Abstract
This work aimed to characterize the antimicrobial compounds obtained from the potential probiotic Lactiplantibacillus plantarum S61, isolated from traditional fermented green olive, involved in their activity against fungi and bacteria responsible for food spoilage and poisonings. Their application as a biopreservative agent was also investigated. The culture of L. plantarum S61 showed substantial antifungal and antibacterial activity against yeasts (Rhodotorula glutinis and Candida pelliculosa), molds (Penicillium digitatum, Aspergillus niger, Fusarium oxysporum, and Rhizopus oryzae), and pathogenic bacteria (Listeria monocytogenes ATCC 19,117, Salmonella enterica subsp. enterica ATCC 14,028, Staphylococcus aureus subsp. aureus ATCC 6538, Pseudomonas aeruginosa ATCC 49,189), with inhibition zones > 10 mm. Likewise, the cell-free supernatant (CFS) of L. plantarum S61 showed an essential inhibitory effect against fungi and bacteria, with inhibition diameters of 12.25–22.05 mm and 16.95–17.25 mm, respectively. The CFS inhibited molds’ biomass and mycelium growth, with inhibition ranges of 63.18–83.64% and 22.57–38.93%, respectively. The antifungal activity of the CFS was stable during 4 weeks of storage at 25 °C, while it gradually decreased during storage at 4 °C. Several antimicrobial compounds were evidenced in the CFS of L. plantarum S61, including organic acids, ethanol, hydrogen peroxide, diacetyl, proteins, and fatty acids. The protein fraction, purified by reversed-phase high-performance liquid chromatography (RP-HPLC), demonstrated important antifungal activity, in relation to the fraction with molecular weight between 2 and 6 kDa. L. plantarum S61 and its CFS, tested in apple and orange fruit biopreservation, demonstrated their protective effect against P. digitatum spoilage. The CFS exhibited effectiveness in reducing Salmonella enterica subsp. enterica ATCC 14,028 in apple juice. L. plantarum S61 and/or its bioactive compounds CFS represent a promising strategy for biocontrol against pathogens and spoilage microorganisms in the agro-industry.
Keywords: Antifungal, Antibacterial, Lactiplantibacillus, Biopreservation, Apple, Orange
Introduction
Spoilage and pathogenic microorganisms can grow in various agricultural products, including fruits, vegetables, and juices, essentials for human nutrition. However, most of these products are easily spoiled by microorganisms, leading to high economic losses and food poisoning. Among annual world food loss, estimated at 13.8%, fruits and vegetables are 21.6% [1]. Furthermore, fruit juices provide an ideal environment for deterioration by microorganisms, due to their nutritional components [2].
The microorganisms involved in fruit and juice spoilage are yeasts, molds, and tolerating acid bacteria [3, 4]. Chemical preservatives are mainly used to achieve their control. However, because of health concerns, consumers demand biological preservatives as an alternative to chemicals [5]. An exciting alternative to chemicals is biological preservatives of microbial origin, including microbial cultures, cell-free supernatant, or purified molecules [6].
The majority of lactic acid bacteria (LAB), especially lactobacilli, could be used as biopreservatives because of their antimicrobial activity, and most of them are recognized for their GRAS status (generally recognized as safe) according to the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) [7–9]. Lactiplantibacillus species can produce various antimicrobial molecules, including organic acids, carbon dioxide, reuterin, diacetyl, bacteriocin-like substances, peptides, hydrogen peroxide, proteinaceous compounds, fatty acids, and cyclic dipeptides [10–15]. L. plantarum species, mostly isolated from fermented vegetables, is known for its genetic diversity and its variable antifungal activity against yeasts and molds [8, 16, 17]. Therefore, selected strains of L. plantarum and/or their purified bioactive compounds may be used to control pathogenic bacteria and other microorganisms involved in food deterioration [13, 18].
In previous works, L. plantarum S61 isolated from traditional fermented green olive demonstrated probiotic properties and essential antibacterial and antifungal activities against pathogenic and spoilage microorganisms [19, 20]. This strain, owing to potentially probiotic properties, GRAS status, and antimicrobial activity, may be used as a biopreservative in foods as an alternative to chemicals. The aim of this study was to characterize the antimicrobial compounds from L. plantarum S61, and to evaluate their effectiveness in apple and orange fruits and apple juice biopreservation against fungal and bacterial spoilage.
Material and methods
Microorganisms and growth conditions
According to the new classification of Lactobacillus [21], the Lactiplantibacillus plantarum S61 (L. plantarum S61) strain was used. This strain was previously isolated from fermented green olives, and it was selected for its antifungal activity and potential probiotic properties [19]. L. plantarum S61 was cultivated in de Man Rogosa and Sharpe Medium (MRS) broth (BIOKAR, France) for 18 h at 30 °C before experimental use.
The target strains used in this work were yeasts (Rhodotorula glutinis and C. pelliculosa) and molds (Aspergillus niger, Penicillium digitatum, Fusarium oxysporum, and Rhizopus oryzae); they were cultured in peptone dextrose agar (PDA, Biokar, France) at 30 °C during 2 days for yeasts and at 25 °C during 5 days for molds. The pathogenic bacteria (Listeria monocytogenes ATCC 19,117, Salmonella enterica subsp. enterica ATCC 14,028, Staphylococcus aureus subsp. aureus ATCC 6538, Pseudomonas aeruginosa ATCC 49,189) were cultured in Muller Hinton broth (MH) at 37° C for 24 h.
Antifungal and antibacterial activity of L. plantarum S61 by overlay method
Antifungal and antibacterial activity of L. plantarum S61 was determined by the overlay method, according to the method of Cavicchioli, et al. [22], with some modifications. The activity was determined against target molds (Penicillium digitatum, Aspergillus niger, Fusarium oxysporum, and Rhizopus oryzae), yeasts (Rhodotorula glutinis (ON209167.1) and Candida pelliculosa), and pathogenic bacteria (Listeria monocytogenes ATCC 19,117 (ID:882,095), Salmonella enterica subsp. enterica ATCC 14,028 (ID:5,618,702), Staphylococcus aureus subsp. aureus ATCC 6538 (ID:1,023,101), Pseudomonas aeruginosa ATCC 49,189 (ID: 1,916,292)). L. plantarum S61 was spot-inoculated on MRS agar plates and incubated at 30 °C for 24 h. Then, the plates were overlaid with 10 mL of nutrient agar containing 105 spores/mL of molds and 105 CFU/mL of yeasts or pathogenic bacteria. After 24 h of incubation at 37 °C for bacteria and 2–5 days at 25 °C for yeasts and molds, the diameter of the inhibition zone was measured. All the tests were done in triplicate.
Preparation of the cell-free supernatant (CFS) from L. plantarum S61
The overnight culture of L. plantarum S61 was inoculated at 1% in MRS broth and incubated at 30 °C for 48 h. Then, the bacterial suspension obtained was centrifuged at 8000 g for 10 min. The CFS obtained was then sterilized using a 0.20 µm pore size filter (Minisart syringe filter, Sartorius, Germany) and stored at − 20 °C for further use.
Antibacterial effect of the CFS of L. plantarum S61
The antibacterial activity of the CFS of L. plantarum S61 was tested against pathogenic bacteria (L. monocytogenes ATCC 19,117, S. enterica subsp. enterica ATCC 14,028, S. aureus subsp. aureus ATCC 6538, and P. aeruginosa ATCC 49,189) according to the method described by Gharbi, et al. [23], with some modifications. Briefly, the overnight culture of each pathogen was mixed with 10 mL of Mueller Hinton (MH) (BIOKAR, France) soft agar (0.7%, w/v) medium at a concentration of 105 CFU/mL. Then, 100 μL of the CFS was added to wells (5 mm in diameter), cut in MH agar previously seeded with the target pathogenic bacteria. The plates were incubated for 24 h at 37 °C; the inhibition zones were measured in millimeter (mm). Sterile MRS medium was used as a negative control. All the experiments were made in triplicate.
Antifungal activity of the CFS of L. plantarum S61
Antifungal activity of the CFS on solid medium
The antifungal activity of the CFS of L. plantarum S61 was tested by a well diffusion assay according to Muhialdin and Hassan [24], with some modifications. Briefly, 100 µL of CFS were added to the wells (5 mm diameter), cut on Potato Dextrose Agar (PDA) (BIOKAR, France), previously inoculated with 105 spores/mL of P. digitatum, A. niger, F. oxysporum, and R. oryzae and 105 CFU/mL of R. glutinis and C. pelliculosa. The sterile MRS medium was used as a negative control. After incubation of the cultures at 25 °C for 2–5 days, the inhibition zones were measured around the wells. All the experiments were made in triplicate.
Fungal mycelium growth inhibition by the CFS
The inhibition of the mycelium growth by the CFS of L. plantarum S61 was determined in Potato Dextrose Agar (PDA) medium (BIOKAR, France). The CFS, sterilized through a 0.20 μm filter, was added at 10% (v/v) to the PDA medium and inoculated in the center with agar discs (5 mm) of colonies of P. digitatum, A. niger, F. oxysporum, and R. oryzae. The plates, made in triplicate, were incubated at 25 °C for 5 days. PDA medium mixed with sterile distilled water (10%, v/v) was used as a control. Diameters (mm) of the colonies of molds were measured, and the percentage of mycelium growth inhibition (MI, %) was calculated using the formula: MI (%) = [(TC-TT)/TC] × 100, where TC is the total fungal colony diameter (mm) obtained with sterile distilled water (control), and TT is the total fungal colony diameter (mm) obtained with CFS.
Fungal biomass growth inhibition by the CFS
The inhibition of the fungal biomass growth was determined in MRS broth, based on the method of Muhialdin and Hassan [24], with some modifications. The sterile CFS was introduced at 10% (v/v) in a flask containing 50 mL of MRS broth (BIOKAR, France), and inoculated with 105 spores/mL of P. digitatum, A. niger, F. oxysporum, or R. oryzae. The cultures were incubated at 25 °C for 5 days. Then, the fungal biomass was collected with Whatman N°1 filter paper (Whatman International, Maidstone, England) and dried in an oven at 100 °C for 18 h. The average fungal biomass was calculated for each fungus tested and compared to the fungal biomass of controls grown in MRS broth. The percentage of biomass growth inhibition (BI, %) was calculated using the formula: BI (%) = [(TC-TT)/TC] × 100; where TC is the total fungal biomass (g) obtained without CFS (control), and TT is the total fungal biomass (g) obtained with CFS.
Minimum inhibitory concentration of the CFS
The minimum inhibitory concentration (MIC) of the CFS of L. plantarum S61 against the target strains was determined by using the liquid dilution in tubes method according to Russo, et al. [25], with some modifications. For this, serial dilutions (2%, 4%, 6%, 8%, 10%, 15%, 20%, 25%, and 30%) of the CFS were prepared in tubes containing liquid culture medium, previously sterilized at 121 °C for 15 min, and inoculated with 105 CFU/mL of R. glutinis or S. enterica subsp. enterica ATCC 14,028, and 105 spores/mL of P. digitatum. After incubation, at 37 °C for 24 h for bacteria and 25 °C for 2–5 days for fungi, the biomass was determined by measuring the optical density at 600 nm for bacteria and yeast strains using a spectrophotometer, compared to a control inoculated with target strains without CFS addition, and the biomass of P. digitatum was determined as described above. The MIC corresponds to the concentration of the CFS where no growth was observed. All the experiments were performed in triplicate.
Minimum bactericidal concentration and minimum fungicidal concentration of CFS
The minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) of the CFS of L. plantarum S61 were determined by pour plating 100 μL of the culture medium, of tubes showing no visible microbial growth (turbidity) (10%, 15%, 20%, 25%, and 30%), on PDA and MH agar for fungi and pathogenic bacteria, respectively. CFS concentrations where no growth of indicator strains was observed after incubation at 37 °C for 24 h for bacteria and 25 °C for 2–5 days for fungi were recorded as MBC and MFC, respectively. All the tests were performed in triplicate.
Determination of antimicrobial compounds from L. plantarum S61
Hydrogen peroxide, diacetyl, reuterin, and proteins determination
The hydrogen peroxide and diacetyl concentrations in the CFS were determined using colorimetric methods according to A.O.A.C. [26]. The protein concentration was determined by a colorimetric assay using the Bradford method Bradford [27], using bovine serum albumin (Sigma-Aldrich, USA) as standard. The reuterin content was determined using the colorimetric method of Circle, et al. [28], using the concentration of Acrolein (Aldrich) ranging from 0.05 to 6 mM prepared in an MRS broth medium, used as a standard solution. All the analyses were performed in triplicate.
HPLC analysis
The organic acids and ethanol were determined in CFS of L. plantarum S61 using a Waters HPLC system equipped with a 1525 binary pump, Breeze™ 2 Software and with both UV diode-array detector (Model 2998, Can) and refractive index (RI) detector (Model 2489), and Rezex-ROA-Organic acid H + column (7.8 mm × 30 cm, Phenomenex, Torrance, CA, USA) was used for the analysis. The elution was carried out at 0.6 mL min−1 using 0.005 N sulfuric acid at 40 °C. The CFS was filtered through a filter of 0.45 μm (Millipore, Bedford, MA, USA), then injected (20 μL) into the HPLC. Calibration curves were used to quantify lactic acid, acetic acid, citric acid, butyric acid, succinic acid, formic acid, oxalic acid, and ethanol. The analyses were conducted in triplicates.
Gas chromatography–mass spectrometry (GC–MS) analysis
The CFS of L. plantarum S61 was subject to extraction with an organic solvent, namely n-hexane 1:1 (v/v). The composition of n-hexane extract obtained from CFS of L. plantarum S61 was analyzed using Shimadzu GC–MS system (Kyoto, Japan) equipped with a fused-silica capillary column (5% phenyl methyl siloxane, 30 m × 0:25 mm, 0.25 μm film thickness) coupled with a mass spectrometer detector (GCMS-QP2010). An inlet temperature of 250 °C with a split ratio of 50 to 80 was employed. Helium was used as the carrier gas at a 1 mL/min constant flow rate. The sample was applied and maintained at 50 °C for 1 min. The temperature was then increased at a rate of 10 °C for a min to 250 °C, at which the temperature was held for 5 min. For MS detection, an electron impact ionization mode was used with ionization energy of 70 eV, ion source temperature of 200 °C, and the mass scan range of 40 to 350 Da. The components were identified based on a comparison of their relative retention time and fragmentation patterns of mass spectra compared to those reported in the literature and the library of the GC–MS system.
Purification of proteinaceous compounds from CFS
The CFS was precipitated by ammonium sulfate at 80% saturation and left in a refrigerator at 4 °C overnight. After centrifugation (10,000 × g, 10 min at 4 °C), the acquired precipitate was recovered and dissolved in phosphate-buffered saline (PBS, pH = 7.4), and then dialyzed using dialysis membranes with 2000, 6000–8000, and 12,000–14,000 MWCO (molecular weight cutoff) at 4 °C for overnight with changes of phosphate buffer (pH 6.8). The dialyzed fractions obtained were then evaluated, using the agar diffusion method, for their antifungal activity against C. pelliculosa. The active dialyzed fraction was purified by reverse-phase high-performance liquid chromatography (RP-HPLC, KNAUER, Germany). The active fraction (20 µL) was injected in a C18 (300 × 4.6 mm) reversed-phase semi-preparative column, which was pre-equilibrated with water containing 5% acetonitrile and 0.1% trifluoroacetic acid (TFA). The elution was performed at a flow rate of 0.6 mL/min using a linear gradient, namely A (99.9% water and 0.1% TFA) and B (99.9% acetonitrile and 0.1% TFA), as follows: from 0 to 5 min (90% A, 10% B), from 5 to 30 min (50% A, 50% B), from 30 to 35 min (20% A, 80% B), from 35 to 50 min (10% A, 90% B), and from 50 to 60 min (90% A, 10% B). The fractions were collected according to their absorbance at 280 nm and then evaluated for their antifungal activity against C. pelliculosa, using the agar diffusion method.
Stability of antifungal compounds from CFS
The stability of antifungal compounds at storage temperature was evaluated by maintaining the CFS at 4 °C and 25 °C for 4 weeks and measuring the antifungal activity against C. pelliculosa, using the well diffusion method. The test was performed in triplicate.
Applications of L. plantarum S61 and its CFS as biopreservative agents
Biopreservation of apple fruit
The L. plantarum S61 and its CFS were tested for their protective effect against P. digitatum on the apple fruit. The test was realized on apples purchased from a supermarket in Oujda, Morocco, using the wound inoculation method described by Crowley, et al. [29], with some modifications. The apple surface was disinfected with ethanol (95%), and exposed to UV for 20 min. After that, small wounds (3 mm wide × 5 mm deep) were made aseptically by pinching sterile paper pins onto apples. Fifty μL of the culture of L. plantarum S61 (107 CFU/mL) or its CFS were introduced into the wound, and then (after 15 min) inoculated with 50 μL of a spore suspension (105 spores/mL) of P. digitatum. The control was maintained using sterile distilled water instead of CFS and inoculated in the same conditions as the assays. The apples were incubated at room temperature (around 25 °C) for 15 days, and the growth of fungi was visually examined. All the treatments were performed in triplicate.
Biopreservation of orange fruit
The biopreservative effect of L. plantarum S61 was assessed on orange fruits purchased from a supermarket in Oujda, Morocco, using a spray method described by Wang, et al. [30], with some modifications. The fruit surface was disinfected with ethanol (95%), and exposed to UV for 20 min. Subsequently, the fruits were sprayed with 105 spores/mL of P. digitatum, and after drying, the fruits were sprayed with 107 CFU/mL of L. plantarum S61 or its CFS. The control was set up using sterile distilled water and then inoculated with P. digitatum in the same conditions as the assays. The fruits were incubated at room temperature (around 25 °C) for 15 days, and the growth of fungi was visually examined. All the treatments were performed in triplicate.
Biopreservation of apple juice
Some apple fruits (700 g), purchased from a local fruit supermarket (Oujda, Morocco), were washed with distilled water, dried with paper, and then crushed in a blender. The apple juice obtained was filtered using Whatman no. 1 filter paper and stored at − 20 °C until use. The storage apple juice (100 mL) with the initial pH of 4.2 was pasteurized at 80 °C for 15 min and then cooled at room temperature (around 25 °C). The MIC (10%, v/v) of sterile CFS of L. plantarum S61 was added to the juice, and then it was inoculated with a concentration of 5 log CFU/mL of the indicator strain (R. glutinis and Salmonella enterica subsp. enterica ATCC 14,028). Apple juices inoculated with indicator strains and not containing the CFS were used as controls. The incubation was done at 25 °C for 48 h for R. glutinis and 37 °C for 48 h for Salmonella enterica subsp. enterica ATCC 14,028. The count level of the indicator strains was determined by pour plating 0.1 mL of serial dilutions, realized in sterile peptone water, on Potato Dextrose Agar (PDA) for R. glutinis and Salmonella-Shigella (SS) Agar (Oxoid, UK) for Salmonella enterica subsp. enterica ATCC 14,028. After 48 h of incubation at 25 °C for R. glutinis and 37 °C for Salmonella, the target colony-forming units (CFU/mL) were recorded. All the experiments were performed in triplicate.
Statistical analysis
The results obtained were presented as means ± standard deviation. The one-way ANOVA analysis was used to compare the means with a significant difference of p < 0.05. The Student–Newman–Keuls (S–N-K) comparison post hoc test was used to identify the groups of means. The analyses were carried out using GraphPad Prism 8 software (San Diego, California USA).
Results
Antifungal and antibacterial activity of L. plantarum S61
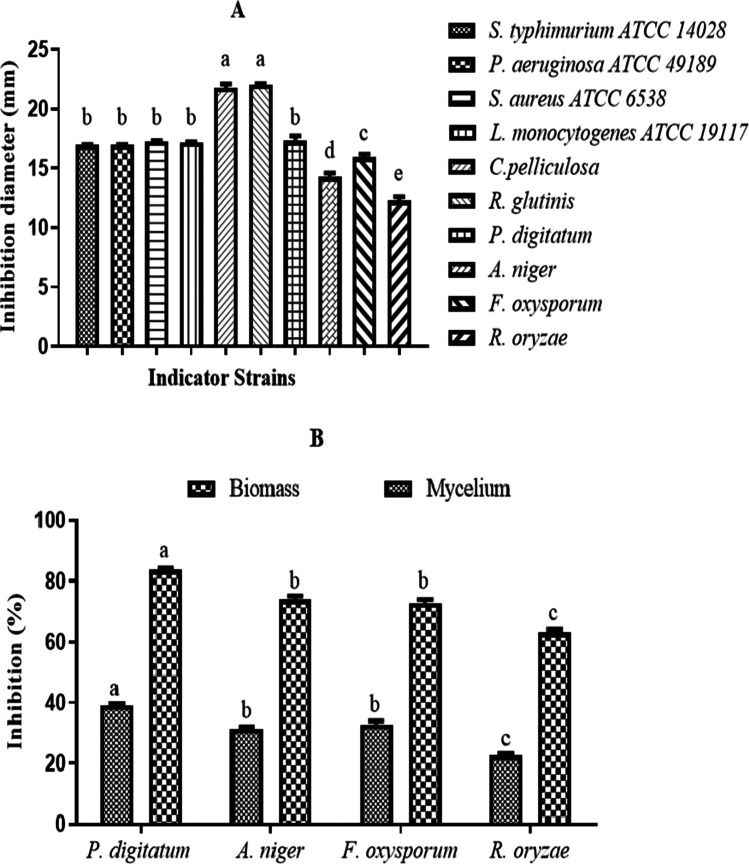
The L. plantarum S61 was examined for its antifungal and antibacterial activity by overlay method, and the results are reported in Fig. 1a. The culture of probiotic L. plantarum S61 demonstrated high inhibition zones (˃ 10 mm) against all target strains, including molds (P. digitatum, A. niger, F. oxysporum, R. oryzae), yeasts (C. pelliculosa, R. glutinis), and Gram-negative and Gram-positive pathogenic bacteria (S. enterica subsp. enterica ATCC 14,028, P. aeruginosa ATCC 49,189, S. aureus subsp. aureus ATCC 6538, L. monocytogenes ATCC 19,117).
Fig. 1.
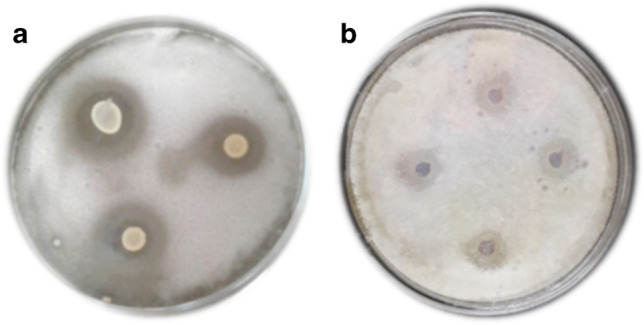
Antifungal activity of culture of L. plantarum S61 (a) and its CFS (b) against P. digitatum
Antifungal activity of CFS
The results of the antifungal activity of CFS of L. plantarum S61 are indicated in Figs. 1b and 2A. The CFS showed antifungal activity with inhibition diameter ranges of 12.25 ± 0.38–17.3 ± 0.42 mm and 21.75 ± 0.35–22.05 ± 0.07 mm against molds (A. niger, P. digitatum, F. oxysporum, Rhizopus sp.) and yeasts (C. pelliculosa, R. glutinis), respectively. The inhibition diameter values obtained against yeasts C. pelliculosa and R. glutinis were significantly (p < 0.05) higher, followed by P. digitatum, F. oxysporum, A. niger, and finally R. oryzae.
Fig. 2.
Antifungal and antibacterial activity (A) and inhibition of mold mycelium and biomass growth (B) of CFS of L. plantarum S61. Values are mean ± standard error of triplicates..a–eMeans in indicator strains with different lowercase letters differed significantly (p < 0.05)
The inhibitory effects of the CFS of L. plantarum S61 against mycelium and biomass growth of fungi are presented in Fig. 2B. The biomass growth inhibition values obtained were 83.64 ± 0.66%, 73.97 ± 0.1%, 72.82 ± 1.63%, and 63.18 ± 0.9%, and the mycelium growth inhibitions values were 38.93 ± 0.66%, 31.25 ± 0.98%, 32.73 ± 1.48%, and 22.57 ± 0.7%, respectively, against P. digitatum, A. niger, F. oxysporum, and R. oryzae. P. digitatum showed the highest significant (p < 0.05) inhibition values, while R. oryzae demonstrated the lowest significant (p < 0.05) inhibition values both for mycelium and biomass growth.
Antibacterial activity of CFS
The antibacterial activity of the CFS of L. plantarum S61 was evaluated by a well diffusion assay against pathogenic bacteria, and the results are reported in Fig. 2A. The results show high antibacterial activity, with inhibition diameter ranges of 17.15 ± 0.21–17.25 ± 0.7 mm and 16.14 ± 1.14–16.95 ± 0.07 mm against Gram-positive bacteria (L. monocytogenes ATCC 19,117 and S. aureus subsp. aureus ATCC 6538) and Gram-negative bacteria (S. enterica subsp. enterica ATCC 14,028 and P. aeruginosa ATCC 49,189), respectively. No significant (p < 0.05) difference was observed between inhibition diameters of all target bacteria.
MIC, MBC, and MFC of the CFS from L. plantarum S61
The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) of the CFS from L. plantarum S61, against the most sensitive spoilage and pathogenic microorganisms (R. glutinis., P. digitatum, and S. enterica subsp. enterica ATCC 14,028), were determined using a liquid culture medium (data not shown). The MIC values obtained were 15% against P. digitatum and 10% against R. glutinis and S. enterica subsp. enterica ATCC 14,028. The MBC value obtained against S. enterica subsp. enterica ATCC 14,028 was 20%; while the MFC values were 25% and 20%, respectively, against P. digitatum and R. glutinis. The MIC, MBC, and MFC values obtained against S. enterica subsp. enterica ATCC 14,028 and R. glutinis were lower than the values obtained against P. digitatum. These results demonstrate that R. glutinis and S. enterica subsp. enterica ATCC 14,028 were more sensitive to the CFS of L. plantarum S61 than P. digitatum.
Determination of antimicrobial compounds of CFS
The results of HPLC determination of organic acids and ethanol in CFS of L. plantarum S61 demonstrated that the compounds detected were lactic acid, acetic acid, citric acid, oxalic acid, and ethanol, with 240.18 mM, 13.66 mM, 1.44 mM, 9.66 mM, and 21.05 mM, respectively. Lactic acid was the major compound in this mixture.
On the other hand, the hydrogen peroxide, diacetyl, reuterin, and proteins were determined in CFS. The hydrogen peroxide, diacetyl, and proteins were 0.0002 µM, 0.0021 µM, and 198.65 µg/mL, respectively. Important content of proteins was obtained. However, hydrogen peroxide and diacetyl were detected in small quantities, while reuterin was not detected.
Gas chromatography–mass spectrometry (GC–MS) analysis of CFS
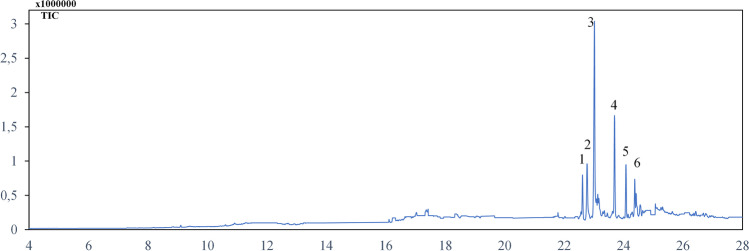
The fatty acids profile of the n-hexane fraction of CFS of L. plantarum S61 was determined by GC–MS analysis, and the results are reported in Fig. 3. The analysis permitted the identification of 6 fatty acids of C16 and C18 carbon chain lengths. They are composed of saturated (palmitic acid (C16:0) and stearic acid (C18:0)), mono-unsaturated (11-hexadecanoic acid (C16:1), 9-octadecenoic acid (C18:1), and oleic acid (C18:1)), and di-unsaturated (linoleic acid (C18:2)) fatty acids. The sequential order of major fatty acids was palmitic acid (47.25%) at 23.012 min, linoleic acid (19.35%) at 23.78 min, and oleic acid (10.43%) at 24.075 min. The other fatty acids, detected at lower levels, included 11-hexadecanoic acid (6.26%), 9-octadecenoic acid (8.78%), and stearic acid (2.99%).
Fig. 3.
GC–MS analysis of n-hexane fraction of L. plantarum S61 showing fatty acids profile. (1) 11-Hexadecanoic acid (C16:1) (22.647 min); (2) 9-octadecenoic acid (C18:1) (22.767 min); (3) palmitic acid (C16:0) (23.012 min); (4) linoleic acid (C18:2) (23.78 min); (5) oleic acid (C18:1) (24.075 min); (6) stearic acid (C18:0) (24.383 min)
Stability of antifungal compounds from CFS
The effect of storage time at 25 °C and 4 °C on the antifungal activity of the CFS of L. plantarum S61 against C. pelliculosa was evaluated, and the results are reported in Table 1. The inhibition diameter values obtained during 4 weeks of storage of the CFS at 25 °C varied, but not significantly (p < 0.05), between 20.63 and 20.1 mm. Differently, during storage at 4 °C, the inhibition diameter values decreased significantly (p < 0.05) from the first week (20.63 mm) to the 4th week (19.2 mm).
Table 1.
Effect of storage time at 25 °C and 4 °C on antifungal compounds from L. plantarum S61
| Storage time | Inhibition diameter (mm) | |
|---|---|---|
| 25 °C | 4 °C | |
| Week 1 | 20.63a ± 0.50 | 20.63a ± 0.52 |
| Week 2 | 20.6a ± 0.42 | 20.3a ± 0.14 |
| Week 3 | 20.5a ± 0.28 | 19.8b ± 0.28 |
| Week 4 | 20.1a ± 0.07 | 19.2c ± 0.14 |
Values are mean ± standard error of triplicates
a,b,cMeans in same column with different lower case letters differed significantly (p < 0.05)
Purification of antifungal compounds from CFS
The CFS of L. plantarum S61 was precipitated with ammonium sulfate at 80% saturation followed by dialysis. The precipitated and dialyzed fractions were tested for their antifungal activity against C. pelliculosa. The results obtained showed the antifungal activity of the precipitated and the dialyzed fraction of 2 KDa MWCO, with inhibition diameters of 14.25 mm and 13 mm, respectively. However, no antifungal activity was obtained with dialyzed fractions of 6–8 KDa and 12–14 KDa.
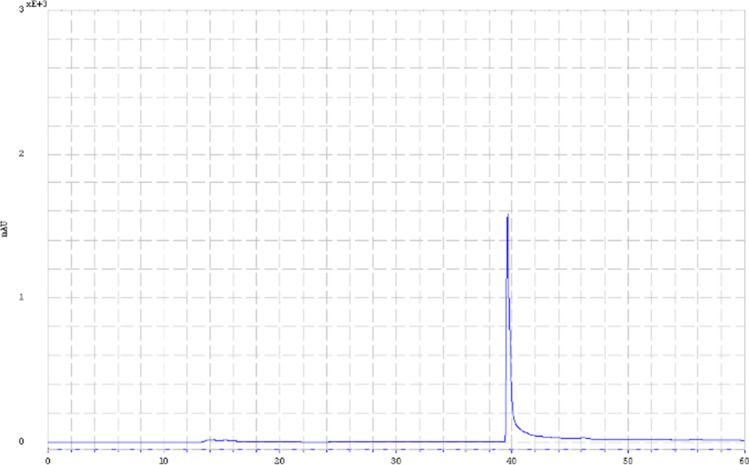
The active 2 KDa dialyzed fraction was further purified using RP-HPLC, and a major peak was observed at 39.65 min (Fig. 4), indicating the high purity of the dialyzed fraction. In addition, the fraction obtained from semi-preparative RP-HPLC exhibited antifungal activity against C. pelliculosa, with an inhibition diameter of 12.65 mm.
Fig. 4.
RP-HPLC chromatogram of the dialyzed fraction (MWCO 2KDa) of CFS of L. plantarum S61
Biopreservation of apple and orange fruits by L. plantarum S61
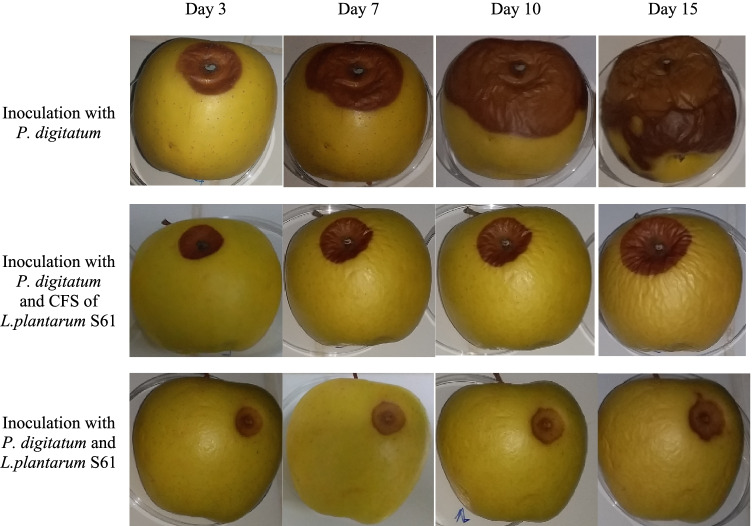
The results of the antifungal activity of L. plantarum S61 and its CFS on apple fruits are shown in Fig. 5. The apple fruits were treated with L. plantarum S61 and its CFS and then inoculated with P. digitatum, while the control was inoculated with P. digitatum only. In the control, the attack on apples by P. digitatum was visible from the 3rd day of incubation, and on the 15th day, the apple fruit was totally infected. However, the apple fruits treated with the CFS showed a delay and lower attack by P. digitatum. The apples inoculated with L. plantarum S61 showed a strong inhibition of P. digitatum, associated with a limited zone of spoilage up to 15 days of the incubation at room temperature (around 25 °C).
Fig. 5.
Biopreservative effect of L. plantarum S61 and its CFS against P. digitatum on apple fruit
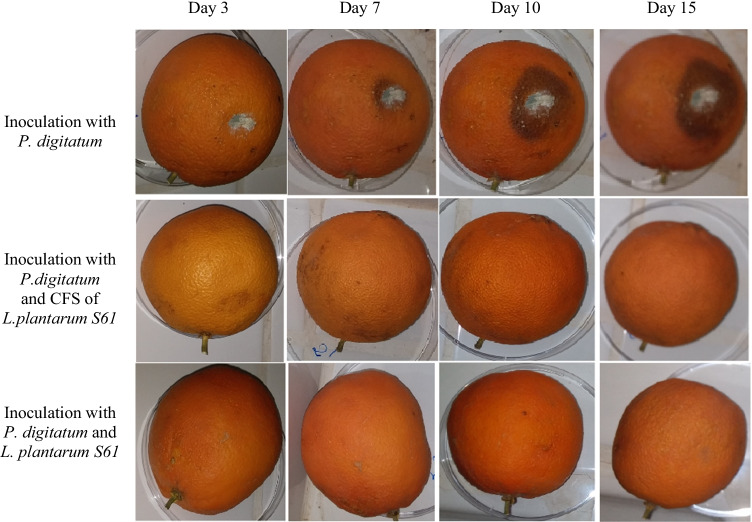
The biopreservative effect of L. plantarum S61 and its CFS on orange fruits was evaluated, and the results are reported in Fig. 6. In the absence of L. plantarum S61 and its CFS (control), the attack of orange fruits by P. digitatum was visible from the 3rd day of incubation, and the surface spoilage of fruits increased substantially on the 15th day of incubation. However, in the presence of L. plantarum S61 and its CFS, total inhibition of P. digitatum was observed on the surface of orange fruits after 15 days of incubation at room temperature (around 25 °C).
Fig. 6.
Biopreservative effect of L. plantarum S61 and its CFS against P. digitatum on orange fruit
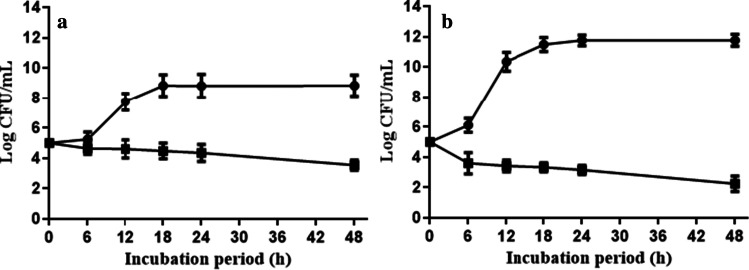
Biopreservation of apple juice by CFS of L. plantarum S61
The effect of CFS from L. plantarum S61 as a biocontrol agent of apple juice against S. enterica subsp. enterica ATCC 14,028 and R. glutinis was evaluated, and the results are reported in Fig. 7. At the MIC value (10%, v/v) obtained against the target strains, the CFS led during 48 h of incubation to an important reduction of the biomass of R. glutinis and S. enterica subsp. enterica ATCC 14,028, from 5 to 2.84 log CFU/mL and from 5 to 3.53 log/mL, respectively. As expected, in the control samples (without CFS addition), the biomass of the target strains increased from 5 to 11.75 log CFU/mL and from 5 to 8.83 log CFU/mL for R. glutinis and S. enterica subsp. enterica ATCC 14,028, respectively. Compared to the control, the biomass of spoilage microorganisms (R. glutinis and S. enterica subsp. enterica ATCC 14,028) was significantly (p < 0.05) reduced in apple juice treated with CFS at 10% (v/v).
Fig. 7.
Biopreservation of apple juice with addition of 10% CFS of L. plantarum S61 (
 ) and without CFS (
) and without CFS (
 ), against S. enterica subsp. enterica ATCC 14,028 (a) and R. glutinis (b)
), against S. enterica subsp. enterica ATCC 14,028 (a) and R. glutinis (b)
Discussion
In this study, L. plantarum S61 and its CFS showed a high and wide inhibitory effect against bacteria, yeasts, and molds used as targets. These results confirmed the results obtained by previous studies [11, 31, 32]. The antibacterial and antifungal activity values obtained with CFS were lower than that obtained with its microbial culture, indicating the continuous release of antimicrobial metabolites in the extracellular compartment by L. plantarum S61. These properties indicate that L. plantarum S61 is an excellent candidate to be studied for food biopreservation purposes.
The inhibition values obtained, against Gram-positive and Gram-negative bacteria, were important and not significantly different. This important and non-selective inhibitory effect should be due to the mixture of antimicrobial compounds in CFS, including organic acids, ethanol, hydrogen peroxide, diacetyl, fatty acids, and proteins highlighted in this work. Stanojević‐Nikolić et al. [33] demonstrated that lactic acid and acetic acid inhibition was higher against pathogenic bacteria (Gram-positive and Gram-negative) more than yeasts (R. glutinis and C. albicans). It was demonstrated that lactic acid, dominating the organic acids in CFS of L. plantarum S61, has a strong and wide inhibitory effect against Gram-positive and Gram-negative bacteria. Consequently, other compounds than organic acids may be responsible for the high antifungal activity against fungi.
The CFS of L. plantarum S61 exerted an important antifungal activity, with inhibition diameter values against yeasts higher than that obtained against molds. Among bacteria and fungi tested as targets, C. pelliculosa and R. glutinis showed the highest susceptibility to the CFS of L. plantarum S61, which may be due to the synergistic effect between multiple compounds in the mixture in CFS. These targets (C. pelliculosa and R. glutinis) strains are reported as emerging pathogens involved in fungemia, particularly for immunocompromised patients [34, 35]. These findings indicate that the probiotic L. plantarum S61 can be used to develop therapeutic agents.
The CFS demonstrated variable inhibition values of mold biomass and mycelium growth, and the highest inhibition values were obtained against P. digitatum. Mold species of Aspergillus, Penicillium, and Fusarium are considered the primary cause of post-harvest spoilage and toxins/food-borne diseases [3]. Moreover, the studies of Deepthi et al. [36] and Gerez et al. [37] demonstrated the ability of CFS from L. plantarum to inhibit the biomass and mycelium growth of fungi. Thus, the bioactive compounds of the CFS of L. plantarum S61 could be used to control post-harvest spoilages of fungi origin, particularly those related to P. digitatum. This funding indicates the possibility of using this strain and its bioactive compounds as a biopesticide.
Various molecules were determined in the CFS of L. plantarum S61. LAB are known for their production capacity of different compounds involved in their antimicrobial activity [38, 39]. Lactic acid was the major organic acid present in CFS, but other organic acids, ethanol, hydrogen peroxide, and diacetyl were detected even in lower amounts. The hydrogen peroxide, diacetyl, and ethanol were demonstrated for their higher inhibitory effect against bacteria than fungi [13]. The important antifungal activity of CFS of L. plantarum S61 obtained against R. glutinis and C. pelliculosa may be due to the synergistic effect between these compounds and antifungal proteins and peptides.
The proteinaceous nature of antifungal compounds from L. plantarum S61 was demonstrated in previous work by the disappearance of antifungal activity in CFS of L. plantarum S61 after the treatment with proteinase K [20]. In the present work, the important content of proteins (198.65 µg mL−1) was determined in the CFS of this strain, and its 2 KDa dialyzed fraction, purified by RP-HPLC, showed antifungal activity against C. pelliculosa, while no antifungal activity was obtained with the other fractions (6–8KDa and 12–14 KDa). These findings indicate that the molecular weight of proteinaceous antifungal compounds from L. plantarum S61 is between 2 and 6 kDa. Higher antifungal activity was previously reported in peptides with low molecular weight (< 10KDa) than that of high molecular weight (> 10KDa) [40], confirming the essential inhibitory effect of the proteinaceous compounds of L. plantarum S61 obtained in this work.
The GC–MS analysis of CFS demonstrated the capacity of L. plantarum S61 to produce fatty acids (di-, mono- and unsaturated) with a chain length of C16 and C18 dominated by palmitic acid. Palmitic acid has higher antifungal activity than unsaturated fatty acids (i.e., oleic and linoleic acids) [41]. The fatty acids (i.e., linoleic acid) are known for their important pharmacological activities, such as anticarcinogenic, anti-obese, antidiabetic, and antihypertensive properties [42]. This finding indicates the possible use of this strain in the production and formulation of health benefits of food products, rich in linoleic acid.
This work demonstrates the capacity of L. plantarum S61 to produce several antimicrobial compounds of hydrophobic (fatty acids, diacetyl), hydrophilic (organic acids, hydrogen peroxide), and hydrophilic/hydrophobic (proteins) nature. The high antimicrobial activity, obtained with the mixture of these different compounds in CFS, may be achieved by the presence of biosurfactants, demonstrated in previous work in L. plantarum S61 [19]. Biosurfactants are amphiphilic molecules allowing the contact between target cells and water-immiscible compounds, and those of lactic acid bacteria are known for their non-toxicity and antimicrobial activity [43–46].
The antifungal compounds from L. plantarum S61 showed their stability at 25 °C for 4 weeks; however, their activity was significantly reduced from the third week at 4 °C onwards, which may be due to the destabilization of the emulsion during storage of CFS. The emulsion destabilization at low temperatures, leading to the separation of aqueous and lipid phases, was reported in foods [47]. The separation of phases in CFS, during storage at 4 °C, should prevent the contact between microbial cells and lipophilic antimicrobials.
The biopreservative effect of L. plantarum S61 and its CFS was further evaluated on apple and orange fruit models against P. digitatum at room temperature (around 25 °C) for 15 days. A strong inhibitory effect of L. plantarum S61 was obtained against P. digitatum on orange fruits, and it was higher than that obtained on apple fruits. This may be due to the synergistic effect of antifungal essential oils of orange peel with antifungal metabolites of the CFS of L. plantarum S61. This study confirms the essential antifungal activity of L. plantarum S61 against fungal attack due to P. digitatum in the apple and pear fruits, and tomato puree, as evaluated in previous works [20]. Moreover, the LAB strains presented a protective effect against spoilage molds in different food models [16, 29, 48]. These findings demonstrate the possibility of using the probiotic L. plantarum S61 and/or its CFS as bioprotective agents in controlling post-harvest spoilage of fruits and vegetables, mainly of fungal origin.
The CFS of L. plantarum S61 demonstrated its effectiveness in reducing the microbial load in contaminated apple juice by R. glutinis and S. enterica subsp. enterica ATCC 14,028, which are reported to be involved in fruit juice spoilages [2]. These results indicate the possible use of CFS of L. plantarum S61 as a decontaminating agent in foods. Lactic acid bacteria (LAB) and their bioactive compounds can exert a broad antibacterial and antifungal activity spectrum; thus, they are important candidates for preserving fruits against pathogens and spoilage microorganisms [8, 49]. Walker and Phillips [50] demonstrated that the addition of bacteriocins inhibited the growth of Alicyclobacillus acidoterrestris spores in apple, grape, and orange juices. Also, LAB strains can remove up to 80% of patulin added to apple juice, without altering the quality of juice [51]. The concentration of CFS used in this study (10% equal to the MIC), although high, was effective in counteracting microbial growth. It, therefore, encourages further investigation aimed at the identification and subsequent concentration of the most active compounds.
In conclusion, the probiotic L. plantarum S61, isolated from naturally fermented olive brine (extreme environment), and its CFS possess high and wide antifungal and antibacterial activity against fungi and pathogenic bacteria. L. plantarum S61 produces serval antibacterial and antifungal compounds, and the molecular weight of active antifungal proteinaceous compounds was found to be between 2 and 6 kDa. L. plantarum S61 and its CFS showed their bioprotective effect against spoilage dues to P. digitatum on apples and oranges and their decontaminating capability against R. glutinis and S. enterica subsp. enterica ATCC 14,028 in apple juice. Further investigation is needed to identify all active compounds produced by L. plantarum S61 and the culture conditions that allow maximum production of these compounds. The best way to apply the bacterium or its CFS or the active fraction should also be investigated, considering the treatment’s cost.
Author contribution
HA and AA selected the study design. HA, IH, YR, RB, and SG conducted the experiments. HA and AA wrote the manuscript. SK, MB, GD, RBS, ES, NG, and AA performed review and editing. All authors read and approved the manuscript.
Funding
The CNRST (PPR/19/2015), CNRST (Morocco)/CNRi (Italy) cooperation and McGill University provided financial support.
Data availability
All data generated or analyzed during this study are included in this article.
Declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO (2019) The state of food and agriculture: moving forward on food loss and waste reduction.http://www.fao.org/3/ca6030en/ca6030en.pdf. Accessed 20 March 2020
- 2.Pandey, A. and P.S. Negi (2018) Use of natural preservatives for shelf life extension of fruit juices. In: (ed) Fruit Juices Academic Press, pp 571–605
- 3.Moss MO. Fungi, quality and safety issues in fresh fruits and vegetables. J Appl Microbiol. 2008;104:1239–1243. doi: 10.1111/j.1365-2672.2007.03705.x. [DOI] [PubMed] [Google Scholar]
- 4.Salomão BdCM (2018) Pathogens and spoilage microorganisms in fruit juice. In: (ed) Fruit Juices, Academic Press, pp 291–308
- 5.Schnürer J, Magnusson J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol. 2005;16:70–78. doi: 10.1016/j.tifs.2004.02.014. [DOI] [Google Scholar]
- 6.Leyva Salas M, Mounier J, Valence F, Coton M, Thierry A, Coton E (2017) Antifungal microbial agents for food biopreservation-a review. Microorganisms 5. 10.3390/microorganisms5030037 [DOI] [PMC free article] [PubMed]
- 7.Tropcheva R, Nikolova D, Evstatieva Y, Danova S. Antifungal activity and identification of Lactobacilli, isolated from traditional dairy product “katak”. Anaerobe. 2014;28:78–84. doi: 10.1016/j.anaerobe.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Sadiq FA, Yan B, Tian F, Zhao J, Zhang H, Chen W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: a comprehensive review. Compr Rev Food Sci Food Saf. 2019;18:1403–1436. doi: 10.1111/1541-4337.12481. [DOI] [PubMed] [Google Scholar]
- 9.EFSA The 2019 updated list of QPS status recommended biological agents in support of EFSA risk assessments. EFSA J. 2020;18:5966. [Google Scholar]
- 10.Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria – potential for control of mould growth and mycotoxins: a review. Food Control. 2010;21:370–380. doi: 10.1016/j.foodcont.2009.07.011. [DOI] [Google Scholar]
- 11.Magnusson J, Schnurer J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol. 2001;67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niku-Paavola M-L, Laitila A, Mattila-Sandholm T, Haikara A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol. 1999;86:29–35. doi: 10.1046/j.1365-2672.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 13.Siedler S, Balti R, Neves AR. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr Opin Biotechnol. 2019;56:138–146. doi: 10.1016/j.copbio.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Sjogren J, Magnusson J, Broberg A, Schnurer J, Kenne L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl Environ Microbiol. 2003;69:7554–7557. doi: 10.1128/aem.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strom K, Sjogren J, Broberg A, Schnurer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phetrans-4-OH-L-Pro) and 3-phenyllactic acid. Appl Environ Microbiol. 2002;68:4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trias R, Baneras L, Montesinos E, Badosa E. Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int Microbiol. 2008;11:231–236. doi: 10.2436/20.1501.01.66. [DOI] [PubMed] [Google Scholar]
- 17.Mora-Villalobos JA, Montero-Zamora J, Barboza N, Rojas-Garbanzo C, Usaga J, Redondo-Solano M, Schroedter L, Olszewska-Widdrat A, López-Gómez JP. Multi-product lactic acid bacteria fermentations: a review. Fermentation. 2020;6:23. doi: 10.3390/fermentation6010023. [DOI] [Google Scholar]
- 18.Arena MP, Silvain A, Normanno G, Grieco F, Drider D, Spano G, Fiocco D. Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front Microbiol. 2016;7:464. doi: 10.3389/fmicb.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abouloifa H, Rokni Y, Bellaouchi R, Ghabbour N, Karboune S, Brasca M, Salah RB, Chihib NE, Saalaoui E, Asehraou A. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob Proteins. 2020;12:683–696. doi: 10.1007/s12602-019-09543-8. [DOI] [PubMed] [Google Scholar]
- 20.Abouloifa H, Gaamouche S, Rokni Y, Hasnaoui I, Bellaouchi R, Ghabbour N, Karboune S, Brasca M, D'Hallewin G, Ben Salah R, Saalaoui E, Asehraou A. Antifungal activity of probiotic Lactobacillus strains isolated from natural fermented green olives and their application as food bio-preservative. Biol Control. 2021;152:104450. doi: 10.1016/j.biocontrol.2020.104450. [DOI] [Google Scholar]
- 21.Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P, O'Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Ganzle MG, Lebeer S. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 22.Cavicchioli VQ, Camargo AC, Todorov SD, Nero LA. Novel bacteriocinogenic Enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J Dairy Sci. 2017;100:2526–2535. doi: 10.3168/jds.2016-12049. [DOI] [PubMed] [Google Scholar]
- 23.Gharbi Y, Fhoula I, Ruas-Madiedo P, Afef N, Boudabous A, Gueimonde M, Ouzari H-I. In-vitro characterization of potentially probiotic Lactobacillus strains isolated from human microbiota: interaction with pathogenic bacteria and the enteric cell line HT29. Ann Microbiol. 2018;69:61–72. doi: 10.1007/s13213-018-1396-1. [DOI] [Google Scholar]
- 24.Muhialdin BJ, Hassan Z. Screening of lactic acid bacteria for antifungal activity against Aspergillus oryzae. Am J Appl Sci. 2011;8:447–451. doi: 10.3844/ajassp.2011.447.451. [DOI] [Google Scholar]
- 25.Russo P, Arena MP, Fiocco D, Capozzi V, Drider D, Spano G. Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. Int J Food Microbiol. 2017;247:48–54. doi: 10.1016/j.ijfoodmicro.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 26.A.O.A.C. (2000) Official Methods of Analysis. The Association of Official Analytical Chemists, Gaithersburg, MD, USA. 17th Edition
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Circle SJ, Stone L, Boruff C. Acrolein determination by means of tryptophane. A colorimetric micromethod. Ind Eng Chem Anal Ed. 1945;17:259–262. doi: 10.1021/i560140a021. [DOI] [Google Scholar]
- 29.Crowley S, Mahony J, van Sinderen D. Broad-spectrum antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol. 2013;58:291–299. doi: 10.1007/s12223-012-0209-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Sun Y, Chen C, Sun Z, Zhou Y, Shen F, Zhang H, Dai Y. Genome shuffling of Lactobacillus plantarum for improving antifungal activity. Food Control. 2013;32:341–347. doi: 10.1016/j.foodcont.2012.12.020. [DOI] [Google Scholar]
- 31.Nayyeri N, Edalatian Dovom MR, Habibi Najafi MB, Bahreini M. A preliminary study on antifungal activity of lactic acid bacteria isolated from different production stages of Lighvan cheese on Penicillium expansum and Rhodotorula mucilaginosa. J Food Meas Charact. 2017;11:1734–1744. doi: 10.1007/s11694-017-9554-x. [DOI] [Google Scholar]
- 32.Nallala V, Sadishkumar V, Jeevaratnam K. Molecular characterization of antimicrobial Lactobacillus isolates and evaluation of their probiotic characteristics in vitro for use in poultry. Food Biotechnol. 2017;31:20–41. doi: 10.1080/08905436.2016.1269289. [DOI] [Google Scholar]
- 33.Stanojević-Nikolić S, Dimić G, Mojović L, Pejin J, Djukić-Vuković A, Kocić-Tanackov S. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J Food Process Preserv. 2016;40:990–998. doi: 10.1111/jfpp.12679. [DOI] [Google Scholar]
- 34.Hof H (2019) Rhodotorula spp. in the gut - foe or friend? GMS Infect Dis 7:Doc02. 10.3205/id000042 [DOI] [PMC free article] [PubMed]
- 35.Lin HC, Lin HY, Su BH, Ho MW, Ho CM, Lee CY, Lin MH, Hsieh HY, Lin HC, Li TC, Hwang KP, Lu JJ. Reporting an outbreak of Candida pelliculosa fungemia in a neonatal intensive care unit. J Microbiol Immunol Infect. 2013;46:456–462. doi: 10.1016/j.jmii.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Deepthi BV, Poornachandra Rao K, Chennapa G, Naik MK, Chandrashekara KT, Sreenivasa MY. Antifungal attributes of Lactobacillus plantarum mys6 against fumonisin producing Fusarium proliferatum associated with poultry feeds. PLoS ONE. 2016;11:e0155122. doi: 10.1371/journal.pone.0155122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerez CL, Torino MI, Rollán G, Font de Valdez G. Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control. 2009;20:144–148. doi: 10.1016/j.foodcont.2008.03.005. [DOI] [Google Scholar]
- 38.Le Lay C, Coton E, Le Blay G, Chobert JM, Haertle T, Choiset Y, Van Long NN, Meslet-Cladiere L, Mounier J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int J Food Microbiol. 2016;239:79–85. doi: 10.1016/j.ijfoodmicro.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Salomskiene J, Jonkuviene D, Macioniene I, Abraitiene A, Zeime J, Repeckiene J, Vaiciulyte-Funk L. Differences in the occurence and efficiency of antimicrobial compounds produced by lactic acid bacteria. Eur Food Res Technol. 2019;245:569–579. doi: 10.1007/s00217-018-03227-3. [DOI] [Google Scholar]
- 40.Muhialdin BJ, Hassan Z, Saari N. In vitro antifungal activity of lactic acid bacteria low molecular peptides against spoilage fungi of bakery products. Ann Microbiol. 2018;68:557–567. doi: 10.1007/s13213-018-1363-x. [DOI] [Google Scholar]
- 41.Liu S, Ruan W, Li J, Xu H, Wang J, Gao Y, Wang J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia. 2008;166:93–102. doi: 10.1007/s11046-008-9124-1. [DOI] [PubMed] [Google Scholar]
- 42.Koba K, Yanagita T. Health benefits of conjugated linoleic acid (CLA) Obes Res Clin Pract. 2014;8:e525–e532. doi: 10.1016/j.orcp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues L, Banat IM, Teixeira J, Oliveira R. Biosurfactants: potential applications in medicine. J Antimicrob Chemother. 2006;57:609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 44.Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci. 2016;17:401. doi: 10.3390/ijms17030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma D, Saharan BS. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol Rep. 2016;11:27–35. doi: 10.1016/j.btre.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma D, Saharan BS, Shailly K (2016) Biosurfactants of lactic acid bacteria. Springer Switzerland
- 47.Degner BM, Chung C, Schlegel V, Hutkins R, McClements DJ. Factors influencing the freeze-thaw stability of emulsion-based foods. Compr Rev Food Sci Food Saf. 2014;13:98–113. doi: 10.1111/1541-4337.12050. [DOI] [PubMed] [Google Scholar]
- 48.Rouse S, Harnett D, Vaughan A, van Sinderen D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J Appl Microbiol. 2008;104:915–923. doi: 10.1111/j.1365-2672.2007.03619.x. [DOI] [PubMed] [Google Scholar]
- 49.Barbosa AAT, Mantovani HC, Jain S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit Rev Biotechnol. 2017;37:852–864. doi: 10.1080/07388551.2016.1262323. [DOI] [PubMed] [Google Scholar]
- 50.Walker M, Phillips CA. The effect of preservatives on Alicyclobacillus acidoterrestris and Propionibacterium cyclohexanicum in fruit juice. Food Control. 2008;19:974–981. doi: 10.1016/j.foodcont.2007.10.003. [DOI] [Google Scholar]
- 51.Hatab S, Yue T, Mohamad O. Removal of patulin from apple juice using inactivated lactic acid bacteria. J Appl Microbiol. 2012;112:892–899. doi: 10.1111/j.1365-2672.2012.05279.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.