Abstract
Non-suicidal self-injury (NSSI) is a highly prevalent transdiagnostic symptom and risk marker for mental health problems among adolescents. Research on the neurobiological mechanisms underlying NSSI is needed to clarify the neural correlates associated with the behavior. We examined resting-state functional connectivity in n = 33 female adolescents aged 12–17 years engaging in NSSI, and in n = 29 age-matched healthy controls using graph theory. Mixed linear models were evaluated with the Bayes Factor to determine group differences on global and regional network measures and associations between network measures and clinical characteristics in patients. Adolescents engaging in NSSI demonstrated longer average characteristic path lengths and a smaller number of weighted hubs globally. Regional measures indicated lower efficiency and worse integration in (orbito)frontal regions and higher weighted coreness in the pericalcarine gyrus. In patients, higher orbitofrontal weighted local efficiency was associated with NSSI during the past month while lower pericalcarine nodal efficiency was associated with suicidal thoughts in the past year. Higher right but lower left pericalcarine weighted hubness was associated with more suicide attempts during the past year. Using a graph-based technique to identify functional connectivity networks, this study adds to the growing understanding of the neurobiology of NSSI.
Keywords: non-suicidal self-injury, functional connectivity, resting-state fMRI, adolescents, graph theory
Introduction
Non-suicidal self-injury (NSSI) is defined as self-injurious behavior in the absence of suicidal intent. In adolescents, the prevalence for single events of NSSI in population-based samples according to a meta-analysis is 17.2% (Swannell et al., 2014). The rising awareness and clinical as well as scientific interest in the phenomenon of NSSI has been reflected by the introduction of the NSSI disorder to the 5th version of the Statistical and Diagnostic Manual of Mental Disorders (DSM-5) under section III, as a research diagnosis requiring further study (American Psychiatric Association, 2013). Criterion A of NSSI disorder is met when a person engages in self-injury without suicidal intent on 5 or more days within the past year. Its prevalence has been found to be 4% in adolescent non-clinical samples (Plener et al., 2016) and around 50% among adolescent in-patient samples (Glenn and Klonsky, 2013; Groschwitz et al., 2015). Previous NSSI history has shown to predict future NSSI and suicide attempts (Asarnow et al., 2011) and increase the risk for suicide (Hawton et al., 2015). NSSI often occurs in the context of psychiatric conditions and is one of the strongest predictors of borderline personality disorder (BPD) development (Ghinea et al., 2019). Importantly, recent research suggests that NSSI is to be seen as a transdiagnostic risk marker or precursor of psychopathology in general (Ghinea et al., 2020).
Up to date, little is known about the neurobiological basis of NSSI. According to the proposed ‘model of distal and proximal trait biology as well as biological states’, the neurobiology of NSSI can be described by ‘distal biological traits’ representing genetic or risk factors predicting the development of NSSI, ‘proximal biological traits’ defined as underlying biological alterations observed in individuals engaging in NSSI and ‘biological states’ immediately causing or precipitated by NSSI, such as reactivity of endocrine and physiological systems (Kaess et al., 2021). In the present study, we aim to contribute to the understanding of proximal traits, namely brain functional alterations in patients with NSSI disorder.
Most studies investigating neurobiological alterations in patients engaging in NSSI used task-based paradigms to examine brain activation: young female adults with NSSI showed higher activation of orbitofrontal brain areas during a gambling task (Vega et al., 2018), decreased prefrontal cortex (PFC) activation during an interference task (Dahlgren et al., 2018) and heightened amygdala responses to negative emotional stimuli (Niedtfeld et al., 2010). Adolescents engaging in NSSI further showed increased activation of the medial PFC and the ventrolateral PFC in a social exclusion paradigm (Groschwitz et al., 2016) and increased amygdala activity in response to negative stimuli (Plener et al., 2012) compared to healthy controls. Studies adopting a network perspective using measures of functional connectivity in adults found diminished connectivity between the left orbitofrontal cortex (OFC) and the right parahippocampal gyrus during a gambling task (Vega et al., 2018) and enhanced amygdala-frontal cortex connectivity following the presentation of negative stimuli (Niedtfeld et al., 2010). In youth with NSSI (n = 13), reduced functional connectivity between right OFC and anterior cingulate cortex (ACC) was found during experimental pain administration (Osuch et al., 2014).
More recent studies have begun to elucidate neural circuits implicated in NSSI using resting-state functional magnetic resonance imaging (rsfMRI) to determine resting-state functional connectivity (RSFC). Female adolescents with NSSI demonstrated higher seed-based RSFC between right amygdala and dorsal ACC as well as supplementary motor area (SMA) and lower negative amygdala RSFC with lateral occipital and angular, frontal pole, as well as inferior and middle temporal regions than healthy controls. Further, NSSI had lower positive left amygdala RSFC with dorsal ACC and SMA (Westlund Schreiner et al., 2017). In a similar seed-based approach, adolescents engaging in NSSI showed reduced amygdala RSFC with the ACC, subcallosal cortex, paracingulate gyrus, right planum temporale and right insula as well as between the medial PFC and pre- and postcentral gyri and left insula, compared to healthy controls (Santamarina-Perez et al., 2019).
Disturbed or altered functional brain networks related to psychiatric disorders or symptoms can be described using graph theory. Graph theoretical analysis of RSFC networks has the potential to quantify global and local patterns of brain connectivity, obtained through rsfMRI. These graph-based analyses yield useful network metrics including network efficiency and organization and illustrate key regions within the network (Bullmore and Sporns, 2009). Complex brain networks are abstractly described as graphs that are composed of nodes, which represent brain regions, linked together by edges that denote connections (Sporns, 2014). When applied to rsfMRI, the nodes identified by graph theory represent brain regions, while the edges represent functional connections corresponding to magnitudes of temporal correlations in blood-oxygen-level-dependent activity between pairs of brain regions (Rubinov and Sporns, 2010). Compared to other methods, graph-based network analysis has the potential to reveal meaningful information about the topological architecture of human brain networks, such as small-worldness, modular organization, and highly connected or centralized hubs. To the best of our knowledge, this approach has not yet been applied to RSFC networks in NSSI.
In the present study, we aimed to investigate brain networks of RSFC in adolescents engaging in NSSI and healthy controls using graph-based analyses. The first aim of the present study was to investigate differences in RSFC between adolescents engaging in NSSI and healthy controls using graph theory on a global brain level. The second aim was to examine group differences on a regional brain level across 76 regions of interest (ROIs) spanning the whole cortex. The third aim of the study was to explore dimensional associations between graph-based RSFC measures and clinical characteristics of NSSI severity, suicidality, depressive symptoms and BPD criteria in adolescent patients with NSSI.
Methods and materials
Participants
The study procedure has been described in detail elsewhere (Reichl et al., 2016, 2019; Ando et al., 2018). Adolescents between 12 and 17 years with NSSI disorder according to DSM-5 were recruited from the specialized outpatient clinic for adolescent risk-taking and self-harm behavior (AtR!Sk; ‘Ambulanz für Risikoverhalten & Selbstschädigung’ (Kaess et al., 2017)) and the inpatient units at the Clinic of Child and Adolescent Psychiatry, University Hospital Heidelberg, Germany. Healthy participants who were age- and sex-matched who had neither received lifetime psychiatric diagnosis nor undergone psychiatric treatment or engaged in NSSI were recruited via public advertisement. Adolescents with acute psychotic symptoms, acute suicidality requiring immediate intervention and poor knowledge of the German language were not included. Informed and written consent was obtained from participants and their parents/caregivers. The study was approved by the institutional ethics committee of the University of Heidelberg (S-449/2013) and was performed in accordance with the Declaration of Helsinki.
Procedure
Included participants underwent a clinical appointment and an MRI exam at the University Hospital Heidelberg. Participants who were pregnant, claustrophobic, had metal implants or a history of brain injury were not included in the MRI part of the study.
Clinical assessment
The sociodemographic information obtained during the clinical assessment included sex, date of birth, medication and drug use. Handedness was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971). All clinical interviews were performed by clinicians trained in the field of child and adolescent psychiatry.
NSSI and suicidal thoughts, plans and attempts
NSSI was assessed using the German version (Fischer et al., 2014) of the Self-Injurious Thoughts and Behavior Interview (SITBI) (Nock et al., 2007). The semi-structured interview assesses the presence, frequency and characteristics of a variety of thoughts and behaviors of NSSI as well as suicidal thoughts, gestures, plans and attempts. Previous research reported good reliability estimates for the German version of the SITBI and good convergent validity in relation to an established questionnaire of self-harming behavior (Fischer et al., 2014).
Psychiatric diagnoses
The Mini-International Neuropsychiatric Interview for Children and Adolescents (M.I.N.I.-KID 6.0), (Sheehan et al., 2010), a semi-structured interview for the assessment of axis I psychiatric disorders according to DSM-IV and ICD-10, was used to assess psychiatric diagnoses of the NSSI group. Additionally, BPD criteria were assessed with the respective module of the German version of the Structured Clinical Interview for DSM-IV Personality Disorders (SKID II) (Fydrich et al., 1997). Depressive symptoms were assessed with the German version of the Beck Depression Inventory II (Hautzinger et al., 1995).
Neuroimaging
The mean number of days between the clinical assessment session and the imaging session was 26.8 days (s.d. = 28.2) for adolescents engaging in NSSI and 28.8 days (s.d. = 32.2) for healthy controls. Participants were scanned using a Magnetom TRIO (Siemens, Erlangen, Germany) 3 Tesla scanner with a 32-channel-head coil. Anatomical T1-weighted images were acquired in the sagittal plane (192 slices, 1 mm slice thickness, 1 × 1 mm2 in-plane resolution, echo time = 2.52 ms, repetition time = 1900 ms, flip angle = 9°). T2*-weighted echo planar images were collected during a resting state (echo time (TE) = 27 ms, repetition time (TR) = 2650 ms, field of view (FoV) = 220 mm, flip angle = 90°, slices = 45), during which participants were asked to keep their eyes open.
Image processing
Standard preprocessing of rsfMRI data was carried out using FMRIB’s Software Library (FSL 5.0, Oxford, 2012) and included motion correction with MCFLIRT, brain extraction using BET, slice time correction, spatial smoothing with a Gaussian kernel of full-width-half-maximum 6 mm and high-pass temporal filtering. Functional images were registered linearly with the processed brain extracted images using the FMRIB linear image registration tool and then co-registered non-linearly with MNI standard space using FNIRT. Automated structural segmentation of the T1-weighted images was carried out using FreeSurfer version 6.0. (Reuter et al., 2010) with the Desikan–Killiany–Tourville atlas (Klein and Tourville, 2012). FreeSurfer performs volume-based segmentations where labeling of brain structures is performed by registration of T1-weighted images to MNI305 space, followed by segmentation based on both a subject-independent probabilistic atlas and subject-specific measured values, and then classification of each voxel to a label. Details of FreeSurfer segmentation have been described previously (Fischl et al., 2002, 2004). After segmentation, all images used in the analyses were visually checked for quality by trained researchers.
Connectivity network
Network construction was performed in R version 3.6.3 (https://www.r-project.org/), using the packages igraph v1.2.1 and brainGraph v2.4.0 (Csardi and Nepusz, 2006; Watson et al., 2018). A total of 76 subject-specific ROIs derived from FreeSurfer were used as nodes. Given the sparse existing literature on RSFC in NSSI, we included ROIs spanning the whole cortex in order to provide the full picture of potential group differences in RSFC. A connectivity matrix consisting of all nodes was created, where the connection of two nodes is represented by the squared partial correlation of the time series with the time series of all other nodes (to control for indirect connections between the connections of interest) and cerebrospinal fluid and white matter partialed out. Graph metric data were extracted and saved for further statistical analyses.
Graph theory metrics
A range of metrics have been developed that are designed to capture different organizational properties of a network (Rubinov and Sporns, 2010). In this study, we explored ‘global’, i.e. whole brain as well as ‘regional’ (nodal) connectivity using standard graph theory metrics as implemented in the brainGraph package (Csardi and Nepusz, 2006; Watson et al., 2018). Measures of unweighted (binary, value of 0 = absence or 1= connection) and weighted graphs (associated with a real number, which indicates strength of the connection) were included. Measures are listed in detail in the Supplementary Material.
Statistical analyses
All network measures and clinical variables were z-transformed to enable comparison of regression coefficients. For the first aim, regression models including global network measures as a dependent variable and group (NSSI vs healthy control) as an independent variable (M1) were compared to null models (M0) containing only the dependent variable as a regressor. For aim two, three mixed linear regression models were compared: M0 included the regional network measure and the factor of hemisphere (left vs right) as a covariate; model M1 additionally included the factor group as an independent variable; model M2 further included the interaction of hemisphere and group to investigate potential effects of laterality. For the third aim, those measures/ROIs showing ‘strong evidence’ for group differences were subjected to further analysis, in patients only. Again, three mixed linear models were compared, where M0 was compared to M1 containing the clinical variable of interest (with hemisphere as a covariate in M0 and M1) and M2 additionally containing the interaction between the clinical variable and hemisphere. Clinical variables included in these analyses were as follows: suicidal thoughts (frequency in days, lifetime, last year and last month), suicidal plans (frequency in days, lifetime and last year), suicide attempts (frequency in attempts, lifetime and last year), acts of NSSI (frequency in days, lifetime, last year, last month and last week), number of BPD criteria and depression severity. A number of variables were not assessed further due to minimal variance (suicidal thoughts last week; suicidal plan last month, last week; suicide attempts last month and last week). Outliers at more than 3 s.d. above the group mean were excluded from these analyses. As most outliers were clinically plausible, additional analyses with less stringent outlier removal are presented alongside the main results (only one extreme data point for NSSI during the past month was removed, while all other data points were retained in the analyses). Instead of using P-value statistics, requiring further adjustment for multiple comparisons, models were compared using the Bayes Factor (BF), where a BF between 3 and 20 can be seen as ‘positive evidence’ and a BF larger than 20 is considered ‘strong evidence’ (Raftery, 1995). Model selection based on Bayesian parameter estimation has several advantages over model selection based on P-values (Freedman, 1983; Freedman et al., 1988; Miller, 1990). While Bayesian model selection does not require P-values for statistical comparison, corresponding P-values have been included in the ‘Results’ section for readers more familiar to the frequentist statistical approach. Statistical analyses were performed using Stata (Version 16; StataCorp LP, College Station, TX, USA). Among the wealth of network measures, it has not been established yet, which measures are most informative for the analysis of (functional) brain networks (Liu et al., 2017). Therefore, we have chosen an explorative approach including all available network measures from the software used. Results with strong evidence (BF > 20) will be emphasized in the main manuscript, while results with ‘positive evidence’ (BF 3–20) will be reported in summary to provide the full picture of results, and details are given in the Supplementary Material.
Results
Demographic and clinical characteristics of participants
Out of n = 67 adolescents engaging in NSSI and n = 47 healthy controls, n = 36 youth with NSSI and n = 31 controls underwent MRI. Of these, n = 1 patient had a space-consuming lesion, which led to unusable T1 images, and n = 2 participants had rsfMRI data that were not usable. For the purpose of the present study, another n = 2 male patients with NSSI were excluded from further analyses due to the known effects of sex on brain development, resulting in a study sample of n = 33 adolescents with NSSI and n = 29 healthy controls.
Demographic and clinical characteristics for both groups are detailed in Table 1. Adolescents were comparable in age, handedness and body mass index between groups. Regarding education, n = 18 adolescents engaging in NSSI and n = 21 controls attended the ‘Gymnasium’ (graduation qualifies for university entrance); n = 4 adolescents from the NSSI group and n = 7 controls attended or completed the ‘Realschule’ (secondary school level certificate) and n = 7 from the NSSI group, and n = 1 controls attended or completed the ‘Hauptschule’ (9 years of elementary school). Within the NSSI group, 12% reported regular use of medication and 30% reported substance use, whereas this was the case in 3.5% (n = 1) of the control group.
Table 1.
Participant characteristics
| NSSI n (%)/mean (s.d.) | Control n (%)/mean (s.d.) | |
|---|---|---|
| N | 33 | 29 |
| Age (years) | 15.84 (1.33) | 16.02 (1.12) |
| Right handedness | 30 (91) | 27 (93) |
| Body mass index | 21.89 (3.48) | 20.73 (2.46) |
| Regular use of medication | 4 (12) | 1 (4) |
| Substance usea | 5 (15) | 1 (4) |
| Acts of NSSI last year | 66.39 (76.80) | 0 |
| Acts of NSSI last month | 3.39 (5.45) | 0 |
| Suicidal thoughts last year | 5.50 (6.46) | 0 |
| Suicidal thoughts last month | 1.13 (0.99) | 0 |
| Suicide attempts lifetime | 1.83 (1.38) | 0 |
| Suicide attempts last year | 1.00 (0.77) | 0 |
| Number of BPD diagnostic criteria | 4.24 (2.33) | 0.07 (0.26) |
| BPD diagnosis | 14 (42) | 0 (0) |
| Depressive symptoms | 29.67 (15.15) | 4.07 (3.59) |
| Depression diagnosis | 25 (76) | 0 (0) |
Notes: NSSI = non-suicidal self-injury; BPD = borderline personality disorder.
On 3 or more days in the last 3 months.
In the NSSI group, the mean frequency of NSSI was 66.39 (s.d. = 76.80) during the last year and 3.39 (s.d. = 5.45) during the last month. The mean reported number of suicide attempts was 1.83 (s.d. = 1.38) across the lifetime and 1.00 (s.d. = 0.77) during the last year.
Group differences in functional connectivity
Global network level
On the global network level, there was no strong evidence that groups differed for any of the network measures. However, there was positive evidence that ‘weighted characteristic path length’ was longer in the NSSI group (MNSSI = 34.9, s.d. = 6.7; Mcontrol = 30.8, s.d. = 4.9; BF = 4.5) and that the ‘weighted number of hubs’ (vertices with hubness score ≥2) was smaller in the NSSI group compared to healthy controls (MNSSI = 13.1, s.d. = 2.4; Mcontrol = 14.5, s.d. = 1.7; BF = 3.1) (see Supplementary Table S1).
Regional network (vertex) level
For the regional network measures, there was strong evidence (BF > 20) of group differences between adolescents engaging in NSSI and controls in three medial frontal and occipital ROIs (Table 2, Figure 1). Medial OFC ‘coreness’, which represents membership in sub-networks of multiple highly interconnected nodes (Hagmann et al., 2008), was lower in the NSSI than in the control group. In contrast, pericalcarine ‘weighted coreness’ was higher in the NSSI group than in the control group. Finally, paracentral ‘degree’, denoting the number of edges, or connections of a vertex, was lower in the NSSI group.
Table 2.
Regression coefficients of models showing strong evidence of group differences in regional functional connectivity (BF > 20)
| ROI | Network measure | NSSI mean (s.d.) | Controls mean (s.d.) | Coefficient (95 % CI) | P | BF |
|---|---|---|---|---|---|---|
| Medial orbitofrontal | Coreness | 1.55 (0.56) | 2.16 (0.92) | −0.76 (−1.18, −0.34) | <0.001 | 25.4 |
| Paracentral | Degree | 2.23 (1.20) | 3.17 (1.65) | −0.51 (−0.80, −0.22) | 0.001 | 21.0 |
| Pericalcarine cortex | W. coreness | 51.05 (12.83) | 38.0 (16.3) | 0.65 (0.30, 1.01) | <0.001 | 36.0 |
Notes: Results from models with BF (= Bayes Factor) >20 are shown. NSSI = non-suicidal self-injury, CI = confidence interval. Coefficients and 95% CI refer to z-standardized variables. W. = Weighted measure includes information about connection strength (as opposed to unweighted measures, which refer to binary graph (connection absent vs present)).
Fig. 1.
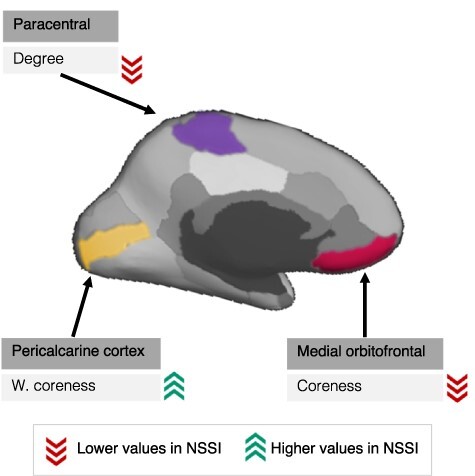
Regional group differences in network measures between adolescents engaging in NSSI vs healthy controls.
Notes: ‘coreness’ = referring to the k-core, which is the largest subnetwork comprising nodes of degree at least k. It reflects membership in sub-networks of multiple highly interconnected nodes. ‘W. (= weighted) coreness’ = refers to the s-core, the largest subnetwork comprising nodes of strength at least s. ‘Degree = number of connections of a node’. Regions of interest are illustrated on the left hemisphere, but models were calculated for left and right hemispheres (with hemisphere as a covariate).
Further, there was positive evidence (BF 3–20) of group differences in a number of regional network measures mainly in frontal ROIs (paracentral, precentral, caudal middle frontal and pars opercularis, known as SMA), where network measures indicated longer path lengths, lower ‘degrees’ (number) and ‘strengths’ of connections, lower ‘local efficiency’ and lower ‘coreness’ (Supplementary Table S2).
Associations between functional connectivity and clinical characteristics in patients
Associations between regional network measures and clinical characteristics for those ROIs which showed strong evidence of group differences between NSSI and controls (BF > 20), namely medial OFC, paracentral and pericalcarine gyrus, are reported in Table 3 and Figure 2. Associations with positive evidence (BF 3–20) in these ROIs are reported in the Supplementary Material (Supplementary Table S3).
Table 3.
Regression coefficients of models showing strong evidence of associations between regional functional connectivity and clinical variables (BF > 20)
| Analyses with conservative outlier management | ||||||
|---|---|---|---|---|---|---|
| Clinical characteristic | ROI | Network measure | Coefficient (95 % CI) | P | BF | |
| Suicidal thoughts | Past year | Pericalcarine | Nodal efficiency | −0.52 (−0.80, −0.25) | <0.001 | 37.2 |
| Suicide attempts | Past year | Pericalcarine | W. hubnessa | 0.77 (0.43, 1.11) | <0.001 | 59.5 |
| NSSI acts | Past month | Medial orbitofrontal | W. local efficiency | 0.31 (0.14, 0.49) | <0.001 | 25.2 |
| Analyses with less conservative outlier management | ||||||
| Suicidal thoughts | Lifetime | Medial orbitofrontal | W. transitivity | 0.30 (0.19, 0.41) | <0.001 | 1136.2 |
| Suicidal thoughts | Lifetime | Medial orbitofrontal | Transitivity | 0.32 (0.21, 0.42) | <0.001 | 16 256.9 |
| Suicidal thoughts | Lifetime | Medial orbitofrontal | W. local efficiency | 0.30 (0.13, 0.47) | <0.001 | 22.6 |
| Suicidal thoughts | Lifetime | Medial orbitofrontal | Local efficiency | 0.33 (0.22, 0.45) | <0.001 | 10 275.4 |
| NSSI acts | Past month | Medial orbitofrontal | W. local efficiency | 0.31 (0.14, 0.49) | <0.001 | 25.2 |
| NSSI acts | Past week | Medial orbitofrontal | W. transitivitya | 0.42 (0.28, 0.57) | <0.001 | 5422.9 |
Notes: Associations between network measures in ROIs where there was strong evidence of group differences in primary analyses. Results from models with BF (= Bayes Factor) > 20 are shown. CI = confidence interval. W. = Weighted measure: includes information about connection strength (as opposed to unweighted measures, which refer to binary graph (connection absent vs present)).
Interaction effect of hemisphere by suicide attempts. Conservative outlier management: outliers ±3 s.d. removed. Less conservative outlier management: clinically plausible but high values included, except for one implausible value in NSSI acts past month.
Fig. 2.
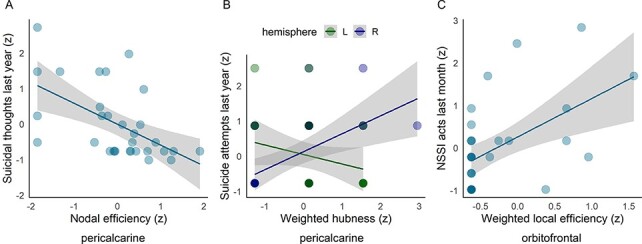
Associations between graph-based measures of regional functional connectivity and clinical characteristics in patients engaging in NSSI.
Notes: Associations of graph-based measures with (A) suicidal thoughts, (B) suicide attempts and (C) acts of NSSI. Results from models with BF (= Bayes Factor) >20 are shown. Each individual measurement is represented by a circle, lines represent linear regression coefficient and grey shaded area represents the standard error. Circles appear darker when multiple measurements are overlaid.
Strong associations between regional network measures and clinical characteristics were only found in OFC and pericalcarine cortex. The more days with suicidal thoughts patients reported during the last year, the lower was ‘nodal efficiency’ in the pericalcarine gyrus. Further, a higher number of reported suicide attempts last year was associated with higher ‘weighted hubness’ in the right pericalcarine gyrus, but with lower ‘weighted hubness’ in the left pericalcarine gyrus. Finally, the higher the number of NSSI acts in the last month, the higher the ‘weighted local efficiency’ in the medial OFC. Additional analyses with less conservative outlier management further showed evidence for strong associations between lifetime suicidal thoughts and medial OFC ‘transitivity’ and ‘local efficiency’ as well as NSSI acts within the past week and medial OFC ‘weighted transitivity’. For these measures, higher severity in clinical characteristics was related to higher ‘transitivity’ and ‘local efficiency’ (Table 3 and Supplementary Figure S1).
Positive evidence of associations between medial OFC measures of higher ‘local efficiency’ and ‘clustering’ but worse integration (i.e. longer ‘characteristic path lengths’) was found in relation to a higher number of suicidal thoughts, plans, suicide attempts and acts of NSSI (Supplementary Table S3). Moreover, results indicated some evidence for higher paracentral ‘participation coefficients’ (indicating that this region has most connections not within the community but with different communities) and lower ‘degree’ (number of links) associated with more suicidal thoughts, plans and acts of NSSI. Finally, lower pericalcarine ‘nodal efficiency’ and ‘average nearest neighbor degree’, which quantifies the average degree of connections of a node, and lower ‘weighted hubness’ were associated with more suicidal thoughts and attempts.
Discussion
This study adds to the limited understanding of brain functional connectivity associated with adolescent NSSI by applying a graph-based network approach. To our knowledge, this is the first study to apply graph theory to investigate the neurobiological underpinnings of NSSI. On a global brain level, adolescents with NSSI exhibited longer ‘shortest characteristic path lengths’ indicating less efficient information transfer. A smaller ‘number of hubs’ further reflects that there are fewer areas of highly interconnected nodes, which are central for information integration, in adolescents with NSSI than in controls. Of note, none of the global group differences described above showed strong evidence (all BF > 3 but < 20). On a regional level, youth with NSSI demonstrated lower ‘coreness’ in the OFC in comparison with healthy controls. ‘Coreness’ is a measure denoting the largest subnetwork of a graph with the highest number of connections in comparison to the surrounding nodes. A lower ‘coreness’ might therefore indicate a less prominent role of the OFC and lower orbitofrontal RSFC in NSSI youths than in controls. This is in line with findings from task-based fMRI, where blunted reward anticipation in the OFC was demonstrated in self-injuring adolescents (Sauder et al., 2016), which has consistently been shown in disorders related with low impulse control and anhedonia or depression. Reduced connectivity between the left OFC and the right parahippocampal gyrus was found in adults with BPD during a gambling task (Vega et al., 2018), and reduced connectivity between right OFC and ACC was found during experimental pain administration in adolescents with NSSI (Osuch et al., 2014). In line with the alterations shown in task-based fMRI studies, our finding of lower ‘coreness’ in youth with NSSI might suggest a deficit of the orbitofrontal regulation of emotional responses and impulsivity normally executed by the OFC on the amygdala. Further, with the OFC involved in evaluating reward values and outcomes (Stalnaker et al., 2015), our findings add evidence of the importance of the brain’s reward circuitry in the context of NSSI (Brunner et al., 2010; Sauder et al., 2016; Poon et al., 2019).
We also found a lower ‘degree’ in the paracentral gyrus, which indicates fewer connections in this region in adolescents engaging in NSSI compared to healthy controls. The paracentral lobule is known for its sensory and motor control of the lower extremities. More recently, it has been implicated in emotion processing in depressive patients with histories of stressful life events (Li et al., 2016). A structural MRI study found that greater impulsivity in depressed adolescents with a history of suicide attempts was related to cortical thickness of the paracentral lobule (Fradkin et al., 2017). To the best of our knowledge, however, the role of the paracentral lobule as a possible correlate of impulsivity or action planning has not previously been discussed in the context of NSSI.
Weaker group differences in several frontal ROIs, namely precentral, caudal middle frontal and pars opercularis, known as SMA, were found, with longer ‘path lengths’, lower ‘degrees (number)’ and ‘strengths’ of connections, lower ‘local efficiency’ and lower ‘coreness’ in patients with NSSI than in controls. These differences seem again to fit the idea of an impaired frontal top-down control that is suggested to play an important role in both emotional dysregulation and impulsive behavior (Etkin et al., 2015). However, given that evidence was positive but not strong, results may need replication in larger samples.
Finally, adolescents with NSSI demonstrated higher ‘weighted coreness’ in the pericalcarine cortex, which suggests a subnetwork of strong connections in this region compared to the healthy control group. As part of the secondary visual cortex, this region is generally implicated in visual information processing. Interestingly, it has been related to the processing of self-related information during social interaction in a group of NSSI and healthy adolescents; however, this effect was not specific to the NSSI group (Perini et al., 2019). While the role of visual processing has been demonstrated in NSSI in the context of face recognition (Seymour et al., 2016), the potential importance of the pericalcarine cortex for NSSI needs to be clarified by further studies. Overall, our results suggesting worse information transfer in youth engaging in NSSI are in line with findings from structural MRI, which indicate widespread white matter microstructural deficits in adolescents with NSSI (Westlund Schreiner et al., 2019a).
A further aim of this study was to determine whether RSFC was related to specific clinical characteristics in the NSSI group. Interestingly, in our study, more acts of NSSI in the last month were related to a higher orbitofrontal ‘weighted local efficiency’. In the context of generally lower OFC efficiency in youth with NSSI (as discussed above), this suggests that the act of NSSI might serve as a compensatory mechanism for emotional dysregulation potentially due to limbic hyperarousal (Plener et al., 2012). This finding is further accompanied by the weaker findings of higher OFC clustering, but worse integration (longer path lengths) associated with more suicidal thoughts, plans, attempts and acts of NSSI and might indicate ‘overactivity’ within the OFC but simultaneously worse information transfer with other regions, such as the amygdala. Similarly, despite higher pericalcarine ‘weighted coreness’ in the NSSI group, those youths who reported more suicidal thoughts in the previous year demonstrated lower ‘nodal efficiency’ in this region. Moreover, fewer connections (‘degree’) and hubs were associated with more suicidal thoughts and attempts in this region. Given that a minority of the studies have demonstrated associations with clinical outcomes in addition to group differences, our findings are difficult to integrate in the current literature. Additionally, our results show that the association between network measures and clinical outcomes may in some brain regions depend on the hemisphere. Our findings therefore reflect that the associations between network measures and clinical characteristics are not straightforward and need to be replicated in larger samples.
A few limitations merit comment. First, the neurobiological and neurophysiological processes underlying graph metrics are still unknown, and these metrics may vary depending on methodological decisions such as the parcellation scheme (Farahani et al., 2019). We have limited our analyses addressing clinical correlates to regions that showed differences between groups. However, RSFC in other brain regions not further examined in these analyses might show meaningful associations with clinical symptoms. For example, the salience network, the default mode network and the central executive network have been involved in the acute stress response, which might contribute to psychopathology when it is inadequate, excessive or prolonged (van Oort et al., 2017). Second, the clinical variables were assessed based on retrospective interviews. More ecologically valid approaches such as ecological momentary assessment with repeated sampling of patients’ experiences or mood have the potential to reduce retrospective bias (Solhan et al., 2009). Third, RSFC changes with continuing maturation of the functional and structural networks in childhood and well into adolescence (Fair et al., 2009; Huang et al., 2013). Therefore, longitudinal studies investigating RSFC networks are needed to elucidate whether processes of neuromaturation play an important role in the pathways to risk behaviors or might reveal neural circuits potentiating the risk for self-injurious behaviors. In particular, the changes related to emotion processing and executive functions taking place during adolescence and puberty (Blakemore, 2008) are likely to be intertwined with the emergence of NSSI. Finally, we acknowledge that first and foremost, the results from this study add to the basic understanding of the neurobiology of NSSI and have limited clinical implications. Nevertheless, critical alterations in functional and structural brain characteristics have previously been associated with pharmacological and psychotherapeutic interventions (Quidé et al., 2012; Cullen et al., 2016). A first study in adolescents with NSSI found stronger negative amygdala-prefrontal connectivity associated with greater post-treatment improvement in NSSI after Dialectical Behavior Therapy for Adolescents and treatment as usual (both treatment arms had to be combined to increase power to investigate RSFC connectivity correlates of psychological treatment) (Santamarina-Perez et al., 2019). Potentially, a better understanding of the neurobiological underpinnings of NSSI may in the future help to identify neurobiological treatment targets and thereby advance treatment development (Westlund Schreiner et al., 2019b).
Conclusion
The current study sheds new light on RSFC associated with NSSI. Using graph theory, we have demonstrated that adolescents engaging in NSSI show altered topological organization of functional networks during rest. The pattern of results was less clear for the clinical associations in patients, with higher and lower network efficiency relating to worse clinical outcomes depending on the brain region and hemisphere. By means of cutting-edge network science and a relatively large sample size, our study adds to elucidating the neurobiological concomitants of adolescent NSSI.
Supplementary Material
Acknowledgements
We would like to extend our thanks to Peter Parzer for his assistance in preprocessing the data and his statistical advice. We would also like to thank all adolescents and their parents for their participation in the study.
Contributor Information
Ines Mürner-Lavanchy, University Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, Bern 60 3000, Switzerland.
Julian Koenig, University Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, Bern 60 3000, Switzerland; Department of Child and Adolescent Psychiatry, Centre for Psychosocial Medicine, University of Heidelberg, Heidelberg 69115, Germany; Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne 50931, Germany.
Corinna Reichl, University Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, Bern 60 3000, Switzerland.
Romuald Brunner, Clinic and Policlinic of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University of Regensburg, Regensburg 93053, Germany.
Michael Kaess, University Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, Bern 60 3000, Switzerland; Department of Child and Adolescent Psychiatry, Centre for Psychosocial Medicine, University of Heidelberg, Heidelberg 69115, Germany.
Funding
This work was supported by the Dres. Majic/Majic-Schelz-Foundation, who funded reimbursement of study participants. The foundation was not involved in the collection, analysis or interpretation of data, in the writing of the manuscript or the decision to submit the article for publication.
Conflict of interest
None of the authors have any financial disclosures to make nor any conflicts of interest to report.
Supplementary data
Supplementary data is available at SCAN online.
References
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Washington, DC: American Psychiatric Association. [Google Scholar]
- Ando A., Reichl C., Scheu F., et al. (2018). Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Research: Neuroimaging, 280, 48–55. [DOI] [PubMed] [Google Scholar]
- Asarnow J.R., Porta G., Spirito A., et al. (2011). Suicide attempts and nonsuicidal self-injury in the treatment of resistant depression in adolescents: findings from the TORDIA study. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9, 267–77. [DOI] [PubMed] [Google Scholar]
- Brunner R., Henze R., Parzer P., et al. (2010). Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: is it disorder specific? Neuroimage, 49, 114–20. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10, 186–98. [DOI] [PubMed] [Google Scholar]
- Csardi G., Nepusz T. (2006). The igraph software package for complex network research. InterJournal, Complex Systems, 1695, 1–9. [Google Scholar]
- Cullen K.R., Klimes-Dougan B., Vu D.P., et al. (2016). Neural correlates of antidepressant treatment response in adolescents with major depressive disorder. Journal ofChild and Adolescent Psychopharmacology, 26, 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren M.K., Hooley J.M., Best S.G., Sagar K.A., Gonenc A., Gruber S.A. (2018). Prefrontal cortex activation during cognitive interference in nonsuicidal self-injury. Psychiatry Research: Neuroimaging, 277, 28–38. [DOI] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. (2015). The neural bases of emotion regulation [no. 11]. Nature Reviews Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., et al. (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5, e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani F.V., Karwowski W., Lighthall N.R. (2019). Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Frontiers in Neuroscience, 13.doi: 10.3389/fnins.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Ameis N., Parzer P., et al. (2014). The German version of the self-injurious thoughts and behaviors interview (SITBI-G): a tool to assess non-suicidal self-injury and suicidal behavior disorder. BMC Psychiatry, 14, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.∼.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–55. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., et al. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Fradkin Y., Khadka S., Bessette K.L., Stevens M.C. (2017). The relationship of impulsivity and cortical thickness in depressed and non-depressed adolescents. Brain Imaging and Behavior, 11, 1515–25. [DOI] [PubMed] [Google Scholar]
- Freedman D.A. (1983). A note on screening regression equations. The American Statistician, 37, 152–5. [Google Scholar]
- Freedman D.A., Navidi W., Peters S.C. (1988). On the impact of variable selection in fitting regression equations. In: Dijkstra, T.K., (editor). On Model Uncertainty and Its Statistical Implications. Berlin, Heidelberg: Springer, 1–16. [Google Scholar]
- Fydrich T., Renneberg B., Schmitz B., Wittchen H.U. (1997). SKID II. Strukturiertes Klinisches Interview Für DSM-IV, Achse II: Persönlichkeitsstörungen. Interviewheft. Eine Deutschsprachige, Erw. Bearb. D. Amerikanischen Originalversion D. SKID-II. In: First, M.B., Spitzer, R.L., Gibbon, M., Williams, J.B.W., Benjamin, L., (editors). Göttingen: Hogrefe. [Google Scholar]
- Ghinea D., Koenig J., Parzer P., et al. (2019). Longitudinal development of risk-taking and self-injurious behavior in association with late adolescent borderline personality disorder symptoms. Psychiatry Research, 273, 127–33. [DOI] [PubMed] [Google Scholar]
- Ghinea D., Edinger A., Parzer P., Koenig J., Resch F., Kaess M. (2020). Non-suicidal self-injury disorder as a stand-alone diagnosis in a consecutive help-seeking sample of adolescents. Journal of Affective Disorders, 274, 1122–5. [DOI] [PubMed] [Google Scholar]
- Glenn, C.R., Klonsky, E.D. (2013). Nonsuicidal self-injury disorder: an empirical investigation in adolescent psychiatric patients. Journal of ClinicalChild & Adolescent Psychology, 42, 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz R.C., Plener P.L., Kaess M., Schumacher T., Stoehr R., Boege I. (2015). The situation of former adolescent self-injurers as young adults: a follow-up study. BMC Psychiatry, 15, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz R.C., Plener P.L., Groen G., Bonenberger M., Abler B. (2016). Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: an fMRI study. Psychiatry Research: Neuroimaging, 255, 43–9. [DOI] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biology, 6, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Worall H., Keller F. (1995). Beck Depressions Inventar, BDI Testhandbuch, 2nd edn. Bern: Hans Huber. [Google Scholar]
- Hawton K., Bergen H., Cooper J., et al. (2015). Suicide following self-harm: findings from the multicentre study of self-harm in England, 2000–2012. Journal of Affective Disorders, 175, 147–51. [DOI] [PubMed] [Google Scholar]
- Huang H., Shu N., Mishra V., et al. (2013). Development of human brain structural networks through infancy and childhood. CerebralCortex, 25, 1389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess M., Ghinea D., Fischer-Waldschmidt G., Resch F. (2017). Die Ambulanz für Risikoverhalten und Selbstschädigung (AtR!Sk) – ein Pionierkonzept der ambulanten Früherkennung und Frühintervention von Borderline-Persönlichkeitsstörungen. Praxis der Kinderpsychologie Und Kinderpsychiatrie, 66, 404–22. [DOI] [PubMed] [Google Scholar]
- Kaess M., Hooley J.M., Klimes-Dougan B., et al. (2021). Advancing a temporal framework for understanding the biology of nonsuicidal self- injury: an expert review. Neuroscience Biobehavioral Reviews, 130, 228–39. doi: 10.1016/j.neubiorev.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Tourville J. (2012). 101 labeled brain images and a consistent human cortical labeling protocol. Frontiers in Neuroscience.doi: 10.3389/fnins.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Ma X., Bian H., et al. (2016). A pilot fMRI study of the effect of stressful factors on the onset of depression in female patients. Brain Imaging and Behavior, 10, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li M., Pan Y., et al. (2017). Complex brain network analysis and its applications to brain disorders: a survey [review article]. Complexity, 2017, e8362741. [Google Scholar]
- Miller A.J. (1990). Subset Selection in Regression. London: Chapman and Hall. [Google Scholar]
- Niedtfeld I., Schulze L., Kirsch P., Herpertz S.C., Bohus M., Schmahl C. (2010). Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biological Psychiatry, 68, 383–91. [DOI] [PubMed] [Google Scholar]
- Nock M., Holmberg E., Photos V., Michel B. (2007). Self-injurious thoughts and behaviors interview: development, reliability, and validity in an adolescent sample. Psychological Assessment, 19, 309–17. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Osuch E., Ford K., Wrath A., Bartha R., Neufeld R. (2014). Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Research: Neuroimaging, 223, 104–12. [DOI] [PubMed] [Google Scholar]
- Perini I., Gustafsson P.A., Hamilton J.P., et al. (2019). Brain-based classification of negative social bias in adolescents with nonsuicidal self-injury: findings from simulated online social interaction. EClinicalMedicine, 13, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plener P.L., Bubalo N., Fladung A.K., Ludolph A.G., Lulé D. (2012). Prone to excitement: adolescent females with non-suicidal self-injury (NSSI) show altered cortical pattern to emotional and NSS-related material. Psychiatry Research: Neuroimaging, 203, 146–52. [DOI] [PubMed] [Google Scholar]
- Plener P.L., Allroggen M., Kapusta N.D., Brähler E., Fegert J.M., Groschwitz R.C. (2016). The prevalence of nonsuicidal self-injury (NSSI) in a representative sample of the German population. BMC Psychiatry, 16, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon J.A., Thompson J.C., Forbes E.E., Chaplin T.M. (2019). Adolescents’ reward-related neural activation: links to thoughts of nonsuicidal self-injury. Suicide & Life-threatening Behavior, 49, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quidé Y., Witteveen A.B., El-Hage W., Veltman D.J., Olff M. (2012). Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neuroscience and Biobehavioral Reviews, 36, 626–44. [DOI] [PubMed] [Google Scholar]
- Raftery A.E. (1995). Bayesian model selection in social research. Sociological Methodology, 25, 111. [Google Scholar]
- Reichl C., Heyer A., Brunner R., et al. (2016). Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology, 74, 203–11. [DOI] [PubMed] [Google Scholar]
- Reichl C., Brunner R., Bender N., et al. (2019). Adolescent nonsuicidal self-injury and cortisol response to the retrieval of adversity: a sibling study. Psychoneuroendocrinology, 110, 104460. [DOI] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. (2010). Highly accurate inverse consistent registration: a robust approach. Neuroimage, 53, 1181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 52, 1059–69. [DOI] [PubMed] [Google Scholar]
- Santamarina-Perez P., Romero S., Mendez I., et al. (2019). Fronto-limbic connectivity as a predictor of improvement in nonsuicidal self-injury in adolescents following psychotherapy. Journal ofChild and Adolescent Psychopharmacology, 29, 456–65. [DOI] [PubMed] [Google Scholar]
- Sauder C.L., Derbidge C.M., Beauchaine T.P. (2016). Neural responses to monetary incentives among self-injuring adolescent girls. Development and Psychopathology, 28, 277–91. [DOI] [PubMed] [Google Scholar]
- Seymour K.E., Jones R.N., Cushman G.K., et al. (2016). Emotional face recognition in adolescent suicide attempters and adolescents engaging in non-suicidal self-injury. EuropeanChild & Adolescent Psychiatry, 25, 247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Sheehan K.H., Shytle R.D., et al. (2010). Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). The Journal of Clinical Psychiatry, 71, 313–26. [DOI] [PubMed] [Google Scholar]
- Solhan M.B., Trull T.J., Jahng S., Wood P.K. (2009). Clinical assessment of affective instability: comparing EMA indices, questionnaire reports, and retrospective recall. Psychological Assessment, 21, 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. (2014). Towards network substrates of brain disorders. Brain, 137, 2117–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker T.A., Cooch N.K., Schoenbaum G. (2015). What the orbitofrontal cortex does not do [no. 5]. Nature Neuroscience, 18, 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swannell S.V., Martin G.E., Page A., Hasking P., St John N.J. (2014). Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide & Life-threatening Behavior, 44, 273–303. [DOI] [PubMed] [Google Scholar]
- van Oort J., Tendolkar I., Hermans E.J., et al. (2017). How the brain connects in response to acute stress: a review at the human brain systems level. Neuroscience and Biobehavioral Reviews, 83, 281–97. [DOI] [PubMed] [Google Scholar]
- Vega D., Ripollés P., Soto À., et al. (2018). Orbitofrontal overactivation in reward processing in borderline personality disorder: the role of non-suicidal self-injury. Brain Imaging and Behavior, 12, 217–28. [DOI] [PubMed] [Google Scholar]
- Watson C.G., Stopp C., Newburger J.W., Rivkin M.J. (2018). Graph theory analysis of cortical thickness networks in adolescents with d-transposition of the great arteries. Brain and Behavior, 8, e00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund Schreiner M., Klimes-Dougan B., Mueller B.A., et al. (2017). Multi-modal neuroimaging of adolescents with non-suicidal self-injury: amygdala functional connectivity. Journal of Affective Disorders, 221, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund Schreiner M., Mueller B.A., Klimes-Dougan B., et al. (2019a). White matter microstructure in adolescents and young adults with non-suicidal self-injury. Frontiers inPsychiatry, 10, 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund Schreiner M., Klimes-Dougan B., Parenteau A., Hill D., Cullen K.R. (2019b). A framework for identifying neurobiologically based intervention targets for NSSI. Current Behavioral Neuroscience Reports, 6, 177–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


