Abstract
Peanut (Arachis hypogaea L.) is an important crop for the family-based systems in the tropics, mainly in Brazil. In the Brazilian drylands, peanuts are cropped in low technological systems, and cheap and efficient technologies are needed to improve crop yield and sustainability. Despite this importance, few data are available on selecting efficient peanut rhizobia in experiments under different edaphoclimatic conditions. This work evaluated the agronomic efficiency and the biological nitrogen fixation (BNF) by two elite Bradyrhizobium strains under four different fields in the Brazilian semiarid region. We compared a new efficient strain Bradyrhizobium sp. ESA 123 with the reference strain B. elkanii SEMIA 6144, currently used in peanut rhizobial inoculants in Brazil. Besides the inoculated treatments, two uninoculated controls were assessed (with and without 80 kg ha−1 of N-urea). The BNF was estimated by the δ15N approach in three out of four field assays. BNF contribution was improved by inoculation of both Bradyrhizobium strains, ranging from 42 to 51% in Petrolina and 43 to 60% in Nossa Senhora da Glória. Peanuts’ yields benefited from the inoculation of both strains and N fertilization in all four assays. Nevertheless, the results showed the efficiency of both strains under different edaphoclimatic conditions, indicating the native strain ESA 123 as a potential bacterium for recommendation as inoculants for peanuts in Brazil, mainly in drylands.
Keywords: Arachis hypogaea, Inoculant, Rhizobia, Seed inoculation, Strain selection
Introduction
Peanut (Arachis hypogaea L.) is a South American native oilseed crop. This species is grown worldwide, and the leading world producer is China. In 2019, China produced more than 17.5 million Mg of shelled peanuts (pods), while Brazil produced around 581 thousand Mg, the 12th world’s producer [1]. Brazilian production is concentrated in São Paulo state, achieving more than 90% of the national output [2]. In the northeast region, peanuts are cropped mainly in the family-based agricultural systems under rainfed conditions in the Brazilian drylands (semiarid climate conditions) [3]. Due to the harsh environmental conditions in the region and the low technological inputs in the agricultural systems, the Brazilian semiarid region yields are below those achieved in the whole country. For these reasons, new eco-friendly and low-cost technologies are urgent to the region.
Among the low-cost, highly efficient technologies, microbial inoculants (for legumes, rhizobial inoculants) are amid the most promising ones [4, 5]. For peanuts, a Zimbabwean strain of Bradyrhizobium elkanii (SEMIA 6144) has been used in commercial inoculants for more than 30 years in Brazil [6]. Despite some experiments showing the agronomic efficiency of this strain under certain field conditions [7–9], the selection of new efficient bradyrhizobia should improve the plant yield and the biological nitrogen fixation (BNF) in Brazil, mainly under semiarid climate conditions, and increase the genetic base of bradyrhizobial strains available for peanut inoculant production. Furthermore, the selection of new efficient bacteria for peanuts should collaborate to increase the adoption of inoculation technology by the farmers since the use of inoculants for peanuts is still small in Brazil.
Screening of peanut rhizobia has been carried out, and newly promising strains have been isolated and characterized in South America, mainly in Argentina [10–12] and Brazil [13–16]. The selection of efficient bradyrhizobial strains should improve inoculants by adding highly efficient bacteria in the current commercial formulations. However, despite some data reported on complete studies characterization and field assessment of peanut’s Bradyrhizobium [17], few reports on their efficiency are taken from experiments in different locations, a critical approach to assure the rhizobial efficiency under several edaphoclimatic conditions [18–20].
Several new peanut bradyrhizobia were isolated in Brazil last decade, including the Bradyrhizobium sp. ESA 123. This strain is indigenous from a soil cropped with peanuts in Barbalha municipality, Ceará state, in the Brazilian semiarid region [21]. ESA 123 stood out regarding the efficiency in potted peanuts experiments with sterile [16] and non-sterile soil substrates [22]. This strain also showed agronomic efficiency in preliminary field assays [7] and improved plant resistance to drought in potted peanuts [23, 24]. More recently, Bradyrhizobium sp. ESA 123 was proven as more compatible with the fungicides commonly used in peanut seed treatments than the commercial strain B. elkanii SEMIA 6144 [25]. Because of these promising characteristics, Bradyrhizobium sp. ESA 123 is a candidate for new peanut inoculant formulations in Brazil.
Therefore, this work aimed to evaluate the agronomic efficiency of Bradyrhizobium sp. ESA 123 in four locations in the Brazilian semiarid region, according to the official standardized protocols determined by the Brazilian Ministry of Agriculture, Livestock, and Food Supply [6]. We hypothesized that Bradyrhizobium sp. ESA 123 inoculation is agronomically efficient to peanuts grown under different edaphoclimatic conditions in the Brazilian drylands, comparable to the current inoculant strain B. elkanii SEMIA 6144.
Materials and methods
Bacterial strains and inoculant preparation
Bradyrhizobial strains SEMIA 6144 and ESA 123 were used in the present study (SisGen authorization: A64C60D). B. elkanii SEMIA 6144 is currently authorized for use in commercial peanut inoculants in Brazil [6]. Bradyrhizobium sp. ESA 123 was isolated from peanut root nodules grown in soil from the Brazilian semiarid [21]. ESA 123 was classified as Bradyrhizobium sp. (16S rRNA GenBank accession MG982490), closely related to B. kavangense [23]. This strain is currently preserved in the Culture Collection of Microorganisms with Agricultural Interests of Embrapa Semiárido (CMISA, ESA 123) and at the Johanna Döbereiner Center of Biological Resources (CRB-JD, BR 14811).
The bacterial strains grew monoxenically in the YM medium [26] for 6 days under constant stirring (120 rpm) at 28 °C. The broths were centrifuged (6000 × g for 10 min), the supernatants were discarded, and the pellet was resuspended in the same volume of sterile NaCl 0.85% (w v−1). The centrifugation/resuspension process was repeated twice for complete cell washing. The cell suspension had the optical density read spectrophotometrically at 600 nm, being adjusted to 0.8. Aliquots of 15 mL of the cell suspensions were softly mixed in plastic bags containing 50 g of sterilized powdered peat (twice autoclaved at 120 °C, 1.2 atm for 60 min), reaching the final concentration of 109 colony-forming unities g−1 of inoculant. The peat-based inoculants were kept at 10 °C until the experiments were set up (no more than 30 days of storage).
Field experiments
Four field assays were conducted between May 2019 and February 2020, in the municipalities of Petrolina and Parnamirim (Pernambuco state), Nossa Senhora da Glória (Sergipe state), and Juazeiro (Bahia state). In Parnamirim, the assay was conducted in the Irrigated Agriculture Experimental Station of Universidade Federal Rural de Pernambuco (UFRPE); in Petrolina, the assay was conducted in the Bebedouro Experimental Field; in Nossa Senhora da Glória, the assay was conducted in the Nossa Senhora da Glória Experimental Field. In Juazeiro, the experiment was implemented in the Mandacaru Experimental Field. The last three experimental fields are in the facilities of Embrapa Semiárido. The description of each experimental site and the characteristics of each assay are detailed in Table 1. The soils were classified according to the Brazilian Soil Classification System [27], and the soil chemical characteristics were assessed according to Teixeira et al. [28]. Finally, soil fertilization was conducted as the technical recommendation for peanuts in Pernambuco state [29].
Table 1.
Experimental conditions, soil characteristics, and fertilization adopted on the experimental sites in Petrolina, Parnamirim, Juazeiro, and Nossa Senhora da Glória
| Petrolina | Parnamirim | Juazeiro | Nossa Senhora da Glória | |
|---|---|---|---|---|
| General experiment information | ||||
| Experiment duration | Jun–Sep (2019) | May–Aug (2019) | Nov 2019–Mar 2020 | Jun–Sep (2019) |
| Latitude/longitude | − 9.3941/ − 40.5097 | − 8.0905/ − 39.5783 | − 9.2716/ − 40.5052 | − 10.2166/ − 37.4241 |
| Peanut genotype | BR1 | L7 Bege | BR1 | BR1 |
| Water supplyα | Drip irrigation | Drip irrigation | Drip irrigation | Rainfed |
| Rainfall (mm accumulated) | 7.0 | 5.2 | 43.1 | 217.7 |
| Average temperature (°C) | 25.7 | 24.9 | 27.9 | 22.5 |
| Soil characteristics | ||||
| Soil typeβ | Ultisol | Luvisol | Vertisol | Oxisol |
|
Electrical conductivity (dS m−1) |
1.06 | 0.63 | 2.07 | 0.53 |
| pH | 6.40 | 7.10 | 6.00 | 5.30 |
| C (g kg−1) | 13.50 | 24.10 | 12.60 | 9.20 |
| P (mg kg−1) | 48.60 | 8.48 | 160.00 | 2.87 |
| K (cmolc kg−1) | 0.30 | 0.59 | 0.60 | 0.45 |
| Na (cmolc kg−1) | 0.04 | 0.20 | 0.50 | 0.16 |
| Ca (cmolc kg−1) | 1.40 | 24.40 | 16.00 | 1.80 |
| Mg (cmolc kg−1) | 0.60 | 5.10 | 10.0 | 1.00 |
| Al (cmolc kg−1) | 0.00 | 0.00 | 0.00 | 0.05 |
| H + Al (cmolc kg−1) | 0.20 | 0.00 | 1.00 | 1.90 |
| Sum of bases (cmolc kg−1) | 2.30 | 30.30 | 25.00 | 3.40 |
| Cation exchange capacity (cmolc kg−1) | 2.50 | 30.30 | 26.00 | 5.40 |
| Base saturation (%) | 91 | 100 | 96 | 64 |
| Peanut nodulating rhizobial population (MPN) γ | 2.48–4.00 | 2.00–3.70 | 3.30–4.17 | 2.30–3.47 |
| Fertilizationδ | ||||
| Simple superphosphate, 20% P2O5 (kg ha−1) | 200 | 200 | 100 | 200 |
| Potassium chloride, 60% K2O (kg ha−1) | 60 | 40 | 40 | 40 |
| Gypsum, 18% Ca; 15% S (kg ha−1) | 500 | 250 | 500 | 500 |
αDrip irrigation system: 16″ hoses, 0.5 m between drippers, and 1.6 L.h−1 flow. Irrigation conducted daily for 3–4 h
βSoil classification: Brazilian Soil Classification System by Santos et al. [27]: Ultisol = “Argissolo”, Luvisol = “Luvissolo”, Vertisol = “Vertissolo”, Oxisol = “Latossolo”. Chemical analysis by Teixeira et al.[28]
γConfidence interval (log10) of the most probable number (MPN) of peanut nodulating rhizobia per gram of soil by Hungria and Araújo[30]
δRecommendations by Cavalcanti et al.[29]
The experiments were implemented according to the standardized determinations to validate the agronomic efficiency of rhizobial strains and other related plant growth–promoting bacteria, as described in the Normative Instruction no. 13/2011 by the Brazilian Ministry of Agriculture, Livestock, and Food Supply [6]. The completely randomized block design was adopted with four replications (blocks) in all assays. The recommended inoculant strain B. elkanii SEMIA 6144 and Bradyrhizobium sp. ESA 123 were the inoculated treatments studied. In addition, two non-inoculated control treatments (with and without urea application) were also included. For the N-fertilized control, 80 kg ha−1 of N-urea was applied. Half of the N fertilizer was amended at the sowing and half 30 days after sowing. Each experimental plot was composed of 6 m rows spaced 0.7 m between rows. In each row, three peanut seeds were sown 0.2 m apart. The peanut-nodulating soil rhizobia populations were assessed by the most probable number (MPN) according to Hungria and Araújo [30].
The peat-based inoculant was mixed with the seeds at 250 g of inoculant to 10 kg of seeds for inoculation. Then, as a sticking agent, a saturated sucrose solution (approximately 40 g L−1) was gently mixed (20 mL kg seed−1) with the seeds and inoculants in a plastic bag. Finally, the inoculated seeds were air-dried for 30–40 min in the shadow before sowing.
The peanuts were sampled 53 days after emergence (DAE) to assess nodulation, biomass production, and BNF. A total of 20 plants were selected per plot (preserving the useful area in the four central lines) and collected. The shoots were separated from the roots, oven-dried (65 ± 2 °C) for 7 days, and weighted for shoot dry mass (SDM) determination. The remaining soil was carefully removed by washing on a sieve to recover fallen nodules from the roots. Nodules were then detached and counted to assess the nodule number per plant (NN) and oven-dried (65 ± 2 °C) for 3 days and weighted afterward to assess the nodule dry mass per plant (NDM).
In Parnamirim, Petrolina, and Nossa Senhora da Glória, the BNF was estimated by 15 N natural abundance (δ15N) approach. As non-fixing references (NFR), sunflower (Helianthus annuus), cotton (Gossypium hirsutum), and castor bean (Ricinus communis) were sown in extra plots in all field blocks in Parnamirim. Non-legume weeds were used as NFR in Petrolina (Amaranthus sp., Cyperus sp., and Cenchrus ciliaris) and Nossa Senhora da Glória (Galinsoga parviflora, Centratherum punctatum, and Portulaca sp.). The weeds were collected within the plots (between two lines). For all NFR, four composite samples formed by the shoots of five plants of each species were collected and oven-dried, as described for peanuts.
All plant samples (peanuts and NFR) were ground and sieved (2 mm mesh), capsuled, and inserted into a mass spectrometer (ThermoQuest-Finnigan MAT Delta Plus, Thermo-Finnigan, USA) interfaced with an elemental analyzer (Carlo Erba 1110, Italy). All analyses were performed at the Laboratory of Isotopic Ecology (USP, Brazil). The total concentrations (%) and the isotopic ratios of N were obtained, and the natural abundances of 15 N were expressed in δ units (‰), which represents the deviation from the standard of the proportions of the masses of 15 N:14 N following the equation:
where Rsample and Rstandard are the ratios 15 N:14 N in both the sample and the standard (atmospheric N2).
When the difference between the averages of the δ15N values of peanut and those of NFR was statistically significant in the F test (p ≤ 0.05), the percentage of nitrogen derived from the atmosphere (%Ndfa) was estimated by the 15 N natural abundance methodology [31], using the equation:
where δ15N(reference) is the average value of the δ15N of the reference plants of each block, δ15N(fixing) is the average value of the δ15N of peanut plants for each plot, and B is the δ15N value for N-fixing plants grown in the absence of nitrogen, assumed to be − 1.35‰ [32]. The %Ndfa was calculated with the average values of the three reference plants in each block in the field. The shoot total N and total fixed N were calculated by multiplying the N concentrations (total and %Ndfa) by the aboveground biomass.
At 95 DAE, at physiological maturity, plants were harvested from the useful area (3 m long of the four inner lines of each plot—around 60 plants per plot). In the field, pods were removed from the plants, placed in paper bags, and transported to the laboratory, where they were kept in the sun for 4 consecutive days and weighted to determine total pod yield (PY). Next, the grains were threshed and kept in a cold chamber (10 ± 2 °C) until processing. Then, the moisture was standardized to 15%, and the grains were to obtain the total grain yield (GY) and weight of 100 grains (W100).
Data analysis
Data were submitted to ANOVA after verifying data normality by the Shapiro–Wilk test using Sisvar v. 5.0 [33]. Four independent analyzes were conducted due to the different characteristics of each assay. After ANOVA, a comparison between 15 N values was conducted independently, comparing all target and reference plants in each experimental site, considering three randomized block experiments. Finally, all averages were compared by the Scott-Knot (p ≤ 0.05) mean range test.
A principal component analysis (PCA) using data from plant growth (shoot dry mass), nodulation (nodule number per plant and nodule dry mass), and yield (pod and grain) was carried out using the correlation matrices with the PaSt 4.10 software [34]. This analysis was conducted to get an integrated view of all experiments conducted at different sites.
Results
ESA 123 improved the peanut SDM in Juazeiro, while no differences were observed in the other assays (Table 2). NN improved with inoculation of ESA 123 in Parnamirim and with both strains in Nossa Senhora da Glória. NDM improved with the inoculation of both strains in Nossa Senhora da Glória. Differences in NDM were also observed in Parnamirim and Nossa Senhora da Glória since N-urea application inhibited the nodulation by the established soil rhizobial population. Rhizobial inoculation and N fertilization positively increased W100 in Nossa Senhora da Glória, but no effects were observed in the other experimental sites.
Table 2.
Shoot dry mass (SDM), number of nodules per plant (NN), nodule dry mass (NDM), pod yield (PY), grain yield (GY), and weight of 100 grains (W100) of peanut (Arachis hypogaea) grown in four experimental sites under inoculation with Bradyrhizobium, nitrogen fertilization (N-urea) or absence of inoculation and N-urea
| Inoculation treatments | SDM | NN | NDM | PY | GY | W100 |
|---|---|---|---|---|---|---|
| g plant−1 | plant−1 | mg plant−1 | kg ha−1 | kg ha−1 | g | |
| Petrolina | ||||||
| ESA 123 | 29.0 | 171 a | 256 | 3930 a | 2654 a | 44.8 |
| SEMIA 6144 | 30.7 | 128 b | 233 | 3925 a | 2537 a | 43.5 |
| N-urea | 33.5 | 192 a | 247 | 4217 a | 2805 a | 41.4 |
| Control | 28.6 | 163 a | 261 | 3592 b | 2319 b | 38.0 |
| F-test (p-value) | ns | ** | ns | * | * | ns |
| CV (%) | 8.3 | 16.3 | 18.3 | 8.9 | 5.5 | 5.4 |
| Parnamirim | ||||||
| ESA 123 | 15.2 | 103 a | 90 a | 3003 a | 2099 a | 51.3 |
| SEMIA 6144 | 12.0 | 69 b | 73 a | 2674 a | 1775 a | 50.2 |
| N-urea | 15.3 | 73 b | 43 b | 2854 a | 1889 a | 58.6 |
| Control | 15.2 | 67 b | 73 a | 2243 b | 1495 b | 49.1 |
| F-test (p-value) | ns | ** | ** | ** | ** | ns |
| CV (%) | 17.3 | 28.7 | 7.3 | 9.6 | 9.1 | 9.5 |
| Juazeiro | ||||||
| ESA 123 | 31.6 a | 164 a | 55 | 4658 b | 2693 b | 39.5 |
| SEMIA 6144 | 23.2 b | 136 a | 48 | 4528 b | 2782 b | 36.7 |
| N-urea | 24.5 b | 73 b | 53 | 5422 a | 3375 a | 39.5 |
| Control | 21.6 b | 117 a | 48 | 4264 c | 2216 c | 34.7 |
| F-test (p-value) | ** | ** | ns | * | * | ns |
| CV (%) | 22.7 | 22.0 | 16.7 | 10.5 | 22.3 | 8.25 |
| Nossa Senhora da Glória | ||||||
| ESA 123 | 13.3 | 42 b | 252 a | 1844 a | 1127 a | 32.8 a |
| SEMIA 6144 | 8.3 | 77 a | 352 a | 1931 a | 1131 a | 36.3 a |
| N-urea | 13.5 | 13 c | 45 c | 1959 a | 1231 a | 36.0 a |
| Control | 9.4 | 25 c | 106 b | 1280 b | 745 b | 25.0 b |
| F-test (p-value) | ns | ** | ** | ** | ** | ** |
| CV (%) | 14.7 | 16.3 | 25.0 | 21.4 | 18.8 | 11.2 |
Means followed by the same letter in the column, within the same experimental site, do not differ by Scott-Knot means range test. ns, not-significant; **p < 0.05; *p < 0.1. Data are means of four replications
CV coefficient of variation
Inoculation with both Bradyrhizobium strains and N-urea fertilization increased the peanut pod and grain yields in all experimental sites. The inoculation with ESA 123, SEMIA 6144, and N fertilization increased the pod/grain yields by 9.3/14.5, 9.4/9.4, and 17.4/21.0%, respectively, in Petrolina. In Parnamirim, the pod/grain yields improved in the plants inoculated with ESA 123, SEMIA 6144, and N-urea by 33.9/40.4, 19.2/18.7, and 27.2/26.4%, respectively. In Juazeiro, ESA 123, SEMIA 6144, and N-urea increased the pod/grain yields by 9.2/21.5, 6.2/18.7, and 27.2/52.3%, respectively. The inoculation with ESA 123, SEMIA 6144, and application of N-urea also increased peanut pod/grain yields in Nossa Senhora da Glória by 44.1/51.3, 50.9/51.8, and 53.0/65.2%, respectively.
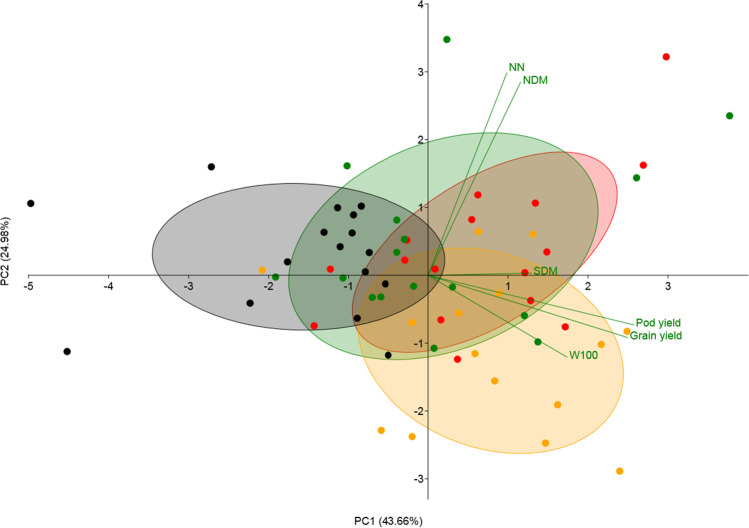
For plant growth, nodulation, and grain yield, in the principal component analysis (PCA), the main components (PC1 and PC2) explained 68.64% of the variance (Fig. 1). The SDM, PY, GY, and W100 variable vectors correlated to each other and separated the treatments inoculated with ESA 123, SEMIA 6144, and with N-urea from the absolute control in the PC1. Likewise, the nodulation variables (NN and NDM) correlated with each other and with the Bradyrhizobium-inoculated treatments, separating the inoculated treatments from the N-urea treatment in the PC2.
Fig. 1.
Principal component analysis (PCA) biplot conducted with the data (all replications) of four field assays using the data of peanut shoot dry mass (SDM), nodule dry mass (NDM), number of nodules per plant (NN), pod yield, grain yield, and the weight of 100 seeds (W100). Colors present the experimental treatments: red: ESA 123; green: SEMIA 6144; yellow: N-fertilization; and black: non-inoculated and non-fertilized control. PC1 and PC2 are the principal components 1 and 2 respectively
The δ15N approach could estimate the BNF since the target peanut plants showed lower 15 N signals than the non-fixing reference species (Table 3). In Nossa Senhora da Glória and Parnamirim, the %Ndfa was higher in plants inoculated with both Bradyrhizobium than in the non-inoculated control treatment. Furthermore, the total fixed N (kg ha−1) until the 53rd day after peanut emergence was higher in the inoculation treatments in Petrolina and Nossa Senhora da Glória; however, no differences were observed in Parnamirim. The BNF in Petrolina was estimated above 60 kg ha−1 under Bradyrhizobium inoculation, and below 49 kg ha−1 of fixed N in association solely with the native established rhizobial community. In Nossa Senhora da Glória, the estimated N fixation ranged between 12 and 13 kg ha−1 in peanut plants inoculated with ESA 123 or SEMIA 6144, while non-inoculated plants associated with native rhizobia fixed 3.2 kg ha−1 of N.
Table 3.
‰δN15, shoot N concentration, % of N nitrogen derived from atmosphere (Ndfa), shoot total N, and N-fixed ha−1 of peanut (Arachis hypogaea) inoculated or not with Bradyrhizobium spp. in three different experimental sites
| Inoculation treatment/non-fixing reference | δ15N | Shoot N concentration | Ndfa | Shoot total N | N-fixed |
|---|---|---|---|---|---|
| ‰ | mg g−1 | % | kg ha−1 | kg ha−1 | |
| Petrolina | |||||
| ESA 123 | 1.8* | 32.5 a | 75.3 a | 79.7 a | 60.0 a |
| SEMIA 6144 | 1.6* | 31.6 a | 77.1 a | 86.1 a | 66.3 a |
| Control | 2.6* | 28.7 a | 69.3 a | 70.5 a | 48.6 b |
| Amaranthus sp. | 11.4 | ||||
| Cyperus sp. | 12.5 | ||||
| Cenchrus ciliaris | 11.1 | ||||
| CV (%) | 5.3 | 8.4 | 12.8 | 8.2 | 13.4 |
| Parnamirim | |||||
| ESA 123 | 3.5* | 26.4 a | 51.4 a | 34.3 a | 17.6 a |
| SEMIA 6144 | 4.4* | 27.7 a | 41.9 a | 28.7 a | 12.0 a |
| Control | 5.7* | 31.5 a | 29.6 b | 37.6 a | 11.4 a |
| Gossypium hirsutum | 9.3 | ||||
| Helianthus annuus | 8.5 | ||||
| Ricinus communis | 7.6 | ||||
| CV (%) | 11.9 | 12.0 | 13.2 | 15.2 | 19.1 |
| Nossa Senhora da Glória | |||||
| ESA 123 | 9.5* | 24.6 a | 43.1 a | 26.1 a | 12.3 a |
| SEMIA 6144 | 6.3* | 29.5 a | 60.1 a | 21.4 a | 12.7 a |
| Control | 12.4* | 18.9 b | 17.5 b | 15.6 a | 3.2 b |
| Galinsoga parviflora | 19.6 | ||||
| Centratherum punctatum | 16.4 | ||||
| Portulaca sp. | 18.2 | ||||
| CV (%) | 7.8 | 10.2 | 20.1 | 12.7 | 16.9 |
Means followed by the same letter in the column, within the same experimental site, do not differ by Scott-Knot means range test (p < 0.05). *Significant difference (Scott-Knot test p < 0.05) of δ15N averages between the target treatments (inoculation with ESA 123 or SEMIA 6144, and control) and the non-fixing references within the same experimental site. Data are means of four replications
CV coefficient of variation
The N concentration in the plant shoots was higher in the inoculated peanuts only in Nossa Senhora da Glória, and no differences were observed in the other sites. Furthermore, the total N content in the experimental area showed no significant differences in the three sites, achieving averages of 78.8, 33.5, and 21.0 kg ha−1 of N in Petrolina, Parnamirim, and Nossa Senhora da Glória, respectively, up to 53 days of peanut growth in the fields.
Discussion
Despite the differences observed among experimental sites, inoculation with both Bradyrhizobium strains significantly increased pod and grain yield in the four experimental sites, compared with non-inoculated plants. Moreover, under three out of four different edaphoclimatic conditions, inoculation with ESA 123 or SEMIA 6144 led to pod/grain yields similar to the plants that received N-urea, which is a promising result. Martins et al. [35] conducted multisite assays that proved the agronomic efficiency of a new cowpea (Vigna unguiculata) Bradyrhizobium strain BR 3267 for use as an inoculant. In the same way, Mercante et al. [19] showed the outstanding agronomic performance of three new common beans (Phaseolus vulgaris) Rhizobium spp. strains in four different locations in central Brazil (Mato Grosso do Sul state). These results indicate that our strategy of assessing the performance of ESA 123 in different fields was right and proved the versatility of the selected strain in divergent edaphoclimatic conditions.
Bradyrhizobium strains from the semiarid region of Brazil are efficient both in the harsh local environments and other edaphoclimatic conditions. For example, the abovementioned cowpea B. yuanmingense BR 3267 showed high agronomic performance in the Brazilian drylands [20, 35, 36] in wet environments in the Brazilian Amazon [37, 38], and in the Brazilian Savanna (Cerrado) biome [39], and Ghana, overseas in the African Continent [40]. The plasticity and adaptation of BR 3267 probably also occur with ESA 123 since, besides the agronomic efficiency observed in the current study, this strain also showed to be agronomically efficient in preliminary field trials in the Brazilian drylands and the tropical rainforest zone in the Brazilian Northeast [7]. In addition, ESA 123 improved peanut resistance to drought stress [23, 24], indicating the versatility of this strain, an essential feature for a commercial inoculant rhizobia. Even in the experiments in the present study, the efficiency of the Brazilian semiarid native strain ESA 123 and the Zimbabwean strain SEMIA 6144 was demonstrated in very different conditions, as described in Table 1. The yield improvements in such different conditions reinforce the efficiency of the recommended strain SEMIA 6144 and support ESA 123 in the Brazilian list of strains used to produce peanut inoculant.
The δ15N technique estimated the capacity of both Bradyrhizobium strains and the native community to fix N2 and incorporate this element into the aboveground peanut biomass. In Petrolina, we found the highest proportions of biologically fixed N in the peanut shoots, with no differences between strains, which indicates the high efficiency of inoculated bacteria compared with the native ones. The efficiency of the N-fixing native community is a common feature in the Brazilian agricultural field [41, 42], even in drylands [43, 44]. Therefore, this character represents a challenge for successful inoculation with elite rhizobial strains. Nevertheless, in all three field assays, the N fixation parameter evaluated by the isotopic technique (%Ndfa and/or total fixed N) was higher in the inoculated compared to the non-inoculated treatments, a desirable characteristic for an inoculant strain. For example, %Ndfa was higher in the inoculated plants in Parnamirim (up to 51.4% for ESA 123 treatment) and in Nossa Senhora da Glória (up to 60.1% for SEMIA 6144 treatment), indicating the outstanding performance of Bradyrhizobium strains in both experimental sites.
When assessing the performance of outstanding soybean (Glycine max) Bradyrhizobium spp. in the Brazilian Amazon, Zilli et al. [42] set up experiments in two different locations with better performance of four Bradyrhizobium strains in a single location, in agreement with our results, reinforcing the need for multisite experiments to assess the efficiency of rhizobial strains on N fixation. Furthermore, both strains performed better than the sole indigenous community in Petrolina and Parnamirim, despite the differences in the experimental conditions (different genotypes and water regimes, for example, Table 1), indicating the responsivity of peanut to inoculation with both strains under different conditions and even so being efficient.
Therefore, the estimate of the total amount of fixed N in the different systems, calculated by the %Ndfa and the total SDM, indicated the high capacity of both Bradyrhizobium strains to fix N in two out of three field conditions. Values higher than 60 kg ha−1 of fixed N were observed in Petrolina under irrigation, and values close to 13 kg ha−1 of fixed N were achieved in Nossa Senhora da Glória under rainfed conditions, showing different rhizobial performance under different environmental conditions. The low values of fixed N in Nossa Senhora da Glória probably result from the high 15 N signals observed in the NFR and target treatments. This data suggests a particular 15 N signal in this specific soil and/or high microbial activity leading to organic matter turnover and increasing mineral N availability in the soil. However, both hypotheses should be addressed in the future.
An isotopic approach to assess the BNF rate in inoculated legume crops has been applied in the Brazilian Northeast region, and values around 60 kg ha−1 of fixed N have been reported in cowpea [44, 45]. For jack-bean (Canavalia ensiformis), Dantas et al. [43] estimated the total fixed N by 90 kg ha−1 of N in a passion-fruit (Passiflora edulis) orchard in Petrolina. However, to the best of our knowledge, there are no data on the efficiency of rhizobial strains assessed under multisite field assays by the δ15N approach for peanuts inoculated with efficient Bradyrhizobium strains in the Brazilian drylands.
In the most common agricultural production system in the Brazilian semiarid, inputs like N fertilizers are low, and the crop residues are either used to feed the cattle or are kept in the soil. Hence, estimating the amount of fixed N in the straw is important [44]. Furthermore, the fixed N represents an essential input of N to the agroecosystem and increases sustainability, mainly in family-based systems.
In Brazil, according to the Ministry of Agriculture, Livestock, and Food Supply [6], the agronomic efficiency of strains in different environments is one of the most critical criteria in selecting new rhizobia for use as inoculants to legume crops. However, studies on selecting new rhizobial strains for peanuts in Brazil are still incipient. The integrated view of results from all experiments in the PCA analysis (Fig. 1) highlighted the efficiency of both rhizobial strains. Thus, our study reports information about the agronomic efficiency of a new Bradyrhizobium sp. strain ESA 123 under different edaphoclimatic conditions in the Brazilian Northeast, comparable to the current B. elkanii strain SEMIA 6144, and supports the indication of the new native strain as a candidate for the official recommendation for peanut inoculant production in Brazil.
Furthermore, the use of elite rhizobial strains as inoculants resulted in peanut grain and pod yields higher than the non-inoculation and, in most cases, similar to N-fertilized treatments. These findings support the inoculation technology for peanuts, with social and economic benefits to the farmers and the planet’s environmental health.
Acknowledgements
We are grateful for the several students and technicians that helped us during the field experiments.
Author contribution
LML, RCS, CERSS, ADSF, LMVM, and PIF-J conceived the research project; RSJ and PIF-J implemented and collected all field assays; RSJ, TRS, RTR, JRSC, and JBAC processed and analyzed all plant material collected in the fields; ADSF and CERSS conducted the isotopic analyzes; PRAR, ADSF, and PIF-J analyzed all data; PRAR and PIF-J wrote the manuscript; all authors read and approved the final manuscript version.
Funding
The Brazilian Council for Scientific and Technological Development (CNPq), INCT–Plant Growth–Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq/Fundação Araucária STI/CAPES INCT-MPCPAgro 465133/2014–4), and the Brazilian Agricultural Research Corporation (Embrapa 23.16.05.016.00.00) provided financial support.
The Pernambuco State Science Foundation (Facepe) awarded scholarships to the second author. Facepe (APQ 0271–5.01/19) and CNPq (316042/2020–0) also awarded the postdoc fellowship to the nineth author. The tenth and last authors are research fellows of CNPq (306812/2018–5 and 311218/2017–2, respectively).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAOSTAT. No title. Agricultural statistics - groundnuts. Published 2020. Accessed March 30, 2021. http://www.fao.org/faostat/en/#data/QC
- 2.CONAB. Séries históricas. Published 2022. Accessed May 10, 2022. https://www.conab.gov.br/info-agro/safras/serie-historica-das-safras?start=20
- 3.dos Santos RC (2005) O Agronegócio do Amendoim no Brasil. Empresa Brasileira de Pesquisa Agropecuária. https://books.google.com.br/books?id=O0K6ZwEACAAJ
- 4.Volpiano CG, Lisboa BB, José JFB de S, Beneduzi A, Granada CE, Vargas LK (2022) Soil-plant-microbiota interactions to enhance plant growth. Rev Bras Ciência do Solo 46:e0210098. 10.36783/18069657RBCS20210098
- 5.de Souza R, Ambrosini A, Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015;38(4):401–419. doi: 10.1590/S1415-475738420150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasil (2011) Instrução Normativa No 13/2011 - Normas Sobre Especificações, Garantias, Registro, Embalagem e Rotulagem Dos Inoculantes Destinados à Agricultura, Das Especificações, Garantias Mínimas e Tolerâncias Dos Produtos. Ministério da Agricultura Pecuária e Abastecimento. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-sda-13-de-24-03-2011-inoculantes.pdf
- 7.Sizenando CIT, Ramos JPC, Fernandes-Junior PI, de Lima LM, Freire RMM, dos Santos RC. Agronomic efficiency of Bradyrhizobium in peanut under different environments in Brazilian Northeast. African J Agric Res. 2016;11(37):3482–3487. doi: 10.5897/AJAR2016.11294. [DOI] [Google Scholar]
- 8.Valetti L, Angelini JG, Taurian T, Anzuay MS, Fabra A, Cerioni GA. Development and field evaluation of liquid inoculants with native bradyrhizobial strains for peanut production. African Crop Sci J. 2016;24(1):1–13. doi: 10.4314/acsj.v24i1.1. [DOI] [Google Scholar]
- 9.Cardoso JD, Gomes DF, Goes KCGP, et al. Relationship between total nodulation and nodulation at the root crown of peanut, soybean and common bean plants. Soil Biol Biochem. 2009;41(8):1760–1763. doi: 10.1016/j.soilbio.2009.05.008. [DOI] [Google Scholar]
- 10.Bogino P, Banchio E, Giordano W. Molecular diversity of peanut-nodulating rhizobia in soils of Argentina. J Basic Microbiol. 2010;50(3):274–279. doi: 10.1002/jobm.200900245. [DOI] [PubMed] [Google Scholar]
- 11.Nievas F, Bogino P, Nocelli N, Giordano W. Genotypic analysis of isolated peanut-nodulating rhizobial strains reveals differences among populations obtained from soils with different cropping histories. Appl Soil Ecol. 2012;53(1):74–82. doi: 10.1016/j.apsoil.2011.11.010. [DOI] [Google Scholar]
- 12.Muñoz V, Ibañez F, Tonelli ML, Valetti L, Anzuay MS, Fabra A. Phenotypic and phylogenetic characterization of native peanut Bradyrhizobium isolates obtained from Córdoba, Argentina. Syst Appl Microbiol Published online. 2011 doi: 10.1016/j.syapm.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Júnior CV, Leite J, Santos CE de R e S et al (2014) Diversity and symbiotic performance of peanut rhizobia from Southeast region of Brazil. African J Microbiol Res 8(6):566–577. 10.5897/AJMR2013.5883
- 14.Santos CER, Stamford NP, Neves MCP, et al. Diversidade de rizóbios capazes de nodular leguminosas tropicais. Rev Bras Ciências Agrárias - Brazilian J Agric Sci. 2007;2(4):249–256. doi: 10.5039/agraria.v2i4a374. [DOI] [Google Scholar]
- 15.Lyra M do CCP de, de Freitas ADS, Silva TA, Santos CERS (2013) Phenotypic and molecular characteristics of rhizobia isolated from nodules of peanut (Arachis hypogaea L.) grown in Brazilian Spodosols. African J Biotechnol 12(17):2147–2156. 10.5897/ajb11.1574
- 16.dos Santos JWM, da Silva JF, Ferreira TDS, et al. Molecular and symbiotic characterization of peanut bradyrhizobia from the semi-arid region of Brazil. Appl Soil Ecol. 2017;121(12):177–184. doi: 10.1016/j.apsoil.2017.09.033. [DOI] [Google Scholar]
- 17.Jain D, Sanadhya S, Saheewala H, et al. Molecular diversity analysis of plant growth promoting rhizobium isolated from groundnut and evaluation of their field efficacy. Curr Microbiol. 2020;77(8):1550–1557. doi: 10.1007/S00284-020-01963-Y/FIGURES/2. [DOI] [PubMed] [Google Scholar]
- 18.Mburu SW, Koskey G, Njeru EM, Ombori O, Maingi JM, Kimiti JM. Differential response of promiscuous soybean to local diversity of indigenous and commercial Bradyrhizobium inoculation under contrasting agroclimatic zones. Int J Plant Prod. 2020;14(4):571–582. doi: 10.1007/S42106-020-00117-1. [DOI] [Google Scholar]
- 19.Mercante FM, Otsubo AA, Brito OR. New native rhizobia strains for inoculation of common bean in the Brazilian savanna. Rev Bras Ciênc Solo. 2017;41:150120. doi: 10.1590/18069657rbcs20150120. [DOI] [Google Scholar]
- 20.Xavier GR, Runjanek NG, Santos CE de R e. S et al (2017) Agronomic effectiveness of rhizobia strains on cowpea in two consecutive years. Aust J Crop Sci 11(9):1154–1160. 10.21475/ajcs.17.11.09.pne715
- 21.Cunha JBA (2014) Diversidade de Isolados de Rizóbio Nodulantes de Acessos de Amendoim (Arachis Hypogaea L.) Cultivados em Solos do Nordeste do Brasil. UNEB
- 22.de Melo EBS, de Lima LM, Fernandes-Júnior PI, et al. Nodulation, gas exchanges and production of peanut cultivated with Bradyrhizobium in soils with different textures. Comun Sci. 2016;7(2):160. doi: 10.14295/cs.v7i2.1449. [DOI] [Google Scholar]
- 23.Barbosa DD, Brito SL, Fernandes PD, Fernandes-Júnior PI, de Lima LM. Can Bradyrhizobium strains inoculation reduce water deficit effects on peanuts? World J Microbiol Biotechnol. 2018;34(12):87. doi: 10.1007/s11274-018-2474-z. [DOI] [PubMed] [Google Scholar]
- 24.Brito SL, Santos AB, Barbosa DD, Fernandes PD, Fernandes-Júnior PI, Lima LM (2019) Bradyrhizobium spp. As attenuators of water deficit stress in runner peanut genotypes based on physiological and gene expression responses. Genet Mol Res 18(4):gmr18379. 10.4238/gmr18379
- 25.dos Santos AB, Gondim TM de S, Fernandes Júnior PI, de Lima LM (2021) Effect of fungicides on the symbiosis between Bradyrhizobium strains and peanut. Pesqui Agropecuária Trop 51:e69089. 10.1590/1983-40632021v5169089.2
- 26.Vincent JM. A manual for the practical study of root nodule bacteria. Oxford: Blackwell Scientific; 1970. [Google Scholar]
- 27.Santos HG, Jacomine PKT, Anjos LHC dos et al (2014) Sistema Brasileiro de Classificação de Solos. doi:ISBN 978–85–7035–198–2
- 28.Teixeira PC, Donagemma GK, Fontana A, Teixeira WG eds (2017) Manual de Métodos de Análise de Solo, 3rd ed. Empresa Brasileira de Pesquisa Agropecuária
- 29.Cavalcanti FJ de A (1998) Recomendações de Adubação Para o Estado de Pernambuco: Segunda Aproximação, 2nd ed. Instituto Agronômico de Pernambuco
- 30.Hungria M, Araujo RS (1994) Manual de Métodos Empregados em Estudos de Microbiologia Agrícola, 1st edn. Empresa Brasileira de Pesquisa Agropecuária
- 31.Shearer G, Kohl DH. N2-fixation in field settings: estimations based on natural 15N abundance. Aust J Plant Physiol. 1986;13:699–756. doi: 10.1071/PP9860699c. [DOI] [Google Scholar]
- 32.Unkovich M, Herridge D, Peoples M et al (2008) Measuring plant-associated nitrogen fixation in agricultural systems. Australian Council for International Agricultural Research (ACIAR)
- 33.Ferreira DF. Sisvar: a computer statistical analysis system. Cienc e Agrotecnologia. 2011;35:1039–1042. doi: 10.1590/S1413-70542011000600001. [DOI] [Google Scholar]
- 34.Hammer Ø, Harper DAT, Ryan PD, Ryan DD, Ryan PD. PaSt: Paleontological statistics software package for education and data analysis. Palaentologia Electron. 2011;4(4):5–7. doi: 10.1016/j.bcp.2008.05.025. [DOI] [Google Scholar]
- 35.Martins LMV, Xavier GR, Rangel FW, et al. Contribution of biological nitrogen fixation to cowpea: a strategy for improving grain yield in the semi-arid region of Brazil. Biol Fertil Soils. 2003;38(6):333–339. doi: 10.1007/s00374-003-0668-4. [DOI] [Google Scholar]
- 36.Marinho R de CN, Nóbrega RSA, Zilli JE et al (2014) Field performance of new cowpea cultivars inoculated with efficient nitrogen-fixing rhizobial strains in the Brazilian Semiarid. Pesqui Agropecu Bras 49(5). 10.1590/S0100-204X2014000500009
- 37.Borges WL, dos Santos FN, da Mota RR, Rumjanek NG. Liming, fertilization, and rhizobia inoculation on cowpea yield in a Brazilian Amazon upland forest environment. Pesqui Agropecuária Bras. 2021;56:1–7. doi: 10.1590/S1678-3921.PAB2021.V56.02191. [DOI] [Google Scholar]
- 38.Zilli JÉ, Marson LC, Marson BF, Rumjanek NG, Xavier GR. Contribuição de estirpes de rizóbio para o desenvolvimento e produtividade de grãos de feijão-caupi em Roraima. Acta Amaz. 2009;39(4):749–758. doi: 10.1590/S0044-59672009000400003. [DOI] [Google Scholar]
- 39.Silva Júnior EB, Favero VO, Xavier GR, Boddey RM, Zilli JE. Rhizobium inoculation of cowpea in Brazilian Cerrado increases yields and nitrogen fixation. Agron J. 2018;110(2):722–727. doi: 10.2134/agronj2017.04.0231. [DOI] [Google Scholar]
- 40.Boddey RM, Fosu M, Atakora WK, et al. Cowpea (Vigna unguiculata) crops in Africa can respond to inoculation with rhizobium. Exp Agric. 2017;53(4):578–587. doi: 10.1017/S0014479716000594. [DOI] [Google Scholar]
- 41.Okito A, José Rodrigues Alves B, Urquiaga S, Michael Boddey R (2004) Nitrogen fixation by groundnut and velvet bean and residual benefit to a subsequent maize crop. Pesqui Agropecuária Bras 39(12):1183–1190
- 42.Zilli JÉ, Pacheco RS, Gianluppi V, Smiderle OJ, Urquiaga S, Hungria M. Biological N2 fixation and yield performance of soybean inoculated with Bradyrhizobium. Nutr Cycl Agroecosystems. 2021;119(3):323–336. doi: 10.1007/S10705-021-10128-7/TABLES/8. [DOI] [Google Scholar]
- 43.Dantas EF, de Freitas ADS, de Lyra M do CCP et al (2019) Biological fixation, transfer and balance of nitrogen in passion fruit (Passiflora edulis Sims) orchard intercropped with different green manure crops. Aust J Crop Sci 13(3):465–471. 10.21475/ajcs.19.13.03.p1559
- 44.Freitas ADS de, Silva AF, Sampaio EV de SB (2012) Yield and biological nitrogen fixation of cowpea varieties in the semi-arid region of Brazil. Biomass Bioenergy 45. 10.1016/j.biombioe.2012.05.017
- 45.Martins JCR, de Freitas ADS, Menezes RSC, Sampaio EV de SB (2015) Nitrogen symbiotically fixed by cowpea and gliricidia in traditional and agroforestry systems under semiarid conditions. Pesqui Agropecu Bras 50(2):178–184. 10.1590/S0100-204X2015000200010