Abstract
STUDY QUESTION
What outcomes should be reported in all studies investigating uterus-sparing interventions for treating uterine adenomyosis?
SUMMARY ANSWER
We identified 24 specific and 26 generic core outcomes in nine domains.
WHAT IS KNOWN ALREADY
Research reporting adenomyosis treatment is not patient-centred and shows wide variation in outcome selection, definition, reporting and measurement of quality.
STUDY DESIGN, SIZE, DURATION
An international consensus development process was performed between March and December 2021. Participants in round one were 150 healthcare professionals, 17 researchers and 334 individuals or partners with lived experience of adenomyosis from 48 high-, middle- and low-income countries. There were 291 participants in the second round.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Stakeholders included active researchers in the field, healthcare professionals involved in diagnosis and treatment, and people and their partners with lived experience of adenomyosis. The core component of the process was a 2-step modified Delphi electronic survey. The Steering Committee analysed the results and created the final core outcome set (COS) in a semi-structured meeting.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 241 outcomes was identified and distilled into a ‘long list’ of 71 potential outcomes. The final COS comprises 24 specific and 26 generic core outcomes across nine domains, including pain, uterine bleeding, reproductive outcomes, haematology, urinary system, life impact, delivery of care, adverse events and reporting items, all with definitions provided by the Steering Committee. Nineteen of these outcomes will apply only to certain study types. Although not included in the COS, the Steering Committee recommended that three health economic outcomes should be recorded.
LIMITATIONS, REASONS FOR CAUTION
Patients from continents other than Europe were under-represented in this survey. A lack of translation of the survey might have limited the active participation of people in non-English speaking countries. Only 58% of participants returned to round two, but analysis did not indicate attrition bias. There is a significant lack of scientific evidence regarding which symptoms are caused by adenomyosis and when they are related to other co-existent disorders such as endometriosis. As future research provides more clarity, the appropriate review and revision of the COS will be necessary.
WIDER IMPLICATIONS OF THE FINDINGS
Implementing this COS in future studies on the treatment of adenomyosis will improve the quality of reporting and aid evidence synthesis.
STUDY FUNDING/COMPETING INTEREST(S)
No specific funding was received for this work. T.T. received a grant (grant number 2020083) from the South Eastern Norwegian Health Authority during the course of this work. T.T. receives personal fees from General Electrics and Medtronic for lectures on ultrasound. E.R.L. is the chairman of the Norwegian Endometriosis Association. M.G.M. is a consultant for Abbvie Inc and Myovant, receives research funding from AbbVie and is Chair of the Women’s Health Research Collaborative. S.-W.G. is a board member of the Asian Society of Endometriosis and Adenomyosis, on the scientific advisory board of the endometriosis foundation of America, previous congress chair for the World Endometriosis Society, for none of which he received personal fees. E.S. received outside of this work grants for two multicentre trials on endometriosis from the National Institute for Health Research UK, the Rosetrees Trust, and the Barts and the London Charity, he is a member of the Medicines and Healthcare Products Regulatory Agency (MHRA), Medicines for Women’s Health Expert Advisory Group, he is an ambassador for the World Endometriosis Society, and he received personal fees for lectures from Hologic, Olympus, Medtronic, Johnson & Johnson, Intuitive and Karl Storz. M.H. is member of the British Society for Gynaecological Endoscopy subcommittee. No other conflict of interest was declared.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: adenomyosis, core outcome set, stakeholder, Delphi, reporting, outcomes, patient centredness, quality of care, quality of life
Introduction
Adenomyosis is a benign condition affecting the uterus, and it can be associated with a significant symptom burden, mainly painful or heavy menstrual periods (Li et al., 2014), chronic pelvic pain and reduced quality of life (QoL) (Li et al., 2014; Choi et al., 2017). The disorder has also been associated with reduced fertility and poor reproductive and obstetric outcomes, including an increased risk of miscarriage, pre-eclampsia, pre-term delivery and postpartum haemorrhage (Tamura et al., 2017; Younes et al., 2017; Bruun et al., 2018; Hashimoto et al., 2018; Bourdon et al., 2020). Adenomyosis is assumed to be present in about 20% of women attending a general gynaecology clinic (Naftalin et al., 2012), and is also common in women undergoing infertility treatment (Puente et al., 2016). In recent years, it became evident that young women also suffer from adenomyosis (Chapron et al., 2020).
Despite the seemingly high prevalence of adenomyosis and its clinical relevance, there is a lack of well-designed clinical trials comparing different options for treating adenomyosis. Furthermore, studies investigating therapeutic interventions for adenomyosis have used many different outcomes and outcome measures, making it challenging to perform a meta-analysis and thus severely curtailing the usefulness of research to inform clinical practice and guidelines (Tellum et al., 2021a). As demonstrated in a previous review, only a few studies on adenomyosis report patient-centred outcomes (Tellum et al., 2021a), which questions their benefit to patients. The selection of appropriate outcomes is crucial when designing clinical trials that evaluate the effects of different interventions. Requiring a standardized set of consensus core outcomes minimizes the risk of bias that results from the investigator ‘cherry-picking’ positive or attractive results for inclusion with the omission of negative or less interesting evidence (Dwan et al., 2013). The development and use of so-called core outcome sets (COS) are widely supported and encouraged in medicine, and has led to the development of a variety of COS under the umbrella of the Core Outcome Measures in Effectiveness Trials (COMET) and Core Outcomes in Women’s and Newborn Health (CROWN) initiatives.
In order to fill this void in the field of adenomyosis, the aim of this consensus study was to develop a COS for adenomyosis research (COSAR) on uterus-sparing interventional studies for symptoms associated with adenomyosis, which was defined as the presence of ectopic endometrial cells and stroma within the myometrium. The scope of the COSAR includes all types of treatment of adenomyosis in premenopausal women and can be applied to all kinds of prospective studies.
We asked the women who participated in the focus group workshops and the patient advocacy members how would they like to be referred to while taking part in this consensus development (lay consumer, public research partner or patient), and ‘patient’ was the term they preferred.
Materials and methods
Protocol/registry entry
The protocol for COSAR was prospectively published (Tellum et al., 2021b) and the project was registered with the COMET initiative (registration number 1649).
Participants, participant recruitment and patient involvement
A Steering Committee was formed comprising specialists with different expertise in the sub-field of adenomyosis (infertility, surgery, diagnostics, basic science) and a patient advocate. In this setting, a patient advocate represents a patient organization and, in contrast to a patient, their focus is not on their personal, lived experience with a condition. The Steering Committee identified three main stakeholder groups to inform the construct of the COS: researchers with expertise in the field, healthcare professionals involved in diagnosis and therapy (doctors, nurses, physical therapists), and patients and their relatives with lived experience of adenomyosis. Potential participants from the personal network of the Steering Committee were contacted directly, as were researchers with highly relevant publications in the field who were identified through a literature search. A systematic web search was used to create a comprehensive, global list of national gynaecological associations and adenomyosis and endometriosis patient advocacy organizations. They were contacted by the managing team with a request to distribute the Delphi survey amongst their members. Participants were further recruited through social media, congresses, and courses and through the network of the World Endometriosis Society (WES) that was represented on the Steering Committee. A website was created to provide information for all participants (www.cosar.org).
Information sources for the long list
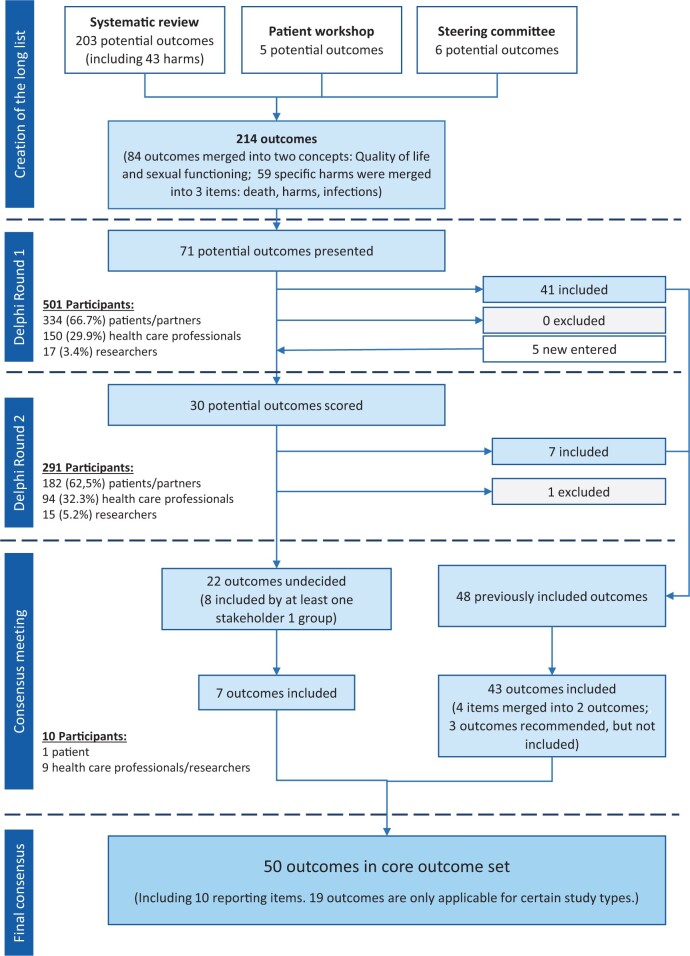
A structured literature review, performed to identify previously reported outcomes (Tellum et al., 2021a), resulted in the creation of a preliminary long list. Additional items were added through patient (focus group) workshops and the Steering Committee (Fig. 1). Finally, using the taxonomy developed and recommended by the COMET initiative (Dodd et al., 2018) the Steering Committee structured the long list into core areas by removing redundant items and merging others according to concepts (Fig. 1). Lay terms were identified for each item on the long list, first in a workshop with patients for whom English was their native language and then the terms were modified in face-to-face meetings with non-native speakers living in other countries.
Figure 1.
Flow chart illustrating the Delphi process with inclusion and exclusion of potential outcomes for studies on uterus-sparing treatments of adenomyosis.
Delphi survey and consensus process
The Delphi technique is a well-established approach to answering a research question through the identification of a consensus view across subject experts, and it is recommended by the COMET initiative for establishing COS (Williamson et al., 2017; Barrett et al., 2020). An electronic Delphi survey was developed on a web-based platform (Nettskjema, University Information Technology Center, University of Oslo, Norway) and piloted with 18 individuals, representing all stakeholder groups, before the launch. After discussion within the Steering Committee and following advice from COMET, it was decided not to perform translations of the survey into languages other than English. Part of the rationale for this decision was time and available funding, and part was the absence of consensus regarding which of the many languages to select. By using non-native English speakers to assess and modify the lay terms of the long list, the Steering Committee tried to ensure that it would be understood by most people.
Items on the long list were presented alphabetically within each core area (McColl et al., 2001). The consensus process was performed as a 2-step modified Delphi procedure, comprising two survey rounds and a final consultation meeting of the Steering Committee. For round 1, the Delphi survey was distributed via a website link or QR-code through presentations at conferences, courses, social media, and member or individual emails to stakeholders and stakeholder organizations. For round 2, all participants from the first round received an invitation by individual email and then three email reminders if they had not responded.
The modified Delphi process allowed participants to leave comments and suggestions for new items in the first round and provided summarized feedback to those who participated in the second round, allowing them to change their score by considering the opinions of others (Fish et al., 2020). The items that did not reach consensus through both survey rounds were discussed in a semi-structured face-to-face consultation meeting within the Steering Committee. Decisions to include or exclude were made by discussion and majority vote.
Outcome scoring
Each item was graded from 1 to 9 (De Meyer et al., 2019), with the additional option “I can’t rate the outcome because I don’t know the outcome”. Written anchors were provided to reduce measurement error (Beckstead, 2014; Remus et al., 2021) (1. Extremely unimportant; 2. Very unimportant; 3. Unimportant; 4. Maybe unimportant; 5. Unsure unimportant or important; 6. Maybe important; 7. Important; 8. Very important; 9. Extremely important). Scores of 1–3 signified an outcome of limited importance, scores of 4–6 signified an outcome as important but not critical and scores of 7–9 signified an outcome as critical, as defined by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (Guyatt et al., 2011).
Consensus definition
A consensus that an outcome should not be included in the COS was defined as 70% or more participants scoring it as 1–3 and fewer than 15% scoring it as 7–9. Consensus for an outcome being included in the COS required 70% or more scoring it as 7–9 and fewer than 15% to score it as 1–3. If an outcome was included by one stakeholder group but not the others, the item was discussed in the Steering Committee consensus meeting (Williamson et al., 2017). If no agreement was to be reached by discussion, the decision was determined by majority opinion.
Data collection and analysis
All data and Delphi scores were collected securely via the ‘Nettskjema’ platform. All fields were mandatory to avoid missing data. Participants entered their email addresses to avoid and identify duplicate entries. Data were reported using ranking orders, percentages and frequencies. A P-value <0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics, Version 22.0 (IBM Corp, Armonk, NY, USA) and Microsoft® Excel®, Version 2111 (Microsoft Corp., Redmond, WA, USA).
Ethics and consent
Institutional review board and Personal Data Officer approval were obtained from the Oslo University Hospital. Owing to the nature of this study, approval from the Regional Committee for medical and health research Ethics system in Norway was waived. The participation in the survey was voluntary, and by participating, the participants gave their consent to be included in the study.
Results
Distribution of Delphi, participants and response rates
The Delphi was piloted between May 10 and 15, 2021, the first round was disseminated and remained open between May 20 and July 30, 2021, and the second round between August 29 and November 04, 2021. The questionnaires and the detailed responses sorted by stakeholder group are provided in Supplementary Data Files S1 and S2. The Steering Committee consensus meeting was held on December 04, 2021.
In the first round, 501 respondents from 48 countries completed the survey, including 327 patients, 7 partners, 17 researchers and 150 health care professionals (HCPs). Figure 2 displays an overview of the countries represented; the participants according to country and stakeholder group are listed in Supplementary Table SI. Amongst HCPs, there were two chiropractors, two physical therapists, six radiologists or ultrasonologists, two midwives and one general practitioner; all other respondents were gynaecologists. In the second round, there were 291 participants from 38 countries resulting in a return rate of 58.1% (Fig. 1). There was no statistically significant difference in distribution between the stakeholder groups between the two rounds (P > 0.2) (Fig. 1). Owing to the high attrition rate, we analysed the results for differences between the two groups (those that did and did not return in round two) and found none.
Figure 2.
World map, colours indicating the number of participants from each country.
Core outcome set
The final COSAR is displayed in Table I and the response rates for the included items provided in Table II. The detailed response rates per outcome are provided in Supplementary Data Files S1 and S2. The discussion leading to the final COS is outlined below. Definitions for each outcome were determined by the Steering Committee, and they can be found in Table III, where the lay terms used are also listed. Ten members attended the final Steering Committee consensus meeting. The Committee recognized that symptoms suggested by patients as outcomes could be caused by concomitant disorders, such as endometriosis, and not necessarily by adenomyosis. After discussion, the Steering Committee decided it would neither be practical nor clinically meaningful to restrict this COS to women with adenomyosis alone and exclude those with endometriosis or fibroids, as the conditions frequently occur together. Furthermore, the Steering Committee noted a lack of evidence to determine which symptoms would be caused by adenomyosis alone. The overall view was that outcomes should not be prejudged and that the example set by the patients should be followed when discussing the inclusion of symptoms unless there was a strong reason not to. If future studies show that some symptoms are not associated with adenomyosis, they could be excluded at that time.
Table I.
Overview of the core outcome sets for studies on uterus-sparing treatments of adenomyosis, structured into core domains.
| Category | Outcome |
|---|---|
| Pain | Cyclic pelvic pain |
| Dyschezia | |
| Dysmenorrhea | |
| Dyspareunia | |
| Non-cyclic, untriggered pelvic pain | |
| Pelvic bulk/pressure symptoms | |
| Radiating pain to lower back and/or extremities during menstruation | |
| Urinary system | Urinary frequency |
| Menstrual bleeding | Blood flow volume |
| Duration of bleeding | |
| Intermenstrual bleeding | |
| Frequency of bleeding/regularity of cycle | |
| Reproductive outcomes* | Infertility Core Outcome Set*: |
| Live, correctly sited (eutopic) pregnancy | |
| Pregnancy loss: | |
| Ectopic pregnancy | |
| Miscarriage | |
| Stillbirth | |
| Termination of pregnancy | |
| Live birth | |
| Gestational age at delivery | |
| Birthweight | |
| Neonatal mortality | |
| Major congenital anomaly | |
| Time to pregnancy leading to live birth | |
| Additional outcomes: | |
| Mode of Conception | |
| Postpartum Haemorrhage | |
| Abnormal placentation | |
| Haematology | Anaemia |
| Life impact | Health-related QOL |
| Sexual function (Including bleeding or pain during or after sexual activity) | |
| Delivery of care | Patient adherence to treatment |
| Patient satisfaction with treatment | |
| Symptom relief rate for most bothersome symptom | |
| Symptom recurrence for any symptom | |
| Symptom recurrence for most bothersome symptom | |
| Lesion size | |
| Uterus volume | |
| Discomfort During Procedure* | |
| Recovery Time* | |
| Need for repeated or other treatment (Need for re-intervention)* | |
| Length of hospital stay* | |
| Premature termination of procedure* | |
| Adverse outcomes | Adverse outcomes (including all harms, adverse reactions and side effects) |
| Infections* | |
| Unplanned/unscheduled bleeding on hormonal medication | |
| Reporting items | Endometriosis present |
| Fibroids present | |
| Chronic pelvic pain present | |
| Wish for future pregnancy | |
| Classification of adenomyosis | |
| Previous treatment for adenomyosis | |
| Recommended outcomes (not mandatory) | |
| Economy | Costs of treatment |
| How much the patient has to pay for a treatment (Patient costs) | |
| Value-for-money of treatment (Cost-utility analysis) | |
Outcome(s) applicable in certain study types only.
Table II.
Rating of the outcomes in the final core outcome set, by stakeholder group.
| Outcome | Rating | Stakeholder group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient n % | Partner n % | HCP n % | Researcher n % | Total n % | |||||||
| Cyclic pelvic pain | I don’t know OC | 3 | 0.9% | 0 | 0.0% | 0 | 0.0% | 1 | 5.9% | 4 | 0.8% |
| Exclude | 8 | 2.4% | 0 | 0.0% | 6 | 4.0% | 1 | 5.9% | 15 | 3.0% | |
| Undecided | 9 | 2.8% | 1 | 14.3% | 6 | 4.0% | 0 | 0.0% | 16 | 3.2% | |
| Include | 307 | 93.9% | 6 | 85.7% | 138 | 92.0% | 15 | 88.2% | 466 | 93.0% | |
| Dyschezia | I don’t know OC | 19 | 5.8% | 2 | 28.6% | 1 | 0.7% | 1 | 5.9% | 23 | 4.6% |
| Exclude | 10 | 3.1% | 0 | 0.0% | 10 | 6.7% | 2 | 11.8% | 22 | 4.4% | |
| Undecided | 49 | 15.0% | 2 | 28.6% | 36 | 24.0% | 3 | 17.6% | 90 | 18.0% | |
| Include | 249 | 76.1% | 3 | 42.9% | 103 | 68.7% | 11 | 64.7% | 366 | 73.1% | |
| Dysmenorrhea | I don’t know OC | 5 | 1.5% | 1 | 14.3% | 1 | 0.7% | 1 | 5.9% | 8 | 1.6% |
| Exclude | 9 | 2.8% | 0 | 0.0% | 4 | 2.7% | 0 | 0.0% | 13 | 2.6% | |
| Undecided | 11 | 3.4% | 1 | 14.3% | 1 | 0.7% | 1 | 5.9% | 14 | 2.8% | |
| Include | 302 | 92.4% | 5 | 71.4% | 144 | 96.0% | 15 | 88.2% | 466 | 93.0% | |
| Dyspareunia | I don’t know OC | 14 | 4.3% | 1 | 14.3% | 1 | 0.7% | 1 | 5.9% | 17 | 3.4% |
| Exclude | 14 | 4.3% | 0 | 0.0% | 5 | 3.3% | 0 | 0.0% | 19 | 3.8% | |
| Undecided | 24 | 7.3% | 0 | 0.0% | 21 | 14.0% | 2 | 11.8% | 47 | 9.4% | |
| Include | 275 | 84.1% | 6 | 85.7% | 123 | 82.0% | 14 | 82.4% | 418 | 83.4% | |
| Non-cyclic. untriggered pelvic pain | I don’t know OC | 6 | 1.8% | 1 | 14.3% | 1 | 0.7% | 0 | 0.0% | 8 | 1.6% |
| Exclude | 13 | 4.0% | 0 | 0.0% | 9 | 6.0% | 1 | 5.9% | 23 | 4.6% | |
| Undecided | 33 | 10.1% | 1 | 14.3% | 29 | 19.3% | 5 | 29.4% | 68 | 13.6% | |
| Include | 275 | 84.1% | 5 | 71.4% | 111 | 74.0% | 11 | 64.7% | 402 | 80.2% | |
| I don’t know OC | 0 | 0.0% | 1 | 50.0% | 0 | 0.0% | 0 | 0.0% | 1 | 0.3% | |
| Pelvic bulk/pressure symptoms | Exclude | 6 | 3.3% | 0 | 0.0% | 3 | 3.2% | 1 | 6.7% | 10 | 3.4% |
| Undecided | 14 | 7.7% | 0 | 0.0% | 18 | 19.4% | 3 | 20.0% | 35 | 12.0% | |
| Include | 161 | 89.0% | 1 | 50.0% | 72 | 77.4% | 11 | 73.3% | 245 | 84.2% | |
| Radiating pain to lower back and/or extremities during menstruation | I don’t know OC | 3 | 1.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 3 | 1.0% |
| Exclude | 10 | 5.5% | 0 | 0.0% | 9 | 9.7% | 0 | 0.0% | 19 | 6.5% | |
| Undecided | 26 | 14.4% | 0 | 0.0% | 40 | 43.0% | 8 | 53.3% | 74 | 25.4% | |
| Include | 142 | 78.5% | 2 | 100.0% | 44 | 47.3% | 7 | 46.7% | 195 | 67.0% | |
| Urinary frequency | I don’t know OC | 18 | 5.5% | 3 | 42.9% | 1 | 0.7% | 1 | 5.9% | 23 | 4.6% |
| Exclude | 13 | 4.0% | 0 | 0.0% | 18 | 12.0% | 2 | 11.8% | 33 | 6.6% | |
| Undecided | 61 | 18.7% | 2 | 28.6% | 59 | 39.3% | 5 | 29.4% | 127 | 25.3% | |
| Include | 235 | 71.9% | 2 | 28.6% | 72 | 48.0% | 9 | 52.9% | 318 | 63.5% | |
| Blood flow volume | I don’t know OC | 8 | 2.4% | 1 | 14.3% | 0 | 0.0% | 1 | 5.9% | 10 | 2.0% |
| Exclude | 8 | 2.4% | 0 | 0.0% | 6 | 4.0% | 0 | 0.0% | 14 | 2.8% | |
| Undecided | 24 | 7.3% | 0 | 0.0% | 4 | 2.7% | 1 | 5.9% | 29 | 5.8% | |
| Include | 287 | 87.8% | 6 | 85.7% | 140 | 93.3% | 15 | 88.2% | 448 | 89.4% | |
| Duration of bleeding | I don’t know OC | 9 | 2.8% | 1 | 14.3% | 0 | 0.0% | 1 | 5.9% | 11 | 2.2% |
| Exclude | 10 | 3.1% | 0 | 0.0% | 8 | 5.3% | 0 | 0.0% | 18 | 3.6% | |
| Undecided | 41 | 12.5% | 0 | 0.0% | 13 | 8.7% | 2 | 11.8% | 56 | 11.2% | |
| Include | 267 | 81.7% | 6 | 85.7% | 129 | 86.0% | 14 | 82.4% | 416 | 83.0% | |
| Intermenstrual bleeding | I don’t know OC | 23 | 7.0% | 1 | 14.3% | 0 | 0.0% | 2 | 11.8% | 26 | 5.2% |
| Exclude | 20 | 6.1% | 0 | 0.0% | 15 | 10.0% | 0 | 0.0% | 35 | 7.0% | |
| Undecided | 71 | 21.7% | 1 | 14.3% | 30 | 20.0% | 7 | 41.2% | 109 | 21.8% | |
| Include | 213 | 65.1% | 5 | 71.4% | 105 | 70.0% | 8 | 47.1% | 331 | 66.1% | |
| I don’t know OC | 8 | 4.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 8 | 2.7% | |
| Frequency of menstruation | Exclude | 7 | 3.9% | 0 | 0.0% | 11 | 11.8% | 3 | 20.0% | 21 | 7.2% |
| Undecided | 46 | 25.4% | 0 | 0.0% | 16 | 17.2% | 5 | 33.3% | 67 | 23.0% | |
| Include | 120 | 66.3% | 2 | 100.0% | 66 | 71.0% | 7 | 46.7% | 195 | 67.0% | |
| I don’t know OC | 8 | 4.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 8 | 2.7% | |
| Regularity of cycle | Exclude | 12 | 6.6% | 0 | 0.0% | 11 | 11.8% | 4 | 26.7% | 27 | 9.3% |
| Undecided | 47 | 26.0% | 0 | 0.0% | 17 | 18.3% | 3 | 20.0% | 67 | 23.0% | |
| Include | 114 | 63.0% | 2 | 100.0% | 65 | 69.9% | 8 | 53.3% | 189 | 64.9% | |
| Fertility Core Outcome Set | I don’t know OC | 66 | 20.2% | 1 | 14.3% | 1 | 0.7% | 0 | 0.0% | 68 | 13.6% |
| Exclude | 13 | 4.0% | 1 | 14.3% | 4 | 2.7% | 0 | 0.0% | 18 | 3.6% | |
| Undecided | 22 | 6.7% | 0 | 0.0% | 5 | 3.3% | 0 | 0.0% | 27 | 5.4% | |
| Include | 226 | 69.1% | 5 | 71.4% | 140 | 93.3% | 17 | 100.0% | 388 | 77.4% | |
| Mode of Conception | I don’t know OC | 70 | 21.4% | 2 | 28.6% | 2 | 1.3% | 0 | 0.0% | 74 | 14.8% |
| Exclude | 11 | 3.4% | 0 | 0.0% | 2 | 1.3% | 1 | 5.9% | 14 | 2.8% | |
| Undecided | 34 | 10.4% | 0 | 0.0% | 20 | 13.3% | 5 | 29.4% | 59 | 11.8% | |
| Include | 212 | 64.8% | 5 | 71.4% | 126 | 84.0% | 11 | 64.7% | 354 | 70.7% | |
| Postpartum Haemorrhage | I don’t know OC | 90 | 27.5% | 2 | 28.6% | 1 | 0.7% | 0 | 0.0% | 93 | 18.6% |
| Exclude | 9 | 2.8% | 0 | 0.0% | 7 | 4.7% | 0 | 0.0% | 16 | 3.2% | |
| Undecided | 45 | 13.8% | 1 | 14.3% | 27 | 18.0% | 5 | 29.4% | 78 | 15.6% | |
| Include | 183 | 56.0% | 4 | 57.1% | 115 | 76.7% | 12 | 70.6% | 314 | 62.7% | |
| I don’t know OC | 34 | 18.8% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 34 | 11.7% | |
| Abnormal placentation | Exclude | 4 | 2.2% | 0 | 0.0% | 3 | 3.2% | 0 | 0.0% | 7 | 2.4% |
| Undecided | 26 | 14.4% | 1 | 50.0% | 6 | 6.5% | 5 | 33.3% | 38 | 13.1% | |
| Include | 117 | 64.6% | 1 | 50.0% | 84 | 90.3% | 10 | 66.7% | 212 | 72.9% | |
| Health-Related Quality of Life | I don’t know OC | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Exclude | 3 | 0.9% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 5 | 1.0% | |
| Undecided | 5 | 1.5% | 0 | 0.0% | 6 | 4.0% | 0 | 0.0% | 11 | 2.2% | |
| Include | 319 | 97.6% | 7 | 100.0% | 142 | 94.7% | 17 | 100.0% | 485 | 96.8% | |
| Sexual Function | I don’t know OC | 7 | 2.1% | 1 | 14.3% | 0 | 0.0% | 0 | 0.0% | 8 | 1.6% |
| Exclude | 5 | 1.5% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 8 | 1.6% | |
| Undecided | 18 | 5.5% | 0 | 0.0% | 9 | 6.0% | 2 | 11.8% | 29 | 5.8% | |
| Include | 297 | 90.8% | 6 | 85.7% | 138 | 92.0% | 15 | 88.2% | 456 | 91.0% | |
| I don’t know OC | 12 | 6.6% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 12 | 4.1% | |
| Bleeding during or after sexual activity | Exclude | 5 | 2.8% | 0 | 0.0% | 11 | 11.8% | 1 | 6.7% | 17 | 5.8% |
| Undecided | 29 | 16.0% | 0 | 0.0% | 27 | 29.0% | 8 | 53.3% | 64 | 22.0% | |
| Include | 135 | 74.6% | 2 | 100.0% | 55 | 59.1% | 6 | 40.0% | 198 | 68.0% | |
| Patient adherence to treatment | I don’t know OC | 12 | 3.7% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 14 | 2.8% |
| Exclude | 5 | 1.5% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 8 | 1.6% | |
| Undecided | 36 | 11.0% | 0 | 0.0% | 6 | 4.0% | 4 | 23.5% | 46 | 9.2% | |
| Include | 274 | 83.8% | 7 | 100.0% | 139 | 92.7% | 13 | 76.5% | 433 | 86.4% | |
| Patient satisfaction with treatment | I don’t know OC | 7 | 2.1% | 1 | 14.3% | 0 | 0.0% | 1 | 5.9% | 9 | 1.8% |
| Exclude | 7 | 2.1% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 10 | 2.0% | |
| Undecided | 24 | 7.3% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 26 | 5.2% | |
| Include | 289 | 88.4% | 6 | 85.7% | 145 | 96.7% | 16 | 94.1% | 456 | 91.0% | |
| Symptom relief rate for most bothersome symptom | I don’t know OC | 14 | 4.3% | 1 | 14.3% | 0 | 0.0% | 0 | 0.0% | 15 | 3.0% |
| Exclude | 3 | 0.9% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 6 | 1.2% | |
| Undecided | 16 | 4.9% | 0 | 0.0% | 5 | 3.3% | 2 | 11.8% | 23 | 4.6% | |
| Include | 294 | 89.9% | 6 | 85.7% | 142 | 94.7% | 15 | 88.2% | 457 | 91.2% | |
| Symptom recurrence for any symptom | I don’t know OC | 15 | 4.6% | 1 | 14.3% | 1 | 0.7% | 0 | 0.0% | 17 | 3.4% |
| Exclude | 6 | 1.8% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 8 | 1.6% | |
| Undecided | 16 | 4.9% | 0 | 0.0% | 5 | 3.3% | 3 | 17.6% | 24 | 4.8% | |
| Include | 290 | 88.7% | 6 | 85.7% | 142 | 94.7% | 14 | 82.4% | 452 | 90.2% | |
| Symptom recurrence for most bothersome symptom | I don’t know OC | 16 | 4.9% | 1 | 14.3% | 0 | 0.0% | 0 | 0.0% | 17 | 3.4% |
| Exclude | 5 | 1.5% | 0 | 0.0% | 5 | 3.3% | 0 | 0.0% | 10 | 2.0% | |
| Undecided | 16 | 4.9% | 0 | 0.0% | 13 | 8.7% | 4 | 23.5% | 33 | 6.6% | |
| Include | 290 | 88.7% | 6 | 85.7% | 132 | 88.0% | 13 | 76.5% | 441 | 88.0% | |
| Lesion size | I don’t know OC | 29 | 8.9% | 2 | 28.6% | 2 | 1.3% | 0 | 0.0% | 33 | 6.6% |
| Exclude | 2 | 0.6% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 5 | 1.0% | |
| Undecided | 39 | 11.9% | 0 | 0.0% | 18 | 12.0% | 4 | 23.5% | 61 | 12.2% | |
| Include | 257 | 78.6% | 5 | 71.4% | 127 | 84.7% | 13 | 76.5% | 402 | 80.2% | |
| Uterus volume | I don’t know OC | 36 | 11.0% | 2 | 28.6% | 2 | 1.3% | 1 | 5.9% | 41 | 8.2% |
| Exclude | 1 | 0.3% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 3 | 0.6% | |
| Undecided | 68 | 20.8% | 1 | 14.3% | 21 | 14.0% | 5 | 29.4% | 95 | 19.0% | |
| Include | 222 | 67.9% | 4 | 57.1% | 125 | 83.3% | 11 | 64.7% | 362 | 72.3% | |
| Discomfort during procedure | I don’t know OC | 12 | 3.7% | 1 | 14.3% | 1 | 0.7% | 1 | 5.9% | 15 | 3.0% |
| Exclude | 5 | 1.5% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 7 | 1.4% | |
| Undecided | 31 | 9.5% | 1 | 14.3% | 12 | 8.0% | 4 | 23.5% | 48 | 9.6% | |
| Include | 279 | 85.3% | 5 | 71.4% | 135 | 90.0% | 12 | 70.6% | 431 | 86.0% | |
| Recovery time | I don’t know OC | 20 | 6.1% | 1 | 14.3% | 0 | 0.0% | 0 | 0.0% | 21 | 4.2% |
| Exclude | 10 | 3.1% | 0 | 0.0% | 2 | 1.3% | 0 | 0.0% | 12 | 2.4% | |
| Undecided | 34 | 10.4% | 0 | 0.0% | 14 | 9.3% | 5 | 29.4% | 53 | 10.6% | |
| Include | 263 | 80.4% | 6 | 85.7% | 134 | 89.3% | 12 | 70.6% | 415 | 82.8% | |
| Need for repeated or other treatment (Need for re-intervention) | I don’t know OC | 33 | 10.1% | 1 | 14.3% | 3 | 2.0% | 0 | 0.0% | 37 | 7.4% |
| Exclude | 4 | 1.2% | 0 | 0.0% | 4 | 2.7% | 0 | 0.0% | 8 | 1.6% | |
| Undecided | 30 | 9.2% | 0 | 0.0% | 10 | 6.7% | 5 | 29.4% | 45 | 9.0% | |
| Include | 260 | 79.5% | 6 | 85.7% | 133 | 88.7% | 12 | 70.6% | 411 | 82.0% | |
| Length of hospital stay | I don’t know OC | 34 | 10.4% | 2 | 28.6% | 2 | 1.3% | 0 | 0.0% | 38 | 7.6% |
| Exclude | 16 | 4.9% | 0 | 0.0% | 6 | 4.0% | 2 | 11.8% | 24 | 4.8% | |
| Undecided | 69 | 21.1% | 0 | 0.0% | 28 | 18.7% | 4 | 23.5% | 101 | 20.2% | |
| Include | 208 | 63.6% | 5 | 71.4% | 114 | 76.0% | 11 | 64.7% | 338 | 67.5% | |
| Death | I don’t know OC | 70 | 21.4% | 2 | 28.6% | 7 | 4.7% | 2 | 11.8% | 81 | 16.2% |
| Exclude | 9 | 2.8% | 0 | 0.0% | 4 | 2.7% | 0 | 0.0% | 13 | 2.6% | |
| Undecided | 20 | 6.1% | 1 | 14.3% | 5 | 3.3% | 1 | 5.9% | 27 | 5.4% | |
| Include | 228 | 69.7% | 4 | 57.1% | 134 | 89.3% | 14 | 82.4% | 380 | 75.8% | |
| Harms | I don’t know OC | 41 | 12.5% | 2 | 28.6% | 4 | 2.7% | 2 | 11.8% | 49 | 9.8% |
| Exclude | 5 | 1.5% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 8 | 1.6% | |
| Undecided | 18 | 5.5% | 0 | 0.0% | 5 | 3.3% | 1 | 5.9% | 24 | 4.8% | |
| Include | 263 | 80.4% | 5 | 71.4% | 138 | 92.0% | 14 | 82.4% | 420 | 83.8% | |
| Infections | I don’t know OC | 39 | 11.9% | 1 | 14.3% | 4 | 2.7% | 1 | 5.9% | 45 | 9.0% |
| Exclude | 7 | 2.1% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 10 | 2.0% | |
| Undecided | 18 | 5.5% | 0 | 0.0% | 10 | 6.7% | 4 | 23.5% | 32 | 6.4% | |
| Include | 263 | 80.4% | 6 | 85.7% | 133 | 88.7% | 12 | 70.6% | 414 | 82.6% | |
| Unplanned bleeding on hormonal medication | I don’t know OC | 12 | 6.6% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 12 | 4.1% |
| Exclude | 11 | 6.1% | 0 | 0.0% | 2 | 2.2% | 1 | 6.7% | 14 | 4.8% | |
| Undecided | 28 | 15.5% | 0 | 0.0% | 16 | 17.2% | 4 | 26.7% | 48 | 16.5% | |
| Include | 130 | 71.8% | 2 | 100.0% | 75 | 80.6% | 10 | 66.7% | 217 | 74.6% | |
| Endometriosis present | I don’t know OC | 4 | 1.2% | 0 | 0.0% | 1 | 0.7% | 1 | 5.9% | 6 | 1.2% |
| Exclude | 5 | 1.5% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 8 | 1.6% | |
| Undecided | 16 | 4.9% | 1 | 14.3% | 3 | 2.0% | 1 | 5.9% | 21 | 4.2% | |
| Include | 302 | 92.4% | 6 | 85.7% | 143 | 95.3% | 15 | 88.2% | 466 | 93.0% | |
| Fibroids present | I don’t know OC | 36 | 11.0% | 3 | 42.9% | 2 | 1.3% | 1 | 5.9% | 42 | 8.4% |
| Exclude | 7 | 2.1% | 0 | 0.0% | 3 | 2.0% | 0 | 0.0% | 10 | 2.0% | |
| Undecided | 34 | 10.4% | 2 | 28.6% | 7 | 4.7% | 3 | 17.6% | 46 | 9.2% | |
| Include | 250 | 76.5% | 2 | 28.6% | 138 | 92.0% | 13 | 76.5% | 403 | 80.4% | |
| Chronic pelvic pain present | I don’t know OC | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Exclude | 5 | 2.8% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 5 | 1.7% | |
| Undecided | 4 | 2.2% | 0 | 0.0% | 2 | 2.2% | 0 | 0.0% | 6 | 2.1% | |
| Include | 172 | 95.0% | 2 | 100.0% | 91 | 97.8% | 15 | 100.0% | 280 | 96.2% | |
| Wish for future pregnancy | I don’t know OC | 14 | 4.3% | 0 | 0.0% | 0 | 0.0% | 1 | 5.9% | 15 | 3.0% |
| Exclude | 10 | 3.1% | 1 | 14.3% | 4 | 2.7% | 0 | 0.0% | 15 | 3.0% | |
| Undecided | 35 | 10.7% | 0 | 0.0% | 6 | 4.0% | 2 | 11.8% | 43 | 8.6% | |
| Include | 268 | 82.0% | 6 | 85.7% | 140 | 93.3% | 14 | 82.4% | 428 | 85.4% | |
| Classification of adenomyosis | I don’t know OC | 50 | 15.3% | 2 | 28.6% | 5 | 3.3% | 1 | 5.9% | 58 | 11.6% |
| Exclude | 1 | 0.3% | 1 | 14.3% | 3 | 2.0% | 0 | 0.0% | 5 | 1.0% | |
| Undecided | 53 | 16.2% | 0 | 0.0% | 8 | 5.3% | 4 | 23.5% | 65 | 13.0% | |
| Include | 223 | 68.2% | 4 | 57.1% | 134 | 89.3% | 12 | 70.6% | 373 | 74.5% | |
| Previous treatment for adenomyosis | I don’t know OC | 12 | 3.7% | 1 | 14.3% | 0 | 0.0% | 1 | 5.9% | 14 | 2.8% |
| Exclude | 7 | 2.1% | 0 | 0.0% | 4 | 2.7% | 0 | 0.0% | 11 | 2.2% | |
| Undecided | 22 | 6.7% | 0 | 0.0% | 10 | 6.7% | 4 | 23.5% | 36 | 7.2% | |
| Include | 286 | 87.5% | 6 | 85.7% | 136 | 90.7% | 12 | 70.6% | 440 | 87.8% | |
| Cost treatment overall | I don’t know OC | 35 | 10.7% | 1 | 14.3% | 3 | 2.0% | 0 | 0.0% | 39 | 7.8% |
| Exclude | 18 | 5.5% | 0 | 0.0% | 6 | 4.0% | 0 | 0.0% | 24 | 4.8% | |
| Undecided | 56 | 17.1% | 0 | 0.0% | 21 | 14.0% | 3 | 17.6% | 80 | 16.0% | |
| Include | 218 | 66.7% | 6 | 85.7% | 120 | 80.0% | 14 | 82.4% | 358 | 71.5% | |
| Personal cost for patient | I don’t know OC | 34 | 10.4% | 1 | 14.3% | 4 | 2.7% | 1 | 5.9% | 40 | 8.0% |
| Exclude | 11 | 3.4% | 0 | 0.0% | 7 | 4.7% | 0 | 0.0% | 18 | 3.6% | |
| Undecided | 44 | 13.5% | 1 | 14.3% | 30 | 20.0% | 3 | 17.6% | 78 | 15.6% | |
| Include | 238 | 72.8% | 5 | 71.4% | 109 | 72.7% | 13 | 76.5% | 365 | 72.9% | |
| Cost utility analysis | I don’t know OC | 43 | 13.1% | 1 | 14.3% | 4 | 2.7% | 0 | 0.0% | 48 | 9.6% |
| Exclude | 11 | 3.4% | 0 | 0.0% | 6 | 4.0% | 0 | 0.0% | 17 | 3.4% | |
| Undecided | 70 | 21.4% | 1 | 14.3% | 16 | 10.7% | 4 | 23.5% | 91 | 18.2% | |
| Include | 203 | 62.1% | 5 | 71.4% | 124 | 82.7% | 13 | 76.5% | 345 | 68.9% | |
Ratings were given on a Likert scale ranging from 1 to 9. ‘Exclusion’ means 70% or more scoring it as 1–3 and fewer than 15% scoring it as 7–9. Consensus for an outcome being included in the core outcome set (COS) required 70% or more scoring it as 7–9 and fewer than 15% to score it as 1–3. HCP, health care professional; OC, outcome.
Table III.
Definitions and lay terms for the outcomes of the core outcome set.
| Outcome (Per category) |
Definition for COSAR | Source/Reference | |
|---|---|---|---|
| Medical term | Lay term | ||
| Pain | |||
| Cyclic pelvic pain | Pain coming at the same time in the menstrual cycle | Cyclic pelvic pain is considered to be a subset of chronic pelvic pain that occurs in relation to the menstrual cycle. This includes pain during ovulation. | (Muse, 1990; Won and Abbott, 2010) |
| Dyschezia | Pain during toilet visit/when opening bowels | Painful or difficult defecation [COSAR: during menstruation] | International Working Group of AAGL, ESGE, ESHRE and WES (Tomassetti et al., 2021) |
| Dysmenorrhea | Painful periods | Painful periods | (RCOG, 2022) |
| Dyspareunia | Pain during sex | Pain associated with sexual activity. | COSAR Steering Committee |
| Non-cyclic, untriggered pelvic pain | Pelvic pain occurring without a trigger | Pain in pelvic area that does not occur in a regular, cyclic fashion and that is not caused by any obvious triggers recognized by the person with adenomyosis. | COSAR Steering Committee |
| Pelvic bulk/pressure symptoms | Feeling tightness or pressure in the pelvic area | Feeling tightness or pressure in the pelvic area | (Spies et al., 2002) |
| Radiating pain | Radiating pain | Radiating pain to the lower back and/or extremities during the menstruation | COSAR Steering committee |
| Urinary system | |||
| Urinary frequency | Needing to urinate often | Abnormally frequent urination (e.g. once every hour or two) is termed urinary frequency. | (Wrenn, 1990) |
| Menstrual bleeding | |||
| Blood flow volume | How heavy the menstrual bleeding is | The amount of vaginal bleeding during menstruation, which is considered heavy >80 ml, normal 5–80 ml and light <5 ml | FIGO (Munro et al., 2018) |
| Duration of bleeding | How many days the menstrual bleeding lasts | Prolonged menstrual flow >8 days, normal 4.5–8 days, shortened <4.5 days | |
| Intermenstrual bleeding | Bleeding in between periods | Experiencing episodes of bleeding that occur between normally timed menstrual periods. (A) cyclic (predictable), (B) non-cyclic | |
| Unscheduled bleeding on hormonal medication | Unplanned bleeding on hormonal medication | Unplanned bleeding on hormonal medication | |
| Length/regularity of cycle | Time between periods | ||
| Reproductive outcomes | |||
| Infertility Core Outcome Set | (Duffy et al., 2020) | ||
| Live, correctly sited (eutopic) pregnancy | Pregnancy with a heartbeat, confirmed by ultrasound | A correctly sited pregnancy diagnosed by ultrasonographic examination of at least one foetus with a discernible heartbeat. | ESHRE (Kirk et al., 2020), (Duffy et al., 2020) |
| Reporting: singleton, twin pregnancy, higher multiple pregnancy and which gestation the ultrasound examination was performed on. A twin pregnancy is counted as one pregnancy event. | |||
| Pregnancy loss, including: | |||
| Ectopic pregnancy | A pregnancy located in the wrong place (outside the cavity of the uterus) | Any pregnancy that is implanted outside the uterine cavity. | ESHRE (Kirk et al., 2020) |
| Miscarriage | Early pregnancy loss | The spontaneous loss of a correctly sited (eutopic) pregnancy prior to 20 completed weeks of gestational age. Miscarriage should be reported after a viable pregnancy has been confirmed by ultrasound. | (Duffy et al., 2020) |
| Stillbirth | When a baby is not alive when born. | The death of a foetus prior to the complete expulsion or extraction from its mother after 20 completed weeks of gestational age. The death is determined by the fact that, after such separation, the foetus does not breathe or show any other evidence of life, such as heartbeat, umbilical cord pulsation or definite movement of voluntary muscles. | |
| Termination of pregnancy | Termination of pregnancy | Intentional loss of a correctly sited (eutopic) pregnancy, through intervention by medical, surgical or unspecified means. | |
| Live birth | Live birth | The complete expulsion or extraction from a woman of a product of fertilization, after 20 completed weeks of gestational age; which, after such separation, breathes or shows any other evidence of life, such as heart beat, umbilical cord pulsation or definite movement of voluntary muscles, irrespective of whether the umbilical cord has been cut or the placenta is attached. A birth weight of 350 g or more can be used if gestational age is unknown. | |
| Gestational age at delivery | At how many weeks of pregnancy the baby is born | The age of a foetus is calculated by the best obstetric estimate determined by assessments which may include early ultrasound, and the date of the last menstrual period, and/or perinatal details. In the case of assisted reproductive techniques, it is calculated by adding 14 days to the number of completed weeks since fertilization. | |
| Birthweight | Birthweight | Birth weight should be collected within 24 h of birth and assessed using a calibrated electronic scale with 10-g resolution. | |
| Neonatal mortality | Death of the baby before, during or shortly after birth | Death of a live born baby within 28 days of birth. This can be sub-divided into early neonatal mortality, if death occurs in the first 7 days after birth and late neonatal mortality, if death occurs between 8 and 28 days after birth. | |
| Major congenital anomaly | A disorder the baby is born with | Structural, functional and genetic anomalies, that occur during pregnancy, and identified antenatally, at birth, or later in life, and require surgical repair of a defect, or are visually evident, or are life-threatening, or cause death. | |
| Time to pregnancy leading to live birth | Time to pregnancy leading to live birth | See detailed definition and measurement in reference. | |
| Additional outcomes | |||
| Mode of conception | Was fertility treatment needed to become pregnant | If a pregnancy occurred spontaneously or through any type of ART. | COSAR Steering Committee |
| Postpartum haemorrhage | Heavy bleeding during and after the delivery. | Postpartum haemorrhage (PPH) is defined as a blood loss of 500 ml or more within 24 h after birth. | (WHO, 2012) |
| Abnormal placentation | Placental complications | Abnormal formation, placental growth or adherence of the placenta in the uterus. | COSAR Steering Committee |
| Haematology | |||
| Anaemia | Low levels of haemoglobin (oxygen carriers) in blood | Anaemia is a condition in which the number of red blood cells or the haemoglobin concentration within them is lower than normal. In non-pregnant women the definitions for anaemia are (at sea level): Mild 110–119 g/l, moderate 80–109 g/l, severe <80 g/l | (WHO, 2011) |
| Life impact | |||
| Health-related QoL | Health impact on quality of life | Quality of life is the individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns | CDC (Centers for Disease Control and Prevention, 2000) (Post, 2014) |
| Health-related QoL was defined as ‘perceived physical and mental health over time’. | |||
| Sexual functioning | Sexual functioning | Sexual functioning is characterized by absence of difficulty moving through the stages of sexual desire, arousal and orgasm, as well as subjective satisfaction with the frequency and outcome of individual and partnered sexual behaviour. | (Masters and Johnson, 1966) |
| Coital bleeding | bleeding during or after sexual activity | Vaginal bleeding during or after sexual activity. | COSAR Steering Committee |
| Delivery of care | |||
| Patient adherence to treatment | How well a patient follows a treatment | Medication compliance (synonym: adherence): refers to the degree or extent of conformity to the recommendations about day-to-day treatment by the provider with respect to the timing, dosage and frequency. It may be defined as ‘the extent to which a patient acts in accordance with the prescribed interval, and dose of a dosing regimen’. | (Cramer et al., 2008) |
| Patient satisfaction with treatment | Patient satisfaction with treatment | Patient satisfaction expresses whether a patient’s expectations about a health encounter were met. | (Rockville, 2021) |
| Symptom relief rate (most bothersome symptom) | How much better the worst symptom gets | Extent to which a treatment relieves a symptom (most bothersome symptom must be pre-defined). | COSAR Steering Committee |
| Symptom Recurrence for any symptom | How long it takes for a symptom to come back | The return of a disease or the signs and symptoms of a disease after a period of improvement. | COSAR Steering Committee |
| Symptom Recurrence for most bothersome symptom | How long it takes for the worst symptom to come back. | The return of a disease or the signs and symptoms of a disease after a period of improvement (most bothersome symptom must be pre-defined). | COSAR Steering Committee |
| Lesion size | Size of adenomyosis lesion | The radiologically estimated size of the primary lesion, measured in three planes perpendicular to each other. | COSAR Steering Committee |
| Discomfort during procedure | Discomfort during procedure | Includes pain or other negative, bodily symptoms that are experienced while a procedure is performed. Is not applicable for procedures that require general anaesthesia. | COSAR Steering Committee |
| Recovery time | Recovery time after procedure | Return to normal activities after a medical procedure was performed. | COSAR Steering Committee |
| Need for re-intervention | Need for repeated or other treatment | Need to repeat a procedure for the same condition, planned or unplanned, or perform a different procedure due to complications or ineffectiveness of the first procedure. | COSAR Steering Committee |
| Length of hospital stay | Length of hospital stay* | Time from admission to discharge of patient. | (WHO and WHO Patient Safety, 2010) |
| Premature termination of procedure | Having to stop a procedure before it was finished | A procedure being stopped before it is finished, either due to patient discomfort, complications or technical problems. | (WHO and WHO Patient Safety, 2010) |
| Uterus volume | Volume (size) of the uterus | The volume of the corpus uteri, excluding the cervix uteri, calculated as d1 (cm)×d2 (cm)×d3 (cm)×0.523, where d1 is the length of the corpus, d2 is the largest anteroposterior diameter and d3 is the largest transverse diameter | MUSA (Van den Bosch et al., 2015) |
| Adverse outcomes | |||
| Harm: impairment of structure or function of the body and/or any deleterious effect arising there from. Harm includes disease, injury, suffering, disability and death. | (WHO and WHO Patient Safety, 2010) | ||
| Adverse reaction: unexpected harm resulting from a justified action where the correct process was followed for the context in which the event occurred. | |||
| Side effect: a known effect, other than that primarily intended, related to the pharmacological properties of a medication. | |||
| We suggest reporting the following incident types within COSAR: Clinical procedure, infections, medication/fluids | |||
| Surgical complications: | |||
| Complication (GRADE I) | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic or radiological interventions. Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics, electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside | (Dindo et al., 2004) | |
| Complication (GRADE II) | Requiring pharmacological treatment with drugs other than such allowed for grade I complications; Blood transfusions; total parenteral nutrition | ||
| Complication (GRADE III) | Requiring surgical, endoscopic or radiological intervention | ||
| Grade IIIa: Intervention not under general anaesthesia | |||
| Grade IIIb: Intervention under general anaesthesia | |||
| Complication (GRADE IV) | Life-threatening complication (including central nervous system complications) requiring IC/ICU management. | ||
| Grade IVa: Single organ dysfunction (including dialysis) | |||
| Grade IVb: Multiorgan dysfunction | |||
| Complication (GRADE V) | Death of a patient | ||
| Infections | Surgical Site Infections have three grades:
|
NICE (Welsh, 2008) CDC (Anderson et al., 2014) | |
| Adverse drug reactions (ADR) |
|
WHO Collaborating Centre for International Drug Monitoring (WHO, 2021) | |
| Reporting items | |||
| Endometriosis present | Terminology regarding location and grade according to working group. | International Working Group of AAGL, ESGE, ESHRE and WES (Tomassetti et al., 2021) | |
| Fibroids present | FIGO Classification | FIGO (Munro et al., 2018) | |
| Chronic pelvic pain present | Chronic pelvic pain can be defined as intermittent or constant pain in the lower abdomen or pelvis of a woman of at least 6 months in duration, not occurring exclusively with menstruation or intercourse and not associated with pregnancy. It is a symptom not a diagnosis. | (RCOG, 2012) | |
| Wish for future pregnancy | If the woman has, at the time of the treatment, an active or future wish to become pregnant. | COSAR steering group. | |
| Classification of adenomyosis | An internationally accepted and accredited system to classify and describe disease. | COSAR steering group. | |
| Previous treatment for adenomyosis | All treatment (including medical, surgical, interventional) that has been used to treat adenomyosis-related symptoms in the past. | COSAR steering group. | |
| Outcomes that are recommended to report, but not mandatory | |||
| Costs of treatment | How much the treatment costs | Costs of treatment | COSAR steering group. |
| Patient costs | How much the patient must pay for a treatment | Direct out of pocket expenses for the patient. | COSAR steering group. |
| Cost-utility analysis | Value-for-money of treatment | Value for money. A specific healthcare treatment is said to be ‘cost-effective’ if it gives a greater health gain than could be achieved by using the resources in other ways. | NICE (Welsh, 2008) |
AAGL, American Association of Gynecologic Laparoscopists; ESGE, European Society for Gynaecological Endoscopy; WES, World Endometriosis Society; RCOG, The Royal College of Obstetricians and Gynaecologists; NICE, National Institute for Health and Clinical Excellence; COSAR, Core Outcome Set in Adenomyosis Research; FIGO, International Federation of Gynecology and Obstetrics; WHO, World Health Organization; CDC, Centers for Disease Control.
Pain outcomes
Dysmenorrhea, cyclic pelvic pain, dyspareunia, non-cyclic untriggered pain (including pain during ovulation) and feeling bulky/pelvic pressure symptoms reached the threshold for agreement and were included (Table II). Dyschezia reached 73% agreement in total, but the threshold for inclusion was only reached among patients when analysed at the stakeholder level. In the final session, there was complete agreement to include this in the final COS.
Two items reached >70% agreement amongst patients, but not overall, and were therefore discussed. Pain radiating to legs was suggested as an additional item in the first round and supported by 78.5% of patients, but only <50% of HCP and researchers. Several Steering Committee members pointed out that their clinical experience showed that this symptom was indeed present in many patients with adenomyosis. A proposed, albeit unproven, mechanism for this was pain radiating through the uterosacral ligaments. Inclusion of the item was strongly advocated by the patient representative. In line with the initial discussion as outlined above, it was agreed to define this item as ‘radiating pain to lower back and/or extremities during menstruation’ and to include it in the COS. However, the group pointed out that this item needed future re-evaluation.
A new item suggested during the Delphi, bloating, reached only 64.6% agreement overall, with a high agreement (78.5%) amongst patients but less than 50% of HCPs and researchers. After discussion, it was decided that this symptom is too non-specific and not a priority for inclusion in this version of the COS, an approach supported by the patient representative.
Several items that were added to the long list during the patient workshop were left undecided after two rounds of the Delphi. These were dysuria, pain when the bladder is full and pain-associated vomiting. In the final session, there was full agreement that all these outcomes were too non-specific, and therefore were not included in the final COS. However, all agreed that these items need more scientific investigation and could be potentially included in the future.
Urinary symptoms
The symptom of urinary frequency achieved 71.9% agreement amongst patients but only 63.5% agreement overall. The Steering Committee determined that this is a specific and easily measurable symptom that might serve as a proxy measure of uterine size or disease severity. There was complete agreement for inclusion.
The other items in this category, urge symptoms, residual urine and urinary incontinence, did not reach the threshold for exclusion or inclusion overall. The patient representative pointed out it might be less clear with urinary symptoms what is normal. After discussion, there was a unanimous agreement to exclude all three items.
Menstrual bleeding
Blood flow volume, and duration of bleeding was supported by all participants through round one and included (Table II).
Other items in this category reached agreement in some stakeholder groups but did not reach the threshold for inclusion overall (Table II, Supplementary Data File S2). However, the Steering Committee pointed out that there is an evidence-based and internationally established system for describing normal and abnormal uterine bleeding (AUB) that should be considered equal to a COS (Munro et al., 2018). Consequently, the Steering Committee unanimously supported the inclusion of the elements of AUB System 1 of the International Federation of Gynecology and Obstetrics (FIGO) that describe the frequency and regularity of the menstrual cycle, duration and suspected volume of the menstrual period, and the presence of intermenstrual bleeding. The Steering Committee considered unscheduled bleeding on medication that suppresses gonadal steroids to be a side effect that should be monitored under the harms category.
The item ‘coital bleeding’ was supported by patients in the second round (74.6% agreement in this group) but not by the participants overall. This item was discussed at length where some Steering Committee members argued for exclusion because the symptom is too non-specific with an uncertain mechanism. However, given the support of patients, the opinion of the patient representative, and the previous example of dyschezia, the item was put to the vote by the Steering Committee, where the majority supported inclusion in principle. However, because the term ‘coital bleeding’ was considered ambiguous, the Steering Committee unanimously supported the alternative and more encompassing description ‘bleeding associated with sexual activity’, in recognition that this symptom could occur outside penetrative sex. Also, most of the Steering Committee voted to move this newly named item to the category ‘quality of life’ under the concept of ‘sexual function’.
Reproductive outcomes
The Steering Committee suggested mandatory reporting of the items in this category for all studies evaluating interventions designed to improve reproductive outcomes and recommended them when the study design includes women wishing for future pregnancy.
There is an existing COS on infertility (Duffy et al., 2018, 2020) (Table I) that received consensus support in the first Delphi round (Table II). While it was acknowledged that not the entire infertility COS might be relevant for adenomyosis research, selective inclusion was an issue, so all items were included in the adenomyosis COS. The Steering Committee, however, decided to modify two of the definitions in the infertility COS (Duffy et al., 2020). The first was to use the terms ‘live’ rather than ‘viable’ for early pregnancies; and to describe eutopic pregnancies as ‘normally sited (eutopic)’ rather than ‘intrauterine’, definitions that agree with the ESHRE terminology on ectopic pregnancies (Kirk et al., 2020).
The Delphi participants supported the inclusion of three additional items to this domain, as they were considered relevant in the context of adenomyosis. Overall, while 72.9% supported the inclusion of placentation disorders, this support rose to 90.3% amongst HCPs and consequently was included in the COS. Although the mode of delivery reached the threshold of consensus only amongst HCPs, and only in the second round (72.8%, 58.1% overall agreement), the Steering Committee considered it highly relevant, particularly following uterine sparing procedural interventions. Consequently, the mode of delivery is included in the COS. The same rationale led to the inclusion of postpartum haemorrhage, an item that reached 76.7% and 70.6% agreement amongst HCPs and researchers, respectively, but only 62.7% in the overall participant cohort (Supplementary Data File S2).
Haematology and laboratory outcomes
Several haematological and hormonal laboratory parameters were presented through the long list, of which only ferritin (71.3% total) and haemoglobin (70.1% total) reached the threshold for consensus support. However, since these items represent iron deficiency or anaemia outcome measures rather than independent outcomes, the Steering Committee unanimously agreed to include anaemia as an outcome and not recommend any specific biochemical outcomes. Of note, both CA 125 and oestrogen levels were the only two items in round one to be clearly excluded by both HCPs and researchers, with 22–35% vote for exclusion (Supplementary Data File S1), and almost reached the exclusion threshold overall in the second round with 14.4%.
Life impact
QoL and health-related QoL (HR QoL) are constructs that comprise several domains and sub-items (Centers for Disease Control and Prevention, 2000). However, during the work on the long list, the Steering Committee considered it counterproductive to let the participants vote on each specific item that was identified in this category through the systematic review (Tellum et al., 2021a), as the length of the list and lack of translation might undermine the validity of the results. Consequently, it was concluded that identification of the disease-specific items relating to HR QoL should be carried out under the scope of a different study and that the concepts of QoL should be presented as one single item.
HR QoL was overwhelmingly supported for inclusion in the first Delphi round (96.8% agreement), as was sexual function, which achieved consensus with 91% of participants indicating support. As stated above, the newly termed item ‘bleeding during sexual activity’ was included in this category, as is dyspareunia, which can be included in both categories, sexual function or pain.
Delivery of care
The Steering Committee recommended the inclusion of all items in this category that the participants in the Delphi process supported. Whereas no item is adenomyosis specific, all are generally considered important for clinical trials (WHO, 2006). Consequently, the following items were included in the COS (with the rate of support): patient adherence to treatment (86.4%), patient satisfaction (91%), discomfort during procedure (86%), recovery time (82.8%) (time to full recovery of normal activities), symptom relief rate (91.2%), any symptom recurrence (90.2%), most bothersome symptom recurrence (88%), length of hospital stay (67.5%) and need for re-intervention or a repeat procedure (82%).
There was discussion regarding the lesion size and uterine volume, each of which was supported for inclusion by 80.2% and 72.3%, respectively, of the participants. Members of the Steering Committee offered that uterine volume is only a proxy for disease burden, with limited available evidence demonstrating an association with symptoms or outcomes. Similarly, and while lesion size may reflect treatment effects, the inter-rater reliability of lesional metrics remains challenging. While all agreed that disease burden should be measured according to a unified classification, lesion size and uterine volume can serve as interim outcomes pending the development and general acceptance of a consensus, imaging-based, adenomyosis reporting system. It was further agreed that outcomes that are applicable to specific types of interventions only, such as procedure time, technical parameters (type and amount of energy used) or weight of removed tissue, should not be included in the COS. Still, they should be reported as appropriate according to current practice in the respective field.
The Steering Committee agreed to exclude health-economic outcomes from the COS as it could be methodologically challenging for many investigators. However, it was acknowledged that, in many environments, the patient-borne treatment costs are an important component of the therapeutic decision-making process and should be reported, despite the difficulty of international comparisons. Consequently, the Steering Committee strongly recommended reporting the results of cost-utility analyses and the overall and patient costs of treatment.
Adverse outcomes
The Steering Committee agreed unanimously that harms, infections, and adverse drug reactions should be monitored and reported systematically according to the nature of the intervention (Table II). Apart from unscheduled bleeding on hormonal medication, which was proposed in the menstrual bleeding category, the Steering Committee decided not to specify a list of adverse outcomes that should be measured as this would be lengthy, possibly leading to under-documentation of rare unlisted events.
Reporting items
Reporting items are not outcomes per se but contain essential information for the interpretation of study results in trials on adenomyosis. The following reporting items were included in the COS (with rates of overall agreement): presence of endometriosis (93%), presence of leiomyomas (80.4%), presence of chronic pelvic pain (96.2%), desire for future pregnancy (85.4%), previous treatment for adenomyosis (87.8%) and classification of adenomyosis (74.5%). The Steering Committee found that recommending a specific classification for endometriosis is outside the project’s mandate but suggests the description of findings according to the recently published expert consensus (Tomassetti et al., 2021). For leiomyomas, the Steering Committee recommends reporting according to the well-established FIGO-fibroid classification (Munro et al., 2018). For adenomyosis, adherence to well-defined terminology is recommended until an internationally accepted and validated reporting system is developed (Van den Bosch et al., 2015; Harmsen et al., 2022). The scope of this work specifically excluded defining adenomyosis diagnostic criteria (imaging, histopathological or other), as the Steering Committee determined that such criteria should be defined by experts based on valid scientific evidence.
Discussion
Summary of findings
Individuals living with adenomyosis and their partners, patient advocates, HCPs and researchers have developed the first COS to standardize outcome selection, collection and reporting for future studies investigating uterus-sparing treatment of adenomyosis in premenopausal women, namely the COSAR. The COSAR is applicable to all uterus-sparing therapeutic interventions, including medical, surgical and other interventional approaches, including those that are guided by imaging techniques. It comprises 50 outcomes, of which 19 are applicable only for certain studies while 10 are reporting items.
Strength and limitations
The strength of this process is the adherence to a recommended and prescriptive methodology, a high number of participants, and a truly global representation of patients and HCPs. Patients were included at all stages of the COSAR development through focus groups and representation on the Steering Committee, ensuring their views were strongly represented. The Steering Committee comprised experts in adenomyosis with different foci of research and clinical interest, such as surgery, imaging, infertility or basic research. This ensured a broad perspective when choosing different outcomes. The COS includes definitions for each outcome to avoid ambiguity in interpretation and includes well-documented existing COS, classifications and definitions (Munro et al., 2018; Vanhie et al., 2016; Duffy et al., 2020) in the COSAR where possible. Such an approach was designed to ensure a high standard of outcomes and facilitate harmonization of outcomes where conditions overlap.
The project and the results are not without limitations. Only 22% of participants were from continents other than Europe, and relatively few patients were from low-income countries. Despite intensive efforts undertaken by the Steering Committee members, it was only possible to engage a small number of participants from Asian countries, a circumstance that may be related to the lack of translation of the survey. As adenomyosis is a benign disorder which requires expert-ultrasound or cost-intensive MRI for diagnosis, the awareness of the condition might be low amongst both patients and health care providers in those regions of the world. Also, a lack of translation of the survey might have limited the active participation of people in non-English speaking countries. If correct, this observation could explain the low rate of engagement with our project and, even for those who did participate, it may have affected their perception of the relevance of some outcomes. However, specific symptoms seem to be universally valid, as the international validation of symptom scoring instruments in gynaecology shows (Nie et al., 2017; Yeung et al., 2019; Schneider et al., 2000). Some of the potential bias may have been addressed by the inclusion of experts from low and middle-income countries on the Steering Committee, voicing their views and opinions.
Another project limitation was the high attrition rate (41.9%), which could weaken the conclusions’ strength. However, our analysis did not indicate that there was an attrition bias.
Another concern relates to the observation that many women have concomitant disorders, especially endometriosis, a circumstance that could influence patient perceptions of relevant outcomes to be those that are not adenomyosis-specific. Also, several included outcomes were chosen based on expert opinions and patient preferences despite an absence of evidence confirming their relevance to adenomyosis. The lack of disease-specific QoL evaluation tools for adenomyosis analogous to those developed for similar conditions is also reflected in this knowledge gap. These issues accentuate the urgent need for this COS and further research to identify additional outcomes of relevance for adenomyosis to be included in a future revision of the COSAR.
Implications for future research
The development of the COSAR is an important step that should improve the quality of future adenomyosis-related clinical investigations, including the performance of systematic review and meta-analysis. Before it can be fully implemented, additional work is needed to define measures for each of the listed outcomes. In addition to dissemination and implementation of the COSAR, it will be necessary to monitor its use in a way that informs future appropriate modifications.
Some important tools for adenomyosis research are still missing, including a disease-specific HR-QoL questionnaire and the validation of generic HR-QoL instruments. There is also a need for studies designed to determine which symptoms are adenomyosis-specific and how they affect people’s QoL.
Conclusion
We have developed a core set of outcomes that should help researchers when designing and reporting the results of future studies on the treatment of adenomyosis. The standardization of reporting will facilitate a better synthesis of evidence and assist patients and clinicians when making decisions regarding the optimal treatment of adenomyosis. The use of a standardized set of outcomes should also stimulate good clinical practice in research and ensure that studies report the outcomes of interest and importance to patients.
Supplementary Material
Acknowledgements
We would like to thank the World Endometriosis Society for contributing to the work on the long list and distribution of the survey. Carla Cressy, Alison Julien, Cherrelle Melton and Karen Havelin for contributing with patient perspectives. Aleksandar Radulovic for assembling a global list of national gynaecological and patient associations.
Authors’ roles
T.T. and J.N. performed the data collection and analysis and wrote the first version of this manuscript. All authors contributed to the conceptualization of the study, data collection, the revision of the manuscript and approval of the final version.
Funding
No specific funding was received for this work. T.T. received a grant (grant number 2020083) from the South-Eastern Norwegian Health Authority during the course of this work.
Conflict of interest
T.T. receives personal fees from General Electrics and Medtronic for lectures on ultrasound. E.R.L. is the chairman of the Norwegian Endometriosis Association. M.G.M. is a consultant for Abbvie inc and Myovant, receives research funding from AbbVie, and is Chair of the Women’s Health Research Collaborative. S.-W.G. is a board member of the Asian Society of Endometriosis and Adenomyosis, on the scientific advisory board of the endometriosis foundation of America, previous congress chair for the World Endometriosis Society, for none of which he received personal fees. E.S. received outside of this work grants for two multicentre trials on endometriosis from the National Institute for Health Research UK, the Rosetrees Trust, and the Barts and the London Charity, he is a member of the Medicines and Healthcare Products Regulatory Agency (MHRA), Medicines for Women’s Health Expert Advisory Group, he is an ambassador for the World Endometriosis Society, and he received personal fees for lectures from Hologic, Olympus, Medtronic, Johnson & Johnson, Intuitive and Karl Storz. M.H. is member of the British Society for Gynaecological Endoscopy subcommittee. No other conflict of interest was declared.
Contributor Information
T Tellum, Department of Gynecology, Oslo University Hospital, Oslo, Norway.
J Naftalin, Institute for Women’s Health, University College Hospital, London, UK.
C Chapron, Department of Obstetrics and Gynecology II and Reproductive Medecine, Université Paris Cité, Faculté de Médecine, CHU Cochin, Paris, France.
M Dueholm, Department of Obstetrics and Gynecology, Aarhus University Hospital, Aarhus, Denmark.
S -W Guo, Shanghai Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China.
M Hirsch, Nuffield Department of Women’s & Reproductive Health, Oxford Endometriosis CaRe Centre, University of Oxford, Oxford, UK.
E R Larby, Norwegian Endometriosis Association, Halden, Norway.
M G Munro, Department of Obstetrics and Gynecology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
E Saridogan, Institute for Women’s Health, University College Hospital, London, UK.
Z M van der Spuy, Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
D Jurkovic, Institute for Women’s Health, University College Hospital, London, UK.
Data Availability
The data underlying this work are available in the article and its online supplementary material.
References
- Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, Nyquist A-C, Saiman L, Yokoe DS, Maragakis LL. et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead JW. On measurements and their quality. Paper 4: verbal anchors and the number of response options in rating scales. Int J Nurs Stud 2014;51:807–814. [DOI] [PubMed] [Google Scholar]
- Bourdon M, Santulli P, Oliveira J, Marcellin L, Maignien C, Melka L, Bordonne C, Millisher AE, Plu-Bureau G, Cormier J. et al. Focal adenomyosis is associated with primary infertility. Fertil Steril 2020;114:1271–1277. [DOI] [PubMed] [Google Scholar]
- Bruun MR, Arendt LH, Forman A, Ramlau-Hansen CH.. Endometriosis and adenomyosis are associated with increased risk of preterm delivery and a small-for-gestational-age child: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2018;97:1073–1090. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Measuring Healthy Days. Atlanta, GA: CDC, 2000. https://www.cdc.gov/hrqol/pdfs/mhd.pdf (1 May 2022, date last accessed). [Google Scholar]
- Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, Gordts S, Guo SW, Just PA, Noël JC. et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update 2020;26:392–411. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Cho SB, Lee SR, Lim YM, Jeong K, Moon HS, Chung H.. Comorbidity of gynecological and non-gynecological diseases with adenomyosis and endometriosis. Obstet Gynecol Sci 2017;60:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK.. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- De Meyer D, Kottner J, Beele H, Schmitt J, Lange T, Van Hecke A, Verhaeghe S, Beeckman D.. Delphi procedure in core outcome set development: rating scale and consensus criteria determined outcome selection. J Clin Epidemiol 2019;111:23–31. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA.. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR.. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018;96:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Bhattacharya S, Bofill M, Collura B, Curtis C, Evers JLH, Giudice LC, Farquharson RG, Franik S. et al. ; Core Outcome Measure for Infertility Trials (COMMIT) Initiative. Standardising definitions and reporting guidelines for the infertility core outcome set: an international consensus development study. Hum Reprod 2020;35:2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Curtis C, Evers JLH, Farquharson RG, Franik S, Khalaf Y, Legro RS, Lensen S, Mol BW. et al. ; COMMIT: Core Outcomes Measures for Infertility Trials. A protocol developing, disseminating and implementing a core outcome set for infertility. Hum Reprod Open 2018;2018:hoy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwan K, Gamble C, Williamson PR, Kirkham JJ; Reporting Bias Group. Systematic review of the empirical evidence of study publication bias and outcome reporting bias – an updated review. PLoS One 2013;8:e66844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish R, MacLennan S, Alkhaffaf B, Williamson PR. “Vicarious thinking” was a key driver of score change in Delphi surveys for COS development and is facilitated by feedback of results. J Clin Epidemiol 2020;128:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schünemann HJ.. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- Harmsen MJ, Van den Bosch T, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L, Hehenkamp W, Groenman F, De Bruyn C, Rasmussen C. et al. Consensus on revised definitions of morphological uterus sonographic assessment (MUSA) features of adenomyosis: results of a modified Delphi procedure. Ultrasound Obstet Gynecol 2022;60:118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Iriyama T, Sayama S, Nakayama T, Komatsu A, Miyauchi A, Nishii O, Nagamatsu T, Osuga Y, Fujii T.. Adenomyosis and adverse perinatal outcomes: increased risk of second trimester miscarriage, preeclampsia, and placental malposition. J Matern Fetal Neonatal Med 2018;31:364–369. [DOI] [PubMed] [Google Scholar]
- Kirk E, Ankum P, Jakab A, Le Clef N, Ludwin A, Small R, Tellum T, Töyli M, Van den Bosch T, Jurkovic D; ESHRE Working Group on Ectopic Pregnancy. Terminology for describing normally sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice. Hum Reprod Open 2020;2020:hoaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu X, Guo SW.. Clinical profiles of 710 premenopausal women with adenomyosis who underwent hysterectomy. J Obstet Gynaecol Res 2014;40:485–494. [DOI] [PubMed] [Google Scholar]
- Masters WH, Johnson VE.. Human Sexual Response. Boston: Little Brown & Co, 1966. [Google Scholar]
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, Thomas R, Harvey E, Garratt A, Bond J.. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5:1–256. [DOI] [PubMed] [Google Scholar]
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet 2018;143:393–408. [DOI] [PubMed] [Google Scholar]
- Muse KN. Cyclic pelvic pain. Obstet Gynecol Clin North Am 1990;17:427–440. [PubMed] [Google Scholar]
- Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D.. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod 2012;27:3432–3439. [DOI] [PubMed] [Google Scholar]
- Nie G, Yang H, Liu J, Zhao C, Wang X.. Psychometric properties of the Chinese version of the menopause-specific quality-of-life questionnaire. Menopause 2017;24:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post MW. Definitions of quality of life: what has happened and how to move on. Top Spinal Cord Inj Rehabil 2014;20:167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente JM, Fabris A, Patel J, Patel A, Cerrillo M, Requena A, Garcia-Velasco JA.. Adenomyosis in infertile women: prevalence and the role of 3D ultrasound as a marker of severity of the disease. Reprod Biol Endocrinol 2016;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus A, Smith V, Wuytack F.. Methodology in core outcome set (COS) development: the impact of patient interviews and using a 5-point versus a 9-point Delphi rating scale on core outcome selection in a COS development study. BMC Med Res Methodol 2021;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockville M. What Is Patient Experience? Agency for Healthcare Research and Quality, 2021. https://www.ahrq.gov/cahps/about-cahps/patient-experience/index.html (1 May 2022, date last accessed).
- Royal College of Obstetricians and Gynaecologists. A to Z of Medical Terms, 2022. https://www.rcog.org.uk/for-the-public/a-z-of-medical-terms/ (1 May 2022, date last accessed).
- Royal College of Obstetricians and Gynecologists. The Initial Management of Chronic Pelvic Pain, Green-Top Guideline No. 41. London, 2012. https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41/ (1 May 2022, date last accessed).
- Schneider HP, Heinemann LA, Rosemeier HP, Potthoff P, Behre HM.. The Menopause Rating Scale (MRS): comparison with Kupperman index and quality-of-life scale SF-36. Climacteric 2000;3:50–58. [DOI] [PubMed] [Google Scholar]
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM.. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300. [DOI] [PubMed] [Google Scholar]
- Tamura H, Kishi H, Kitade M, Asai-Sato M, Tanaka A, Murakami T, Minegishi T, Sugino N.. Complications and outcomes of pregnant women with adenomyosis in Japan. Reprod Med Biol 2017;16:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellum T, Omtvedt M, Naftalin J, Hirsch M, Jurkovic D.. A systematic review of outcome reporting and outcome measures in studies investigating uterine-sparing treatment for adenomyosis. Hum Reprod Open 2021a;2021:hoab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellum T, Naftalin J, Hirsch M, Saridogan E, Jurkovic D.. A protocol for developing, disseminating, and implementing a core outcome set for adenomyosis research. Facts Views Vis Obgyn 2021b;13:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassetti C, Johnson NP, Petrozza J, Abrao MS, Einarsson JI, Horne AW, Lee TTM, Missmer S, Vermeulen N, Zondervan KT et al; International Working Group of AAGL, ESGE, ESHRE and WES. An international terminology for endometriosis, 2021. Hum Reprod Open 2021;2021:hoab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, Van Schoubroeck D, Landolfo C, Installe AJ, Guerriero S. et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46:284–298. [DOI] [PubMed] [Google Scholar]
- Vanhie A, Meuleman C, Tomassetti C, Timmerman D, D’Hoore A, Wolthuis A, Van Cleynenbreugel B, Dancet E, Van den Broeck U, Tsaltas J. et al. Consensus on recording deep endometriosis surgery: the CORDES statement. Hum Reprod 2016;31:1219–1223. [DOI] [PubMed] [Google Scholar]
- Welsh A. Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. London: RCOG Press, 2008. [PubMed] [Google Scholar]
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, Clarke M, Gargon E, Gorst S, Harman N. et al. The COMET handbook: version 1.0. Trials 2017;18:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won HR, Abbott J.. Optimal management of chronic cyclical pelvic pain: an evidence-based and pragmatic approach. Int J Womens Health 2010;2:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). Quality of Care: A Process for Making Strategic Choices in Health Systems. Geneva: World Health Organization, 2006. [Google Scholar]
- World Health Organization (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization, 2011. [Google Scholar]
- World Health Organization (WHO). WHO Recommendations for the Prevention and Treatment of Post-Partum Haemorrhage. Geneva: World Health Organization, 2012. [Google Scholar]
- World Health Organization (WHO). Adverse Reaction Terminology. Geneva: World Health Organization, 2021. [Google Scholar]
- World Health Organization, WHO Patient Safety. Conceptual Framework for the International Classification for Patient Safety Version 1.1: Final Technical Report January 2009. Geneva: World Health Organization, 2010. [Google Scholar]
- Wrenn K. Dysuria, frequency, and urgency. In: Walker HK, Hall WD, Hurst JW (eds). Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworths, 1990, 842–844. [PubMed] [Google Scholar]
- Yeung SY, Kwok JWK, Law SM, Chung JPW, Chan SSC.. Uterine fibroid symptom and health-related quality of life questionnaire: a Chinese translation and validation study. Hong Kong Med J 2019;25:453–459. [DOI] [PubMed] [Google Scholar]
- Younes G, Tulandi T.. Effects of adenomyosis on in vitro fertilisation treatment outcomes: a meta-analysis. Fertil Steril 2017;108:483–490.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this work are available in the article and its online supplementary material.