Abstract
COVID-19 has disrupted cervical screening in several countries, due to a range of policy-, health-service and participant-related factors. Using three well-established models of cervical cancer natural history adapted to simulate screening across four countries, we compared the impact of a range of standardised screening disruption scenarios in four countries that vary in their cervical cancer prevention programs. All scenarios assumed a 6- or 12-month disruption followed by a rapid catch-up of missed screens. Cervical screening disruptions could increase cervical cancer cases by up to 5–6%. In all settings, more than 60% of the excess cancer burden due to disruptions are likely to have occurred in women aged less than 50 years in 2020, including settings where women in their 30s have previously been offered HPV vaccination. Approximately 15–30% of cancers predicted to result from disruptions could be prevented by maintaining colposcopy and precancer treatment services during any disruption period. Disruptions to primary screening had greater adverse effects in situations where women due to attend for screening in 2020 had cytology (vs. HPV) as their previous primary test. Rapid catch-up would dramatically increase demand for HPV tests in 2021, which it may not be feasible to meet because of competing demands on the testing machines and reagents due to COVID tests. These findings can inform future prioritisation strategies for catch-up that balance potential constraints on resourcing with clinical need.
Keywords: Cervical screening, Cancer screening, Cervical cancer, COVID-19, Coronavirus, Modelling
1. Background
Since the onset of the global SARS-CoV-2 (COVID-19) pandemic, there have been reports from several countries of drops in attendance for screening, disruptions to screening or diagnostic services, and a reduction in cancer diagnoses (Dinmohamed et al., 2020; Cancer Australia, 2020; Scottish Government, 2020; Maringe et al., 2020; Wilkinson, 2020; Australian Institute of Health and Welfare, 2020; Feletto et al., 2020; Cancer Research UK, 2020). Initial reductions in cancer diagnoses could be partially the result of disruptions or reduced attendance for screening/ diagnosis, and/or due to delays in symptoms being investigated. This has led to concerns that there could be a substantial burden of current and future disease, including cancer, indirectly caused by the COVID-19 pandemic, as well as its direct effects.
Cervical cancer prevention programs, encompassing cervical screening and vaccination against human papillomavirus (HPV), are well-established in many high-income countries. They are also being implemented to varying extents in low and middle income countries, spurred on by a call for global action to eliminate cervical cancer as a public health problem, and the 2020 launch of the World Health Organization's elimination strategy, which specifies 2030 targets for HPV vaccination, cervical screening, and precancer and cancer treatment (World Health Organization, 2020a). Cervical cancer prevention programs have been disrupted to various extents in high-income countries (Scottish Government, 2020; Australian Institute of Health and Welfare, 2020; National Institute for Public Health and the Environment (RIVM), 2020). There are likely multiple causes of this, including not only formal pauses to programs or reminders, but also women being less likely to attend for screening, or a reduction in capacity for screening and follow-up. The latter two factors could potentially be caused by personal illness, caring for someone with an illness, reluctance or inability of women to attend appointments during ‘lockdowns’, concerns about being exposed to SAR-CoV-2 infection, or competing priorities. Furthermore, screening and preventive health may have reduced saliency in the context of the pandemic, or changes in or loss of employment could create financial barriers.
The impact of reduced screening attendance could be expected to vary between countries, not only due to the extent of the disruption, but also due to variability in the design of prevention programs between settings. This variability includes differences in when HPV vaccination began, the birth cohorts offered vaccination during catch-up, and vaccination coverage (since vaccination will offer some protection against cervical cancer; Lei et al., 2020); and primary screening test technology, target age range and interval, and screening participation (since these will impact the duration of effective protection conferred on women via screening at the start of the pandemic). The feasible or preferred strategies to recover from disruptions could also vary due to the level of program organization (for example, whether or not it is feasible to identify and contact individual women who become overdue) and differing constraints on resources. Constraints on resources could include a reduced capacity for HPV testing due to competing demands on consumables, machines and reagents for COVID-19 testing; and reduced capacity of primary and/or secondary care due to competing demands on services, reduced throughput due to physical distancing requirements and COVID-19-related protocols, or reduced workforce due to illness or caring responsibilities.
Therefore, key questions to address include what the impact of screening disruptions will be, which women are at highest risk, and what are the most appropriate recovery strategies, in light of any resourcing constraints and available information. All of these will likely be highly setting specific particularly as the COVID-19 situation remains highly dynamic. Vaccines preventing COVID-19 are starting to become available: more than 50 countries have commenced vaccination (Ritchie et al., 2021), typically focussing on groups at higher risk of infection or serious COVID-19 disease in the initial phase. Nevertheless, it will take some time for widespread vaccination to be achieved in all countries (global coverage on 25th January 2020 estimated as 0.6%) (Ritchie et al., 2021), and there will be a backlog of missed screening visits to catch up. Additionally, there is a lag in the availability of data on screening and related outcomes.
In the context of these data delays and uncertainty, simulation modelling can provide some insights and consider a range of scenarios ahead of data being available, to allow for planning. This study therefore aims to identify some general principles, and how these might vary between settings with different cervical cancer prevention program designs (in settings with longstanding screening activities; in practice, high-income countries). In particular, we aim to identify if there are particular age groups at highest risk, as this could provide insight into prioritisation strategies that could be used in recovery; and to identify the resourcing implications of a rapid recovery. More broadly, we also aim to examine which program characteristics are associated with greater resilience to disruptions, since these would apply beyond the specific example of COVID-19.
2. Methods
2.1. Model platforms
This work was done under the auspices of the COVID-19 and Cancer Global Modelling Consortium, which aims to connect modelling teams and other experts to support decision-making in cancer control during and after the pandemic (www.ccgmc.org). The analysis used three well-established modelling platforms (Policy1-Cervix (Cancer Council NSW), MISCAN (Erasmus MC), and Harvard) that simulate cervical screening and HPV vaccination for four countries (Australia, The Netherlands, Norway, USA; five country-level models total). The models incorporate detailed local data and model predictions are consistent with local epidemiological data across a range of disease endpoints, including HPV prevalence, precancer detection and cancer incidence by age and HPV type group (Burger et al., 2020a; Cancer Council NSW, 2019; Smith et al., 2020a; Portnoy et al., 2021; Jansen et al., 2021; Smith et al., 2021b). Models reflect current and historical HPV vaccination uptake, and cervical screening recommendations and behaviour in the modelled countries, all of which have longstanding screening activities and have been offering HPV vaccination for more than 10 years. These countries vary in their vaccination programs (including start date, extent and timing of catch-up offered, and coverage), their screening programs (including primary test, screening interval, target age range, and coverage), and the extent to which cohorts offered vaccination overlap with those age-eligible for screening (Table 1 ). Results are presented separately for different primary screening approaches for Norway (cytology vs HPV) and the USA (cytology vs co-testing), because neither country currently has a single screening approach that is used consistently for all women screened. In Norway, this is due to the country being partway through a transition from cytology to primary HPV screening that began in some regions in 2015 but will not be complete nationally until 2025 (Portnoy et al., 2021). In the USA, this is due to guidelines providing multiple options, and a resulting variation in clinical practice (US Preventive Services Task Force, 2018; Fontham et al., 2020; Watson et al., 2018). The results from Norway and the USA can be interpreted as the expected outcomes in women screened with cytology throughout their lives; or alternatively outcomes in women who are initially screened with cytology, then at the recommended age switch to primary HPV screening (Norway; switching from age 34 assumed to occur from 2015) or co-testing (USA; from age 30) and thereafter continue to be screened with that approach. Information on screening programs including switching is included in Table 1.
Table 1.
Summary of cervical cancer prevention in modelled countries.
| Country | HPV vaccination |
Screening |
|||||
|---|---|---|---|---|---|---|---|
| Begana | Screening ages affected in 2020 | Approx coverageb | Primary test | Recommended interval/agesc | Participationd | Organization | |
| Australia | 2007 | 25–39 y | 57% [25–29y] 30% [30–39y] |
HPV with 16/18 genotyping (since Dec 2017)e | 5y/25–74 | 54% | National register sends an invitation at age 24y 9 months and reminders when overdue for routine screening or surveillance |
| Netherlands | 2009 | Not eligible until 2023 | 0% | HPV (since Jan 2017)f | 5y/30–60g | 82% | Regional organization sends invitations at fixed ages (30,35,40,45,50,55,60y) or when additional follow-up required |
| Norway | 2009 | 25–30 y | 56–62% [25–29y] | Cytology HPVh |
3y/25–69 5y/34–69 |
71% | National register sends an invitation at age 25 and reminders when due/ overdue for routine screening or surveillance |
| USA | 2006 | 21–40 y | 65% [21-24y] 58% [25–29y] 19% [30–39y] |
Cytology Co-testingh HPV |
3y/21–65 5y/30–65 5y/30–65 |
81% | No national level organization. Health provider organizations provide varying levels of organization within their system. |
Started for target age.
Restricted to cohorts offered vaccination age-eligible for screening; age in 2020 (Portnoy et al., 2021; Smith et al., 2021a; Gefenaite et al., 2012; Reagan-Steiner et al., 2016; Reagan-Steiner et al., 2015; Centers for Disease Control and Prevention, 2013; Centers for Disease Control and Prevention, 2011; Centers for Disease Control and Prevention., 2010; Centers for Disease Control and Prevention, 2009).
End age is not a hard stop in Australia, Norway or USA, ie women with an abnormal test at around the end age or without a consistent history of negative tests in the period leading up to the recommended end age are typically kept under surveillance until they meet exit criteria (eg: the abnormality is cleared or treated). In Norway and the USA, HPV-based screening is recommended from a certain start age, and cytology screening recommended for women age-eligible for screening but below the minimum age recommended for HPV-based screening.
Participation at the recommended interval.
Colposcopy referral for women with HPV16/18 detected or both HPV (oncogenic type but not 16/18) detected and LBC ≥ ASC-H or glandular abnormalities; women with HPV (not 16/18) detected and LBC < ASC-H are referred for 12 m repeat HPV testing.
Colposcopy referral for women with both HPV detected (any oncogenic type) and LBC ≥ ASC-US; women with normal LBC are referred for 6 m repeat LBC testing.
Screening recommended at 65 for women who are HPV-positive at age 60; women who are HPV-negative at age 40 or 50 are recommended to return in 10 years rather than 5.
Colposcopy referral for women with both HPV detected (any oncogenic type) and LBC ≥ ASC-US; HPV-positive women with normal LBC are referred for 12 m repeat HPV testing.
2.2. Scenarios
Standardised screening disruption scenarios were modelled for all countries (Table 2 ). Standardised scenarios were used for two reasons. Firstly, because real-world data on disruptions are limited and delayed, and the situation remains highly dynamic; and secondly because using standardised scenarios in all countries allowed us to gain insight into how differences in historical vaccination uptake and screening program characteristics influence the impact of disruptions (by removing variability in the disruptions themselves). The simplest scenarios assumed disruptions occurred to routine primary screening visits only, but did not extend to later steps in the follow-up and diagnostic pathway. Additional scenarios incrementally included disruptions to later steps in the follow-up and diagnostic pathway (surveillance visits for women with a recent abnormal screening test; colposcopy and treatment visits for women with a screen-detected abnormality), and to women presenting with symptoms. This was done in order to isolate the effects of disrupting different points on the pathway, to inform prioritisation strategies, and because disruptions may not in practice affect all points on the pathway. Disruptions to surveillance visits, colposcopy and precancer treatment would be expected to affect additional women compared to those already affected by disruptions to primary screening. This is because some of the surveillance and colposcopy visits and precancer treatments would have been due to an abnormality detected prior to the disruption period (for example follow-up testing of women who were triage-negative in 2019; women who were referred for colposcopy before the disruption but had not yet attended). Disruptions were assumed to last for a fixed period of time (6 or 12 months), during which time the affected services were unavailable but delays in the management pathway were not cumulative (that is, for example, there was not a 6-month disruption to screening, followed by a 6-month delay for colposcopy; rather there was a 6-month period when both services were disrupted, and after this period affected services were restored). All disruption scenarios assumed that women who missed visits or tests in 2020 would return after the disruption period ended, in order to estimate the resourcing implications of a rapid recovery.
Table 2.
Scenarios modelled.
| Scenario | Duration | Extent of disruption to: |
|||
|---|---|---|---|---|---|
| Routine primary screening | Surveillance visits | Colposcopy/precancer treatment | Symptomatic detection | ||
| S0 | None | None | None | None | None |
| S1 | 6 months | 100% ↓ | None | None | None |
| S2 | 100% ↓ | 100% ↓ | None | None | |
| S3 | 100% ↓ | 100% ↓ | 100% ↓ | None | |
| S4 | 100% ↓ | 100% ↓ | 100% ↓ | 100% ↓ | |
| S5 | 12 months | 100% ↓ | None | None | None |
| S6 | 100% ↓ | 100% ↓ | None | None | |
| S7 | 100% ↓ | 100% ↓ | 100% ↓ | None | |
| S8 | 100% ↓ | 100% ↓ | 100% ↓ | 100% ↓ | |
Disruptions are assumed to occur across all affected services for the duration, followed by rapid recovery of missed visits when the disruption period ends. The exact timing of the disruption differs between the Australian model and other models due to differences in the time-step used in the models (one year for Australia; smaller in other models). Two models (Australia, USA-Policy1) assume the 12-month disruption occurs over the full year of 2020 (recovery from January 2021); other models assume the 12-month disruption occurs from March 2020 – February 2021 (recovery from March 2021). All models assume the 6-month disruption occurs entirely within 2020. In the 6-month disruption scenarios, recovery is assumed to commence from September 2020 in all models apart from the Australian model, where it is assumed to commence from January 2021.
2.3. Outcomes
The outcomes we considered were additional diagnosed cervical cancers (screen- or symptomatically-detected, at any stage); cancers diagnosed at a later stage (upstaged); additional cervical cancer deaths due to additional/ upstaged cancers; and the level of demand for health service resources that would be created by a relatively quick recovery. Additional and upstaged cancers were also stratified by age (based on women's age in 2020, when the disruption occurred), and by the type of disruption, to gain insight into which groups might be most affected. The absolute number of additional cases was estimated for Australia and the Netherlands, as in both countries there is a single screening modality used across the entire country. As there is not a single screening modality used in either Norway or the USA, in order to have metrics that could be compared across countries, outcomes were additionally calculated per million women aged 20 years or older in 2020, as an approximation of the population affected by screening (since the settings considered had different start and end ages for screening).
Upstaged cancers cannot be simply calculated as the difference in cancers detected at every stage relative to the comparator scenario, because there are also additional cancers that need to be taken into account. Therefore, upstaged cancers were calculated by assuming: additional cancers detected at distant stage were the result of upstaging from regional to distant; additional cancers relative to the comparator scenario (ie those arising due to a missed screening visit, follow-up visit, +/−colposcopy and precancer treatment during the disruption) were detected at localized stage (since recovery after the disruption was rapid and all women who missed a visit re-attended within two years); and differences in the number of cancers detected at localized and regional stage not explained by the first two assumptions were the result of upstaging from local to regional. Further details on how upstaged cancers and additional cancer deaths were calculated are included in an Appendix (A.1.2, Table A1).
Demand for resources was considered in the context of the typical volumes for those tests and procedures, to consider the extent to which they may exceed the available capacity (including usual capacity and if there is reduced capacity created by COVID-19), and for how long. Some models were not able to accommodate every strategy or stratification of results, due to differences in their underlying structure.
In a secondary analysis, we also considered whether disruptions due to COVID-19 could delay when these countries might expect to achieve cervical cancer elimination (ie incidence rates of fewer than 4 new cases per 100,000 women/year, age-standardised using the 2015 WHO female population; World Health Organization, 2020a; Canfell et al., 2020), or whether this could be avoided by the rapid recovery strategy that was modelled. Based on previous work, the existing burden of disease, and pre-pandemic prevention programs in place, among these four countries, only Australia was predicted to reach the cervical cancer elimination threshold prior to 2030 (Hall et al., 2019; Burger et al., 2020b; Portnoy et al., 2021).
3. Results
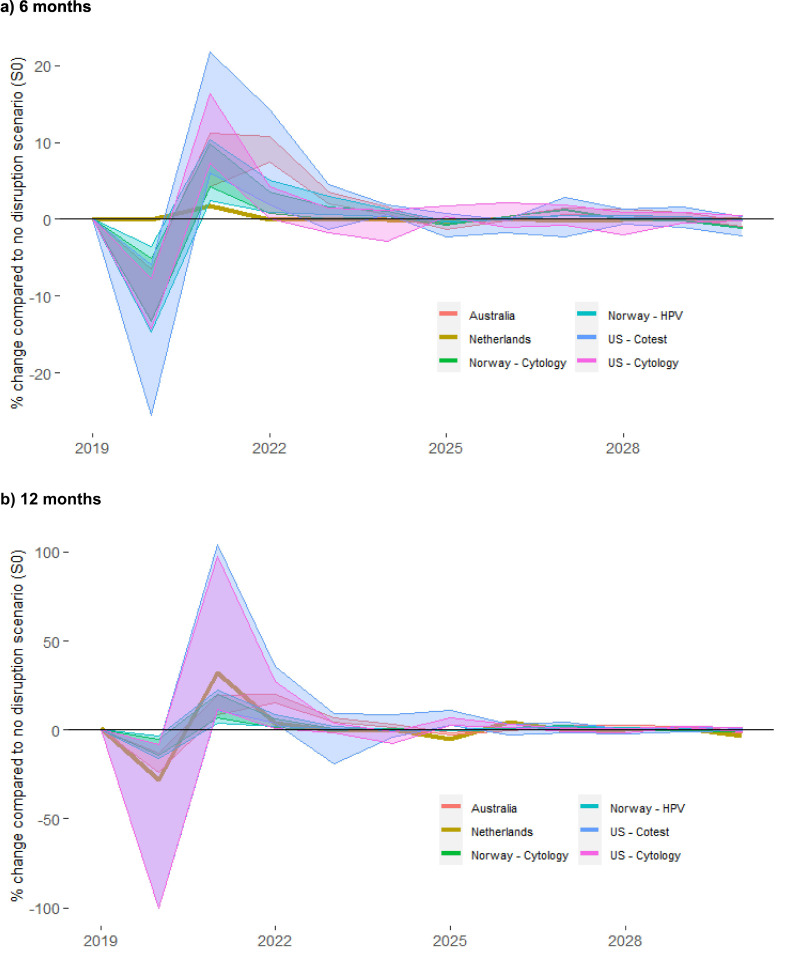
In the absence of disruptions to screening, the number of cervical cancer cases over 2020–2030 per million women aged 20+ years was predicted to range from 310.6 (USA – co-testing; midpoint between predictions from Harvard and Policy1 models) to 1510.1 (Norway–cytology)(Table 3 ). The impact of disruptions followed a similar pattern in all settings: there was an initial decrease in diagnoses in 2020, as cancers that would otherwise have been detected through screening were missed; this was followed by an increase in diagnoses in the following years (Fig. 1 ). The increase would include a shift in timing for screen-detected cancers missed in 2020, and additional cancers due to progression of some precancers that would otherwise have been treated in 2020. The number of additional cancers predicted over 2020–2030 varied widely between the countries, due to pre-existing differences in their disease burden and population size (12-month scenarios in Table 3; 6-month scenarios in Table A5), but ranged from around 0–27.0 additional cancers per million women aged 20 years or older across the scenarios considered. This represented an up to 5.7% increase in the age-standardised rate (data not shown). The number of cancers was predicted to increase by up to 5.3% compared to the no disruption scenario. The number of cancers predicted to be upstaged due to disruptions was smaller, ranging from 0.0–10.2 cancers detected at a later stage per million women aged 20+ years. The relative increase was higher when disruptions extended throughout the clinical pathway and when the burden of disease in the absence of disruptions was lower (Table 3; Fig. 1). In settings where we looked at different screening approaches in the same population (Norway, USA), the absolute increase in cases due to a standard-length disruption was in some cases similar to or slightly larger in the context of HPV-based screening compared to cytology screening when surveillance or colposcopy and precancer treatment were disrupted. Even where the absolute increase in cases was slightly higher though, the total number of cases in the context of a standard disruption period was still predicted to be lower in the context of HPV-based screening than in the context of cytology-based screening (Fig. A1). Additional deaths in the longer term resulting from these additional and upstaged cancer cases ranged from 0.0–16.6 per million women aged 20+. Rates of additional cancers, upstaged cancers, and additional deaths over the longer term resulting from these two factors, were generally predicted to be higher in Norway than in Australia, the Netherlands, and the US (potentially due to the higher burden of disease expected in Norway in the absence of a disruption). An exception was in the case of disruptions to primary screening only (and no disruptions to surveillance or other services), where there were more additional cancers per million women aged 20+ predicted in Australia than in Norway. Australia and the Netherlands were the only settings modelled where results could be extrapolated to make national-level estimates for additional cancer cases, upstaged cancers, and additional deaths. In the absence of disruptions there were predicted to be 7510 and 7770 cervical cancer cases over 2020–2030 in Australia and the Netherlands, respectively. In Australia, disruptions were predicted to result in 41–196 additional cervical cancers, 15–96 upstaged cancers, and 8–68 additional deaths over the longer term, with the upper end representing 12-month disruptions to primary screening, surveillance, colposcopy and precancer treatment. In the Netherlands, disruptions to primary screening only were predicted to result in 8–27 additional cancer cases, and 2–10 upstaged cancers (Table A6).
Table 3.
Predicted impact of disruptions on women screened, and cancer diagnoses over 2020–2030 and related deaths, by setting: 12-month scenarios.
| Setting | Disruptions include | Women predicted to miss screening visitsa | Cervical cancer cases (2020−2030)a |
Predicted additional deaths due to additional/upstaged cancers in 2020–2030a | |||
|---|---|---|---|---|---|---|---|
| Expected (no disruptions) | Additional due to disruptions | % increase | Detected at higher stage | ||||
| Australia | Primary Scr (S5): | 107,130.0 | 791.5 | 8.7 | 1.1 | 3.1 | 1.8–2.7 |
| Surveillance (S6): | 116,019.2 | 14.4 | 1.8 | 6.8 | 3.4–4.9 | ||
| Colp/Tx (S7): | 116,019.8 | 20.6 | 2.6 | 10.2 | 5.1–7.2 | ||
| Netherlands | Primary Scr (S5): | 67,340.3 | 1144.1 | 4.0 | 0.4 | 1.4 | na |
| Norway (cytology) | Primary Scr (S5): | 178,046.6 | 1510.1 | 6.1 | 0.4 | 3.7 | 5.2 |
| Surveillance (S6): | 178,046.6 | 19.6 | 1.3 | 8.8 | 13.3 | ||
| Colp/Tx (S7): | 178,046.6 | 23.1 | 1.5 | 9.2 | 14.6 | ||
| Norway (primary HPV) | Primary Scr (S5): | 130,191.9 | 1321.6 | 5.3 | 0.4 | 2.8 | 3.6 |
| Surveillance (S6): | 130,191.9 | 22.9 | 1.7 | 9.6 | 14.9 | ||
| Colp/Tx (S7): | 130,191.9 | 27.0 | 2.0 | 10.2 | 16.6 | ||
| USA (cytology) Harvard | Primary Scr (S5): | 215,086.1 | 788.7 | 5.3 | 0.7 | 1.7 | 0.1 |
| Surveillance (S6): | 215,086.1 | 9.3 | 1.2 | 2.6 | 0.1 | ||
| Colp/Tx (S7): | 215,086.1 | 14.1 | 1.8 | 3.7 | 0.1 | ||
| USA (co-testing) Harvard | Primary Scr (S5): | 214,200.7 | 236.3 | 1.3 | 0.6 | 0.0 | – |
| Surveillance (S6): | 214,200.7 | 7.5 | 3.2 | 1.1 | – | ||
| Colp/Tx (S7): | 214,200.7 | 11.3 | 4.8 | 1.8 | 0.1 | ||
| USA (cytology) Policy1 | Primary Scr (S5): | 198,171.6 | 606.8 | 7.3 | 1.2 | 1.4 | 1.1–1.8 |
| Surveillance (S6): | 214,630.0 | 14.8 | 2.4 | 3.8 | 2.4–4.2 | ||
| Colp/Tx (S7): | 214,630.0 | 19.2 | 3.2 | 5.2 | 3.1–5.5 | ||
| USA (co-testing) Policy1 | Primary Scr (S5): | 184,924.3 | 384.9 | 4.8 | 1.2 | 1.0 | 0.8–1.3 |
| Surveillance (S6): | 227,511.4 | 14.7 | 3.8 | 3.8 | 2.3–4.1 | ||
| Colp/Tx (S7): | 227,511.4 | 20.4 | 5.3 | 4.7 | 3.1–5.5 | ||
na = not available. Lower disease level in Harvard US model in the no disruption scenario is partially due to the model reflecting squamous cell carcinoma only. Results for 6-month disruption scenarios are presented in Table A5.
Values are per million women aged 20+ in 2020.
Fig. 1.
percentage change in total cancer cases over 2020–2030, by year and setting.
6 months chart represents the range across scenarios S1 to S3 and the 12 months chart represents the range across scenarios S5 to S7 (in both cases, the percent change is relative to S0). Results for the US represent the range across the two included models (Harvard and Policy1-Cervix). Model-specific US results are included in an Appendix (Fig. A3). Results in Table 3 represent the aggregated percentage change compared to S0 across the period 2020–2030.
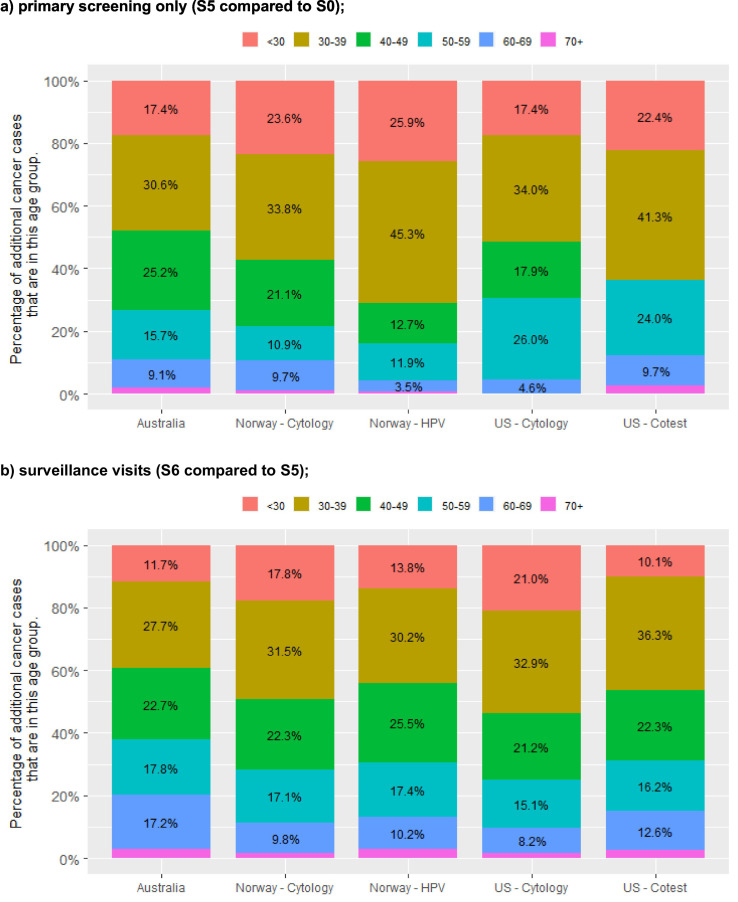
We considered results for the 12-month scenarios by age (Fig. 2 ). Results by age are shown for each incremental step in the screening, follow-up and diagnostic pathway, to provide insight into how disruptions to that particular step affects women of different ages (panels a to c) and also summarised to show the aggregated impact of disruption across all steps (panel d). For additional context, panel e shows the age distribution of cancers in each setting in the absence of disruptions. For all three of the countries where results were available by age (Australia, Norway and the USA), around half or more (42.3–71.2%) of all additional cancer cases were predicted to occur in women aged less than 40 years in 2020, and more than half (63.7–83.9%) in women aged less than 50 years in 2020. In all three of these countries, women aged 30–39 years in 2020 were predicted to be the group with the most additional cancers over 2020–2030, with 29.0–45.3% of the additional cancers resulting from disruptions expected to be diagnosed in women in this age group. Relatively few of the additional cancers were predicted to occur in women aged 70 years or more in 2020 (none to 3.9%), as in most countries women in this age group are not routinely screened (although they may remain under surveillance as a result of a test when they were younger than 70). The predicted age distribution of upstaged cancers was broadly similar to that for additional cancers, including that women aged younger than 50 were generally the most affected, but women in their 50s or 60 were relatively more affected by upstaging than by additional cancers in Australia and the USA (Fig. A2).
Fig. 2.
percentage of additional cancer cases over 2020–2030 in each age group*, by setting and extent of disruption (12-month scenarios).
* Age = age in 2020, not necessarily at the time of cancer diagnosis.
Results for the US represent the midpoint of results for the two included models (Harvard and Policy1-Cervix). Model-specific US results are included in an Appendix (Fig. A4). All Norway models reflect the recommendation that women aged 25–33y be screened with 3y cytology; all US models reflect the recommendation that women aged 21–29y be screened with 3y cytology.
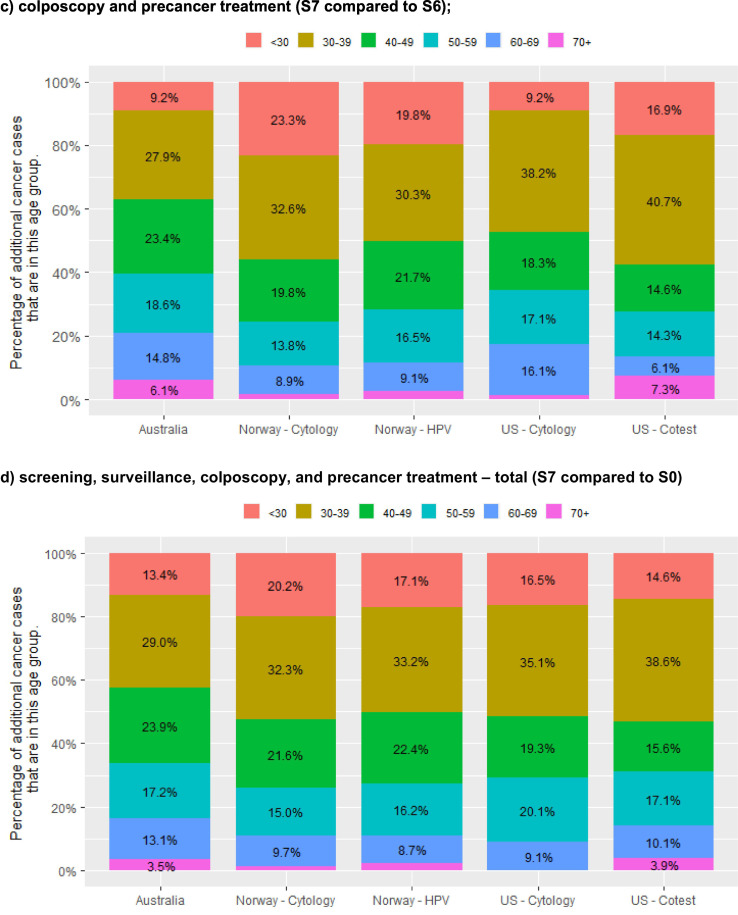
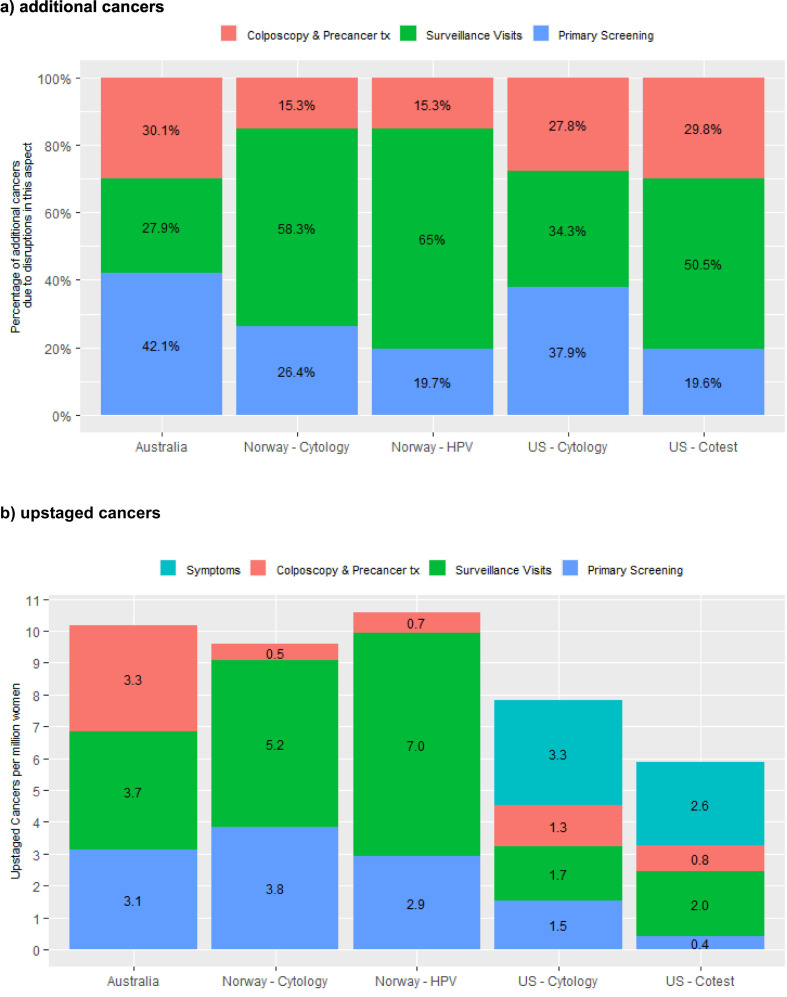
In the three countries where results were available for increasing disruptions across the screening pathway (Australia, Norway and the USA), the percentage of additional cancers that were due to disruptions to primary screening was larger in settings where the women due to attend for screening in 2020 had cytology as their last screening test (range 26.4–42.1% of the predicted additional cancers) (Table 3, Fig. 3 ). Australia is included in the group of settings where the women due to attend for screening in 2020 had cytology as their last screening test, as only women whose last screening test was cytology were expected to attend for primary screening in 2020; women who had already had their first primary HPV screening test were not due to re-attend for primary screening until at least late 2022. Disruptions to surveillance visits were relatively more important in settings where a woman's last test was HPV (accounting for 50.5–65.0% of additional cancers), and there was more variability in this proportion where a woman's last test was cytology (27.9–58.3% of additional cancers). Disruptions to colposcopy and precancer treatment accounted for 15.3–30.1% of additional cancers, and there was less variability in this proportion based on a woman's last test type than for the other two sources of disruption. Similar to the findings for additional cancers, disruptions to primary screening tended to result in more upstaged cancers in settings where the women due to attend for screening in 2020 had cytology as their last screening test, and conversely disruptions to surveillance visits led to relatively more upstaged cancers in the context of HPV-based screening than for cytology. Disruptions to investigation of symptoms had a relatively larger effect on upstaged cancers in the USA, but smaller effect on upstaged cancers in Australia (Fig. 3b; Fig. A5).
Fig. 3.
percentage of additional cancer cases and rate of upstaged cancers over 2020–2030 due to type of disruption, by setting and extent of disruption (12-month scenarios).
Results for the US represent the midpoint of results for the two included models (Harvard and Policy1-Cervix). Model-specific US results are included in an Appendix (Fig. A5). All Norway models reflect the recommendation that women aged 25–33y be screened with 3y cytology; all US models reflect the recommendation that women aged 21–29y be screened with 3y cytology.
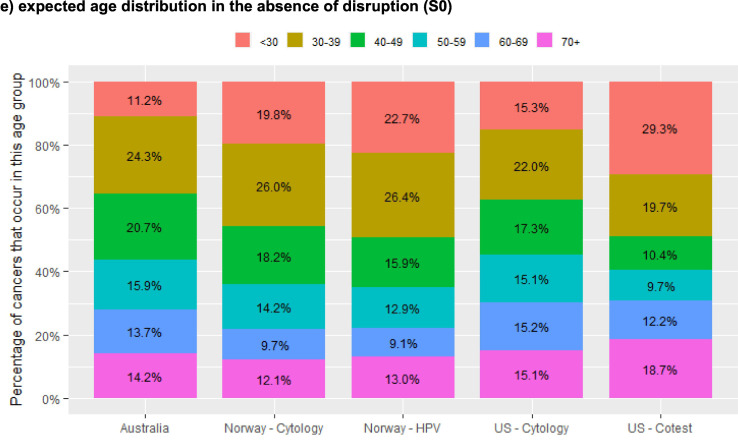
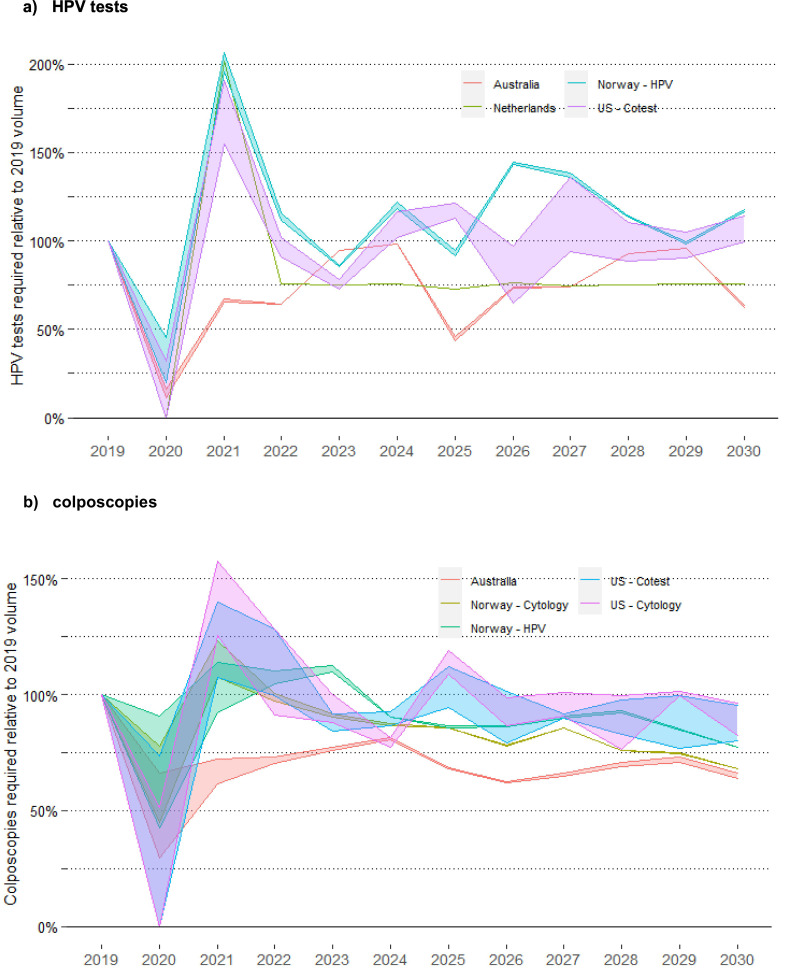
In the context of a rapid unconstrained recovery after a 12-month disruption, demand for HPV tests in the US, the Netherlands and Norway would be 50–100% higher in 2021 than the volumes required in 2019 (assumed to be indicative of usual capacity) but returned to closer to 2019 levels in 2022 (Fig. 4 ). Demand was lower in Australia as 2020 and 2021 were the third and fourth year after the transition from a 2-year to a 5-year interval (and most women would have attended in the first two years post-transition; the effect of extending the interval from 5 to 10 years for some HPV-negative women in Netherlands can be seen from 2022). Demand for colposcopy was predicted to follow a broadly similar pattern (a large decrease in 2020 compared to the no disruption scenario, generally followed by an increase in 2021), but the increased demand in 2021 (up to 57.6% higher) was less extreme than that for HPV tests, and in some instances there was no increase relative to demand in 2019 (Fig. 4). Resource demand by year was not considered for the 6-month scenarios, as the disruption finished and recovery had started before the end of 2020, so there was generally little difference in resource demand at the level of a year.
Fig. 4.
relative demand for resources over 2020–2030 to achieve the modelled rapid recovery, by year and setting.
Resource demand is referenced to 2019 (pre-disruption) volumes, as estimate of usual achievable capacity. Results for the US represent the range across the two included models (Harvard and Policy1-Cervix). Model-specific US results are included in an Appendix (Fig. A6). Results for HPV test demand are restricted to settings with HPV-based screening (Norway – cytology and US – cytology scenarios are excluded due to relatively smaller demand for HPV tests in the context of cytology-based screening).
Our secondary analysis found that, in the context of a rapid recovery, even the most extreme disruption scenario (S7; all screening and precancer treatment services were disrupted for 12 months) did not delay when cervical cancer elimination was predicted to occur at the national level in Australia (2026 in both the no disruption and most extreme disruption scenarios). The elimination year of 2026 differs slightly from the base case estimate of 2028 in a previously published analysis (Hall et al., 2019), because the base case estimate in the earlier analysis used the Australian Standard population for age -standardising, compared to the methodology and standard population since recommended for cervical cancer elimination reporting (Canfell et al., 2020) (although the previous analysis additionally explored a range of populations for age-standardising, and 2026 falls within the range reported for these of 2021–2035) (Hall et al., 2019).
4. Discussion
Our results suggest that that the absolute impact of disruptions to primary screening tends to be largest where women expected to attend in 2020 had cytology as their previous primary screening test, but that if disruptions to surveillance visits, colposcopy and precancer treatment also occur, the rate of additional cancers could be similar or greater in the context of HPV screening. Disruptions to surveillance visits generally had a larger effect in the context of HPV-based screening than they did for cytology, although this is partly due to more women being placed under surveillance in the context of HPV-based screening than for cytology. Disruptions to colposcopy seemed to be similarly important regardless of the primary screening test used. The overall effect, though, was that there were fewer cancers over 2020–2030 in the context of HPV-based screening than there were for cytology-based screening, either with or without a disruption; in fact, cancer rates remained lower in the context of disrupted HPV-based screening with a rapid recovery than in the context of uninterrupted cytology-based screening. This suggests that HPV-based programs are likely more resilient to disruptions than cytology-based programs (provided there is relatively rapid catch-up of missed screens), as women are better protected overall. The percentage increase in cancer was approximately 5% or less across countries, but tended to be largest when the absolute burden was lowest (for example in the context of HPV-based screening compared to cytology; or in Australia and the US, compared to Norway and the Netherlands), as this generally reflected more effective pre-pandemic screening programs being in place. The finding that disruptions to primary screening played a relatively larger role in Australia than in other settings with HPV-based screening is likely driven by timing: although all women had already been recommended to attended for their first primary HPV test, the model reflected that by the time disruptions were assumed to occur, only just over half of all women had done so (Smith et al., 2020a). Therefore, the only women expected to attend for routine screening in 2020 and who would therefore be affected by disruptions were women who were last screened with cytology more than two years earlier, and so the effect was more comparable to settings using cytology. In contrast, the Norway HPV model assumed that the transition to HPV screening began in 2015, and so a much higher proportion of women had switched before 2020, and the US co-testing models assumed women aged 30 years or older had switched at age 30 years.
We found that in all settings, the women most affected by additional cancers will be those aged in their 30s in 2020 (some of whom may be in their 40s when a cancer is diagnosed, or by 2030), even assuming a rapid catch-up of women who missed screening or other services in 2020. This occurred even though in Australia and the USA, women in their 30s in 2020 had some level of vaccine protection (Table 1). The proportion of additional cancers and upstaged cancers that occurred in women aged less than 50 was consistently lower in Australia than in Norway or the USA however, especially in women aged 30–39 years, an age group where Australia's vaccine coverage was higher than in the other two countries (Table 1). Additional cancers in women aged less than 30 in 2020 were lower in the US and Norway in the context of HPV-based screening than they were in the context of cytology, even though women in this age group who missed screening in 2020 would all have had cytology as their most recent primary test (since women do not switch to HPV-based screening until age 30 in the US and age 34 in Norway). This is potentially because the women who missed screening eventually had a more sensitive HPV test at a later screening visit, which was more likely to detect precancer and allow it to be treated, whereas this may have been missed by continued cytology screening. Findings were broadly similar in terms of the age distribution of cancers that would be detected at a later stage due to the disruption, except that women in their 50s or 60s were relatively more affected by upstaging than by additional cancers in Australia and the USA.
4.1. Study strengths and limitations
Strengths of our analysis include that it used well-established models that have informed policy in their respective settings (Burger et al., 2020b; Lew et al., 2017; Simms et al., 2016; Kim et al., 2018; Burger et al., 2015; Burger et al., 2012; Matthijsse et al., 2018; Kaljouw et al., 2021; Kim et al., 2021), and that by examining standardised disruption scenarios in a range of different settings with different prevention program characteristics, we have been able to identify some common themes. This approach has enabled us to identify groups at most risk, even in the context of a rapid recovery, and who therefore could be prioritised if there are constraints on resources. By using multiple independently-developed models (with different natural history assumptions and underlying model structures, but each consistent with observed data), we have encompassed a broad range of parameter and structural uncertainty. Our analysis also has some limitations. Our results are not intended to directly represent the expected outcomes of COVID-19 related disruptions in individual countries, because standardised disruption scenarios were used and rapid recovery was assumed. Data are not yet available to inform setting-specific estimates for impact, and the situation is continuing to change over time. These findings are instead intended to be indicative of the groups who are potentially at highest risk, and of whether these groups are consistent across settings or vary depending on design or other characteristics of the screening program. The findings provide some insights into the likely effects of a shorter or longer disruption, however, as the outcomes for the 12-month disruption scenarios are generally around double those of the 6-month disruption scenarios. This suggests that there is some degree of linearity and so these results could be used to estimate outcomes for disruptions that were somewhat shorter or longer than the hypothetical scenarios modelled. This would also suggest that, for example, a 6-month disruption followed by a slower 6-month recovery (rather than rapid recovery, as modelled here) would have outcomes intermediate between our 6- and 12-month disruption scenarios. Additionally, we assumed a complete disruption; outcomes are likely to be smaller if screening attendance and services were reduced, rather than completely stopped. Linearity may not be conserved over extended periods however: a similar recent analysis for the USA comparing 6- and 24-month disruptions using two of the models included here found that the effects of a 24-month disruption were more than four times higher than the 6-month disruption (Burger et al., 2021). We designed the analysis to isolate the impact of certain factors on cervical cancer and the likely resourcing demands during recovery, particularly step in the screening pathway and primary screening modality; however, other factors such as differences in screening intensity cannot not be isolated. Evaluating the impact of routine screening history and frequency will be the focus of a future analysis. We also did not take into account the competing risk of death due to COVID-19, which varies markedly by setting, age, and sex (with females and younger people generally being less likely to die), with additional variation in the sex differential over time in some settings (Alkhouli et al., 2020; Ahrenfeldt et al., 2021; The Sex, Gender and COVID-19 Project, 2021). We were also unable to provide more detailed estimates on upstaged cancers, due to the relatively small number of cases in the simulated population in most models. Additional deaths due to upstaging are therefore uncertain, and also assume that stage-specific survival for women diagnosed with cancer would remain stable over the next decade. This analysis focussed on disruptions to screening, and did not additionally consider disruptions to HPV vaccination programs. Disruptions to vaccination programs would be unlikely to affect our conclusions for cervical cancer diagnoses over 2020–2030 and resulting deaths due to the relatively long period between acquiring an HPV infection (that might otherwise have been prevented by vaccination) and cancer. Herd effects from the well-established vaccination programs in each of these countries would also provide some protection for those who miss vaccine doses. We were also unable to model outcomes in specific population subgroups, for example stratified by ethnicity or socioeconomic status, although our findings can provide insight into the extent to which disparities could widen if groups within the population face differential disruptions or delays in resuming screening. Prior to COVID-19, women in an ethnic minority group, or living in areas that are more economically disadvantaged or more remote, were less likely to have optimal screening and treatment and so were at higher risk of cervical cancer. These same groups may also be more affected by COVID-19, and to face additional barriers to screening due to COVID-19 (Castanon et al., 2021; Hawkins et al., 2020). Women who are overdue for screening are known to be at higher risk for cancer, so prioritisation strategies should also take into account any population groups that are more likely to be under-screened, to avoid widening inequalities.
4.2. Implications for policy, practice, and future research
Our findings that rapid catch-up of missed screens can keep the impact of disruptions fairly small is consistent with findings for colorectal cancer screening (de Jonge et al., 2021; Kregting et al., 2021). Based on estimates from other studies, delays in cancer diagnoses will likely have a greater impact on lives lost than disruptions to cervical screening (Maringe et al., 2020; Degeling et al., 2021), although comparisons were limited by variation between settings, and differences in methodology and reporting.
Our findings on the likely demand for HPV tests, and some information suggesting that capacity to perform these tests could be impaired by demand for COVID tests that use overlapping resources, suggests that some prioritisation during the recovery phase may be required in most or all of these settings, unless there is substantial spare capacity. Equally though, we have identified some groups at higher risk and so propose that recovery strategies should take these findings into consideration, rather than relying on opportunistic or demand-driven approaches to recovery. Prioritisation may also be required if the unconstrained demand for colposcopy exceeds capacity. In the absence of quantified capacity constraints, we have not assessed prioritisation strategies here. Further research is therefore required, first to quantify any constraints on resources, and then to identify the optimal prioritisation approach given those constraints and the extent of the disruption - all of which will be setting-specific. The prioritisation or recovery strategies that are feasible will also potentially vary by setting, as would approaches to implementing them. For example, some settings will have the ability to target women based on time since their last test or their recent test results, and to send reminders to individuals. Other settings will not have this capability and so broader demographic approaches could be used, for example targeting particular age groups, or subgroups or regions with lower screening coverage. Additionally, demographic characteristics such as age would be highly informative in settings that plan to use media campaigns as part of their recovery strategy, since demographics can directly inform content design and media purchasing decisions (as has occurred in Australia, for example). In all three countries where results by age were available for this analysis, close to half or more of additional cervical cancers would be expected to occur in those aged less than 40 in 2020, and more than 63% in those aged less than 50 in 2020. This suggests that a quite different group needs to be targeted for missed screening/ follow-up visits in cervical screening programs than in breast or colorectal screening programs (which typically do not start until at least age 50). Recovery plans also need to consider the extent to which the barriers to attendance relate to different parts of the health system (constraints due to workforce vs equipment; affecting primary vs secondary care) and also to barriers relating to the women themselves (for example reluctance to attend, financial barriers, or reduced saliency of screening).
Our findings on the groups most vulnerable to missed visits and the comparative resilience of different program designs apply beyond the specific example of disruptions due to COVID-19. These findings suggest that it is important to minimise loss to follow-up in women who are under surveillance, as well as those requiring colposcopy and treatment; that on-time screening is more critical if a woman was last screened with cytology than if she was last screened with an HPV test; and that lower or falling screening participation in women younger than 40 should be cause for concern (as for example has been seen in Australia, New Zealand and Norway), even if some of these women may have previously been offered vaccination (Smith et al., 2019; Australian Institute of Health and Welfare, 2018; Engesæter et al., 2021).
There is potential for HPV testing on a self-collected sample (self-sampling) to address some barriers to recovery. It enables a woman to be screened without attending a healthcare setting – for example kits could be mailed out directly or following a telephone or video consultation with a screening provider (VCS Foundation, 2020). Self-sampling is also more acceptable to under- and never-screened women than clinician collection, and so provides a tool to reach the women most vulnerable to screening disruptions (Arbyn et al., 2018). Self-sampling is already available to women who are overdue for screening in the Netherlands and Australia. Preliminary information suggests this has facilitated screening in the context of COVID-19 in the Netherlands (Castanon et al., 2021); however in Australia, where women cannot access self-sampling until they are aged 30+ and at least two years overdue, uptake has been relatively limited (Smith et al., 2020b). In addition to facilitating and expediting recovery, a move to offer self-sampling more widely, and to consider more flexible models of screening that are more accessible to women and do not require a clinic visit, could help address long-standing inequities in screening participation, and consequently cervical cancer burden, that exist in many countries. Scaling up self-sampling as an option will be more straightforward in countries that already offer primary HPV-based screening. Some activities that would facilitate it further in those settings include allowing self-collection devices that are low cost and readily available; HPV test manufacturers listing swabs and other self-sampling devices as collection devices that are suitable for use with their test technologies, expediting regulatory approval and validation of self-collection in individual countries; and automation of the pre-analytic process (currently self-collected samples require more hands-on processing in this phase compared to clinician-collected samples). Clinical management guidelines and pathways may also need to be updated. Regardless of the sample type used, HPV-based screening provides a stronger level and greater duration of protection for women who are screen-negative than cytology (Ronco et al., 2014; Gage et al., 2014; Dillner et al., 2008) that would be advantageous during recovery, as the overwhelming majority of women who are screen-negative would not need to be screened again for at least five years, allowing a focus on those who are screen-positive or overdue for screening. Self-collection would not resolve any capacity issues which may occur at colposcopy services though, and our findings indicate demand for these services would be expected to increase if there is rapid catch-up of missed visits. In contrast to self-collection, it is unlikely that HPV vaccination in adult women would aid in recovery, since the women affected by missed visits over a relatively short time period are those with precancers requiring treatment, or even undiagnosed cancer, and prophylactic vaccines will have no effect on these cases. Past efforts to vaccinate adolescent girls has potentially contributed to keeping the impact of screening disruptions relatively small in these countries, but vaccinating adult women has been shown to have very marginal benefits and high costs (including opportunity costs, in the context of HPV vaccine supply shortages expected to continue until 2024) (Kim et al., 2021; Laprise et al., 2020; World Health Organization, 2020b).
Our findings suggest that provided there is a rapid catch-up of missed visits, the timing of achieving cervical cancer elimination in high-income countries would not necessarily be delayed. We were limited in our ability to examine this aspect, since only Australia was predicted to have national rates fall below 4 per 100,000 in the coming decade, however, reassuringly, cervical cancer incidence rates are predicted to return to pre-disruption levels relatively soon after a rapid recovery strategy is implemented. More significantly, COVID-19-related disruptions could delay the full introduction of primary HPV screening in some settings where this was planned or partially underway, including both high- and low- and middle-income countries. This represents an important opportunity cost. Previous work has found that a delay of even one year would be associated with measurable loss, even in a setting with an existing high-quality organised cytology screening program (Castanon et al., 2019). In low- and middle-income countries the burden of cervical cancer is substantially higher than in high-income countries, but this is mostly due to the lack of effective prevention programs. Consequently, the effect of disruptions to screening programs in these settings is likely to be very small, since these programs are generally not in place or having limited impact. The more critical effect on cervical cancer prevention from COVID-19 in these settings is likely to come from delays in efforts to scale up screening and other measures to meet WHO targets. Delays in scaling up cervical cancer prevention would be associated with substantial opportunity costs, and exploring the impacts of COVID-19-related disruptions on cervical cancer prevention and elimination in low and middle income countries is the subject of ongoing work (Simms et al., 2020; Canfell, 2020; Ginsburg et al., 2021).
5. Conclusions
Rapid recovery of missed visits can keep the impact of disruptions to cervical screening and related services relatively small. Women whose last primary screening test was cytology, who are already in surveillance or follow-up, or who are aged 30–39 years appear to be the most vulnerable to disruptions. These groups could be prioritised during recovery, especially if resourcing is constrained. There were fewer cancers in the context of HPV-based screening than there were for cytology-based screening, either with or without a disruption.
Funding
This work was supported by the National Health and Medical Research Council (Australia; grant APP1159491 to MAS), Cancer Council NSW (MH, XO), Cancer Research UK (grant number C8162/A27047 to AC and MR), Cancer Institute NSW (ECF181561 to MAS), National Institutes of Health (USA; U01CA199334 to EAB and JK), Norwegian Cancer Society (#198073 to EAB).
These funders had no role in the study design; collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit.
Conflict of interest statement
MR reports that Public Health England provided financing for the epidemiological evaluation of the English HPV pilot; being a member of Public Health England's Laboratory Technology Group, HPV Self-sampling Operational Steering Group, and HPV Self-sampling Project Board; having attended meetings with various HPV assay manufacturers; fee for lecture from Hologic paid to employer (2018).
KC reports that she is co-principal investigator of an unrelated investigator-initiated trial of cervical screening in Australia (Compass; ACTRN12613001207707 and NCT02328872), which is conducted and funded by the VCS Foundation (VCS), a government-funded health promotion charity. The VCS Foundation received equipment and a funding contribution from Roche Molecular Systems USA. However, neither KC nor her institution on her behalf (Cancer Council NSW) receives direct funding from industry for this trial or any other project.
All other authors: no conflicts to declare.
CRediT authorship contribution statement
Megan A. Smith: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - original draft, Visualization, Supervision, Project administration, Funding acquisition. Emily A. Burger: Conceptualization, Methodology, Software, Investigation, Writing - review & editing, Funding acquisition. Alejandra Castanon: Conceptualization, Writing - review & editing. Inge M.C.M. de Kok: Conceptualization, Methodology, Writing - review & editing. Sharon Hanley: Conceptualization, Writing - review & editing. Matejka Rebolj: Conceptualization, Writing - review & editing. Michaela T. Hall: Methodology, Software, Investigation, Writing - review & editing. Erik E.L. Jansen: Methodology, Software, Investigation, Writing - review & editing. James Killen: Methodology, Software, Investigation, Writing - review & editing. Xavier O'Farrell: Software, Formal analysis, Writing - review & editing, Visualization. Jane J. Kim: Methodology, Writing - review & editing, Funding acquisition. Karen Canfell: Conceptualization, Supervision, Writing - review & editing, Funding acquisition.
Acknowledgments
We gratefully acknowledge Stephen Sy, Mary Caroline Regan, and Catherine Regan for assisting with submitting and processing model runs. This work was performed on behalf of the Screening Working Group (WG2) of the COVID-19 and Cancer Global Modelling Consortium (CCGMC.org) and we acknowledge all members of the CCGMC Steering Committee, Secretariat, and Working Group 2.
Editor: Eduardo Franco
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106623.
Appendix A. Supplementary data
References
- Ahrenfeldt L.J., Otavova M., Christensen K., Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Wien. Klin. Wochenschr. 2021;133:393–398. doi: 10.1007/s00508-020-01793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouli M., Nanjundappa A., Annie F., et al. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin. Proc. 2020;95:1613–1620. doi: 10.1016/j.mayocp.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbyn M., Smith S.B., Temin S., et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare . AIHW; Canberra, ACT: 2018. Cervical screening in Australia 2018. Cat. no. CAN 111.https://www.aihw.gov.au/reports/cancer-screening/cervical-screening-in-australia-2018/contents/table-of-contents [Google Scholar]
- Australian Institute of Health and Welfare . AIHW; Canberra, ACT: 2020. Cancer screening and COVID-19 in Australia, Cat. no: CAN 136.https://www.aihw.gov.au/reports/cancer-screening/cancer-screening-and-covid-19-in-australia/contents/how-has-covid-19-affected-australias-cancer-screening-programs (accessed 13th January 2021) [Google Scholar]
- Burger E.A., Ortendahl J.D., Sy S., et al. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br. J. Cancer. 2012;106:1571–1578. doi: 10.1038/bjc.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E.A., Sy S., Nygard M., et al. Too late to vaccinate? The incremental benefits and cost-effectiveness of a delayed catch-up program using the 4-valent human papillomavirus vaccine in Norway. J. Infect. Dis. 2015;211:206–215. doi: 10.1093/infdis/jiu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E., de Kok I., Groene E., et al. Estimating the natural history of cervical carcinogenesis using simulation models: a CISNET comparative analysis. J. Natl. Cancer Inst. 2020;112(9):955–963. doi: 10.1093/jnci/djz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E.A., Smith M.A., Killen J., et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):e213–e222. doi: 10.1016/S2468-2667(20)30006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E.A., Jansen E.E., Killen J., et al. Impact of COVID-19-related care disruptions on cervical cancer screening in the United States. J. Med. Screen. 2021 doi: 10.1177/09691413211001097. 9691413211001097, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Australia . Cancer Australia; Surry Hills, NSW: 2020. Review of the Impact of COVID-19 on Medical Services and Procedures in Australia Utilising MBS Data: Lung and Prostate Cancers.https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/review-impact-covid-19-medical-services-and-procedures-australia-utilising-mbs-data [Google Scholar]
- Cancer Council NSW Policy1-Cervix Documentation. 2019. https://www.policy1.org/models/cervix/documentation/policy1-cervix-v1_0.pdf (accessed 14th February 2020)
- Cancer Research UK How coronavirus is impacting cancer services in the UK. 2020. https://scienceblog.cancerresearchuk.org/2020/04/21/how-coronavirus-is-impacting-cancer-services-in-the-uk/ (accessed 13th January 2021)
- Canfell K. HPV Prevention Board online technical meeting: Impact of COVID-19 on Cervical Cancer Screening, Treatment and Vaccination, 12–13 November 2020. HPV Prevention Board; 2020. Overview of the COVID-19 and Cancer Global Modelling Consortium (CCGMC) efforts in relation to cervical cancer.https://www.uantwerpen.be/en/projects/hpv-prevention-and-control-board/meetings/technical-meeting-november-2020 Antwerp/Virtual. [Google Scholar]
- Canfell K., Kim J.J., Brisson M., et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon A., Rebolj M., Sasieni P. Is a delay in the introduction of human papillomavirus-based cervical screening affordable? J. Med. Screen. 2019;26:44–49. doi: 10.1177/0969141318800355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon A., Rebolj M., Burger E.A., et al. Optimal cervical screening COVID-19 recovery strategies in high-income countries depend on context of current programme organisation. Lancet Public Health. 2021 doi: 10.1016/S2468-2667(21)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National, state and local area vaccination coverage among adolescents aged 13–17 years - United States 2008. Morb. Mortal. Wkly Rep. 2009;58:997–1001. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National, state and local area vaccination coverage among adolescents aged 13 through 17 years - United States 2010. Morb. Mortal. Wkly Rep. 2011;60:1117–1123. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National and state vaccination coverage among adolescents aged 13-17 years--United States, 2012. MMWR Morb. Mortal. Wkly Rep. 2013;62:685–693. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National, state and local area vaccination coverage among adolescents aged 13–17 years - United States 2009. Morb. Mortal. Wkly Rep. 2010;59:1018–1023. [PubMed] [Google Scholar]
- de Jonge L., Worthington J., van Wifferen F., et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol. Hepatol. 2021;6:304–314. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degeling K., Baxter N.N., Emery J., et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia-Pacific J. Clin. Oncol. 2021 doi: 10.1111/ajco.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Rebolj M., Birembaut P., et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinmohamed A.G., Visser O., Verhoeven R.H.A., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesæter B., Baadstrand Skare G., Groeneveld L., Tropé A. 2021. Annual Report 2019: Screening Activity and Results from the Cervical Program (in Norwegian) Oslo. [Google Scholar]
- Feletto E., Grogan P., Dickson C., et al. How has COVID-19 impacted cancer screening? Adaptation of services and the future outlook in Australia. Public Health Res. Pract. 2020;30(4):3042026. doi: 10.17061/phrp3042026. [DOI] [PubMed] [Google Scholar]
- Fontham E.T.H., Wolf A.M.D., Church T.R., et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020;70:321–346. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- Gage J.C., Schiffman M., Katki H.A., et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J. Natl. Cancer Inst. 2014;106(8):dju153. doi: 10.1093/jnci/dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefenaite G., Smit M., Nijman H.W., et al. Comparatively low attendance during human papillomavirus catch-up vaccination among teenage girls in the Netherlands: insights from a behavioral survey among parents. BMC Public Health. 2012;12:498. doi: 10.1186/1471-2458-12-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg O., Basu P., Kapambwe S., Canfell K. Eliminating cervical cancer in the COVID-19 era. Nat. Cancer. 2021;2:133–134. doi: 10.1038/s43018-021-00178-9. [DOI] [PubMed] [Google Scholar]
- Hall M.T., Simms K.T., Lew J.B., et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19–e27. doi: 10.1016/S2468-2667(18)30183-X. [DOI] [PubMed] [Google Scholar]
- Hawkins R.B., Charles E.J., Mehaffey J.H. Socio-economic status and COVID-19-related cases and fatalities. Public Health. 2020;189:129–134. doi: 10.1016/j.puhe.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E., Naber S., Aitken C., et al. Cost-effectiveness of HPV-based cervical screening based on first year results in the Netherlands: a modelling study. BJOG Int. J. Obstet. Gynaecol. 2021;128:573–582. doi: 10.1111/1471-0528.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljouw S., EEL Jansen, Aitken C.A., et al. Reducing unnecessary referrals for colposcopy in hrHPV-positive women within the Dutch cervical cancer screening programme: a modeling study. Gynecol. Oncol. 2021;160(3):713–720. doi: 10.1016/j.ygyno.2020.12.038. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Burger E.A., Regan C., Sy S. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706–714. doi: 10.1001/jama.2017.19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Simms K.T., Killen J., et al. Human papillomavirus vaccination for adults aged 30–45 years in the United States: a cost-effectiveness analysis. PLoS Med. 2021;18(3):e1003534. doi: 10.1371/journal.pmed.1003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregting L.M., Kaljouw S., de Jonge L., et al. Effects of cancer screening restart strategies after COVID-19 disruption. Br. J. Cancer. 2021;124:1516–1523. doi: 10.1038/s41416-021-01261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise J.F., Chesson H.W., Markowitz L.E., et al. Effectiveness and cost-effectiveness of human papillomavirus vaccination through age 45 years in the United States. Ann. Intern. Med. 2020;172:22–29. doi: 10.7326/M19-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J., Ploner A., Elfström K.M., et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- Lew J.B., Simms K.T., Smith M.A., et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health. 2017;2:e96–e107. doi: 10.1016/S2468-2667(17)30007-5. [DOI] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijsse S.M., Naber S.K., Hontelez J.A.C., et al. The health impact of human papillomavirus vaccination in the situation of primary human papillomavirus screening: a mathematical modeling study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Public Health and the Environment (RIVM) Temporary stop to population screening [in Dutch] 2020. https://www.rivm.nl/nieuws/tijdelijke-stop-bevolkingsonderzoeken (accessed 28th January 2021)
- Portnoy A., Pedersen K., Trogstad L., et al. Impact and cost-effectiveness of strategies to accelerate cervical cancer elimination: a model-based analysis. Prev. Med. 2021;144:106276. doi: 10.1016/j.ypmed.2020.106276. [DOI] [PubMed] [Google Scholar]
- Reagan-Steiner S., Yankey D., Jeyarajah J., et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years--United States, 2014. MMWR Morb. Mortal. Wkly Rep. 2015;64:784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Steiner S., Yankey D., Jeyarajah J., et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2016;65:850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- Ritchie H., Ortiz-Ospina W., Beltekian D., et al. Our World in Data: Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations
- Ronco G., Dillner J., Elfstrom K.M., et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- Scottish Government. Health screening programmes paused. 2020. https://www.gov.scot/news/health-screening-programmes-paused/ (accessed 13th January 2021).
- Simms K.T., Laprise J.-F., Smith M.A., et al. Cost-effectiveness of the next generation nonavalent human papillomavirus vaccine in the context of primary human papillomavirus screening in Australia: a comparative modelling analysis. Lancet Public Health. 2016;1:e66–e75. doi: 10.1016/S2468-2667(16)30019-6. [DOI] [PubMed] [Google Scholar]
- Simms K., Keane A., Canfell K. HPV Prevention Board online technical meeting: Impact of COVID-19 on Cervical Cancer Screening, Treatment and Vaccination, 12–13 November 2020. HPV Prevention Board; 2020. Modelled impact of delays in elimination scale-up on cervical cancer deaths averted for 78 LMICs.https://www.uantwerpen.be/en/projects/hpv-prevention-and-control-board/meetings/technical-meeting-november-2020/ Antwerp/ virtual. [Google Scholar]
- Smith M., Rumlee L., Canfell K. National Screening Unit; Wellington: 2019. National Cervical Screening Programme Monitoring Report Number 48 (1 July – 31 December 2017)https://www.nsu.govt.nz/system/files/page/master_monitoringreport48.pdf [Google Scholar]
- Smith M.A., Hall M.T., Simms K.T., et al. Modelled analysis of hypothetical impacts of COVID-19 related disruptions to the National Cervical Screening Program. 2020. https://www.health.gov.au/resources/publications/modelled-analysis-of-hypothetical-impacts-of-covid-19-related-disruptions-to-the-national-cervical-screening-program
- Smith M.A., Saville M., Canfell K. Response to: HPV swab self-collection and cervical cancer in women who have sex with women. Med. J. Aust. 2020;213:239. doi: 10.5694/mja2.50736. [DOI] [PubMed] [Google Scholar]
- Smith M.A., Winch K., Canfell K., Brotherton J.M.L. Effective HPV vaccination coverage in Australia by number of doses and two-dose spacing: what if one or two doses are sufficient? Tumour Virus Res. 2021;11(2):200216. doi: 10.1016/j.tvr.2021.200216. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Hall M., Saville M., et al. Could HPV testing on self-collected samples be routinely used in an organized cervical screening program? A modeled analysis. Cancer Epidemiol. Biomarkers Prev. 2021;30(2):268–277. doi: 10.1158/1055-9965.EPI-20-0998. [DOI] [PubMed] [Google Scholar]
- The Sex, Gender and COVID-19 Project The COVID-19 Sex-Disaggregated Data Tracker. 2021. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/?explore=country (accessed 19th April 2021)
- US Preventive Services Task Force Screening for cervical Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674–686. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- VCS Foundation Home-Based HPV Self-Collection. 2020. https://www.vcs.org.au/pathology/hpv-self-collection/home-based-hpv-self-collection/
- Watson M., Benard V., Flagg E.W. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003–2014. Prev. Med. Rep. 2018;9:124–130. doi: 10.1016/j.pmedr.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson E. How cancer services are fighting to counter COVID-19’s impact. BMJ. 2020;370:m2747. doi: 10.1136/bmj.m2747. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Global strategy to accelerate the elimination of cervical cancer as a public health problem.https://www.who.int/publications/i/item/9789240014107 (accessed 13th January 2021) [Google Scholar]
- World Health Organization Global market study: HPV. 2020. https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/HPV_Global_Market_Study_Public_Summary-Nov2020.pdf?ua=1 (accessed 25th January 2021)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.