Abstract
Background
A randomized, double-blind, placebo-controlled phase 2b trial of the tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine was conducted in patients with resected stage III/IV melanoma. Dendritic cells (DCs) were harvested with and without granulocyte-colony stimulating factor (G-CSF). This analysis investigates differences in clinical outcomes and RNA gene expression between DC harvest methods.
Methods
The TLPLDC vaccine is created by loading autologous tumor lysate into yeast cell wall particles (YCWPs) and exposing them to phagocytosis by DCs. For DC harvest, patients had a direct blood draw or were pretreated with G-CSF before blood draw. Patients were randomized 2:1 to receive TLPLDC or placebo. Differences in disease-free survival (DFS) and overall survival (OS) were evaluated. RNA-seq analysis was performed on the total RNA of TLPLDC + G and TLPLDC vaccines to compare gene expression between groups.
Results
144 patients were randomized: 103 TLPLDC (47 TLPLDC/56 TLPLDC + G) and 41 placebo (19 placebo/22 placebo + G). Median follow-up was 27.0 months. Both 36-month DFS (55.8% vs. 24.4% vs. 30.0%, p = 0.010) and OS (94.2% vs. 69.8% vs. 70.9%, p = 0.024) were improved in TLPLDC compared to TLPLDC + G or placebo, respectively. When compared to TLPLDC + G vaccine, RNA-seq from TLPLDC vaccine showed upregulation of genes associated with DC maturation and downregulation of genes associated with DC suppression or immaturity.
Conclusions
Patients receiving TLPLDC vaccine without G-CSF had improved OS and DFS. Outcomes remained similar between patients receiving TLPLDC + G and placebo. Direct DC harvest without G-CSF had higher expression of genes linked to DC maturation, likely improving clinical efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03272-8.
Keywords: Cancer vaccine, Melanoma, Dendritic cell, Immunotherapy, Personalized medicine
Introduction
Immunotherapy, particularly with the introduction of checkpoint inhibitor (CPI) therapy, has drastically changed the management of advanced stage melanoma [1–3]. Additional immunotherapeutic options, such as cancer vaccines, may also have a role in the management of melanoma. Cancer vaccines, in particular, may have a benefit when administered in combination with CPIs [4–6]. Multiple vaccination approaches exist, including dendritic cell (DC)-based vaccines, with promising results [7].
DC-based vaccines capitalize on the active cellular immune response using the most potent antigen-presenting cell (APC), the DC, to prime both cytotoxic T-cell (CTL) and T-helper cell responses specific to tumor antigens. The only FDA-approved dendritic cell-based cancer vaccine, sipuleucel-T (Provenge), targets metastatic castration-resistant prostate cancer by priming DCs ex vivo with the tumor-associated antigen prostatic-acid phosphatase (PAP) linked to granulocyte–macrophage colony-stimulating factor (GM-CSF) [8, 9].
The tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine uses the same potent DC delivery mechanism but loads DCs ex vivo with yeast cell wall particles (YCWPs) containing autologous tumor lysate from an individual’s tumor. This utilizes the highly immunogenic properties of YCWPs to create a personalized vaccine targeting each patient’s unique tumor antigens. The TLPLDC vaccine strategy was first studied in a phase 1/2a trial, which demonstrated safety and potential for efficacy in a variety of solid tumors [10]. This led to the current phase 2b trial of TLPLDC used to prevent recurrence in patients with resected stage III/IV melanoma. The primary analysis of this trial showed no difference in the primary endpoint of 24-month disease-free survival (DFS) by intention-to-treat (ITT) analysis at median follow-up of 19.1 months (38.5 vs 27.0%, p = 0.974). However, in a pre-specified per-treatment (PT) analysis of patients completing the primary vaccine series (PVS) without early recurrence in the first six months, the TLPLDC vaccine group had significantly increased 24-month DFS (62.9 vs 34.8%, p = 0.041) [11].
A key aspect of creating each patient’s individualized TLPLDC vaccine creation is the harvest of DCs. In the current trial, this was accomplished by two methods: a 120 cc blood draw for harvest or a lower volume blood draw (50–70 cc) 24–48 h after administering G-CSF. In previous trials, G-CSF may be used to augment DC collection for production of DC-based vaccines [3, 12, 13]. The protocol allowed for investigators or patients to choose between these two methods. Here, we report the final outcomes from the phase 2b trial comparing placebo to the TLPLDC vaccine, with 36-month disease-free and overall survival. In addition, as prespecified in the trial statistical analysis plan, we compare outcomes of patients vaccinated using the two different DC harvest methods.
Methods
Patient Selection and Randomization
The protocol was approved by the Western Institutional Review Board. Patients with stage IIIA-C or stage IV resectable melanoma were identified prior to definitive surgery and screened for inclusion criteria including anticipation for being clinically disease-free after surgery and other standard of care (SoC) therapies, ability to provide approximately 1cm3 (or 1 mg minimum) of tumor for vaccine production, and ECOG 0–1 performance. Due to a change in standard of care therapy for advanced melanoma, the protocol was amended partway through the trial to allow enrollment of patients on CPI therapy, and to allow treatment with vaccine or placebo concurrently with CPI therapy; patients were required to tolerate CPI therapy alone for 3 months prior to starting study inoculations. Patients were excluded if they had evidence of residual disease after surgery or SoC therapies, immune deficiency such as HIV, active HBV or HCV, or were taking steroids or immunosuppressants. Patients were screened and approached by a research nurse and/or study coordinator. Patients were initially consented prior to definitive surgery during the process of tissue collection by a research nurse coordinator, study coordinator, and/or principal investigator. Patients were consented again after completion of SoC therapy, prior to beginning the vaccination series. Patients, treating physicians, study personnel, and clinical and medical monitors were blinded to treatment groups.
Beginning in February 2015 patients were randomized 2:1 to vaccine or control, with computer-generated randomization tables and a site-balancing algorithm. Initial sample size was calculated to require at least 120 patients with an estimated 2-year recurrence rate of 60% and treatment effect corresponding to a hazard ratio of 0.50, in order to reach a power of 80% to detect a statistical difference between treatment arms with a two-sided alpha of 0.05. Sample size was increased to 140 when a blinded assessment of the first 75 patients found an early recurrence rate of 12% prior to completion of the primary vaccine series (within the first six months). Once patients recurred in either treatment arm, they were offered open-label TLPLDC vaccination as previously described, but were followed for overall survival [14].
Vaccine Production
Vaccine production has been described in greater detail elsewhere [10, 14]. Briefly, tissue collection occurred at time of surgical resection. After completion of SoC therapy, peripheral blood was collected for dendritic cell (DC) generation. Based on patient and provider preference, patients either had 120 ml of peripheral blood drawn, or they received a single injection of 300 μg of subcutaneous G-CSF for pre-DC mobilization with a 50–70 ml peripheral blood draw 24–48 h later. Peripheral blood was sent to a central facility for extraction of peripheral blood monocytes and derivation of immature DCs after exposure to IL-4 and GM-CSF.
Tumor lysate (TL) was created through freeze/thaw cycling of tumor tissue and loaded into yeast cell wall particles (YCWP) derived from S. cerevisae. The YCWPs were loaded with TL, stock CpG oligonucleotides, and tetanus helper peptide (amino acids 948–968, sequence FNNFTVSFWLRVPKVSASHLE). TL-loaded YCWPs were then exposed to immature DCs at day two of incubation for phagocytosis, in the presence of cytokines TNF-α, IL-1β, IL-6, and PGE2. Successful production of the TLPLDC vaccine was confirmed by tests for sterility, DC counts, and DC viability. The process of vaccine production was the same regardless of the blood draw method (with or without G-CSF). For control patients, empty YWCPs were phagocytosed by autologous DCs generated in the same fashion, and the placebo vaccines were similarly tested for sterility and viability.
The vaccine contained 1–1.5 × 106 TLPLDC per dose, while the placebo contained the same number of empty YWCP-loaded DCs. Each dose was provided in individual vials with 250 μl of freezing media. A batch of six doses per patient, to cover the full series, was created and sent to the site regardless of treatment arm. Each dose was thawed and diluted with 500 μl of sterile saline for intradermal injection. These doses were administered at months 0, 1, 2, 6, 12, and 18 months. The inoculations were given in the same lymph node draining area for each respective patient. The primary vaccine series (PVS) was defined as the first three doses. The 6-month, 12-month, and 18-month vaccines were considered boosters.
Analysis Plan
Survival analysis was performed using Kaplan–Meier (K-M) analysis. The primary endpoint of this study was 24-month disease-free survival (DFS), which was previously reported [11]. The secondary endpoints included 36-month DFS and overall survival (OS), as well as safety. Time to recurrence was defined as date of randomization to time of confirmed recurrence. The pre-specified analysis plan included a three-arm subgroup analysis of DFS and OS, analyzing patients receiving G-CSF for vaccine production (TLPLDC + G), those who did not (TLPLDC), and placebo. We additionally performed an exploratory four-arm analysis of DFS and OS comparing TLPLDC, TLPLDC + G, placebo, and placebo with G-CSF (placebo + G). Other exploratory outcomes included investigation of immunologic response to vaccination and correlation of vaccine immune response to clinical outcome.
Statistics were calculated with SPSS (version 22, IBM Corp, Released 2013, Armonk, NY). Demographic data were analyzed using student’s t-test if quantitative and Chi-square if categorical. Comparison of DFS and OS between groups was performed with log-rank analysis with Mantel-Cox log rank test.
Phenotypic Characterization of the TLPLDC Vaccine
RNA-sequencing analysis on the total RNA of patients’ prepared TLPLDC and TLPLDC + G vaccines was performed by ROSALIND® (San Diego, CA; https://rosalind.onramp.bio/) using the TruSeq Stranded mRNA Library Prep Kit High Throughput. Equal samples of randomly selected TLPLDC and TLPLDC + G vaccine doses were analyzed. Data were analyzed with a HyperScale architecture developed by ROSALIND®, reads were trimmed using cutadapt, and quality scores were assessed using FastQC [15, 16]. After aligning reads to the Homo sapiens genome build hg19, individual sample reads were quantified using HTseq and normalized with relative log expression using DESeq2 R library [17–19]. Quality control was performed using RSeQC [20]. DEseq2 was used to calculate fold changes and p-values, and perform covariate correction [19]. Relative RNA expression (RRE) was compared between TLPLDC and TLPLDC + G. These results were correlated with clinical outcomes.
Results
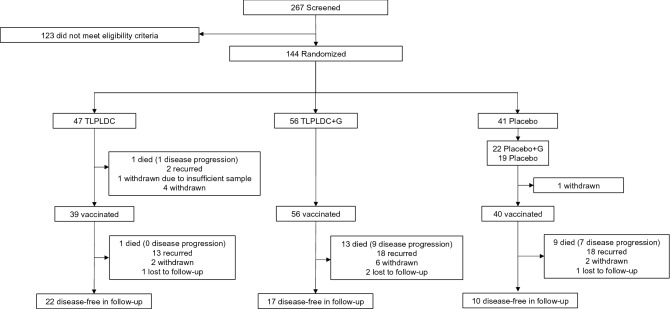
A total of 267 patients were screened and 144 patients were enrolled and randomized. A total of 103 patients were in the vaccine group (47 received TLPLDC, 56 received TLPLDC + G), and 41 in the control group (19 received placebo, 22 received placebo + G) (Fig. 1). Patients in the vaccine group were significantly older than those in the placebo group (median age 65.5 vs. 58.7, p = 0.037), as well as in the three-arm analysis of TLPLDC vs. TLPLDC + G vs. placebo (median age 69.5 vs. 61.7 vs. 58.7, p = 0.042). There were no other significant clinicopathologic or treatment differences between the vaccine and placebo groups or between the three treatment arms when comparing TLPLDC vs. TLPLDC + G vs. placebo (Tables 1, 2, 3). While individual M stage was significantly different between TLPLDC vs. TLPLDC + G vs. placebo vs. placebo + G on 4-arm analysis (p = 0.014), no differences in overall AJCC stage III vs. IV (p = 0.120) or other factors were identified between groups.
Fig. 1.
CONSORT diagram
Table 1.
Demographic and pathologic data with comparison between Vaccine vs. Placebo, and in the 3-arm analysis (TLPLDC vs TLPLDC + G vs placebo)
| Category | Intention to treat analysis | Three-arm analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine (%) | Placebo (%) | p-value | TLPLDC (%) | TLPLDC + G (%) | Placebo (%) | p-value | ||
| Age | ||||||||
| Median | 65.5 | 58.7 | 0.037 | 69.5 | 61.7 | 58.7 | 0.042 | |
| IQR | 55.6 ˗ 73.0 | 49.5˗67.8 | 57.2˗75.2 | 55.5˗70.1 | 49.5˗67.8 | |||
| Race | ||||||||
| Asian | 0 (0) | 1 (2.4) | 0.320 | 0 (0) | 0 (0) | 1 (2.4) | 0.583 | |
| Black | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) | 1 (2.4) | |||
| Hispanic | 3 (2.9) | 1 (2.4) | 1 (2.1) | 2 (3.5) | 1 (2.4) | |||
| Native American | 1 (1.0) | 0 (0) | 0 (0) | 1 (1.8) | 0 (0) | |||
| Unknown | 1 (1.0) | 0 (0) | 0 (0) | 1 (1.8) | 0 (0) | |||
| White | 98 (95.1) | 38 (92.7) | 46 (97.9) | 52 (92.9) | 38 (92.7) | |||
| Ulceration | ||||||||
| Present | 17 (16.5) | 12 (29.3) | 0.316 | 7 (14.9) | 10 (17.9) | 12 (29.3) | 0.316 | |
| Absent | 22 (21.4) | 7 (17.1) | 11 (23.4) | 11 (19.6) | 7 (17.1) | |||
| Not available | 64 (62.1) | 22 (53.7) | 29 (61.7) | 35 (62.5) | 22 (53.6) | |||
| TILS | ||||||||
| Brisk | 3 (2.9) | 2 (4.9) | 0.462 | 1 (2.1) | 2 (3.6) | 2 (4.9) | 0.247 | |
| Mild | 2 (1.9) | 0 (0) | 0 (0) | 2 (3.6) | 0 (0) | |||
| Non-Brisk | 14 (13.6) | 10 (24.4) | 9 (19.1) | 5 (8.9) | 10 (24.4) | |||
| Absent | 8 (7.8) | 1 (2.4) | 5 (10.6) | 3 (5.4) | 1 (2.4) | |||
| Not available | 76 (73.8) | 28 (68.3) | 32 (68.1) | 44 (78.6) | 28 (68.3) | |||
| Biopsy Margins | ||||||||
| Positive | 26 (25.2) | 13 (31.7) | 0.756 | 16 (34.0) | 10 (17.9) | 13 (31.7) | 0.330 | |
| Negative | 46 (44.7) | 19 (46.3) | 18 (38.3) | 28 (50.0) | 19 (46.3) | |||
| Not available | 31 (30.1) | 9 (22.0) | 13 (27.7) | 18 (32.1) | 9 (22.0) | |||
| BRAF Mutation | ||||||||
| Yes | 18 (17.5) | 6 (14.6) | 0.648 | 10 (21.3) | 8 (14.3) | 6 (14.6) | 0.768 | |
| No | 31 (30.1) | 10 (24.5) | 13 (27.7) | 18 (32.1) | 10 (24.4) | |||
| Not available | 54 (52.4) | 25 (61.0) | 24 (51.1) | 30 (53.6) | 25 (61.0) | |||
Table 2.
Staging data with comparison between Vaccine vs. Placebo, and in the 3-arm analysis (TLPLDC vs TLPLDC + G vs placebo)
| Category | Intention to treat analysis | Three-arm analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine (%) | Placebo (%) | p-value | TLPLDC(%) | TLPLDC + G(%) | Placebo (%) | p-value | ||
| AJCC Stage | ||||||||
| III | 82 (79.6) | 32 (78.0) | 0.835 | 42 (89.4) | 40 (71.4) | 32 (78.0) | 0.081 | |
| IV | 21 (20.4) | 9 (22.0) | 5 (10.6) | 16 (28.6) | 9 (22.0) | |||
| T stage | ||||||||
| T0 | 2 (1.9) | 0 (0) | 0.354 | 0 (0) | 2 (3.6) | 0 (0) | 0.587 | |
| Tis | 1 (1.0) | 0 (0) | 0 (0) | 1 (1.8) | 0 (0) | |||
| T1 | 7 (6.8) | 7 (17.1) | 4 (8.5) | 3 (5.4) | 7 (17.1) | |||
| T2 | 21 (20.4) | 4 (9.8) | 10 (21.3) | 11 (19.6) | 4 (9.7) | |||
| T3 | 23 (22.3) | 8 (19.5) | 12 (25.5) | 11 (19.6) | 8 (19.5) | |||
| T4 | 24 (23.3) | 7 (17.1) | 10 (21.3) | 14 (25.0) | 7 (17.1) | |||
| TX | 11 (10.7) | 7 (17.1) | 5 (10.6) | 6 (10.7) | 7 (17.1) | |||
| Unavailable | 14 (13.6) | 8 (19.5) | 6 (12.8) | 8 (14.3) | 8 (19.5) | |||
| N stage | ||||||||
| N1 | 18 (17.5) | 14 (34.1) | 0.168 | 12 (25.5) | 6 (10.7) | 14 (34.1) | 0.296 | |
| N2 | 26 (25.2) | 9 (22.0) | 11 (23.4) | 15 (26.8) | 9 (22.0) | |||
| N3 | 32 (31.1) | 7 (17.1) | 13 (27.7) | 19 (33.9) | 7 (17.1) | |||
| Unavailable | 27 (26.2) | 11 (26.8) | 11 (23.4) | 16 (28.6) | 11 (26.8) | |||
| M stage | ||||||||
| M0 | 71 (68.9) | 26 (63.4) | 0.667 | 40 (85.1) | 31 (55.4) | 26 (63.4) | 0.111 | |
| M1a | 5 (4.9) | 4 (9.8) | 1 (2.1) | 4 (7.1) | 4 (9.8) | |||
| M1b | 6 (5.8) | 1 (2.4) | 1 (2.1) | 5 (8.9) | 1 (2.4) | |||
| M1c | 8 (7.8) | 3 (7.3) | 2 (4.3) | 6 (10.7) | 3 (7.3) | |||
| Unavailable | 13 (12.6) | 7 (17.1) | 3 (6.4) | 10 (17.9) | 7 (17.1) | |||
Table 3.
Prior treatment data with comparison between Vaccine vs. Placebo in the ITT and PT cohorts, and in the 3-arm analysis (TLPLDC vs TLPLDC + G vs placebo)
| Category | Intention to treat analysis | Three-arm analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine (%) | Placebo (%) | p-value | TLPLDC (%) | TLPLDC + G (%) | Placebo (%) | p-value | ||
| Wide local excision | ||||||||
| Yes | 100 (97.1) | 40 (97.6) | 0.876 | 46 (97.9) | 54 (96.4) | 40 (97.6) | 0.895 | |
| No | 3 (2.9) | 1 (2.4) | 1 (2.1) | 2 (3.6) | 1 (2.4) | |||
| Lymph node surgery | ||||||||
| SLNB | 17 (16.5) | 6 (14.6) | 0.077 | 7 (14.9) | 10 (17.9) | 6 (14.6) | 0.267 | |
| LND | 39 (37.9) | 9 (22.0) | 17 (36.2) | 22 (39.3) | 9 (22.0) | |||
| SLNB and LND | 20 (19.4) | 16 (39.0) | 11 (23.4) | 9 (16.1) | 16 (39.0) | |||
| None | 27 (26.2) | 10 (24.4) | 12 (25.5) | 15 (26.8) | 10 (24.4) | |||
| Immunotherapy | ||||||||
| Yes | 41 (39.8) | 15 (36.6) | 0.721 | 19 (40.4) | 22 (39.3) | 15 (36.6) | 0.931 | |
| No | 62 (60.2) | 26 (63.4) | 28 (59.6) | 34 (60.7) | 26 (63.4) | |||
| Checkpoint inhibitor | ||||||||
| Yes | 34 (33.0) | 9 (22.0) | 0.191 | 16 (34.0) | 18 (32.1) | 9 (22.0) | 0.416 | |
| No | 69 (67.0) | 32 (78.0) | 31 (66.0) | 38 (67.9) | 32 (78.0) | |||
| Chemotherapy | ||||||||
| Yes | 7 (6.8) | 1 (2.4) | 0.303 | 2 (4.3) | 5 (8.9) | 1 (2.4) | 0.346 | |
| No | 96 (93.2) | 40 (97.6) | 45 (95.7) | 51 (91.1) | 40 (97.6) | |||
| Radiation therapy | ||||||||
| Yes | 26 (25.2) | 11 (26.8) | 0.844 | 14 (29.8) | 12 (21.4) | 11 (26.8) | 0.615 | |
| No | 77 (74.8) | 30 (73.2) | 33 (70.2) | 44 (78.2) | 30 (73.2) | |||
As previously described, the vaccine was well-tolerated, and production was successful in all patients with a median delivery time of 18 days. The vaccine resulted in significantly improved estimated 24-month DFS by PT analysis as reported by our group [11].
Clinical Results
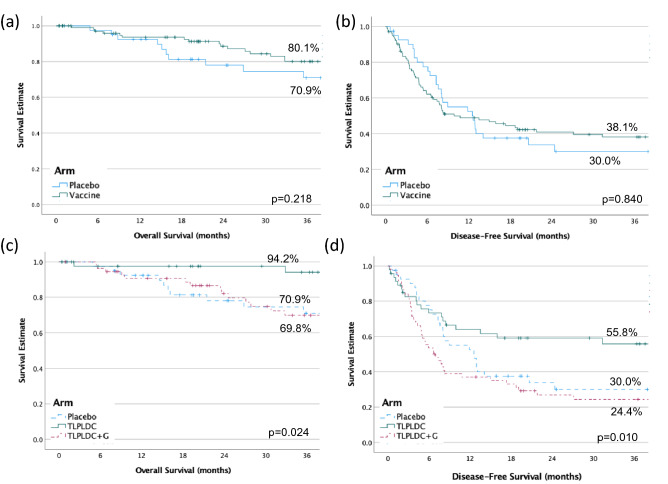
Secondary endpoints included 36-month DFS and OS. At a median follow-up of 27.0 months, by ITT analysis, there was no difference in 36-month estimated survival between the overall vaccine and placebo groups. 36-month estimated DFS was 38.1% for the vaccine group and 30.0% for placebo (HR 0.95 p = 0.840). The 36-month estimated OS was 80.1% for the vaccine group and 70.9% for placebo (HR 0.61, p = 0.218; Fig. 2A/B).
Fig. 2.
36-month overall survival A and disease-free survival B comparing vaccine to placebo, and overall survival C and disease-free survival D comparing TLPLDC, TLPLDC + G, and placebo
By pre-specified three-arm analysis comparing TLPLDC vs. TLPLDC + G vs. placebo, patients receiving TLPLDC had significantly improved OS and DFS, while survival between TLPLDC + G and placebo remained similar. Comparing TLPLDC vs. TLPLDC + G vs. placebo by ITT analysis, respectively, 36-month estimated DFS was 55.8% vs. 24.4% vs. 30.0% (p = 0.010). Similarly, 36-month estimated OS was 94.2% vs. 69.8% vs. 70.9% (p = 0.024; Fig. 2C/D).
A four-arm analysis was additionally performed comparing TLPLDC vs. TLPLDC + G vs. placebo vs. placebo + G, demonstrating similar results. The 36-month estimated DFS was 55.8% vs. 24.4% vs. 30.7% vs. 28.6% (p = 0.025), while 36-month estimated OS was 94.2% vs. 69.8% vs. 76.6% vs. 64.8% (p = 0.042) (Suppl. Figure 1).
Phenotypic Characterization of the TLPLDC Vaccine Results
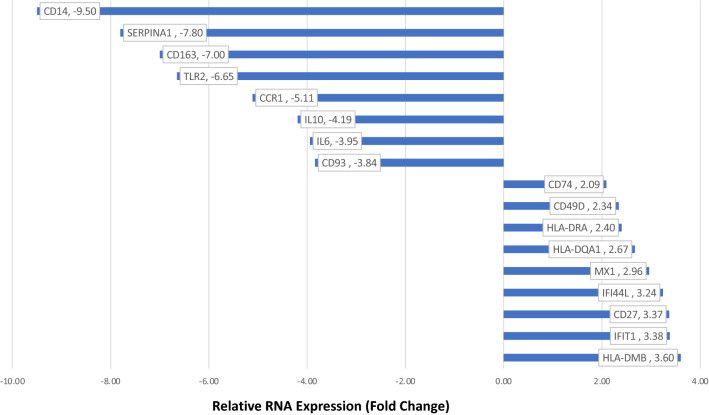
When compared to TLPLDC + G vaccine, RNA-seq from the TLPLDC vaccine showed upregulation of genes associated with DC maturation and antigen presentation, including HLA-DMB (RRE: 3.60), IFIT1 (3.38), CD27 (3.37), IFI44L (3.24), MX1 (2.96), HLA-DQA1 (2.67), HLA-DRA (2.40), CD49D (2.34) and CD74 (2.09). Slight upregulation in CD83 (RRE: 1.14), CD86 (1.05), CD40 (1.05), CD40 ligand (1.43), and PD1 (1.02) were additionally noted.
Downregulated genes were associated with DC suppression or immaturity including CD14 (RRE: 9.50), SERPINA1 (7.8), CD163 (7.00), TLR2 (6.65), CCR1 (5.11), IL10 (4.19), IL6 (3.95), and CD93 (3.84). Figure 3 displays up- and downregulated genes with associated fold changes.
Fig. 3.
Relative RNA expression of genes in the TLPLDC vs. TLPLDC + G vaccine, with associated fold changes
Discussion
In this randomized, double-blind, placebo-controlled phase 2b trial, the 36-month DFS and OS did not differ between the TLPLDC vaccine vs. placebo by ITT analysis. In a pre-specified three-arm analysis and exploratory four-arm analysis; however, patients receiving the vaccine created without G-CSF stimulation at the time of DC harvest had significantly improved DFS and OS compared to the TLPLDC + G and placebo groups. In these patients, TLPLDC vaccine demonstrated up-regulated genes associated with antigen presentation and DC maturation, and down-regulated genes associated with DC immaturity compared to TLPLDC + G vaccine.
While the overall results of this trial were negative, we discovered a profound impact of G-CSF stimulation prior to DC harvest on clinical outcomes, with implications for dendritic cell vaccine production previously unrecognized. The effect of DC maturity on immune response has been demonstrated in some preclinical work. Jonuleit, et al. conducted an in vitro study comparting two DC populations, CD1a + immature DCs cultured in GM-CSF and IL-4, and terminally differentiated, or mature, CD83 + DCs stimulated with select cytokines. When stimulated with melanoma peptides MelanA/MART-1, the mature DCs showed rapid activation and expansion of CD8 + T cells with strong antigen-specific cytotoxicity, while no CD8 + expansion was seen with the immature DCs [21]. This effect was subsequently studied in vivo with stage IV melanoma patients, comparing the peptide-pulsed mature DCs versus immature DCs as described above. The mature DC-based vaccines induced recall antigen-specific CD4 + T-cell responses in 87.5% of patients, compared to only 37.5% of patients receiving DC-based vaccines using immature DCs [22]. The importance of DC maturity has also been described in DC-based vaccines for prostate cancer [23].
While this early data provided some information regarding the effect of DC maturity on immune response, little was known about the specific effects of stimulation with G-CSF in vivo on dendritic cell maturity. G-CSF was included in our clinical trial protocol to increase a patient’s white blood cell count in order to reduce the overall blood volume required for isolation of sufficient monocytes for maturation into DCs. However, G-CSF functions by increasing the number of immature cells in circulation [24]. The vaccine production strategy utilizes a 72-h rapid maturation process, which only permits sufficient time for DC differentiation from mature, but not immature, monocytes as demonstrated by RNA-seq analysis we conducted to investigate differences in observed outcomes [10, 25]. Our current work suggests that the combination of GCS-F stimulation with our protocol timing leads to harvest of relatively immature DCs, which does not promote a tumor-specific immunologic response and, therefore, results in a less effective vaccine [26].
While DC maturity is clearly a significant contributor to clinical efficacy, there may be additional factors that are also important. The TLPLDC + G had relatively higher expression of IL-10, CD14 and TLR2, suggesting a skewed response towards a Th1 phenotype. Specifically, steady-state IL-10 producing DCs may induce tolerance instead of a stimulatory immune response and induce T cell anergy and Tregs [26]. This phenomenon of immature DCs producing tolerance, or even exacerbation of disease, has been demonstrated in pre-clinical models [27, 28]. To avoid inducing tolerance, it is therefore important to ensure that DCs used for vaccines are mature [26]. Given the effects of G-CSF stimulation that we have documented on DC maturity, cells collected 24–48 h after pre-treatment with G-CSF may require additional time for maturation than previously known. Protocols utilizing G-CSF and similar production timing to ours should be cautioned that this combination may result in an ineffective vaccine. These results represent the most robust clinical data demonstrating the significant difference in efficacy based on the maturation of DCs used to create dendritic cell-based cancer vaccines and provide important lessons for future protocols.
This study had several limitations. The study protocol had to be amended midway through the investigation to accommodate for changes to standard of care treatments, namely CPI therapy. Additionally, the use of additional immunotherapy was not randomized, but rather given at the treatment team’s discretion. Exploratory outcomes included study of the immunologic response to vaccination which we have discussed above; however, given the nature of this investigation and the limited number of patients who underwent RNA sequencing, definitive conclusions cannot be made. Protein expression confirmation and detailed immunologic assessment from bio-banked specimens correlating to outcomes and DC function will be the subject of future investigations.
Conclusions
In pre-specified three-arm analysis, the TLPLDC vaccine appears to be clinically effective when produced without G-CSF pre-treatment for DC harvest. This difference between vaccine formulation efficacy is attributable to utilization of DCs with a mature phenotype, and merits further investigation. Confirmatory testing and detailed immunologic assessment correlating outcomes and DC function is forthcoming. A phase III trial is planned, which will standardize vaccine production without G-CSF and compare CPI therapy alone to CPI plus vaccine combination therapy. This trial will not only provide a comparison of the vaccine to modern SoC therapy for melanoma, but also evaluate for synergy between the two treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbrevations
- TLPLDC
Tumor lysate, particle-loaded, dendritic cell
- YCWPs
Yeast cell wall particles
Author contributions
AA, EC, GC, TV, RC, AS, PM, PB, AH, FV, and AT contributed to analysis and interpretation of the results. JH, AB, JJ, JS, MS, XY, TW, MF, and GP contributed to data acquisition and interpretation of results. TW, MF, and GP contributed to the conception and design and interpretation of results. AA, EC, GC, and TV contributed to the drafting of the manuscript. RC, AO, PM, PB, AH, FV, AT, DH, JH, AB, JJ, JS, MS, XY, TW, BF, and GP contributed to revisions of the manuscript. All authors have approved the final version of this manuscript.
Funding
The study was sponsored by Elios Therapeutics, LLC, Greenville, SC.
Declarations
Conflict of interest
The protocol was approved by the Western Institutional Review Board and all patients provided informed consent for participation. Dr. Faries is an advisor for Bristol-Myers Squibb, Sanofi, Array Bioscience and Pulse Bioscience. Dr. Wagner is an employee of Orbis Health Solutions. Dr. Peoples is employed by Orbis Health Solutions and Cancer Insight; is a consultant for Rapamycin Holdings, Heat Biologics, Abexxa Biologics, and Pelican Therapeutics; and has received funding from the above as well as Sellas Life Sciences and Genentech. Dr Jakub served on a Novartis Melanoma Surgical Oncology Advisory Board. Dr. Clifton is employed by Parthenon Therapeutics. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the Department of the Army, Department of the Air Force, Department of Defense, or the US Government. The voluntary, fully informed consent of the subjects used in this research was obtained as required by 32 CFR 219 and DODI 3216.02_AFI40-402.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davar D, Tarhini AA, Kirkwood JM. Adjuvant immunotherapy of melanoma and development of new approaches using the neoadjuvant approach. Clin Dermatol. 2013;31(3):237–250. doi: 10.1016/j.clindermatol.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2013;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J hematol oncol. 2018;1:1–25. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vreeland TJ, et al. Gaining ground on a cure through synergy: combining checkpoint inhibitors with cancer vaccines (in eng) Expert Rev Clin Immunol. 2016;12(12):1347–1357. doi: 10.1080/1744666X.2016.1202114. [DOI] [PubMed] [Google Scholar]
- 5.Kleponis J, Skelton R, Zheng L. Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol Med. 2015;12(3):201–208. doi: 10.7497/j.issn.2095-3941.2015.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madan RA, Heery CR, Gulley JL. Combination of vaccine and immune checkpoint inhibitor is safe with encouraging clinical activity. OncoImmunology. 2014;1(7):1167–1168. doi: 10.4161/onci.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. "Clinical use of dendritic cells for cancer therapy," (in eng) Lancet Oncol. 2014;15(7):e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.Small EJ, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18(23):3894–3903. doi: 10.1200/jco.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 10.Herbert GS, et al. "Initial phase I/IIa trial results of an autologous tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine in patients with solid tumors," (in eng) Vaccine. 2018;36(23):3247–3253. doi: 10.1016/j.vaccine.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 11.Vreeland TJ, Clifton GT, Hale DF, Chick RC, Hickerson AT, Cindass JL, Adams AM, Bohan PM, Andtbacka RH, Berger AC, Jakub JW. A phase IIb randomized controlled trial of the TLPLDC vaccine as adjuvant therapy after surgical resection of Stage III/IV Melanoma: a primary analysis. Annal surg oncol. 2021;11:6126–6137. doi: 10.1245/s10434-021-09709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen WS, Svrivastava AK, Batra L, Barsoumian H, Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev Vaccines. 2018;17(3):207–215. doi: 10.1080/14760584.2018.1434000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams AM, Chick RC, Vreeland TJ, Clifton GT, Hale DF, McCarthy PM, O’Shea AE, Bohan PM, Hickerson AT, Park H, Sloan AJ. Safety and efficacy of autologous tumor lysate particle-loaded dendritic cell vaccination in combination with systemic therapies in patients with recurrent and metastatic melanoma. Melanoma res. 2021;31(4):378–388. doi: 10.1097/CMR.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 15.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j. 2011;17(1):10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 16.S. Andrews, "FastQC: a quality control tool for high throughput sequence data.," 2010.
- 17.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2014;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Gen biol. 2014;12:1–21. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28(16):2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 21.Jonuleit H, Giesecke A, Kandemir A, Paragnik L, Knop J, Enk AH. Induction of tumor peptide-specific cytotoxic T cells under serum-free conditions by mature human dendritic cells. Arch dermatol res. 2000;292(7):325–32. doi: 10.1007/s004030000144. [DOI] [PubMed] [Google Scholar]
- 22.Jonuleit H, et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93(2):243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 23.Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS ONE. 2011;6(4):e18801. doi: 10.1371/journal.pone.0018801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, et al. "Activities of granulocyte-macrophage colony-stimulating factor and interleukin-3 on monocytes," (in eng) Am J Hematol. 2004;75(4):179–189. doi: 10.1002/ajh.20010. [DOI] [PubMed] [Google Scholar]
- 25.Obermaier B, Dauer M, Herten J, Schad K, Endres S, Eigler A. "Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes," (in eng) Biol Proced Online. 2003;5:197–203. doi: 10.1251/bpo62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sittig SP, de Vries IJM, Schreibelt G. Primary Human Blood Dendritic Cells for Cancer Immunotherapy-Tailoring the Immune Response by Dendritic Cell Maturation. Biomedicines. 2015;3(4):282–303. doi: 10.3390/biomedicines3040282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234(1):142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, et al. DNA array and biological characterization of the impact of the maturation status of mouse dendritic cells on their phenotype and antitumor vaccination efficacy. Cell Immunol. 2001;214(1):60–71. doi: 10.1006/cimm.2001.1883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.