To the Editor—We read with interest the findings of Smith et al 1 in which genomic sequencing was utilized to clarify transmission events among healthcare-workers (HCWs) and patients. Results of genomic sequencing and epidemiological investigations of in-hospital exposure were combined to identify putative nosocomial transmission events. However, information on potential exposures of HCWs and patients to coronavirus disease 2019 (COVID-19) in the community was unavailable. Both community and intrahospital exposures contribute to severe acute respiratory coronavirus virus 2 (SARS-CoV-2) acquisition among HCWs. 2 Rapid transmission and a relatively slowly evolving pathogen limits the value of sequencing when it is not augmented with exposure data. 3 In the absence of information on community exposures, relying on genomic sequencing may misclassify coincidental infections of HCWs and/or patients as an intrahospital transmission cluster, particularly during periods of low viral diversity, such as during the emergence of a SARS-CoV-2 variant. 4 Here, we share our experience regarding the limitations of sequencing in investigating nosocomial transmission during initial emergence of the SARS-CoV-2 δ (delta) variant. Despite the availability of ample data from sequencing and intrahospital outbreak investigations, additional epidemiological evidence from communitywide contact tracing was still required to thoroughly evaluate potential nosocomial transmission.
In Singapore, a large nosocomial COVID-19 outbreak attributed to the SARS-CoV-2 δ (delta) variant in April 2021 5 provided impetus for inpatient and HCW surveillance via weekly, routine, rostered, PCR testing at our institution, the largest tertiary-care hospital in Singapore (1,785 beds). 6 Given that our hospital is located in downtown Singapore, a densely populated city, there was a risk of spillover from COVID-19 outbreaks in the surrounding community (Fig. 1a). 6 Intensive community surveillance of COVID-19 clusters was conducted by the Singapore Ministry of Health using digital contact-tracing tools made mandatory to register entry or exit to or from areas of high human traffic or enclosed indoor spaces. This surveillance allowed retrospective contact tracing when community COVID-19 clusters were detected. A history of having visited an area with a COVID-19 community cluster was considered a significant epidemiological risk factor, and all inpatients were routinely asked to provide this history on admission triage. 7 Similarly, HCWs who were retrospectively identified as having visited these clusters were required to notify our hospital’s epidemiology department. 8 Information on community exposure (visiting an area with a known COVID-19 community clusters) could thus be integrated into the outbreak investigation of nosocomial COVID-19 cases. Our institution reported its first potential nosocomial-onset COVID-19 case in September 2021 (defined as PCR positive ≥7 days from initial admission). Soon after, a cluster of nosocomial-onset COVID-19 cases was detected on a renal ward. 9 We utilized contact tracing and genomic sequencing to investigate nosocomial-onset COVID-19 cases. 10 All inpatient COVID-19 cases and HCW cases over 1 month (August 20–September 17, 2021) with a cycle threshold value (Ct) <31 were sent for sequencing using the ARTIC protocol on Oxford Nanopore minION sequencers (Oxford Nanopore Technologies, Oxford, UK). Contact tracing was performed for all nosocomial-onset COVID-19 cases, all community-onset COVID-19 cases initially managed outside isolation areas, as well as all HCWs at work during their infective periods. Epidemiological outbreaks were defined as ≥2 cases of COVID-19 in patients and HCWs with significant close contact, defined as contact within 2 m of the index case for ≥15 minutes, during the infectious period of the index case. Genomic clusters were detected based on whole-genome similarity analysis (ie, when sequences are ≤3 single-nucleotide polymorphisms different and fall in the same branch of the genome similarity tree). 10
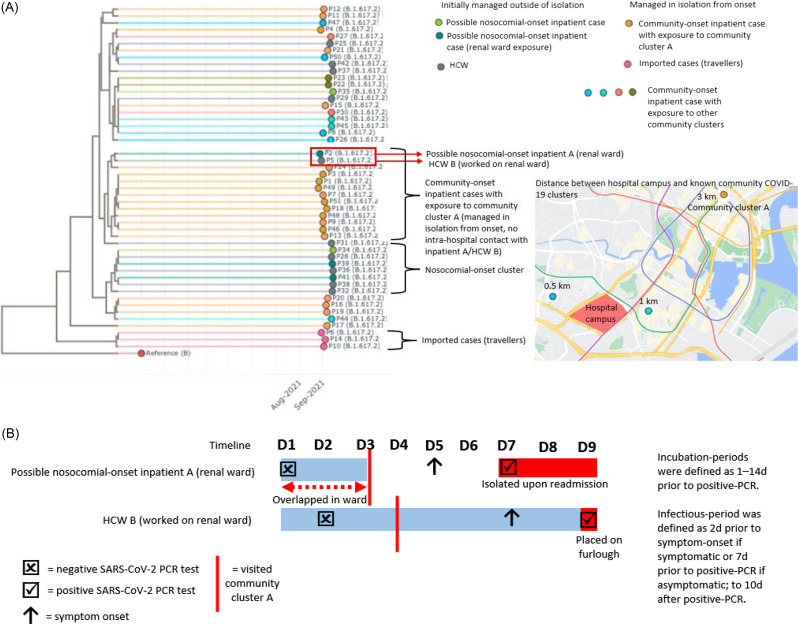
Fig. 1.
Combining genomic sequencing and epidemiological investigation of intrahospital and community exposure to COVID-19 cases.
As revealed by genomic sequencing, most nosocomial-onset and HCW cases clustered on a separate phylogenetic branch from community-onset COVID-19 cases managed in isolation from the onset (Fig. 1a), suggesting disparate introductions. However, an identical sequence match was observed between a possible nosocomial-onset COVID-19 case and an HCW who had both been on the renal ward. Inpatient A was initially admitted to the renal ward for 3 days and tested positive upon readmission 4 days after discharge. HCW B tested positive 2 days later. HCW B had worked daily on the renal ward 2 weeks prior to diagnosis (during the period of inpatient A’s initial admission), although HCW B did not directly care for inpatient A (Fig. 1b). In total, 191 HCWs and 41 inpatients were additionally identified as having had significant close contact and were placed on enhanced surveillance (ie, PCR tests on day 1, day 4, day 7, and day 10 after exposure). None subsequently tested positive. Based on genomic analysis and intrahospital outbreak investigation alone, nosocomial transmission could not be ruled out given overlap in space and time. However, when information on community exposures was considered, both HCW B and inpatient A had visited community cluster A (inpatient A between discharge and readmission; HCW B, after work) (Fig. 1b). Indeed, based on genomic analysis, inpatient A and HCW B clustered together with other community-onset inpatient cases with reported exposure to community cluster A who were managed in isolation from the onset. Inpatient A and HCW B did not cluster with other nosocomial-onset and HCW COVID-19 cases (Fig. 1a). The sequencing linkage between inpatient A and HCW B more likely reflected acquisition from a common community source rather than nosocomial transmission. However, this information would not have been readily apparent without information on their community exposures.
Despite the potential for genomic sequencing in clarifying nosocomial transmission of SARS-CoV-2, possible pitfalls in interpretation still exist. Our experience highlights that thorough epidemiological investigation, including both intrahospital and community exposures, remains important in investigating nosocomial COVID-19 outbreaks.
Acknowledgments
Financial support
No financial support was provided relevant to this article. Funding for consumables was provided by the Singapore General Hospital.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1. Smith L, Morris CP, Jibowu MH, et al. SARS-CoV-2 exposure investigations using genomic sequencing among healthcare workers and patients in a large academic center. Infect Control Hosp Epidemiol 2022;28:612.e1–612.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lenggenhager L, Martischang R, Sauser J, et al. Occupational and community risk of SARS-CoV-2 infection among employees of a long-term care facility: an observational study. Antimicrob Resist Infect Control 2022;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abbas M, Robalo Nunes T, et al. Explosive nosocomial outbreak of SARS-CoV-2 in a rehabilitation clinic: the limits of genomics for outbreak reconstruction. J Hosp Infect 2021;117:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leducq V, Jary A, Bridier-Nahmias A, et al. Nosocomial transmission clusters and lineage diversity characterized by SARS-CoV-2 genomes from two large hospitals in Paris, France, in 2020. Sci Rep 2022;12:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chow A, Guo H, Kyaw WM, Li AL, Lim RHF, Ang B. Rostered routine testing for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare personnel—is there a role in a tertiary-care hospital with enhanced infection prevention and control measures and robust sickness-surveillance systems? Infect Control Hosp Epidemiol 2021. doi: 10.1017/ice.2021.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wee LE, Conceicao EP, Aung MK, Oo AM, Yong Y, Venkatachalam I, Sim JX. Rostered routine testing for healthcare workers and universal inpatient screening: the role of expanded hospital surveillance during an outbreak of COVID-19 in the surrounding community. Infect Control Hosp Epidemiol 2021. doi: 10.1017/ice.2021.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wee LE, Hsieh JYC, Phua GC, et al. Respiratory surveillance wards as a strategy to reduce nosocomial transmission of COVID-19 through early detection: the experience of a tertiary-care hospital in Singapore. Infect Control Hosp Epidemiol 2020;41:820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wee LE, Sim XYJ, Conceicao EP, et al. Containment of COVID-19 cases among healthcare workers: the role of surveillance, early detection, and outbreak management. Infect Control Hosp Epidemiol 2020;41:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wee LE, Conceicao EP, Sim JX, et al. Sporadic outbreaks of healthcare-associated COVID-19 infection in a highly vaccinated inpatient population during a community outbreak of the B.1.617.2 variant: the role of enhanced infection-prevention measures. Am J Infect Control 2022;50:465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wee LE, Ko KKK, Conceicao EP, et al. Linking sporadic hospital clusters during a community surge of the SARS-CoV-2 delta variant (B.1.617.2): the utility of whole-genome sequencing. Infect Control Hosp Epidemiol 2022. doi: 10.1017/ice.2022.106. [DOI] [PMC free article] [PubMed] [Google Scholar]