Abstract
Undifferentiated osteoclast-like giant cell pancreatic carcinoma (UC-OGC) is a rare pancreatic carcinoma that is composed of osteoclast-like giant cells among other cells and is hardly described in literature due to its infrequent presentation. With that, a rare symptom of pancreatic cancers is upper gastrointestinal (GI) bleeding. We report a 76-year-old African American male who presented with one episode of bloody emesis associated with intermittent episodes of severe abdominal pain and a 25 lbs of unintentional weight loss secondary to metastatic UC-OGC. The patient was stabilized and referred to an oncologist for further treatment. We present this case to add to the existing literature on UC-OGC of the pancreas.
Keywords: gastrointestinal cancer, metastatic, giant cell tumors, gastrointestinal bleed, mucinous cystic neoplasms, pancreatic cancer, osteoclast-like giant cell pancreatic carcinoma, undifferentiated osteoclast-like giant cell pancreatic carcinoma
Introduction
Mucinous cystic neoplasms (MCNs) are rare lesions of the tail of the pancreas, accounting for 2%-5% of all exocrine pancreatic lesions [1]. They are typically formed in the tail of the pancreas and are usually noninvasive; however, they can undergo malignant changes. When invasive, MCNs can transform into a variety of other types of malignant lesions but less commonly develop into undifferentiated osteoclast-like giant cell tumors [2].
Histologically, it is suggested that undifferentiated osteoclast-like giant cell pancreatic carcinoma (UC-OGC) comes from both epithelial and mesenchymal origin, with positive immunohistochemical staining for CEA and keratin favoring epithelial origin and positive CD68 and vimentin favoring mesenchymal derivation. Compared to its counterpart, pleomorphic giant cell tumor and adenocarcinoma, UC-OGC has a better prognosis because it is histologically less aggressive with slow metastasis. The survival rate can vary from person to person from four months to 10 years. It has a similar outcome to ductal pancreatic cancer, however, with a median patient survival after diagnosis of 11 months. This is most likely due to it being diagnosed late in the cancer process [3,4]. The following case demonstrates a 76-year-old male with a rare presentation of rare cancer.
Case presentation
We report on a 76-year-old African American male with no relevant medical history throughout his life who was brought to the emergency department by his wife for a syncopal episode. For the past two months, he has been having intermediate episodes of severe upper abdominal pain, nausea, dry heaves, non-bloody emesis, early satiety, increased frequency of stooling, and a 25-lb unintentional weight loss over a two-month period. At the time, he denied diarrhea, melena, and hematochezia. The abdominal examination was positive for tenderness to palpation of both upper quadrants, and the rest of the physical examination was unremarkable. Blood work and imaging were ordered on admission, and the laboratory results are shown in Table 1.
Table 1. Pertinent laboratory results during the first admission.
L: laboratory value below the reference range, H: laboratory value above the reference range, TIBC: total iron-binding capacity
| Collected parameter | Value | Reference range |
| WBC | 7.06 | 4.50-10 × 103/uL |
| RBC | 2.79 L | 4.40-5.60 × 106/uL |
| HBG | 8.2 L | 13-17 g/dL |
| HCT | 24.5 L | 39.6%-50% |
| MCV | 87.8 | 80-97 fL |
| MCH | 29.4 | 27-33.9 pg |
| MCHC | 33.5 | 33.2-35.4 g/dL |
| RDW | 14.4 | 11.5%-14.5% |
| PLT | 190 | 140-440 × 103/uL |
| Iron | 29 L | 65-175 ug/dL |
| TIBC | 153 L | 228-460 ug/dL |
| Iron saturation | 18.95 | 15%-50% |
| Na+ | 141 | 135-147 mmol/L |
| K+ | 2.90 L | 3.50-5.30 mmol/L |
| Cl- | 104 | 96-112 mmol/L |
| BUN | 7 | 7-20 mg/dL |
| CRE | 0.730 | 0.5550-1.300 mg/dL |
| BUN/CR | 9.59 L | 12-20 |
| Ca2+ | 8.80 | 8.20-10.60 mg/dL |
| Direct bilirubin | 1.02 H | 0-0.40 mg/dL |
| Total bilirubin | 2.10 H | 0.20-1 mg/dL |
| Indirect bilirubin | 1.08 H | 0-1 mg/dL |
| Alkaline phosphatase | 107 | 41-126 U/L |
| ALT | 70 H | 10-49 U/L |
| AST | 68 H | 14-35 U/L |
A computed tomography (CT) scan of the abdomen and pelvis with contrast revealed several pancreatic lesions along with ductal dilation within the tail of the pancreas, dilation of the common bile duct (CBD), and intrahepatic biliary ducts. One left kidney lesion and multiple hepatic lesions were also visualized. This prompted an endoscopic retrograde cholangiopancreatography (ERCP) with cold snare removal of a protruding bile duct stone, sphincterotomy, brushing of the CBD for malignancy, and placement of a metal stent with adequate drainage. Cytology report from CBD brushing revealed no malignant cells. A magnetic resonance imaging (MRI) with and without contrast revealed an 8.2 × 5.1 cm expansile pancreatic body/tail mass with pancreatic ductal dilation, a 3.1 × 2.9 cm pancreatic head mass, a 2.1 × 2 cm left kidney mass, and multiple hepatic lesions consistent with metastatic disease. Interventional radiology was consulted, and the patient underwent an ultrasound-guided biopsy of liver lesions. Tumor cells were positive for CD68, CD163, and vimentin histiocytic markers in keeping with monocytic differentiation of osteoclastic giant cells. KRAS, ARID1A, CDKN2A, DKM6A, NF1, and TP53 mutations were observed in the tumor cells. Histochemical and morphologic features were consistent with osteoclast-like giant cell tumors of the pancreas. Once stabilized, the patient was discharged with instructions to follow up with an oncologist.
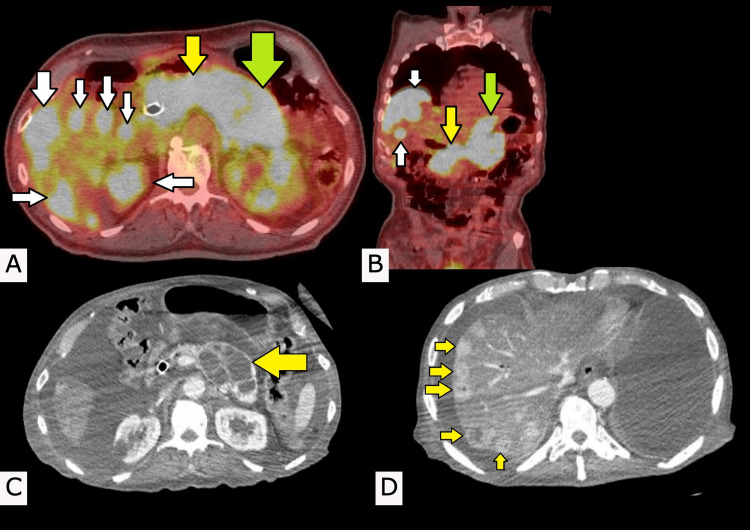
One month later, he then presented to the emergency department for two syncopal episodes and was accompanied by hematemesis with a hemoglobin of 6.8 gm/dL and reticulocytes at 3.16%. The patient was transfused one unit of blood for low hemoglobin and underwent esophagogastroduodenoscopy, which was unremarkable. The upper gastrointestinal (GI) bleed was presumed to be due to the pancreatic masses. Furthermore, a positron emission tomography-computed tomography (PET-CT) was done, and it revealed an innumerable amount of lesions throughout the liver along with abnormal metabolism with the standardized uptake values (SUVs) in the 14-18 range. There was also a large mass involving the body and tail of the pancreas with considerable metabolism, with a maximum SUV of about 18. Additionally, a bile duct stent was seen in the distal common bile duct region, placed a few weeks prior (Figure 1A, 1B). The patient was eventually deemed stable by all teams and was discharged home with instructions to follow up with oncology for further treatment.
After his discharge, the patient further underwent treatment with the chemotherapy regimen of Folfirinox. During his treatment at our facilities, CT images with contrast were taken (Figure 1C, 1D). The radiologist reported diffuse hepatic steatosis with numerous enhancing lesions, many of which have central cystic/necrotic components scattered throughout the liver. There is pneumobilia with a common bile duct stent in place. Multi-lobular cystic enlargement involves the body and tail of the pancreas, which contain multiple thickened internal septations. There was a hypoattenuating lesion in the left kidney. He continued his regime for six months with lesions mildly decreasing in size, until he transitioned to hospice care.
Figure 1. Positron emission tomography (PET) scans and computed tomography (CT) scans.
(A) Axial and (B) coronal PET scans displaying a large mass involving the body (yellow arrows) and tail (green arrows) of the pancreas with innumerable masses throughout the liver (white arrows). These lesions with considerate metabolism are indicated by hyperlucent (bright white) areas on the scan. (C) Multicystic enlargement of the body and tail of the pancreas (yellow arrow). (D) Multiple enhanced lesions of the liver, some of which are cystic or necrotic in nature (yellow arrows).
Discussion
UC-OGC is difficult to diagnose due to its similar presentation to pancreatic ductal adenocarcinoma. Additionally, UC-OGC can be described as “pure” or as a “mixed” carcinoma associated with other types of pancreatic cancers. The genetic pathways of pure and mixed carcinoma are described in the literature. Some differentiating signs between ductal adenocarcinoma and UC-OGC are on imaging; typically, UC-OGC is larger and has cystic components compared to ductal adenocarcinoma. UC-OGC can produce an intraductal polypoid mass causing dilation of the bile and pancreatic duct, leading to hyperbilirubinemia and/or jaundice and associated symptoms, similar to our case. Also, calcification, necrotic areas, venous tumor thrombosis, and hemorrhage have been described in these tumors as well. However, the best way to diagnose UC-OGC is through biopsy and immunohistochemical staining [5].
On histology, UC-OGC appears as benign giant cell tumors of bone that contain a combination of osteoclast-like multinucleated and mononuclear cells. Histologically, it is suggested that UC-OGC comes from both epithelial and mesenchymal origin, staining positive for CEA and keratin favoring epithelial origin and positive for CD68 and vimentin favoring mesenchymal derivation, and can or cannot contain mutations against p53. However, it has a similar outcome to common ductal pancreatic cancer, with a median patient survival after diagnosis of 12 months [3-6]. In our patient’s case, tumor cells were positive for KRAS, ARID1A, CDKN2A, DKM6A, NF1, and TP53 mutations. Other literature describes mutations in KRAS, TP53, CDKN2A, and SMAD4, but as far as we know, none describe DKM6A and NF1 mutations correlating with UC-OGC [5].
There is no standardized treatment for UC-OGC because of how rare the occurrence of this disease is. Thus, chemotherapy and/or radiation therapy for cancer is not well documented [5,7]. However, some literature suggests using the standardized treatment regime for ductal pancreatic carcinoma because UC-OGC is a variant. The first-line treatment for ductal pancreatic carcinoma is surgical resection with or without conjunctive chemotherapy and/or radiation therapy. A few articles also proposed using monoclonal antibodies against PD-1 and PD-L1 due to their effectiveness against a wide variety of cancers, including giant cell cancers in other organs [5,8-13]. Finally, when taking the approach to treating this and other rare cancers, the providers need to have flexible thinking and creative use of their knowledge of the pathophysiology of these cancers to treat them properly.
Gastrointestinal bleeding is a rare complication of pancreatic cancer. Only 2.6% of patients with pancreatic cancer experience an episode of upper GI bleeding [14]. The diagnosis can be established using endoscopic or surgical methods, although conventional endoscopic evaluation has been unsuccessful in some cases, including the case in point. Physicians should be aware that the severity of the bleeding in these patients can vary from occult bleeding to severe hemorrhagic bleeding, leading to hypovolemic shock. Treatment options include endoscopic hemostasis, arterial embolization, and surgery. Failure to achieve hemostasis results in an extremely poor prognosis [14].
Conclusions
We present this case to add to the existing literature on UC-OGC of the pancreas. Due to the rarity of this condition, it renders the possibility of cohort studies unlikely, leaving case reports and meta-analyses as the majority of literature on this topic. With that, long-term follow-up along with thorough and detailed history-taking of these patients is important to guide management and understand the prognosis of this cancer. In the existing literature, the number one treatment option is surgery. However, in this case, due to metastasis, chemoradiation or immunotherapy may be considered as initial management or conjunctive treatment with surgery. Finally, to our best knowledge, no case of UC-OGC described in the literature presented with an upper gastrointestinal bleed.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.An osteoclast-like giant cell tumor embedded in the mural nodule of a pancreatic mucinous cystic neoplasm: a case report and literature review. Fan X, Wang W, Li C, Tang T, Han Y, An K. http://org.proxy2.cl.msu.edu/10.1097/MD.0000000000015246. Medicine (Baltimore) 2019;98:0. doi: 10.1097/MD.0000000000015246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucinous cystic neoplasms of pancreas. Naveed S, Qari H, Banday T, Altaf A, Para M. http://org.proxy2.cl.msu.edu/10.14740/gr600e. Gastroenterology Res. 2014;7:44–50. doi: 10.14740/gr600e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osteoclastic giant cell tumor of the pancreas. Temesgen WM, Wachtel M, Dissanaike S. http://org.proxy2.cl.msu.edu/10.1016/j.ijscr.2014.01.002. Int J Surg Case Rep. 2014;5:175–179. doi: 10.1016/j.ijscr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osteoclastic giant cell tumor of the pancreas: case report and literature review. Leighton CC, Shum DT. https://journals-lww-com.proxy2.cl.msu.edu/amjclinicaloncology/Fulltext/2001/02000/Osteoclastic_Giant_Cell_Tumor_of_the_Pancreas_.14.aspx. Am J Clin Oncol. 2001;24:77–80. doi: 10.1097/00000421-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: what do we know so far? Demetter P, Maréchal R, Puleo F, et al. http://org.proxy2.cl.msu.edu/10.3389/fonc.2021.630086. Front Oncol. 2021;11:630086. doi: 10.3389/fonc.2021.630086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. Luchini C, Pea A, Lionheart G, et al. http://org.proxy2.cl.msu.edu/10.1002/path.4941. J Pathol. 2017;243:148–154. doi: 10.1002/path.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. http://www.serena.unina.it/index.php/jop/article/view/3349/3596. JOP. 2011;12:170–176. [PubMed] [Google Scholar]
- 8.PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. http://org.proxy2.cl.msu.edu/10.1056/NEJMoa1411087. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Hamid O, Robert C, Daud A, et al. http://org.proxy2.cl.msu.edu/10.1093/annonc/mdz011. Ann Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. Brahmer JR, Tykodi SS, Chow LQ, et al. http://org.proxy2.cl.msu.edu/10.1056/NEJMoa1200694. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerging role of immunotherapy in advanced urothelial carcinoma. Koshkin VS, Grivas P. http://org.proxy2.cl.msu.edu/10.1007/s11912-018-0693-y. Curr Oncol Rep. 2018;20:48. doi: 10.1007/s11912-018-0693-y. [DOI] [PubMed] [Google Scholar]
- 12.Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Schmid P, Rugo HS, Adams S, et al. https://www-sciencedirect-com.proxy2.cl.msu.edu/science/article/pii/S1470204519306898?via%3Dihub. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 13.Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Colevas AD, Bahleda R, Braiteh F, et al. http://org.proxy2.cl.msu.edu/10.1093/annonc/mdy411. Ann Oncol. 2018;29:2247–2253. doi: 10.1093/annonc/mdy411. [DOI] [PubMed] [Google Scholar]
- 14.Upper gastrointestinal bleeding - initial manifestation of pancreatic head carcinoma. Obleagă CV, Vere CC, Mogoanţa SŞ, et al. http://org.proxy2.cl.msu.edu/10.12865/CHSJ.43.03.09. Curr Health Sci J. 2017;43:236–240. doi: 10.12865/CHSJ.43.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]