Abstract
One in six nursing home residents and staff with positive SARS-CoV-2 tests ≥90 days after initial infection had specimen cycle thresholds (Ct) <30. Individuals with specimen Ct<30 were more likely to report symptoms but were not different from individuals with high Ct value specimens by other clinical and testing data.
Prior severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection is associated with a reduced risk of repeated infection for nearly 6 months. However, reinfections are frequently reported, particularly after 90 days from prior infection. 1,2 Nucleic acid may be intermittently positive for months after infection, which makes diagnosing reinfection difficult. 3 Cycle threshold value (Ct), viral culture, and whole-genome sequencing are tools to help differentiate noninfectious remnant nucleic acid from infectious virus due to reinfection, but they are often unavailable to providers.
Given the high volume of asymptomatic testing in nursing homes, these settings may benefit from understanding whether routinely available testing and clinical data can distinguish individuals with repeated positive test results who have a higher likelihood of being infectious from those who are less likely to transmit virus. 4,5 Early in the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) recommended that nursing home residents and healthcare personnel (HCP) previously diagnosed with SARS-CoV-2 infection should not be retested within 90 days following an initial infection, unless symptomatic. The CDC also recommended that, after 90 days, individuals should be tested if they develop COVID-19 symptoms, have been exposed to persons with COVID-19, were associated with a COVID-19 outbreak, or as part of routine HCP screening based on community incidence and vaccination status. 4 We described the testing and clinical characteristics of nursing home individuals with a repeated positive SARS-CoV-2 test. We used RT-PCR, Ct values, and viral culture of specimens to determine potential infectivity.
Methods
Through direct consultation with nursing homes, 4 US public health jurisdictions - Nebraska, Kentucky, the District of Columbia, and Pennsylvania - identified a convenience sample of nursing home residents and HCP with a positive SARS-CoV-2 test from July 2020 through March 2021 who had also had a positive SARS-CoV-2 test ≥90 days prior.
Individual-level data included demographics, days from initial to repeated positive test, testing information between initial and repeated positive test, and testing indication, including presence of symptoms, COVID-19 exposure, or an ongoing outbreak in a nursing home. When available, repeated positive respiratory specimens were sent to the CDC for retesting using the CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. 6 Specimens were categorized as low Ct value (<30) or high Ct value (≥30 or RT-PCR negative at retesting). Specimens with a Ct value of <34 at retesting were assessed for replication-competent virus in cell culture. Each specimen with a Ct value <30 underwent Next Generation sequencing.
Data were collected using REDCap electronic data capture tools hosted at the CDC and analyzed using SAS version 9.4 software (SAS institute, Cary, NC). Continuous variables were compared using Wilcoxon signed-rank tests. Proportions were compared using the χ2 test or the Fisher exact test. The correlation between time from initial infection to repeated positive test and Ct value was assessed using the Spearman rank coefficient. This study was determined to be non–human-subjects research as part of a public health response, consistent with applicable federal law and CDC policy (eg, §45 CFR part 46.102(l)(2), 21 CFR part 56; 42 USC §241(d); 5 USC §552a; 44 USC §3501 et seq).
Results
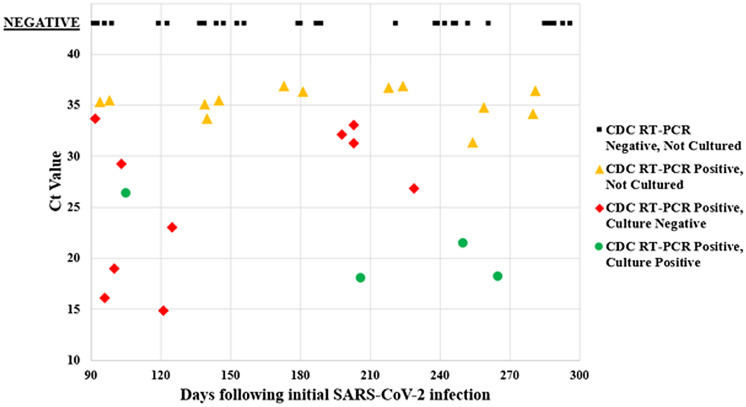
Among participating jurisdictions, 1,534 individuals were reported to have had a repeated positive test ≥90 days after their initial positive test; 1,437 (94%) came from a query of the Pennsylvania Department of Health National Election Disease Surveillance System (Supplementary Table online). Among the total reported, 64 individuals unvaccinated against COVID-19 at the time of collection, representing 61 unique nursing homes, had a repeated positive respiratory specimen available. Overall, 37 specimens (58%) were RT-PCR negative at the CDC and 27 (42%) were positive (Fig. 1). Of the positive specimens, 15 had Ct values <34 and 14 (22%) were cultured. Ten (16%) of 64 specimens had low Ct values (<30), of which 4 specimens (6%) were viral-culture positive at 105, 206, 250, and 265 days following initial SARS-CoV-2 infection. There was no correlation between Ct value and time from initial infection (P = .34).
Fig. 1.
RT-PCR Ct values for repeated positive SARS-CoV-2 specimens retested at the CDC and days to specimen collection from initial infection, July 2020–March 2021 (n = 64). (One specimen with Ct = 31 was mistakenly not sent for viral culture.)
Even though 6 of 10 individuals with low Ct values were HCP, the proportion was not significantly different than the number of HCP among individuals with high-Ct specimens (18 of 54; P = .16) (Table 1). Median days to repeated positive test result was not different for low-Ct specimens (median, 122 days; IQR, 103–229) and high-Ct specimens (median, 201 days; IQR, 139–254; P = .13). At the time of repeated positive test, 5 (50%) of 10 individuals with low-Ct specimens reported COVID-19 symptoms compared to 1 (4%) of 27 individuals with a high-Ct specimens (P < .01). We detected no significant difference in indication for testing (eg, following a known or suspected COVID-19 exposure during an active outbreak) between individuals with low-Ct and high-Ct specimens (P = .18). No variants of concern or variants being monitored were identified among sequenced specimens.
Table 1.
Characteristics of Nursing Home Individuals With Repeated Positive SARS-CoV-2 Test Specimens, 4 US Jurisdictions, July 2020–November 2021
| Characteristic | Total | High RNA Viral Load (Ct < 30) |
Low RNA Viral Load (Ct ≥30 or Negative at Retesting) a | P Value b |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| 64 | 10 (16) | 54 (84) | ||
| Age, median y [IQR] | 67 [51–80] | 62 [47–79] | 68 [53–80] | .56 |
| Days from initial infection to repeated positive test, median [IQR] | 194 [124–251] | 122 [103–229] | 201 [139–254] | .13 |
| Nursing home role, no. (%) | .16 | |||
| Resident | 40 (63) | 4 (40) | 36 (67) | |
| HCP | 24 (38) | 6 (60) | 18 (33) | |
| Testing between initial and repeated positive test, no. (%) | ||||
| At least 2 negative SARS-CoV-2 RT-PCR or antigen test result | 30 (47) | 6 (60) | 24 (44) | .49 |
| Had a negative SARS-CoV-2 RT-PCR or antigen test result 14 d prior to repeated positive test | 32 (50) | 7 (70) | 25 (46) | .30 |
| Days from most recent SARS-CoV-2 RT-PCR or antigen test to repeated positive test result, median [IQR] | 7 [6–14] | 4 [4–7] | 8 [6–14] | .05 |
| Symptom status at time of repeated positive test, no. (%) c | <.01 | |||
| Asymptomatic | 31 (84) | 5 (50) | 26 (96) | |
| Symptomatic | 6 (16) | 5 (50) | 1 (4) | |
| Known or suspected COVID-19 exposure in nursing home outbreak at repeated positive episode, no. (%) d,e | .18 | |||
| Yes | 46 (79) | 9 (100) | 37 (76) | |
| No | 12 (21) | 0 (0) | 12 (24) | |
| Symptomatic or confirmed or suspected COVID-19 exposure, no. (%) f | .57 | |||
| Yes | 48 (91) | 10 (100) | 38 (88) | |
| No | 5 (9) | 0 (0) | 5 (12) | |
| SARS-CoV-2 viral sequence of repeated positive specimen, no. (%) g,h | … | |||
| B.1.1.186 | … | 1 (10) | … | |
| B.1.234 | … | 2 (20) | … | |
| B.1.296 | … | 1 (10) | … | |
| B.1.2 | … | 3 (30) | … | |
| B.1.1369 | … | 1 (10) | … | |
| B.1.1.434 | … | 1 (10) | … | |
| B.1.1.207 | … | 1 (10) | … | |
Note. Ct, cycle threshold value; HCP, healthcare personnel.
37 specimens had an undetectable SARS-CoV-2 RNA when retested.
Categories were compared for statistical significance by Wilcoxon signed-rank test or χ2 test when indicated.
n=37. Symptom status at time of repeated positive test is unknown for 27 individuals (0 with Ct < 30 and 27 with Ct ≥ 30).
n=58. Confirmed COVID-19 exposure is unknown for 58 individuals (5 with Ct <30 and 53 with Ct ≥ 30).
n=58. Suspected COVID-19 exposure during a nursing home outbreak at the time of repeated positive test is unknown for 6 individuals (1 with Ct < 30 and 5 with Ct ≥ 30).
n=53. Unknown exposure status for 11 individuals (11 with Ct ≥ 30).
No specimens had genetic sequences matching variants of interest or concern per SARS-CoV-2 variant classifications and definitions as of the update on August 26, 2021. 9
n=10. SARS-CoV-2 sequencing was performing for specimens with Ct < 30 only.
Discussion
In this study, ∼1 in 6 individuals with repeated positive SARS-CoV-2 specimens ≥90 days after initial infection demonstrated a Ct value <30, 40% of whom also had culturable virus, suggesting potential infectivity and risk of transmission. Individuals with low-Ct specimens were more likely to report COVID-19 symptoms but were otherwise difficult to distinguish from individuals with high-Ct specimens using clinical information, including prior testing results, timing since initial infection, or SARS-CoV-2 exposure history. These findings support the CDC recommendations at the time of the study for testing individuals ≥90 days after their initial infection.
Individuals with specimens with low Ct values may be infectious and pose a risk for transmission. Although the CDC RT-PCR assay does not quantify the relationship between Ct value and RNA viral load, lower Ct values among SARS-CoV-2 RT-PCR–positive specimens have been correlated with larger amounts of viral RNA, greater likelihood of recovering replication-competent virus, and worse clinical outcomes, which can guide inferences of potential infectivity at a population level. 7,8 However, a negative viral culture may not definitively indicate that an individual is noninfectious, particularly when specimen handling during transport may degenerate viable virus. 9 Therefore, we analyzed our results by categorizing specimens as low Ct if <30 and as high Ct if ≥30 instead of solely relying on viral culture positivity.
This study had several limitations. We lacked viral sequencing data from the initial positive SARS-CoV-2 specimen, which limited an assessment of reinfection by different viral lineages. Second, 58% of specimens were negative at retesting, suggesting that many specimens may have degraded in transit to the CDC or may have been falsely positive at initial testing. Comparisons between state public health laboratory and CDC PCR assays or Ct values were not possible. Third, our study included a convenience sample of unvaccinated nursing home residents, which may have biased our population to include those more likely to be tested and may reduce generalizability to other populations. Fourth, the study was completed prior to the emergence of SARS-CoV-2 Delta and Omicron variants, which may have affected the infectiousness of individuals with repeated positivity. 9
Determining infectivity of individuals with repeated positive SARS-CoV-2 tests by viral culture or viral sequencing is not practical in most clinical settings. However, clinical and testing information that were available to providers were not sufficient to distinguish the potential infectiousness of nursing home residents and HCP with repeated positive SARS-CoV-2 tests. Further development of readily available laboratory methods associated with infectivity may greatly aid the clinical assessment of individuals with repeated positive tests.
REPOST-90 Study Team: Patricia Shewmaker, Magdalena Medrzycki, Shannon Rogers, Nhien Wynn, Phili Wong, Shilpi Jain, Brian Emery, Houping Wang, John Michael Metz, Xiaoling Tang, Marla Petway, Srinivasan Velusamy, Anna Kelleher, Peter Cook, Jing Zhang, Brian Lynch, Han Jia Ng, Raydel Anderson, Bettina Bankamp, Gimin Kim, Benton Lawson, Congrong Miao, Kay Radford, Dexter Thompson, Suganthi Suppiah, Leslie Barclay, Theresa K. Bessey, Michael D. Bowen, Rashi Gautam, Michelle Honeywood, Slavica Mijatovic Rustempasic, Sung-Sil Moon, Kenny Nguyen, Sarah Smart and Courtnee N. Wright.
Acknowledgments
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.62.
click here to view supplementary material
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1. Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative healthcare workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lutgring JD, Tobolowsky FA, Hatfield KM, et al. Evaluating the presence of replication-competent SARS-CoV-2 from nursing home residents with persistently positive RT-PCR Results. Clin Infect Dis 2022;74:525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fontana LM, Villamagna AH, Sikka MK, McGregor JC. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): review of current literature. Infect Control Hosp Epidemiol 2021;42:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Interim Infection prevention and control recommendations to prevent SARS-CoV-2 spread in nursing homes. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html. Published 2021. Accessed April 23, 2021.
- 5. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. US Food and Drug Administration website. https://www.fda.gov/media/134922/download. Published 2021. Accessed May 20, 2022.
- 7. Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID-19 infectious potential assessment—a systematic review. Clin Infect Dis 2021;73:e3884–e3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhoads D, Peaper DR, She RC, et al. College of American Pathologists (CAP) microbiology committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin Infect Dis 2021;72:e685–e686. [DOI] [PubMed] [Google Scholar]
- 9. SARS-CoV-2 variant classifications and definitions. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html. Published 2021. Accessed September 20, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.62.
click here to view supplementary material