Abstract
Vibrio parahaemolyticus can degrade insoluble chitin with the help of chitinase enzymes that generate soluble N-acetyl glucosamine oligosaccharides (GlcNAcn) to induce a state of natural competence for the uptake of extracellular DNA. In this study, we had evaluated the role of various regulatory factors such as TfoX, CytR, OpaR, and RpoS during natural transformation of V. parahaemolyticus. The results suggest that TfoX regulates natural competence via CytR in a chitin-dependent manner. CytR controls the release of GlcNAc6 from insoluble chitin and conversion of GlcNAc6 into smaller GlcNAc residues inside the periplasm by modulating the expression of endochitinase and periplasmic chitinases. In addition, CytR was also responsible for GlcNAc6-mediated upregulation of competence-related genes such as pilA, pilB, comEA, and qstR. Next, we found that the quorum sensing regulator OpaR affects the natural transformation through its regulation of extracellular nuclease Dns. The ΔopaR mutant showed increased expression of Dns, which might degrade the eDNA. As a consequence, the transformation efficiency was decreased and eDNA-dependent growth was hugely enhanced. However, when Dns-containing DASW was substituted with fresh DASW, the transformation was detectable in ΔopaR mutant and eDNA-dependent growth was less. These results suggest that the occurrence of natural transformation and eDNA-dependent growth were inversely related to each other. Lastly, the general stress regulator RpoS was required for neither quorum-sensing dependent nor chitin-dependent regulation of natural competence in V. parahaemolyticus.
Keywords: Chitin, Natural transformation, Competence, Regulators
Introduction
Several members of Vibrionaceae family such as Vibrio cholerae, Vibrio vulnificus, Vibrio fischeri, and Vibrio parahaemolyticus have been found to become naturally competent in the presence of chitin and can uptake extracellular DNA (eDNA) from its surroundings [1–4]. Once inside the bacterial system, this eDNA might serve as a source of nutrition or incorporate into the chromosome by homologous recombination [5]. The regulatory system for chitin-dependent competence and transformation has been extensively studied in V. cholerae [6]. The complex regulatory network coordinates components of chitin utilization (TfoX), quorum sensing (HapR), nucleoside scavenging (CytR), and general stress response (RpoS) for the activation of competence pathway in V. cholerae. The chitin oligosaccharides are the key molecule for the induction of natural competence state in V. cholerae [1]. The chitin oligosaccharides activate two transmembrane regulators, ChiS and TfoS [7, 8], which cumulatively trigger the master competence regulator, TfoX, for the induction of genes required for DNA uptake [1]. The sigma factor RpoS, a general stress response regulator, is also reported to be required for chitin-dependent natural transformation via regulation of chitinase activity [9]. The cytidine repressor, CytR, has been identified as a positive regulator of competence gene expression in V. cholerae. CytR can increase the expression of chitinase chiA1 and competence genes, comEA and pilA [10]. The quorum sensing regulator, HapR, is produced only at high cell densities to repress the expression of extracellular nuclease, dns, and activate the transcription of comEA. This cell density–dependent counter-regulation of dns and comEA through the same regulator, i.e., HapR, corroborates the availability of non-degraded extracellular DNA for bacterial transformation [11].
The regulators of V. cholerae competence machinery are mostly conserved among Vibrio species, including V. parahaemolyticus. It is a causative agent of human acute gastroenteritis and might cause wound infection or septicemia in individuals with pre-existing medical conditions. It was reported that GlcNAcn acts as the inducer of natural transformation in V. parahaemolyticus [12]. The generation of GlcNAcn≥2 from chitin was mediated by the action of extracellular chitinases such as VPA0055 (ChiA2), VPA1177 (ChiA), VP0619 (ChiB), and VP2338 (ChiA1). Further breakdown of GlcNAcn≥4 inside the periplasm might depend on activities of periplasmic chitinases such as VPA0832 (chitodextrinase, Cdx), VP0755 (N,N′-diacetylchitobiase, Chb), and VP2486 (β-N-acetyl hexosaminidase, BNh) [13].
As most of the components of competence system are conserved among Vibrio species, we speculate that the regulation of natural competence in V. parahaemolyticus might be more or less similar to V. cholerae. In this study, the homologous factors of V. cholerae such as TfoX, CytR, OpaR, and RpoS were analyzed for their role in the utilization of chitin, GlcNAcn, utilization of eDNA as a nutrient, and natural transformation in V. parahaemolyticus. We found that in the presence of chitin, TfoX-dependent regulation of GlcNAc6 utilization and natural competence was mediated through CytR. The transformation of V. parahaemolyticus also depends on the HapR homolog, OpaR, which mainly controls the expression of dns. Unlike V. cholerae, RpoS was found to be redundant in V. parahaemolyticus.
Materials and methods
Bacterial strains, plasmids used, and growth conditions
V. parahaemolyticus strain RIMD2210633 was obtained from the Laboratory for Culture Collection, Research Institute for Microbial Diseases, Osaka University [14]. This strain was used for the construction of deletion mutants. The genomic DNA isolated from streptomycin-resistant (SmR) mutant of V. parahaemolyticus strain RIMD2210633 (VPS) was used as extracellular DNA (eDNA) for natural transformation experiments [12]. The bacterial strain was grown aerobically at 30 °C in MLB (Luria–Bertani broth containing 3% NaCl).
The isogenic deletion mutants were constructed by double-crossover allelic exchange using the R6K-ori suicide vector pXAC623 [15] and transformed in Escherichia coli β2155 λ pir. The Escherichia coli β2155 λ pir transformants were selected on LB plates containing 0.5 mM DAP and 20 μg ml−1 of chloramphenicol. We used pGEMT easy vector (Promega, USA) for TA cloning and transformed in E. coli JM109 and selected on LB plates containing 100 μg ml−1 of ampicillin. E. coli strains JM109 and β2155 were routinely cultured in LB broth at 37 °C and 0.5 mM DAP was added for the growth of E. coli β2155. The medium was supplemented with appropriate antibiotics as required.
Construction of isogenic deletion mutants
The isogenic deletion was created in genes VP1241 (tfoX), VP0252 (cytR), VP2516 (opaR), and VP2553 (rpoS) using splicing by overlap extension PCR and allelic exchange (Table 1). The V. parahaemolyticus RIMD2210633 genome sequence [16] was used as the template for primer designing (Table 2). The double-crossover deletion mutants were generated as mentioned earlier [12]. Briefly, the truncated version of the desired genes was cloned into the suicide vector pXAC623 and transformed into the E. coli β2155 λpir (donor). The resulting plasmid was then mobilized into WT strain (recipient) by filter mating on MLB plate. The transconjugants were selected on MLB plates containing 20 µg ml−1 of chloramphenicol but no DAP, followed by sucrose counter selection on LB-1% plates with 10% sucrose at 25 °C. Double-crossover deletion mutants were screened by PCR-based assay using specific primers.
Table 1.
List of strains and plasmids used in this study
| Strains or plasmids | Description | Source |
|---|---|---|
| V. parahaemolyticus | ||
| V. parahaemolyticus RIMD22106333 | Clinical isolate of serotype O3:K6; wild-type (WT) strain | [14] |
| VPS | Spontaneous streptomycin-resistant (SmR) mutant | [12] |
| ΔtfoX | Deletion in VP1241 of WT | This study |
| ΔcytR | Deletion in VP0252 of WT | This study |
| ΔopaR | Deletion in VP2516 of WT | This study |
| ΔrpoS | Deletion in VP2553 of WT | This study |
| Escherichia coli | ||
| β2155 | thrB1004 pro thi strA hsdS Δ(lacZ)ΔM15 [F′ Δ(lacZ)M15 lacIq traD36 proA+proB+] ΔdapA::erm(Emr), pir::RP4; kan (Kmr) from SM10 | [15] |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 (rK–,mK +) relA1 supE44 ∆(lac-proAB) [F´, traD36, proAB, lacIqZ∆M15] | Promega |
| Plasmids | ||
| pGEM-T easy | TA-cloning vector; AmpR | Promega |
| pXAC623 | Suicide vector derived from pKTN701 containing the sacB gene of Bacillus subtilis; CmR | [15] |
AmpR, ampicillin-resistant; Cm.R, chloramphenicol-resistant; SmR, streptomycin-resistant
Table 2.
List of primers used in this study
| Deletion | Primer | Sequence (5′ → 3′) |
|---|---|---|
| ∆vp1241 | tfoX_F1 | GGTACCGATGGGTGGTAACGAAAT |
| tfoX_R1 | ACGTCAACATCATTGCCGTCATCGCTAAACAAGC | |
| tfoX_F2 | GCTTGTTTAGCGATGACGGCAATGATGTTGACGT | |
| tfoX_R2 | GAGCTCTCTGCAGCAGGTTTTGGC | |
| tfoX_Int | CTTGCAGAAGACTTTTGG | |
| ∆vp0252 | cytR_F1 | GAGCTCTGGGATTTTAGATACTTCG |
| cytR_R1 | AGGTTAAACACTATGGCGGCCTAAGCGTTCGGTAGT | |
| cytR_F2 | ACTACCGAACGCTTAGGCCGCCATAGTGTTTAACCT | |
| cytR_R2 | GAATTCAAACGCGCAGGCAAATAC | |
| cytR_Int | CCATAAACGCATAGCAC | |
| ∆vp2516 | opaR_F1 | GTCGACATGAGTCAGTTCAATAGC |
| opaR_R1 | CAAATCTGAGCTTTAGTGGTCCATATCCATTTTCCT | |
| opaR_F2 | AGGAAAATGGATATGGACCACTAAAGCTCAGATTTG | |
| opaR_R2 | TCTAGAGAGCCAGAAGTGGCTTG | |
| opaR_Int | ATCACGAGTTGATGCGC | |
| ∆vp2553 | rpoS_F1 | GTCGACACCGAATTACCGAACTTG |
| rpoS_R1 | GGAGGCGTTATGAGTATCTCCTAATTAAACAAGGCC | |
| rpoS_F2 | GGCCTTGTTTAATTAGGAGATACTCATAACGCCTC | |
| rpoS_R2 | TCTAGAAACCTACTAAACCGAAGC | |
| rpoS_Int | AATTCGATCCAGAAAGAG |
Growth analysis in chitin flakes, colloidal chitin, and chitin oligosaccharide
The overnight culture was freshly inoculated and grown until mid-log phase. The defined artificial seawater (DASW) supplemented with 1% shrimp shell chitin flakes (Sigma-Aldrich, USA) was inoculated with approximately 104 cfu ml−1 using mid-log phase culture. The cultures were incubated at 30 °C and growth was enumerated by the plate count method at different time points. Similarly, 2% colloidal chitin broth was inoculated and incubated at 30 °C under shaking of 100 rpm for 72 h and 2% colloidal chitin plates were spotted with 10 μl of mid-log phase culture and kept at 30 °C for 5 days and the zone of chitin clearing for each colony was recorded. In the case of chitin oligosaccharides, 5 mM GlcNAc2 and 2.5 mM GlcNAc6 supplemented DASW were used and growth was checked at 0 h, 2 h, 4 h, 8 h, 16 h, 24 h, 32 h, 40 h, and 48 h by colony count method.
Chitin-induced natural transformation assays on chitin flakes and GlcNAc6
The natural transformation assay was performed by growing mid-log phase cultures in DASW media supplemented with either 5% chitin flake or 2 mM GlcNAc6 as mentioned earlier [12]. A total of 12.5 μg ml−1 of gDNA isolated from VPS was added as the source of eDNA. In negative control, only dH2O was added. The transformation efficiency was calculated as the number of colonies in antibiotic plates (streptomycin 200 μg ml−1) divided by the number of colonies on plates without antibiotic.
The utilization of eDNA as nutrient and genetic material
Overnight grown culture of WT and ∆opaR mutant was diluted in 1:100 ratio with MLB broth and grown until the OD600 reached 0.3–0.4. The bacterial pellet was then washed twice with DASW and diluted in DASW so that the OD600 reached 0.2. This 4 ml of bacterial suspension was added to the conical flask having 200 mg sterilized chitin (5%) flakes and incubated statically at 30 °C for 24 h. The next day, 12.5 μg ml−1 of gDNA was added without removing the spent media (planktonic phase) and incubated at 30 °C for 8 h under static condition. This sample was marked as ( +) DNA. In negative control, only dH2O was added and marked as ( −) DNA. After an 8-h incubation period, the conical flask was vigorously vortexed to release the attached bacteria. The appropriate dilutions were then plated onto MLB plates with or without streptomycin (200 µg ml−1). The transformation efficiency was calculated as the number of colonies in antibiotic plates divided by the number of colonies on plates without antibiotic.
Total RNA isolation and real-time PCR
Overnight grown culture of WT and isogenic mutant strains were 1:100 diluted in MLB broth and grown until the OD600 reaches 0.3–0.4. The bacterial pellet was diluted in 0.2% lactate supplemented DASW so that the OD600 become 0.2. This bacterial suspension was added to the conical flask having 100 mg of sterilized chitin flakes and incubated statically at 30 °C for 24 h. The bacterial cells were detached by vigorous vortexing and the RNeasy kit (QIAGEN, Germany) was used for RNA extraction according to the manufacturer’s protocol. The enzymatic lysis of V. parahaemolyticus was performed with lysozyme (5 mg ml−1) for 15 min at room temperature. In column DNase treatment was performed for 15 min at room temperature.
The mRNA expression analysis in the presence of purified GlcNAc6 was done using mid-log phase bacterial suspension diluted in 0.2% lactate and 0.625 mM GlcNAc6 supplemented DASW. This culture was incubated at 30 °C for 5 h, followed by RNA isolation. The primers were designed by Primer Quest tool of Integrated DNA Technologies (IDT, USA). We used Luna® Universal One-Step kit RT-qPCR kit (NEB, MA, USA) for real-time RT-PCR using 50 ng of RNA in a Mini Opticon Real-time PCR system (BIO-RAD, CA, USA). The relative expression (2−∆∆Ct) of the target transcripts was calculated using pvsA as an internal control.
Statistical analysis
The data were analyzed by Student’s t-test using GraphPad Prism. A probability level (P) value of ≤ 0.05 was considered statistically significant. Three independent experiments were done and the data represents mean ± SE of these independent events.
Results
Role of transcription regulators TfoX, CytR, OpaR, and RpoS during natural transformation of V. parahaemolyticus under environmental conditions
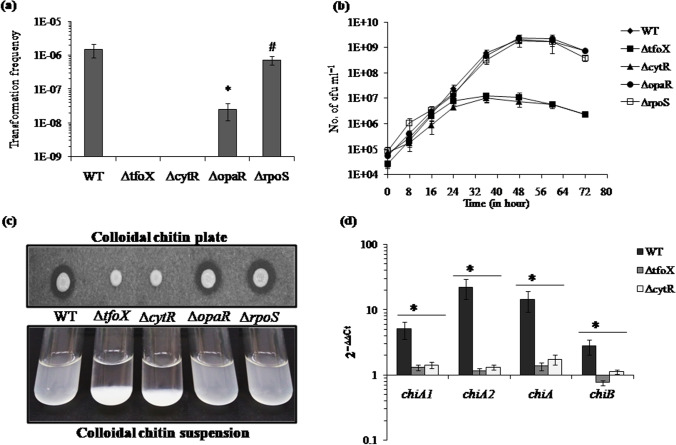
In V. cholerae, the co-ordinated action of transcription regulators TfoX (VC1153), CytR (VC2677), HapR (VC0583), and RpoS (VC0534) determines the proper functioning of competence machinery for natural transformation. The TfoX of V. parahaemolyticus shares 68% identity with TfoX of V. cholerae. Similarly, CytR shares 76%, OpaR shares 75%, and RpoS shares 78% identity with their respective homolog present in V. cholerae. Here, the role of these regulators in V. parahaemolyticus was determined using the isogenic mutant ΔtfoX, ΔcytR, ΔopaR, and ΔrpoS. In the presence of insoluble chitin, ΔcytR and ΔtfoX mutants were non-transformable (Fig. 1a), ΔopaR mutant showed 60-fold reduction (2.5 × 10−8), and ΔrpoS mutant (7.3 × 10−7) did not show any significant change in transformation frequency compared to WT (1.5 × 10−6).
Fig. 1.
Role of transcription regulators TfoX, CytR, OpaR, and RpoS during chitin-dependent natural transformation. a WT and isogenic mutant strains ΔtfoX, ΔcytR, ΔopaR, and ΔrpoS were naturally transformed on shrimp chitin flakes with 12.5 μg/ml extracellular DNA. The average of at least three independent experiments was represented. *Statistically significant difference between transformation frequencies (p < 0.05); #non-significant difference between the transformation frequency of WT and ΔrpoS mutant. b Growth curve of indicated isogenic mutants and WT in DASW with chitin as a sole carbon source was recorded. Each point represents the mean± SE of three independent experiments. c Wild-type and indicated isogenic mutants of V. parahaemolyticus were assayed for chitinase activity using DASW plate and broth containing 2% colloidal chitin. d The relative expression of four chitinase genes at mRNA level was checked for ΔtfoX and ΔcytR mutant compared to WT strain. pvsA was used as an internal control. Each bar indicates the mean ± SE of three independent experiments
The release of chitin oligosaccharides from crude chitin is the pre-requisite condition for the induction of competence state. Therefore, to determine the reason behind transformation phenotype showed by these mutants, we had analyzed the chitinase activity by checking growth on chitin flakes and colloidal chitin along with assessment of mRNA expression level of chitinase genes. The isogenic mutant’s ΔtfoX, ΔcytR, ΔopaR, ΔrpoS, and WT were used for growth curve analysis in the presence of 1% chitin flakes. After 48 h of incubation, the maximum growth was observed and the viable counts of WT, ΔtfoX, ΔcytR, ΔopaR, and ΔrpoS were 2.1 × 109, 1.1 × 107, 7.5 × 106, 2.3 × 109, and 1.8 × 109 cfu ml−1, respectively (Fig. 1b). Therefore, ΔtfoX and ΔcytR mutant showed 180-fold and 280-fold reduced growth compared to WT strain, but ΔopaR and ΔrpoS mutant showed WT-like growth. A similar result was obtained when colloidal chitin was used as growth supplement in either plate assay or liquid suspension (Fig. 1c).
Next, the mRNA expression of four endo-chitinase genes, i.e., chiA1, chiA2, chiA, and chiB, was checked in growth defective mutants, i.e., ΔcytR and ΔtfoX. The result showed downregulation of all these genes in both of the mutants (Fig. 1d). This suggests that the transcription factors TfoX and CytR regulate natural transformation in a chitin-dependent manner.
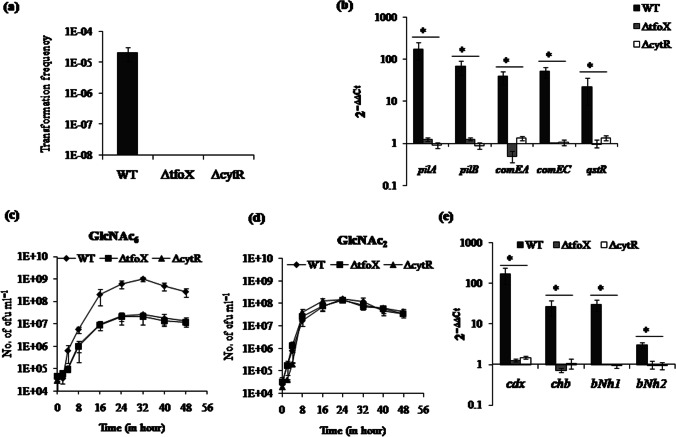
TfoX-dependent regulation of periplasmic chitinase and competence genes controls the GlcNAc6 utilization and natural transformation
As ΔcytR and ΔtfoX mutants were unable to generate GlcNAcn due to a lack of chitinase activity, we used purified GlcNAc6 for the induction of natural competence. However, even the presence of GlcNAc6 could not induce natural transformation in these two mutants (Fig. 2a). This observation leads us to check the mRNA expression of competence-related genes such as pilA, pilB, comEA, comEC, and qstR. The Δtfox mutant showed 142.3-, 52.8-, 77.9-, 50.1-, and 21.9-fold downregulation in pilA, pilB, comEA, comEC, and qstR, respectively (Fig. 2b). The ΔcytR mutant showed 194.7-, 75.4-, 29-, 48.2-, and 16.1-fold downregulation in pilA, pilB, comEA, comEC, and qstR, respectively (Fig. 2B).
Fig. 2.
TfoX-dependent regulation of periplasmic chitinase and competence genes controls the GlcNAc6 utilization and natural transformation. a GlcNAc6 used for natural transformation in WT, ΔtfoX, and ΔcytR. Data point represents the mean ± SE of three independent experiments. b The relative mRNA level expression of five competence genes pilA, pilB, comEA, comEC, and qstR was checked for ΔtfoX and ΔcytR mutant compared to WT strain. pvsA was used as an internal control. Each bar indicates the mean ± SE of three independent experiments. c Growth curve of indicated isogenic mutants and WT in DASW in the presence of purified GlcNAc6. Each point represents the mean ± SE of three independent experiments. d Growth curve of indicated isogenic mutants and WT in DASW in the presence of purified GlcNAc2. Each point represents the mean ± SE of three independent experiments. e The relative mRNA expression of cdx (VPA0832), chb (VP0545), bNh1 (VP2486), and bNh2 (VP0755) was checked for WT and ΔtfoS mutant in the presence of GlcNAc6. Each bar indicates the mean ± SE of three independent experiments
When the growth of ΔcytR and ΔtfoX mutant was studied in the presence of GlcNAc6, we found that these two mutants were incapable to utilize GlcNAc6 (Fig. 2c). However, ΔcytR and ΔtfoX mutant could grow like WT in the presence of GlcNAc2 (Fig. 2c). Therefore, we compared the mRNA expression of four periplasmic chitinases that were responsible for the conversion of GlcNAc6 into GlcNAc/GlcNAc2 between WT and mutants. The ΔtfoX mutant showed 131.9-, 36.7-, 29.5-, and threefold downregulation in cdx, chb, bNh1, and bNh2, respectively (Fig. 2E). Similarly, ΔcytR mutant showed 111.7-, 24.8-, 32.2-, and 3.2-fold downregulation in cdx, chb, bNh1, and bNh2, respectively (Fig. 2e). The results suggest that TfoX controls chitin utilization via downstream regulator CytR.
The absence of OpaR leads to better utilization of eDNA as a nutrient
DNA is a source of phosphate, carbon, and nitrogen and abundantly present in the biofilm matrix in environment. The DNA acquired through natural transformation has been suggested as a significant source of nutrition along with providing genetic material for recombination [5]. Here, we found that natural transformation and eDNA-dependent growth might occur simultaneously in V. parahaemolyticus.
We have compared the growth and natural transformation frequency without removing the spent media before the addition of gDNA. The WT strain showed 2.2 × 108 cfu ml−1 in ( −) DNA condition and 1.9 × 109 cfu ml−1 in ( +) DNA condition, i.e., ninefold higher growth in the presence of eDNA (Fig. 3a) and transformation frequency of 2 × 10−7. Interestingly, ∆opaR mutant showed 3.1 × 108 cfu ml−1 in ( −) DNA condition and 5.4 × 1010 cfu ml−1 in ( +) DNA condition, i.e., 174-fold higher growth in the presence of eDNA but no transformation was observed. This might depend on faster degradation of eDNA by extracellular nuclease such as Dns; and thus, we compared the mRNA expression of dns between WT and ∆opaR mutant. We found 25-fold higher expression of dns in ∆opaR mutant compared to WT (data not shown).
Fig. 3.
The absence of OpaR leads to better utilization of eDNA as a nutrient. a The growth of WT and ΔopaR mutant was compared with eDNA (+ DNA) and without eDNA (-DNA) in spent DASW. Asterisk (*) represents p < 0.05. b The growth of WT and ΔopaR mutant was compared with eDNA (+ DNA) and without eDNA (-DNA) in spent DASW. Each bar represents the mean ± SE of three independent experiments
When this similar experiment was performed by substituting the spent media with fresh DASW, the result was different. The WT strain did not show any significant change in growth with or without DNA (Fig. 3b). However, the ΔopaR mutant showed twofold higher growth in ( +) DNA compared to ( −) DNA. This growth difference was significant, but not that much huge as in the presence of spent media. Moreover, in this experimental condition, WT and ΔopaR mutant showed transformation frequency of 1.5 × 10−6 and 2.5 × 10−8, respectively, as mentioned earlier. This result clearly indicates that the presence of nuclease in the spent media completely abolishes the natural transformation of ∆opaR mutant. However, when spent media were replaced with fresh DASW, the removal of nuclease led to detectable transformation in ∆opaR mutant.
Discussion
The natural competence in Vibrios is stringently associated with chitin utilization (Fig. 4). In Vibrio cholerae, TfoX, CytR, and RpoS have been showed to control the natural competence in a chitin-dependent manner. The alternative sigma factor RpoS in V. cholerae controls natural transformation through the modulation of quorum sensing regulator HapR [1]. However, later it was reported that RpoS is also required for the liberation of soluble chitin oligosaccharides that serve as an inducing cue for competence [9]. In V. parahaemolyticus, we found that the deletion of RpoS did not affect the natural transformation ability at all. We also observed that the deletion of quorum sensing regulator, OpaR, drastically reduced the transformation ability. These two observations can be justified by two possible explanations. First, unlike V. cholerae, RpoS in V. parahaemolyticus does not affect the quorum sensing regulation. A similar effect was observed in V. harveyi, where quorum sensing is reported to be independent of RpoS regulation [17]. Second, RpoS in V. parahaemolyticus did not regulate the expression of chitinases like in V. cholerae. Thus, we concluded that RpoS was required for neither quorum-sensing dependent nor chitin-dependent regulation of natural competence in V. parahaemolyticus.
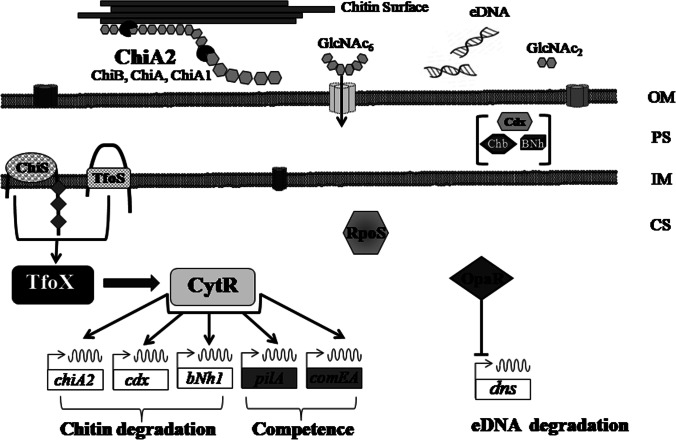
Fig. 4.
Regulators of natural competence in V. parahaemolyticus: a schematic representation. The polymeric chitin is digested predominantly by chitinases to release GlcNAc2-6 moieties which then enter the periplasmic space through porin. GlcNAc4-6 are then further converted into GlcNAc2-3 inside the periplasm with the assistance of periplasmic chitinase. The interaction of sensor histidine kinase, ChiS, and membrane-bound transcriptional regulator, TfoS, with these chitin oligosaccharides activates both of them. ChiS and TfoS cumulatively upregulate the expression of master regulator of competence, i.e., TfoX. Subsequently, TfoX upregulates the expression of cytR, which in turn upregulates the expression of numerous genes such as chiA2, cdx, pilA, and comEA. Another regulator that controls quorum sensing, i.e., OpaR, represses the transcription of dns, a nuclease, due to which the degradation of eDNA is prohibited and promotes natural transformation. The general stress regulator, RpoS, did not play any significant role in natural transformation of V. parahaemolyticus. OM, outer membrane; PS, periplasmic space; IM, inner membrane; CS, cytosol
In V. cholerae, ChiS and TfoS were illustrated as transmembrane regulators that sense the presence of chitin in the environment and induce the state of natural competence by activating TfoX [7, 8, 18]. TfoX was also shown to positively regulate the expression of nucleoside scavenging cytidine repressor, CytR, which in turn control the expression of chitinase chiA1 and competence genes such as pilA and comEA [10]. In V. parahaemolyticus, we found TfoX-induced CytR positively regulates the expression of ChiA2 instead of ChiA1, thus controlling the release of GlcNAc6 from extracellular chitin degradation. In addition, CytR also regulates the expression of periplasmic chitinases such as Cdx, Chb, and BNh which were required for the further degradation of GlcNAc6 inside the periplasm for its uptake through inner membrane transporters. This conclusion was interpreted from the observation that ∆cytR mutant was incapable of growth in purified GlcNAc6 and mRNA expression level of periplasmic chitinases, so it seems that TfoX-dependent transcription factor CytR regulates chitin degradation in a two-step process: first, release of GlcNAc6 from insoluble chitin by controlling the expression of endo-chitinases; and second, breakdown of GlcNAc6 into GlcNAc2/3 inside the periplasm by controlling the expression of periplasmic chitinases. We found that ΔcdxΔchbΔbNh mutant (periplasmic chitinase deficient) was not able to utilize GlcNAc6 like ΔcytR mutant, but was able to uptake eDNA (data not shown). However, the ∆cytR mutant was unable to uptake eDNA in the presence of purified GlcNAc6 due to low-level expression of competence-related genes such as pilA, pilB, comEA, and qstR. Both ∆tfoX and ∆cytR mutants produced similar results in all the experiments. Therefore, we conclude that TfoX regulates chitin-dependent natural competence via CytR. The regulatory mechanism of TfoX and CytR (downstream regulators of transmembrane regulator TfoS) was found to be similar between V. parahaemolyticus and V. cholerae. However, the chitin oligosaccharide that is required for the induction of natural transformation by these two regulators was found to be different in these two Vibrio species. GlcNAc6 can induce ChiS-dependent GlcNAc2 catabolic operon as well as TfoX-dependent natural competence, but GlcNA2 can induce only ChiS-dependent GlcNAc2 catabolic operon in V. parahaemolyticus.
Quorum sensing regulator HapR plays a critical role in modulating natural competence in V. cholerae [1]. The lack of HapR abolishes the DNA uptake by downregulation of chiA1, pilA, and comEA expression and upregulation of dns [1, 11]. In this study, the deletion of OpaR (HapR homolog) in V. parahaemolyticus showed 60-fold reduction in transformation frequency but did not affect the expression of chitinase genes at all, unlike V. cholerae. Therefore, it seems that OpaR might affect the natural transformation in V. parahaemolyticus through its regulation of dns and competence machinery. The obtained result showed that when Dns was present in high concentration in the medium (spent DASW), it could completely abolish transformation in ∆opaR mutant. In the presence of a low amount of Dns (fresh DASW), ∆opaR mutant could not attain WT-like transformation frequency. This observation indicates the possible involvement of OpaR either directly or indirectly with the regulation of competence machinery. In addition, we also found that natural transformation and eDNA-dependent growth could occur simultaneously but inversely related to each other, i.e., the higher the eDNA-dependent growth, the lower the transformation frequency.
Earlier, we found that natural competence can only be induced by the largest chitin oligosaccharide GlcNAc6 in V. parahaemolyticus [12] which makes this process much more specific because relatively few other microbes can take up long chitin oligosaccharides as GlcNAc1-2. However, in the case of V. cholerae, even the smallest chitin oligosaccharide, i.e., GlcNAc2, could induce the state of competence. This is one of the major variations between these two species. In this study, we found some other variations in terms of competence regulation such as redundancy of RpoS in V. parahaemolyticus and OpaR regulates competence by controlling the expression of endonuclease, but not competence genes comEA. Altogether, the phenomenon of natural competence is conserved among Vibrios with slight variations between the species.
Funding
This investigation is supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID; JP20wm0125004) from Ministry of Education, Culture, Sport, Science and Technology in Japan (MEXT) and Japan Agency for Medical Research and Development (AMED). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Cristiano Gallina Moreira
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 2.Gulig PA, Tucker MS, Thiaville PC, Joseph JL, Brown RN. USER friendly cloning coupled with chitin-based natural transformation enables rapid mutagenesis of Vibrio vulnificus. Appl Environ Microbiol. 2009;75:4936–4949. doi: 10.1128/AEM.02564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollack-Berti A, Wollenberg MS, Ruby EG. Natural transformation of Vibrio fischeri requires tfoX and tfoY. Environ Microbiol. 2010;12:2302–2311. doi: 10.1111/j.1462-2920.2010.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Dai J, Morris JG, Jr, Johnson JA. Genetic analysis of the capsule polysaccharide (K antigen) and exopolysaccharide genes in pandemic Vibrio parahaemolyticus O3:K6. BMC Microbiol. 2010;10:274. doi: 10.1186/1471-2180-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vorkapic D, Pressler K, Schild S. Multifaceted roles of extracellular DNA in bacterial physiology. Curr Genet. 2016;62(1):71–79. doi: 10.1007/s00294-015-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Bernardy EE, Hammer BK, Miyashiro T. Competence and natural transformation in Vibrios. Mol Microbiol. 2013;89(4):583–595. doi: 10.1111/mmi.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto S, Mitobe J, Ishikawa T, Wai SN, Ohnishi M, Watanabe H, Izumiya H. Regulation of natural competence by the orphan two-component system sensor kinase ChiS involves a non-canonical transmembrane regulator in V. cholerae. Mol Microbiol. 2014;91:326–347. doi: 10.1111/mmi.12462. [DOI] [PubMed] [Google Scholar]
- 8.Dalia AB, Lazinski DW, Camilli A. Identification of a membrane-bound transcriptional regulator that links chitin and natural competence in Vibrio cholerae. mBio. 2014;5:e01028–13. doi: 10.1128/mBio.01028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalia AB. RpoS is required for natural transformation of Vibrio cholerae through regulation of chitinases. Environ Microbiol. 2016;18(11):3758–3767. doi: 10.1111/1462-2920.13302. [DOI] [PubMed] [Google Scholar]
- 10.Watve SS, Thomas J, Hammer BK. CytR is a global positive regulator of competence, type VI secretion, and chitinases in Vibrio cholerae. PLoS ONE. 2015;10(9):e0138834. doi: 10.1371/journal.pone.0138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blokesch M, Schoolnik GK. The extracellular nuclease Dns and its role in natural transformation of Vibrio cholerae. J Bacteriol. 2008;190(21):7232–7240. doi: 10.1128/JB.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debnath A, Mizuno T, Miyoshi SI. Regulation of chitin-dependent growth and natural competence in Vibrio parahaemolyticus. Microorganisms. 2020;8:E1303. doi: 10.3390/microorganisms8091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano T, Okubo M, Tsuda H, Yokoyama M, Hakamata W, Nishio T. Chitin heterodisaccharide, released from chitin by chitinase and chitin oligosaccharide deacetylase, enhances the chitin-metabolizing ability of Vibrio parahaemolyticus. J Bacteriol. 2019;201:e00270–e319. doi: 10.1128/JB.00270-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong HC, Liu SH, Wang TK, Lee CL, Chiou CS, Liu DP, Nishibuchi M, Lee BK. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl Environ Microbiol. 2000;66:3981–3986. doi: 10.1128/AEM.66.9.3981-3986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroda T, Mizushima T, Tsuchiya T. Physiological roles of three Na+/H+ antiporters in the halophilic bacterium Vibrio parahaemolyticus. Microbiol Immunol. 2005;49:711–719. doi: 10.1111/j.1348-0421.2005.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 16.Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y, Kimura S, Yasunaga T, Honda T, Shinagawa H, Hattori M, Iida T. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet. 2003;361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 17.Lin YH, Miyamoto C, Meighen EA. Cloning, sequencing, and functional studies of the rpoS gene from Vibrio harveyi. Biochem Biophys Res Commun. 2002;293:456–462. doi: 10.1016/S0006-291X(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Izumiya H, Mitobe J, Morita M, Arakawa E, Ohnishi M, Watanabe H. Identification of a chitin-induced small RNA that regulates translation of tfoX gene, encoding a positive regulator of natural competence in V. cholerae. J Bacteriol. 2011;193:1953–1965. doi: 10.1128/JB.01340-10. [DOI] [PMC free article] [PubMed] [Google Scholar]