Abstract
Streptococcus mutans glucan-binding protein A (GbpA) has sequence similarity in its carboxyl-terminal domain with glucosyltransferases (GTFs), the enzymes responsible for catalyzing the synthesis of the glucans to which GbpA and GTFs can bind and which promote S. mutans attachment to and accumulation on the tooth surface. It was predicted that this C-terminal region, comprised of what have been termed YG repeats, represents the GbpA glucan-binding domain (GBD). In an effort to test this hypothesis and to quantitate the ligand-binding specificities of the GbpA GBD, several fusion proteins were generated and tested by affinity electrophoresis or by precipitation of protein-ligand complexes, allowing the determination of binding constants. It was determined that the 16 YG repeats in GbpA comprise its GBD and that GbpA has a greater affinity for dextran (a water-soluble form of glucan) than for mutan (a water-insoluble form of glucan). Placement of the GBD at the carboxyl terminus was necessary for maximum glucan binding, and deletion of as few as two YG repeats from either end of the GBD reduced the affinity for dextran by over 10-fold. Interestingly, the binding constant of GbpA for dextran was 34-fold higher than that calculated for the GBDs of two S. mutans GTFs, one of which catalyzes the synthesis of water-soluble glucan and the other of which catalyzes the synthesis of water-insoluble glucan.
Cariogenic bacteria such as Streptococcus mutans cause tooth decay in the presence of sucrose due to their acidogenicity, aciduricity, and ability to synthesize extracellular polysaccharides called glucans (7, 16). Lactic acid is the major end product of fermentation by S. mutans and, under cariogenic conditions, results in a persistent reduction in plaque pH, which leads to tooth demineralization. The extracellular glucans promote adherence and are critical for increasing the proportions of S. mutans in plaque. S. mutans possesses three glucosyltransferases (GTFs) capable of catalyzing the synthesis of glucan. GTF-I (product of gtfB) produces a water-insoluble glucan called mutan, which is rich in alpha-1,3-glucosidic linkages (25). The glucan produced by GTF-S (product of gtfD) is similar to dextran in that it is mostly water soluble and is rich in alpha-1,6-linkages (9). GTF-SI (product of gtfC) produces a mixture of water-soluble and insoluble glucans (8). All three enzymes consist of an amino-terminal enzymatic domain and a carboxyl-terminal glucan-binding domain (GBD) (11, 15, 24). S. mutans also synthesizes at least three nonenzymatic glucan-binding proteins; among them is GbpA, which contains a C-terminal GBD homologous to the GBDs of the GTFs (2). A loss of GbpA is associated with an increase in caries in a rat model and an accompanying accumulation of mutants that have undergone recombination between the gtfB and gtfC genes (10). The GBDs of the three GTFs and GbpA consist of repeating units of 21 amino acids, which were termed YG repeats by Giffard and Jacques (4), due to the consistent presence of one or more tyrosine residues near the beginning of the repeat and a highly conserved glycine in the middle of the repeat. It was shown that the repeats are homologous not only to each other within a protein but also to the repeats found in the GTFs from other oral streptococci, toxins A and B from Clostridium difficile, and the autolysins from Streptococcus pneumoniae and its bacteriophages (28).
Several studies have found that GTFs and GbpA can bind to both dextran and mutan (5, 15, 21, 26). Glucan binding appears to be specific for alpha-1,6-linkages, and this is true even for mutan, which consists predominantly of alpha-1,3-linkages (5, 21). Despite the apparent similarities in the glucan-binding properties of each of these proteins, the relative affinities of the individual GBDs for dextran and mutan have not been compared. In this study, we propose to determine if the YG repeats of GbpA represent its full GBD, determine whether its affinity for water-soluble glucan matches its affinity for water-insoluble glucan, and determine if the GbpA GBD has an affinity for glucan similar to that of the GBDs of GTFs.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli JM109 (20) was grown aerobically at 37°C in 2×YT medium (Difco, Detroit, Mich.) and was used in all cloning and protein purification procedures. S. mutans 3209 serotype c is a human isolate which is more efficient at producing mutan and adhering to surfaces than other laboratory strains (21). S. mutans was grown aerobically at 37°C in brain heart infusion (Difco).
Cloning of fusion proteins.
All DNA-modifying enzymes were purchased from Promega (Madison, Wis.) and used according to the instructions provided by the manufacturer. Cloning and plasmid transformation were done by standard procedures (1, 22). Taq DNA polymerase (Promega) or ULTma DNA polymerase (Perkin-Elmer, Branchburg, N.J.) was used in PCRs according to the instructions supplied by the manufacturer. Primers were supplied by GIBCO BRL (Grand Island, N.Y.).
The DNA region encoding the GBD beginning with amino acid 158 and proceeding to the carboxyl terminus (2) of S. mutans GbpA was amplified by PCR with the 5′ phosphorylated primers 5′-TCTCTCCAACCAATAGCTTCTTT-3′ and 5′-CCGCCATATTTACCGTTTTCAA-3′. The PCR product was ligated to the EcoRV site of the PinPoint Xa1 vector (Promega), yielding plasmid pDPL4. The plasmid expressed a fusion protein (B-GBD) containing the biotinylated 1.3S transcarboxylase subunit (TCS) from Propionibacterium shermanii and the GBD. B-GBD could be detected with anti-GbpA antibody or with avidin-horseradish peroxidase on a Western immunoblot.
Plasmids pDPL4-108 and pDPL4-78 were generated the same way as pDPL4, except that the phosphorylated oligonucleotides 5′-TATCTTTCCTAGATCCTGACACC-3′ and 5′-TAAGTGATTCACCTTCAGTTTTG-3′, respectively, were used as upstream PCR primers, while the downstream primer was the same as that used to generate pDPL4. After induction, pDPL4-108 expressed a fusion protein, B–GBD-10n, which was truncated by six YG repeats at the N terminus of the domain. B–GBD-14n was expressed by pDPL4-78 and had a deletion of two YG repeats at the N terminus.
To generate fusion proteins with deletions at the carboxyl terminus, the GBD in pDPL4 was PCR amplified with the primers 5′-CAGGGTCTCATCAAGATCGGGG-3′ and 5′-GTGCACTAGTGATTCTTAACAACTTGTCCACT-3′. The PCR product was cut with the restriction enzymes BamHI and SpeI and ligated into the 3.4-kb fragment of pDPL4 cut with the same endonucleases. This procedure made codon 410 of GbpA the new stop codon and left intact all downstream regulatory sequences and the junction between TCS and GBD. The resulting plasmid, pDPL-10c, expressed a fusion protein, B–GBD-10c, which had six YG repeats removed from its C terminus. pDPL4-14c was cloned in the same fashion, except that the downstream primer was replaced by 5′-ATAGACTAGTAGCCTGATACACGTTTGCCA-3′, resulting in a stop codon at position 520 of GbpA. The protein expressed by this plasmid, B–GBD-14c, had a deletion of two YG repeats at the C terminus.
The TCS was fused to the N terminus as well as to the C terminus to construct pDPL-B. The SpeI restriction site in pDPL4, including the stop codon in GBD, was replaced by an XbaI site, and a new NsiI restriction site was introduced further upstream by use of the phosphorylated primer 5′-GAAGTTATGCATAGTATCTAGATGGTAAA-3′ and the U.S.E. Mutagenesis Kit (Pharmacia Biotech, Uppsala, Sweden) as recommended by the supplier. The new plasmid, pDPL-NX, was cut with NsiI and XbaI to allow subcloning of the TCS gene. The transcarboxylase gene in PinPoint Xa1 was amplified with primers 5′-TCACATGCATCTGAAGGTAACAGTC-3′ and 5′-CGATCTAGATGACACTATAGAACCAG-3′ and cut with NsiI and XbaI. The ligation product, pDPL-B, expressed a 75-kDa protein, B-GBD-B.
A GBD-streptokinase C (SKC) fusion protein was generated by PCR amplification and ligation of the GBD from GbpA upstream of the SKC gene in pKM2 (12), yielding pGS1.
The GBDs of the three GTFs from S. mutans were fused to TCS as follows. The GBD of GTF-I was PCR amplified with primers 5′-GTTGGGTACCCTTATGACGGTAAAG-3′ and 5′-AAGAGATCTAAAAAACAATTAGTTAATCCG-3′. These primers created restriction sites for KpnI and BglII, which allowed ligation into the PinPoint Xa1 vector in frame with TCS. The resulting plasmid, pGB, expressed a fusion protein (B-GB) containing TCS and the GBD of GTF-I. The GBD of GTF-SI was ligated to TCS in plasmid pGC, expressing B-GC, by the same PCR and cloning approach (primers: 5′-GTAACTGGGTACCTATTTGATGGTAAAGG-3′ and 5′-ATAATCAAAATACAGATCTTCTAAGATAAG-3′). The GBD of GTF-S was subcloned as follows. Primers 5′-CTGAAATCAAAAATAGGATCCATGCTGCC-3′ and 5′-GGTCAATGCGGATCCGTCTTCCAT-3′ generated a PCR product that contained BamHI restriction sites at either end. The BamHI-digested PCR product was ligated into the BamHI site of the PinPoint Xa1 vector to yield plasmid pGD and fusion protein B-GD.
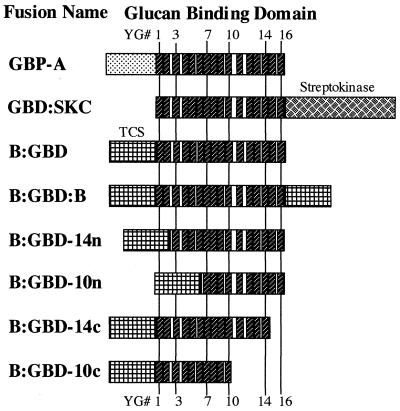
Table 1 and Fig. 1 list and schematically represent, respectively, the various fusion proteins.
TABLE 1.
Plasmids and proteins used in this study
| Plasmid | Protein | Size (kDa) | Feature | Reference or source |
|---|---|---|---|---|
| pMLG43 | GbpA | 59.0 | Native protein, secreted by S. mutans | 21 |
| GBD | 47.8 | GBD from GBP-A (unless noted otherwise) | ||
| PinPoint Xa1 | TCS | 13.1 | TCS from P. shermanii, biotinylated in vivo | Promega |
| pDPL4 | B-GBD | 60.9 | Fusion protein between TCS and GBD from GbpA, 16 YG repeats, biotinylated | This study |
| pDPL-B | B-GBD-B | 75.5 | Same as B-GBD, but TCS at both N and C termini | This study |
| pDPL4-78 | B–GBD-14n | 53.8 | Same as B-GBD, but two YG repeats deleted from the N terminus | This study |
| pDPL-108 | B–GBD-10n | 42.6 | Same as B-GBD, but six YG repeats deleted from the N terminus | This study |
| pDPL-14c | B–GBD-14c | 55.6 | Same as B-GBD, but two YG repeats deleted from the C terminus | This study |
| pDPL-10c | B–GBD-10c | 42.9 | Same as B-GBD, but six YG repeats deleted from the C terminus | This study |
| pKM2 | SKC | 47 | SKC from Streptococcus equisimilis | 12 |
| pGS1 | GBD-SKC | 95 | Fusion between GBD at the N terminus and SKC at the C terminus | This study |
| pGB | B-GB | 60.8 | Fusion between TCS and the GBD from GTF-I from S. mutans (similar to B-GBD) | This study |
| pGD | B-GD | 62.4 | Fusion between TCS and the GBD from GTF-S from S. mutans (similar to B-GBD) | This study |
FIG. 1.
Schematic representation of fusion proteins containing a GBD. The positions of individual YG repeats (small hatched boxes) were kept constant in the figure. Numbers indicate how many repeats were still present in the truncated protein, and “n” or “c” indicates the terminus where the deletion occurred. GbpA carries its native N-terminal domain, while GBD-SKC contains the enzyme SKC at the C terminus of the GBD. All other fusion proteins were made with TCS and GBD. B-GB and B-GD (not shown) were similar to B-GBD, except that the GBD was derived from GTF-I and GTF-S, respectively.
Protein purification.
E. coli JM109 cells containing a PinPoint Xa1-based plasmid were grown at 37°C in 2×YT medium (1) containing 100 μg of ampicillin per ml and 2 μM biotin. Overnight cultures were diluted 1:100 in fresh medium and allowed to grow for 1 h before expression of the fusion protein was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 100 μM. The cells were harvested after 4 to 5 h, resuspended in 10 volumes (weight/volume) of TEN3 buffer (20 mM Tris-HCl [pH 7.2], 1 mM EDTA, 300 mM NaCl, 100 μM phenylmethylsulfonyl fluoride), and disrupted by two passes through a French pressure cell at 20,000 lb/in2; cellular debris was removed by centrifugation.
The supernatant was incubated for 2 h at 4°C with Sephacryl S1000 (Sigma), which acted as an affinity matrix for proteins containing a GBD. The matrix was washed extensively with TEN3 buffer and 0.5 M guanidinium hydrochloride. Bound protein was eluted with 6 M guanidinium hydrochloride and dialyzed at 4°C overnight against 0.1 M phosphate buffer (pH 6.8) with multiple changes of buffer.
In a second purification step, the protein sample was incubated for 2 h at 4°C with an avidin resin (SoftLink; Promega) which specifically binds to biotinylated proteins, such as B-GBD. The matrix was washed with 0.1 M phosphate buffer, and proteins were eluted with the same buffer containing 5 μM biotin. The proteins were dialyzed overnight at 4°C against a buffer of choice and used for analysis on the following day. Six to eight micrograms of B-GBD was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, visualized with a silver stain kit from Boehringer Mannheim Biochemicals (Indianapolis, Ind.), and found to be free of other proteins.
Affinity electrophoresis.
Protein affinities were measured with native polyacrylamide gels as described previously (14) with some modifications. Discontinuous 10% polyacrylamide gels were made by the method of Laemmli (13). Dextran (Sigma or Fisher) or mutan was added to the separating gel solution before the gels were poured, resulting in a polyacrylamide-polysaccharide matrix that retarded the migration of proteins that bound to the glucan present in the gels. Mutan was produced by S. mutans 3209 and prepared as described previously (17, 21). The amounts of ammonium persulfate and N,N,N′,N′-tetramethylethylenediamine in the gels were doubled to prevent settling of the water-insoluble mutan. Gels were run in a Hoefer SE250 gel apparatus at 200 V for 90 min at 10°C. Proteins were then blotted onto Optitran nitrocellulose (Schleicher & Schuell, Inc., Keene, N.H.), incubated with a rabbit anti–B-GBD antibody (Biodesign International, Kennebunk, Maine), and visualized with a secondary anti-rabbit horseradish peroxidase- or alkaline phosphatase-conjugated immunoglobulin G antibody (Sigma). Retardation units (RU) were calculated as follows: RU = (B/A) − (B0/A0), with A0 being the distance between the 84-kDa molecular mass (Bio-Rad, Hercules, Calif.) marker and the 207-kDa marker, B0 being the distance between the 84-kDa marker and the sample in gels without any added polysaccharides, and A and B being the equivalent of A0 and B0 in gels with polysaccharides added.
PEG precipitation.
Binding constants for dextran were determined by utilizing the highly hydrophilic nature of the proteins as follows. Protein samples in TN buffer (20 mM Tris-HCl, 100 mM NaCl [pH 7.2]) at a final concentration of 2 to 2.5 μM were incubated with fluorescein isothiocyanate (FITC)-labeled dextran (FD4; Sigma) at a final concentration of 0.1 to 20 mM. After 30 min at room temperature, protein and protein–FITC-dextran complexes were precipitated by the addition of an equal volume of cold 50% polyethylene glycol (PEG; molecular weight, 3,350; Sigma) for 5 min at 4°C. Precipitates were removed by centrifugation at high speed in an Eppendorf microcentrifuge for 30 min at 4°C. The supernatant, containing only the unbound FITC-dextran, was immediately separated from the pellet after centrifugation. FITC-dextran concentrations in the supernatant were measured on a Perkin-Elmer LS50B luminescence spectrometer with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. The slit width was adjusted according to the FITC-dextran concentration. A standard curve was generated each time by measuring various concentrations of FITC-dextran in TN buffer containing 25% PEG. The amount of bound FITC-dextran was determined by subtracting the amount of free FITC-dextran from the initial concentration. The same values (although slightly lower) were obtained when the pellet fraction was brought back to its initial volume and then heated to 50°C for 10 min to denature the protein. Values for free and bound FITC-dextran were divided by the concentration of protein (all concentrations were micromolar) to adjust all data to a 1 μM protein concentration. Data were analyzed by the method of Scatchard (23).
RESULTS
Previous experiments indicated that the GbpA GBD was able to bind to the alpha-1,6-glucosidic linkages within dextran and mutan (5, 21). However, questions concerning the affinity for the ligand, the role of the repeating units, and the significance of the C-terminal location of the binding domain were left unanswered. To address these questions, several fusion proteins were constructed. Figure 1 and Table 1 provide an overview of all the fusion proteins that were tested.
GbpA GBD.
The YG repeat domain within GbpA was predicted to represent the GBD based on reports of similar GBDs in the GTFs (11, 15, 19). To confirm this prediction, we constructed fusion proteins which contained all 16 YG repeats of GbpA. Their glucan-binding properties were quantitatively compared to those of native GbpA in an affinity electrophoresis assay in which the migration of proteins capable of binding dextran or mutan was retarded relative to that on a gel that did not contain either of these glucans. Preliminary experiments in which the concentration or molecular weight of dextran added to the gels was varied allowed the determination of optimal assay conditions to ensure that any retardation of protein migration was due to a specific interaction with dextran or mutan and not to nonspecific physical effects of the addition of glucans to the gel. The data are presented in RU, as described in Materials and Methods, where an RU of 0 indicates that the protein does not bind at all at the indicated glucan concentration.
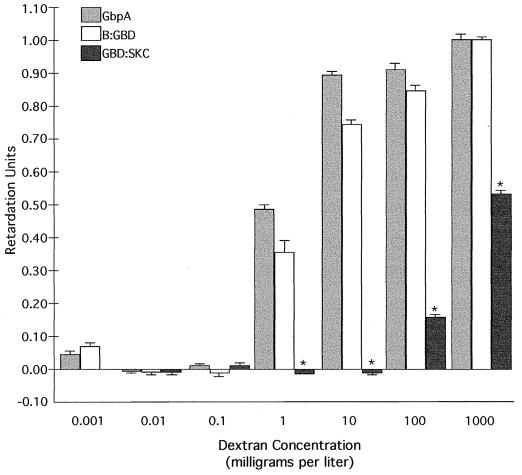
The results for GbpA, B-GBD, and GBD-SKC tested in gels containing dextran are shown in Fig. 2. GbpA and B-GBD required dextran concentrations of greater than 0.1 mg/liter within the gel matrix in order for their migration to be inhibited. Retardation then increased with increasing dextran concentration up to the largest amount tested. The results for GbpA and B-GBD were similar whether purified protein or an E. coli lysate was used and were statistically identical except at a dextran concentration of 10 mg/liter. In contrast, GBD-SKC, although able to bind dextran, required at least a 100-fold-higher dextran concentration to reach an RU value comparable to those for GbpA and B-GBD.
FIG. 2.
Binding of GbpA, B-GBD, and GBD-SKC to various concentrations of dextran in the gel retardation assay. Purified proteins or cellular lysates containing the protein of interest were run on native gels containing different amounts of dextran (molecular weight, 500,000). Proteins were blotted onto nitrocellulose and stained with an anti-GBD antibody. RU were calculated as described in the text. Asterisks indicate that the migration of GBD-SKC was reduced significantly compared to those of both GbpA and B-GBD, based on a Student t test (P, <0.01). The migration of GbpA and that of B-GBD did not differ statistically, except at a dextran concentration of 10 mg/liter. Error bars show standard deviations.
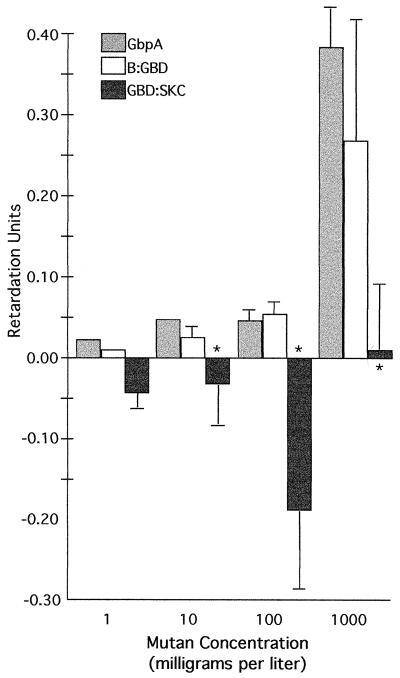
When tested in gels containing mutan, GbpA and B-GBD had equal affinities, although a concentration of 100 mg/liter or higher was required to detect binding (Fig. 3). GBD-SKC did not bind mutan in any detectable manner at the concentrations tested.
FIG. 3.
Binding of GbpA, B-GBD, and GBD-SKC to various concentrations of mutan in the gel retardation assay. Purified proteins or cellular lysates containing the protein of interest were run on native gels containing different amounts of mutan. Proteins were blotted onto nitrocellulose and stained with an anti-GBD antibody. RU were calculated as described in the text. The asterisks indicate that the migration of GBD-SKC was reduced significantly compared to those of both GbpA and B-GBD (except at a mutan concentration of 10 mg/liter, where the standard deviation of GbpA was zero and therefore could not be statistically tested), based on a Student t test (P, <0.03). The migration of GbpA and that of B-GBD did not differ statistically. Error bars show standard deviations.
Since the glucan-binding properties of B-GBD and GbpA were nearly identical, the B-GBD fusion was used for further study. The diminished glucan binding of GBD-SKC was striking and may have been due to the amino-terminal rather than the carboxyl-terminal location of the GBD. The placement of the GBD within a protein was therefore addressed.
C-terminal location of the GBD.
All naturally occurring proteins that contain the YG repeat motif have this series of repeats at the carboxyl terminus of the protein (4, 28). Since the comparison of the glucan-binding properties of B-GBD and GBD-SKC could potentially be complicated by inhibitory effects unique to the presence of SKC, we constructed an additional fusion protein in which a second TCS was placed C terminal to B-GBD to yield B-GBD-B. Since B-GBD demonstrated full glucan-binding capacity, a comparison to B-GBD-B would indicate to what extent a free carboxyl terminus was necessary for optimal binding. Both B-GBD and B-GBD-B could be easily purified by passage over affinity columns. Therefore, the binding of these fusion proteins to dextran was determined by calculation of dissociation constants with FITC-labeled dextran instead of affinity electrophoresis. The resulting Kd values were 8.5 × 10−8 M for B-GBD and 7.3 × 10−6 M for B-GBD-B. Thus, the dissociation constant for B-GBD-B and FITC-dextran was more than 100-fold lower than that for B-GBD and FITC-dextran.
Effects of truncating the GBD.
Work by other investigators (11, 15, 26) has shown that the addition of YG repeats to a GBD resulted in an increased affinity for glucan, while the deletion of YG repeats was associated with a decrease in glucan-binding affinity. Since these deletions were made at the carboxyl terminus of the GBD, it was of interest to determine if the observed decrease in glucan binding was due to disruption of the native carboxyl terminus or whether the deletion of YG repeats anywhere within the GBD would be equivalent. Several fusion proteins were constructed based on the deletion of either 2 or 6 of the YG repeats from the 16 normally found in the GBD of GbpA and B-GBD. The fusion proteins had the deletions at either the amino terminus (B–GBD-14n and B–GBD-10n) or the carboxyl terminus (B–GBD-14c and B–GBD-10c) of the GBD (Fig. 1 and Table 1). Purification of these truncated fusion proteins proved difficult in some cases, perhaps due to protein instability. Therefore, the dextran-binding affinities of the truncated fusion proteins were tested by binding to FITC-dextran when they could be purified efficiently or by affinity electrophoresis of a cellular lysate when purification was difficult. No matter which method was used, the loss of just two YG repeats from either the C terminus or the N terminus reduced glucan binding by over 10-fold, and the loss of six YG repeats reduced binding by over 100-fold.
GBDs of the GTFs.
The GTFs of S. mutans have GBDs similar to the GBD of GbpA. However, the GTFs synthesize glucans that can vary dramatically in the proportion of alpha-1,6-linkages, and these glucans bind the GBD during their synthesis. It was therefore of interest to ascertain whether fusion proteins containing the GBDs of the GTFs would differ in their affinities for glucan compared to each other and compared to the GBD of GbpA. Fusion proteins were constructed with the TCS and the GBD from each of the GTFs, as had been done with the GbpA GBD. These were designated B-GB (GBD encoded by gtfB), B-GC (GBD encoded by gtfC), and B-GD (GBD encoded by gtfD). Despite the fact that each protein contained the complete GBD of a GTF, only B-GD was stable and easily purified in large enough quantities for the determination of a dissociation constant. B-GB was more difficult to obtain in large quantities, but the dissociation constant could be estimated. The dissociation constant calculated for B-GD was 2.5 × 10−7 M, and that calculated for B-GB was 2.4 × 10−7 M. Interestingly, the dissociation constants of B-GB and B-GD for FITC-dextran were nearly identical, even though B-GB contained the GBD of a GTF that synthesizes glucan with predominantly alpha-1,3-linkages and B-GD contained the GBD of a GTF that synthesizes glucan with predominantly alpha-1,6-linkages. The affinity of these fusion proteins was 34-fold lower than that of B-GBD, a result which might reflect differences in the functions of the binding domains in each of these proteins.
DISCUSSION
The nonenzymatic glucan-binding proteins of S. mutans have been thought to contribute to plaque formation, but the precise details of their contributions are unknown. We therefore undertook an investigation of the glucan-binding properties of GbpA which focused on the YG repeats as the GBD, the relative binding to dextran versus mutan, and a comparison of its binding affinity with those of other glucan-binding proteins.
The data in this report were generated by two different glucan-binding assays. The comparisons made by affinity electrophoresis provided semiquantitative data for binding to either dextran or mutan. However, the sensitivity and range over which retardation could be measured were limited. Therefore, the alternative method, which measured bound and free FITC-dextran, was used for the calculation of dissociation constants. By its nature, this assay was limited to measuring binding to dextran and could not be adapted to measuring binding to the water-insoluble mutan.
The inclusion of the YG repeat domain from GbpA in a fusion with the TCS to form B-GBD and the ability of the fusion protein to bind glucan with an affinity identical to that of native GbpA in all but one instance provided evidence that the YG repeat domain comprised the complete GBD of GbpA. Thereafter, similar fusions with variations of the GbpA GBD or GBDs from other proteins were constructed and provided the basis for the comparisons in this study. However, some of the fusion proteins were unstable, even in the presence of protease inhibitor cocktails. Kato and Kuramitsu (11) reported similar problems with proteins containing parts of the GBD of GTF-I, and the tendency of GbpA and the GTFs to degrade when isolated from S. mutans culture supernatants was described by Russell (21).
It was found that a much higher concentration of mutan than of dextran was required for the retardation of B-GBD migration. Since B-GBD is specific for alpha-1,6-linkages (no binding is observed to pseudonigeran, a polymer homogeneous in terms of alpha-1,3-linkages), the lower proportion of alpha-1,6-linkages in mutan may be the simple explanation for the decreased affinity. Nonetheless, this observation is interesting, since the ability of S. mutans to synthesize mutan is essential for efficient colonization and caries development (3, 20), and it might have been predicted that a higher binding affinity for mutan would better facilitate plaque development.
Fusion proteins that contained the GBD in the center (B-GBD-B) or at the N terminus of the protein (GBD-SKC) were able to bind dextran, but their affinities were at least 100-fold lower than that of B-GBD. Since it has been reported that YG repeats could be added to the carboxyl terminus of a GTF GBD with a resulting increase in affinity for dextran (26), it is clear that fusions can be made to the C terminus without disrupting glucan binding. However, if a fusion consists of something other than YG repeats, there may be a disruption of the predicted beta-sheet structure (6) of the GBD, resulting in a reduction in the ability of the GBD to interact with glucan. Therefore, a free carboxyl terminus may be necessary for the GBD to engage in optimal glucan binding and may explain why all proteins that have the YG motif have the repeats located at the C terminus of the protein. It is also possible that the B-GB and B-GC fusion proteins were less stable because sequences upstream of the GBD in these GTFs may be necessary for proper folding of the GBD.
Proteins that contained a truncated GBD had a lower affinity for dextran and mutan than did B-GBD. This reduction in affinity increased with increasing loss of YG repeats, but the 90% reduction associated with the removal of just 2 of the 16 repeats indicates that the contribution of individual YG repeats is not additive. The affinity of B-GD for dextran was 1 order of magnitude lower than that of B-GBD, even though the GBD of B-GD contains one more YG repeat than the GBD of B-GBD. Mooser et al. (18) reported that GTF-I from Streptococcus sobrinus had a higher affinity for dextran than GTF-S from the same organism and that GTF-I contained six more YG repeats than GTF-S. Taken together, the findings are consistent with the proposal by von Eichel-Streiber et al. (27) and Giffard and Jacques (4) that YG repeats are the functional units of glucan binding. However, these data and the heterogeneity in the primary sequence of the YG repeats indicate that not all repeats contribute equally to glucan binding. Work previously done in our laboratory indicated that the secondary structure of the GBD was almost completely beta sheet (6). However, regions within the GBD were found to be able to undergo a transition to alpha helices which was associated with a loss of dextran binding. It is possible that the deletion of individual YG repeats can result in the formation of alpha helices instead of beta sheets, ultimately affecting the tertiary structure of the protein and its glucan binding. The magnitude of the transition might depend on the number and location of the YG repeats that are deleted.
We conclude that the YG repeat domain of GbpA constitutes its GBD and that this domain has at least a 100-fold-higher affinity for dextran than for mutan. Even a relatively minor deletion of two YG repeats or the placement of the GBD anywhere other than at the carboxyl terminus of the protein can substantially reduce the glucan-binding ability of the GBD. The GBD of GbpA also has over a 30-fold-higher affinity for dextran than both GTF-I and GTF-S from S. mutans.
ACKNOWLEDGMENTS
We gratefully acknowledge the help and advice of Peter Weber and Tom Andersen, Albany Medical College.
This work was supported by grant DE10058 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Ausubel F M, et al. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 2.Banas J A, Russell R R B, Ferretti J J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990;58:667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara T, Tamesada M, Bian Z, Kawabata S, Kimura S, Hamada S. Deletion and reintroduction of glucosyltransferase genes of Streptococcus mutans and role of their gene products in sucrose dependent cellular adherence. Microb Pathog. 1996;20:225–233. doi: 10.1006/mpat.1996.0021. [DOI] [PubMed] [Google Scholar]
- 4.Giffard P M, Jacques N A. Definition of a fundamental repeating unit in streptococcal glucosyltransferase glucan-binding regions and related sequences. J Dent Res. 1994;73:1133–1141. doi: 10.1177/00220345940730060201. [DOI] [PubMed] [Google Scholar]
- 5.Haas W, Banas J A. The glucan binding domain of the Streptococcus mutans glucan binding protein. Adv Exp Med Biol. 1997;418:707–708. doi: 10.1007/978-1-4899-1825-3_165. [DOI] [PubMed] [Google Scholar]
- 6.Haas W, MacColl R, Banas J A. Circular dichroism analysis of the glucan binding domain of Streptococcus mutans glucan binding protein-A. Biochim Biophys Acta. 1998;1384:112–120. doi: 10.1016/s0167-4838(98)00005-3. [DOI] [PubMed] [Google Scholar]
- 7.Hamada S, Slade H D. Biology, immunology, and cariogenicity of Streptococcus mutans. Biol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988;56:1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989;57:2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazlett K R, Michalek S M, Banas J A. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect Immun. 1998;66:2180–2185. doi: 10.1128/iai.66.5.2180-2185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato C, Kuramitsu H K. Carboxyl-terminal deletion analysis of the Streptococcus mutans glucosyltransferase-I enzyme. FEMS Microbiol Lett. 1990;72:299–302. doi: 10.1016/0378-1097(90)90321-g. [DOI] [PubMed] [Google Scholar]
- 12.Klessen C, Schmidt K H, Ferretti J J, Malke H. Tripartite streptokinase gene fusion vectors for gram-positive and gram-negative prokaryotes. Mol Gen Genet. 1988;212:295–300. doi: 10.1007/BF00334699. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Landale E C, McCabe M M. Characterization by affinity electrophoresis of an alpha-1,6-glucan-binding protein from Streptococcus sobrinus. Infect Immun. 1987;55:3011–3016. doi: 10.1128/iai.55.12.3011-3016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lis M, Shiroza T, Kuramitsu H K. Role of C-terminal direct repeating units of the Streptococcus mutans glucosyltransferase-S in glucan binding. Appl Environ Microbiol. 1995;61:2040–2042. doi: 10.1128/aem.61.5.2040-2042.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loesche W L. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto K, Yamashita K, Yoshida A, Hayashi S, Machida Y, Nagai T. Production and physicochemical properties of water-insoluble glucan from Streptococcus mutans. Chem Pharm Bull. 1987;35:3813–3821. doi: 10.1248/cpb.35.3813. [DOI] [PubMed] [Google Scholar]
- 18.Mooser G, Shur D, Lyou M, Watanabe C. Kinetic studies on dextransucrase from the cariogenic oral bacterium Streptococcus mutans. J Biol Chem. 1985;260:6907–6915. [PubMed] [Google Scholar]
- 19.Mooser G, Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect Immun. 1988;56:880–884. doi: 10.1128/iai.56.4.880-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munro C L, Michalek S M, Macrina F L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell R R B. Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol. 1979;112:197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci. 1949;51:660–672. [Google Scholar]
- 24.Shimamura A, Nakano Y J, Mukasa H, Kuramitsu H K. Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. J Bacteriol. 1994;170:810–816. doi: 10.1128/jb.176.16.4845-4850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiroza T, Ueda S, Kuramitsu H K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987;169:4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickerman M M, Sulavik M C, Minick P E, Clewell D B. Changes in the carboxyl-terminal repeat region affect extracellular activity and glucan products of Streptococcus gordonii glucosyltransferase. Infect Immun. 1996;64:5117–5128. doi: 10.1128/iai.64.12.5117-5128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Eichel-Streiber C, Sauerborn M, Kuramitsu H K. Evidence for a modular structure of the homologous repetitive C-terminal carbohydrate-binding sites of Clostridium difficile toxins and Streptococcus mutans glucosyltransferases. J Bacteriol. 1992;174:6707–6710. doi: 10.1128/jb.174.20.6707-6710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wren B W. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol Microbiol. 1991;5:797–803. doi: 10.1111/j.1365-2958.1991.tb00752.x. [DOI] [PubMed] [Google Scholar]