Abstract
Phellinus Quél is one of the largest genera of Hymenochaetaceae; it comprises about 220 species widely distributed on Earth. Most Phellinus species are lignicolous mushrooms that accumulate bioactive compounds. This research studied the phenolic composition of Phellinus spp. and their relationship with antibacterial and antiviral capacity. Phenolics were extracted from Phellinus badius, P. fastuosus, and P. grenadensis; their antiviral and antibacterial activities were evaluated against Listeria monocytogenes, Staphylococcus aureus, Salmonella enterica, and Escherichia coli O157: H7; and the bacteriophages MS2 and Φ- × 174. Gallic acid, chlorogenic acid, caffeic acid, epicatechin, ferulic acid, catechin, 1,3-dicaffeoylquinic acid, p-coumaric acid, and rutin were found in different proportions among Phellinus spp. Total phenolic content ranged from 96 to 209 mg GAE/g, and total flavonoids from 10 to 27 QE/g. The minimum inhibitory concentrations of P. badius, P. grenadensis, and P. fastuosus against E. coli O157: H7 were 13, 20, and 27 mg/mL, against S. enterica were 20, 30, and 15 mg/mL, and against L. monocytogenes were 10, 15, and 25 mg/mL, respectively. The phenolic content was better correlated with the antibacterial effect against E. coli O157: H7 and L. monocytogenes (r = 0.8–0.9), but not against S. enterica (r = 0.05). The antiviral activity of the extracts (0.9 mg/mL) was 29 to 41% against MS2 and 27 to 38% for Φ-X174 virus (r = 0.8–0.9). In silico analysis showed binding energy values of − 7.9 and − 4.8 kcal/mol between the identified phenolic compounds and the M and G proteins of each virus. The antibacterial and antiviral properties of Phellinus species were correlated with the phenolic content.
Keywords: Medicinal mushrooms, Viral agents, Pathogenic bacteria, Gallic acid
Introduction
Recently, the search for antiviral and antibacterial properties in fungi species has increased [1]. Fungi are organisms with higher biodiversity; more than 80,000 species have been identified only in the soil [2], and their bioactive properties have been recognized by traditional medicine. In addition, the content of active compounds in fungi is being used by the pharmaceutical, medical, agriculture, and food industries. Despite this, the proportion of studied bioactive metabolites from fungi and their properties is relatively low; therefore, these organisms remain an untapped resource with enormous industrial potential [1]. Phellinus Quél is one of the largest genera of Hymenochaetaceae, which is comprised of about 220 species. Most Phellinus macro-fungi are perennial lignicolous mushrooms widely distributed on Earth. Several species of Phellinus have pharmacologically important bioactive metabolites like polysaccharides, phenols, and flavonoids, which are traditionally used to treat different diseases such as cancer, diabetes, and hepatitis allergy inflammation [3]. Some Phellinus fungi are historically recorded as traditional medicines used to treat various diseases in eastern Asian countries, especially China, Japan, and Korea [4]. Polysaccharides and polyphenols are responsible for the therapeutic potential of Phellinus spp. An array of pharmacological activities was found in the mycelia, submerged culture, and fruiting bodies of Phellinus species, which includes antioxidant, anti-inflammatory, anti-diabetic, reduction in triglyceride absorption and obesity, system protection, on dermatological conditions like eczema, antimicrobial, and anticancer activity [5]. Phenolic compounds are also considered potent antioxidant, antimicrobial, and antiviral agents, among other properties [6].
Phellinus species have been studied as a source of bioactive compounds with antioxidant and antibacterial properties [7]. For example, P. merrillii (Murrill) Rivarden, P. fastuosus (Lév.) S. Ahmad, P. grenadensis (Murrill) Ryvarden, and P. badius (Cooke) G. Cunn. from Sonora, Mexico, showed high values of total phenolics, flavonoids, and antioxidant capacity [8]. Also, the methanolic extract of P. gilvus (Schwein.) Pat., P. rimosus (Berk.) Pilát, and P. badius showed total phenolic contents of 44.7–49.31 mg of gallic acid equivalents (GAE)/g, and total flavonoid contents of 26.48–30.58 mg of quercetin equivalents (QE)/g; these values were positively correlated with their antioxidant activity [9]. Meanwhile, the methanolic extract of P. gilvus, rich in phenolic compounds, showed antioxidant properties and exhibited antibacterial properties against Gram-positive (Staphylococcus aureus and Streptococcus mutans) and Gram-negative bacteria (Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa) [10]. Also, the extracts of P. linteus obtained by sequentially organic solvent extraction (petroleum ether, chloroform, and methanol) presented antibacterial activity. The methanol extracts showed the best antibacterial activity against Xanthomonas campestris, Pseudomonas syringae, Agrobacterium tumefaciens, Klebsiella pneumoniae, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptomyces pneumoneae, followed by chloroform, petroleum ether. The phytochemical characterization of methanolic extracts revealed the presence of alkaloids, tannins, flavonoids, steroids, glycosides, and phenolic compounds [11]. However, the molecular identification of the active compounds and mechanisms of action still needs to be addressed.
Despite the recognized antioxidant and antibacterial activity of Phellinus spp. extracts, their antiviral properties have been scarcely investigated. Viral diseases are a significant problem impacting the world, and the production of broad-spectrum antiviral substances is a challenge in the pharmaceutical industry since they must offer protection against emerging and re-emerging diseases [12]. Therefore, the search for novel and efficient antiviral compounds is continuously needed. In this context, phenolic compounds are recognized as antiviral compounds, and their mode of action includes multiple targets in the infectious cycle, such as affecting the viral attachment to the host cell, their entry, replication, and assembly [13]. For example, delphinidin (an anthocyanidin) impaired the attachment and entry of the hepatitis C virus into the host cell surface [14]. Meanwhile, epigallocatechin gallate (commonly found in the green tea plant) showed a broad-spectrum activity against enveloped and non-enveloped viruses; its activity was attributed to the interactions with the virion surface proteins [15]. Also, dicaffeoylquinic acid can bind the HIV-1 integrase and inhibit its activity [16]. Nevertheless, when phenolic compounds receive attention as antiviral agents, most of the studied molecules come from plant tissues, leaving aside the potential of fungi as a source of antiviral compounds [17]. Therefore, the present study aimed to characterize the phenolic composition of P. badius, P. grenadensis, and P. fastuosus extracts and their relation with the antiviral and antibacterial activity against the bacteriophages MS2 (ssRNA), Φ- × 174 (ssDNA), Listeria monocytogenes, Staphylococcus aureus, Salmonella enterica serovar Choleraesuis, and Escherichia coli O157: H7.
Materials and methods
Phellinus badius, P. grenadensis, and P. fastuosus collection and identification
The Phellinus species were collected in the Sierra de Álamos-Río Cuchujaqui Biosphere Reserve, Sonora, Mexico (27°03′18.3″N, 109°05′29.3″W), and transported to the CIAD laboratories in Hermosillo. The mushrooms were dried in an oven at 60 °C for 3 days, and the taxonomic identification (Table 1) was carried out in the Laboratory of Fungal Biotechnology of CIAD according to Gilbertson and Ryvarden [18], Larsen and Cobb-Poulle [19], and the information supplied by the Species Index Fungorum database [20].
Table 1.
Morphologic characteristics used for the identification of Phellinus badius, P. grenadensis, and P. fastuosus (Basidiomycota: Hymenochaetaceae)
| Phellinus badius | Phellinus grenadensis | Phellinus fastuosus | |
|---|---|---|---|
| Hymenial setae | Lacking | Lacking | Lacking |
| Basidiospore | Subglobose-globose | Oval-ellipsoid | Ellipsoid-subglobose |
| Basidiocarp | Pileate | Pileate | Pileate |
| Spore color in KOH | Yellow–brown | Dark brown | Brown |
| Spore size (µm) | 5 × 4 | 3 × 6 | 5 × 6 |
| Pores per mm | 3 | 6 | 8 |
| Shape | Ungulate | Ungulate | Flat-ungulate |
Extraction of phenolic compounds
Phenolic compounds were extracted from the Phellinus spp. with methanol, as previously described by Vazquez-Armenta et al. [21]. The fruiting bodies of Phellinus were mechanically ground, and 10 g were placed in 100 mL of methanol: water (7:3) solution; subsequently, the ground mushrooms were macerated for 10 days at 25 °C in darkness. The extracts were then filtered (Whatman No. 1, Springfield Mill, Maidstone Kent, UK), and methanol was removed in a rotatory evaporator (63 rpm and 45 °C) at reduced pressure. Then the residues were hydrolyzed with 10 mL of NaOH (4 M) for 4 h, and the pH was adjusted to 2 with HCl (4 M) and lyophilized for further analysis. The yield of extraction was expressed as a percent.
Total phenolic and flavonoid content
The total phenolic content was measured with the colorimetric method described by Çayan et al. [22]. In a microplate well, 75 μL of Folin–Ciocalteu reagent (1:10) and 60 μL of 7.5% Na2CO3 were added to 15 μL of each mushroom extract. The microplate was incubated at 25 °C in darkness for 30 min, and the optical density (OD) was measured at 765 nm with a microplate reader FLUOstar Omega (BMG Labtech, Chicago, IL, USA). Gallic acid was used as standard, and results were expressed in mg of GAE per gram of dry extract. All of the samples were determined by triplicate.
The flavonoid content was determined based on the method described by Zhishen et al. [23], with some modifications. Each mushroom extract (100 µL) was mixed with 430 µL of mixture A (1.8 mL of 5% NaNO2 with 24 mL of distillate water) and incubated for 5 min. Then, 30 µL of 10% anhydrous AlCl3 was added and incubated for 1 min. Later, 440 µL of the mixture B (12 mL of NaOH 1 M with 14.4 mL of distillate water) was added, and 150 µL of this reaction was taken and read at 496 nm with a microplate reader FLUOstar Omega (BMG Labtech, Chicago, IL, USA). The samples were analyzed by triplicate, and results were expressed in mg QE/g.
Phenolic compound identification
The ultra-performance liquid chromatography (UPLC) analysis was carried out by using an ACQUITY Ultra Performance LCTM system (Waters) linked simultaneously to a PDA 2996 photodiode array detector (Waters) as previously reported by Fratianni et al. [24]. This analysis was performed at 30 °C by using a reversed-phase column (BEH C18, 1.7-μm, 2.1 × l00-mm; Waters). The used mobile phase consisted of solvent A (7.5 mM acetic acid) and solvent B (acetonitrile) at a flow rate of 250 μL/min. Gradient elution was used starting at 50/0 solvent B for 0.8 min, 5–20% solvent B for 5.2 min, isocratic 20% solvent B for 0.5 min, 20–30% solvent B for 1 min, isocratic 30% solvent B for 0.2 min, 30–50% solvent B for 2.3 min, 50–100% solvent B for 1 min, isocratic 100% solvent B for 1 min, and finally 100–5% solvent B for 0.5 min. At the end of this sequence, the column was equilibrated under the initial conditions for 2.5 min. The applied pressure during the chromatographic run ranged from 6,000 to 8,000 psi. The effluent carrying the phenolic compounds was introduced to a liquid chromatography detector (scanning range, 210–400 nm; resolution, 1.2 nm). Each extract was injected (10 μL) by duplicate, and phenolic compounds were identified and quantified with standard substances and calibration curves. The ultraviolet-detection wavelength was set at 280 nm. The Empower software (Waters) was used to control the instruments and data acquisition and processing.
Antibacterial activity
The antibacterial activity of each Phellinus extract was evaluated against Listeria monocytogenes (ATCC 7644), Staphylococcus aureus (ATCC 6538), Salmonella enterica subsp. enterica serovar Choleraesuis (ATCC 14,028), and Escherichia coli O157: H7 (ATCC 43,890). The broth microdilution method was used to determine the minimum inhibitory concentration (MIC) of each extract against each bacterium. Stock solutions of each Phellinus extract were prepared in trypticase soy broth (TSB), and several concentrations were prepared (0–35 mg/mL). The treatment consisted of mixing 5 µL of bacterial inoculum (1 × 108 CFU/mL) with 295 µL of each extract dilution in sterile 96-well microplates (Costar 96). The inoculum of each bacterium was prepared from overnight cultures; the microplate included a positive (it contained the inocula but not the extract) and negative controls (they contained the extracts but not the inoculum), and then they were incubated at 37 °C for 24 h. The extracts’ MIC values were considered the lowest concentrations needed to inhibit the growth of the tested bacteria and confirmed by counting the viable bacteria on agar plates [25]. Also, the growth curves of the tested bacteria (108 CFU/mL) were recorded in the presence of the fungi extracts by applying the same experimental conditions. The microplates were incubated at 37 °C for 16 h with intermittent shaking, and the OD readings at 600 nm were recorded every 30 min. The experiment was replicated three times, and the experimental growth data were fitted to the Baranyi function [26] with a complementary tool for Microsoft Excel (D-model, J. Baranyi, Institute of Food Research, Norwich, UK) and used to calculate the adaptation or lag time (λ; h), maximum growth rate (μmax; OD/h), and maximum growth Ymax (Ymax; OD) for each growth curve.
Antiviral activity against MS2 and Φ-X174
The antiviral activity was evaluated by exposing the bacteriophages MS2 (ATCC 15,597-B1) and Φ- × 174 (ATCC 13,706-B1) to the presence of the Phellinus extracts. The viral dispersions (2 logs of plaque-forming units, PFU/mL) were exposed to 0.9 mg/mL of each extract, mixed and agitated for 10 min; a control mixture of the untreated virus was included. Subsequently, the viral infection was quantified using the double agar layer method. The treated and control virus were added to 105 CFU/mL of their respective bacterial host [Escherichia coli strain C-3000 (ATCC 15,597) for MS2 and Escherichia coli strain C (ATCC 13,706) for Φ- × 174]. First, the mixtures were added to a top layer of melted agar (3% tryptic soy broth, 0.5% NaCl, 0.6% agar) and poured onto a bottom layer of solid agar (3% tryptic soy broth, 0.5% NaCl, 1.2% agar). The plates were solidified and incubated 24 h at 37 °C. The PFU in treatments and control were counted, and the percentage of activity was determined by subtracting the titer values of the treated samples from the untreated control [27]. The experiment was replicated three times.
Molecular docking between viral proteins and the identified phenolic compounds
The molecular docking analysis was simulated between each identified phenolic compound of Phellinus extracts with the M protein of the MS2 bacteriophage (PDB: 5TC1; chain M) and the G protein of Φ-X174 (PDB: 1CD3; chain G) searching for physicochemical interactions. The M and G proteins are considered responsible for the initial viral attachment to the F-pili and lipopolysaccharides of the host cells, respectively [28, 29]. This analysis was done with the AutoDock Vina application in the UCSF Chimera version 1.13 software (Resource for Biocomputing, Visualization, and Informatics, University of California, San Francisco, USA). The affinity energies (kcal/mol) were selected as those with the lowest root-mean-square deviation (RMSD, Å) between the selected proteins and each of the identified phenolic compounds. Ten binding modes with a 3 level of exhaustiveness search and a 3 kcal/mol level of maximum energy difference were set as basic parameters during the analysis.
Statistical analysis
A completely randomized design was applied to estimate significant differences among Phellinus species extracts. A one-way analysis of variance (ANOVA) was used to evaluate the effect of the fungal species on the responses being assessed. Furthermore, the means comparisons (extracts and controls) were made by the Tukey–Kramer test. The Pearson correlation analysis was used to evaluate the relationships between the content of total phenols and total flavonoids vs. the antibacterial and antiviral activity capacity, respectively. The analyses were performed in the statistical package NCSS version 2007 (NCSS, LLC, Utah, USA).
Results and discussion
Identification and quantification of phenolic compounds
The weight yield of the extracts showed values around 18% w/w (Table 2), and they were similar among them (p > 0.05); but the P. badius had the highest content of total phenolic compounds and flavonoids with values of 209.76 mg GAE/g and 27.61 mg QE/g, respectively. In contrast, the total phenolic and flavonoid contents were not different (p > 0.05) between P. grenadensis (111.4 mg GAE/g and 13.12 mg QE/g) and P. fastuosus (96.96 mg GAE/g and 10.4 mg QE/g). For P. badius, the main phenolic compounds were gallic acid (134.15 μg/g), chlorogenic acid (264.15 μg/g), caffeic acid (67.9 μg/g), epicatechin (71.05 μg/g), and ferulic acid (82.45 μg /g) (Fig. 1). The extract of P. grenadensis showed five phenolic compounds: gallic acid (28.4 μg/g), catechin (26.95 μg/g), 1,3-dicaffeoylquinic acid (14.3 μg/g), rutin (587.5 μg/g), and ferulic acid (464.75 μg/g). Meanwhile, the extract of P. fastuosus showed gallic acid (47.95 μg/g), chlorogenic acid (193.70 μg/g), catechin (26.15 μg/g), epicatechin (116.6 μg/g), 1,3-dicaffeoylquinic acid (27.13 μg/g), p-coumaric acid (4.45 μg/g), and ferulic acid (75.85 μg/g). p-Coumaric acid was found only in P. fastuosus, caffeic acid only in P. badius, and rutin only in P. grenadensis; the latter was the most abundant phenolic molecule identified by UPLC.
Table 2.
Identified phenolic compounds, total phenolic, flavonoids, and weight yield of P. badius, P. grenadensis, and P. fastuosus extracts
| Compound | Retention time (mins) | Concentration (µg/g) | ||
|---|---|---|---|---|
| P. badius | P. grenadensis | P. fastuosus | ||
| Gallic acid | 1.04 | 134.15 | 28.4 | 47.95 |
| Chlorogenic acid | 3.14 | 264.15 | ND | 193.70 |
| Catechin | 3.43 | ND | 26.95 | 26.15 |
| Caffeic acid | 3.81 | 67.9 | ND | ND |
| Epicatechin | 4.03 | 71.05 | ND | 116.6 |
| 1,3-Dicaffeoylquinic acid | 4.54 | ND | 14.3 | 27.3 |
| p-Coumaric acid | 5.14 | ND | ND | 4.45 |
| Rutin | 5.28 | ND | 587.5 | ND |
| Ferulic acid | 5.47 | 82.45 | 464.75 | 75.85 |
| Extract weight yield (%) | 18.4a* | 17.5a | 18a | |
| Total phenols (mg GAE/g) | 209.76a | 111.4b | 96.96b | |
| Total flavonoids (mg QE/g) | 27.61a | 13.12b | 10.4b | |
*Different letters in the same row indicate significant differences among extracts (p < 0.05)
Fig. 1.
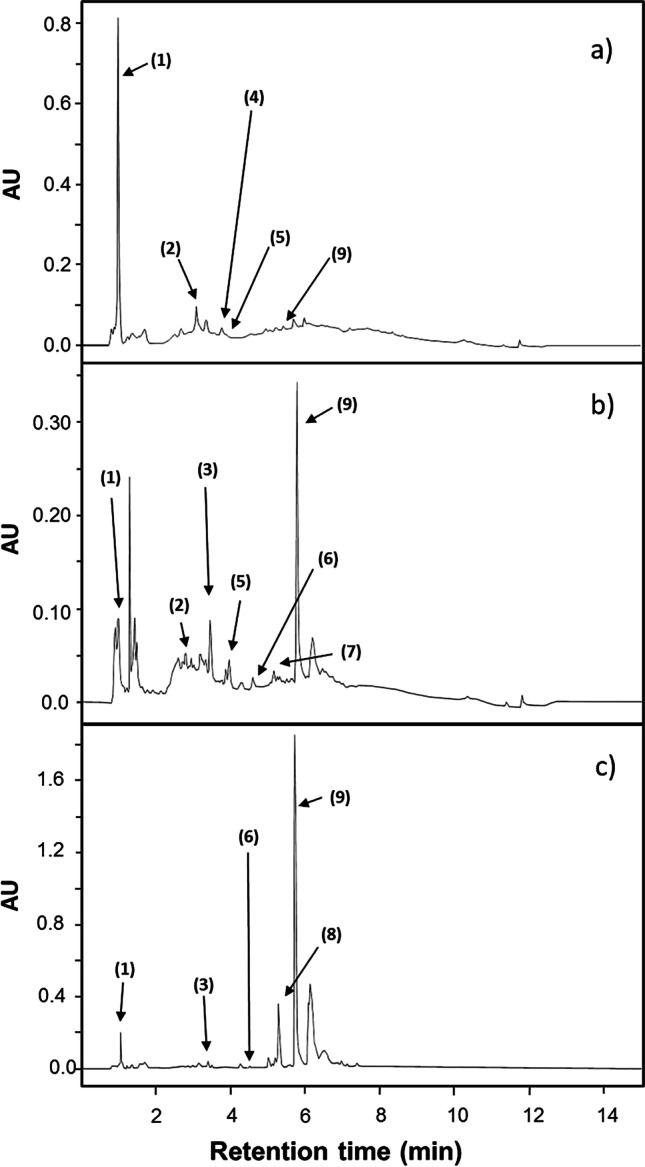
UPLC-DAD chromatograms of a P. badius, b P. grenadensis, and c P. fastuosus extract showing the identified phenolic compounds: (1) gallic acid, (2) chlorogenic acid, (3) catechin, (4) caffeic acid, (5) epicatechin, (6) 1,3-dicaffeoylquinic acid, (7) p-coumaric acid, (8) rutin, and (9) ferulic acid
Wood-degrading fungi such as Phellinus produce lignin-degrading enzymes, for example, lignin peroxidases, manganese-dependent peroxidases, and laccases [30]. Then, the degraded lignin could be used to synthesize phenolic compounds accumulated in the fungal fruiting body. However, enzymatic expression and activity are influenced by several biotic and abiotic factors [30], and it has been demonstrated that the ligninolytic activity of Phellinus is also affected by the species [31]. Therefore, the differences in the phenolic composition observed among the studied specimens could be caused by the differential expression of enzymes in charge of degrading lignin or phenolic compounds’ biosynthesis.
Previous studies highlighted the genus Phellinus as a source of phenolic compounds. The production of flavonoids by Phellinus sp. was increased with naphthaleneacetic acid and coumarin at 0.03 and 0.02 g/L, respectively [32]. Similarly, Wang et al. [33] improved the production of flavonoids in P. igniarius by adding wheat bran, corn silk, yeast extract, KH2PO4, and MgSO4. Besides, Ayala-Zavala et al. [9] showed that methanolic extracts from P. gilvus, P. rimosus, and P. badius showed 49.31, 46.51, and 44.7 mg GAE/g, respectively, and total flavonoid contents of 30.58, 28, and 26.48 mg QE/g, respectively. The values of total phenolic contents found in the present study were 2 to fourfold higher than those reported in the studies mentioned above, while the total flavonoid contents were similar. These differences could be attributed to the extraction method because, in the present work, a methanolic maceration for 10 days plus alkaline and acid hydrolysis were employed, which could explain the higher amount of extracted phenolic compounds.
On the other hand, the methanolic extracts of P. merrillii showed a total phenolic content of 913.91 mg GAE/g and total flavonoids of 563.83 mg QE/g [34], but this extract was obtained with the same technique used in our work. Therefore, the observed differences could be explained by biotic and abiotic factors surrounding each species [35, 36]. In this way, the results reported here help broaden the knowledge about the phenolic content of Phellinus and extraction methods.
Although Phellinus species are recognized as a source of phenolic compounds, little is known about their specific composition. Sułkowska-Ziaja et al. [37] identified four phenolic acids (3,4-dihydroxy-phenylacetic, gallic, protocatechuic, and syringic acids) in the methanolic extracts of P. igniarius, P. pini, P. pomaceus, and P. robustus, varying their concentration among the species, with protocatechuic acid as the common compound. Similarly, Heleno et al. [38] reported gallic acid, p-hydroxybenzoic acid, protocatechuic acid, and 3, 4, or 5-O-caffeoylquinic acid in Phellinus linteus. Gallic and chlorogenic acids and rutin were also identified in Ganoderma lucidum extracts but not in Trametes hirsuta, which showed catechol and cinnamic acid [39]. This family of compounds is widely recognized for its hepatoprotective, antioxidant, and antiviral activities [40]. To the extent of our literature review, this is the first report of 1,3-dicaffeoylquinic acid in mushrooms, even when it is commonly found in coffee beans, cherries, apples, kiwifruit, and apricots [41]. Therefore, P. fastuosus and P. grenadensis can be considered sources of this hydroxycinnamic acid derivative.
Antibacterial activity
The antibacterial activities of the extracts are shown in Table 3. P. badius showed the lowest MIC (13 mg/mL) against the growth of E. coli O157: H7, followed by P. grenadensis (20 mg/mL) and P. fastuosus extracts (27 mg/mL). The same pattern was observed against L. monocytogenes, but this bacterium was more sensitive to the presence of the extracts than E. coli O157: H7 (MIC = 10–25 mg/mL). Meanwhile, S. enterica ser. Choleraesuis was more susceptible to P. fastuosus extract than P. badius (MIC = 20 mg/mL) and P. grenadensis (MIC = 30 mg/mL). Nevertheless, no inhibitory activity was observed of all the extracts against S. aureus at the tested concentrations (0–35 mg/mL).
Table 3.
Minimum inhibitory concentrations (MIC) of P. badius, P. grenadensis, and P. fastuosus extracts against pathogenic bacteria, and Pearson correlations of the antibacterial activity with the total phenol and flavonoid contents
| Bacteria | MIC (mg/mL) | ||
| P. badius | P. grenadensis | P. fastuosus | |
| E. coli O157: H7 | 13 | 20 | 27 |
| S. Choleraesuis | 20 | 30 | 15 |
| L. monocytogenes | 10 | 15 | 25 |
| S. aureus | > 35 | > 35 | > 35 |
| Correlated responses | Pearson coefficient (r) | p-value | |
|
Polyphenols Vs |
E. coli O157: H7 | 0.888 | 0.001 |
| S. Choleraesuis | 0.055 | 0.888 | |
| L. monocytogenes | 0.805 | 0.009 | |
|
Flavonoids Vs |
E. coli O157: H7 | 0.904 | 0.001 |
| S. Choleraesuis | 0.029 | 0.941 | |
| L. monocytogenes | 0.826 | 0.006 | |
Pearson’s correlations showed a positive relationship with the phenolic content and the antibacterial capacity (Table 3). The total phenol content showed r values of 0.88 with the dose needed to inhibit the growth of E. coli O157: H7 (p = 0.0013), with L. monocytogenes of 0.8050 (p = 0.0088), and with S. Choleraesuis of 0.0552 (p = 0.880). Meanwhile, the flavonoid content showed r values of 0.904 with the doses needed to inhibit the growth of E. coli O157: H7 (p = 0.0008), L. monocytogenes of 0.8260 (p = 0.0061), and S. Choleraesuis of 0.0291 (p = 0.9410). The high correlation coefficient with the MICs against E. coli O157: H7 and L. monocytogenes with a low probability (p < 0.05) indicates the significant relationship between the phenolic compounds and the antibacterial capacity.
The growth kinetics evidenced how the studied Phellinus extracts affected bacterial growth (Table 4). P. badius extended the lag phase of E. coli O157: H7 about 5.5 times compared to non-treated bacteria, while for S. Choleraesuis and L. monocytogenes, the extension was 1.8 and 2.3 times, respectively. P. fastuosus caused the lag phase extension by 3.7, 3.1, and 2.0 times for E. coli O157: H7, L. monocytogenes, and S. Choleraesuis, respectively, compared with untreated bacteria. Similarly, E. coli O157: H7 was more susceptible to P. grenadensis extending the lag phase 3.3 times, followed by S. Choleraesuis (2.1 times) and L. monocytogenes (2.0 times). The extracts also affected the maximum growth rates (μmax) of the tested bacteria. For example, E. coli O157: H7 showed a reduction of 62% on this parameter in the presence of P. badius extract, compared with the control, followed by P. grenadensis (57%) and P. fastuosus (52%). For S. Choleraesuis, max values were more drastically affected by the extracts, with a 99% reduction caused by P. badius, 90% by P. grenadensis, and 80% by P. fastuosus. Finally, max of L. monocytogenes was reduced 84 and 81% by P. grenadensis and P. fastuosus extracts, but to a lesser extent by P. badius (43%). The lag phase extension and the max reduction caused by the Phellinus extracts affected the maximum bacterial growth (ymax). Considering that the lag phase (λ) is the time taken by bacteria in adapting to a new environment, therefore, the extracts affected the ability of bacteria to adapt to the growing media; usually, this occurs by altering the membrane functionality and nutrient absorption. The influence of the treatments on the growth rate indicated that the extracts affected the treated bacteria’s reproductive capacity, possibly by inhibiting the activity and production of enzymes necessary for cellular reproduction.
Table 4.
Effect of extracts of P. badius, P. grenadensis, and P. fastuosus on the growth parameters of E. coli O157: H7, S. Choleraesuis, and L. monocytogenes
| Bacteria | Treatment | λ (h) | μmax (OD/h) | ymax (OD) | R2* |
|---|---|---|---|---|---|
| E. coli O157: H7 | Control | 3.4 ± 0.10d | 0.209 ± 0.006d | 2.46 ± 0.074d | 0.98 |
| P. badius | 18.5 ± 0.55ª | 0.079 ± 0.002ª | 0.37 ± 0.014a | 0.96 | |
| P. grenadensis | 11.2 ± 0.34c | 0.100 ± 0.003c | 0.59 ± 0.018c | 0.81 | |
| P. fastuosus | 12.6 ± 0.38b | 0.089 ± 0.003b | 0.48 ± 0.011b | 0.96 | |
| S. Choleraesuis | Control | 9.8 ± 0.29d | 0.383 ± 0.011d | 1.29 ± 0.039d | 0.99 |
| P. badius | 18.0 ± 0.54c | 0.005 ± 0.001ª | 0.08 ± 0.002ª | 0.98 | |
| P. grenadensis | 21.0 ± 0.63ª | 0.079 ± 0.002c | 0.29 ± 0.009c | 0.92 | |
| P. fastuosus | 19.8 ± 0.59b | 0.037 ± 0.001b | 0.16 ± 0.005b | 0.99 | |
| L. monocytogenes | Control | 4.5 ± 0.13d | 0.230 ± 0.007d | 2.35 ± 0.070c | 0.98 |
| P. badius | 10.6 ± 0.32b | 0.131 ± 0.004c | 0.41 ± 0.012ª | 0.84 | |
| P. grenadensis | 9.2 ± 0.28c | 0.037a ± 0.001ª | 0.40 ± 0.012ª | 0.85 | |
| P. fastuosus | 13.8 ± 0.41a | 0.063b ± 0.002b | 0.59 ± 0.018b | 0.98 |
Data are the mean of three independent experiments. Different letters in each column indicate differences (p < 0.05) among treatments for each bacterium. λ corresponds to the lag phase, μmax is the maximum growth rate, and ymax is the maximum growth of bacteria
*R2 = Coefficient of determination
The antibacterial activity of Phellinus extracts can be attributed to the presence of phenolic compounds. It has been reported that chlorogenic acid found in the extracts of P. badius and P. fastuosus showed bacteriostatic and bactericidal effects against E. coli O157: H7. This effect was dose-dependent and associated with exposure time [42]. Vazquez-Armenta et al. [21] reported that L. monocytogenes was more susceptible to ferulic, caffeic, and gallic acids (MIC = 3–4 mg/mL) than to catechin and rutin (MIC > 7 mg/mL). Meanwhile, gallic and ferulic acids, found in all Phellinus extracts, inhibited the bacterial growth of P. aeruginosa, S. aureus, and L. monocytogenes by damaging their cytoplasmic membranes [43]. A similar effect was observed in the cytoplasmic membrane of uropathogenic E. coli treated with catechin, protocatechuic, and vanillic acids [44]. Catechin was more effective than protocatechuic and vanillic acids at disturbing membrane permeability. These results were confirmed using a phospholipids membrane model monolayer, where catechin also showed the most fluidizing effect on the monolayers, followed by protocatechuic and vanillic acids [44].
The disruption of the cytoplasmic membrane could lead to the internalization of phenolic compounds to reach multiple targets. For example, the flavonoid quercetin (aglycone form of rutin) at 0.8 mM increased the permeability of L. monocytogenes membrane ~ 80% after 30 min of treatment [25]. Quercetin is an inhibitor of the bacterial DNA gyrase [45] and d-alanine:d-alanine ligase [46] implicated in nucleic acid synthesis and assembly of cell wall precursors. Whereas rutin selectively promoted the cleavage of topoisomerase IV, an essential enzyme related to bacterial survival, this result evidences phenolic compounds’ intercellular action [47]. On the other hand, caffeoylquinic acid was also identified in P. fastuosus and P. grenadensis extracts. However, this compound exhibited weakly or no antimicrobial activity against Gram-positive and Gram-negative bacteria; it also inhibited the efflux pump systems of S. aureus, E. faecalis, and B. cereus, in charge of expelling antimicrobial activity agents, including phenolic compounds [48, 49]. In this way, the presence of 1,3-dicaffeoylquinic acid in Phellinus extracts could enhance the other phenolic acids and flavonoids’ antimicrobial activity. Consequently, the variety of phenolic compounds in the extracts of Phellinus could act via multiple modes of action; nevertheless, more studies must be conducted to get a deeper insight into this idea.
Previous studies explored the antibacterial properties of Phellinus species using different extraction techniques. Yoon and Jang [10] obtained a methanol extract from P. gilvus with values of total phenolic and flavonoid contents of 31.17 mg GAE/g and 15.29 mg QE/g, respectively; this extract inhibited the growth of S. aureus, Streptococcus mutans, Bacillus subtilis, E. coli, and Pseudomonas aeruginosa, with MIC values ranging from 50 to 200 mg/mL. Those MIC values are higher than the MIC values obtained in the present work (10–35 mg/mL), and this could be attributed to the lower phenolic and flavonoid content of P. gilvus compared with P. badius, P. fastuosus, and P. grenadensis.
Kodiyalmath and Krishnappa [11] evaluated the antimicrobial activity of P. linteus by using different solvents (petroleum ether, chloroform, and methanol) and tested against Salmonella typhi, S. aureus, and E. coli using the disk diffusion technique. The tested bacteria were more sensitive to methanol > chloroform > petroleum ether extract; a comparison of these results with those of the present study is difficult considering the different antimicrobial tests used, but it can be highlighted that the methanolic and chloroform extracts of P. linteus showed phenolic compounds, while the petroleum ether extracts did not show phenolic compounds. Leyva et al. [34] evaluated the antibacterial activity of fractionated (polar and non-polar fractions) methanolic extracts of P. merrillii. The non-fractionated methanolic extract showed the highest phenolic content (913.91 mg GAE/g), flavonoids (563.83 mg QE/g), and the highest inhibition of S. aureus, E. coli O157: H7, Salmonella Choleraesuis, and L. monocytogenes, followed by the non-polar fraction and the polar fraction. However, this study did not determine the extracts’ MICs nor evaluated the changes in bacterial growth parameters as the present work did. It is important to mention that the studies discussed above did not identify the phenolic compounds.
Antiviral activity
Phellinus extracts at 0.9 mg/mL showed antiviral activity against both bacteriophages (Table 5), and P. badius showed the highest activity (p < 0.05) by inhibiting 41.1 and 37.7% of the plaque formation of MS2 and Φ-X174 compared with their respective controls. Meanwhile, P. grenadensis and P. fastuosus inhibited 37.38 and 28.97% for MS2, and 33.02 and 26.41% for Φ-X174, respectively. Pearson’s coefficient between total phenols and the antiviral activity against MS2 was 0.837 (p = 0.005), and against Φ -X174, it was 0.887 (p = 0.001). Besides, Pearson’s coefficient between total flavonoid contents and the antiviral activity against MS2 was 0.852 (p = 0.004), and 0.902 (p = 0.001) against Φ -X174. The significant coefficients (p < 0.05) indicated that these compounds are potentially responsible for antiviral activity.
Table 5.
Antiviral activity of P. badius, P. grenadensis, and P. fastuosus extracts, and Pearson correlation of the antiviral activity with the total phenol and flavonoid contents
| Extract (0.9 mg/mL) | Plaque inhibition (%) | ||
| MS2 | Φ-X174 | ||
| P. badius | 41.12 ± 0.67ª | 37.73 ± 0.07ª | |
| P. grenadensis | 37.38 ± 0.14b | 33.02 ± 0.08b | |
| P. fastuosus | 28.97 ± 0.17c | 26.41 ± 0.15c | |
| Correlated responses | Pearson coefficient (r) | p-value | |
|
Polyphenols Vs |
MS2 | 0.837 | 0.005 |
| Φ-X174 | 0.887 | 0.001 | |
|
Flavonoids Vs |
MS2 | 0.852 | 0.004 |
| Φ-X174 | 0.902 | 0.001 | |
Previous studies evidenced the antiviral properties of phenolic compounds obtained from natural sources; for example, Miguel et al. [50] reported that the infectivity of Qβ bacteriophage, an enteric viral surrogate, was reduced by a honey-based product named água-mel. The higher antiviral activity was observed in the samples that showed the highest polyphenol content. Similarly, Gescher et al. [51] tested procyanidins against the herpes simplex virus and observed a significant effect of the molecular size; the bigger the size, the higher antiviral effect, gallic acid having the lowest antiviral activity because of the simplicity of its structure. The extracts of the present study were hydrolyzed, and a similar effect cannot be discarded. In the case of mushrooms, the study of antiviral properties has been increased in recent years [1]. It has been reported that extracts from Fomes fomentarius, P. igniarius, and P. pini exerted antiviral activity against enveloped viruses such as Herpes simplex 1(HSV-1) [52]. Also, mycelial extracts of Pleurotus ostreatus, F. fomentarius, Auriporia aurea, and Trametes versicolor demonstrated activity against HSV-2 [53]. Among the studied Phellinus, Lee et al. [54] reported that P. igniarius extracts affected the influenza virus’s replication by interfering with the viral attachment to the host cell surface. Nevertheless, the studies of antiviral activity of Phellinus against non-enveloped viruses are limited; in this regard, these results showed that P. badius, P. fastuosus, and P. grenadensis were effective against this kind of viruses.
On the other hand, the binding energies of phenolic compounds with the M and G proteins of the MS2 and Φ- × 174 bacteriophages are shown in Table 6. Phenolic compounds showed binding energy values ranging from − 7.9 to − 4.8 kcal/mol. Rutin showed the highest binding affinity with both proteins (− 7.9 and − 7.3 kcal/mol with the G and M protein, respectively), followed by 1,3-dicaffeoylquinic acid (− 7.2 kcal/mol with the M protein and − 6.4 kcal/mol with the G protein), and chlorogenic acid (− 6.4 kcal/mol with the M protein and − 6.3 kcal/mol with the G protein). Meanwhile, p-coumaric acid showed the lowest affinity (− 4.8 kcal/mol with the M protein), similar to gallic acid with the G protein (− 4.8 kcal/mol). As can be seen in Table 7, strong and significant coefficients (r) were found among the number of hydrogen bond donors (nOHNH), number of hydrogen bond acceptors (nON), molecular polar surface area (TPSA), number of rotatable bonds (Nrotb), and the binding energies of phenolic compounds with the viral proteins. The number of hydrogen bond donors showed an r = − 0.9315, explaining the higher affinity of rutin and 1,3-dicaffeoylquinic acid with both proteins; both phenolics and proteins have more –OH groups (hydrogen donors) in their molecules. Therefore, the antiviral properties of the Phellinus extracts studied here could be attributed to their phenolic content.
Table 6.
Binding free energy and physicochemical characteristics of phenolic compounds found in P. badius, P. grenadensis, and P. fastuosus extracts with the M and G proteins of MS2 and Φ- × 174
| Compound | LogP | TPSA (Molecular polar surface area) | Hydrogen bonds donors (nOHNH) | Hydrogen bonds acceptors (nON) | Number of rotatable bonds (Nrotb) | Binding Free Energy (Kcal/mol) | |
|---|---|---|---|---|---|---|---|
| M | G | ||||||
| Rutin | − 1.06 | 269.43 | 10 | 16 | 6 | − 7.3 | − 7.9 |
| 1,3-Dicaffeoylquinic acid | 1.42 | 211.28 | 7 | 12 | 9 | − 7.2 | − 6.4 |
| Chlorogenic acid | − 0.45 | 164.74 | 6 | 9 | 5 | − 6.3 | − 6.4 |
| Epicatechin | 1.37 | 110.37 | 5 | 6 | 1 | − 6.0 | − 6.1 |
| Catechin | 1.37 | 110.37 | 5 | 6 | 1 | − 5.9 | − 6.2 |
| Ferulic acid | 1.25 | 66.76 | 2 | 4 | 3 | − 5.4 | − 5.0 |
| Caffeic acid | 0.94 | 77.75 | 3 | 4 | 2 | − 5.3 | − 5.1 |
| Gallic acid | 0.59 | 97.98 | 4 | 5 | 1 | − 5.0 | − 4.8 |
| p-Coumaric acid | 1.43 | 57.53 | 2 | 3 | 2 | − 4.8 | − 4.9 |
Table 7.
Correlation between the physicochemical properties of phenolic compounds identified in Phellinus spp. extracts, and their respective binding energy
| Parameter | Correlation coefficient (r) | p-value |
|---|---|---|
| LogP | 0.5496 | 0.0181 |
| TPSA | − 0.9254 | 0.0000 |
| Hydrogen bonds donors | − 0.9315 | 0.0000 |
| Hydrogen bonds acceptors | − 0.9285 | 0.0000 |
| Rotatable bonds | − 0.6823 | 0.0018 |
Conclusions
Phellinus extracts showed antibacterial activity against E. coli O157: H7, L. monocytogenes, and S. Choleraesuis; this activity was higher for P. badius than P. grenadensis and P. fastuosus. Besides, Phellinus extracts showed antiviral activity against the MS2 bacteriophage and Φ-X174; the extract of P. badius had the highest antiviral activity. Moreover, the evaluated bioactivities were well correlated with the phenolic content of Phellinus extracts. Finally, the in silico analysis showed that rutin, 1,3-dicaffeoylquinic acid, and chlorogenic acid were the most probable active phenolics in binding the M and G proteins of the MS2 and Φ- × 174 bacteriophages. Therefore, this research indicates the potential uses of P. badius, P. grenadensis, and P. fastuosus as a source of antibacterial and antiviral agents.
Acknowledgements
The authors thank the Mexican Council for Science and Technology CONACYT (Project number CB-2008-01-103105) for their financial support.
Author contribution
FJVA: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. JML: investigation, writing—review and editing. VMH: funding acquisition, supervision, writing—review and editing. GAGA: funding acquisition, supervision, writing—review and editing. MRCV: formal analysis, funding acquisition, writing—review and editing. ME: funding acquisition, supervision, writing—review and editing. AG: funding acquisition, investigation, writing—review and editing. FN: formal analysis, funding acquisition, investigation, writing—review and editing. FF: formal analysis, investigation, writing—review and editing. RGH: formal analysis, visualization, writing—review and editing. JFAZ: Conceptualization, funding acquisition, project administration, resources, supervision, validation, visualization, writing—review and editing. All authors discussed and agreed with the submitted version of the manuscript.
Funding
Consejo Nacional de Ciencia y Tecnología, CB-2008-01-103105, Jesus Fernando Ayala-Zavala
Data availability
Data can be found on the institution repository (https://ciad.repositorioinstitucional.mx/jspui/handle/1006/346) or requested to the corresponding author. The used fungal material can be located at the fungal collection “Dr. Martín Esqueda Valle” at the herbarium of the Sonoran State University.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Responsible Editor: Melissa Fontes Landell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hyde KD, Xu J, Rapior S, et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- 2.Shirouzu T, Matsuoka S, Doi H, Nagata N, Ushio M, Hosaka K. Complementary molecular methods reveal comprehensive phylogenetic diversity integrating inconspicuous lineages of early-diverged wood-decaying mushrooms. Sci Rep. 2020;10:e3057. doi: 10.1038/s41598-020-59620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawashte G, Sonawane H, Mittal S, Borde M 2021 Medicinal fungi: a natural source of pharmacologically important metabolites. In Recent Developments in Microbial Technologies. Ed. Springer. p 379 – 394. 10.1007/978-981-15-4439-2_18
- 4.He P, Zhang Y, Li N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: a review. Food Funct. 2021;12:1856–1881. doi: 10.1039/D0FO02342F. [DOI] [PubMed] [Google Scholar]
- 5.Varghese R, Dalvi YB, Lamrood PY, Shinde BP, Nair CKK. Historical and current perspectives on therapeutic potential of higher basidiomycetes: an overview. 3 Biotech, 9:362. 10.1007/s13205-019-1886-2 [DOI] [PMC free article] [PubMed]
- 6.Sandargo B, Chepkirui C, Cheng T, Chaverra-Muñoz L, Thongbai B, Stadler M, Hüttel S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol Adv. 2019;37:e107344. doi: 10.1016/j.biotechadv.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, Tan Y, Sun Z, Ren A, Zhu J, Zhao M. Exogenous salicylic acid (SA) promotes the accumulation of biomass and flavonoid content in Phellinus igniarius (Agaricomycetes) Int J Med Mushrooms. 2019;21:955–963. doi: 10.1615/IntJMedMushrooms.2019032557. [DOI] [PubMed] [Google Scholar]
- 8.Ayala-Zavala JF, Pérez-Carlón JJ, Esqueda M, et al. Polar fractionation affects the antioxidant properties of methanolic extracts from species of genus Phellinus Quél. (higher Basidiomycetes) Int J Med Mushrooms. 2012;14:563–573. doi: 10.1615/intjmedmushr.v14.i6.40. [DOI] [PubMed] [Google Scholar]
- 9.Ayala-Zavala JF, Silva-Espinoza BA, Cruz-Valenzuela MR, et al. Antioxidant and antifungal potential of methanol extracts of Phellinus spp. from Sonora. Mexico Rev Iberoam Micol. 2012;29:132–138. doi: 10.1016/j.riam.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Yoon KN, Jang HS. Antioxidant and antimicrobial activities of fruiting bodies of Phellinus gilvus collected in Korea. Korean J Clin Lab Sci. 2016;48(4):355–364. doi: 10.15324/kjcls.2016.48.4.355. [DOI] [Google Scholar]
- 11.Kodiyalmath JK, Krishnappa M. Evaluation of antimicrobial activity of Phellinus linteus (Berk. & MA Curtis.) with their wild collections from Western Ghats of India. Tropical Plant Res. 2017;4(2):351–7. doi: 10.22271/tpr.2017.v4.i2.046. [DOI] [Google Scholar]
- 12.Andersen PI, Ianevski A, Lysvand H, et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int J Infec Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A. Antiviral effect of phytochemicals from medicinal plants: applications and drug delivery strategies. Drug Delivery Transl Res. 2020;10:354–367. doi: 10.1007/s13346-019-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calland N, Sahuc ME, Belouzard S, et al. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J Virol. 2015;89:10053–10063. doi: 10.1128/JVI.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury P, Sahuc ME, Rouillé Y, et al. Theaflavins, polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture. PLoS ONE. 2018;13:e0198226. doi: 10.1371/journal.pone.0198226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makola MM, Dubery IA, Koorsen G, et al. The effect of geometrical isomerism of 3,5-Dicaffeoylquinic acid on its binding affinity to HIV-integrase enzyme: a molecular docking study. J Evidence-Based Complementary Altern Med. 2016;2016:e4138263. doi: 10.1155/2016/4138263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhusseiny SM, El-Mahdy TS, Awad MF, Elleboudy NS, Farag MMS, Aboshanab KM, Yassien MA. Antiviral, cytotoxic, and antioxidant activities of three edible agaricomycetes mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J of Fungi. 2021;7(8):645. doi: 10.3390/jof7080645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbertson RL, Ryvarden L 1987 North American Polypores. Vol. 2. Megasporoporia – Wrightoporia. Fungiflora, Oslo.
- 19.Larsen MJ, Cobb-Poulle LA 1989 Phellinus (Hymenochaetaceae). A survey of the world taxa. Fungiflora, Oslo.
- 20.Index Fungorum 2019 Richmond, Royal Botanic Gardens Kew. http://indexfungorum.org. Accessed 10 November 2019
- 21.Vazquez-Armenta FJ, Bernal-Mercado AT, Lizardi-Mendoza J, et al. Phenolic extracts from grape stems inhibit Listeria monocytogenes motility and adhesion to food contact surfaces. J Adhes Sci Technol. 2018;32:889–907. doi: 10.1080/01694243.2017.1387093. [DOI] [Google Scholar]
- 22.Çayan F, Deveci E, Tel-Çayan G, Duru ME. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC-DAD. J Food Meas Charact. 2020;14:1690–1698. doi: 10.1007/s11694-020-00417-0. [DOI] [Google Scholar]
- 23.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 24.Fratianni F, Coppola R, Nazzaro F. Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar Annurca. J Med Food. 2011;14:957–963. doi: 10.1089/jmf.2010.0170. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez-Armenta FJ, Bernal-Mercado AT, Tapia-Rodriguez MR, et al. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control. 2018;90:266–273. doi: 10.1016/j.foodcont.2018.02.041. [DOI] [Google Scholar]
- 26.Baranyi J, McClure PJ, Sutherland PJ, Roberts TA. Modeling bacterial growth responses. J Ind Microbiol. 1993;12:190–194. doi: 10.1007/BF01584189. [DOI] [Google Scholar]
- 27.Bae J, Schwab KJ. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol. 2008;74:477–484. doi: 10.1128/AEM.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy ME. Norovirus protein structure and function. FEMS Microbiol Lett. 2005;253:1–8. doi: 10.1016/j.femsle.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Roznowski AP, Tokuda JM, et al. Structural changes of tailless bacteriophage Φx174 during penetration of bacterial cell walls. Proc Natl Acad Sci USA. 2017;114:13708–13713. doi: 10.1073/pnas.1716614114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cajthaml T. Biodegradation of endocrine-disrupting compounds by ligninolytic fungi: mechanisms involved in the degradation. Environ Microbiol. 2015;17:4822–4834. doi: 10.1111/1462-2920.12460. [DOI] [PubMed] [Google Scholar]
- 31.Okino LK, Machado KM, Fabris C, Bononi VL. Ligninolytic activity of tropical rainforest basidiomycetes. World J Microbiol Biotechnol. 2000;16:889–893. doi: 10.1023/A:1008983616033. [DOI] [Google Scholar]
- 32.Ma XK, Li L, Peterson EC, Ruan T, Duan X. The influence of naphthaleneacetic acid (NAA) and coumarin on flavonoid production by fungus Phellinus sp.: modeling of production kinetic profiles. Appl Microbiol Biotechnol. 2015;99:9417–9426. doi: 10.1007/s00253-015-6824-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Yuan Z, Tan X, Ran Z, Jin H 2018 Optimization of medium components for the production of flavonoids and soluble protein with Phellinus igniarius in liquid culture. In: Liu H, Song C, Ram A (eds) Advances in applied biotechnology. Springer, Singapore, pp 421–431 10.1007/978-981-10-4801-2_43
- 34.Leyva JM, Pérez-Carlón JJ, González-Aguilar GA, Esqueda M, Ayala-Zavala JF. Funcionalidad antibacteriana y antioxidante de extractos hidroalcohólicos de Phellinus merrillii. Rev Mex Micol. 2013;37:11–17. doi: 10.33885/sf.2013.3.1108. [DOI] [Google Scholar]
- 35.Li J-M, Liang H-Q, Qiao P, Su K-M, Liu P-G, Guo S-X, Chen J. Chemical composition and antioxidant activity of Tuber indicum from different geographical regions of China. Chem Biodivers. 2019;16(3):e1800609. doi: 10.1002/cbdv.201800609. [DOI] [PubMed] [Google Scholar]
- 36.Fogarasi M, Socaci SA, Dulf FV, Diaconeasa ZM, Fărcaș AC, Tofană M, Semeniuc CS. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules. 2018;23(12):3272. doi: 10.3390/molecules23123272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sułkowska-Ziaja K, Maślanka A, Szewczyk A, Muszyńska B. Physiologically active compounds in four species of Phellinus. Nat Prod Commun. 2017;12:363–366. doi: 10.1177/1934578X1701200313. [DOI] [PubMed] [Google Scholar]
- 38.Heleno SA, Martins A, Queiroz MJ, Ferreira IC. Bioactivity of phenolic acids: Metabolites versus parent compounds: a review. Food Chem. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 39.Sheikh IA, Vyas D, Ganaie MA, Dehariya K, Singh V. HPLC determination of phenolics and free radical scavenging activity of ethanolic extracts of two polypore mushrooms. Int J Pharm Pharm Sci. 2014;6:679–684. [Google Scholar]
- 40.Deshpande S, Matei MF, Jaiswal R, Bassil BS, Kortz U, Kuhnert N. Synthesis, structure, and tandem mass spectrometric characterization of the diastereomers of quinic acid. J Agric Food Chem. 2016;64:7298–7306. doi: 10.1021/acs.jafc.6b02472. [DOI] [PubMed] [Google Scholar]
- 41.Nagy Á, Abrankó L. Profiling of hydroxycinnamoylquinic acids in plant extracts using in-source CID fragmentation. J Mass Spectrom. 2016;51:1130–1145. doi: 10.1002/jms.3847. [DOI] [PubMed] [Google Scholar]
- 42.Kabir F, Katayama S, Tanji N, Nakamura S. Antimicrobial effects of chlorogenic acid and related compounds. J Korean Soc Appl Biol Chem. 2014;57:359–365. doi: 10.1007/s13765-014-4056-6. [DOI] [Google Scholar]
- 43.Borges A, Ferreira C, Saavedra M, Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 44.Bernal-Mercado AT, Gutierrez-Pacheco MM, Encinas-Basurto D, et al. Synergistic mode of action of catechin, vanillic and protocatechuic acids to inhibit the adhesion of uropathogenic Escherichia coli on silicone surfaces. J Appl Microbiol. 2020;128:387–400. doi: 10.1111/jam.14472. [DOI] [PubMed] [Google Scholar]
- 45.Wu T, Zang X, He M, Pan S, Xu X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J Agric Food Chem. 2013;61:8185–8190. doi: 10.1021/jf402222v. [DOI] [PubMed] [Google Scholar]
- 46.Wu D, Kong Y, Han C, et al. D-Alanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int J Antimicrob Agents. 2008;32:421–426. doi: 10.1016/j.ijantimicag.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Bernard FX, Sable S, Cameron B, et al. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob Agents Chemother. 1997;41:992–998. doi: 10.1128/AAC.41.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco P, Hernando-Amado S, Reales-Calderon JA, et al. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms. 2016;4:1–19. doi: 10.3390/microorganisms4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiamegos YC, Kastritis PL, Exarchou V, et al. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS ONE. 2011;6:e18127. doi: 10.1371/journal.pone.0018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miguel MG, Faleiro L, Antunes MD, Aazza S, Duarte J, Silvério AR. Antimicrobial, antiviral and antioxidant activities of “água-mel” from Portugal. Food Chem Toxicol. 2013;56:136–144. doi: 10.1016/j.fct.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Gescher K, Hensel A, Hafezi W, Derksen A, Kühn J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res. 2011;89:9–18. doi: 10.1016/j.antiviral.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Doğan HH, Karagöz S, Duman R. In vitro evaluation of the antiviral activity of some mushrooms from Turkey. Int J Med Mushrooms. 2018;20:201–212. doi: 10.1615/IntJMedMushrooms.2018025468. [DOI] [PubMed] [Google Scholar]
- 53.Krupodorova T, Rybalko S, Barshteyn V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol Sin. 2014;29:284–290. doi: 10.1007/s12250-014-3486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Kim JI, Heo J, et al. The anti-influenza virus effect of Phellinus igniarius extract. J Microbiol. 2013;51:676–681. doi: 10.1007/s12275-013-3384-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be found on the institution repository (https://ciad.repositorioinstitucional.mx/jspui/handle/1006/346) or requested to the corresponding author. The used fungal material can be located at the fungal collection “Dr. Martín Esqueda Valle” at the herbarium of the Sonoran State University.
Not applicable.


