Abstract
Objective:
Eligibility for clinical trials in osteoarthritis (OA) is usually limited to Kellgren-Lawrence (KL) grades 2 and 3 knees. Our aim was to describe the prevalence and severity of cartilage damage in KL 2 and 3 knees by compartment and articular subregion.
Design:
The Multicenter Osteoarthritis (MOST) study is a cohort study of individuals with or at risk for knee OA. All baseline MRIs with radiographic disease severity KL2 and 3 were included. Knee MRIs were read for cartilage damage in 14 subregions. We determined the frequencies of no, any and widespread full-thickness cartilage damage by knee compartment, and the prevalence of any cartilage damage in 14 articular subregions.
Results:
665 knees from 665 participants were included (mean age 63.8 ± 7.9 years, 66.5% women). 372 knees were KL2 and 293 knees were KL3. There was no cartilage damage in 78 (21.0%) medial tibio-femoral joint (TFJ), 157 (42.2%) lateral TFJ and 62 (16.7%) patello-femoral joint (PFJ) compartments of KL2 knees, and 17 (5.8%), 115 (39.3%) and 35 (12.0%) compartments, respectively, of KL3 knees. There was widespread full-thickness damage in 94 (25.3%) medial TFJ, 36 (9.7%) lateral TFJ and 176 (47.3%) PFJ compartments of KL2 knees, and 217 (74.1%), 70 (23.9%) and 104 (35.5%) compartments, respectively, of KL3 knees. The subregions most likely to have any damage were central medial femur (80.5%), medial patella (69.8%) and central medial tibia (69.9).
Conclusions:
KL2 and KL3 knees vary greatly in cartilage morphology. Heterogeneity in the prevalence, severity and location of cartilage damage in in KL2 and 3 knees should be considered when planning disease modifying trials for knee OA.
Keywords: Osteoarthritis, MRI, Radiography, Kellgren-Lawrence, Frequencies, Cartilage, Knee, Frequency, Clinical Trial
Introduction
Imaging plays an important role in defining structural osteoarthritis (OA) disease severity and potential suitability of patients to be recruited to disease-modifying OA drug (DMOAD) trials (1). A common target tissue of DMOADs is cartilage, and structural success of a clinical trial is usually defined by reduced cartilage loss or increase in cartilage thickness in joint areas with prevalent cartilage surface damage compared to placebo, commonly in the medial tibio-femoral joint (TFJ) (2). Change in cartilage morphology over time in DMOAD trials is commonly assessed quantitatively by magnetic resonance imaging (MRI) and used as an outcome measure (3).
To be eligible for inclusion into a DMOAD trial from a structural perspective, knees with radiographic disease severity Kellgren-Lawrence (KL) grades 2 and 3 are typically included (4). These knees exhibit definite structural disease, are at risk for progression, but are not considered end-stage (5). However, one reason of failure of clinical trials in the past may have been due to inclusion based on the heterogeneity and wide spectrum of structural tissue damage of KL2 and 3 knees (1). KL2 knees are defined primarily by the presence of osteophytes, and may exhibit absence of cartilage damage and thus, are unlikely to benefit from cartilage-anabolic approaches. On the other hand, both KL2 and KL3 knees may have widespread cartilage loss to bone, making them potentially less responsive to a treatment focused on preserving cartilage. Understanding the proportion of knees and compartments without cartilage damage or with end-stage cartilage loss in a sample of KL2 and 3 knees will inform the decision-making regarding patient eligibility considerations.
In radiographically normal knees, the anatomical distribution of quantitative cartilage measures has been presented (6) as well as distribution of denuded areas (equivalent to full-thickness damage) (7). However, from a morphologic (i.e. semi-quantitative grading) perspective anatomical patterns and distribution of cartilage damage have not been well described. Stefanik et al. presented detailed data on the compartmental distribution of cartilage damage regardless of KL grade in a population-based cohort focusing on the patellofemoral joint (PFJ) and found that the prevalence of isolated PFJ damage was greater than isolated TFJ damage (8). This pattern was similar between males and females and among body mass index (BMI) categories. However, details on subregional involvement of cartilage damage were not presented. Furthermore, it remains unclear whether cartilage damage patterns are similarly comparable across BMI and age groups in the weight-bearing compartments.
Beyond cartilage damage, subchondral bone marrow lesions (BMLs) are strongly associated with pain cross-sectionally and in a longitudinal fashion regarding fluctuation of BMLs and pain in the same direction (9, 10). Subregions with both, - BMLs and cartilage damage -, have an increased risk of structural progression compared to those with cartilage damage only (11). Knees or compartments exhibiting both cartilage damage and BMLs in the same subregion may be particularly relevant for targeted treatment approaches and inclusion into clinical DMOAD trials as these may be considered at risk for faster progression compared to those knees without BMLs.
Thus, the aims of this study are threefold. First, we will describe the frequencies of compartments in knees with radiographic disease severity KL2 and 3 without any or only minimal cartilage damage, Secondly, we wish to describe the compartmental frequencies of any and of widespread full-thickness cartilage damage. Finally, for KL2 and KL3 knees combined, we will describe the prevalence of any and full-thickness cartilage damage and of any and full-thickness cartilage damage with adjacent BMLs in the same articular subregion. The latter aspect will be particularly helpful defining a subgroup of potential fast progressors, which are knees likely to benefit most from pharmacologic intervention as subregions with cartilage damage and adjacent BMLs are those at high risk for further cartilage damage (12).
Methods
Study design and subjects
Subjects were participants in the Multicenter Osteoarthritis Study (MOST), a prospective longitudinal study of 3026 people aged 50 to 79 years with a goal of identifying risk factors for incident and progressive knee OA in a population either with or at high risk of developing OA. Factors considered to contribute to a high risk of knee OA included being overweight or obese, having either knee pain, aching, or stiffness on most of the preceding 30 days, a prior knee injury that made it difficult to walk for at least 1 week, or previous knee surgery. They were recruited from two US communities, Birmingham, Alabama and Iowa City, Iowa through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. The Health Insurance Portability and Accountability Act-compliant study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama at Birmingham, University of California at San Francisco and Boston University School of Medicine. We obtained written informed consent from all patients. Subjects were not eligible to participate in MOST if they screened positive for rheumatoid arthritis had ankylosing spondylitis, psoriatic arthritis, reactive arthritis, renal insufficiency that required haemodialysis or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without assistance, or were planning to move out of the area in the next 3 years (13).
Radiographs
At baseline, all subjects underwent weight-bearing posteroanterior fixed flexion knee radiographs using a plexiglass positioning frame (SynaFlexer) (14). A musculoskeletal radiologist and two rheumatologists all with over 10 years’ experience reading study radiographs and blinded to clinical data, graded the x-rays according to the KL scale (4). Radiographs were presented sequentially with readers blinded to all clinical data and to MRI. Radiographic tibiofemoral OA was considered present if KL grade ≥2. The inter-rater reliability for pairs of readers among the three readers ranged from a weighted-κ of 0.77 to 0.80. In addition, long limb radiographs were acquired in all MOST subjects at the baseline visit. Mechanical alignment was measured to the nearest 0.1° on these x-rays with high inter-reader reproducibility (ICC = 0.98). We defined malalignment as a mechanical axis of 2° or more in either varus or valgus direction on a long limb x-ray. Neutral alignment was defined as anything less than 2° varus or valgus (15). In the present study we included all knees from participants with available baseline MRIs with radiographic OA grades KL 2 and 3.
MRI acquisition and assessment
MRIs were obtained in both knees at baseline with a 1.0 T dedicated extremity unit (OrthOne, GE Healthcare, Wilmington, Massachusetts, USA) with a circumferential extremity coil using a fat-suppressed (FS) fast spin-echo intermediate-weighted sequence in the sagittal plane (repetition time (TR)=4800 ms, echo time (TE)=35 ms, 3 mm slice thickness, 32 slices, 288×192 pixel matrix, 2 excitations number of acquisitions (NEX), 140×140 mm field of view (FOV), echo train length (ETL)=8), a proton density-weighted sequence in the axial plane (TR=4680 ms, TE=13 ms, 3 mm slice thickness, 20 slices, 288×192 pixel matrix, 2 NEX, 140×140 mm FOV, ETL=8), and a short tau inversion recovery (STIR) sequence in the coronal plane (TR=6650 ms, TE=15 ms, inversion time (TI) =100 ms, 3 mm slice thickness, 28 slices, 256×192 pixel matrix, 2 NEX, 140 mm2 FOV, ETL=8).
Two musculoskeletal radiologists, with 12 and 14 years experience in standardized semiquantitative MRI assessment of knee OA at the time of reading, blinded to radiographic OA grade, and clinical data, graded cartilage status and BMLs according to the whole organ MRI score system (WORMS) (16). Cartilage status and BMLs were scored in each of the five subregions in the medial and lateral TFJ and for four subregions in the PFJ, for a total of 14 subregions per knee. Cartilage morphology and signal were scored semiquantitatively from 0 to 6 in each subregion: 0 = normal thickness and signal; 1 = normal thickness but increased signal on PDw or STIR images; 2.0 = partial-thickness focal defect <1 cm in greatest width; 2.5 = full-thickness focal defect <1 cm in greatest width ; 3 = multiple areas of partial-thickness defects intermixed with areas of normal thickness, or a grade 2.0 defect wider than 1 cm but <75% of the region; 4 = diffuse (>75% of the region) partial thickness loss; 5 = multiple areas of full-thickness loss or a grade 2.5 lesion wider than 1 cm but <75% of the region; 6 = diffuse (>75% of the region) full-thickness loss.
For the purpose of this study we defined compartments that did not show any cartilage damage as those with grades 0 in all 14 articular subregions and those with only minor cartilage damage as those with a maximum of grade 2.5, i.e. a small full thickness defect in any of the subregions. Any cartilage damage was defined as all WORMS scores from 2 to 6 and widespread full-thickness cartilage damage was defined as a maximum WORMS grade of 5 or 6 in a given compartment.
BML size was scored from 0 to 3 based on the extent of regional involvement: 0 = none; 1 = <25% of the subregion, 2 = 25-50% of the subregion; 3 = >50% of the subregion. BMLs were defined as poorly-delineated areas of hyperintensity directly adjacent to the subchondral plate on the STIR and PDw FS images.
The weighted κ coefficients of inter-observer reliability (30 knees randomly selected read by both readers) were 0.66 (95% CI 0.58-0.73) for the readings of BMLs (comparing 0–3 scores in each subregion), and 0.78 (95% CI 0.76–0.81) for cartilage morphology (comparing 0–6 scores in each subregion). .
Statistical analysis
One knee per person was included in the analyses. When a participant had data for two knees, one knee was randomly selected. Descriptive statistics were used to tabulate the proportion of KL2 and 3 knees on a compartmental basis that did not show any or only minor cartilage damage. The compartmental involvement of any and widespread full-thickness cartilage damage for KL2 and 3 knees separately and combined was assessed for compartments for the entire sample and stratified for sex and BMI. Risk differences and their respective Wald intervals were used to compare differences in frequencies between subgroups. Finally, the frequencies of affected subregions for KL2 and 3 knees combined were assessed for all 14 articular subregions as well as for those knees with any cartilage damage and concomitant BMLs in the same subregion. All statistical calculations were performed using SAS software (V.9.4 for Windows; SAS Institute; Cary, North Carolina, USA).
Results
After exclusion of 875 participants due to missing baseline MRIs or X-rays, there were 762 knees from 665 subjects who met our inclusion criteria for this study. Only one knee per person was included leaving 665 knees for analysis. 372 (55.9%) knees had a radiographic disease severity of KL2 and 293 (44.1%) knees were KL3 at the MOST baseline visit. On average the subjects were elderly (mean age 63.8 ± 7.9 years) and overweight (mean BMI 30.9 ± 5.0), and 66.5% were women. 139 (37.4%) of KL2 knees fulfilled the criteria of symptomatic OA (defined as knee pain on most days in the last 30 days and radiographic OA K/L≥2) and 136 (46.4%) of KL3 knees. For KL2 there were 152 (41.4%) limbs with varus malalignment and for KL3 there were 194 (67.1%). 81 (22.1%) of KL2 knees and 49(17.0%) of KL3 knees had valgus malalignment. For 9 knees alignment measures were not available.
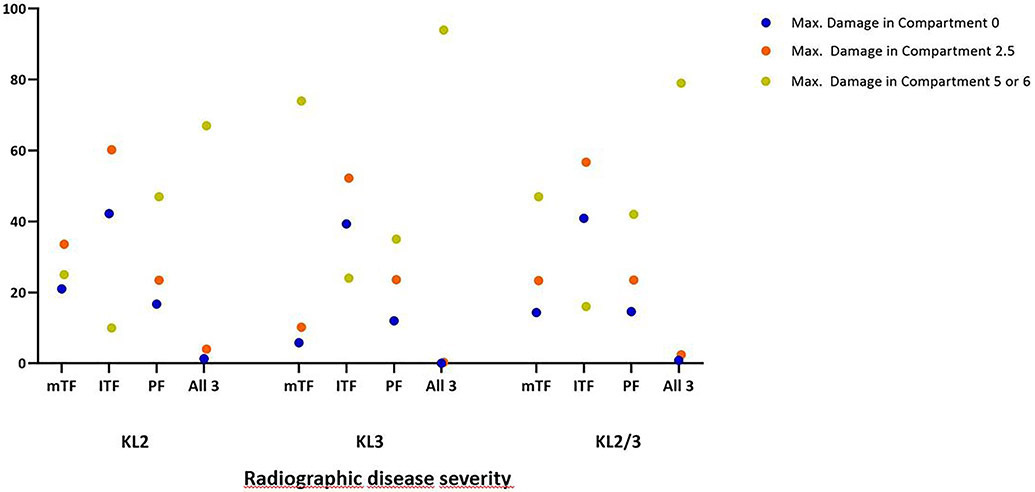
Regarding absence of cartilage damage for KL2 knees, 78 (21.0%) had no damage in the medial TFJ, 157 (42.2%) in the lateral TFJ and 62 (16.7%) in the PFJ. For KL3 knees the respective numbers were 17 (5.8%) for the medial TFJ, 115 (39.3%) for the lateral FTJ and 35 (12.0%) for the PFJ. Including knees with minimal cartilage damage (i.e. a maximum grade of 2.5 on the WORMS scale) for KL2 these numbers changed to 125 (33.6%) for the medial TFJ, 224 (60.2%) for the lateral TFJ and 87 (23.4%) for the PFJ. For KL3 the respective numbers were 30 (10.2%), 153 (52.2%) and 69 (23.6%). Further details regarding no or only minimal damage including those that did not exhibit any or showed only minimal damage in both weight-bearing compartments are presented graphically in Figure 1.
Figure 1.
Percentage of knees without any (blue dots), minimal (orange dots) cartilage damage or widespread full-thickness damage (green dots) by compartment. Minimal cartilage damage is defined as a maximum grade of 2.5, i.e. focal or fissure-like full thickness lesions. Widespread full thickness damage is defined as a maximum grade of 5 or 6 in a compartment.
mTF: medial tibiofemoral compartment; lTF: lateral tibiofemoral compartment; PF: patellofemoral compartment. All 3: medial and lateral tibiofemoral compartments and patellofemoral compartment (i.e. no or only minimal or widespread full thickness cartilage damage in the medial AND lateral tibiofemoral compartments AND the patellofemoral compartment). KL: Kellgren-Lawrence.
Regarding presence of any (all WORMS scores from grade 2 to grade 6) cartilage damage 294 (79.0%) KL2 knees had any damage in the medial TFJ, 215 (57.8%) in the lateral TFJ and 310 (83.3%) in the PFJ. For KL3 these numbers were 276 (94.2%), 178 (60.8%) and 258 (88.1%). Of note, for KL2 knees women had less cartilage damage medially (74.9% vs. 88.0%), while for the lateral TFJ, men showed less damage (62.0% vs. 48.7%). For KL3 knees differences regarding sex were only observed for the PFJ (92.5% females vs. 80.2% males). Regarding BMI categories those participants with KL3 and a BMI <25 kg/m2 showed less damage in the medial TFJ compared to those in the other categories. Table 1 gives an overview of any cartilage damage for KL2 and KL3 knees and both combined.
Table 1:
Any cartilage damage in KL2 and 3 knees by compartment
| KL Grade | Compartment | ANY N (proportion) |
95% CI for Proportion of any Cartilage Damage |
Female N (proportion) |
Male N (proportion) | Difference in Proportions by Sex (95% CI) |
BMI <25 N (proportion) |
BMI 25-30 N (proportion) |
BMI >30 N (proportion) |
Difference in Proportions by BMI ≥ 30 and BMI < 30 (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | mTFJ | 294 (0.79) | (0.75, 0.83) | 191 (0.75) | 103 (0.88) | 0.13 (0.05, 0.21)** | 29 (0.69) | 123 (0.82) | 142 (0.79) | −0.0028 (−0.086, 0.08) |
| 2 | lTFJ | 215 (0.58) | (0.53, 0.63) | 158 (0.62) | 57 (0.49) | −0.13 (−0.24, −0.024)** | 24 (0.57) | 80 (0.53) | 111 (0.62) | 0.075 (−0.025, 0.18) |
| 2 | PFJ | 310 (0.83) | (0.80, 0.87) | 220 (0.86) | 90 (0.77) | −0.094 (−0.18, −0.0063)** | 34 (0.81) | 118 (0.79) | 158 (0.88) | 0.086 (0.011, 0.16)** |
| 2 | All three combined | 367 (0.99) | (0.97, 1.00) | 252 (0.99) | 115 (0.98) | −0.0053 (−0.032, 0.022) | 41 (0.98) | 150 (1.00) | 176 (0.98) | −0.017 (−0.041, 0.0068) |
| 3 | mTFJ | 276 (0.94) | (0.92, 0.97) | 173 (0.93) | 103 (0.97) | −0.047 (−0.096, 0.0026) | 18 (0.86) | 85 (0.91) | 173 (0.97) | −0.063 (−0.12, −0.0027)** |
| 3 | lTFJ | 178 (0.61) | (0.55, 0.66) | 119 (0.64) | 59 (0.56) | 0.080 (−0.037, 0.20) | 13 (0.62) | 61 (0.66) | 104 (0.58) | 0.068 (−0.046, 0.18) |
| 3 | PFJ | 258 (0.88) | (0.84, 0.92) | 173 (0.93) | 85 (0.80) | 0.12 (0.039, 0.21)** | 16 (0.76) | 83 (0.89) | 159 (0.89) | −0.020 (−0.097, 0.058) |
| 3 | All three combined | 293 (1.00) | (1.00, 1.00) | 187 (1) | 106 (1.00) | 0.11 (0.037, 0.17)** | 21 (1.00) | 93 (1.00) | 179 (1.00) | NA |
| 2 and 3 | mTFJ | 570 (0.86) | (0.83, 0.88) | 364 (0.82) | 206 (0.92) | 0.100 (0.051, 0.15)** | 47 (0.75) | 208 (0.86) | 315 (0.88) | 0.044 (−0.0097, 0.098) |
| 2 and 3 | lTFJ | 393 (0.59) | (0.55, 0.63) | 277 (0.63) | 116 (0.52) | −0.107 (−0.19, −0.027)** | 37 (0.59) | 141 (0.58) | 215 (0.60) | 0.017 (−0.058, 0.092) |
| 2 and 3 | PFJ | 568 (0.85) | (0.83, 0.88) | 393 (0..89) | 175 (0.79) | −0.104 (−0.17, −0.043)** | 50 (0.79) | 201 (0.83) | 317 (0.88) | 0.063 (0.0084, 0.12)** |
| 2 and 3 | All three combined | 660 (0.99) | (0.99, 1.00) | 439 (0.99) | 221 (0.99) | −0.0022 (−0.017, 0.012) | 62 (0.98) | 243 (1.00) | 355 (0.99) | −0.0079 (−0.021, 0.0047) |
KL Grade – Kellgren and Lawrence Grade; mTFJ – medial tibio-femoral joint; lTFJ – lateral tibio-femoral joint, PFJ – patello-femoral joint, BMI-body mass index in kg/m2
N=665 KL2: n= 372 KL3: n= 293 KL2 and 3: female: n=442 Male: n=223, KL2: female n= 255 male n=117. KL3: female n=187 male n=106. For Both kl2 and 3 combined: BMI <25 =63, 25-30 =243, >30 =359
statistically significant at p < 0.05
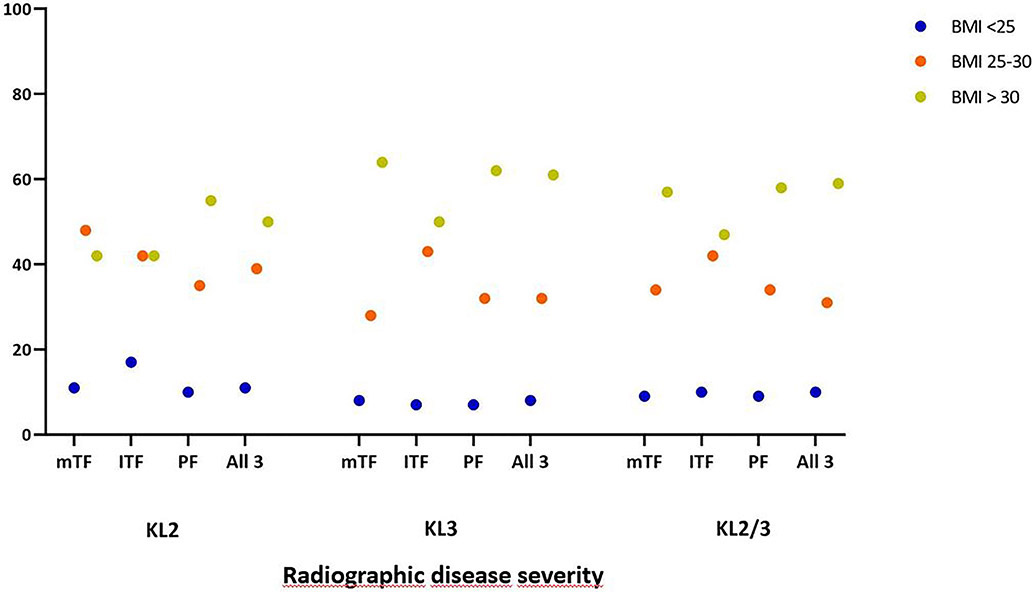
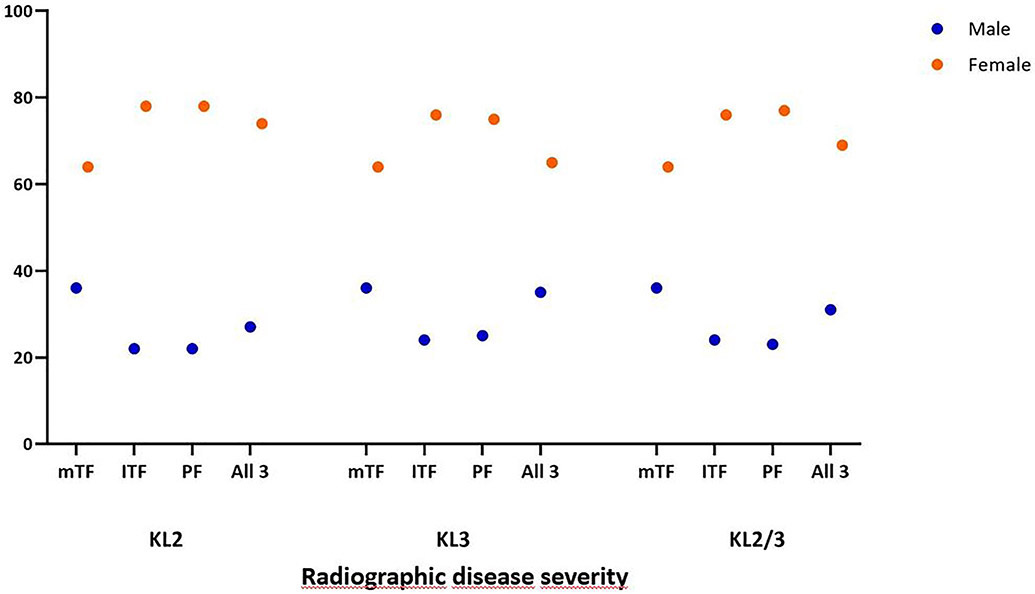
Regarding widespread full-thickness damage (i.e. WORMS grade 5 and 6), 94 (25.3%) knees with KL2 had a maximum cartilage damage score of 5 or 6 in the medial TFJ, 36 (9.7%) in the lateral TFJ and 176 (47.3%) in the PFJ. Of KL3 knees 217 (74.1%) knees had a maximum cartilage damage score of 5 or 6 in the medial TFJ, 70 (23.9%) in the lateral TFJ and 104 (35.5%) in the PFJ. Of all knees, 185 (27.8%) had a maximum score of 5 medially, 55 8.3%) laterally and 152 (22.9%) in the PFJ. For a maximum grade of 6 these numbers were 126 (18.9%), 51 (7.7%) and 128 (19.2%). For all knees, 523 (78.6%) had widespread full-thickness damage in at least one of the three compartments. 58 (18.7%) knees with widespread full thickness cartilage damage (WORMS 5 and 6) had ipsicompartmental BMLs in any of the 5 lateral subregions. 307 (78.8%) knees with full thickness damage medially (WORMS 5 and 6) had BMLs in any of the 5 medial subregions and 55 (17.7%) knees with WORMS 5 and 6 in any of the 4 patellofemoral subregions had ipsicompartmental BMLs. Details for widespread full-thickness damage are presented in Table 2. Additional graphical information about wide spread full-thickness damage regarding stratification by BMI and sex categories is shown in Figures 2a and 2b, while further details are presented in Appendix 1. Considering all knees, the subregion most frequently affected by any cartilage damage was the central medial femur (80.5%), followed by the central medial tibia (69.9%) and medial patella (69.8%) subregion.
Table 2:
Widespread full thickness (grade 5 and 6) cartilage damage in KL2 and 3 knees
| KL Grade | Compartment | Max. damage 5 N (proportion*) |
95% CI for Proportion of Max. Damage 5 |
Max. damage 6 N (proportion*) |
95% CI for Proportion of Max. Damage 6 |
Max. damage in compartment 5 or 6 N (proportion*) |
95% CI for Proportion of Max. Damage 5 or 6 |
|---|---|---|---|---|---|---|---|
| 2 | mTFJ | 86 (0.29) | (0.24, 0.34) | 8 (0.27) | (0.23, 0.32) | 94 (0.32) | (0.27, 0.37) |
| 2 | lTFJ | 30 (0.14) | (0.11, 0.18) | 6 (0.28) | (0.23, 0.33) | 36 (0.17) | (0.13, 0.21) |
| 2 | PFJ | 86 (0.28) | (0.23, 0.33) | 90 (0.29) | (0.24, 0.34) | 176 (0.57) | (0.52, 0.62) |
| 2 | All three combined | 147 (0.40) | (0.35, 0.45) | 102 (0.28) | (0.23, 0.33) | 249 (0.68) | (0.63, 0.73) |
| 3 | mTFJ | 99 (0.36) | (0.31, 0.42) | 118 (0.43) | (0.37, 0.49) | 217 (0.79) | (0.74, 0.84) |
| 3 | lTFJ | 25 (0.14) | (0.10, 0.18) | 45 (0.25) | (0.20, 0.30) | 70 (0.39) | (0.33, 0.45) |
| 3 | PFJ | 66 (0.26) | (0.21, 0.31) | 38 (0.15) | (0.11, 0.19) | 104 (0.40) | (0.34, 0.46) |
| 3 | All three combined | 99 (0.34) | (0.29, 0.39) | 175 (0.60) | (0.54, 0.66) | 274 (0.94) | (0.91, 0.97) |
| 2 and 3 | mTFJ | 185 (0.33) | (0.29, 0.37) | 126 (0.22) | (0.19, 0.25) | 311 (0.55) | (0.51, 0.59) |
| 2 and 3 | lTFJ | 55 (0.14) | (0.11, 0.17) | 51 (0.13) | (0.10, 0.16) | 106 (0.27) | (0.24, 0.30) |
| 2 and 3 | PFJ | 152 (0.27) | (0.24, 0.30) | 128 (0.23) | (0.20, 0.26) | 280 (0.49) | (0.45, 0.53) |
| 2 and 3 | All three combined | 246 (0.37) | (0.33, 0.41) | 277 (0.42) | (0.38, 0.46) | 523 (0.79) | (0.76, 0.82) |
KL2: n=372; KL3 n= 293. KL Grade – Kellgren and Lawrence Grade; mTFJ – medial tibio-femoral joint; lTFJ – lateral tibio-femoral joint, PFJ – patello-femoral joint
proportion summed to 1
Figure 2a.
Percentage of knees with wide spread full-thickness cartilage damage by compartment and BMI. Wide spread full-thickness cartilage damage is defined as a maximum grade of 5 or 6, i.e. multiple areas of full-thickness loss or a grade 2.5 lesion wider than 1 cm but <75% of the region or diffuse (>75% of the region) full-thickness loss.
mTF: medial tibiofemoral compartment; lTF: lateral tibiofemoral compartment; PF: patellofemoral compartment; All 3: medial and lateral tibiofemoral compartments and patellofemoral compartment (i.e. wide spread full-thickness cartilage damage in the medial AND lateral tibiofemoral compartments AND the patellofemoral compartment); BMI: body mass index in kg/m2.
Figure 2b.
Percentage of knees with wide spread full-thickness cartilage damage by compartment and sex. Wide spread full-thickness cartilage damage is defined as a maximum grade of 5 or 6, i.e. multiple areas of full-thickness loss or a grade 2.5 lesion wider than 1 cm but <75% of the region or diffuse (>75% of the region) full-thickness loss.
mTF: medial tibiofemoral compartment; lTF: lateral tibiofemoral compartment; PF: patllofemoral compartment; All 3: medial and lateral tibiofemoral compartments and patellofemoral compartment (i.e. wide spread full-thickness cartilage damage in the medial AND lateral tibiofemoral compartments AND the patellofemoral compartment); BMI: body mass index in kg/m2.
When considering those subregions with full thickness damage only (i.e., grades 2.5, 5 and 6 on the WORMS scale) the most commonly affected ones were the central medial tibia (41.1%) and central medial femur (40.9%). For those with full thickness damage and adjacent BMLs the two most common subregions were identical, i.e. also the central medial tibia (23.9%) and central medial femur (20.5%). The third most commonly affected subregion were the lateral patella (24.5%) for those without BMLs in the same subregion, and for those with BMLs in the same subregion it was the anterior lateral femur (11.7%). The frequencies for all subregions and details of the ranking for those subregions without and with BMLs in the same subregion are presented in Table 3.
Table 3.
Any cartilage damage and any cartilage damage with adjacent BML, full thickness cartilage damage and full thickness cartilage damage with adjacent BML all subregions KL2 and 3 combined
| ANY Cartilage Damage | ANY Cartilage Damage AND BML |
Full Thickness Cartilage Damage |
Full Thickness Cartilage Damage AND BML |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Compartment | Subregion | n/N (proportion) |
95% CI | n/N (proportion) |
95% CI | n/N (proportion) |
95% CI | n/N (proportion) |
95% CI |
| mTFJ | cmT | 465/665 (0.70) | (0.67, 0.74) | 197/665 (0.30) | (0.27, 0.34) | 273/665 (0.41) | (0.37, 0.45) | 159/665 (0.24) | (0.21, 0.27) |
| cmF | 535/665 (0.81) | (0.78, 0.84) | 183/665 (0.28) | (0.25, 0.31) | 272/665 (0.41) | (0.37, 0.45) | 136/665 (0.21) | (0.18, 0.24) | |
| pmF | 360/665 (0.54) | (0.50, 0.58) | 111/665 (0.17) | (0.14, 0.20) | 54/665 (0.08) | (0.06, 0.10) | 28/665 (0.04) | (0.03, 0.06) | |
| pmT | 143/665 (0.22) | (0.19, 0.25) | 21/665 (0.03) | (0.02, 0.04) | 22/665 (0.03) | (0.02, 0.04) | 8/665 (0.01) | (0.002, 0.02) | |
| amT | 177/665 (0.27) | (0.24, 0.30) | 48/665 (0.07) | (0.05, 0.09) | 42/665 (0.06) | (0.04, 0.08) | 18/665 (0.03) | (0.02, 0.04) | |
| lTFJ | clT | 247/665 (0.37) | (0.33, 0.41) | 84/665 (0.13) | (0.10, 0.16) | 98/665 (0.15) | (0.12, 0.18) | 61/665 (0.09) | (0.07, 0.11) |
| clF | 296/665 (0.45) | (0.41, 0.49) | 50/665 (0.08) | (0.06, 0.10) | 89/665 (0.13) | (0.10, 0.16) | 26/665 (0.04) | (0.03, 0.06) | |
| plF | 152/665 (0.23) | (0.20, 0.26) | 47/665 (0.07) | (0.05, 0.09) | 46/665 (0.07) | (0.05, 0.09) | 25/665 (0.04) | (0.03, 0.06) | |
| plT | 198/665 (0.30) | (0.27, 0.34) | 39/665 (0.06) | (0.04, 0.08) | 76/665 (0.11) | (0.09, 0.13) | 22/665 (0.03) | (0.02, 0.04) | |
| alT | 54/665 (0.08) | (0.06, 0.10) | 4/665 (0.006) | (0.0001, 0.012) | 21/665 (0.03) | (0.02, 0.04) | 3/665 (0.005) | (−0.0004, 0.01) | |
| PFJ | mP | 464/665 (0.70) | (0.67, 0.74) | 37/665 (0.06) | (0.04, 0.08) | 169/665 (0.25) | (0.22, 0.28) | 24/665 (0.04) | (0.03, 0.06) |
| amF | 323/665 (0.49) | (0.45, 0.53) | 26/665 (0.04) | (0.03, 0.06) | 57/665 (0.09) | (0.07, 0.11) | 12/665 (0.02) | (0.01, 0.03) | |
| lP | 369/665 (0.56) | (0.52, 0.60) | 62/665 (0.09) | (0.07, 0.11) | 163/665 (0.25) | (0.22, 0.28) | 51/665 (0.08) | (0.06, 0.10) | |
| alF | 271/665 (0.41) | (0.37, 0.45) | 95/665 (0.14) | (0.11, 0.17) | 137/665 (0.21) | (0.18, 0.24) | 78/665 (0.12) | (0.10, 0.15) | |
mTFJ –medial tibio-femoral joint; lTFJ – lateral tibio-femoral joint, PFJ – patello-femoral joint, BML – bone marrow lesion; cMT –central medial tibia; cmF –central medial femur, pmF –posterior medial femur; pmT – posterior medial tibia; amT –anzerior medial tibia; clT –central lateral tibia, clF –central lateral femur, plF –posterior lateral femur, plT posterior lateral tibia, alT –anterior lateral tibia, mP –medial patella, amF –anterior medial femur, lP –lateral patella, alF –anterior lateral femur
Discussion
Knees with structural disease severity KL2 and 3 show a wide spectrum of subregional structural involvement and subregional disease burden from no damage to advanced full-thickness cartilage loss. Almost a quarter of KL2 knees exhibit only minimal cartilage damage in the medial TFJ. A quarter of KL2 and almost three quarters of KL3 knees exhibit widespread full-thickness damage in the medial TFJ. Considering the detailed subregional division, any cartilage damage was observed most commonly in the central medial femur subregion (~80%) followed by the medial patella and the central medial tibia subregions (both ~70%). The most commonly affected subregion with cartilage damage and concomitant BMLs in the same subregion is the central medial tibia followed by the central medial femur and the posterior medial femur.
The fact that around 20% of KL2 knees do not have any and one third has only minimal cartilage damage in the medial TFJ with even higher rates for the lateral TFJ was unexpected. Given that the large majority of knees without radiographic OA show OA-specific tissue alterations on MRI including cartilage damage lead to the assumption that once radiographic OA is present, from a structural perspective the disease has to be considered advanced (17). This was also reflected in our cohort where 85% and 60% of all knees showed any damage in the medial and lateral TFJ compartment, respectively. The fact that medial cartilage damage was more commonly observed than lateral is in line with previous work on prevalence of compartmental dominance of radiographic OA with medial OA being more common. However, the focus on accurate identification of medial narrowing on X-ray may sacrifice our ability to correctly identify knees with lateral narrowing(18). Nonetheless, given that joint space width (on X-ray) or cartilage thickness (on MRI) in the medial TFJ is the most common structural outcome in DMOAD trials it is unlikely that any cartilage-anabolic molecule will have an effect on these outcome parameters when no cartilage damage is present. Thus, inclusion of such patients likely may be reconsidered.
While almost 80% of KL2 knees showed any damage in the medial TFJ, for KL3 knees the number was as high as 94%, while differences between KL2 and 3 were much less pronounced for the lateral and PFJ compartments. Not unexpected and given that KL3 is primarily characterized by definite joint space narrowing, in comparison to KL2, the proportion of widespread full-thickness loss was much higher in KL3 (74%) than KL2 knees (25%). This may be a relevant fact for consideration in patient inclusion given the mode of action of a specific DMOAD. Particularly for anti-catabolic, cartilage-preserving treatment approaches less structural effects may be observed once widespread full-thickness damage is already present compared to knees where cartilage damage is only superficial. However, it has to be acknowledged that KL3 knees show faster progression than KL2 knees when considering cartilage thickness loss as the outcome (19).
Using MRI data from a population-based cohort Stefanik et al. focused on cartilage damage and BMLs particularly in the PFJ using different definitions of structural involvement (8). Authors found that isolated PFJ damage was more common than isolated TFJ damage (cartilage and bone) and when mixed disease was the most common pattern, the PFJ had more severe damage. We did not focus on isolated compartmental damage but rather described the compartmental frequencies for any and full-thickness damage.
The radiographic definition of knee OA relies on the presence of a definite osteophyte on the anterior-posterior or posterior-anterior radiograph (i.e. KL2), while the presence of definite joint space narrowing commonly defines a knee as KL3 (20, 21). Joint space narrowing (JSN) is an indirect surrogate measure of cartilage and meniscal damage (including extrusion) and tibiofemoral JSN over time is strongly and independently influenced by progressive worsening of cartilage damage, meniscal damage and meniscal extrusion (22). We did not include the meniscus in our analysis and this was a cross-sectional description only, intended mainly to inform patient inclusion to clinical trials. The success of pharmacological cartilage restoration or preservation may strongly be influenced by local biomechanics, and meniscal pathology is one of the main drivers of OA incidence and progression (23). In addition, different structural phenotypes may progress differently and may differ in their response to DMOAD effects (24).
While in radiographically normal knees the anatomical distribution of quantitative cartilage measures has been presented (6) as well as distribution of denuded areas (similar to full thickness damage using scoring approaches) (7), from a morphologic (i.e. semi-quantitative) perspective anatomical patterns and distribution of cartilage damage have not been well described. Location-independent so-called ordered values approaches have been introduced for analyzing the magnitude of subregional involvement in cartilage thickness changes and is ranking these regardless of pre-specified subregions or anatomic location (25). This approach was more efficient for discriminating longitudinal rates of change between healthy knees and those with different radiographic OA grades compared with radiography and predefined anatomic subregions (26). Compared with several clinical, radiographic, and molecular measures, the relatively strongest predictor of longitudinal MRI-defined cartilage thinning was reduced baseline cartilage thickness in the medial femur reflecting the mentioned fact that more advanced cartilage damage progresses faster than low grade damage (27). To date, still no pharmacologic agent has been approved by regulatory agencies despite ongoing and promising efforts, including a report that intra-articular administration of sprifermin resulted in an improvement in total TFJ joint cartilage thickness after 2 years (28). Whether KL2 knees responded differently to KL3 knees was not reported in that trial. Recently, a post-hoc subgroup analysis focusing on “patients at risk” defined by low minimum joint space width and moderate-to-high pain at baseline demonstrated translation of structure modification into symptomatic benefit (29).
Our study has several limitations. We did not include the entire spectrum of structural disease in our analysis and acknowledge that other joint tissues are highly relevant concerning structural progression, which also may have an impact on treatment effects. These include the meniscus, inflammation or ligamentous integrity. We employed 1.0 T extremity MRI, which has been questioned to yield inferior image quality when compared to 1.5 T or 3 T large bore systems. These issues, to the extent they exist, seem not to affect semi-quantitative scoring of knee OA. In a comparative exercise scoring knees of subjects, which had received a 1.0 T extremity MRI scan and a 1.5 T large bore examination of the same knee on the same day, we could show good agreement, sensitivity and specificity for all assessed features (30). Finally, we focused on one time point only as the main aim was a characterization of knees that are commonly considered eligible to be included to clinical trials. We acknowledge that beyond baseline local structural tissue changes many other factors influence rates of progression including but not limited to limb alignment, comorbidities or pain (31).
In summary, 20% of KL2 and 6% of KL3 knees do not exhibit any cartilage damage in the medial TFJ and one third of KL2 knees exhibits only minimal cartilage damage in the medial TFJ. Thus, these compartments without or only minimal damage are likely not amenable for anabolic cartilage DMOAD effects. On the other hand, about 25% of KL2 and >70% of KL3 knees show widespread full-thickness cartilage damage in the medial TFJ and are likely not ideal candidates for anti-catabolic treatment approaches. Between 20% and 30% of medial compartments exhibit additional BMLs in the same subregions where cartilage damage occurs and thus, have to be considered at high risk for progression and may be considered for enrichment of clinical trial populations. As neither cartilage nor BMLs are visualized on X-rays and given the heterogeneity of cartilage damage in KL2 and 3 knees, using only radiography as an instrument to define structural eligibility needs to be reconsidered depending on mode of action of a specific DMOAD compound.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the MOST study at the clinical sites in Birmingham, AL and Iowa City, IA and at the MOST Coordinating Centers at University of California San Francisco, San Francisco, CA and Boston University, Boston, MA.
Funding and role of the funding source
Supported by NIH grants from the National Institute of Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820).
Appendix 1. Widespread full-thickness (grade 5 and 6) cartilage damage in KL2 and 3 knees. Risk Difference by Sex and BMI
| KL Grade | Compartment | Female (proportion*) |
Male (proportion*) | Risk Difference (95% CI) | BMI <25 (proportion*) | BMI 25-30 (proportion*) |
BMI >30 (proportion*) |
Risk Difference BMI < 30 and BMI >= 30 (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 2 | mTFJ | 60 (0.64) | 34 (0.36) | 0.02 (−0.34, 0.37) | 10 (0.11) | 45 (0.48) | 39 (0.42) | −0.09 (−0.45, 0.27) |
| 2 | ITFJ | 28 (0.78) | 8 (0.22) | −0.27 (−0.42, −0.11) ** | 6(0.17) | 15 (0.42) | 15 (0.42) | 0.10 (−0.32, 0.52) |
| 2 | PFJ | 137(0.78) | 39 (0.22) | −0.11 (−0.23, 0.01) | 18(0.10) | 61 (0.35) | 97(0.55) | −0.08 (−0.22, 0.07) |
| 2 | All three combined | 183 (0.73) | 66 (0.27) | −0.15 (−0.26, −0.04) ** | 28(0.11) | 96 (0.39) | 125 (0.50) | −0.10 (−0.22, 0.03) |
| 3 | mTFJ | 138 (0.64) | 79 (0.36) | −0.09 (−0.22, 0.04) | 17 (0.08) | 61 (0.28) | 139 (0.64) | 0.12 (−0.004, 0.25) |
| 3 | ITFJ | 53 (0.76) | 17 (0.24) | −0.06 (−0.27, 0.16) | 5 (0.07) | 30 (0.43) | 35 (0.50) | −0.09 (−0.34, 0.15) |
| 3 | PFJ | 78 (0.75) | 26 (0.25) | −0.10 (−0.27, 0.06) | 7 (0.07) | 33 (0.32) | 64 (0.62) | −0.06 (−0.25, 0.14) |
| 3 | All three combined | 177(0.65) | 97 (0.35) | −0.16 (−0.28, −0.04) ** | 21 (0.08) | 87 (0.32) | 166(0.61) | 0.11 (−0.01, 0.23) |
| 2 and 3 | mTFJ | 198 (0.64) | 113 (0.36) | −0.06 (−0.17, 0.04) | 27 (0.09) | 106 (0.34) | 178 (0.57) | −0.01 (−0.12, 0.10) |
| 2 and 3 | ITFJ | 81 (0.76) | 25 (0.24) | −0.08 (−0.24, 0.08) | 11 (0.10) | 45 (0.42) | 50 (0.47) | 0.040 (−0.15, 0.23) |
| 2 and 3 | PFJ | 215 (0.77) | 65 (0.23) | −0.11 (−0.21, −0.01) ** | 25 (0.09) | 94 (0.34) | 161 (0.58) | −0.02 (−0.14, 0.10) |
| 2 and 3 | All three combined | 360 (0.69) | 163 (0.31) | −0.13 (−0.21, −0.05) ** | 49 (0.10) | 156 (0.31) | 291 (0.59) | −0.01 (−0.10, 0.07) |
mTFJ –medial tibio-femoral joint; lTFJ – lateral tibio-femoral joint, PFJ – patello-femoral joint, BMI –body mass index in kg/m2.
Proportions summed to 1
statistically significant at p<0.05
Footnotes
Conflict of interests
AG has received consultancies, speaking fees, and/or honoraria from Sanofi-Aventis, Merck Serono, and TissuGene and is President and shareholder of Boston Imaging Core Lab (BICL), LLC a company providing image assessment services.
FWR is Chief Medical Officer and shareholder of BICL, LLC. and has received consultancies, speaking fees, and/or honoraria from Calibr –California Institute of Biomedical Research.
References
- 1.Roemer FW, Kwoh CK, Hayashi D, Felson DT, Guermazi A. The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA. Nat Rev Rheumatol. 2018;14(6):372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oo WM, Yu SP, Daniel MS, Hunter DJ. Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expert Opin Emerg Drugs. 2018;23(4):331–47. [DOI] [PubMed] [Google Scholar]
- 3.Eckstein F, Peterfy C. A 20 years of progress and future of quantitative magnetic resonance imaging (qMRI) of cartilage and articular tissues-personal perspective. Semin Arthritis Rheum. 2016;45(6):639–47. [DOI] [PubMed] [Google Scholar]
- 4.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter DJ, Altman RD, Cicuttini F, Crema MD, Duryea J, Eckstein F, et al. OARSI Clinical Trials Recommendations: Knee imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):698–715. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein F, Yang M, Guermazi A, Roemer FW, Hudelmaier M, Picha K, et al. Reference values and Z-scores for subregional femorotibial cartilage thickness--results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthritis Cartilage. 2010;18(10):1275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frobell RB, Wirth W, Nevitt M, Wyman BT, Benichou O, Dreher D, et al. Presence, location, type and size of denuded areas of subchondral bone in the knee as a function of radiographic stage of OA - data from the OA initiative. Osteoarthritis Cartilage. 2010;18(5):668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanik JJ, Niu J, Gross KD, Roemer FW, Guermazi A, Felson DT. Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage.21(5):695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63(3):691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68(9):1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roemer FW, Felson DT, Wang K, Crema MD, Neogi T, Zhang Y, et al. Co-localisation of non-cartilaginous articular pathology increases risk of cartilage loss in the tibiofemoral joint--the MOST study. Ann Rheum Dis. 2013;72(6):942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. [DOI] [PubMed] [Google Scholar]
- 14.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage. 2013;21(6):789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. [DOI] [PubMed] [Google Scholar]
- 17.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felson DT, Nevitt MC, Zhang Y, Aliabadi P, Baumer B, Gale D, et al. High prevalence of lateral knee osteoarthritis in Beijing Chinese compared with Framingham Caucasian subjects. Arthritis Rheum. 2002;46(5):1217–22. [DOI] [PubMed] [Google Scholar]
- 19.Maschek S, Wirth W, Ladel C, Hellio Le Graverand MP, Eckstein F. Rates and sensitivity of knee cartilage thickness loss in specific central reading radiographic strata from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22(10):1550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer FW, Demehri S, Omoumi P, Link TM, Kijowski R, Saarakkala S, et al. State of the Art: Imaging of Osteoarthritis-Revisited 2020. Radiology. 2020;296(1):5–21. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325(6):568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crema MD, Nevitt MC, Guermazi A, Felson DT, Wang K, Lynch JA, et al. Progression of cartilage damage and meniscal pathology over 30 months is associated with an increase in radiographic tibiofemoral joint space narrowing in persons with knee OA-the MOST study. Osteoarthritis Cartilage. 2014;22(10):1743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50(9):2811–9. [DOI] [PubMed] [Google Scholar]
- 24.Roemer FW, Collins JE, Neogi T, Crema MD, Guermazi A. Association of knee OA structural phenotypes to risk for progression: a secondary analysis from the Foundation for National Institutes of Health Osteoarthritis Biomarkers study (FNIH). Osteoarthritis Cartilage. 2020;28(9):1220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Does the use of ordered values of subregional change in cartilage thickness improve the detection of disease progression in longitudinal studies of osteoarthritis? Arthritis Rheum. 2009;61(7):917–24. [DOI] [PubMed] [Google Scholar]
- 26.Wirth W, Buck R, Nevitt M, Le Graverand MP, Benichou O, Dreher D, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography--data from the OA initiative. Osteoarthritis Cartilage. 2011;19(6):689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckstein F, Le Graverand MP, Charles HC, Hunter DJ, Kraus VB, Sunyer T, et al. Clinical, radiographic, molecular and MRI-based predictors of cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2011;70(7):1223–30. [DOI] [PubMed] [Google Scholar]
- 28.Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of Intra-Articular Sprifermin vs Placebo on Femorotibial Joint Cartilage Thickness in Patients With Osteoarthritis: The FORWARD Randomized Clinical Trial. JAMA. 2019;322(14):1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guehring H, Moreau F, Daelken B, Ladel C, Guenther O, Bihlet AR, et al. The effects of sprifermin on symptoms and structure in a subgroup at risk of progression in the FORWARD knee osteoarthritis trial. Semin Arthritis Rheum. 2021;51(2):450–6. [DOI] [PubMed] [Google Scholar]
- 30.Roemer FW, Lynch JA, Niu J, Zhang Y, Crema MD, Tolstykh I, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2010;18(2):168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage. 2017;25(12):1926–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.