132:100–108. doi: 10.1016/j.ijfoodmicro.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Kailasapathy, K. Fermented Foods Beverages of the World (CRC Press, 2010).
- 139.Galati A, Oguntoyinbo FA, Moschetti G, Crescimanno M, Settanni L. The cereal market and the role of fermentation in cereal-based food production in Africa. Food Rev. Int. 2014;30:317–337. doi: 10.1080/87559129.2014.929143. [DOI] [Google Scholar]
- 140.Alsharif, A. M., Choo, Y.-M. & Tan, G.-H. Detection of five mycotoxins in different food matrices in the Malaysian market by using validated liquid chromatography electrospray ionization triple quadrupole mass spectrometry. Toxins10.3390/toxins11040196 (2019). [DOI] [PMC free article] [PubMed]
- 141.Puangkham S, et al. Monitoring and health risk of mycotoxins in imported wines and beers consumed in Thailand. World Mycotoxin J. 2017;10:401–409. doi: 10.3920/WMJ2017.2216. [DOI] [Google Scholar]
- 142.Chuaysrinule C, Maneeboon T, Roopkham C, Mahakarnchanakul W. Occurrence of aflatoxin- and ochratoxin A-producing Aspergillus species in Thai dried chilli. J. Agri. Food Res. 2020;2:100054. [Google Scholar]
- 143.Wikandari, R. et al. Assessment of Microbiological quality and mycotoxin in dried chili by morphological identification, molecular detection, and chromatography analysis. Int. J. Environ. Res. Public Health10.3390/ijerph17061847 (2020). [DOI] [PMC free article] [PubMed]
- 144.Jalili M, Jinap S. Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control. 2012;24:160–164. doi: 10.1016/j.foodcont.2011.09.020. [DOI] [Google Scholar]
- 145.Shiratori, N. et al. Occurrence of penicillium brocae and penicillium citreonigrum, which produce a mutagenic metabolite and a mycotoxin citreoviridin, respectively, in selected commercially available rice grains in Thailand. Toxins10.3390/toxins9060194 (2017). [DOI] [PMC free article] [PubMed]
- 146.Huong BTM, et al. Aflatoxins and fumonisins in rice and maize staple cereals in Northern Vietnam and dietary exposure in different ethnic groups. Food Control. 2016;70:191–200. doi: 10.1016/j.foodcont.2016.05.052. [DOI] [Google Scholar]
- 147.Panrapee I, Phakpoom K, Thanapoom M, Nampeung A, Warapa M. Exposure to aflatoxin B 1 in Thailand by consumption of brown and color rice. Mycotoxin Res. 2016;32:19–25. doi: 10.1007/s12550-015-0236-4. [DOI] [PubMed] [Google Scholar]
- 148.Lim CW, Yoshinari T, Layne J, Chan SH. Multi-mycotoxin screening reveals separate occurrence of Aflatoxins and Ochratoxin A in Asian rice. J. Agric. Food Chem. 2015;63:3104–3113. doi: 10.1021/acs.jafc.5b00471. [DOI] [PubMed] [Google Scholar]
- 149.Ebarvia, M. L., dela Cruz, J. A. & dela Cruz, T. E. E. P71: Molecular Identification and Aflatoxin Screening of Aspergillus Isolated from Philippine Dried Fish Products (IMEKOFOODS, 2017).
- 150.Borzekowski, A. et al. Formation of Zearalenone metabolites in tempeh fermentation. Molecules10.3390/molecules24152697 (2019). [DOI] [PMC free article] [PubMed]
- 151.Reiter EV, Razzazi-Fazeli E, Zentek J. A limited survey of aflatoxin B1 contamination in Indonesian palm kernel cake and copra meal sampled from batches. Mycotoxin Res. 2013;29:135–139. doi: 10.1007/s12550-013-0168-9. [DOI] [PubMed] [Google Scholar]
- 152.Yibadatihan S, Jinap S, Mahyudin NA. Simultaneous determination of multi-mycotoxins in palm kernel cake (PKC) using liquid chromatography-tandem mass spectrometry (LC-MS/MS) Food Addit. Contam. Part A. 2014;31:2071–2079. doi: 10.1080/19440049.2014.978396. [DOI] [PubMed] [Google Scholar]
- 153.Singkong W, Ratanapol H, Liamkaew W. Zearalenone contamination in corn for human consumption in Kamphaengphet, Thailand. Chiang Mai. J. Sci. 2013;40:534–539. [Google Scholar]
- 154.Ali N, Sardjono, Yamashita A, Yoshizawa T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 1998;15:377–384. doi: 10.1080/02652039809374655. [DOI] [PubMed] [Google Scholar]
- 155.Yamashita A, et al. Fusarium mycotoxins (Fumonisins, Nivalenol, and Zearalenone) and Aflatoxins in corn from Southeast Asia. Biosci. Biotechnol. Biochem. 1995;59:1804–1807. doi: 10.1271/bbb.59.1804. [DOI] [PubMed] [Google Scholar]
- 156.Yue CS, Selvi C, Tang AN, Chee KN, Ng HY. Determination of biogenic amines in Malaysian traditional wine by High-Performance Liquid Chromatography (HPLC) Anal. Lett. 2021;54:1968–1994. doi: 10.1080/00032719.2020.1831008. [DOI] [Google Scholar]
- 157.AMBARITA MTD, Raes K, De Meulenaer B. Identification of key sensory attributes of Sambal-Terasi, impact of different type of terasi, chemical characteristics and salt addition. Sains Malays. 2020;49:561–571. doi: 10.17576/jsm-2020-4903-11. [DOI] [Google Scholar]
- 158.Dalin, Ly., Mayrhofer, S., Schmidt, J. -M., Zitz, U. & Domig, K. J. Biogenic amine contents and microbial characteristics of Cambodian fermented foods. Foods10.3390/foods9020198 (2020). [DOI] [PMC free article] [PubMed]
- 159.Pilapil AR, et al. Chemical quality assessment of traditional salt-fermented shrimp paste from Northern Mindanao, Philippines. J. Sci. Food Agric. 2016;96:933–938. doi: 10.1002/jsfa.7167. [DOI] [PubMed] [Google Scholar]
- 160.Ezzat MA, Zare D, Karim R, Ghazali HM. Trans- and cis-urocanic acid, biogenic amine and amino acid contents in ikan pekasam (fermented fish) produced from Javanese carp (Puntius gonionotus) and black tilapia (Oreochromis mossambicus) Food Chem. 2015;172:893–899. doi: 10.1016/j.foodchem.2014.09.158. [DOI] [PubMed] [Google Scholar]
- 161.Zare D, Muhammad K, Bejo MH, Ghazali HM. Determination of urocanic acid, a compound implicated in histamine toxicity, and assessment of biogenic amines relative to urocanic acid content in selected fish and fish products. J. Food Composit. Anal. 2015;37:95–103. doi: 10.1016/j.jfca.2014.06.014. [DOI] [Google Scholar]
- 162.Vallejos MJM, Pham LJ, Barraquio VL. Biogenic amines in some natural and processed cheeses sold in Laguna Province, Philippines. Philipp. J. Sci. 2012;141:111–115. [Google Scholar]
- 163.Saaid M, Saad B, Hashim NH, Mohamed Ali AS, Saleh MI. Determination of biogenic amines in selected Malaysian food. Food Chem. 2009;113:1356–1362. doi: 10.1016/j.foodchem.2008.08.070. [DOI] [Google Scholar]
- 164.Qiao N, et al. Investigation of biogenic amines in dried bonito flakes from different countries using high-performance liquid chromatography. Food Anal. Methods. 2020;13:2213–2221. doi: 10.1007/s12161-020-01830-3. [DOI] [Google Scholar]
- 165.Nout MJR, Ruikes MMW, Bouwmeester HM, Beljaars PR. Effect of processing conditions on the formation of biogenic amines and ethyl carbamate in soybean tempe. J. Food Saf. 1993;13:293–303. doi: 10.1111/j.1745-4565.1993.tb00114.x. [DOI] [Google Scholar]
- 166.Riebroy S, Benjakul S, Visessanguan W, Kijrongrojana K, Tanaka M. Some characteristics of commercial Som-fug produced in Thailand. Food Chem. 2004;88:527–535. doi: 10.1016/j.foodchem.2004.01.067. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We declare that data sharing does not apply to our manuscript. This is a review article with no data analyzed during the study.