Abstract
Vernonia anthelmintica Willd (VA) is a popular medicinal plant used in local and traditional medicine to manage various disorders. In order to explore the phytochemical profile, antioxidant and enzyme modulatory activities of extracts prepared from the seeds of VA, different extraction methodologies, including modern (accelerated-ASE, ultrasound-UAE, and tissue smashing-TSE extractions) and traditional (maceration and Soxhlet) extractions, were employed and their effects on the activities of the extracts were investigated. The chemical compounds of the extracts were qualitatively analyzed by ultra-high-pressure liquid chromatography-tandem mass spectrometry (UPLC-Orbitrap-MS) technique. Among them, 11 compounds were undoubtedly identified by comparison with reference substance, while 13 compounds were tentatively identified by comparison with literature data, including 8 phenolic acids, 14 flavonoids and 2 esters were identified in the extracts. Additionally, the quantitative analysis found that ASE showed the highest extraction efficiency. The antioxidant activity was determined in vitro via six standard assays. Two key enzymes related to the diseases of vitiligo (tyrosinase) and type II diabetes (α-glucosidase) were adopted to assess the activity of VA extracts against them. All extracts showed potent antioxidant ability with a predominance for that obtained by ASE, which corroborated with the high phenolic (22.62 ± 0.23 mg gallic acid equivalent (GAE)/g extract) and flavonoid contents (68.85 ± 0.25 mg rutin equivalent (RE)/g extract). The extracts obtained by ASE, UAE and SE could increase the tyrosinase activity and all the extracts displayed remarkable inhibitory activity against α-glucosidase. This study demonstrated that the VA extracts obtained by novel extraction techniques such as ASE, could be considered as a positive candidate to be utilized by the food and medical industries, not only for obtaining bioactive compounds to be used as natural antioxidants, but possibly also for its health benefits for therapeutic bio-product development.
Keywords: Vernonia anthelmintica Willd., Chemical profile, Antioxidant and enzyme modulatory activities, Extraction methods
Vernonia anthelmintica Willd.; Chemical profile; Antioxidant and enzyme modulatory activities; Extraction methods.
1. Introduction
Natural products (NPs) are used for managing different diseases by humankind because of their powerful therapeutic effect and fewer toxicity [1]. However, due to their complex characteristics, the use of NPs as a medicine requires a complicated process [2]. Preliminary identification of the compounds and examination of antioxidant activity as well as enzymatic modulator activity of NPs are often a critical step to perform any further studies [3]. And, the extraction of active ingredients from NPs is a prerequisite for all analyses, a suitable extraction method may not only enhance the activity, but also greatly help to improve its clinical therapeutic effect.
However, it is well known that the activity and yield of active compounds extracted from NPs vary due to different extraction methods [4, 5]. So far, the traditional extraction methods including maceration, percolation and soxhlet extraction have been widely used by researchers to obtain biologically active substances from various NPS. However, these conventional extraction methods suffer from some shortcomings such as low efficiency, high solvent consumption and environmental pollution [6]. In recent years, with the popularity of environmental chemistry, more environmentally friendly extraction methods have received more and more attention. As a result, some emerging green extraction methods such as accelerated solvent extraction (ASE), ultrasound-assisted extraction (UAE) and tissue-smashing extraction (TSE) were developed and widely used in current research due to their advantages of high efficacy, low consumption of solvents and materials and time-saving [7]. Considering that different extraction methods will lead to different chemical compositions and functions of the extracts, it is necessary to investigate the effects of different extraction methods in order to better develop and utilize a natural product.
According to Germplasm Resources Information [8], Vernonia anthelmintica Willd (VA), an annual herb, is a member of the Compositae family and mostly inhabits the continents of Africa, Asia temperate (China), and Asia tropical (Sri Lanka, Laos, Myanmar, Nepal, India, and Pakistan). It is frequently used to adorn a room or the surroundings and is also known as Purple Fleabane because of its lovely purple blossoms. In addition, VA seeds have a long history of traditional use in China, namely in the treatment of vitiligo for thousands of years [9, 10]. These problems include those of the skin, central nervous system, kidney, gynecological, gastrointestinal, metabolic, and general health. Pharmacological research has demonstrated that VA seed extracts have a variety of effects, including anti-vitiligo [11], anti-diabetic [12], antioxidant [13], anti-inflammatory [14], and neuroprotective effects [15], etc. Many phenolic compounds, including phenolic acids (chlorogenic acid, isochlorogenic acid A) and flavonoids (isorhamnetin, luteolin), have been found to exist in VA, according to a number of studies [16, 17, 18]. These compounds have been identified as the main contributors toward the antioxidant, anti-vitiligo, and anti-diabetic activities. To the best of our knowledge, previous studies have not yet compared various extraction methods with the composition of chemical ingredients, antioxidant and enzyme modulatory activity of the extracts as evaluation indicators, instead focusing primarily on the extraction efficiency of the active ingredients by a single extraction method.
Therefore, in the present study, in order to well understand the extracts of VA seeds, and to in-depth explore VA as functional food ingredients or medical products for prevention of vitiligo and type II diabetes, the chemical characteristics, antioxidant properties, and enzymatic modulator activity of five kinds of different extracts were investigated and compared.
2. Materials and methods
2.1. Plant material
The seeds of VA were purchased from the local farm market of Xinjiang province, China, in 3 September 2020. A voucher specimen was deposited at the Second Hospital of Hebei Medical University. The plants were powdered with a laboratory mill and then extracted with different methods.
2.2. Chemicals and regents
All references for qualitative and quantitative analysis were purchased from Chengdu DeSiTe Biotechnology Co., Ltd (Chengdu, China). 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2′-Azino-bis (3-ethylbenzothiazolie-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), neocuproine, and rutin were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Tyrosinase, α-Glucosidase, p-Nitrophenyl-α-D-glucopyranoside (pNPG), L-Dopa were obtained from Shanghai Baomanbio Technology Co., Ltd (Shanghai, China). Gallic acid, Folin-Ciocalteu's phenol reagent and 2,4,6-Tripyridyl-s-Triazine (TPTZ) were purchased from Beijing Solarbio Technology Co., Ltd (Beijing, China). Ultrapure water was obtained from a Milli-Q system (Millipore, Billerica, MA, USA). Acetonitrile, methanol, ethanol and formic acid (both HPLC grade) were purchased from Merck (Darmstadt, Germany). All other chemicals and reagents were of analytical grade.
2.3. Preparation of the extracts
In this research, all samples were prepared using 50% aqueous methanol with a ratio of 30 mL per gram. Maceration extraction (ME), soxhlet extraction (SE), ultrasound assisted extraction (UAE), tissue smashing extraction (TSE), and accelerated solvent extraction (ASE) were selected to extract the active ingredients. After extraction, centrifugation (10000 rpm) for 5 min at 4 °C was performed, and the supernatant was stored at 4 °C for further analysis. Meanwhile, in order to study the extraction yield, partial supernatant was subjected to rotary evaporation.
2.3.1. Ultrasound assisted extraction (UAE)
UAE extraction was performed in a SB-5200DT ultrasonic water bath (Ningbo, Zhejiang, China). 1.0 g powder was ultrasonically extracted by 30 mL of 50% methanol for 30 min (40 kHz, 300 W).
2.3.2. Maceration extraction (ME)
1.0 g powder of VA was soaked and extracted with 30 mL of 50% methanol as solvent for 24 h at room temperature without light exposure.
2.3.3. Tissue smashing extraction (TSE)
0.2 g of plant materials were extracted by a dispersing machine using 50% aqueous ethanol (1:30 ratio of plant material to aqueous ethanol, w/v) as extraction solvent with 25600 rpm for 1 min to prepare TSE samples.
2.3.4. Accelerated solvent extraction (ASE)
ASE process was performed with BUCHI SpeedExtractor E-916 instrument (Flawil, Switzerland). 2.5 g of herbal sample was mixed with diatomic earth thoroughly in 40 mL extraction cell and extracted with 50% methanol. The extractions were performed at 100 °C with a pressure of 100 bar, then heated for 1 min maintaining 5 min for two cycles. The extraction solvent (2 min) and N2 (5 min) were used to flush the extraction cell and extracts obtained were collected into the collection flask finally. The extract was collected and diluted to 75 mL by the solvent.
2.3.5. Soxhlet extraction (SE)
The powdered VA (3.0 g) were placed to the filter paper and extracted with 50% methanol solution (90 mL, 1:30 ratio) in a Soxhlet apparatus for 4 h at 100 °C.
2.4. Total flavonoid and phenolics contents
For the determination of the total contents of flavonoid and phenolic, colorimetric assays was used. Specially noting, the samples were highly active, so the samples of each extraction method were diluted 10 times with 50% methanol before the determination. For total phenolic content (TPC), Folin-Ciocalteu reagent (FCR) was employed following Zhang et al. [19], and the process was slightly modified. Briefly, 0.2 mL of each extract and 0.5 mL FCR (1 mM) were mixed and allowed to stand for 3 min. Then, 2 mL of Na2CO3 solution (15%) was added and finally water was added to make the solution to be 10 mL. After 1 h, the absorbance was measured at 765 nm. The result was expressed as equivalents of gallic acid (GAE). The total flavonoid content (TFC) was determined by AlCl3 method described by Dai et al. [20] with some modification. 0.15 mL of extract was added to 0.5 mL of NaNO2 (5%). After 6 min, 0.5 mL of Al (NO3)3 (10%) was added to the solution and kept for 6 min. Then, 10 mL of NaOH (4%) was added and the absorbance was measured at 510 nm after 15 min. The result was expressed as equivalents of rutin (mg/g).
2.5. Antioxidant and enzyme modulatory activities
The following methods were employed to determine the antioxidant properties of VA extracts: total antioxidant capacity by phoshomolybdenum method, total reducing power by potassium ferricyanide method, reducing power by cupric ion (CUPRAC) and ferric ion (FRAP) and free radicals scavenging ability by towards DPPH, ABTS+. The antioxidant ability was expressed as equivalents of Trolox (mg/g).
For the determination of in vitro enzyme activities, the effects of different extracts on tyrosinase and α-glucosidase were assayed based on previously reported methods with slightly modifications. The enzyme activity was expressed as EC50 values or IC50 values.
2.5.1. Cupric ion reducing (CUPRAC) method
The CUPRAC ability was measured in line with the method of Puangbanlang et al. [21] with some modifications. 0.1 mL of the extraction solution (1 mL) was added to a mixture that containing 1 mL of CuCl2 (10 mM), 1 mL of neocuproine (7.5 mM) and 1 mL NH4Ac buffer (1 M, pH 7.0). The absorbance of the mixture was determined after 1 mL of water was added and reaction 30 min at 25 °C. Similarly, a blank sample was prepared by adding extract solvent instead of extract.
2.5.2. Ferric ion reducing (FRAP) method
The determination of FRAP was conducted by the procedure as described by Liyanaarachchi et al. [22]. Firstly, the working FRAP solution was prepared by mixing acetate buffer (0.3 M, pH 3.6), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) (10 mM) and ferric chloride (20 mM) in 40 mM HCl at 10:1:1 (v/v/v) and reaction 10 min at 37 °C. Then, 0.02 mL of extract was mixed in 0.18 mL FRAP and reaction for 30 min at room temperature. The absorbance of the solution was measured at 593 nm.
2.5.3. 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity
The DPPH free radical scavenging activity was obtained following the steps described by Sun et al [23]. Briefly, 0.02 mL of extract was mixed with 0.18 mL of DPPH-ethanol solutions (0.2 mM) and kept away from light for 30 min. The absorbance was measured at 517 nm. The scavenging percentage was obtained from the following Eq. (1):
| (1) |
Where A0 is the absorbance value of the blank without extract, A1 is the absorbance value of extract, A2 is the absorbance value of the control without DPPH.
2.5.4. ABTS+ radical scavenging capacity
The ABTS+ radical scavenging ability of VA was determined by the steps of Sarikurkcu et al. [24] with minor modifications. Firstly, ABTS+ radical cation was formed by mixed the equal volumes of 2 mM ABTS solution and 2.45 mM potassium persulphate at 25 °C for 12 h. Then methanol was used to dilute the ABTS+ solution until its absorbance to 0.700 ± 0.02 at 734 nm. 0.02 mL extracts were mixed with 0.18 mL ABTS+ solution, and then reacted for 6 min at 25 °C. 734 nm was used as the measuring wavelength. The scavenging ability of ABTS+ was calculated as that of DPPH radical scavenging capacity.
2.5.5. Reducing power assay (PF)
The reducing power of extract was assessed as described previously with slight modifications [25]. First, a mixture including 1 mL of the extract, 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL potassium ferricyanide (PF) (1%, w/v) was prepared and kept for 20 min in water bath at 50 °C. Then centrifuge was at 3000 rpm for 10 min after adding trichloroacetic acid to stop the reaction. Subsequently, 2.5 mL of the supernatant was mixed with the same volume of deionized water and 1 mL of FeCl3 (0.1%, w/v). The absorbance of the reaction mixture was measured at 700nm. Extraction solvent was used to eliminate the impact as blank.
2.5.6. Antioxidant capacity assay
Phosphomolybdenum assay was performed to determine total antioxidant ability of the extracts of VA [26]. Firstly, a mixed solution with 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate was prepared. Then 1 mL extract and 4 mL of that mixed solution was reacted. The solution was incubated 90 min at 95 °C. Then, the absorbance of the solution was measured at 695 nm after cooling to room temperature. Extraction solvent replacement of extract was used as the blank.
2.5.7. Tyrosinase activity assay
Tyrosinase activation ability of different extracts was assessed as Suganya et al. [27] with slight modifications. Determination was carried out by using 3,4-3dihydroxyphenylalanine (L-DOPA) as the substrate. The mixed solution was reacted for 1 h on room temperature, which was prepared by mixing 0.04 mL of extracts, 0.04 mL of PBS (50 mM, pH 6.8), 0.04 mL of L-DOPA (1 mg/mL) solution and 0.04 mL of tyrosinase (800 U/mL). 475 nm was used to measure. Activation of tyrosinase was obtained from the following Eq. (2):
| (2) |
Where, A1 represents the result of the reaction solution, A2 is the PBS without enzyme solution, A3 is the reagent blank without extracts, A4 is the control solution that using solvents and PBS replaced of extracts and enzyme solution. EC50 value was calculated by the GraphPad Prism 8 software.
2.5.8. α-Glucosidase inhibition assay
The inhibition of α-glucosidase was assayed as described by Grochowski et al. [26] with some modifications. After reacted at 37 °C for 15 min, the reaction system which consisted of 20 μL of extracts, 20 μL of PBS (50 mM, pH 6.8), 20 μL of pNPG (5 mM) and 20 μL of α-glucosidase (2.5 U/mL) was terminated with 80 μL sodium carbonate (0.2 M). At last, 475 nm was employed to determine the absorptions. Inhibition ability of α-glucosidase was obtained from the following Eq. (3):
| (3) |
where, A1 represents the result of the reaction solution, A2 is the PBS without enzyme solution, A3 is the reagent blank without extracts, A4 is the control solution that using solvents and PBS replaced of extracts and enzyme solution. IC50 value was calculated by the GraphPad Prism 8 software.
2.6. Qualitative analysis by HPLC-orbitrap-MS
Qualitative analysis of extracts was performed on a Thermo ultimate 3000 liquid chromatography connected to Thermo orbitrap elite mass spectrometer. XSelect HSS T3 (4.6 × 150 mm) 3.5 μm was employed as the column. Water with 0.1% formic acid (A) and acetonitrile (B) were employed as mobile phase. The flow rate was 0.3 mL/min and the injection volume was 10 μL. The gradient was: 0–10 min, 12–20% B; 10–22 min, 20–25% B; 22–40 min, 25–30% B; 40–70 min, 30–35% B; 70–85 min, 35–45% B; 85–90 min, 45–60% B; 90–95 min, 60–95% B; 95–100 min, 95% B; 100–101 min, 95-12 % B; 101–125 min, 12% B. MS spectra were scanned in negative ion mode in the range of 50–1000 Da using electro spray ionization (ESI) ion source. The compounds were identified by comparing their mass spectrometry information with those obtained from the standards or in the literature.
2.7. Quantitative analysis by HPLC-MS/MS
Thirteen main compounds were quantitatively determined by using a LC-20AD (Shimadzu) liquid chromatography, equipped with a vacuum degasser, a binary pump, and an autosampler, connected to an analyst software and a 6500 Qtrap system from AB SCIEX, a hybrid triple quadrupole linear ion trap mass spectrometer equipped with Turbo V sources. A XSelect HSS T3 column (4.6 × 150 mm, 3.5 μm) was employed and the column temperature was set as 30 °C. The mobile phase consisted of water containing 0.05% trifluoroacetic acid (A) and acetonitrile (B). The gradient program was as follow: 0–4 min, 15–28% B; 4–8 min, 28–35% B; 8–13 min, 35–37% B; 13–15 min, 37–95 B; 15–17 min, 95% B at a flow rate of 1.0 mL/min. The auto-sampler was conditioned at 4 °C and the injection volume was 5 μL for analysis.
The ESI was used and the ion spray voltage was set at -4500 V, the turbo spray temperature was maintained at 500 °C. Nebulizer gas (gas 1) and heater gas (gas 2) was set at 50 and 50 psi, respectively. The instrument was calibrated every 5 days using the manufacturer’s calibration solutions. The precursor-to-production pair, declustering potential (DP), and collision energy (CE) for them were described in Table 1.
Table 1.
MS conditions for the determination of 13 main compounds in VA.
| Compounds | MW | tR (min) | MS1 (m/z) | MS2 (m/z) | DP(V) | CE(V) |
|---|---|---|---|---|---|---|
| Chlorogenic acid | 354 | 3.6 | 353.2 | 191.0 | 133 | 31 |
| Caffeic acid | 180 | 4.7 | 179.0 | 135.0 | 25 | 20 |
| Rutin | 610 | 5.2 | 609.0 | 300.2 | 145 | 52 |
| Isochlorogenic acid B | 516 | 6.1 | 515.3 | 353.3 | 50 | 8 |
| Isochlorogenic acid A | 516 | 6.6 | 515.3 | 353.3 | 50 | 8 |
| Isochlorogenic acid C | 516 | 6.9 | 515.3 | 353.3 | 50 | 8 |
| Scutellarin | 286 | 8.5 | 285.0 | 117.0 | 125 | 46 |
| Saccharol | 288 | 10.1 | 287.0 | 151.0 | 50 | 20 |
| Luteolin | 286 | 10.1 | 285.1 | 133.0 | 101 | 20 |
| Quercetin | 302 | 10.4 | 301.0 | 151.0 | 85 | 27 |
| Butochalcone | 272 | 12.3 | 271.0 | 135.0 | 47 | 8 |
| Apigenin | 270 | 13.0 | 269.0 | 117.0 | 99 | 44 |
| Isorhamnetin | 316 | 14.5 | 315.0 | 300.0 | 133 | 31 |
2.8. Statistical analysis
Mean value and standard deviation ( ±SD) were employed to describe the results. The experiment was carried out all three times. The normalities of variables were tested at first by Shapiro-Wilk test. According to whether variables were normality or nonnormally, One-way ANOVA or Kruskal-Wallis was employed to confirm any difference between the various extraction technologies. p < 0.05 is considered statistically significant. In order to observe the possible correlation between different extraction methods and antioxidant and enzymatic modulator activities, orthogonal projections to latent structure-discriminant analysis (OPLS-DA) was used to process the experiment data. Hierarchical clustering analyses were done to classify different extraction methods.
3. Results and discussion
3.1. Extraction yields and the contents of total flavonoid (TFC) and phenolic (TPC) of VA extracts
Five kinds of extraction methods have been used to extract bioactive compounds from plants as the choice of extraction method is very significant for the extraction of bioactive phytochemicals. Different yields of VA extracts were obtained by using different extraction methods (Table 2). The highest yield obtained was the ASE extract (1.71%), followed by the SE extract (1.53%) and UAE extract (1.29%).
Table 2.
Yields, TPC, TFC and in vitro antioxidant enzymatic modulator activities of the VA extracts using different extraction methods.
| Extraction methods | Yields∗∗ (%) | TPC∗ (mg GAE/g) | TFC∗∗ (mg RE/g) | DPPH∗∗ (mg TE/g extract) | ABTS ∗∗ (mg TE/g extract) | CUPRAC ∗ (mg TE/g extract) | FRAP∗ (mg TE/g extract) | PF ∗∗ (mg TE/g extract) | Antioxidant capacity assay∗∗ (mg TE/g extract) | Tyrosinase activation ∗∗ (EC50: mg/mL) | α- glucosidase inhibition ∗∗ (IC50: mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UAE | 1.24 ± 0.06c | 19.97 ± 0.45c | 56.32 ± 1.15c | 20.88 ± 0.65a | 22.43 ± 0.60c | 86.51 ± 1.48c | 47.22 ± 1.63a | 65.11 ± 2.17a | 107.23 ± 5.23a | 6.62 ± 0.09a | 1.07 ± 0.05a |
| ME | 0.92 ± 0.04e | 10.04 ± 0.24e | 30.92 ± 0.38e | 11.63 ± 0.87d | 12.13 ± 1.16e | 35.57 ± 0.41e | 23.29 ± 0.56c | 30.85 ± 1.29e | 40.06 ± 2.18e | 30.35 ± 0.81c | 7.51 ± 0.23e |
| TSE | 1.14 ± 0.05d | 13.19 ± 0.17d | 41.19 ± 0.63d | 13.39 ± 0.17c | 17.32 ± 0.28d | 51.45 ± 2.51d | 27.39 ± 0.63bc | 38.47 ± 0.54d | 59.76 ± 1.16d | 15.96 ± 1.24b | 1.39 ± 0.02b |
| ASE | 1.72 ± 0.04a | 22.62 ± 0.23a | 68.85 ± 0.25a | 21.25 ± 0.35a | 34.11 ± 0.98a | 109.37 ± 0.24a | 49.51 ± 1.48a | 48.20 ± 0.44b | 84.85 ± 3.02b | 5.72 ± 0.08a | 2.37 ± 0.08c |
| SE | 1.53 ± 0.04b | 21.22 ± 0.20b | 60.83 ± 0.75b | 17.63 ± 0.09b | 25.63 ± 0.79b | 101.57 ± 1.66b | 38.29 ± 1.31b | 43.60 ± 1.80c | 72.65 ± 1.72c | 6.34 ± 0.29a | 4.06 ± 0.06d |
The result was described as means ± S.D. ∗Statistical evaluation was performed by Kruskal-Wallis test. ∗∗Statistical evaluation was performed by ANOVA test.
a, b, c, d, e represented the groups with significant differences on yields, TPC, TFC, antioxidant and enzyme modulatory activities of tested extracts (p < 0.05).
For statistical data to each graph, p value or others, please see the supplementary material (Part 1).
Furthermore, as shown in Table 2, the extract of VA obtained using the ASE were found to have the highest TFC (68.85 ± 0.25 GAE/g extract) and TPC (22.62 ± 0.23 RE/g extract), followed by SE. The time of ASE was much shorter, which should be considered if time-saving and high efficiency were required simultaneously. The compounds including phenol acids and flavonoids are considered with high antioxidant activity, playing a significant effect in many diseases by maintaining the oxidation balance in the body. Previous reports demonstrated that VA extracts contain a wide range of phenolic compounds with the majority of phenolic acid and flavonoids. In order to have a better understanding of the nature of the extracts yielded, the phytochemical assessment of TFC and TPC of the extracts obtained by ME, SE, UAE, ASE and TSE were performed. The result of TFC was described as the equivalent of rutin, and the result of TPC was described as the equivalent of gallic acid.
3.2. Antioxidant and enzymatic modulator activities of VA extracts in vitro
In the determination of antioxidant activity, all assays clearly demonstrated that ME showed the lowest activity, followed by TSE. It can be seen from Table 2 that different extraction methods had a greater impact on the antioxidant activity of plant extracts, which was consistent with the result of dos Santos et.al that the activities of Brazilian “pacová” were varies from different extraction methods [28]. In order to compare the extraction methods comprehensive, 6 kinds of methods based on different principles were employed to evaluate the antioxidant ability, such as DPPH, ABTS based on free radical scavenging ability, CUPRAC, FRAP, total reducing power based on reducing ability, and total antioxidant capacity based on oxidative capacity determination and the experimental results were expressed as Trolox equivalent. In the result of scavenging activity of DPPH and ABTS free radical, extract of VA obtained by ASE possess the powerful activity, that might be attributed to its higher TPC results. Because most phenolic compounds have hydroxyl groups that are hybridized with aromatic rings in their structures, they can become a source of hydrogen donors for rapid reaction with oxidative groups, so phenolic compounds have strong antioxidant activity. And previous study also showed that there was a close relationship between TPC and free radical scavenging [29]. For the determination of reducing power, in addition to total reducing power, CUPRAC and FRAP were also commonly used. The results showed that all extracts showed strong activity, of which ASE had the highest reducing power (35.57–109.37 and 23.29–49.51 mg TE/g extract for CUPRAC and FRAP assays, respectively). It can conclude that different antioxidant activity assays are different depending on the extraction methods. Due to the wide application of natural antioxidants in many industries, people are increasingly interested in finding natural antioxidants [30]. For example, natural antioxidants not only can be used for the prevention of food oxidation and the treatment of many diseases but also have the advantages of safety and less side effects, so it is more important to find them. While the determination of antioxidant activity is often used for preliminary screening of natural products, the strong antioxidant capacity of plant extracts may be due to different reasons, such as scavenging or absorbing oxygen free radicals [31]. Frankly speaking, it was difficult to compare as different antioxidant assays were used, and the principle of each measurement was different. For all the antioxidant assays, ASE extract possesses strong antioxidant activity and this extraction technique improved the extraction efficiency, saved extraction time, and reduced the use of solvents, more in line with the current concept of green chemistry. Compared with other extraction methods, several advantages of ASE increase the efficiency of extracting active compounds from plant materials, which enables the sample to be extracted under high temperature and high pressure. High temperature of the solvent improves its diffusion capacity, ensures the penetration of the solvent in the extracts, and improves the efficiency of the extraction of active ingredient [32]. All in all, these results showed that VA extracts had strong antioxidant capacity and it can be thought as a precious source of antioxidants.
In this study, a comparison of the activities of VA extracts by different methods on tyrosinase and α-glucosidase was completed and the results were expressed as EC50 and IC50 values. As shown in Table 1, a variation in tyrosinase activity was highlighted for the different extraction techniques. The extract obtained by ASE showed the highest activation against tyrosinase (5.72 ± 0.08 mg/mL), followed by SE extract (6.34 ± 0.29 mg/mL). The ME extract possessed the lowest activity (30.35 ± 0.81 mg/mL). The result was consistent with Zhou [33] and Tuerxuntayi [11] et.al who determinate the tyrosinase activity of the extract of VA seeds. Gawali's results also confirmed the inhibitory effect of VA seed extract on α-glucosidase [34]. Overall, the activity of the extracts was basically correlated with TFC and TPC, indicating that the phytochemicals generated by each extraction technique are responsible for this activity. Interestingly, the TSE extract had weak activation effect on tyrosinase, but strong inhibitory effect on α-glucosidase, which suggested that compounds with α-glucosidase inhibitory activity might be extracted with higher content in TSE extraction. The experimental results pointed out the most suitable extraction method for possible clinical application, which undoubtedly can enhance the effect of the drug.
3.3. Identification of ingredients in VA extract
The determination of TFC and TPC provided an initial result about the constituents and the detailed information was obtained through UPLC-Orbitrap-MS analysis. Figure 1 showed the representative chromatogram of VA extract and the identified compounds were listed in Table 3. 24 compounds including 8 phenolic acids, 14 flavonoids and 2 esters were identified in the extracts. Among them, 11 compounds were undoubtedly identified by comparison with reference substance, while 13 compounds were tentatively identified by comparison with literature data.
Figure 1.
HPLC chromatogram of Vernonia anthelmintica Willd. extract at 360 nm.
Table 3.
Phytochemicals identified in VA extracts by UPLC-Orbitrap-MS.
| Peaks | RT (min) | Formula | [M-H]− (m/z) | MS/MS (m/z) | Identification | References |
|---|---|---|---|---|---|---|
| 1 | 15.26 | C16H18O9 | 353 | 191, 179, 135 | Chlorogenic acid | -a |
| 2 | 17.87 | C16H18O9 | 353 | 191,179, 135 | 4-O-caffeoylquinic acid/3-O-caffeoylquinic acid/5-O-caffeoylquinic acid | [35] |
| 3 | 18.23 | C21H18O12 | 461 | 285 | luteolin-7-O-β-glucuronide | [36] |
| 4 | 20.75 | C21H22O12 | 465 | 303, 297, 329 | 5,7,3',4'-tetrahydroxy-flavanone-7-O-glucoside/5,7,3',4'-tetrahydroxy-flavanone-3-O-glucoside | [35] |
| 5 | 23.07 | C21H22O12 | 465 | 303, 297, 329 | 5,7,3',4'-tetrahydroxy-flavanone-7-O-glucoside, 5,7,3',4'-tetrahydroxy-flavanone-3-O-glucoside | [35] |
| 6 | 25.03 | C15H12O6 | 287 | 151, 135 | isomer of saccharol | [39] |
| 7 | 29.30 | C25H24O12 | 515 | 353, 335, 191 | 1,3-O-dicaffeoylquinic acid/1,5-O-dicaffeoylquinic acid | [35] |
| 8 | 30.18 | C16H18O9 | 353 | 191, 179, 135 | 4-O-caffeoylquinic acid/3-O-caffeoylquinic acid/5-O-caffeoylquinic acid | [35] |
| 9 | 32.74 | C15H10O7 | 301 | 257, 193 | Quercetin | - |
| 10 | 33.91 | C25H24O12 | 515 | 353, 335, 191 | Isochlorogenic acid B | - |
| 11 | 34.14 | C25H24O12 | 515 | 353, 179, 191 | Isochlorogenic acid A | - |
| 12 | 34.20 | C25H24O12 | 515 | 353, 335, 191 | Isochlorogenic acid C | - |
| 13 | 35.07 | C15H12O5 | 271 | 135 | Butin/2',3',4,4'-tetrahydroxychalcone | [36] |
| 14 | 36.88 | C35H60O6 | 577 | 414 | β-daucosterol | [39] |
| 15 | 36.14 | C22H26O8 | 417 | 181 | Syringaresinol | [38] |
| 16 | 38.79 | C15H12O5 | 271 | 135 | Butin/2',3',4,4'-tetrahydroxychalcone | [35] |
| 17 | 42.79 | C15H10O6 | 285 | 215 | Scutellarin | - |
| 18 | 42.87 | C15H10O6 | 285 | 151, 267, 257, 241 | Luteolin | - |
| 19 | 45.90 | C15H12O4 | 255 | 119, 135 | Liquiritigenin | [37] |
| 20 | 46.95 | C15H10O5 | 269 | 251, 241, 225 | Apigenin | - |
| 21 | 52.54 | C15H12O6 | 287 | 151, 135 | Saccharol | - |
| 22 | 59.03 | C16H12O7 | 315 | 315 | Isorhamnetin | - |
| 23 | 65.86 | C15H12O5 | 271 | 135 | Butochalcone | - |
| 24 | 87.12 | C26H26O12 | 529 | 367, 191, 179, 161 | 3,5-O-dicaffeoylquinic acid methyl ester/3,4-O-dicaffeoylquinic acid methyl ester | [35] |
Note: a Qualitation by comparing with the reference substance.
There were many isomers in the extracts, which causes certain difficulties in characterization. For phenolic acids, chlorogenic acid (peak 1 [M-H]− m/z 353), isochlorogenic acid B (peak 10 [M-H]− m/z 515), isochlorogenic acid A (peak 11 [M-H]− m/z 515), isochlorogenic acid C (peak 12, [M-H]− m/z 515) were undoubtedly identified by matching the information with those of reference standards. The remaining four compounds were tentatively identified by matching the data with those previously described by Wang et. al [35]. Regrettably, it was difficult to accurately characterize them without reference materials as the existence of isomers. Peak 2 and peak 8 showed [M-H]− at m/z 353 and fragment ions are generated at m/z 191, 179, 135. The deprotonated quinic acid group produced a fragment ion at m/z 191, the caffeoyl group produced an ion at m/z 179, and [caffeoyl-CO2]− resulted in an ion at m/z 135. Therefore, peak 2 and peak 8 were tentatively identified as 5-O-caffeoylquinic acid, 4-O-caffeoylquinic acid or 3-O-caffeoylquinic acid, and further confirmation required the reference substance to distinguish. Peak 24 had the [M-H]− at m/z 529, and fragment ions generated at m/z 367, 191, 179, and 161 are considered to be caffeoylquinic acid methyl ester. The peak 7 related to caffeoylquinic acid showed [M-H]− at m/z515, and fragment ions of m/z 353 were generated, so peak 7 can be preliminarily identified as caffeoylquinic acid.
Seven flavonoids were definitely identified in VA extract by matching their information with those of references, including quercetin (peak 9 [M-H]−m/z 301), scutellarin (peak 17 [M-H]− m/z 285), luteolin (peak 18 [M-H]- m/z 285), apigenin (peak 20 [M-H]- m/z 269), saccharol (peak 21 [M-H]- m/z 151), isorhamnetin (peak 22 [M-H]- m/z 315), butochalcone (peak 23 [M-H]- m/z 271). Peak 3 ([M-H]- m/z 461) was identified as luteolin-7-O-β-glucuronide, because its information was consistent with the data previously describe by Yin et al. [36]. Peak 19 showed [M-H]- at m/z 255 and MS/MS ions at m/z 119 and 135 were generated, and it was considered as liquiritigenin as compared with previous report [37]. The data of Peak 6 was same as peak 21, and it was preliminarily identified to be the isomer of saccharol.
3.4. Quantitative analysis of 13 main compounds in VA by HPLC-MS/MS
3.4.1. Validation of the HPLC-MS/MS method
The calibration curves were obtained by plotting the relationship between the peak area and concentration of the analytes, and coefficient of determination (r2) of the calibration curves were used to evaluated the linearity. Results showed that all 13 compounds within the measurement range had good linear correlation coefficients ranging from 0.9944 to 0.9998 (Table 4). Mixed standard stock solution was then diluted to determine the limits of detection (LOD) and quantification (LOQ), which were defined as the analyte mass resulting in a signal-to-noise (S/N) ratio of 3 and 10, respectively.
Table 4.
Validation of the HPLC-MS/MS quantitative method.
| Analytes | Equations | Linear ranges (ng/mL) | Correlation Coefficients (r2) | LOQs (ng/mL) | LODs (ng/mL) | Precision (RSD, %) | Repeatability (RSD, %) | Stability (RSD, %) |
|---|---|---|---|---|---|---|---|---|
| Isorhamnetin | y = 840.81x + 561.14 | 2.34–112.5 | 0.9996 | 75.0 | 20.0 | 1.24 | 4.89 | 3.78 |
| Caffeic acid | y = 785.15x + 330242 | 125–6000 | 0.9944 | 44.5 | 10.0 | 1.23 | 1.3 | 1.14 |
| Luteolin | y = 50.535x + 17314 | 187.5–3000 | 0.9983 | 150.0 | 40.0 | 2.49 | 3.67 | 1.01 |
| Quercetin | y = 8706.7x + 20124 | 3.13–100 | 0.9993 | 2.0 | 0.5 | 1.7 | 1.98 | 1.66 |
| Apigenin | y = 46.859x + 1144.8 | 31.25–1000 | 0.9988 | 500.0 | 100.0 | 3.04 | 5.4 | 2.91 |
| Chlorogenic acid | y = 282.75x + 27232 | 156.25–3750 | 0.9998 | 25.0 | 10.0 | 3.32 | 3.83 | 4.11 |
| Isochlorogenic acid C | y = 122x + 19604 | 156.25–3750 | 0.9958 | 14.0 | 5.0 | 3.15 | 5.77 | 3.95 |
| Butochalcone | y = 323.76x + 350697 | 625–5000 | 0.9951 | 28.0 | 10.0 | 1.36 | 0.97 | 0.89 |
| Saccharol | y = 164.24x + 44313 | 1250–7500 | 0.9997 | 1000.0 | 200.0 | 0.66 | 4.33 | 1.14 |
| Scutellarin | y = 43.933x + 19252 | 1250–10000 | 0.9986 | 335.0 | 150.0 | 1.53 | 1.12 | 2.1 |
| Rutin | y = 7530.6x + 10341 | 0.63–60 | 0.9967 | 2.7 | 1,0 | 2.29 | 2.2 | 1.28 |
| Isochlorogenic acid B | y = 38.016x + 5726.1 | 312.5–7500 | 0.9996 | 41.3 | 15.0 | 4.15 | 2.64 | 2.05 |
| Isochlorogenic acid A | y = 171.76x + 15075 | 312.5–5000 | 0.9978 | 4.2 | 1.5 | 4.4 | 4.58 | 2.68 |
Precision was evaluated by analyzing the mixed standard stock solution six times successively and the relative standard deviation (RSD) was taken as a measure of precision. Repeatability was investigated by preparation and analysis six sample solutions. Stability was tested with the same sample solution that stored at 4 °C and analyzed at 0, 12, 24, 36, 48 and 72 h. The results (Table 4) showed that the developed method was good, suitable for the determination 13 compounds in VA extracts.
3.4.2. Contents of main compounds in the extracts of VA
HPLC-QTRAP-MS was employed to determine the main components in the extracts obtained by different extraction methods, five phenolic acids (caffeic acid, chlorogenic acid, isochlorogenic acid A, B, and C), and eight flavonoids (isorhamnetin, luteolin, quercetin, rutin, butochalcone, saccharol, apigenin, scutellarin) were selected for quantification analysis. As shown in Table 5, chlorogenic acid, butochalcone, isochlorogenic acid A, B and C were almost the five richest ingredients in VA extracts. Besides, SE possess the highest extraction efficiency for the compounds, including isorhamnetin, caffeic acid, quercetin, isochlorogenic acid C and butochalcone, while the highest contents of luteolin, chlorogenic acid, saccharol, scutellarin were derived from the extracts of UAE. Overall, ME and TSE showed the lowest extraction efficiency. However, it is worth noting that apigenin and rutin from ME and isochlorogenic acid A from TSE possessed the largest contents. While, rutin and scutellaria had the lowest content under ASE and SE methods, which suggested that these two components may be unstable, and high temperature and high pressure caused their destruction. In terms of extraction principles, for more stable components, high temperature and pressure extraction had higher extraction efficiency, while for those more unstable, the impregnation extraction method may be more suitable.
Table 5.
The contents of 13 compounds in the extracts of VA seeds by different extraction methods.
| Compounds | Extraction methods |
||||
|---|---|---|---|---|---|
| UAE | ME | TSE | ASE | SE | |
| Isorhamnetin∗∗ (ng/g) | 200.22 ± 18.59d | 262.81 ± 25.40b | 220.18 ± 6.13cd | 246.78 ± 12.47bc | 530.40 ± 23.02a |
| Caffeic acid∗∗ (μg/g) | 14.22 ± 0.59b | 13.36 ± 0.22c | 12.22 ± 0.36d | 13.24 ± 0.28c | 18.73 ± 0.21a |
| Luteolin∗∗ (μg/g) | 15.66 ± 0.55a | 9.044.82 ± 0.80b | 6.92 ± 0.48c | 6.51 ± 0.36c | 9.11 ± 0.39b |
| Quercetin∗ (ng/g) | 230.53 ± 5.96d | 196.60 ± 20.43c | 244.69 ± 0.22c | 515.27 ± 8.47b | 617.72 ± 15.48a |
| Apigenin∗∗ (μg/g) | 3.11 ± 0.21bc | 3.47 ± 0.09a | 2.99 ± 0.18c | 1.05 ± 0.12d | 3.33 ± 0.102.74ab |
| Chlorogenic acid∗ (mg/g) | 1.13 ± 0.00a | 0.67 ± 0.07d | 0.60 ± 0.02e | 0.79 ± 0.01b | 0.83 ± 0.04c |
| Isochlorogenic acid C∗∗ (mg/g) | 1.52 ± 0.10b | 1.08 ± 0.09c | 0.89 ± 0.04d | 1.62 ± 0.05b | 2.22 ± 0.04a |
| Butochalcone∗ (mg/g) | 0.46 ± 0.01b | 0.26 ± 0.72c | 0.18 ± 0.00e | 0.22 ± 0.00d | 0.56 ± 0.01a |
| Saccharol∗ (mg/g) | 0.22 ± 0.00a | 0.17 ± 0.01c | 0.13 ± 0.00e | 0.15 ± 0.01d | 0.20 ± 0.01b |
| Scutellarin∗∗ (mg/g) | 0.11 ± 0.00a | 0.06 ± 0.00c | 0.07 ± 0.00b | 0.055 ± 0.00d | 0.06 ± 0.00c |
| Rutin∗ (ng/g) | 1.55 ± 0.01d | 52.63 ± 4.68a | 8.04 ± 0.71b | 2.37 ± 0.33c | 1.37 ± 0.32d |
| Isochlorogenic acid B∗ (mg/g) | 0.28 ± 0.02b | 0.23 ± 0.00c | 0.45 ± 0.03bc | 0.33 ± 0.00a | 0.44 ± 0.00a |
| Isochlorogenic acid A∗∗ (mg/g) | 0.16 ± 0.01a | 136.31 ± 0.33b | 15.94 ± 0.48a | 14.95 ± 1.09ab | 14.93 ± 0.83ab |
a, b, c, d, e represented the groups with significantly different contents of each component by different extraction methods (p < 0.05).
For statistical data to each compound, please see the supplementary material (Part 2).
Statistical evaluation was performed by Kruskal-Wallis test.
Statistical evaluation was performed by ANOVA test.
3.5. Multivariate analysis
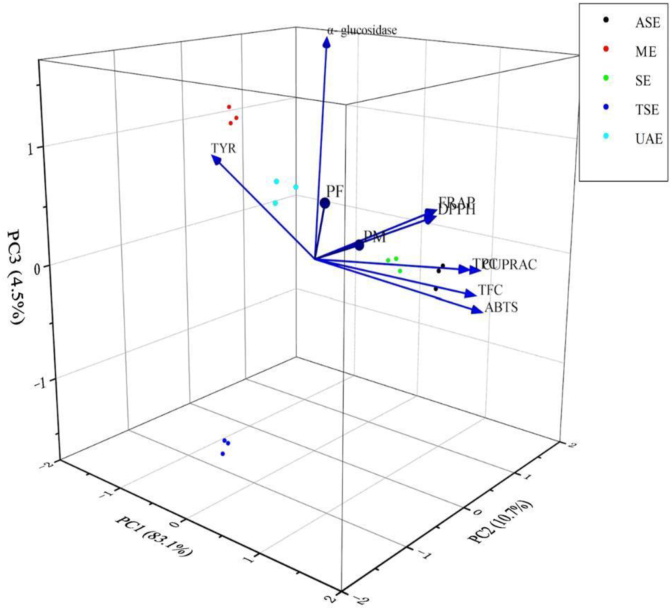
In the current study, in order to compare the five extraction techniques in terms of pharmacological properties, the antioxidant and enzymatic modulator activities dataset of VA samples were subjected to supervised orthogonal projections to latent structure-discriminant analysis (OPLS-DA) and hierarchical cluster analysis. Figure 2 showed the obtained OPLS-DA biplot for antioxidant and enzymatic modulator activities (loadings), determined in extracts of different extraction methods (scores). The total variance explained was 98.3%, of which the first component accounting for 83.1%, the second for 10.7%, and the third for 1%. From the biplot, it was evident that the ASE and SE had a high correlation with ABTS, CUPRAC, TFC and TPC. However, the UAE showed strong activity in the determination of total reducing power and total antioxidant capacity. VIP map was also used to find activity detection methods with high recognition potential. As shown in Figure 3, among the ten measured activities, four of them have high discrimination abilities, which were α-glucosidase inhibitory activity, total reducing activity, total antioxidant activity, and ABTS. The hierarchical cluster analysis was also performed on these dates in order to reveal the influences of different extraction methods on each matrix. The different extraction methods were divided into 2 main groups according to Figure 4. ASE, SE, and UAE were classified into one category, while ME and TSE were considered to have similar extraction effects. All experimental results showed that UAE, ASE and SE methods were considered to be the most valid methods.
Figure 2.
Biplot (scores and loadings) obtained from the OPLS-DA on data set of different extracts and varies activity. In the Figure: ASE, accelerated solvent extraction; ME, maceration extraction; SE, soxhlet extraction; TSE, tissue-smashing extraction UAE, ultrasound-assisted extraction.
Figure 3.
Variable importance in the projection showing the best discriminating variables in the OPLS-DA model (variable with VIP was highest than 1, was considered to be important).
Figure 4.
Dendrogram obtained from cluster analysis based on the in vitro antioxidant and enzymatic modulator activities of different extraction methods.
4. Conclusion
In this work, the activities including antioxidant and enzymatic modulation of the seeds of VA extracts obtained by different extraction technologies were assessed. The results indicated that choice of extraction technique had great effect. Modern extraction methods including ASE and UAE and conventional extraction SE showed strong activity. The effect on tyrosine and α-glucosidase of the VA extracts had also been studied, which provided a new option to the drug development for the treatment of vitiligo and diabetes. In general, this study was of great significance for the further applications as a positive candidate to be utilized by the food and medical industries.
Declarations
Author contribution statement
Guang-li Bian: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ya-lin Hu: Analyzed and interpreted the data; Wrote the paper.
Kai Yan: Contributed reagents, materials, analysis tools or data.
Xin-jie Cheng: Performed the experiment; Analyzed and interpreted the data.
De-qiang Li: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr Deqiang Li was supported by National Natural Science Foundation of China [81603066], Natural Science Foundation of Hebei Province of China [H2020206091], the foundation of the Second Hospital of Hebei Medical University [2HN202101].
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Martins N., Ferreira I.C.F.R., Barros L., Carvalho A.M., Henriques M., Silva S. Plants used in folk medicine: the potential of their hydromethanolic extracts against Candida species. Ind. Crop. Prod. 2015;66:62–67. [Google Scholar]
- 2.Unuofin J.O., Lebelo S.L. UHPLC-QToF-MS characterization of bioactive metabolites from Quercus robur L. grown in South Africa for antioxidant and antidiabetic properties. Arab. J. Chem. 2021;14 [Google Scholar]
- 3.Palmieri S., Pellegrini M., Ricci A., Compagnone D., Lo Sterzo C. Chemical composition and antioxidant activity of Thyme, Hemp and Coriander extracts: a comparison study of maceration, soxhlet, UAE and RSLDE Techniques. Foods. 2020;9:1221. doi: 10.3390/foods9091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirinos R., Rogez H., Campos D., Pedreschi R., Larondelle Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Separ. Purif. Technol. 2007;55:217–225. [Google Scholar]
- 5.Cvetanović A., Švarc-Gajić J., Zeković Z., Gašić U., Tešić Z., Zengin G., Mašković P., Mahomoodally M.F., Durović S. Subcritical water extraction as a cutting edge technology for the extraction of bioactive compounds from chamomile: influence of pressure on chemical composition and bioactivity of extracts. Food Chem. 2018;266:389–396. doi: 10.1016/j.foodchem.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed, Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 2013;117:426–436. [Google Scholar]
- 7.Espino Fernández M., de los Á.M., Gomez F.J.V., Boiteux J., Silva M.F. Green analytical chemistry metrics: towards a sustainable phenolics extraction from medicinal plants. Microchem. J. 2018;141:438–443. [Google Scholar]
- 8.Rakhymbay L., Turak A., Zhenis Z., Aisa H.A. Phenolic compounds from Vernonia anthelmintica seeds. Chem. Nat. Compd. 2019;55:732–733. [Google Scholar]
- 9.Dogra N.K., Kumar S., Kumar D. Vernonia anthelmintica (L.) Willd.: an ethnomedicinal, phytochemical, pharmacological and toxicological review. J. Ethnopharmacol. 2020;256 doi: 10.1016/j.jep.2020.112777. [DOI] [PubMed] [Google Scholar]
- 10.Mamat N., Lu X.Y., Kabas M., Aisa H.A. Potential anti-vitiligo properties of cynarine extracted from. Vernonia anthelmintica (L.) Willd., Int. J. Mol. Med. 2018;42:2665–2675. doi: 10.3892/ijmm.2018.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuerxuntayi A., Liu Y.Q., Tulake A., Kabas M., Eblimit A., Aisa H.A. Kaliziri extract upregulates tyrosinase, TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC Compl. Alternative Med. 2014;14:166. doi: 10.1186/1472-6882-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocvirk S., Kistler M., Khan S., Talukder S.H., Hauner H. Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh - an ethnobotanical survey. J. Ethnobiol. Ethnomed. 2013;9:43. doi: 10.1186/1746-4269-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., Pinlaor S., Yongvanit P., Kawanishi S., Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A., Dash D., Kela S., Dwivedi S., Tiwari P. Analgesic and anti-inflammatory properties of the fruits of Vernonia anthelmintica (L) Willd. Asian Pac. J. Trop Dis. 2014;4:S874–S878. [Google Scholar]
- 15.Kadiyala M., Ponnusankar S., Elango K. Screening of siddha medicinal plants for its in-vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity. Phcog. Mag. 2014;10:294–298. doi: 10.4103/0973-1296.133281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L., Shao Y.L., Hua L., Li Y., Hussain S.H., Arfan M., Gao K. Guaianolides and elemanolides from Vernonia anthelmintica. Phytochem. Lett. 2014;7:14–18. [Google Scholar]
- 17.Cai R., Yuan Y., Cui L., Wang Z., Yue T. Cyclodextrin-assisted extraction of phenolic compounds: current research and future prospects. Trends Food Sci. Technol. 2018;79:19–27. [Google Scholar]
- 18.Gowd V., Karim N., Shishir M.R.I., Xie L., Chen W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019;93:81–93. [Google Scholar]
- 19.Zhang X.X., Shi Q.Q., Ji D., Niu L.X., Zhang Y.L. Determination of the phenolic content, profile, and antioxidant activity of seeds from nine tree peony (Paeonia section Moutan DC.) species native to China. Food Res. Int. 2017;97:141–148. doi: 10.1016/j.foodres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Dai C.Y., Liu P.F., Liao P.R., Qu Y., Wang C.X., Yang Y., Cui X.M. Optimization of flavonoids extraction process in Panax notoginseng stem leaf and a study of antioxidant activity and its effects on mouse melanoma B16 cells. Molecules. 2018;23:2219. doi: 10.3390/molecules23092219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puangbanlang C., Sirivibulkovit K., Nacapricha D., Sameenoi Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta. 2019;198:542–549. doi: 10.1016/j.talanta.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Liyanaarachchi G.D., Samarasekera J.K.R.R., Mahanama K.R.R., Hemalal K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crop. Prod. 2018;111:597–605. [Google Scholar]
- 23.Sun D., Chen X., Zhu C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: a comparison of different extraction methods. Int. J. Biol. Macromol. 2020;158:1239–1247. doi: 10.1016/j.ijbiomac.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Sarikurkcu C., Andrade J.C., Ozer M.S., de Lima Silva J.M.F., Ceylan O., de Sousa E.O., Coutinho H.D.M. LC-MS/MS profiles and interrelationships between the enzyme inhibition activity, total phenolic content and antioxidant potential of Micromeria nervosa extracts. Food Chem. 2020;328 doi: 10.1016/j.foodchem.2020.126930. [DOI] [PubMed] [Google Scholar]
- 25.Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT--Food Sci. Technol. 2007;40:344–352. [Google Scholar]
- 26.Grochowski D.M., Uysal S., Aktumsek A., Granica S., Zengin G., Ceylan R., Locatelli M., Tomczyk M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017;20:365–372. [Google Scholar]
- 27.Suganya P., Jeyaprakash K., Mallavarapu G.R., Murugan R. Comparison of the chemical composition, tyrosinase inhibitory and anti-inflammatory activities of the essential oils of Pogostemon plectranthoides from India. Ind. Crop. Prod. 2015;69:300–307. [Google Scholar]
- 28.dos Santos L.C., Álvarez-Rivera G., Sánchez-Martínez J.D., Johner J.C.F., Barrales F.M., de Oliveira A.L., Cifuentes A., Ibáñez E., Martínez J. Comparison of different extraction methods of Brazilian “pacová” (Renealmia petasites Gagnep.) oilseeds for the determination of lipid and terpene composition, antioxidant capacity, and inhibitory effect on neurodegenerative enzymes. Food Chem. X. 2021;12 doi: 10.1016/j.fochx.2021.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T., Jónsdóttir R., Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009;116:240–248. [Google Scholar]
- 30.Tomaino A., Cimino F., Zimbalatti V., Venuti V., Sulfaro V., De Pasquale A., Saija A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005;89:549–554. [Google Scholar]
- 31.Deng Y., Yang G., Yue J., Qian B., Liu Z., Wang D., Zhong Y., Zhao Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control. 2014;38:184–191. [Google Scholar]
- 32.Kellogg J.J., Wallace E.D., Graf T.N., Oberlies N.H., Cech N.B. Conventional and accelerated-solvent extractions of green tea (camellia sinensis) for metabolomics-based chemometrics. J. Pharm. Biomed. Anal. 2017;145:604–610. doi: 10.1016/j.jpba.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J., Shang J., Ping F., Zhao G. Alcohol extract from Vernonia anthelmintica (L.) willd seed enhances melanin synthesis through activation of the p38 MAPK signaling pathway in B16F10 cells and primary melanocytes. J. Ethnopharmacol. 2012;143:639–647. doi: 10.1016/j.jep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Gawali N.B., Nagmoti D.M., Kothavade P.S., Bulani V.D., Juvekar A.R. Inhibitory activities of semi-purified fractions of Vernonia Anthelmintica Willd seeds against carbohydrate hydrolyzing enzymes (A-Amylase and A-Glucosidase) Indian J. Pharmacol. 2013;45:B9. [Google Scholar]
- 35.Wang J., Tian J., Gu J., Sun Q., Guo X., Zhang J., Wang C., Liu Y. Rapid identification of polyphenolic compounds in the Vernonia anthelmintica by UPLC-LTQ-Orbitrap-MS. Modern Chinese Med. 2019;21:881–887. [Google Scholar]
- 36.Yin R., Han F., Tang Z., Liu R., Zhao X., Chen X., Bi K. UFLC-MS/MS method for simultaneous determination of luteolin-7-O-gentiobioside, luteolin-7-O-beta-D-glucoside and luteolin-7-O-beta-D-glucuronide in beagle dog plasma and its application to a pharmacokinetic study after administration of traditional Chinese medicinal preparation: kudiezi injection. J. Pharm. Biomed. Anal. 2013;72:127–133. doi: 10.1016/j.jpba.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Dong S.Q., Fan H.R., Li Q.S., Wei G.L., Li Y.Z., Liu C.X., Si D.Y. LC-MS/MS method for quantification of liquiritigenin in rat plasma: application to pharmacokinetic study of liquiritin. Chin. Herb. Med. 2016;8:53–60. [Google Scholar]
- 38.Sim H.J., Kim J.H., Lee K.R., Hong J. Simultaneous determination of structurally diverse compounds in different fangchi species by UHPLC-DAD and UHPLC-ESI-MS/MS. Molecules. 2013;18:5235–5250. doi: 10.3390/molecules18055235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turak A., Liu Y.Q., Aisa H.A. Elemanolide dimers from seeds of Vernonia anthelmintica. Fitoterapia. 2015;104:23–30. doi: 10.1016/j.fitote.2015.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.