Abstract
Fusariosis has presented a significant increase in their incidence in the last years. This epidemiological panorama probably is due to the increasing profile of refractory susceptibility of Fusarium spp. to available drugs, especially in immunocompromised individuals. Thus, the development of new compounds with effectiveness on these organisms is a necessity. This study evaluated the antifungal potential of a chloroacetamide derivative (4-BFCA) against resistant Fusarium strains. As a result, the compound was effective against all strains (MIC range 12.5–50 μg/mL). The time kill assay demonstrated that 4-BFCA presents a concentration-dependent fungicidal action. Although its action mechanism has not yet been elucidated, it was possible to observe its efficacy through damages and alterations provoked along the hyphae of Fusarium spp. 4-BFCA maintained a high survival rate of Tenebrio molitor larvae, suggesting that it does not cause acute systemic toxicity on this host at the concentration evaluated. In addition, 4-BFCA was 83.33% effective in combating a fungal infection in vivo on the chorioallantoid membrane of embryonated eggs. Our results are very promising and arouse interest to investigate the action of 4-BFCA on Fusarium strains since it acts as a possible candidate for the development of new therapies for the treatment of fusariosis.
Keywords: Fusarium, Antifungal resistance, Chloroacetamide derivatives, Antifungal therapy
Introduction
Fusarium spp. are organisms saprophytic and ubiquitous considered opportunistic pathogens, capable to cause infections in a human known as fusariosis [1–3]. In recent years, the incidences of these infections have increased considerably, since these fungi represent the second most common cause of infections due to filamentous fungi in humans, after only Aspergillus spp. [4–9]. Fusariosis can be localized, with clinical manifestations in the skin, nails (onychomycosis), and eyes (keratitis or endophthalmitis)—mainly related to healthy individuals—or invasive and disseminated—mainly reported in immunocompromised individuals [10].
Among the main species related to fusariosis, Fusarium solani appears as most prevalent, followed by F. oxysporum and F. verticillioides [11]. Cases of multidrug resistance are the main problem related to Fusarium species, since fusariosis is usually refractory to the antifungal therapy available, especially in the cases related to immunocompromised patients [7, 12]. Due to this, in Brazil, the options for the treatment of fusariosis are restricted to amphotericin B, natamicin, and voriconazole [7, 12]. However, there is no consensus for establishing the best antifungal therapy, since there are variations of susceptibility among species that also depend on the related clinical manifestation [6, 8, 13].
When compared to advances in the development of new antibacterial agents, studies related to the discovery of new antifungal agents have disadvantages [14, 15]. Thus, the prospection of new therapeutic options capable of overcoming the cases of multi-resistance in Fusarium spp. is essential. In this scenario, chloroacetamides are used widely globally as herbicides for the control of weeds mainly in corn, soybean, and cotton crops [16, 17]. In addition to the action on plants, studies have shown that these agents and their derivatives are biologically active. Thus, can be mentioned the presence of antileishmania action, antibacterial and antifungal [16, 18, 19]. In addition, according to Machado [20], chloroacetamide derivatives, such as N-4-bromophenyl-2-chloroacetamide (4-BFCA), showed anti-Candida activity, efficacy to inhibit the growth of these yeasts, and fungicide action against dermatophyte. However, no studies are evaluating the antifungal potential of these derivatives against Fusarium spp.
Thus, this study evaluated the antifungal potential and toxicological parameters of 4-BFCA against multi-resistant Fusarium strains.
Material and methods
Preparation of compound
The N-(4-bromophenyl)-2-chloroacetamide (4-BFCA—MW: 248.5 g/mol) (Fig. 1) was synthesized by Lavorato et al. [21]. The compound was fully characterized by its melting points and IR, 1H, and 13C through nuclear magnetic resonance spectroscopy (NMR) spectra and its demonstrated melting point consistent with a previous report [22, 23].
Fig. 1.
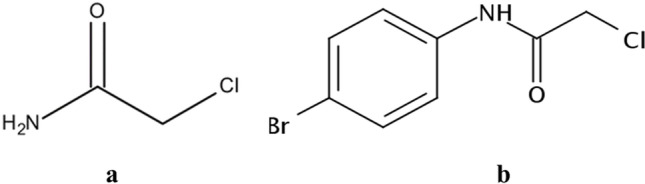
Chemical structure of the chloroacetamide (a) and of N-(4-bromophenyl)-2-chloroacetamide (4-BFCA) (b)
For biological assays, 4-BFCA was diluted in 2% DMSO (Sigma-Aldrich, St. Louis, Missouri, USA) and the remaining volume was completed with RPMI 1640 (Gibco, Grand Island, NY, USA) to obtain the required concentrations for each biological assay. The antifungal amphotericin B (AMB—Cristália, São Paulo, Brazil) was prepared according to CLSI [24]. Thus, an AMB stock solution was initially diluted in 2% DMSO and the remaining volume supplemented with RPMI medium to obtain a solution at 128 µg/mL, which was used as a control drug for the test.
Fungal strains
In this study, a total of 16 Fusarium spp. strains were evaluated: F. falciforme (F 9, F 20, and HCF 19), F. keratoplasticum (F 21, HCF 17, and HCF 26), F. oxysporum (F 24, F 35, HCF 22, and HCF 46), F. proliferatum (F2), and F. solani (ATCC 16,031, F 28, F 33, F 34, and HCF 41). All strains are from the mycology collection of yeasts of the Federal University of Rio Grande do Sul (Porto Alegre, Rio Grande do Sul, Brazil). Fusarium spp. strains were first identified through DNA sequence analysis, which were compared to Basic Local Alignment Search Tool (BLAST) for these sequences in the Database Multilocus Sequence Typing (MLST) for Fusarium genus. As a control, the standard ATCC 36,031 strain obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) was used.
Antifungal susceptibility testing
The broth microdilution assay was performed to determine the minimum inhibitory concentration (MIC) of 4-BFCA against multi-resistant Fusarium strains. The compound was prepared at 100 μg/mL, with concentrations ranging between 0.09 and 50 μg/mL. AMB solution at 128 μg/mL was used as control. A suspension containing each fungal inoculum in saline solution (NaCl 0.85%) was prepared. The suspensions were homogenized in a vortex and after 5 min the supernatant containing the fungal conidia was removed and transferred to a new sterile tube. Then, conidial density was read in a spectrophotometer at 530 nm, and a 1:50 dilution of the fungal inoculum was performed to obtain a concentration of approximately 0.5–4 × 104 UFC/mL. The microplates were incubated at 32 °C for 48 h, without shaking. The minimum inhibitory concentration (MIC) was determined as the lowest concentration of 4-BFCA capable of inhibiting 100% of fungal growth visually. The methodology followed the one proposed by document M38-A2 [24].
Time kill assay
The 4-BFCA solutions at MIC, MICx2, MICx4, and MICx8 concentrations were prepared in RPMI 1640 and evaluated against F. solani (ATCC 31,036) strain. A fungal inoculum in sterile saline solution (NaCl 0.85%) was prepared and the suspension reading was performed in a spectrophotometer at 530 nm. An aliquot of this fungal suspension was added in each 4-BFCA solution to obtain approximately 0.5–4 × 104 UFC/mL. As a control, solutions containing only fungal inoculum in RPMI 1640 were used. All 4-BFCA solutions were made in triplicate. The determination of kinetics curves was performed in the times 0, 3, 6, 12, 24, and 48 h at 32 °C without shaking. During each time, an aliquot of 100 μL of each sample and control was removed and diluted to 10−3 to allow counting of the Fusarium colonies. Then, 20 μL of each sample were plated on sabouraud dextrose agar (SDA) using a Drigalski spreader through spreading-plate method, making it possible to reliably count the filamentous colonies. The plates were incubated at 32 °C at 48 h. After growth, colony count was performed, and the results were expressed by plotting mean log10 CFU/mL. The presence of fungicidal action was defined when a decrease of ≥ 99.9% in the log10 CFU/mL was observed. When the reduction in the log10 CFU/mL was < 99.9%, the 4-BFCA action is considered fungistatic [25].
Scanning electron microscopy
After determination of MIC values for 4-BFCA and AMB against F. solani (ATCC 31,036) strain, an aliquot at MIC/2 concentration of both samples was collected for scanning electron microscopy (SEM). Firstly, the samples were prepared through several steps, including washing, fixing, and centrifugation [26]. Thus, pellets containing the samples were obtained and adhered to coverslips previously treated with poly-L-lysine for 1 h. Then, the coverslips were dehydrated in a series of acetone solutions: 30, 50, 70, 95 (5 min), and 100% (10 min) (Dinâmica, Diadema, São Paulo, Brazil). Lastly, the samples were subject to critical point drying (MS CPD 300, Leica), coated with gold and palladium for SEM viewing (Carl Zeiss EVO® MA10—Carl, Oberkochen, Germany). A current of 10 kV was used.
Acute toxicity tests in mealworms
Tenebrio molitor larvae (Coleoptera: Tenebrionidae) were anesthetized by cooling (2 °C) during 2 min. Then, 50 µL of 4-BFCA solution at 100 μg/mL was applied in each larva with support of a micro syringe into the hemocoel, at the second or third visible sternite above the legs, in the ventral portion. This was the maximum volume that did not cause larvae death at 48 h, and thus used in this assay. The equivalent dose was calculated as 5 µg/200 mg; so, this dose is below of the recommended limit of chloroacetamides for most sensitive mammals. The larvae were incubated at 37 °C in Petri dishes containing a rearing diet. The number of dead larvae was recorded at 4 h intervals during 48 h. The assay was performed in triplicate with groups of seven larvae, with a total of twenty-one larvae per treatment. The methodology followed the one proposed by De Souza et al. and Usui et al. [27, 28].
Fungal infection model on the chorioallantoic membrane (CAM)
White fertile eggs fresh Lohmann (Lohmann selected Leghorn, LSL) for this infection model were used. Firstly, optimal conditions of incubation temperature (38 to 39 °C) and humidity (55 to 60%) were established for the eggs’ maintenance during 12 days. In parallel, the F. solani ATCC 31,036 strain was grown on SDA for 48 h at 32 °C. Thus, a fungal inoculum suspension was prepared in 5 mL of 0.85% sterile saline solution, according to M38-A2 protocol [24]. The resulting suspension was vortexed, and the fungal cell density was adjusted in a spectrophotometer at 530 nm wavelength. Thus, a final suspension of the ATCC strain was prepared at 1:100 dilution by counting Neubauer chamber resulting in approximately 1–4 × 103 cells/mL [29]. From the fourth day of incubation onward, the eggs were turned four times a day until they were infected with 100 µL of the fungal inoculum, the holes were sealed with paraffin, and embryo viability was checked daily. In brief, on developmental day 8, 100 µL of 4-BFCA at 100 µg/mL was added in each egg (negative control—0.85% sterile saline solution). On day 12, the eggs were opened again and 100 µL of the 4-BFCA solution and of the negative control was removed, and spread with Drigalski strap on Petri dishes containing SDA. Thus, the plates were incubated at 30 °C for 72 h [30, 31].
Statistical analysis
Statistical analysis was performed for CAM test, through non-parametric Student’s t-test, and two-way ANOVA followed by Dunnett’s test to assess the fungicidal effect of 4-BFCA at different concentrations when compared to the control (untreated control). Both analyses consider P < 0.05 significant (*), and GraphPad Prism 7.0 was used. The experiments were performed in triplicate.
Results
The 4-BFCA was effective against all Fusarium spp. strains evaluated, presenting a similar susceptibility profile among species, with MIC values ranging from 12.5 to 50 μg/mL. The F. keratoplasticum specie was the most sensitive to 4-BFCA, since this had the lowest MIC value (12.5 μg/mL). The strains HCF 17, HCF 22, and HCF 41 showed low susceptibility to AMB (MIC > 16 μg/mL) (Table 1).
Table 1.
Minimum inhibitory concentration (MIC µg/mL) values refer to 4-BFCA and amphotericin B (AMB) against Fusarium spp. strains
| Fusarium spp. | Strains | 4-BFCA | AMB |
|---|---|---|---|
|
F. falciforme (n = 3) |
F 9 F 20 HCF 19 |
25 25 50 |
4 4 8 |
|
F. keratoplasticum (n = 3) |
F 21 HCF 17 HCF 26 |
12.5 25 50 |
8 > 16 4 |
|
F. oxysporum (n = 4) |
F 24 F 35 HCF 22 HCF 46 |
25 25 50 50 |
2 4 > 16 8 |
| F. proliferatum | F2 | 25 | 4 |
|
F. solani (n = 5) |
ATCC 36,031 F 28 F 33 F 34 HCF 41 |
25 25 50 25 25 |
4 8 2 8 > 16 |
| MIC range | - | 12.5–50 | 2– > 16 |
| Geometric mean | - | 31.04 | 4.45 |
Resistant strains at the highest concentration tested for 4-BFCA (MIC > 50 µg/mL) and amphotericin B (AMB—MIC > 16 µg/mL)
The evaluation of time kill kinetics indicates that the 4-BFCA has fungicidal action (reduction ≥ 99.9% in the log10 CFU/mL) against Fusarium spp. (Fig. 2). As a result, this action is dependent on the 4-BFCA concentration and elapsed time. At higher concentrations, such as MICX2, MICx4, and MICx8, the fungicidal action starts from 24 h with significant reduction of the Fusarium cells (*P < 0.05) when compared to the untreated control. At MIC, the 4-BFCA is not able to promote a cell kill ≥ 99.9% in the log10 CFU/mL, not showing a significant reduction when compared to the control, acting fungistatically on Fusarium strain.
Fig. 2.
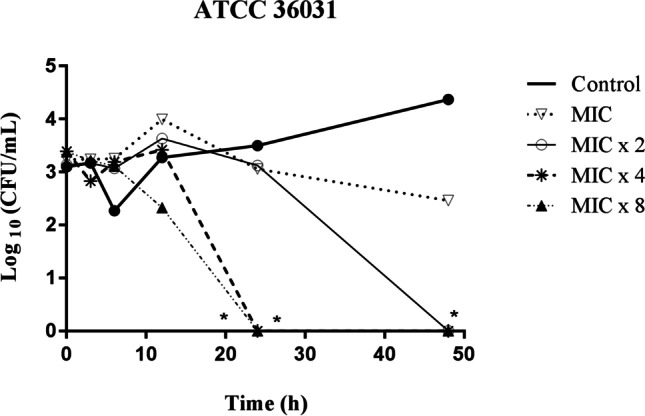
Log plots of killing kinetics for 4-BFCA against F. solani (ATCC 31,036) strain at MIC, MICx2, MICx4, and MICx8 concentrations and control untreatment. The concentrations ranged from 25 to 200 μg/mL (MIC = 25 μg/mL). The asterisks (*) indicate statistical differences when compared to the untreated control (*P < 0.05)
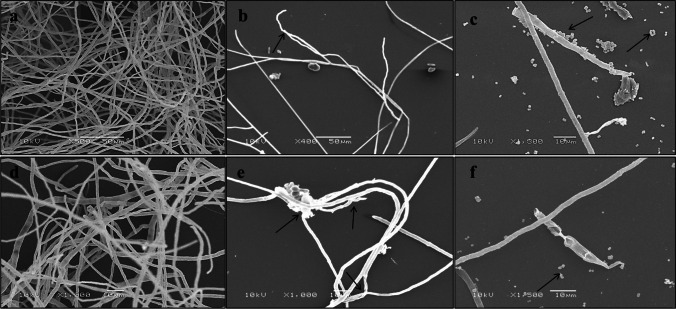
The antifungal effect of the 4-BFCA on F. solani ATCC 31,036 cells can be revealed through microphotographs of SEM. For untreated cells, the qualitative presence of the fungal mycelium formed by a large amount of branched and elongated hyphae can be observed (Fig. 3a and d). On the other hand, 4-BFCA appears to reduce the presence of Fusarium hyphae when the microphotographs are compared to the untreated control. (Fig. 3b and e).
Fig. 3.
Microphotographs of SEM analysis of F. solani (ATCC 36,031) strain in the presence of 4-BFCA at MIC/2 concentration, amphotericin B (AMB). Untreated cells were used as a control: a and d ATCC 36,031—untreated cells at different magnifications; b and e ATCC 36,031—cells treated with 4-BFCA at different magnifications; c and f ATCC 36,031—cells treated with AMB at different magnifications. The changes provoked on F. solani cell structure after treatment with 4-BFCA and AMB are indicated by the arrows. Currently used: 10 kV
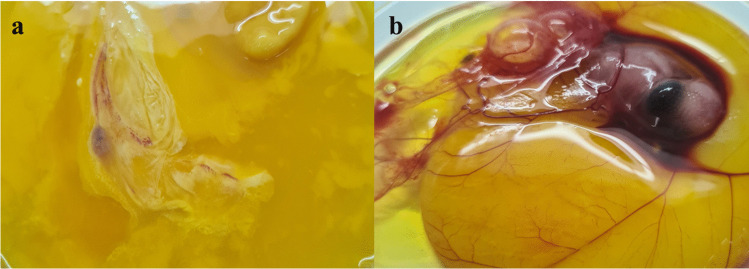
The Kaplan–Meier plot showed that all T. molitor larvae survived the first 24 h of exposure to 4-BFCA tested and the PBS control. From 24 h, the survival rate remained at 71.4% up to 48 h, for both treatments. There was no significant difference among the groups (p > 0.5), confirming that the 4-BFCA does not present toxicity to larvae (Fig. 4). In parallel, the results of the CAM model infection showed eggs with fungal growth in which embryos were dead and eggs without fungal growth in which embryos were still alive at the time of hatching (Table 2). The eggs treated with 4-BFCA solution at 100 µg/mL showed the absence of fungal growth, while the untreated eggs in the F. solani growth were visualized. The 4-BFCA was 83.33% effective in combating the fungal infection in vivo (Fig. 5).
Fig. 4.
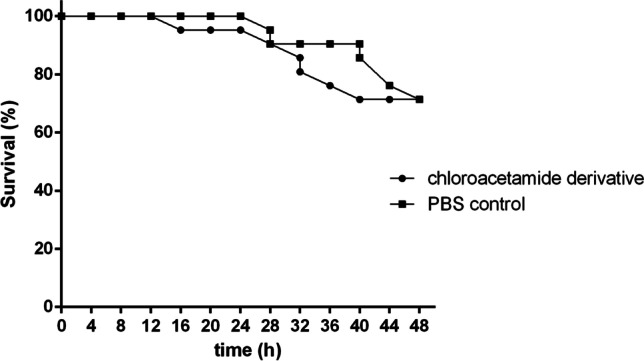
Kaplan–Meier plots showing survival curve of mealworms after of exposition to 4-BFCA at 100 μg/mL (■) and phosphate buffer solution (PBS) (●)
Table 2.
Chorioallantoic membrane (CAM) infection model by F. solani showing the antifungal activity of 4-BFCA at 100 μg/mL on eggs and embryos tested
| Strain | Eggs (n = 12) | Embryos (n = 12) | 4-BFCA efficiency |
||
|---|---|---|---|---|---|
| With fungal growth | Without fungal growth | Deads | Lives | ||
|
F. solani ATCC 31,036 |
2 | 10 | 1 | 11 | 83.33% |
Total number of eggs and embryos tested: n = 12. F. solani ATCC 31,036 inoculum at 1–4 × 103 CFU/mL
Fig. 5.
Macroscopic changes in infected embryonated eggs. Eggs were infected on developmental day 4. a Control, no treatment (embryo dead). b Treatment with 4-BFCA at 100 µg/mL (embryo live)
Discussion
The antimicrobial efficacy of chloroacetamides has been proven by the use of 2-chloroacetamide compound as a preservative in hygiene and cosmetic products for human use. This substance is authorized by Food and Drug Administration (FDA) as a preservative in these products, since it has no dermal toxicity, irritation, and ocular sensitivity at low concentrations, as demonstrated in an in vivo study conducted with mammalian animals [32, 33]. In Brazil, the Agência Nacional de Vigilância Sanitária (ANVISA) determines that cosmetics and hygiene products for human use contain a maximum of 0.3% of this preservative, suggesting its safety for topical use at low concentrations [34]. Despite preservative action of chloroacetamides, studies evaluating the antifungal action of this class are scarce. The findings show only information about the susceptibility profile of a Candida species, dermatophytes, A. niger, and F. oxysporum strain against these derivatives [16, 19, 20]. In this study, we suggest that the anti-Fusarium action presented by 4-BFCA is probably due to its lipophilic characteristics.
According to Lavorato et al. [21], studies of quantitative structure–activity relationship (QSAR) had shown a high value of the partition coefficient (LogP 2.52) for 4-BFCA, relating their liposolubility to its antileishmanial action, since the higher the LogP of a compound, the greater its lipophilic character. In this context, the lipophilicity can facilitate the penetration of 4-BFCA through the Fusarium cell membrane, leading to fungal death. Thus, we suggest that the 4-BFCA anti-Fusarium activity is also related to its physicochemical characteristics, such as its liposolubility. In addition, according to Machado et al. [20] the 4-BFCA presented strong anti-dermatophytic and anti-Candida action (MIC range 6.25–50 μg/mL), highlighting the greater effectiveness of this compound against filamentous fungi. The presence of bromine at the para position of the phenyl ring has improved the activity of the phenylchloroacetamide derivatives. This is in agreement with the data observed for the antifungals used in clinical therapy that have halogens in their structure, such as fluorine-fluconazole [35] and chloro-clotrimazole [36]. These substituents present characteristic that apparently enhances the potency of these drugs against fungal cells.
Results of analysis of time kill curves indicate that at concentrations higher than MIC, the 4-BFCA provoked a dose-dependent fungicidal action on Fusarium strain. According to Machado et al. [20], 4-BFCA showed only a fungistatic effect on Candida strains. However, on dermatophytes, a fungicidal action at concentrations higher than MIC and dependent on the elapsed time was also observed, similar to the behavior against Fusarium. Both fungistatic and fungicidal behavior also occurs in the action of commercial antifungal, such as fluconazole, amphotericin B, terbinafine, and echinocandins, since the fungicidal effect of the same will depend on their concentration [35–37]. Our findings are very promising, since the presence of fungicidal action is one of the essential characteristics for choosing a new antifungal candidate. Although the use of fungistatic antifungals can be considered, they only reduce the growth and reproduction of fungi, keeping the residual cells that can become resistant from successive expositions to these agents, causing therapeutic failures [37].
The 4-BFCA causes extravasation of the intracellular material and changes in the F. solani cells (Fig. 3b and e). This same behavior was observed by Machado et al. [20] when 4-BFCA was evaluated against Candida species and dermatophytes. However, against dermatophytes, as well as against Fusarium spp., 4-BFCA effect is more pronounced, which suggests a greater efficacy of this compound against filamentous fungi when compared to yeasts. AMB action on F. solani cells (Fig. 3c and f) demonstrates the presence of numerous F. solani conidia. Although further studies are needed, we suggest that the antifungal mechanism of 4-BFCA on Fusarium is similar to its anti-dermatophytic effect, as observed by Machado et al. [20].
Alternative models to the use of mammalian animals have been widely applied as screening tests for evaluation of acute systemic toxicity and efficacy of new compounds. Larvae of T. molitor appear as a new model of promising invertebrate host for evaluation of this parameter, avoiding the use of vertebrate animals in preliminary tests [27, 28]. Likewise, the in vivo infection model using the chorioallantoic membrane is also effective for evaluating the antifungal efficacy of new compounds. In this study, embryonic death in the treated eggs may be related to infection by F. solani strain. According to Jacobsen et al. [30], the fungal infection dose, 1–4 × 103 CFU/mL, is considered to be low. Thus, the infection would not be assigned to the dominant as one cause of the non-viability of the embryos. Our results are promising, although in vivo studies in mammalian models are needed to confirm these data.
Conclusion
The 4-BFCA was effective against multi-resistant Fusarium strains, showing a dose-dependent fungicidal action and able to damage the structure of fungal cells. In addition, the compound suggests safety for possible systemic use, although in vivo studies with mammalian animal models are needed. These findings are very promising, since this compound has a high potential as a candidate for the development of a new therapy for the treatment of fusariosis.
Acknowledgements
The authors thank the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS – EDITAL 04/2016 – PRONUPEQ 2016) for financial support and research fellowships. Alexandre Meneghello Fuentefria is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the PQ fellowships (Edital Universal 2018).
Author contribution
Gabriella da Rosa Monte Machado participated in the conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, and writing—review and editing of the text. Stefânia Neiva Lavorato participated in the methodology, validation, formal analysis, and data investigation. William Lopes and Mário Teixeira participated in the methodology, data curation, and formal analysis. The professors Marilene Henning Vainstein, Ricardo José Alves, Saulo Fernandes de Andrade, and Alexandre Meneghello Fuentefria participated in the visualization, supervision, project administration, and acquisition of the financial support.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Carlos Pelleschi Taborda
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nucci F, Nouér SA, Capone D, Anaissie E, Nucci M. Fusariosis. Semin Respir. Crit Care. 2015;36:706–714. doi: 10.1055/s-0035-1562897. [DOI] [PubMed] [Google Scholar]
- 2.Debourgogne A, Dorin J, Machouart M. Emerging infections due to filamentous fungi in humans and animals: only the tip of the iceberg? Environ Microbiol. 2016;8:332–342. doi: 10.1111/1758-2229.12404. [DOI] [PubMed] [Google Scholar]
- 3.Diongue K, Ndiaye M, Seck MC, Diallo MA, Badiane AS, Ndiaye D (2017) Onychomycosis caused by Fusarium spp. in Dakar, Senegal: epidemiological, clinical, and mycological study. Dermatol Res Pract 2017:4. [DOI] [PMC free article] [PubMed]
- 4.Gupta C, Jongman M, Das S, Snehaa K, Bhattacharya SN, Seyedmousavi S, Van Diepeningen AD. Mycopathologia. 2016;181:497–504. doi: 10.1007/s11046-016-0014-7. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hatmi AMS, Curfs-Breuker I, De Hoog GS, Meis JF, Verweij PE. Antifungal susceptibility testing of Fusarium: a practical approach. J Fungi. 2017;3:19. doi: 10.3390/jof3020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batista BG, Dalla Lana DF, Silveira GP, SA MM, Ferreira M, Russo TVC, Canto RFS, Barbosa FAR, Braga AL, Kaminski TFA, De Oliveira LFS, Machado MM, Lopes W, Vainstein MH, Teixeira ML, Andrade SF, Fuentefria AM. Allylic selenocyanates as new agents to combat Fusarium involved with human infections. ChemistrySelecty. 2017;2:11926–11932. doi: 10.1002/slct.201702338. [DOI] [Google Scholar]
- 7.Taj-Aldeen SJ. Reduced multidrug susceptibility profile is a common feature of opportunistic fusarium species: Fusarium multi-drug resistant pattern. J Fungi. 2017;3:18. doi: 10.3390/jof3020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosa PD, Heidrich D, Corrêa C, Scroferneker ML, Vettorato G, Fuentefria AM, Goldani LZ. Genetic diversity and antifungal susceptibility of Fusarium isolates in onychomycosis. Mycoses. 2017;60:616–622. doi: 10.1111/myc.12638. [DOI] [PubMed] [Google Scholar]
- 9.Costa MI, Vilugron Rodrigues FAV, Vieira FF, Jarros IC, Kischkel B, Negri M, Becker TCA, Svidzinski TIE. Effects of intratracheal Fusarium solani inoculation in immunocompetent mice. Microb Pathog. 2019;128:317–322. doi: 10.1016/j.micpath.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Van Diepeningen AD, Al-Hatmi AMS, Brankovics B, De Hoog GS. Taxonomy and clinical spectra of Fusarium species: where do we stand in 2014? Curr Clin Microbiol Rep. 2014;1:10–18. doi: 10.1007/s40588-014-0003-x. [DOI] [Google Scholar]
- 11.Dabas Y, Bakhshi S, Xess I. Fatal cases of bloodstream infection by Fusarium solani and review of published literature. Mycopathologia. 2016;181:291–296. doi: 10.1007/s11046-015-9960-8. [DOI] [PubMed] [Google Scholar]
- 12.Stempel JM, Hammond SP, Sutton DA, Weiser LM, Marty FM. Invasive fusariosis in the voriconazole era: single-center 13-year experience. Open Forum Infect Dis. 2015;2:ofv099. doi: 10.1093/ofid/ofv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersal T, Al-Hatmi ASM, Cilo DB, Curfs-Breuker I, Meis JF, Özkalemkaş F, Ener B, Van Diepeningen AD. Fatal disseminated infection with Fusarium petroliphilum. Mycopathologia. 2015;179:119–124. doi: 10.1007/s11046-014-9813-x. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy MW, Denning DW, Walsh T. Future research priorities in fungal resistance. J Infec Dis. 2017;216:484–492. doi: 10.1093/infdis/jix103. [DOI] [PubMed] [Google Scholar]
- 15.Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist. 2017;10:249–259. doi: 10.2147/IDR.S124918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katke SA, Amrutkar SV, Bhor RJ, Khairnar MV. Synthesis of biologically active 2-chloro-N-alkyl/arylacetamide derivatives. Int J Pharm Sci Res. 2011;2:148–156. [Google Scholar]
- 17.Karier P, Kraus G, Kolbe I. Metazachlor traces in the main drinking water reservoir in Luxembourg: a scientific and political discussion. Environ Sci Eur. 2017;29:25. doi: 10.1186/s12302-017-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marco-Contelles J, Gomez-Sanchez E. New agents with antimycobacterial activity. Arch Pharm. 2005;338:562–563. doi: 10.1002/ardp.200500133. [DOI] [PubMed] [Google Scholar]
- 19.Aschale M. Synthesis and antimicrobial evaluation of some novel substituted 2-chloroacetanilides. Int J Chemtech Res. 2012;4:437–1441. [Google Scholar]
- 20.Machado GRM, De Andrade SF, Pippi B, Bergamo VZ, Berlitz SJ, Lopes W, Lavorato SN, Külkamp-Guerreiro IC, Vainstein MH, Teixeira ML, Alves RJ, Fuentefria AM. Chloroacetamide derivatives as a promising topical treatment for fungal skin infections. Mycologia. 2019;111:612–623. doi: 10.1080/00275514.2019.1620550. [DOI] [PubMed] [Google Scholar]
- 21.Lavorato SN, Duarte MC, De Andrade PHR, Coelho EAF, Alves RJ. Synthesis, antileishmanial activity and QSAR studies of 2-chloro-N-arylacetamides. Braz J Pharm Sci. 2016;53:e16067. [Google Scholar]
- 22.Harte AJ. Gunnlaugsson T. Synthesis of α-chloroamides in water. Tetrahedron Lett. 2006;47:6321–6324. doi: 10.1016/j.tetlet.2006.06.090. [DOI] [Google Scholar]
- 23.Singh DCP, Hashim SR, Singhal RG. Synthesis and antimicrobial activity of some new thioether derivatives of quinoxaline. E-J Chem. 2011;8:635–642. doi: 10.1155/2011/482831. [DOI] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute (CLSI) (2008) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved Standard, 2ed edn. Wayne, PA: CLSI Document M38-A2. Clinical Laboratory Standards Institute.
- 25.Gill TA, Li J, Saenger M, Scofield SR. Thymol-based submicron emulsions exhibit antifungal activity against Fusarium graminearum and inhibit Fusarium head blight in wheat. J Appl Microbiol. 2016;121:1103–1116. doi: 10.1111/jam.13195. [DOI] [PubMed] [Google Scholar]
- 26.Joubert LM, Ferreira JAG, Stevens DA, Cegelski L. Aspergillus fumigates biofilms: a comparison of processing techniques for scanning electron microscopy of fungal mycelium and extracellular matrix. Microsc Microanal. 2015;21:935–936. doi: 10.1017/S1431927615005474. [DOI] [Google Scholar]
- 27.De Souza PC, Morey AT, Castanheira GM, Bocate KP, Panagio LA, Ito FA, Almeida RS. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J Microbiol Methods. 2015;118:182–186. doi: 10.1016/j.mimet.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Usui K, Nishida S, Sugita T, Ueki T, Matsumoto Y, Okumura H, Sekimizu K. Acute oral toxicity test of chemical compounds in silkworms. Drug Discov Ther. 2016;10:57–61. doi: 10.5582/ddt.2016.01025. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues A, Pina Vaz C, Fonseca AF, De-Oliveira JM. Evaluating the concentration of a Candida albicans suspension. Infect Dis Obstet Gyneco. 1993;1:134–136. doi: 10.1155/S1064744993000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen ID, Grobe K, Slesiona S, Hube B, Berndt A, Brock M. Embryonated eggs as an alternative infection model to investigate Aspergillus fumigatus virulence. Infect Immun. 2010;78:2995–3006. doi: 10.1128/IAI.00268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalla Lana DF, Giuliani LM, Reolon JB, Lopes W, Vainstein MH, Danielli LJ, et al. Nanoemulsion improves the antifungal activity of allylic thiocyanates against yeasts and filamentous pathogenic fungi. ChemistrySelect. 2018;3:11663–11670. doi: 10.1002/slct.201802204. [DOI] [Google Scholar]
- 32.Liebert MA. Final report on the safety assessment of chloroacetamide. J Am Coll Toxicol. 1991;10:21–22. doi: 10.3109/10915819109078620. [DOI] [Google Scholar]
- 33.Food and Drug Administration (FDA) (1987) Cosmetic product formulation data for Chloroacetamide. FDA Computer Printout.
- 34.ANVISA website. Available in http://portal.anvisa.gov.br/documents/10181/3285739/RDC_29_2012_.pdf/c74fbb1a c98b 4899–81ae-7ad9e18d807e. [accessed 25 March 2020].
- 35.Corrêa JCR, Salgado HRN. Review of fluconazole properties and analytical for its determination. Crit Ver Anal Chem. 2011;4:270–279. doi: 10.1080/10408347.2011.588924. [DOI] [Google Scholar]
- 36.Crowley PD, Gallagher HC. Clotrimazole as a pharmaceutical: past, present and future. J Appl Microbiol. 2014;1:611–617. doi: 10.1111/jam.12554. [DOI] [PubMed] [Google Scholar]
- 37.Kovacic P, Cooksy A. Novel, unifying mechanism for amphotericin B and other polyene drugs: electron affinity, radicals, electron transfer, autoxidation, toxicity, and antifungal action. MedChemComm. 2012;3:274–328. doi: 10.1039/C2MD00267A. [DOI] [Google Scholar]