Abstract
Background $ Aims:
Etiological risk factors for cirrhosis have changed in the last decade. It remains unclear to what extent these trends in cirrhosis risk factors have changed hepatocellular cancer (HCC) risk.
Approach & Results:
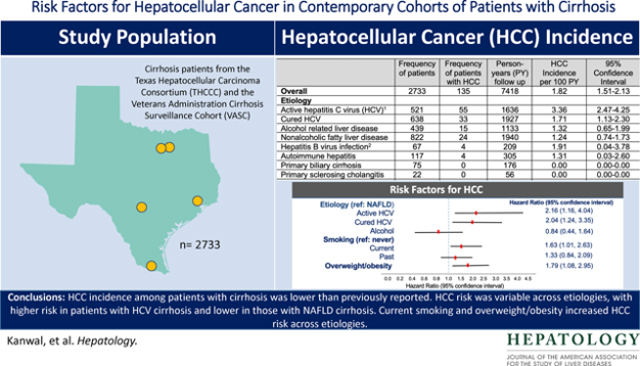
We used data from two contemporary, prospective multiethnic cohorts of patients with cirrhosis: the Texas Hepatocellular Carcinoma Consortium Cohort and the Houston Veterans Administration Cirrhosis Surveillance Cohort. Patients with cirrhosis were enrolled from seven U.S. centers and followed until HCC diagnosis, transplant, death or June 30, 2021. We calculated the annual incidence rates for HCC and examined the effects of etiology, demographic, clinical, and lifestyle factors on the risk of HCC. We included 2733 patients with cirrhosis (mean age 60.1 years, 31.3% women). At enrollment, 19.0% had active HCV, 23.3% cured HCV, 16.1% had alcoholic liver disease, and 30.1% had nonalcoholic fatty liver disease (NAFLD). During 7,406 person-years follow up, 135 patients developed HCC at an annual incidence rate of 1.82% (95% confidence interval [CI]=1.51–2.13). The annual HCC incidence rate was 1.71% in patients with cured HCV, 1.32% in patients with alcoholic liver disease, and 1.24% in patients with NAFLD cirrhosis. Compared to patients with NAFLD, the risk of progression to HCC was 2-fold higher in patients with cured HCV (hazard ratio [HR]=2.04, 95% CI, 1.24–3.35). Current smoking (HR=1.63, 95%CI, 1.01–2.63) and overweight/obesity (HR=1.79, 95% CI 1.08, 2.95) were also associated with HCC risk.
Conclusions.
HCC incidence among patients with cirrhosis was lower than previously reported. HCC risk was variable across etiologies, with higher risk in patients with HCV cirrhosis and lower in those with NAFLD cirrhosis. Current smoking and overweight/obesity increased HCC risk across etiologies.
Keywords: Risk stratification, epidemiology, hepatitis C, nonalcoholic fatty liver disease, alcohol
Graphical Abstract
BACKGROUND
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide and has one of the fastest increasing cancer-related mortality rates in the U.S.1,2 Most cases of HCC arise in the background of cirrhosis. Underlying etiological risk factors for cirrhosis have dramatically changed in the U.S. in the last decade.3–5 Metabolic dysfunction traits (e.g., obesity, diabetes) and the associated non-alcoholic fatty liver disease (NAFLD) have become the dominant cirrhosis risk factors whereas active untreated hepatitis C virus (HCV) and hepatitis B virus (HBV) infection have decreased in prevalence.6 In contrast, the rising rates of alcohol use disorders in the U.S. have resulted in an increase in alcoholic liver disease and alcoholic cirrhosis.2 These trends in cirrhosis risk factors are expected to translate into parallel shifts in the overall HCC risk in patients with cirrhosis, but data from well conducted prospective studies are lacking.
There is a knowledge gap about the absolute risk of HCC among newer cohorts of patients with cirrhosis from different etiological risk factors. Although cirrhosis is the main precursor lesion in HCC, most patients with cirrhosis do not progress to HCC. Predicting progression by better understanding risk factors would allow clinicians to more effectively plan secondary prevention efforts and HCC surveillance in cirrhosis. Most previous studies that examined HCC risk and risk factors in patients with cirrhosis were retrospective in design with incomplete ascertainment of important exposures and outcomes and included patients with a single etiological risk factor.7–10
We combined and harmonized data from two large prospective, contemporary multiethnic cohorts of patients with cirrhosis from multiple etiologies who were seen in routine clinical practice across seven centers in the U.S. to examine the risk of HCC overall and in key subgroups. We also examined the effects of etiology, demographic, clinical, and lifestyle factors on the risk of HCC.
METHODS
Study Cohorts
We used data from two prospective cohort studies of patients with cirrhosis: the Texas Hepatocellular Carcinoma Consortium Cohort (THCCC)6,11 and the Houston Veterans Administration Cirrhosis Surveillance Cohort (HVASC).
In the THCCC, we prospectively recruited patients with cirrhosis from seven institutions in four cities (Michael E DeBakey VA Medical Center and Baylor St. Lukes Medical Center in Houston; University of Texas Southwestern, Parkland Health & Hospital System, and Baylor Scott & White Research Institute in Dallas; Doctor’s Hospital at Renaissance in McAllen; and Texas Liver Institute in San Antonio). Recruitment started on December 2016 and is still ongoing.
Cirrhosis diagnosis was based on predefined criteria for liver histology, radiology, liver elastography, or serum biomarkers (see Supplementary Materials). Patients with uncontrolled hepatic decompensation, history of HCC, or presence of non-hepatic cancer were excluded. At the time of consent, patients completed surveys including medical history, alcohol, and tobacco use. We also abstracted data from the patients’ electronic medical records (EMR) including: (1) clinician recorded diagnoses including cirrhosis etiology (e.g., HCV, HBV), severity (ascites, varices; hepatic encephalopathy), and liver-related treatments; and (2) liver imaging and liver biopsy results, and (3) laboratory data within 12 months of enrollment.
Patients were scheduled for 6-month visits as part of routine clinical care and followed until HCC diagnosis, liver transplantation, or death. They received liver ultrasound, CT or MRI for HCC surveillance per the decision of treating physician. For the current analysis, we included THCCC participants enrolled between December 2016 and April 2020, with follow up until June 30, 2021.
The HVASC is a cohort of patients with cirrhosis in active HCC surveillance recruited from hepatology clinics at the Michael E DeBakey VA Medical Center between August 2014 and December 2016 with follow-up until June 2021. HVASC used similar eligibility, inclusion and exclusion criteria, and recruitment procedures as those described for THCCC; indeed, most procedures for THCCC were adopted from the HVASC study. HVASC participants completed a shorter survey, although most items in HVASC overlapped with those included in the THCCC surveys.
We harmonized data from THCCC and HVASC into one common dataset to use for the current analysis using the steps described by Rolland et al12. This harmonization was possible given similarities in data collection procedures and survey instruments. We went back to the source documents and EMR for both cohorts to complete missing data items.
Primary Outcome
Our primary outcome was incident HCC that developed following enrollment. EMR reviews were conducted for all participants at 3-month intervals to capture incident HCCs, liver transplantation and death dates. We defined HCC according to AASLD criteria including histological or radiological diagnosis using characteristic appearance (arterial enhancement and delayed washout) on triple phase CT or MRI (LI-RAD 5) or those with suspicious lesions (LI-RAD 4) that were reviewed in multidisciplinary tumor boards and empirically treated as HCC. All study sites have multidisciplinary HCC tumor boards. For our analysis, we used the date of final confirmation as the date of HCC diagnosis.
Possible HCC Risk Factors
Socio-demographic variables included age at enrollment, sex, and a constructed variable from self-reported race and ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, and other groups). We defined HCV as active, cured, or none. Active HCV was based on a positive HCV ribonucleic acid test, and cured HCV was based on documentation of treatment and subsequent sustained virological response (SVR) in the EMR.13 Because most HCV patients received antiviral treatment with direct acting antiviral agents during follow up, we modeled HCV status (active, cured, none) as a time varying covariate. HBV was based on a positive hepatitis B surface antigen.14,15 All HBV patients were on antiviral treatment. We used a validated survey for ascertaining alcohol use that classified alcohol use status as lifetime abstention (never), former light to moderate use, former heavy use, current light to moderate and current heavy use, as defined by the Centers for Disease Control and Prevention.16 We defined alcohol related cirrhosis based on a combination of clinician recorded diagnosis of alcoholic liver disease and patients’ self-report of former heavy (8 or more alcoholic beverages per week for women or 15 or more alcoholic beverages per week for men) or any current use of alcohol. We used clinician recorded diagnosis to define other etiologies, including autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hereditary hemochromatosis, and Wilson’s Disease. The diagnosis of NAFLD requires documentation of hepatic steatosis on liver histology or imaging. Given that hepatic steatosis cannot be reliably ascertained in the clinical setting of liver cirrhosis, we defined NAFLD as the possible etiology of cirrhosis for patients without HCV (active/untreated or resolved HCV), HBV, alcoholic liver disease, or other clinician documented etiologies (such as autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hereditary hemochromatosis, Wilson’s Disease, including few patients who were classified as having cryptogenic cirrhosis). Most (>90%) of patients classified as NAFLD also had clinician recorded diagnosis of NAFLD.
We defined diabetes, hypertension, and dyslipidemia based on patient’s medical history (survey or EMR) or self-reported treatment with diabetes medications (oral hypoglycemic medications or insulin), anti-hypertensives, or treatments for dyslipidemia (bile acid–binding resins, HMG-CoA reductase inhibitors, nicotinic acid and fibric acid derivatives) at any time before enrollment. We defined smoking status as never, past, and current smoker based on self-report using the baseline survey. We used height and weight values at enrollment to calculate body mass index (BMI). We defined Child Pugh Class based on physician documentation of presence and severity of ascites, hepatic encephalopathy, and laboratory data at enrollment into the study cohort.
Statistical Analyses
We estimated follow up of patients from the date of enrollment (index date) to the development of HCC, liver transplantation, death, or end of follow up (June 30, 2021). We calculated the annual and cumulative incidence rates for HCC in the overall cohort and in subgroups defined based on etiological risk factors. We generated cumulative incidence function curves, accounting for competing risks of transplant and death, to illustrate and compare the cumulative incidence of HCC by common etiological risk factors (cured HCV, NAFLD, alcohol) and used the Gray’s test to evaluate the difference between curves.
We constructed univariate and multivariable Cox proportional hazards models for competing risks data, using Fine and Gray method17 to the examine the effects of etiological, demographic, clinical, and lifestyle factors on the risk of HCC. We focused on common etiological risk factors. Some etiology-based groups (such as autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, HBV, etc.), had few HCC cases in each group; therefore, we combined these smaller groups into one group (Other) for the purposes of the regression analyses. We accounted for competing risks of transplantation and death in the models. Because all variables (see Possible HCC Risk Factors) were selected a priori based on clinical considerations and literature, we included all in our multivariable model in lieu of arbitrarily selecting a subset based on statistical significance alone.
RESULTS
Of 2733 patients with cirrhosis, 2381 (87.1%) participants were enrolled in THCCC and 352 (12.9%) in HVASC. The mean age of the cohort was 60.1 years (standard deviation, SD 9.95 years) and 856 (31.3%) were women. The cohort included 50.2% non-Hispanic white, 27.4% Hispanics, and 19.5% non-Hispanic Blacks. Among 748 Hispanics, 410 (54.8%) were born in the U.S., 122 born in Mexico (16.3%), and the rest were born in other regions (Central America, South America, and the Caribbean). HCV was the leading etiological risk factor (42.4%) followed by NAFLD (30.1%) and alcohol-related liver disease (16.1%). In total, 19.1% had active HCV and 23.3% cured HCV at baseline. These proportions changed to 9.2% and 33.2%, respectively by the end of study follow up. In total, 2.4% of the cohort had HBV, 4.3% autoimmune hepatitis, and 2.7% had primary biliary cholangitis. Other etiological risk factors were rare (0.8% primary sclerosing cholangitis, 0.5% hereditary hemochromatosis, 0.1% alpha 1 antitrypsin deficiency, and 0.2% Wilson disease). More than 22% of patients were current smokers, while 6.7% reported current heavy alcohol use, 43.1% had diabetes, 52.2% hypertension, and most were overweight or obese (body mass index ≥25). Most patients (63.6%) had a baseline Child-Pugh Class A, and less than 5% had Child Pugh C class.
Patients with active HCV were more likely to be non-Hispanic Black, men, and current smokers than the rest of the cohort; those with NAFLD more likely to be women, Hispanic, overweight, and have diabetes, hypertension, and dyslipidemia; whereas patients with alcoholic liver disease were more likely to be younger and have Child Class B or C cirrhosis than the rest of the cohort (Supplementary Table 1).
The overall adherence to HCC surveillance was high. In total, 80% of participants underwent at least one HCC surveillance imaging during follow up. Most patients (64.1%) received ultrasound-based screening, 21.4% patients received at least one abdominal computerized tomography, and 24.9% had at least one abdominal magnetic resonance imaging during follow up. There were no clinically meaningful differences in the frequency and type of surveillance tests among patients with the key etiological risk groups (Supplementary Table 1).
Risk of HCC in patients with cirrhosis
During a total of 7,406 person-years follow up, 62 patients received transplantation, another 344 died during follow-up, and 135 patients developed HCC at an annual incidence rate of 1.82% (95% CI 1.50, 2.17%). Of all HCC cases, the diagnosis was based on imaging in 92.5%, histology in 6.6% of patients, while one patient (0.74%) had HCC diagnosed on explant at the time of liver transplantation.
Table 2 displays the annual incidence rates of HCC in key subgroups by etiological risk factors and race/ethnicity. The annual HCC incidence rate was the highest in patients with active HCV (3.36%, 95% CI, 2.47, 4.25%) followed by patients with cured HCV (1.71%, 95% CI 1.13, 2.30%), those with alcoholic liver disease (1.32%, 95% CI, 0.65, 1.99%), and patients with NAFLD cirrhosis (1.24%, 95% CI, 0.74, 1.73%). In total, 67 patients had HBV. Of these, 24 patients were co-infected with HCV and 11 had co-existing alcohol related liver disease; HCC developed at an annual incidence rate of 1.91% (95% CI, 0.04, 3.78%). Only 32 patients had HBV infection without other risk factors; the annual HCC incidence rate was 1.13% (95% CI, 0.00, 3.35%) in this subgroup. Few patients progressed to HCC in other risk groups (see Table 2).
Table 2.
HCC incidence overall and in key subgroups defined based on etiology and race/ethnicity.
| Frequency of patients | Frequency of patients with HCC | Person-years (PY) follow up | HCC Incidence per 100 PY | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Overall | 2733 | 135 | 7418 | 1.82 | 1.51–2.13 |
| Etiology 1 | |||||
| Active hepatitis C virus (HCV)1 | 521 | 55 | 1636 | 3.36 | 2.47–4.25 |
| Cured HCV | 638 | 33 | 1927 | 1.71 | 1.13–2.30 |
| Alcohol related liver disease | 439 | 15 | 1133 | 1.32 | 0.65–1.99 |
| Nonalcoholic fatty liver disease | 822 | 24 | 1940 | 1.24 | 0.74–1.73 |
| Hepatitis B virus infection2 | 67 | 4 | 209 | 1.91 | 0.04–3.78 |
| Autoimmune hepatitis | 117 | 4 | 305 | 1.31 | 0.03–2.60 |
| Primary biliary cirrhosis | 75 | 0 | 176 | 0.00 | 0.00–0.00 |
| Primary sclerosing cholangitis | 22 | 0 | 56 | 0.00 | 0.00–0.00 |
| Other etiologies2 | 81 | 2 | 179 | 1.12 | 0.00–2.68 |
| Missing | 10 | 1 | 45 | 2.24 | 0.00–6.64 |
| Race/ethnicity | |||||
| Non-Hispanic White | 1373 | 72 | 3711 | 1.94 | 1.49–2.38 |
| Hispanics | 748 | 31 | 1879 | 1.65 | 1.07–2.23 |
| Non-Hispanic Black | 533 | 28 | 1618 | 1.73 | 1.09–2.38 |
HCV status was defined based on data at the time of cohort enrollment.
Only, 32 patients had HBV infection without other risk factors, with an annual HCC incidence rate of 1.13% (95% CI, 0.00, 3.35%).
See footnote for Table 1.
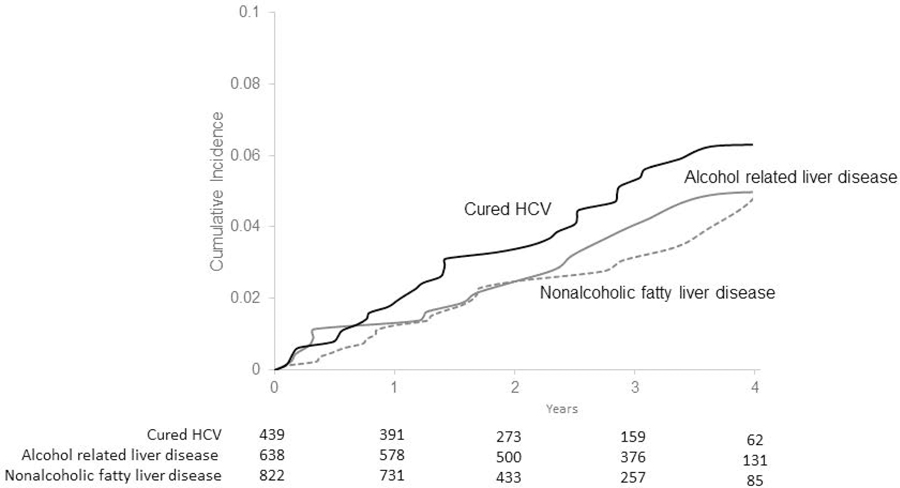
The 3-year cumulative incidence rate of HCC was 5.3% (95% CI, 4.4, 6.3%) in the overall cohort and ranged in subgroups based on etiological risk factors from 3.1% (95% CI, 1.9, 4.7%) in patients with NAFLD, 3.6% (95% CI, 2.0, 6.0%) in patients with alcoholic liver disease to 5.1% (95% CI, 3.5, 7.2%) in patients with cured HCV (Figure 1). The annual HCC incidence rates were not different among non-Hispanic whites (1.94%), non-Hispanic Blacks (1.73%) and Hispanics (1.65%) (Gray test, p= 0.71).
Figure 1.
Cumulative incidence of hepatocellular cancer. Hepatitis C virus infection (HCV), nonalcoholic fatty liver disease, alcohol liver disease.
Associations between etiological, demographic, clinical, and lifestyle factors and HCC
In the multivariable model adjusting for other etiological and demographic risk factors, compared to patients with NAFLD, those with active HCV had 2.1-fold higher risk of HCC (HR=2.16, 95% CI, 1.16, 4.04). The relative risk of HCC persisted in patients with cured HCV and remained over 2-fold higher than the risk in patients with NAFLD (HR=2.04, 95% CI, 1.24, 3.35). There was no statistically significant difference in the risk of HCC between patients with alcohol and NAFLD related cirrhosis.
Current smokers had a higher risk of HCC than non-smokers (HR, 1.63, 95% CI, 1.01–2.63) (Table 3). Being overweight was associated with higher risk of HCC (HR, 1.79, 95% CI, 1.08–2.95) whereas dyslipidemia was associated with lower HCC risk (HR=0.62, 95% CI, 0.40–0.94).
Table 3.
Associations between demographic, etiological, and life factors and risk of incident hepatocellular cancer. NAFLD – nonalcoholic fatty liver disease. BMI, body mass index
| Variables | Unadjusted Hazard Ratio | Adjusted Hazard Ratio |
|---|---|---|
| Age in years | ||
| < 55 | 1.00 | 1.00 |
| 55–64 | 1.38 (0.83, 2.28) | 1.11 (0.66, 1.88) |
| 65+ | 1.89 (1.15, 3.11) | 1.92 (1.14, 3.25) |
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 2.61 (1.61, 4.24) | 2.22 (1.25, 3.65) |
| Race/ethnicity | ||
| Non-Hispanic (NH) white | 1.00 | 1.00 |
| NH Black | 0.94 (0.61, 1.46) | 0.66 (0.42, 1.03) |
| Hispanic | 0.83 (0.55, 1.27) | 0.98 (0.63, 1.53) |
| Other | 0.97 (0.35, 2.67) | 1.27 (0.45, 3.59) |
| Etiology | ||
| NAFLD (reference) | 1.00 | 1.00 |
| Active HCV | 2.32 (1.18, 4.59) | 2.16 (1.16, 4.04) |
| Cured HCV | 2.02 (1.16, 3.54) | 2.04 (1.24, 3.35) |
| Alcoholic liver disease | 0.92 (0.48, 1.74) | 0.84 (0.44, 1.64) |
| Other etiologies1 | 0.79 (0.18, 3.47) | 0.90 (0.40, 2.03) |
| Smoking | ||
| Never | 1.00 | 1.00 |
| Current | 2.22 (1.41, 3.49) | 1.63 (1.01, 2.63) |
| Past | 1.72 (1.12, 2.64) | 1.33 (0.84, 2.09) |
| Diabetes | ||
| No | 1.00 | 1.00 |
| Yes | 1.02 (0.73, 1.43) | 1.21 (0.84, 1.73) |
| Overweight/obesity | ||
| BMI <25 | 1.00 | 1.00 |
| BMI 25 and more | 1.60 (0.97, 2.63) | 1.79 (1.08, 2.95) |
| Hypertension | ||
| No | 1.00 | 1.00 |
| Yes | 1.15 (0.82, 1.62) | 1.33 (0.94, 1.87) |
| Dyslipidemia | ||
| No | 1.00 | 1.00 |
| Yes | 0.68 (0.47, 1.00) | 0.62 (0.40, 0.94) |
| Child Pugh Class | ||
| A | 1.00 | 1.00 |
| B | 1.47 (0.99, 2.18) | 1.85 (1.22, 2.80) |
| C | 2.26 (1.15, 4.43) | 3.25 (1.58, 6.67) |
Includes all other etiologies, including hepatitis B, autoimmune liver disease, hemochromatosis, Wilson’s disease, alpha 1 anti-trypsin deficiency, cryptogenic cirrhosis, and few patients with missing etiology.
Risk of HCC increased with age; patients older than 65 had 92% higher risk of HCC than 55 year and younger (HR, 1.92, 95% CI, 1.14, 3.25). The risk of HCC was 2.2-fold higher in men vs. women (HR, 2.26, 95% CI, 1.25–3.25). Non-Hispanic Blacks were at a lower risk of developing HCC than non-Hispanic Whites (HR=0.66, 95% CI=0.42–1.03), although this trend did not reach statistical significance. Higher Child Pugh Class was associated with a significantly increased HCC risk (Table 3).
DISCUSSION
In this study, we have provided contemporary data about the magnitude of HCC risk in relation to key etiological risk factors for HCC using data from two U.S. based prospective cohorts of patients with cirrhosis. The absolute risk (i.e., incidence rate) of HCC in our multi-etiology cohort of patients with cirrhosis was lower than the risk reported in previous older studies.18–21 The etiological risk factors for cirrhosis, especially HCV, had the strongest effect on HCC risk; the highest risk was in active HCV, followed by HBV, cured HCV, alcoholic liver disease and lowest in NAFLD. However, health behaviors such as smoking and being overweight were associated with a further increase in HCC risk.
The annual incidence rate of HCC in our cohort overall was 1.82% (95% CI, 95% CI 1.50, 2.17%). Previous studies, mostly of patients with untreated HCV or HBV found higher annual incidence rates that fell between 2% to 8%.5,19–21 Our results show that the shift in cirrhosis etiological risk factors from HCV to NAFLD have resulted in a downward shift in the magnitude of HCC risk in patients with cirrhosis. The results of our study are largely consistent with data from recent retrospective cohort studies. We previously reported an annual HCC incidence of 1.81% in Veterans with HCV who had cirrhosis at the time of virological cure and an annual incidence of 1.06% in patients with NAFLD cirrhosis.7,8,13 Our current study extends these data to show that the pretest probability of HCC in the newer cohorts of patients with cirrhosis is lower than expected based on historic cohorts. However, HCC risk approached or exceeded the currently accepted threshold beyond which HCC surveillance may be cost-effective in all identified subgroups; none of the subgroups had a markedly lower risk. However, the current surveillance recommendations are based on studies in patients with active HCV and HBV with no direct evidence relating to the newer cohorts of patients with cirrhosis, most of whom have cured HCV or NAFLD (as in our study). Both the effectiveness and cost-effectiveness of surveillance depend on the risk of HCC (incidence rate). Our data on HCC incidence could guide future cost-effectiveness analysis and HCC surveillance in cirrhosis.
We found that HCC risk in cirrhosis was driven primarily by etiological risk factors. HCC risk was the highest in the subgroup with active HCV cirrhosis which constituted a relatively small proportion overall, especially with the evolution of time during follow up. This risk was followed by cured HCV which accounted for over a third of our cohort, demonstrating that HCV (active or cured) will continue to be an important contributor to the burden of HCC in the next decade. Our findings also underscore the importance of ongoing HCC surveillance in patients with cirrhosis following SVR. HCC risk was lower in patients with NAFLD-cirrhosis (1.24%) than in patients with HCV cirrhosis in our cohort. Although HCV was the strongest risk factor for HCC, NAFD could eventually be responsible for more cases of HCC than HCV given the higher prevalence of NAFLD in the general population and among patients with cirrhosis including our cohort. Indeed, a recent study estimated NAFLD will result in 135,000 HCC cases in the U.S. between 2015 and 2030.22
Our data may also inform secondary prevention of HCC in cirrhosis. In our cohort, approximately 60% of patients were current or former smokers; smoking was associated with a higher risk of HCC. Several constituents of tobacco smoke can promote hepatocarcinogenesis.23–27 Overall, our observed association provides support to the recent guidance that cirrhosis patient should be counseled on abstaining from smoking as a risk reduction strategy in HCC.28 Our study provides novel evidence that obesity is associated with increased HCC risk among patients with cirrhosis. Obesity has been associated with 1.5–2.0-fold increase in HCC risk in general population studies.29 But whether and to what extend overweight or obesity modifies HCC risk in patients with established cirrhosis has been unknown. Our finding of a 79% higher risk of HCC in overweight/obese patients compared to those with normal weight also have important implications for secondary prevention of HCC. The mechanisms of obesity related hepatocarcinogenesis need to be examined.
Studies have shown substantial differences among racial and ethnic groups in the distribution of etiological risk factors for cirrhosis and HCC, with HCV being more common in Blacks and NAFLD more common in Hispanics.30 We found that Blacks had a trends toward lower risk of developing HCC compared with non-Hispanic Whites after adjusting for etiological risk factors, especially HCV and NAFLD. The race effect in our study is similar to that observed in previous studies that controlled for etiology.31,32 Although, Hispanic ethnicity is associated in some studies with a higher risk of cirrhosis and HCC,33 we did not find evidence for an association between ethnicity and HCC risk. Ethnicity-HCC relationship will require a larger cohort with longer follow up (and more accrued HCC cases) to support a comprehensive evaluation.
Ours is one of the few multicenter, prospective cohort studies that involved U.S. patients of all ages, multiple racial and ethnic groups and both sexes with cirrhosis from multiple etiologies, and minimal loss to follow-up. However, the study has limitations. Despite following strict protocols for follow-up, with structured EMR reviews to ascertain outcomes, we may have missed HCC events in some patients who sought care outside of the healthcare systems where recruitment for the study took place. Although we used validated surveys for alcohol use and smoking, we cannot exclude measurement bias and we did not systematically collect medication information. NAFLD diagnosis was a constructed variable defined based on absence of other active risk factors (HCV or alcohol) but not based on demonstration of fatty liver disease, which is difficult to do in cirrhosis, potentially resulting in misclassification bias. However, most patients classified as NAFLD had physician documented diagnosis of NAFLD and had at least one metabolic risk factor, providing support to our definition. We likely underestimated the presence of NAFLD because we did not account for the possibility that some patients with viral hepatitis may also have metabolic dysfunction associated fatty liver disease. Few patients in our cohort had HBV infection or other etiological risk factors (such as primary sclerosing cholangitis) and hence the results cannot be generalized to cohorts with high proportions of patients with these etiological risk factors. We also followed patients in hepatology specialty clinics care; this setting could limit generalizability to broader population of patients with cirrhosis.
In conclusion, in this large prospective contemporary cirrhosis cohort study, we found that the incidence rates of HCC were lower than that previously reported. The magnitude of HCC risk varied depending on the underlying etiology, being highest in patients with active HCV cirrhosis, followed by HBV, cured HCV, and lowest in those with alcohol or NAFLD cirrhosis. The proportions of these etiological risk factors were highest for cured HCV and NAFLD and lowest for active HCV or HBV. Smoking and overweight were associated with an increased HCC risk. Our results have implications for studies of cost-effectiveness of HCC surveillance and for secondary prevention of HCC.
Supplementary Material
Table 1.
Descriptive statistics for the study cohort
| Variables | N=2733 (%) |
|---|---|
| Age in years | |
| < 55 | 644 (23.6) |
| 55–64 | 1148 (42.0) |
| 65+ | 941 (34.4) |
| Sex | |
| Female | 856 (31.3) |
| Male | 1877 (68.7) |
| Race/ethnicity | |
| Non-Hispanic (NH) white | 1373 (50.2) |
| NH-Black | 533 (19.5) |
| Hispanic | 748 (27.4) |
| Other | 79 (2.9) |
| Etiology of liver disease 1 | |
| Active hepatitis C virus (HCV)2 | 521 (19.0) |
| Cured HCV2 | 638 (23.3) |
| Alcoholic liver disease | 439 (16.1) |
| Nonalcoholic fatty liver disease | 822 (30.1) |
| Hepatitis B virus infection3 | 67 (2.4) |
| Autoimmune hepatitis | 117 (4.3) |
| 0Primary biliary cirrhosis | 75 (2.7) |
| Primary sclerosing cholangitis | 22 (0.8) |
| Other etiologies4 | 81 (2.9) |
| Missing | 10 (0.36 |
| Alcohol use | |
| Never | 873 (31.9) |
| Current heavy | 183 (6.7) |
| Current but not heavy | 191 (7.0) |
| Past Heavy | 903 (33.0) |
| Past Not Heavy | 583 (21.4) |
| Smoking | |
| Never | 1087 (39.8) |
| Current | 616 (22.5) |
| Past | 1030 (37.7) |
| Diabetes | |
| No | 1555 (56.9) |
| Yes | 1178 (43.1) |
| Body mass index (kg/m 2 ) | |
| < 25 | 547 (20.0) |
| 25–29 | 864 (31.6) |
| 30–34 | 725 (26.5) |
| 35+ | 597 (21.9) |
| Hypertension | |
| No | 1305 (47.8) |
| Yes | 1428 (52.2) |
| Dyslipidemia | |
| No | 1800 (65.9) |
| Yes | 933 (34.1) |
| Child Pugh Class | |
| A | 1738 (63.6) |
| B | 685 (25.1) |
| C | 119 (4.4) |
| Missing | 191 (6.9) |
Some patients had more than one etiological risk factor. Where possible, we relied on the primary etiology assigned by the treating clinician. Specifically, patients with HCV and excessive alcohol use were classified as patients with HCV. Alcohol related cirrhosis was classified as the underlying risk factor when alcohol was defined as the only risk factors. Nonalcoholic fatty liver disease was defined as the etiology in patients without HCV (active/untreated or resolved HCV), HBV, alcohol, or other etiological risk factors. There could be overlap in etiology in patients with autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis.
HCV status was defined based on data at the time of cohort enrollment. In total, 908 patients achieved sustained virological response during follow up.
Of the 67 patients with HBV, 24 patients were co-infected with HCV and 11 had co-existing alcohol related liver disease. Only, 32 patients had HBV infection without other risk factors.
Includes patients with hemochromatosis, Wilson’s disease, alpha 1 anti-trypsin deficiency, cryptogenic cirrhosis
What is already known about this subject?
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide, and it develops in the setting of liver cirrhosis.
The underlying etiological risk factors for cirrhosis have dramatically changed in the last decade.
What are the new findings?
In this multicenter, prospective multiethnic cohort study, HCC incidence among patients with cirrhosis was lower than previously reported, showing that the trends in cirrhosis risk factors have shifted the overall HCC risk in patients with cirrhosis.
Among patients with cirrhosis, hepatitis C virus infection (active and cured) had the strongest effect on HCC risk.
Health behaviors such as smoking and being overweight were associated with a further increase in HCC risk
How might it impact on clinical practice in the foreseeable future?
Our results have implications for studies of cost-effectiveness of HCC surveillance and for secondary prevention of HCC in patients with cirrhosis who are seen in current clinical practice.
Acknowledgments
Grant Support: This work was supported by the National Cancer Institute (NCI U01 CA23099, U01 CA230694, and R01CA186566), Cancer Prevention & Research Institute of Texas grant (RP150587), and in part by Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338). Drs. Kanwal and El-Serag are investigators at the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, Texas.
Abbreviations:
- (HCC)
hepatocellular cancer
- (NAFLD)
nonalcoholic fatty liver disease
- (HR)
hazard ratio
- (CI)
confidence interval
- (HCV)
hepatitis C virus
- (HBV)
hepatitis B virus
- (THCCC)
Texas Hepatocellular Carcinoma Consortium Cohort
- (HVASC)
Houston Veterans Administration Cirrhosis Surveillance Cohort
- (EMR)
electronic medical record
- (SVR)
sustained virological response
References
- 1.Ryerson AB EC, Altekruse SF. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. Nov 2020;18(12):2650–2666. doi: 10.1016/j.cgh.2019.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: a Danish nationwide cohort study. Ann Intern Med. Jun 19 2012;156(12):841–7, W295. doi: 10.7326/0003-4819-156-12-201206190-00004 [DOI] [PubMed] [Google Scholar]
- 4.Nathalie Ganne-Carrié 1 CC, Bourcier 3 Valérie, Archambeaud 4 Isabelle, Perarnau 5 Jean-Marc, Oberti 6 Frédéric, Roulot 7 Dominique, Moreno 8 Christophe, Louvet 9 Alexandre, Dao 10 Thông, Moirand 11 Romain, Goria 12 Odile, Nguyen-Khac 13 Eric, Carbonell 14 Nicolas, Antonini 15 Térésa, Pol 16 Stanislas, de Ledinghen 17 Victor, Ozenne 18 Violaine, Henrion 19 Jean, Péron 20 Jean-Marie, Tran 21 Albert, Perlemuter 22 Gabriel, Amiot 23 Xavier, Zarski 24 Jean-Pierre, Beaugrand 3 Michel, Chevret 2 Sylvie. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69(6):1274–1283. doi:10.1016 [DOI] [PubMed] [Google Scholar]
- 5.Li DK, Ren Y, Fierer DS, et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. Jun 2018;67(6):2244–2253. doi: 10.1002/hep.29707 [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Kanwal F, Feng Z, et al. Risk Factors for Cirrhosis in Contemporary Hepatology Practices-Findings From the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology. Jul 2020;159(1):376–377. doi: 10.1053/j.gastro.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. Dec 2018;155(6):1828–1837 e2. doi: 10.1053/j.gastro.2018.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. Oct 2017;153(4):996–1005 e1. doi: 10.1053/j.gastro.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 9.Tansel A, Katz LH, El-Serag HB, et al. Incidence and Determinants of Hepatocellular Carcinoma in Autoimmune Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. Aug 2017;15(8):1207–1217 e4. doi: 10.1016/j.cgh.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natarajan Y, Tansel A, Patel P, et al. Incidence of Hepatocellular Carcinoma in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig Dis Sci. Jul 2021;66(7):2439–2451. doi: 10.1007/s10620-020-06498-7 [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, Marrero JA, Khaderi S, et al. Design of the Texas Hepatocellular Carcinoma Consortium Cohort Study. Am J Gastroenterol. Mar 2019;114(3):530–532. doi: 10.14309/ajg.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolland B, Reid S, Stelling D, et al. Toward Rigorous Data Harmonization in Cancer Epidemiology Research: One Approach. Am J Epidemiol. Dec 15 2015;182(12):1033–8. doi: 10.1093/aje/kwv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology. Jan 2020;71(1):44–55. doi: 10.1002/hep.30823 [DOI] [PubMed] [Google Scholar]
- 14.Kruse RL, Kramer JR, Tyson GL, et al. Clinical outcomes of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology. Dec 2014;60(6):1871–8. doi: 10.1002/hep.27337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasiha Kanwal TJT, Kramer Jennifer R, Cao Yumei, Smith Donna, Gifford Allen L, El-Serag Hashem B, Naik Aanand D, Asch Steven M Development, Validation, and Evaluation of a Simple Machine Learning Model to Predict Cirrhosis Mortality. JAMA Network Open. 2020;11doi:10.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevention. CfDCa. Excessive Alcohol Use.
- 17.He P, Eriksson F, Scheike TH, Zhang MJ. A Proportional Hazards Regression Model for the Sub-distribution with Covariates Adjusted Censoring Weight for Competing Risks Data. Scand Stat Theory Appl. Mar 2016;43(1):103–122. doi: 10.1111/sjos.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. Nov 2004;127(5 Suppl 1):S35–50. doi: 10.1053/j.gastro.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 19.Thiele M, Gluud LL, Fialla AD, Dahl EK, Krag A. Large variations in risk of hepatocellular carcinoma and mortality in treatment naive hepatitis B patients: systematic review with meta-analyses. PLoS One. 2014;9(9):e107177. doi: 10.1371/journal.pone.0107177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. Oct 2007;5(10):1221–8. doi: 10.1016/j.cgh.2007.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. Jul 2012;57(1):69–76. doi: 10.1016/j.jhep.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. Jan 2018;67(1):123–133. doi: 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ, Nonalcoholic Steatohepatitis Clinical Research N. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. Apr 2011;54(4):753–9. doi: 10.1016/j.jhep.2010.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archambeaud I, Auble H, Nahon P, et al. Risk factors for hepatocellular carcinoma in Caucasian patients with non-viral cirrhosis: the importance of prior obesity. Liver Int. Jul 2015;35(7):1872–6. doi: 10.1111/liv.12767 [DOI] [PubMed] [Google Scholar]
- 25.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. Dec 2009;38(6):1497–511. doi: 10.1093/ije/dyp280 [DOI] [PubMed] [Google Scholar]
- 26.Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. May 2018;27(3):205–212. doi: 10.1097/CEJ.0000000000000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. Apr 2018;118(7):1005–1012. doi: 10.1038/s41416-018-0007-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. May 2020;158(6):1822–1830. doi: 10.1053/j.gastro.2019.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Das A, Majumder K, et al. Obesity is Independently Associated With Increased Risk of Hepatocellular Cancer-related Mortality: A Systematic Review and Meta-Analysis. Am J Clin Oncol. Sep 2018;41(9):874–881. doi: 10.1097/COC.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol. Jan 2016;14(1):5–12; quiz e9–10. doi: 10.1016/j.cgh.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 31.El-Serag HB, Kramer J, Duan Z, Kanwal F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. Sep 2014;109(9):1427–35. doi: 10.1038/ajg.2014.214 [DOI] [PubMed] [Google Scholar]
- 32.Mittal S, Kramer JR, Omino R, et al. Role of Age and Race in the Risk of Hepatocellular Carcinoma in Veterans With Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. Feb 2018;16(2):252–259. doi: 10.1016/j.cgh.2017.08.042 [DOI] [PubMed] [Google Scholar]
- 33.Rich NE, Oji S, Mufti AR, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. Feb 2018;16(2):198–210 e2. doi: 10.1016/j.cgh.2017.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.