Abstract
Background
Psychiatric disorders have seriously affected human life, one of the risk genes related to psychosis is the methylenetetrahydrofolatereductase (MTHFR) gene. This gene has a potential role in psychiatric disorders. Therefore, a meta-analysis is conducted to investigate the correlations between two prevalent MTHFR single nucleotide polymorphisms (SNPs), MTHFR C677T, A1298C, severe psychological disorders (schizophrenia, major depression, bipolar disorder).
Methods
A total of 81 published studies were screened and selected by a search of electronic databases up to April 2022. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the association between MTHFR polymorphism and psychiatric disorders susceptibility by using random effect models.
Results
We found that MTHFR C677T polymorphism is significantly related to schizophrenia and major depression in the overall population. MTHFR C677T has been linked to an increased risk of bipolar disorder in the recessive model (TT vs. CT + CC). Ethnic subgroup analysis shows that schizophrenia and major depression significantly correlate with MTHFR C677T and A1298C in Asian populations but not Caucasians. Besides, schizophrenia is correlated substantially with MTHFR C677T in the African population. However, the MTHFR A1298C polymorphism is only marginally linked to major depression.
Conclusion
Findings of the current study revealed that MTHFR may contribute to the common pathogenesis of psychiatric diseases and that its variants may be essential in controlling the expression of psychosis-related genes. This study could help the researchers and health specialists in the early diagnosis and treatment of psychiatric disorders.
Keywords: MTHFR C677T, MTHFR A1298C, disorders, meta-analysis, gene variants
Introduction
Mental disorders have seriously affected human life, causing considerable familial and social burden (1). They are among the leading causes of disability globally and have been related to an increase in premature mortality (2). Major psychiatric disorders include schizophrenia (SZ), major depression (MD), bipolar disorder (BPD), and others (3). These mental disorders are more likely to occur in families, suggesting that they are related to genetic factors (4, 5). Many susceptible genes have been found through unbiased genome-wide association studies (GWAS), a kind of analysis comparing allele frequencies of all available polymorphic markers with specific symptoms or disease states (6, 7). GWAS and many other follow-up replication studies have suggested that methylenetetrahydrofolatereductase (MTHFR) polymorphisms are associated with psychiatric disorders.
The MTHFR is a crucial enzyme in the one-carbon metabolism (OCM) process, which involves folate and homocysteine (Hcy) metabolisms. It transforms 5,10-methylenetetrahydrofolate (5,10-methylene THF) to 5-methyltetrahydrofolate (5-methyl THF), and it is involved in folate and homocysteine conversion, which is linked to DNA methylation (8–10). A number of mutations in the MTHFR gene have been found, and the most common mutations are C677T (rs1801133) and A1298C (rs1801131), which are correlated with enzyme deficiency (11–14). In addition, MTHFR polymorphism may significantly decrease MTHFR activity, affect the concentration of Hcy in plasma, and lead to a wide range of mental, neurological, and vascular dysfunction (15).
The human Methylenetetrahydrofolatereductase (MTHFR) gene is located in chromosomal region 1p36.3 (16). The MTHFR gene has 14 common or rare single nucleotide polymorphisms linked with enzyme defects, the most prevalent of which are C677T and A1298C. The C677T gene location is one of the most researched and clinically significant variants in exon 4. The variation in C677T is due to the replacement of cytosine by thymine, which leads to the conversion of valine to alanine at codon 222 (11). The polymorphism of A1298C is due to the adenine substitution by cytosine, leading to the conversion of glutamic acid to alanine at residue 429 (10). The replacement of 677 and 1,298 nucleotides C-T and A-C in the MTHFR gene reduces enzyme activity, and this decrease in MTHFR activity may affect the OCM cycle (17). Abnormal OCM might impair cortical and hippocampal neurogenesis during development and affect brain maturation and function (18–20).
The association between MTHFR polymorphism and mental illnesses has already been explored, but the influence of MTHFR on psychiatric disorders is still disputed, and limited studies have been found (21–23). These inconsistencies might be attributed to limited sample sizes, ethnic heterogeneity, and differences in population substructure. So, in current study these limitations have been overcome and summarized the conflicting data. A meta-analysis is performed to explore the connection of MTHFR C677T and A1298C polymorphisms with major mental disorders (including SZ, MD, and BPD). We also assessed whether ethnicity would affect the results. Therefore, it will provide more powerful evidence of whether MTHFR variants influence psychiatric diseases.
Materials and methods
Search strategy
We initially searched PubMed, Embase, Proquest, Web of Science, CNKI (Chinese National Knowledge Infrastructure), VIP (Chinese) database, and Wanfang (Chinese) database for the following terms: MTHFR (methylenetetrahydrofolatereductase), gene (gene or genetic or polymorphism or variants or variation), and psychiatric disorders (psychiatry disorders or mental illness or mental disorders or psychosis). We discovered that most research concentrated on MTHFR C677T and MTHFR A1298C. The researchers investigated the relationships between MTHFR gene variants and susceptibility to mental diseases such as schizophrenia, bipolar disorder, and depression. To guarantee that we missed no studies, we searched these databases again using these gene terms (MTHFR C677T and A1298C) and major mental disorders such as “schizophrenia,” “bipolar disorder,” “depression,” and so on. All of the research was completed and published by April 2022. After that, we selected relevant papers and examined their bibliographies to find additional references.
Study selection
Selection of articles for analysis purposes was made based on the following criteria: (1) case-control studies; (2) giving comprehensive data of formally diagnosed patients with unrelated healthy control subjects for generating an odds ratio (OR) with a 95% confidence interval (CI); (3) Case status was classified as having a DSM-IV-diagnosed mental condition, with control patients having no history of psychiatric disorders or other neurological abnormalities; (4) the studies used samples that did not overlap with other studies; (5) the use of internationally recognized loci gene polymorphism detection techniques (such as polymerase chain reaction-restriction fragment length polymorphism, real-time quantitative polymerase chain reaction, or amplification block mutation system-polymerase chain reaction); and (6) the demographic characteristics of the control group, such as gender and age, were comparable to those of the case group. In addition, articles were excluded if they (1) not reported the target genotype frequencies, (2) were reviews, letters, or commentaries, or (3) were duplicate reports.
Data extraction and management
Two reviewers independently extracted the following information from all eligible studies: author, year of publication, country, ethnicity (categorized as Asian, Caucasian, and African populations), and the number of distinct genotypes in cases and controls for C677T or A1298C genotype. In the case of a disagreement, a discussion was held, and if no agreement could be achieved, a third person was consulted for consensus.
Statistical analysis
We investigated the potential of conducting a meta-analysis of all eligible studies. The odds ratio (OR) and associated 95% confidence intervals (CIs) were used to examine the strength of the connection between MTHFR polymorphism and mental disorders: the allele model (T vs. C, C vs. A), the dominant model (TT + CT vs. CC, CC + AC vs. AA), the homozygote model (TT vs. CC, CC vs. AA) and the recessive model (TT vs. CT + CC, CC vs. AC + AA). The Chi-square test was used to analyze the genotype distribution in the control groups for Hardy Weinberg equilibrium (HWE). The Cochran’s (Q) X2 test and I2 statistic were used to assess the heterogeneity between individual studies (24). Considering the heterogeneity of studies, this meta-analysis adopted a random effect model (25). Subgroup analyses were performed using ethnicity stratification, and sensitivity analyses were undertaken by excluding papers from the meta-analysis that were not in HWE. The funnel plots were displayed and evaluated using Egger’s linear regression test to control publication bias (26). Stata 14.0 was used to conduct all statistical analyses (StataCorp, College Station, TX, United States). A P-value of less than 0.05 was regarded as statistically significant. The article mainly showed the forest plots of T vs. C of MTHFR C677T and C vs. A of MTHFR A1298C; the other results were shown in the tables.
Results
Characteristics of eligible studies
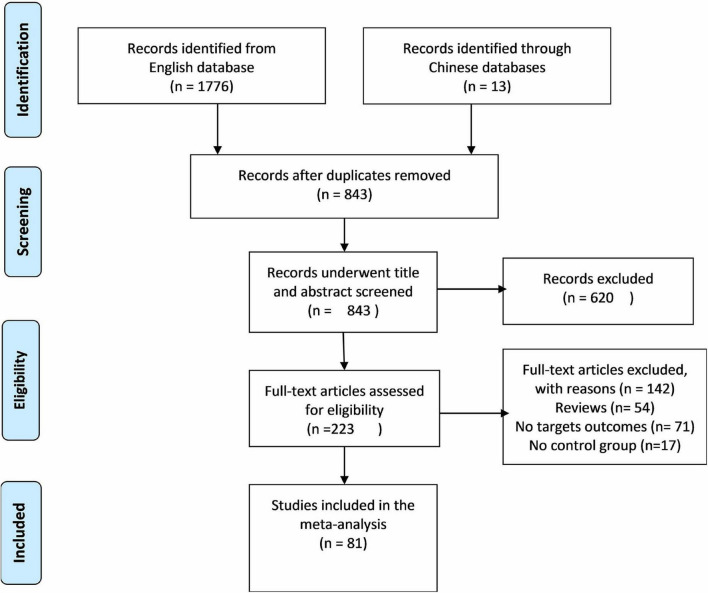
Out of screened articles, 843 unduplicated association studies were found. Figure 1 depicts a flow chart of the research process, the eliminated studies, and the reasons for their exclusion. Following an initial literature search and further screening, 81 (27–106) publications were retrieved. Our meta-analysis comprised 49,775 subjects (20,981 patients and 28,794 controls) with MTHFR C677T genotyping and 16,058 subjects (6,690 patients and 9,368 controls) with MTHFR A1298C genotyping. Detailed information (first author, year of publication, country, ethnicity, case/control, genotype, and PHWE) of included articles are summarized in Tables 1, 2.
FIGURE 1.
Flow diagram of the study selection process.
TABLE 1.
Overview of MTHFR C677T genotype distribution of psychosis patients and controls, with information about country, ethnicity, and disease.
| References | Year | Country | Ethnicity | Case | Control | Case |
Control |
P
HWE
|
||||
| CC | CT | TT | CC | CT | TT | |||||||
| Schizophrenia | ||||||||||||
| Arinami et al. (27) | 1997 | Japanese | Asian | 297 | 419 | 96 | 138 | 63 | 154 | 214 | 51 | 0.074 |
| Kunugi et al. (28) | 1998 | Japanese | Asian | 343 | 258 | 121 | 168 | 54 | 95 | 129 | 34 | 0.342 |
| Virgos et al. (29) | 1999 | Spain | Caucasian | 210 | 218 | 81 | 98 | 31 | 79 | 106 | 33 | 0.793 |
| Joober et al. (30) | 2000 | Canada | Caucasian | 105 | 90 | 30 | 52 | 23 | 41 | 36 | 13 | 0.278 |
| Sazci et al. (31) | 2003 | Turkey | Caucasian | 130 | 226 | 59 | 49 | 22 | 106 | 103 | 17 | 0.236 |
| Tan et al. (32) | 2004 | Singapore | Asian | 236 | 120 | 136 | 84 | 16 | 80 | 33 | 7 | 0.165 |
| Yu et al. (33) | 2004 | China | Asian | 230 | 251 | 91 | 96 | 43 | 85 | 126 | 40 | 0.554 |
| Yu et al. (33) | 2004 | Scotland | Caucasian | 426 | 628 | 199 | 186 | 41 | 306 | 260 | 62 | 0.535 |
| Sazci et al. (34) | 2005 | Turkey | Asian | 297 | 341 | 144 | 115 | 38 | 161 | 156 | 24 | 0.093 |
| Vilella et al. (35) | 2005 | Spain | Caucasian | 158 | 234 | 58 | 75 | 25 | 85 | 85 | 39 | 0.952 |
| Kempisty et al. (36) | 2006 | Poland | Caucasian | 200 | 300 | 113 | 68 | 19 | 210 | 79 | 11 | 0.303 |
| Philibert et al. (37) | 2006 | United States | Caucasian | 206 | 359 | 107 | 83 | 16 | 176 | 137 | 46 | 0.021* |
| Lee et al. (38) | 2006 | South Korea | Asian | 235 | 235 | 74 | 128 | 33 | 99 | 115 | 21 | 0.009* |
| Yang et al. (39) | 2007 | China | Asian | 100 | 100 | 33 | 51 | 16 | 52 | 40 | 8 | 0.937 |
| Jonsson et al. (40) | 2008 | Denmark | Caucasian | 419 | 1006 | 200 | 177 | 42 | 490 | 413 | 103 | 0.249 |
| Jonsson et al. (40) | 2008 | Norway | Caucasian | 163 | 177 | 75 | 70 | 18 | 80 | 75 | 22 | 0.501 |
| Jonsson et al. (40) | 2008 | Sweden | Caucasian | 258 | 293 | 137 | 104 | 17 | 156 | 113 | 24 | 0.581 |
| Muntjewerff (41) | 2008 | Netherlands | Caucasian | 252 | 405 | 110 | 111 | 31 | 205 | 165 | 35 | 0.61 |
| Roffman et al. (42) | 2008 | United States | Caucasian | 79 | 75 | 41 | 27 | 11 | 35 | 32 | 8 | 0.865 |
| Feng et al. (43) | 2009 | China | Asian | 123 | 123 | 17 | 67 | 39 | 40 | 65 | 18 | 0.308 |
| Betcheva et al. (44) | 2009 | Bulgaria | Caucasian | 185 | 182 | 76 | 85 | 24 | 84 | 76 | 22 | 0.457 |
| García-Miss et al. (45) | 2010 | Mexico | Caucasian | 105 | 108 | 29 | 45 | 31 | 22 | 54 | 31 | 0.864 |
| Kang et al. (46) | 2010 | Korean | Asian | 360 | 348 | 125 | 176 | 59 | 130 | 158 | 60 | 0.317 |
| Ye et al. (47) | 2010 | China | Asian | 104 | 56 | 12 | 58 | 34 | 14 | 32 | 10 | 0.266 |
| Bouaziz et al. (48) | 2010 | Tunisia | African | 25 | 25 | 18 | 4 | 3 | 19 | 5 | 1 | 0.397 |
| Arzaghi et al. (49) | 2011 | Iran | Asian | 66 | 94 | 35 | 27 | 4 | 54 | 38 | 2 | 0.11 |
| Kim et al. (50) | 2011 | Korean | Asian | 201 | 350 | 62 | 101 | 38 | 112 | 167 | 71 | 0.313 |
| Muntjewerff et al. (51) | 2011 | Netherlands | Caucasian | 739 | 886 | 334 | 319 | 86 | 405 | 389 | 92 | 0.921 |
| Tsutsumi et al. (52) | 2011 | Japan | Asian | 413 | 385 | 160 | 184 | 69 | 138 | 183 | 64 | 0.8 |
| Zhang et al. (53) | 2012 | China | Asian | 235 | 102 | 96 | 113 | 26 | 52 | 45 | 5 | 0.225 |
| Lochman et al. (54) | 2013 | Czechia | Caucasian | 186 | 209 | 72 | 90 | 24 | 105 | 86 | 18 | 0.948 |
| Zhang et al. (55) | 2013 | China | Asian | 1002 | 1036 | 166 | 450 | 384 | 213 | 505 | 318 | 0.63 |
| Kontis et al. (56) | 2013 | Greece | Caucasian | 90 | 55 | 40 | 37 | 13 | 21 | 22 | 12 | 0.187 |
| El-Hadidy et al. (57) | 2014 | Egypt | African | 103 | 149 | 52 | 36 | 15 | 114 | 30 | 5 | 0.103 |
| Hei et al. (58) | 2014 | China | Asian | 130 | 80 | 17 | 65 | 48 | 24 | 38 | 18 | 0.029 |
| Nishi et al. (59) | 2014 | Japan | Asian | 621 | 486 | 220 | 309 | 92 | 174 | 239 | 73 | 0.532 |
| Nishi et al. (59) | 2014 | Japan | Asian | 1,149 | 2,742 | 417 | 530 | 202 | 1,072 | 1,260 | 410 | 0.207 |
| Foroughmand et al. (60) | 2015 | Iran | Asian | 200 | 200 | 104 | 76 | 20 | 123 | 64 | 13 | 0.244 |
| Misiak et al. (61) | 2016 | Poland | Caucasian | 135 | 146 | 64 | 52 | 16 | 71 | 53 | 22 | 0.786 |
| Takano et al. (62) | 2016 | Japan | Asian | 45 | 30 | 17 | 18 | 10 | 12 | 14 | 4 | 0.62 |
| Wang et al. (63) | 2017 | China | Asian | 254 | 339 | 79 | 129 | 46 | 109 | 175 | 55 | 0.26 |
| Oniki et al. (64) | 2017 | Japan | Asian | 256 | 194 | 89 | 135 | 32 | 64 | 93 | 37 | 0.207 |
| Debost et al. (65) | 2017 | Denmark | Caucasian | 1699 | 1681 | 839 | 704 | 156 | 829 | 724 | 128 | 0.08 |
| Zhilyaeva et al. (66) | 2018 | Russia | Caucasian | 500 | 499 | 245 | 212 | 43 | 280 | 188 | 31 | 0.057 |
| Ota et al. (67) | 2019 | Japan | Asian | 538 | 1263 | 181 | 255 | 102 | 458 | 604 | 201 | 0.937 |
| Wan et al. (68) | 2019 | China | Asian | 97 | 92 | 24 | 47 | 26 | 24 | 43 | 25 | 0.532 |
| Wan, L (69) | 2019 | China | Asian | 242 | 234 | 45 | 122 | 75 | 71 | 113 | 50 | 0.687 |
| Major depression | ||||||||||||
| Arinami et al. (27) | 1997 | Japanese | Asian | 32 | 419 | 9 | 14 | 9 | 154 | 214 | 51 | 0.074 |
| Kunugi et al. (28) | 1998 | Japanese | Asian | 71 | 258 | 10 | 31 | 30 | 95 | 129 | 34 | 0.342 |
| Tan et al. (32) | 2004 | Singapore | Asian | 88 | 120 | 49 | 34 | 5 | 80 | 33 | 7 | 0.165 |
| Kelly et al. (70) | 2004 | United Kingdom | Caucasian | 100 | 89 | 30 | 56 | 14 | 40 | 37 | 12 | 0.467 |
| Reif et al. (71) | 2005 | Germany | Caucasian | 46 | 176 | 23 | 17 | 6 | 75 | 80 | 21 | 0.962 |
| Yuan et al. (72) | 2005 | China | Asian | 60 | 80 | 22 | 27 | 11 | 27 | 38 | 15 | 0.801 |
| Chen-Sheng et al. (73) | 2005 | China | Asian | 39 | 20 | 22 | 15 | 2 | 11 | 9 | 0 | 0.194 |
| Yuan (74) | 2007 | China | Asian | 60 | 80 | 22 | 27 | 11 | 27 | 38 | 15 | 0.801 |
| Słopien et al. (75) | 2008 | Poland | Caucasian | 83 | 89 | 26 | 38 | 19 | 46 | 36 | 7 | 0.991 |
| Zhao (76) | 2008 | China | Asian | 77 | 85 | 12 | 37 | 28 | 21 | 48 | 16 | 0.219 |
| Yuan et al. (77) | 2008 | China | Asian | 116 | 80 | 46 | 48 | 22 | 27 | 38 | 15 | 0.801 |
| Hong et al. (78) | 2009 | China | Asian | 178 | 85 | 75 | 84 | 19 | 32 | 44 | 9 | 0.28 |
| Kim et al. (79) | 2009 | China | Asian | 63 | 458 | 16 | 28 | 19 | 84 | 248 | 126 | 0.63 |
| Pan et al. (80) | 2009 | United States | Caucasian | 170 | 83 | 72 | 79 | 19 | 30 | 44 | 9 | 0.598 |
| Cao et al. (81) | 2010 | China | Asian | 50 | 59 | 9 | 23 | 18 | 24 | 27 | 8 | 0.926 |
| Zeman et al. (82) | 2010 | Czechia | Caucasian | 42 | 41 | 15 | 18 | 9 | 16 | 17 | 8 | 0.377 |
| Feng et al. (83) | 2010 | China | Asian | 152 | 152 | 32 | 66 | 54 | 51 | 81 | 20 | 0.167 |
| Li et al. (84) | 2010 | China | Asian | 402 | 600 | 132 | 192 | 78 | 156 | 343 | 101 | <0.001* |
| Song (85) | 2010 | China | Asian | 156 | 123 | 33 | 68 | 55 | 35 | 74 | 14 | 0.008* |
| Lizer et al. (86) | 2011 | United States | Caucasian | 82 | 74 | 31 | 34 | 17 | 33 | 28 | 13 | 0.114 |
| Zhao et al. (87) | 2011 | China | Asian | 94 | 98 | 24 | 43 | 27 | 36 | 45 | 17 | 0.651 |
| Chojnicka et al. (88) | 2012 | Poland | Caucasian | 710 | 2547 | 342 | 300 | 68 | 1213 | 1081 | 253 | 0.593 |
| Evinova et al. (89) | 2012 | Slovak | Caucasian | 134 | 143 | 70 | 54 | 10 | 58 | 73 | 12 | 0.1 |
| Qiao et al. (90) | 2012 | China | Asian | 94 | 98 | 24 | 43 | 27 | 36 | 45 | 17 | 0.651 |
| Shen et al. (91) | 2014 | China | Asian | 368 | 219 | 88 | 259 | 21 | 113 | 91 | 15 | 0.563 |
| Sayadi et al. (92) | 2016 | Tunisia | African | 208 | 187 | 105 | 80 | 23 | 80 | 93 | 14 | 0.066 |
| Mei et al. (93) | 2016 | China | Asian | 37 | 65 | 9 | 26 | 2 | 32 | 27 | 6 | 0.59 |
| Huang et al. (94) | 2017 | China | Asian | 80 | 80 | 20 | 36 | 24 | 30 | 38 | 12 | 0.995 |
| Li et al. (95) | 2017 | China | Asian | 218 | 582 | 97 | 93 | 28 | 461 | 89 | 32 | <0.001* |
| Mei et al. (96) | 2018 | China | Asian | 106 | 175 | 25 | 75 | 6 | 90 | 73 | 12 | 0.59 |
| Saraswathy et al. (97) | 2019 | India | African | 91 | 206 | 78 | 12 | 1 | 183 | 22 | 1 | 0.68 |
| Bipolar disorder | ||||||||||||
| Arinami et al. (27) | 1997 | Japanese | Asian | 40 | 419 | 15 | 20 | 5 | 154 | 214 | 51 | 0.074 |
| Kunugi et al. (28) | 1998 | Japanese | Asian | 143 | 258 | 41 | 74 | 28 | 95 | 129 | 34 | 0.342 |
| Tan et al. (32) | 2004 | Singapore | Asian | 167 | 120 | 99 | 60 | 8 | 80 | 33 | 7 | 0.165 |
| Reif et al. (71) | 2005 | Germany | Caucasian | 92 | 176 | 48 | 34 | 10 | 75 | 80 | 21 | 0.962 |
| Kempisty et al. (36) | 2006 | Poland | Caucasian | 200 | 300 | 108 | 73 | 19 | 210 | 79 | 11 | 0.303 |
| Zhao et al. (98) | 2008 | China | Asian | 61 | 73 | 12 | 28 | 21 | 18 | 40 | 15 | 0.404 |
| Ozbek et al. (99) | 2008 | Turkey | Caucasian | 197 | 238 | 104 | 76 | 17 | 116 | 97 | 25 | 0.603 |
| Jonsson et al. (40) | 2008 | Norway | Caucasian | 117 | 177 | 58 | 49 | 10 | 80 | 75 | 22 | 0.501 |
| Chen et al. (100) | 2009 | China | Asian | 501 | 461 | 178 | 231 | 92 | 153 | 235 | 73 | 0.272 |
| Ezzaher et al.(101) | 2011 | Tunisia | African | 92 | 170 | 41 | 40 | 11 | 94 | 62 | 14 | 0.411 |
| Arzaghi et al. (49) | 2011 | Iran | Asian | 90 | 94 | 52 | 34 | 4 | 54 | 38 | 2 | 0.11 |
| El-Hadidy et al. (57) | 2013 | Egypt | African | 134 | 149 | 46 | 70 | 18 | 114 | 30 | 5 | 0.239 |
| Permoda-Osip et al. (102) | 2014 | Poland | Caucasian | 112 | 164 | 51 | 50 | 11 | 66 | 82 | 16 | 0.657 |
| Wang et al. (103) | 2015 | China | Asian | 531 | 447 | 287 | 206 | 38 | 215 | 199 | 33 | 0.16 |
| Rahimi et al. (104) | 2016 | Iran | Caucasian | 150 | 148 | 69 | 67 | 14 | 81 | 62 | 5 | 0.093 |
*P < 0.05.
TABLE 2.
Overview of MTHFR A1298C genotype distribution of psychosis patients and controls, with information about country, ethnicity, and disease.
| First author | Year | Country | Ethnicity | Case | Control | Case |
Control |
P HWE | ||||
| AA | AC | CC | AA | AC | CC | |||||||
| Schizophrenia | ||||||||||||
| Sazci et al. (31) | 2003 | Turkey | Caucasian | 130 | 226 | 57 | 59 | 14 | 114 | 93 | 19 | 0.996 |
| Yu et al. (33) | 2004 | China | Asian | 230 | 251 | 130 | 78 | 22 | 154 | 81 | 16 | 0.235 |
| Scotland | Caucasian | 426 | 628 | 177 | 209 | 40 | 292 | 272 | 64 | 0.955 | ||
| Sazc et al. (34) | 2005 | Turkey | Caucasian | 297 | 341 | 130 | 129 | 38 | 159 | 155 | 27 | 0.201 |
| Vilella et al. (35) | 2005 | Spain | Caucasian | 158 | 234 | 76 | 68 | 14 | 124 | 97 | 13 | 0.286 |
| Lee et al. (38) | 2006 | South Korea | Asian | 235 | 236 | 157 | 7 | 71 | 145 | 14 | 77 | <0.001* |
| Kempisty et al. (105) | 2007 | Poland | Caucasian | 200 | 300 | 109 | 74 | 17 | 185 | 105 | 10 | 0.29 |
| Jonsson et al. (40) | 2008 | Denmark | Caucasian | 418 | 1004 | 184 | 186 | 48 | 462 | 419 | 123 | 0.052 |
| 2008 | Norway | Caucasian | 163 | 177 | 89 | 60 | 14 | 82 | 79 | 16 | 0.625 | |
| 2008 | Sweden | Caucasian | 258 | 293 | 110 | 113 | 35 | 122 | 129 | 42 | 0.406 | |
| Betcheva et al. (44) | 2009 | Bulgaria | Caucasian | 181 | 183 | 91 | 72 | 18 | 80 | 79 | 24 | 0.406 |
| Kang et al. (46) | 2010 | Korean | Asian | 360 | 348 | 248 | 105 | 7 | 239 | 100 | 9 | 0.703 |
| Zhang et al. (106) | 2010 | China | Asian | 379 | 380 | 230 | 127 | 22 | 260 | 108 | 12 | 0.848 |
| Kim et al. (50) | 2011 | Korean | Asian | 201 | 350 | 129 | 67 | 5 | 240 | 105 | 5 | 0.083 |
| Zhang et al. (53) | 2012 | China | Asian | 235 | 102 | 126 | 91 | 18 | 62 | 33 | 7 | 0.376 |
| Foroughmand et al. (60) | 2015 | Iran | Asian | 200 | 200 | 65 | 108 | 27 | 60 | 89 | 51 | 0.126 |
| Misiak et al. (61) | 2016 | Poland | Caucasian | 135 | 146 | 55 | 64 | 13 | 55 | 72 | 19 | 0.64 |
| Takano et al. (62) | 2016 | Japan | Asian | 45 | 30 | 34 | 8 | 3 | 21 | 9 | 0 | 0.2 |
| Oniki et al. (64) | 2017 | Japan | Asian | 256 | 194 | 173 | 75 | 8 | 124 | 65 | 5 | 0.597 |
| Ota et al. (67) | 2019 | Japan | Asian | 537 | 1262 | 358 | 163 | 16 | 820 | 395 | 47 | 0.947 |
| Wan et al. (68) | 2019 | China | Asian | 97 | 92 | 66 | 29 | 2 | 69 | 22 | 1 | 0.603 |
| Wan et al. (69) | 2019 | China | Asian | 242 | 234 | 174 | 63 | 5 | 171 | 58 | 5 | 0.975 |
| Major depression | ||||||||||||
| Reif et al. (71) | 2005 | Germany | Caucasian | 46 | 184 | 16 | 21 | 9 | 75 | 96 | 13 | 0.016* |
| Zeman et al. (82) | 2010 | Czechia | Caucasian | 42 | 41 | 22 | 17 | 3 | 20 | 18 | 3 | 0.495 |
| Feng et al. (83) | 2010 | China | Asian | 152 | 152 | 122 | 28 | 2 | 115 | 35 | 2 | 0.716 |
| Evinova et al. (89) | 2012 | Slovak | Caucasian | 134 | 143 | 49 | 65 | 20 | 70 | 61 | 12 | 0.801 |
| Li et al. (95) | 2017 | China | Asian | 218 | 582 | 86 | 75 | 57 | 396 | 144 | 42 | <0.001* |
| Bipolar disorder | ||||||||||||
| Reif et al. (71) | 2005 | Germany | Caucasian | 92 | 184 | 30 | 47 | 15 | 75 | 96 | 13 | 0.016* |
| Kempisty et al. (105) | 2007 | Poland | Caucasian | 200 | 300 | 99 | 78 | 23 | 185 | 105 | 10 | 0.29 |
| Jonsson et al. (40) | 2008 | Norway | Caucasian | 115 | 177 | 47 | 56 | 12 | 82 | 79 | 16 | 0.624 |
| Ozbek et al. (99) | 2008 | Turkey | Caucasian | 197 | 238 | 91 | 84 | 22 | 113 | 101 | 24 | 0.848 |
| Permoda-Osip et al. (102) | 2014 | Poland | Caucasian | 111 | 156 | 51 | 50 | 10 | 60 | 74 | 22 | 0.915 |
*P < 0.05.
Methylenetetrahydrofolatereductase C677T/A1298C and psychiatric disorders
Association between the methylenetetrahydrofolatereductase C677T/A1298C polymorphisms and schizophrenia
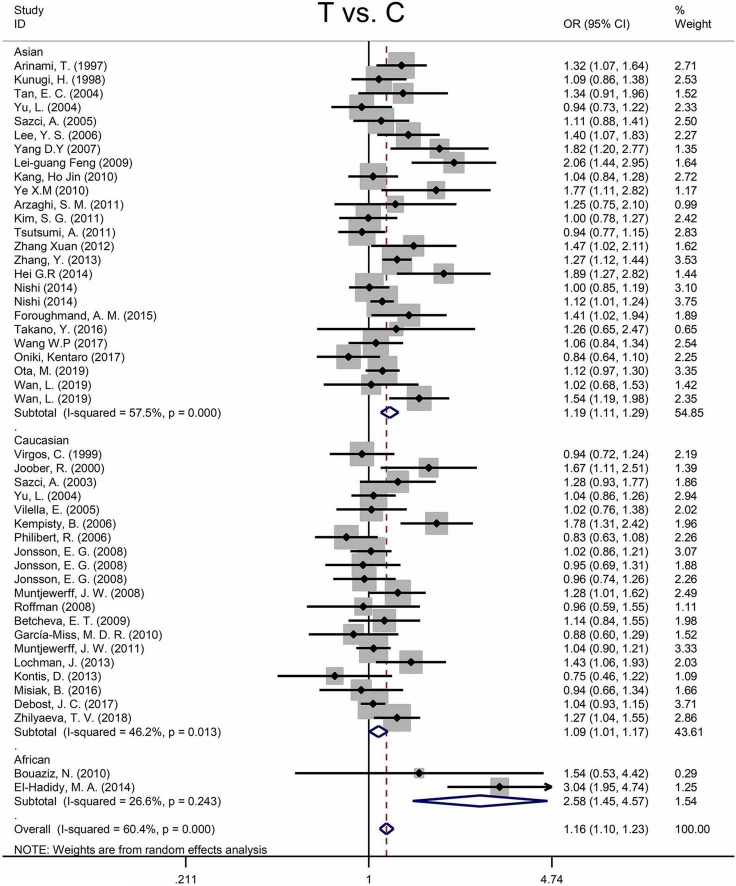
Findings of the association and the heterogeneity test is shown in Table 3. MTHFR C677T polymorphism was shown to be highly associated with an increased risk of developing SZ in all statistical models (for T vs. C, OR = 1.16, 95% CI = 1.10–1.23, P < 0.001; for TT + CT vs. CC: OR = 1.18, 95% CI = 1.10–1.27, P < 0.001; for TT vs. CT + CC: OR = 1.25, 95% CI = 1.13–1.37, P < 0.001; for TT vs. CC: OR = 1.35, 95% CI = 1.19–1.52, P < 0.001) (Figure 2 and Table 3).
TABLE 3.
Odds ratios and heterogeneity results for the 4 genetic models of the MTHFR C677T and A1298C for SZ.
| MTHFR | Comparison model | OR (95% CI) | P OR | Heterogeneity |
|||
| Q within | P-value | I2 (%) | |||||
| MTHFRC677T | All studies | T vs. C | 1.16(1.10–1.23) | <0.001 | 116.30 | <0.001 | 60.4 |
| TT + CT vs. CC | 1.18(1.10–1.27) | <0.001 | 93.38 | <0.001 | 50.7 | ||
| TT vs. CT + CC | 1.25(1.13–1.37) | <0.001 | 80.44 | 0.001 | 42.8 | ||
| TT vs. CC | 1.35(1.19–1.52) | <0.001 | 103.78 | <0.001 | 55.7 | ||
| Asian | T vs. C | 1.19(1.11–1.29) | <0.001 | 56.46 | <0.001 | 57.5 | |
| TT + CT vs. CC | 1.22(1.10–1.35) | <0.001 | 48.36 | 0.002 | 50.4 | ||
| TT vs. CT + CC | 1.31(1.16–1.48) | <0.001 | 41.63 | 0.014 | 42.3 | ||
| TT vs. CC | 1.46(1.24–1.72) | <0.001 | 57.48 | <0.001 | 58.2 | ||
| Caucasian | T vs. C | 1.09(1.01–1.17) | 0.036 | 35.29 | 0.013 | 46.2 | |
| TT + CT vs. CC | 1.11(1.01–1.21) | 0.034 | 28.48 | 0.075 | 33.3 | ||
| TT vs. CT + CC | 1.12(0.97–1.29) | 0.132 | 27.76 | 0.088 | 31.6 | ||
| TT vs. CC | 1.16(0.98–1.37) | 0.082 | 32.08 | 0.031 | 40.8 | ||
| African | T vs. C | 2.58(1.45–4.57) | 0.001 | 1.36 | 0.243 | 26.6 | |
| TT + CT vs. CC | 2.37(1.00–5.64) | 0.050 | 1.84 | 0.175 | 45.6 | ||
| TT vs. CT + CC | 4.59(1.77–11.92) | 0.002 | 0.10 | 0.756 | 0 | ||
| TT vs. CC | 5.81(1.20–15.32) | <0.001 | 0.31 | <0.001 | 0 | ||
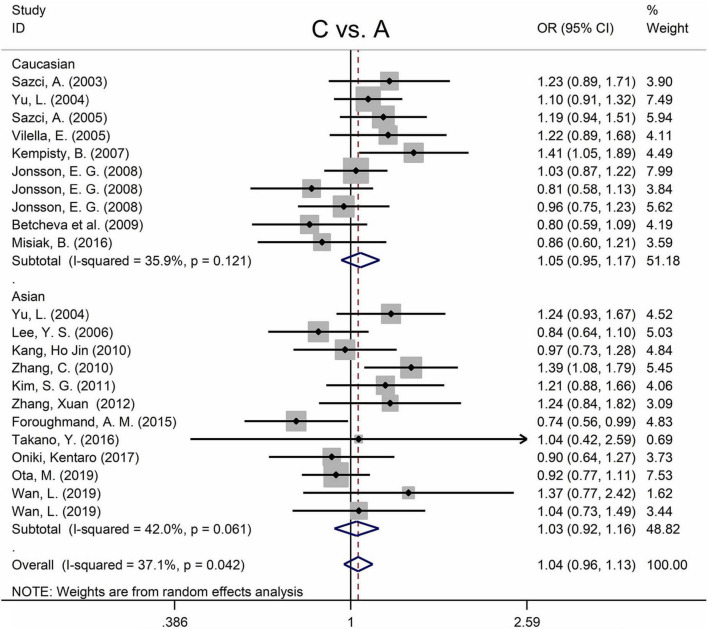
| MTHFR A1298C |
All studies | C vs. A | 1.04(0.96–1.13) | 0.305 | 33.40 | 0.042 | 37.1 |
| CC + AC vs. AA | 1.06(0.98–1.15) | 0.165 | 23.60 | 0.313 | 11.0 | ||
| CC vs. AC + AA | 1.05(0.88–1.25) | 0.622 | 31.24 | 0.07 | 32.8 | ||
| CC vs. AA | 1.08(0.89–1.29) | 0.438 | 31.32 | 0.069 | 32.9 | ||
| Caucasian | C vs. A | 1.05(0.95–1.17) | 0.327 | 14.04 | 0.121 | 35.9 | |
| CC + AC vs. AA | 1.07(0.95–1.20) | 0.289 | 11.04 | 0.273 | 18.5 | ||
| CC vs. AC + AA | 1.09(0.87–1.37) | 0.434 | 13.54 | 0.14 | 33.5 | ||
| CC vs. AA | 1.12(0.88–1.44) | 0.357 | 14.85 | 0.095 | 39.4 | ||
| Asian | C vs. A | 1.03(0.92–1.16) | 0.602 | 18.98 | 0.061 | 42.0 | |
| CC + AC vs. AA | 1.05(0.94–1.18) | 0.418 | 12.42 | 0.333 | 11.5 | ||
| CC vs. AC + AA | 1.00(0.74–1.34) | 0.981 | 16.80 | 0.114 | 34.5 | ||
| CC vs. AA | 1.02(0.77–1.37) | 0.870 | 15.70 | 0.153 | 29.9 | ||
FIGURE 2.
Forest plots for the associations between MTHFR C677T polymorphisms and SZ for the allele model with random effect model.
An ethnic subgroup analysis revealed a substantial association between MTHFR C677T polymorphism and SZ among Asian populations (for T vs. C: OR = 1.19, 95% CI = 1.11–1.29, P < 0.001; for TT + CT vs. CC: OR = 1.22, 95% CI = 1.10–1.35, P < 0.001; for TT vs. CT + CC: OR = 1.31, 95% CI = 1.16–1.48, P < 0.001; for TT vs. CC: OR = 1.46, 95% CI = 1.24–1.72, P < 0.001); in Caucasian populations, a significant association was found with the allele model (for T vs. C: OR = 1.09, 95% Cl = 1.01–1.17, P = 0.036) and the dominant model (for TT + CT vs. CC: OR = 1.11, 95% Cl = 1.01–1.21, P = 0.034); in African populations, there was a significant association with the allele model (for T vs. C: OR = 2.58, 95% Cl = 1.45–4.57, P = 0.001), the recessive model (TT vs. CT + CC: OR = 4.59, 95% CI = 1.77–11.92, P = 0.002) and the homozygote model (for TT vs. CC: OR = 5.81, 95% Cl = 1.20–15.32, P < 0.001). All these findings are summarized in Table 3. Subgroup analysis reveals that the association between MTHFR C677T polymorphism and SZ exists in Asian (all genetic models) and African populations (allele models, recessive models, and homozygous models) but not in Caucasian (only allele models and dominant models).
The MTHFR A1298C polymorphism was not statistically correlated with SZ in all models (Figure 3 and Table 3). Moreover, subgroup analysis revealed no correlation between the MTHFR A1298C polymorphism and SZ in Asian or Caucasian populations (Figure 3 and Table 3). African populations were not included in the study because of the small number of studies.
FIGURE 3.
Forest plots for the associations between MTHFR A1298C polymorphisms and SZ for the allele model with random effect model.
There were two articles not in Hardy–Weinberg equilibrium (37, 38) (Tables 1, 2). Sensitivity analysis revealed that the overall association between MTHFR C677T polymorphism and SZ remained unchanged after omitting these two samples from the meta-analysis (for T vs. C: OR = 1.17, 95% CI = 1.10–1.24, P < 0.001, Supplementary Figure 6; for TT + CT vs. CC: OR = 1.18, 95% CI = 1.10–1.28, P < 0.001; for TT vs. CT + CC: OR = 1.25, 95% CI = 1.14–1.38, P < 0.001; for TT vs. CC: OR = 1.35, 95% CI = 1.20–1.53, P < 0.001). Sensitivity analysis for the MTHFR A1298C polymorphism revealed that excluding Lee et al. (38) had no impact on the conclusion of the meta-analysis (Supplementary Figure 7).
Association between the methylenetetrahydrofolatereductase C677T/A1298C polymorphisms and major depression
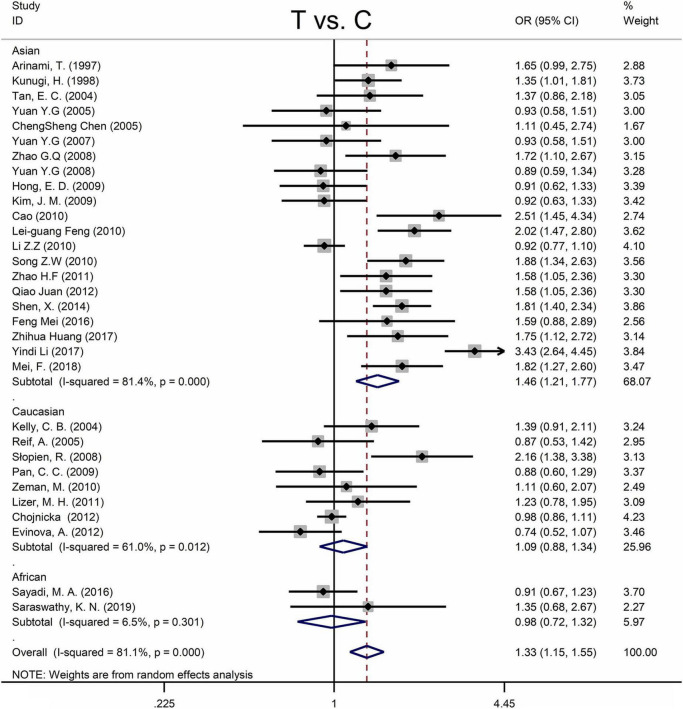
Table 4 shows the main results as well as the heterogeneity test. MTHFR C677T polymorphism was shown to be highly associated with an increased risk of developing MD in all statistical models (for T vs. C: OR = 1.33, 95% CI = 1.15–1.55, P < 0.001; for TT + CT vs. CC: OR = 1.35, 95% CI = 1.08–1.70, P = 0.009; for TT vs. CT + CC: OR = 1.58, 95% CI = 1.28–1.95, P < 0.001; for TT vs. CC: OR = 1.66, 95% CI = 1.31–2.11, P < 0.001) (Figure 4 and Table 4).
TABLE 4.
Odds ratios and heterogeneity results for the 4 genetic models of the MTHFR C677T and A1298C for MD.
| MTHFR | Comparison model | OR (95% CI) | P OR | Heterogeneity |
|||
| Q within | P-value | I2 (%) | |||||
| MTHFRC677T | All studies | T vs. C | 1.33(1.15–1.55) | <0.001 | 159.05 | <0.001 | 81.1 |
| TT + CT vs. CC | 1.35(1.08–1.70) | 0.009 | 183.95 | <0.001 | 83.7 | ||
| TT vs. CT + CC | 1.58(1.28–1.95) | <0.001 | 75.2 | <0.001 | 60.1 | ||
| TT vs. CC | 1.66(1.31–2.11) | <0.001 | 80.47 | <0.001 | 62.7 | ||
| Asian | T vs. C | 1.46(1.21–1.77) | <0.001 | 107.45 | <0.001 | 81.4 | |
| TT + CT vs. CC | 1.52(1.11–2.08) | 0.009 | 135.03 | <0.001 | 85.2 | ||
| TT vs. CT + CC | 1.75(1.34–2.28) | <0.001 | 54.54 | <0.001 | 63.3 | ||
| TT vs. CC | 1.89(1.40–2.57) | <0.001 | 56.63 | <0.001 | 64.7 | ||
| Caucasian | T vs. C | 1.09(0.88–1.34) | 0.445 | 17.97 | 0.012 | 61.0 | |
| TT + CT vs. CC | 1.08(0.81–1.44) | 0.616 | 18.02 | 0.012 | 61.2 | ||
| TT vs. CT + CC | 1.07(0.86–1.34) | 0.527 | 7.11 | 0.417 | 1.6 | ||
| TT vs. CC | 1.20(0.83–1.73) | 0.337 | 11.59 | 0.115 | 39.6 | ||
| African | T vs. C | 0.98(0.72–1.32) | 0.879 | 1.07 | 0.301 | 6.5 | |
| TT + CT vs. CC | 0.91(0.52–1.59) | 0.735 | 1.95 | 0.162 | 48.8 | ||
| TT vs. CT + CC | 1.57(0.80–3.09) | 0.189 | 0.07 | 0.788 | 0 | ||
| TT vs. CC | 1.30(0.65–2.63) | 0.460 | 0.18 | 0.669 | 0 | ||
| MTHFRA1298C | All studies |
C vs. A | 1.44(0.84–2.48) | 0.191 | 35.80 | <0.001 | 88.8 |
| CC + AC vs. AA | 1.42(0.77–2.61) | 0.263 | 26.32 | <0.001 | 84.8 | ||
| CC vs. AC + AA | 2.63(1.49–4.65) | 0.001 | 7.55 | 0.109 | 47 | ||
| CC vs. AA | 2.83(1.39–5.77) | 0.004 | 10.27 | 0.036 | 61 | ||
| Caucasian | C vs. A | 1.40(1.08–1.82) | 0.011 | 1.83 | 0.4 | 0 | |
| CC + AC vs. AA | 1.39(0.97–1.98) | 0.073 | 1.74 | 0.418 | 0 | ||
| CC vs. AC + AA | 2.14(1.23–3.71) | 0.007 | 1.68 | 0.433 | 0 | ||
| CC vs. AA | 2.36(1.31–4.26) | 0.004 | 1.58 | 0.454 | 0 | ||
| Asian | C vs. A | 1.61(0.42–6.17) | 0.484 | 23.67 | <0.001 | 95.8 | |
| CC + AC vs. AA | 1.61(0.39–6.68) | 0.513 | 20.23 | <0.001 | 95.1 | ||
| CC vs. AC + AA | 2.93(0.76–11.29) | 0.118 | 2.16 | 0.142 | 53.7 | ||
| CC vs. AA | 3.13(0.53–18.66) | 0.210 | 3.34 | 0.068 | 70 | ||
FIGURE 4.
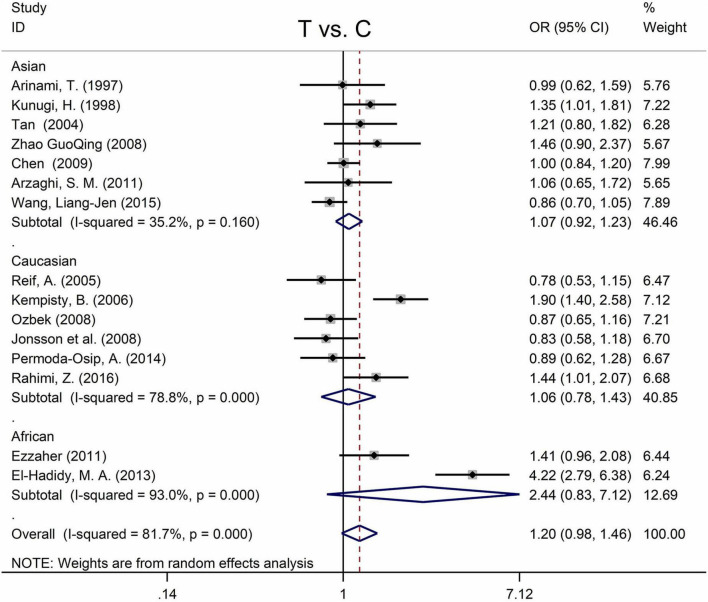
Forest plots for the associations between MTHFR C677T polymorphisms and MD for the allele model with random effect model.
Subgroup analysis by ethnicity revealed a substantial correlation between the MTHFR C677T polymorphism and MD in Asian populations (for T vs. C: OR = 1.46, 95% CI = 1.21–1.77, P < 0.001; for TT + CT vs. CC: OR = 1.52, 95% CI = 1.11–2.08, P = 0.009; for TT vs. CT + CC: OR = 1.75, 95% CI = 1.34–2.28, P < 0.001; for TT vs. CC: OR = 1.89, 95% CI = 1.40–2.57, P < 0.001), but not in Caucasian and African populations (Figure 4 and Table 4).
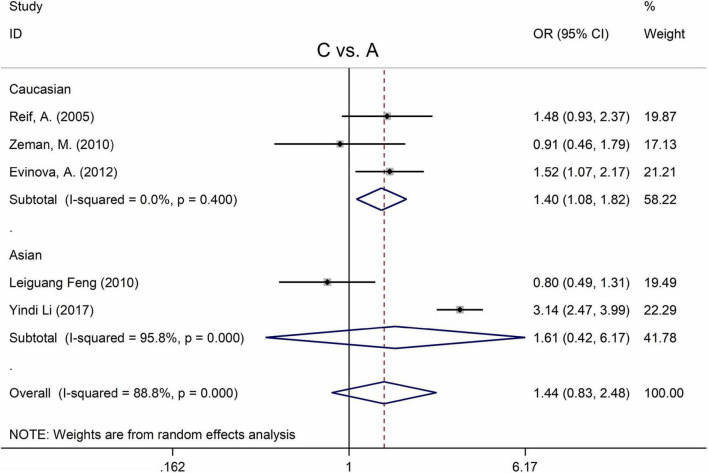
The MTHFR A1298C polymorphism was found to be highly associated with MD in the recessive model (for CC vs. AC + AA: OR = 2.63, 95% CI: 1.49–4.65, P = 0.001) and the homozygote model (for CC vs. AA: OR = 2.83, 95% Cl = 1.39–5.77, P = 0.004) (Table 4). Moreover, subgroup analysis demonstrated a positive correlation between the MTHFR A1298C polymorphism and MD in the Caucasian population (for C vs. A: OR = 1.40, 95% CI = 1.08–1.82, P = 0.011; for CC vs. AC + AA: OR = 2.14, 95% CI = 1.23–3.71, P = 0.007; for CC vs. AA: OR = 2.36, 95% CI = 1.31–4.26, P = 0.004) (Figure 5 and Table 4). Nonetheless, there was no statistical correlation between A1298C polymorphism and MD in Asian populations (Figure 5 and Table 4). Subgroup analysis shows that the correlation between MTHFR C677T polymorphism and MD exists in the Asian population (all genetic models) but not in Caucasian and African populations.
FIGURE 5.
Forest plots for the associations between MTHFR A1298C polymorphisms and MD for the allele model with random effect model.
Four articles were not found in Hardy–Weinberg equilibrium (71, 84, 85, 95) (Tables 1, 2). Sensitivity analysis revealed that the overall correlation between MTHFR C677T polymorphism and MD remained unchanged after eliminating these data from the meta-analysis (Supplementary Figure 8). Sensitivity analyses for MTHFR A1298C polymorphism revealed that excluding Reif A. et al. (71) and Li et al. (95) resulted in a decreasing statistical correlation with MD; nonetheless, all statistical models revealed that MTHFR A1298C polymorphism was not significantly correlated with MD (Supplementary Figure 9).
Association between the methylenetetrahydrofolatereductase C677T/A1298C polymorphisms and bipolar disorder
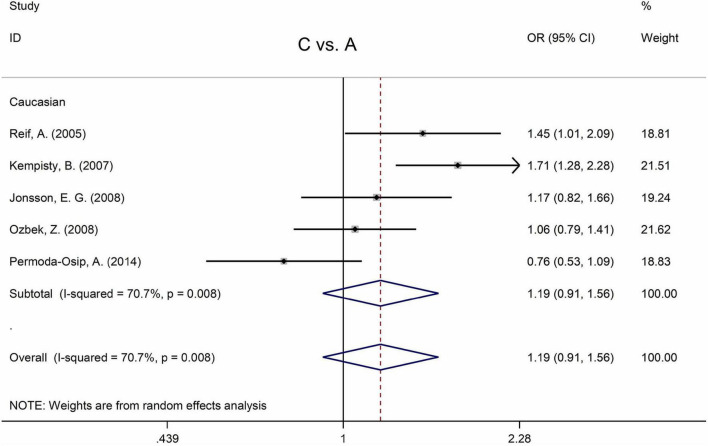
Table 5 displays the main results and the heterogeneity test. There was a marginal correlation between the MTHFR C677T polymorphism and BPD in the recessive model (for TT vs. CT + CC: OR = 1.31, 95% CI: 1.03–1.67, P = 0.028) and the homozygote model (for TT vs. CC: OR = 1.40, 95% Cl = 1.00–1.94, P = 0.049) (Table 5). Moreover, subgroup analysis indicated no statistical correlation between the MTHFR C677T polymorphism and BPD in Asian, African, or Caucasian populations (Figure 6 and Table 5). Additionally, all models revealed that the MTHFR A1298C polymorphism was not statistically correlated with BPD (Figure 7 and Table 5).
TABLE 5.
Odds ratios and heterogeneity results for the 4 genetic models of the MTHFR C677T and A1298C for BPD.
| MTHFR | Comparison model | OR (95% CI) | P OR | Heterogeneity |
|||
| Q within | P-value | I2 (%) | |||||
| MTHFRC677T | All studies | T vs. C | 1.20(0.98–1.46) | 0.073 | 76.32 | <0.001 | 81.7 |
| TT + CT vs. CC | 1.21(0.93–1.57) | 0.161 | 74.99 | <0.001 | 81.3 | ||
| TT vs. CT + CC | 1.31(1.03–1.67) | 0.028 | 22.71 | 0.065 | 38.4 | ||
| TT vs. CC | 1.40(1.00–1.94) | 0.049 | 36.79 | 0.001 | 61.9 | ||
| Asian | T vs. C | 1.07(0.92–1.24) | 0.399 | 9.26 | 0.160 | 35.2 | |
| TT + CT vs. CC | 1.01(0.83–1.23) | 0.926 | 8.6 | 0.197 | 30.2 | ||
| TT vs. CT + CC | 1.23(0.99–1.54) | 0.063 | 4.61 | 0.60 | 0 | ||
| TT vs. CC | 1.17(0.91–1.49) | 0.216 | 6.03 | 0.42 | 0.4 | ||
| Caucasian | T vs. C | 1.06(0.78–1.43) | 0.711 | 23.61 | <0.001 | 78.8 | |
| TT + CT vs. CC | 1.03(0.73–1.46) | 0.862 | 18.96 | 0.002 | 73.6 | ||
| TT vs. CT + CC | 1.20(0.73–1.98) | 0.468 | 11.67 | 0.04 | 57.2 | ||
| TT vs. CC | 1.19(0.65–2.18) | 0.566 | 15.96 | 0.007 | 68.7 | ||
| African | T vs. C | 2.44(0.83–7.12) | 0.104 | 14.29 | <0.001 | 93.0 | |
| TT + CT vs. CC | 3.09(0.79–12.18) | 0.106 | 14.15 | <0.001 | 92.9 | ||
| TT vs. CT + CC | 2.50(0.87–7.19) | 0.09 | 2.59 | 0.107 | 61.4 | ||
| TT vs. CC | 3.90(0.81–18.69) | 0.089 | 5.29 | 0.021 | 81.1 | ||
| MTHFRA1298C | All studies (Caucasian) |
C vs. A | 1.19(0.91–1.56) | 0.208 | 13.67 | 0.008 | 70.7 |
| CC + AC vs. AA | 1.19(0.91–1.56) | 0.200 | 7.54 | 0.110 | 0.110 | ||
| CC vs. AC + AA | 1.50(0.81–2.77) | 0.200 | 13.66 | 0.008 | 70.7 | ||
| CC vs. AA | 1.58(0.79–3.16) | 0.200 | 15.85 | 0.003 | 74.8 | ||
FIGURE 6.
Forest plots for the associations between MTHFR C677T polymorphisms and BPD for the allele model with random effect model.
FIGURE 7.
Forest plots for the associations between MTHFR A1298C polymorphisms and BPD for the allele model with random effect model.
Only one study was not in Hardy Weinberg equilibrium (71) (Table 2), and there was no statistical association between A1298C polymorphism and BPD after removing this study (Supplementary Figure 10).
Association between the methylenetetrahydrofolatereductase C677T/A1298C polymorphisms and psychiatric disorders
Significant publication biases were found when all diseases were considered (Supplementary Figure 5 and Supplementary Table 2). Therefore, analyses between MTHFR C677T and mental disorders were unsuitable here. However, the main results and the heterogeneity tests between MTHFR C677T and mental disorders were shown in Supplementary Table 1. Furthermore, the forest plots indicated that MTHFR C677T was strongly associated with psychiatric disorders, and sensitivity analysis did not affect the results (Supplementary Figures 1, 2).
Most studies were not in Hardy–Weinberg equilibrium when all diseases were considered. Moreover, analysis between MTHFR A1298C and psychiatric disorders was also unsuitable. Significant correlations were detected between the MTHFR A1298C polymorphism and psychiatric disorders (Supplementary Figure 3). However, sensitivity analysis revealed that excluding did change the conclusion (Supplementary Figure 4).
Publication bias
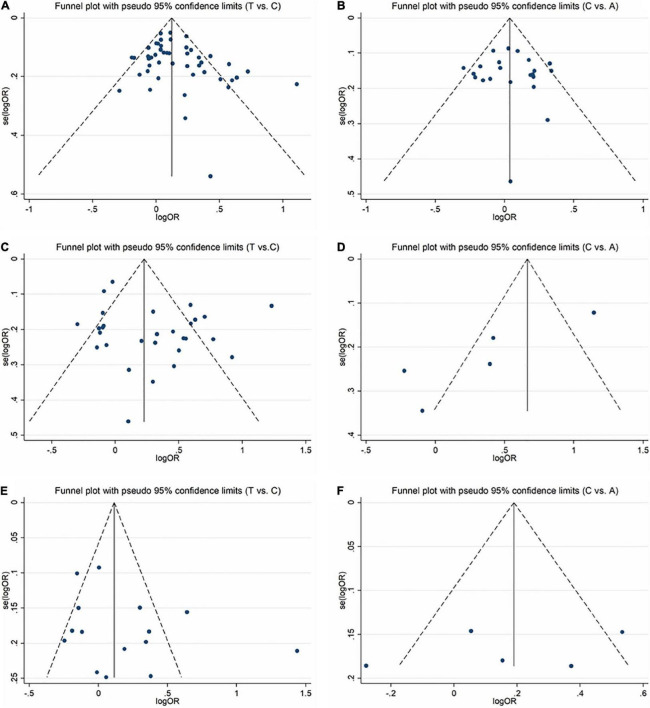
In order to evaluate publication bias, we used formal statistical methods (Egger’s regression test). Table 6 and Figure 8 presented the funnel plots for the meta-analysis. We observed that for SZ, no publication bias could be observed except in the dominant model (TT + CT vs. CC, PEgger = 0.01). The Egger’s test results for MD were substantial in two genetic models of overall populations (allele model: C vs. A, PEgger = 0.03; homozygote model: CC vs. AA, PEgger = 0.02). And there was no publication bias for BPD. Publication bias may correlate to the editor’s decision for publication. However, it is common that only the positive results are published, and negative findings are unavailable. So, we could not exclude this kind of possibility.
TABLE 6.
Publication bias risk in this meta-analysis.
| Disease | MTHFR | P Egger | 95% CL | |
| Schizophrenia | C677T | T vs. C | 0.05 | 0.02-2.10 |
| TT + CT vs. CC | 0.01 | 0.27-2.08 | ||
| TT vs. CT + CC | 0.24 | −0.34-1.32 | ||
| TT vs. CC | 0.06 | −0.05-1.86 | ||
| A1298C | C vs. A | 0.73 | −1.60-2.25 | |
| CC + AC vs. AA | 0.66 | −2.03-1.31 | ||
| CC vs. AC + AA | 0.09 | −0.17-2.39 | ||
| CC vs. AA | 0.14 | −0.35-2.32 | ||
| Major depression | C677T | T vs. C | 0.18 | −0.64-3.33 |
| TT + CT vs. CC | 0.35 | −1.09-3.00 | ||
| TT vs. CT + CC | 0.40 | −0.80-1.97 | ||
| TT vs. CC | 0.09 | −0.21-2.52 | ||
| A1298C | C vs. A | 0.03 | −12.82–1.28 | |
| CC + AC vs. AA | 0.08 | −13.00-1.10 | ||
| CC vs. AC + AA | 0.05 | −4.48-0.02 | ||
| CC vs. AA | 0.02 | −4.78–0.81 | ||
| Bipolar disorder | C677T | T vs. C | 0.19 | −1.45-6.66 |
| TT + CT vs. CC | 0.21 | −1.62-6.66 | ||
| TT vs. CT + CC | 0.31 | −0.95-2.81 | ||
| TT vs. CC | 0.18 | −0.82-4.02 | ||
| A1298C | C vs. A | 0.54 | −30.72-19.79 | |
| CC + AC vs. AA | 0.54 | −17.02-10.90 | ||
| CC vs. AC + AA | 0.75 | −24.98-31.11 | ||
| CC vs. AA | 0.82 | −26.75-31.41 | ||
FIGURE 8.
Funnel plots for assessing the publication bias risk in this meta-analysis. (A) Funnel plot for allele contrast (T vs. C) of C677T polymorphism in SZ. (B) Funnel plot for allele contrast (C vs. A) of A1298C polymorphism in SZ. (C) Funnel plot for allele contrast (T vs. C) of C677T polymorphism in MD. (D) Funnel plot for allele contrast (C vs. A) of A1298C polymorphism in MD. (E) Funnel plot for allele contrast (T vs. C) of C677T polymorphism in BPD. (F) Funnel plot for allele contrast (C vs. A) of A1298C polymorphism in BPD.
Discussion
A mental disorder is a neurological disease with complicated etiology, which may be closely related to genetic factors. A great number of research on the susceptibility to mental illnesses (including SZ, MD, and BPD) have been undertaken using MTHFR gene polymorphism. Some studies supported the susceptibility variation of MTHFR in mental diseases (27, 31, 70, 96, 105, 69), whereas other studies showed a negative correlation (28, 29, 33, 46, 102, 46). These variations might be due to the type of disease, ethnicity, or sample size. Our meta-analysis incorporates all previous research and provides more reliable evidence for the association between mental illness and MTHFR SNPs.
For Sz, our meta-analysis found a substantial association between MTHFR C677T polymorphism and higher incidence of SZ, which is consistent with research by Hu et al. (22) and Peerbooms et al. (23). In addition, we found that MTHFR A1298C polymorphism was not correlated with increased SZ risk, which is consistent with Peerbooms et al. (23). However, Hu et al. (22) discovered a marginal correlation between the MTHFR A1298C polymorphism and SZ. The inconsistency may be mainly owing to the limited sample size in the previous meta-analyses. For MTHFR C677T and A1298C, the sensitivity analysis has no substantial change to the results. As a result, the study’s findings are relatively consistent.
For MD, our meta-analysis’s results showed a significant correlation between MTHFR C677T polymorphism and increased risk of MD. The meta-analysis of Wu et al. (107) supports our view, whereas Gaysina et al. (108) and Peerbooms et al. (23) discovered no association between the C677T and MD. These discrepancies might be due to ethnicity, sample size, and other factors. Sensitivity analysis showed no change in the overall correlation between C677T polymorphism and MD. Also, we found a correlation between the A1298C polymorphism and MD (in recessive models and homozygous models). However, after excluding two studies not in Hardy–Weinberg equilibrium (38, 95), we discovered that A1298C polymorphism was not correlated with depression. We suspect that the reason for this is the insufficient number of studies included.
For BPD, meta-analysis reveals that MTHFR C677T polymorphism is weakly related to the occurrence of diseases (in recessive models and homozygous models). The meta-analysis of Hu et al. (22) found a marginal connection of C677T with an elevated risk of BPD (the recessive model), but some studies (21, 109, 110) found no associations. Different numbers of studies included may cause the inconsistency. Our meta-analysis included all current research, providing more reliable evidence for the association between MTHFR C677T polymorphism and BPD. As for A1298C, we only found studies in Caucasian people, and we did not find any association in these studies. The sensitivity analysis has no change to the results. Therefore, the results of this study are generally robust.
Many researchers have discovered that MTHFR is closely related to cognitive function, such as verbal fluency, visual-motor coordination, attention selectivity, and distribution (111–113). MTHFR polymorphism may also cause central nerve injury and microvascular injury, affect the synthesis of central neurotransmitters and the methylation of central neural system amines and phospholipids, and eventually lead to various mental diseases (114). All these impairments are not specific to one disease; therefore, we guess MTHFR may work on the common pathogenesis of these psychiatric disorders.
Some limitations of this meta-analysis should be considered when interpreting the findings. Firstly, we can only search for English and Chinese articles with some language limitations. Second, publication bias cannot be ignored in the current study since Egger test findings are substantial in several SZ and MD genetic models. It may correlate to the editor’s decision for publication and so on. However, it is common that only the positive results are published, and negative findings are unavailable. And we could not exclude this kind of possibility. Furthermore, the number of articles on A1298C polymorphism with MD are insufficient to provide conclusive evidence. More original research is required to validate our results. Despite some limitations, our current research also has some value. First of all, our meta-analysis includes a large sample size, which can reduce errors. Secondly, we fully considered and analyzed the impact of race on the disease.
Conclusion
Our meta-analysis findings demonstrate that MTHFR C677T polymorphism increases the risk of schizophrenia and severe depression in the general population, and a marginal correlation of MTHFR C677T with a higher risk of bipolar disorder has also been reported for the recessive model. More original research and a bigger sample size are required to validate our results. Nevertheless, the findings of our meta-analysis imply that MTHFR may play a significant role in the common pathogenesis of mental illness and that its variation may be involved in controlling the expression of genes associated with it. It would help in the early diagnosis and treatment of related mental disorders. Moreover, studies on risk factor analysis could be performed on psychiatric disorders to better prevent these mental health problems.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Y-XZ: conceptualization, software, data curation, and writing – original draft preparation. DH: conceptualization, methodology, and funding acquisition. L-PY: data curation and validation. CG: visualization and investigation. C-CC: software and validation. Z-YG: writing – reviewing and editing. H-MS: project administration and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81803857) and Autonomous Subject of Beijing University of Chinese Medicine (Grant No. 2018-JYBZZ-XJSJJ002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.976428/full#supplementary-material
References
- 1.Barnett R. Depression. Lancet (London, England). (2019) 393:2113. 10.1016/s0140-6736(19)31151-1 [DOI] [PubMed] [Google Scholar]
- 2.Adorjan K, Falkai P. Premature mortality, causes of death, and mental disorders. Lancet (London, England). (2019) 394:1784–6. 10.1016/s0140-6736(19)32521-8 [DOI] [PubMed] [Google Scholar]
- 3.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. (2003) 54:515–28. 10.1016/s0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- 4.Malhi GS, Mann JJ. Depression. Lancet (London, England). (2018) 392:2299–312. 10.1016/s0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- 5.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet (London, England). (2016) 388:86–97. 10.1016/s0140-6736(15)01121-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennison CA, Legge SE, Pardinas AF, Walters JTR. Genome-wide association studies in schizophrenia: Recent advances, challenges, and future perspective. Schizophr Res. (2019) 217:4–12. 10.1016/j.schres.2019.10.048 [DOI] [PubMed] [Google Scholar]
- 7.Schwab SG, Wildenauer DB. Genetics of psychiatric disorders in the GWAS era: An update on schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2013) 263(Suppl. 2):S147–54. 10.1007/s00406-013-0450-z [DOI] [PubMed] [Google Scholar]
- 8.Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci U S A. (1998) 95:13217–20. 10.1073/pnas.95.22.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Födinger M, Hörl WH, Sunder-Plassmann G. Molecular biology of 5,10-methylenetetrahydrofolate reductase. J Nephrol. (2000) 13:20–33. [PubMed] [Google Scholar]
- 10.Wan L, Li Y, Zhang Z, Sun Z, He Y, Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. (2018) 8:242. 10.1038/s41398-018-0276-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. (1995) 10:111–3. 10.1038/ng0595-111 [DOI] [PubMed] [Google Scholar]
- 12.Lewis SJ, Lawlor DA, Smith GD, Araya R, Timpson N, Day INM, et al. The thermolabile variant of Mthfr is associated with depression in the British Women’s heart and health study and a meta-analysis. Mol Psychiatry. (2006) 11:352–60. 10.1038/sj.mp4001790 [DOI] [PubMed] [Google Scholar]
- 13.Lewis SJ, Zammit S, Gunnell D, Smith GD. A meta-analysis of the MTHFR C677t polymorphism and schizophrenia risk. Am J Med Genet Part B, Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2005) 135b:2–4. 10.1002/ajmg.b.30170 [DOI] [PubMed] [Google Scholar]
- 14.van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? Am J Hum Genet. (1998) 62:1044–51. 10.1086/301825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism: Epidemiology, metabolism and the associated diseases. Eur J Med Genet. (2015) 58:1–10. 10.1016/j.ejmg.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Goyette P, Pai A, Milos R, Frosst P, Tran P, Chen Z, et al. Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome Off J Int Mamm Genome Soc. (1998) 9:652–6. 10.1007/s003359900838 [DOI] [PubMed] [Google Scholar]
- 17.Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H. One-carbon metabolism and schizophrenia: Current challenges and future directions. Trends Mol Med. (2009) 15:562–70. 10.1016/j.molmed.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr Rev. (2008) 66:250–5. 10.1111/j.1753-4887.2008.00031.x [DOI] [PubMed] [Google Scholar]
- 19.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. (2005) 10:40–68; image 5. 10.1038/sj.mp.4001558 [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, et al. Brain surface contraction mapped in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Mol Psychiatry. (2009) 14:976–86. 10.1038/mp.2008.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen-Woods S, Craig I, Gaysina D, Gray J, Gunasinghe C, Craddock N, et al. The bipolar association case-control study (BACCS) and meta-analysis: No association with the 5,10-methylenetetrahydrofolate reductase gene and bipolar disorder. Am J Med Genet Part B Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2010) 153b:1298–304. 10.1002/ajmg.b.31101 [DOI] [PubMed] [Google Scholar]
- 22.Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: An updated meta-analysis. J Neural Transmission (Vienna, Austria 1996). (2015) 122:307–20. 10.1007/s00702-014-1261-8 [DOI] [PubMed] [Google Scholar]
- 23.Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L. MTHFR in Psychiatry Group et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain Behav Immun. (2011) 25:1530–43. 10.1016/j.bbi.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. (2014) 20:123–9. 10.1111/1469-0691.12494 [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clin Trials. (2015) 45(Pt A):139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, Graphical Test. BMJ (Clin Res Ed). (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet. (1997) 74:526–8. 10.1002/(sici)1096-8628(19970919)74:53.0.co;2-e [DOI] [PubMed] [Google Scholar]
- 28.Kunugi H, Fukuda R, Hattori M, Kato T, Tatsumi M, Sakai T, et al. C677t polymorphism in methylenetetrahydrofolate reductase gene and psychoses. Mol Psychiatry. (1998) 3:435–7. 10.1038/sj.mp.4000390 [DOI] [PubMed] [Google Scholar]
- 29.Virgos C, Martorell L, Simó JM, Valero J, Figuera L, Joven J, et al. Plasma homocysteine and the methylenetetrahydrofolate reductase C677t gene variant: Lack of association with schizophrenia. Neuroreport. (1999) 10:2035–8. 10.1097/00001756-199907130-00008 [DOI] [PubMed] [Google Scholar]
- 30.Joober R, Benkelfat C, Lal S, Bloom D, Labelle A, Lalonde P, et al. Association between the Methylenetetrahydrofolate reductase 677c–>T missense mutation and schizophrenia. Mol Psychiatry. (2000) 5:323–6. 10.1038/sj.mp.4000724 [DOI] [PubMed] [Google Scholar]
- 31.Sazci A, Ergül E, Güzelhan Y, Kaya G, Kara I. Methylenetetrahydrofolate reductase gene polymorphisms in patients with schizophrenia. Brain Res Mol Brain Res. (2003) 117:104–7. 10.1016/s0169-328x(03)00327-9 [DOI] [PubMed] [Google Scholar]
- 32.Tan EC, Chong SA, Lim LC, Chan AO, Teo YY, Tan CH, et al. Genetic analysis of the thermolabilemethylenetetrahydrofolate reductase variant in schizophrenia and mood disorders. Psychiatric Genet. (2004) 14:227–31. 10.1097/00041444-200412000-00012 [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Li T, Robertson Z, Dean J, Gu NF, Feng GY, et al. No association between polymorphisms of methylenetetrahydrofolate reductase gene and schizophrenia in both chinese and scottish populations. Mol Psychiatry. (2004) 9:1063–5. 10.1038/sj.mp.4001566 [DOI] [PubMed] [Google Scholar]
- 34.Sazci A, Ergul E, Kucukali I, Kara I, Kaya G. Association of the C677t and A1298c polymorphisms of methylenetetrahydrofolate reductase gene with schizophrenia: Association is significant in men but not in women. Progr Neuro Psychopharmacol Biol Psychiatry. (2005) 29:1113–23. 10.1016/j.pnpbp.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 35.Vilella E, Virgos C, Murphy M, Martorell L, Valero J, Simó JM, et al. Further evidence that hyperhomocysteinemia and methylenetetrahydrofolate reductase C677t and A1289c polymorphisms are not risk factors for schizophrenia. Progr Neuro Psychopharmacol Biol Psychiatry. (2005) 29:1169–74. 10.1016/j.pnpbp.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 36.Kempisty B, Mostowska A, Gorska I, Luczak M, Czerski P, Szczepankiewicz A, et al. Association of 677c>T polymorphism of methylenetetrahydrofolate reductase (Mthfr) gene with bipolar disorder and schizophrenia. Neurosci Lett. (2006) 400:267–71. 10.1016/j.neulet.2006.02.055 [DOI] [PubMed] [Google Scholar]
- 37.Philibert R, Gunter T, Hollenbeck N, Adams WJ, Bohle P, Packer H, et al. No association of the C677t methylenetetrahydrofolate reductase polymorphism with schizophrenia. Psychiatric Genet. (2006) 16:221–3. 10.1097/01.ypg.0000242192.28526.fa [DOI] [PubMed] [Google Scholar]
- 38.Lee YS, Han DH, Jeon CM, Lyoo IK, Na C, Chae SL, et al. Serum Homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients. Neuroreport. (2006) 17:743–6. 10.1097/01.wnr.0000215777.99473.52 [DOI] [PubMed] [Google Scholar]
- 39.Yang D-Y, Lu X-B, Li Y-H, Tong Z-S, Fu Y, Yang M-X, et al. The association of methylenetetrahydrofolate reductase gene polymorphism, plasma homocysteine level and first-episode schizophrenia [in Chinese]. Chin Hehavioral Med Sci. (2007) 16:901–2. 10.3760/cma.j.issn.1674-6554.2007.10.013 30704229 [DOI] [Google Scholar]
- 40.Jonsson EG, Larsson K, Vares M, Hansen T, Wang AG, Djurovic S, et al. Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: An association study. Am J Med Genet Part B Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2008) 147B:976–82. 10.1002/ajmg.b.30671 [DOI] [PubMed] [Google Scholar]
- 41.Muntjewerff J-W. Meta-analysis of plasma homocysteine and methylenetetrahydrofolate reductase (MTHFR) polymorphisms in schizophrenia. Biol Psychiatry. (2008) 63:134S–S. [DOI] [PubMed] [Google Scholar]
- 42.Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, et al. MTHFR 677c –> T genotype disrupts prefrontal function in schizophrenia through an interaction with Comt 158val –> Met. Proc Natl Acad Sci U S A. (2008) 105:17573–8. 10.1073/pnas.0803727105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng LG, Song ZW, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677t gene variant with schizophrenia: A chinese han population-based case-control study. Psychiatry Res. (2009) 168:205–8. 10.1016/j.psychres.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 44.Betcheva ET, Mushiroda T, Takahashi A, Kubo M, Karachanak SK, Zaharieva IT, et al. Case-control association study of 59 candidate genes reveals the DRD2 SNP Rs6277 (C957t) as the only susceptibility factor for schizophrenia in the bulgarian population. J Hum Genet. (2009) 54:98–107. 10.1038/jhg.2008.14 [DOI] [PubMed] [Google Scholar]
- 45.García-Miss MR, Pérez-Mutul J, López-Canul B, Solís-Rodríguez F, Puga-Machado L, Oxté-Cabrera A, et al. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677t polymorphism, are risk factors for schizophrenia. J Psychiatric Res. (2010) 44:441–6. 10.1016/j.jpsychires.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 46.Kang HJ, Choe BM, Kim SH, Son SR, Lee KM, Kim BG, et al. No association between functional polymorphisms in COMT and MTHFR and schizophrenia risk in Korean population. Epidemiol Health. (2010) 32:e2010011. 10.4178/epih/e2010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye X, Zhang X, Wang H. Study association of MTHFR C677t polymorphism and schizophrenia [in Chinese]. Heilongjiang Med J. (2010) 34:641–5. [Google Scholar]
- 48.Bouaziz N, Ayedi I, Sidhom O, Kallel A, Rafrafi R, Jomaa R, et al. Plasma homocysteine in schizophrenia: Determinants and clinical correlations in tunisian patients free from antipsychotics. Psychiatry Res. (2010) 179:24–9. 10.1016/j.psychres.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 49.Arzaghi SM, Hossein-Nezhad A, Shariat SV, Ghodsipour A, Shams J, Larijani B. C677t methylenetetrahydrofolate reductase (MTHFR) gene polymorphism in schizophrenia and bipolar disorder: An association study in Iranian population. Iranian J Psychiatry. (2011) 6:1–6. [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SG, Song JY, Joo EJ, Jeong SH, Kim SH, Lee KY, et al. No Association of functional polymorphisms in methlylenetetrahydrofolate reductase and the risk and minor physical anomalies of schizophrenia in Korean population. J Korean Med Sci. (2011) 26:1356–63. 10.3346/jkms.2011.26.10.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muntjewerff JW, Ophoff RA, Buizer-Voskamp JE, Strengman E, den Heijer M, Consortium G. Effects of season of birth and a common MTHFR gene variant on the risk of schizophrenia. Eur Neuropsychopharmacol J Eur College Neuropsychopharmacol. (2011) 21:300–5. 10.1016/j.euroneuro.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 52.Tsutsumi A, Glatt SJ, Kanazawa T, Kawashige S, Uenishi H, Hokyo A, et al. The genetic validation of heterogeneity in schizophrenia. Behav Brain Funct BBF. (2011) 7:43. 10.1186/1744-9081-7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Liu T, Yang Y, Deng X, Yan X, Yang K, et al. Association analysis of methylenetetrahydrofolate reductase (MTHFR) gene polymorphism and treatment-resistant schizophrenia [in Chinese]. J Clin Psychiatry. (2012) 22:293–7. [Google Scholar]
- 54.Lochman J, Plesník J, Janout V, Povová J, Míšek I, Dvořáková D, et al. Interactive effect of MTHFR and Adra2a gene polymorphisms on pathogenesis of schizophrenia. Neuro Endocrinol Lett. (2013) 34:792–7. [PubMed] [Google Scholar]
- 55.Zhang Y, Yan H, Tian L, Wang F, Lu T, Wang L, et al. Association of MTHFR C677t polymorphism with schizophrenia and its effect on episodic memory and gray matter density in patients. Behav Brain Res. (2013) 243:146–52. 10.1016/j.bbr.2012.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kontis D, Theochari E, Fryssira H, Kleisas S, Sofocleous C, Andreopoulou A, et al. Comt and MTHFR polymorphisms interaction on cognition in schizophrenia: An exploratory study. Neurosci Lett. (2013) 537:17–22. 10.1016/j.neulet.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 57.El-Hadidy MA, Abdeen HM, Abd El-Aziz SM, Al-Harrass M. MTHFR gene polymorphism and age of onset of schizophrenia and bipolar disorder. BioMed Res Int. (2014) 2014:318483. 10.1155/2014/318483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hei G, Pang L, Chen X, Zhang W, Zhu Q, Lü L, et al. [Association of serum folic acid and homocysteine levels and 5, 10-methylenetetrahydrofolate reductase gene polymorphism with schizophrenia]. Zhonghuayixuezazhi. (2014) 94:2897–901. [PubMed] [Google Scholar]
- 59.Nishi A, Numata S, Tajima A, Kinoshita M, Kikuchi K, Shimodera S, et al. Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677t polymorphism in schizophrenia. Schizophrenia Bull. (2014) 40:1154–63. 10.1093/schbul/sbt154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foroughmand AM, Galehdari H, Pooryasin A, Ajam T, Kazemi-Nezhad SR. Additive effect of MTHFR and GRIN1 genetic polymorphisms on the risk of schizophrenia. Mol Biol Res Commun. (2015) 4:33–42. [PMC free article] [PubMed] [Google Scholar]
- 61.Misiak B, Laczmanski L, Sloka NK, Szmida E, Piotrowski P, Loska O, et al. Metabolic dysregulation in first-episode schizophrenia patients with respect to genetic variation in one-carbon metabolism. Psychiatry Res. (2016) 238:60–7. 10.1016/j.psychres.2016.01.077 [DOI] [PubMed] [Google Scholar]
- 62.Takano Y, Ozeki Y, Sekine M, Fujii K, Watanabe T, Okayasu H, et al. Multi-regression analysis revealed a relationship between l-serine and methionine, a component of one-carbon metabolism, in the normal control but not in the schizophrenia. Ann Gen Psychiatry. (2016) 15:23. 10.1186/s12991-016-0113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W-P, Fan W, Shi B, Tong C, Wang X, Cai J, et al. Effect of MTHFR gene on the schizophrenia and its cognitive function [in Chinese]. Chin J Med Genet. (2017) 34:905–8. [DOI] [PubMed] [Google Scholar]
- 64.Oniki K, Ishioka M, Osaki N, Sakamoto Y, Yoshimori Y, Tomita T, et al. Association between oxidative stress-related genes polymorphisms and metabolic abnormalities among schizophrenia patients. Clin Neuropsychopharmacol Ther. (2017) 8:25–37. 10.5234/cnpt.8.25 [DOI] [Google Scholar]
- 65.Debost JC, Debost M, Grove J, Mors O, Hougaard DM, Borglum AD, et al. Comt Val158met and MTHFR C677t moderate risk of schizophrenia in response to childhood adversity. ActapsychiatricaScandinavica. (2017) 136:85–95. 10.1111/acps.12761 [DOI] [PubMed] [Google Scholar]
- 66.Zhilyaeva TV, Sergeeva AV, Blagonravova AS, Kasimova LN, Kuznetsov KV, Golovanova VI, et al. Association study of methylenetetrahydrofolate reductase genetic polymorphism 677c>T with schizophrenia in hospitalized patients in population of European Russia. Asian J Psychiatry. (2018) 32:29–33. 10.1016/j.ajp.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 67.Ota M, Sato N, Yoshida F, Hattori K, Hidese S, Teraishi T, et al. A polymorphism of the methylenetetrahydrofolate reductase gene confers susceptibility to schizophrenia and related brain changes. Schizophr Res. (2019) 208:462–4. 10.1016/j.schres.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 68.Wan L, Li Y, Zhou Y, Li R, Zheng Y. Age matters: An atypical association between polymorphism of MTHFR and clinical phenotypes in children with schizophrenia. J Mol Neurosci MN. (2019) 69:485–93. 10.1007/s12031-019-01382-0 [DOI] [PubMed] [Google Scholar]
- 69.Wan L, Zhang G, Liu M, Wang C, Li Y, Li R. Sex-specific effects of methylenetetrahydrofolate reductase polymorphisms on schizophrenia with methylation changes. Compr Psychiatry. (2019) 94:152121. 10.1016/j.comppsych.2019.152121 [DOI] [PubMed] [Google Scholar]
- 70.Kelly CB, McDonnell AP, Johnston TG, Mulholland C, Cooper SJ, McMaster D, et al. The MTHFR C677t polymorphism is associated with depressive episodes in patients from northern Ireland. J Psychopharmacol (Oxford, England). (2004) 18:567–71. 10.1177/0269881104047285 [DOI] [PubMed] [Google Scholar]
- 71.Reif A, Pfuhlmann B, Lesch KP. Homocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychoses. Progr Neuro Psychopharmacol Biol Psychiatry. (2005) 29:1162–8. 10.1016/j.pnpbp.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 72.Yuan Y-G, Li H, Wu R. The association of plasma homocysteine level, methylene tetrahydrofolate reductase gene polymorphism and depression in the elderly [in Chinese]. Chin J Psychiatry. (2005) 38:150. [Google Scholar]
- 73.Cheng-Sheng C, Tsai J-C, Hin-Yeung T, Yu-Ting K. Homocysteine levels, MTHFR C677t genotype, and mrihyperintensities in late-onset major depressive disorder. Am J Geriatric Psychiatry. (2005) 13:869–75. 10.1176/appi.ajgp.13.10.869 [DOI] [PubMed] [Google Scholar]
- 74.Yuan YG. Plasma homocysteine levels and MTHFR polymorphisms in senile depression and mild Alzheimer’s disease [in Chinese]. Chin J Geriatr. (2007) 26:767–69. [Google Scholar]
- 75.Slopien R, Jasniewicz K, Meczekalski B, Warenik-Szymankiewicz A, Lianeri M, Jagodzinski PP. Polymorphic variants of genes encoding MTHFR, MTR, and MTHFD1 and the risk of depression in postmenopausal women in Poland. Maturitas. (2008) 61:252–5. 10.1016/j.maturitas.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 76.Zhao G-Q. Association of polymorphism of homocysteine and MTHFR gene C677t with major depression [in Chinese]. Chin J Nerv Ment Dis. (2008) 34:378–80. [Google Scholar]
- 77.Yuan YG, Zhang ZJ, Li JJ. Plasma homocysteine but not MTHFR gene polymorphism is associated with geriatric depression in the Chinese Population. Actaneuropsychiatrica. (2008) 20:251–5. 10.1111/j.1601-5215.2008.00290.x [DOI] [PubMed] [Google Scholar]
- 78.Hong ED, Taylor WD, McQuoid DR, Potter GG, Payne ME, Ashley-Koch A, et al. Influence of the MTHFR C677t polymorphism on magnetic resonance imaging hyperintensity volume and cognition in geriatric depression. Am J Geriatric Psychiatry Off J Am Assoc Geriatric Psychiatry. (2009) 17:847–55. 10.1097/JGP.0b013e3181aad5b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Modification by two genes of associations between general somatic health and incident depressive syndrome in older people. Psychosomatic Med. (2009) 71:286–91. 10.1097/PSY.0b013e3181990fff [DOI] [PubMed] [Google Scholar]
- 80.Pan CC, McQuoid DR, Taylor WD, Payne ME, Ashley-Koch A, Steffens DC. Association analysis of the COMT/MTHFR genes and geriatric depression: An MRI study of the putamen. Int J Geriatric Psychiatry. (2009) 24:847–55. 10.1002/gps.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao L, Liu Z, Zhao L. Correlation analysis of plasma homocysteine and MTHFR gene polymorphism with post-stroke depression [in Chinese]. Guangdong Med J. (2010) 31:2946–9. [Google Scholar]
- 82.Zeman M, Jachymova M, Jirak R, Vecka M, Tvrzicka E, Stankova B, et al. Polymorphisms of genes for brain-derived neurotrophic factor, methylenetetrahydrofolate reductase, tyrosine hydroxylase, and endothelial nitric oxide synthase in depression and metabolic syndrome. Folia Biol. (2010) 56:19–26. [PubMed] [Google Scholar]
- 83.Feng L-G, Shao C, Liu Y, Tao Y, Hao X. The detection of genepolymorphisms of MTHFR C677t and A1298c in severe depression patients [in Chinese]. Basic Clin Med. (2010) 30:811–4. [Google Scholar]
- 84.Li ZZ, Yu SY, Zhang C, Yuan CM, Hong W, Wang Y, et al. Association of MTHFR gene C677t polymorphisms and depression in han populations. J Shanghai Jiaotong Univ (Med Sci). (2010) 30:624–7. [Google Scholar]
- 85.Song Z-W. Association of Methylenetetrahydrofolate reductase gene polymorphism and plasma homocysteine with major depression [in Chinese]. J Med Postgraduates. (2010) 23:934–7. [Google Scholar]
- 86.Lizer MH, Bogdan RL, Kidd RS. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism in depressed versus nondepressed patients. J Psychiatric Pract. (2011) 17:404–9. 10.1097/01.pra.0000407963.26981.a6 [DOI] [PubMed] [Google Scholar]
- 87.Zhao H-F, Qiao J, Zhu X-H. The association study of MTHFR gene polymorphism with depression [in Chinese]. J Psychiatry. (2011) 24:435–7. 10.3969/j.issn.1009-7201.2011.06.011 [DOI] [Google Scholar]
- 88.Chojnicka I, Sobczyk-Kopciol A, Fudalej M, Fudalej S, Wojnar M, Waskiewicz A, et al. No association between MTHFR C677t polymorphism and completed suicide. Gene. (2012) 511:118–21. 10.1016/j.gene.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 89.Evinova A, Babusikova E, Straka S, Ondrejka I, Lehotsky J. Analysis of genetic polymorphisms of brain-derived neurotrophic factor and methylenetetrahydrofolate reductase in depressed patients in a slovak (Caucasian) population. Gen Physiol Biophys. (2012) 31:415–22. 10.4149/gpb_2012_049 [DOI] [PubMed] [Google Scholar]
- 90.Qiao J, Zhao H-F, Zhu X-H, Geng D-Q. The study of MTHFR Gene Polymorphism in Depression [in Chinese]. J Clin Psychiatry. (2012) 22:92–4. [Google Scholar]
- 91.Shen X, Wu Y, Guan T, Wang X, Qian M, Lin M, et al. Association analysis of COMT/MTHFR polymorphisms and major depressive disorder in Chinese Han population. J Affect Disord. (2014) 161:73–8. 10.1016/j.jad.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 92.Sayadi MA, Achour O, Ezzaher A, Hellara I, Omezzine A, Douki W, et al. Ct Genotype of 5,10-methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism is protector factor of major depressive disorder in the Tunisian Population: A case control study. Ann Gen Psychiatry. (2016) 15:18. 10.1186/s12991-016-0103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mei F, Wu Y, Ding G. Association analysis of the MTHFR polymorphism and post-stroke depression in the elderly [in Chinese]. Geriatrics Health Care. (2016) 22:363–5+9. 30174331 [Google Scholar]
- 94.Huang Z-H, Zhou H-Y, Iv W-C, Cheng X-Y, Chen F. The correlation between MTHFR gene, Erαgene Polymorphism and Postpartum Depression Susceptibility [in Chinese]. J Med Theory Pract. (2017) 30:1108–9+22. [Google Scholar]
- 95.Li Y, Yue H, Li H. Effect of folic acid intake during pregnancy and MTHFR polymorphism on postpartum depression [in Chinese]. Chin J Woman Child Health Res. (2017) 28:632–5. 10.1038/ejcn.2011.136 [DOI] [PubMed] [Google Scholar]
- 96.Mei F, Wu Y, Ding G, Pan F, Chen L, Wu J. Association of methylenetetrahydrofolate reductase Gene 677c>T polymorphism with post-stroke depression risk and antidepressant treatment response in Han Chinese. JPMA J Pakistan Med Assoc. (2018) 68:888–92. [PubMed] [Google Scholar]
- 97.Saraswathy KN, Ansari SN, Kaur G, Joshi PC, Chandel S. Association of vitamin B12 mediated hyperhomocysteinemia with depression and anxiety disorder: A cross-sectional study among bhil indigenous population of India. ClinNutr ESPEN. (2019) 30:199–203. 10.1016/j.clnesp.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 98.Zhao G-Q, Jiao Z-A, Wang S-M, Liu Z-X, Shi H. An association analysis of methylenetetrahydrofolate dehydrogenase polymorphism, plasma homocysteine levels and bipolar affective disorder [in Chinese]. J Psychiatry. (2008) 21:130–2. [Google Scholar]
- 99.Ozbek Z, Kucukali CI, Ozkok E, Orhan N, Aydin M, Kilic G, et al. Effect of the methylenetetrahydrofolate reductase gene polymorphisms on homocysteine, folate and vitamin B12 in patients with bipolar disorder and relatives. Progr Neuro Psychopharmacol Biol Psychiatry. (2008) 32:1331–7. 10.1016/j.pnpbp.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, Liu Y, Zhang D, Liu Z, Wang P, Zhou D, et al. C677t methylenetetrahydrofolate reductase gene polymorphisms in bipolar disorder: An association study in the Chinese population and a meta-analysis of genetic association studies. Neurosci Lett. (2009) 449:48–51. 10.1016/j.neulet.2008.10.077 [DOI] [PubMed] [Google Scholar]
- 101.Ezzaher A, Mouhamed DH, Mechri A, Omezzine A, Neffati F, Douki W, et al. Hyperhomocysteinemia in Tunisian Bipolar I patients. Psychiatry Clin Neurosci. (2011) 65:664–71. 10.1111/j.1440-1819.2011.02284.x [DOI] [PubMed] [Google Scholar]
- 102.Permoda-Osip A, Dmitrzak-Weglarz M, Hauser J, Rybakowski JK. Are genes connected with homocysteine metabolism associated with bipolar disorder? Neuropsychobiology. (2014) 69:107–11. 10.1159/000358091 [DOI] [PubMed] [Google Scholar]
- 103.Wang LJ, Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, et al. A potential interaction between COMT and MTHFR genetic variants in Han Chinese patients with Bipolar II disorder. Sci Rep. (2015) 5:8813. 10.1038/srep08813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rahimi Z, Kakabaraee K, Garavand A, Rahimi Z. The T allele of MTHFR C.C677t and Its synergism with G (Val 158) allele of COMT C.G472a polymorphism are associated with the risk of Bipolar I disorder. Genet Test Mol Biomarkers. (2016) 20:510–5. 10.1089/gtmb.2016.0061 [DOI] [PubMed] [Google Scholar]
- 105.Kempisty B, Bober A, Luczak M, Czerski P, Szczepankiewicz A, Hauser J, et al. Distribution of 1298a>C polymorphism of methylenetetrahydrofolate reductase gene in patients with bipolar disorder and schizophrenia. Eur Psychiatry J Assoc Eur Psychiatrists. (2007) 22:39–43. 10.1016/j.eurpsy.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 106.Zhang C, Xie B, Du Y, Cheng W, Fang Y, Yu S. Further evidence that methylenetetrahydrofolate reductase A1298c polymorphism is a risk factor for schizophrenia. J Neural Transm (Vienna). (2010) 117:1115–7. 10.1007/s00702-010-0442-3 [DOI] [PubMed] [Google Scholar]
- 107.Wu YL, Ding XX, Sun YH, Yang HY, Chen J, Zhao X, et al. Association between MTHFR C677t polymorphism and depression: An updated meta-analysis of 26 Studies. Progr Neuro-Psychopharmacol Biol Psychiatry. (2013) 46:78–85. 10.1016/j.pnpbp.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 108.Gaysina D, Cohen S, Craddock N, Farmer A, Hoda F, Korszun A, et al. No Association with the 5,10-methylenetetrahydrofolate reductase gene and major depressive disorder: Results of the Depression Case Control (DECC) study and a meta-analysis. Am J Med Genet Part B Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2008) 147B:699–706. 10.1002/ajmg.b.30665 [DOI] [PubMed] [Google Scholar]
- 109.Rai V. Evaluation of methylenetetrahydrofolate reductase gene variant (C677t) as risk factor for bipolar disorder. Cell Mol Biol (Noisy-le-Grand, France). (2011) 57:OL1558–66. [PubMed] [Google Scholar]
- 110.Zintzaras E. C677t and A1298c methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: A meta-analysis of genetic association studies. Psychiatric Genet. (2006) 16:105–15. 10.1097/01.ypg.0000199444.77291.e2 [DOI] [PubMed] [Google Scholar]
- 111.Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism on executive function in schizophrenia. Schizophr Res. (2007) 92:181–8. 10.1016/j.schres.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 112.Wang X, Wang Z, Wu Y, Yuan Y, Hou Z, Hou G. Association analysis of the Catechol-O-Methyltransferase/methylenetetrahydrofolate reductase genes and cognition in late-onset depression. Psychiatry Clin Neurosci. (2014) 68:344–52. 10.1111/pcn.12133 [DOI] [PubMed] [Google Scholar]
- 113.Zhilyaeva TV, Sergeyeva AV, Kasimova LN, Blagonravova AS. Cognitive function dynamics during folate augmented therapy in patients with schizophrenia carrying MTHFR677c>T gene polymorphism: A pilot study. Sovremennyetehnologii Med. (2015) 7:147–53. 10.17691/stm2015.7.4.20 [DOI] [Google Scholar]
- 114.Liu WW, Dong XZ, Liu P. Mthfr 677c/T polymorphism-new ideas about depressive disorder treatment. Chin Pharmacol Bull. (2015) 31:915–9. 10.3969/j.issn.1001-1978.2015.07.006 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.