Abstract
Here we asked whether, similar to visual and auditory event-related potentials (ERPs), somatosensory ERPs reflect affect. Participants were stroked on hairy or glabrous skin at five stroking velocities (0.5, 1, 3, 10 and 20 cm/s). For stroking of hairy skin, pleasantness ratings related to velocity in an inverted u-shaped manner. ERPs showed a negativity at 400 ms following touch onset over somatosensory cortex contra-lateral to the stimulation site. This negativity, referred to as sN400, was larger for intermediate than for faster and slower velocities and positively predicted pleasantness ratings. For stroking of glabrous skin, pleasantness showed again an inverted u-shaped relation with velocity and, additionally, increased linearly with faster stroking. The sN400 revealed no quadratic effect and instead was larger for faster velocities. Its amplitude failed to significantly predict pleasantness. In sum, as was reported for other senses, a touch’s affective value modulates the somatosensory ERP. Notably, however, this ERP and associated subjective pleasantness dissociate between hairy and glabrous skin underscoring functional differences between the skin with which we typically receive touch and the skin with which we typically reach out to touch.
Keywords: ultra-late positive potential, LPP, sN400, affective touch, social touch, C-tactile, CT
Introduction
Of the many stimuli that excite our sensory systems, only a subset reaches awareness and of those only a few significantly alter the way we think, feel or behave. Critical in this filtering process is a stimulus’ motivational salience. Highly salient stimuli, characterized by their potential to be beneficial or harmful, trigger an affective response that helps amplify emerging stimulus representations from early sensory to later conceptual stages, ensuring that these stimuli stand out among less salient ones. Much research has examined this temporal unfolding for associated neural processes in the visual and auditory modalities, but relatively little is known about touch. Here we sought to address this issue by identifying potential electrophysiological markers of cortical tactile affect in the event-related potential (ERP).
Early and late ERP indexes of affect
Whether an event elicits an affective response depends on a combination of factors including stimulus inherent properties that have been relevant in the course of human evolution as well as an individual’s mental state characteristics. For example, in general, humans have a biological predisposition to perceive happy faces as affectively positive. Yet, whether seeing a happy face makes one feel positive will depend on other variables including the probability of encountering a happy face, one’s current mood and level of extraversion, among others (Somerville et al., 2011). This combination of stimulus- and state-driven adaptive processes is supported by an integration of the so-called bottom-up and top-down information (Hajcak and Nieuwenhuis, 2006; Grandjean et al., 2008).
The advent of neuroimaging has made it possible to examine these processing streams as they unfold in time. Particularly useful has been the electroencephalogram (EEG), which measures post-synaptic cortical activity with millisecond resolution and enables the differentiation of early sensory and later conceptual processing stages. In the visual modality, for example, an early lateralized occipital ERP called the C1 serves as a direct marker of primary visual cortex activity. Comparing the C1 elicited to affective and neutral images has highlighted that the former enhance stimulus perception as early as 65 ms following stimulus onset (Stolarova et al., 2006). In the auditory modality, neural responses are a bit more extended in time because stimuli are necessarily dynamic. Here, a negative response measured around 200 ms over temporal cortex has been linked to stimulus affect. Specifically, an enhanced auditory cortex response elicited by rare relative to frequent sounds is further amplified for rare sounds that are positive or negative when compared with rare neutral sounds (Schirmer et al., 2005; Schirmer and Escoffier, 2010). Although these early sensory modulations may be influenced by higher-order mental processes, they are typically considered more stimulus-driven or bottom-up when compared with later ERPs (Schirmer and Kotz, 2006; Paulmann and Kotz, 2008; Jiang and Pell, 2015).
Early sensory modulations are followed by a family of positive deflections referred to as late positive potentials (LPPs) (Schupp et al., 2000; Moran et al., 2013). They have been reported to emerge after 500 ms and possibly later with a midline and typically posterior scalp topography irrespective of stimulus modality. Much research has shown larger LPP amplitudes to affectively charged when compared with neutral stimuli. For example, positive and negative words, objects, faces or voices increase the LPP relative to neutral controls (Hajcak et al., 2010; Brown et al., 2012). Notably, such late effects are driven by a range of processes including sensory stimulus features (e.g. sound intensity), stimulus probability or task relevance. Physically salient, rare and imperative events elicit a larger LPP than events that are perceptually weak, frequent and mere distractors (Polich, 2007; Weinberg and Hajcak, 2011). As such LPP amplitude modulations may index a combination of bottom-up and top-down processes.
The brain’s temporal signature of tactile affect
When compared with the visual and auditory modalities, the tactile EEG/ERP has received relatively little attention. Moreover, existing studies have focused primarily on non-affective matters related, for example, to sensory discrimination or object recognition via the glabrous skin of the palm. To facilitate temporal synchronization in the EEG, tactile stimuli typically comprised small square-shaped electric pulses rather than an actual mechanical stimulus (Nierhaus et al., 2015; Forschack et al., 2017; Perri et al., 2019). Popular dependent measures included a reduction in the power of Rolandic or mu rhythms (i.e. alpha/beta band) with stimulus intensity as well as early deflections in the ERP over somatosensory cortex contra-lateral to the stimulation site.
To date, few EEG/ERP studies have pursued the affective processing of touch. Some of these studies addressed the issue indirectly by examining how touch shapes responses to a foreground stimulus (Schirmer and Gunter, 2017; Spapé et al., 2017) or by presenting pictures of positive tactile interactions (Peled-Avron et al., 2016; Adler and Gillmeister, 2019; Schirmer and McGlone, 2019). Together, this work confirmed that touch has affective value but offered limited insights into the underlying somatosensory processes and how they unfold in time.
Less than a handful of labs have directly recorded the EEG to affective touch (Singh et al., 2014; Ravaja et al., 2017; Haggarty et al., 2020). Of particular interest here is a study by Ackerley and colleagues examining the ERP elicited to soft brush strokes (Ackerley et al., 2013). This study revealed a positive deflection over a frontal midline channel (Fz) tracking both stimulus onset and offset with a ∼700 ms delay. Despite its frontal topography, the authors suggested an association with other LPPs and affect based on the deflection’s timing and polarity. Moreover, based on the conduction velocity of different somatosensory nerve fibers, they speculated about a possible role of unmyelinated and hence slow conducting C-tactile (CT) afferents, which were previously shown to support tactile pleasure (Löken et al., 2009). In line with this, a subsequent magnetoencephalography (MEG) study identified a signal modulation around 700 ms following CT-targeted touch with sources in the posterior insula (Hagberg et al., 2019), a known projection target for CT input (Olausson et al., 2002; Morrison, 2016).
Taken together, existing work highlights that touch, like stimuli from other sensory modalities, can be affectively relevant. Moreover, it implies a possible role of CT afferents in modulating an ERP component of the LPP family and raises the possibility that this component marks tactile affect as a function of both early bottom-up and later top-down processes. However, many questions remain and two are of particular interest here.
The present study
A first question concerns the link between the LPP and tactile affect. At present, this link is speculative as research has not yet probed an involvement of CTs or correlated this component with subjective pleasure. CTs are known to be present in hairy but absent or extremely rare, if they exist at all, in glabrous skin (Olausson et al., 2010; McGlone et al., 2012, 2014). Moreover, CT firing relates to velocity in an inverted u-shaped manner; it is maximal to stroking velocities between 1 and 10 cm/s and declines for faster and slower stroking (Löken et al., 2009). Like CTs, touch pleasantness ratings show an inverted u-shaped response as a function of stroking velocity and indeed are linearly predicted by CT activity (Essick et al., 1999, 2010). Thus, both CT-targeted manipulations and pleasantness ratings could be useful to further examine the LPP. Note, however, that an inverted u-shaped relation between stroking velocity and pleasantness also manifests for glabrous skin (Löken et al., 2011; Luong et al., 2017), highlighting that this relation is necessary but not sufficient to infer CT involvement.
A second question concerns potential early sensory or bottom-up effects. As detailed above, such effects have been reported for other modalities, yet whether they exist for touch is still unknown. Indeed, if tactile affect, as aroused by CT stimulation, strictly depends on C-fiber signaling, then one may not expect a modulation of somatosensory components prior to the LPP. This is because C-fiber information travels at a speed of 2 m/s or slower (Vallbo et al., 1993; Watkins et al., 2017) and targets the insula rather than the somatosensory cortex (Olausson et al., 2002). If, however, tactile affect also involves myelinated Aβ fibers, as has been suggested recently (McGlone et al., 2012; Abraira et al., 2017; Marshall et al., 2019; Cruciani et al., 2021), it could modulate early somatosensory processes.
The present study probed the above questions in two experiments. In both experiments, participants were stroked with a soft brush and rated touch pleasantness. We introduced two manipulations in an effort to dissociate CT signaling from that of myelinated somatosensory afferents. Within each experiment, we manipulated stroking velocity as CTs show an inverted u-shaped response in spike discharge at the receptor level, whereas myelinated afferents show monotonically increasing responses with faster stroking (Löken et al., 2009). Between experiments, we manipulated the stimulated skin area. In Experiment 1, stroking targeted the forearm, which is densely innervated by CTs, but less so with myelinated mechanoreceptors, whereas in Experiment 2, stroking targeted the palm, which is densely innervated by myelinated mechanoreceptors but none or very few CTs (Vallbo et al., 1993; Ackerley, 2022).
Based on extant research, we expected an LPP at Fz. Moreover, if this LPP marks tactile affect it should positively predict pleasantness ratings. If its modulation depends on CT input it should show an inverted u-shaped relation with stroking velocity and emerge for stroking to the arm but be smaller or absent for stroking to the palm. Lastly, we speculated that, prior to the LPP, tactile affect might modulate activity of somatosensory cortex contra-lateral to the stimulation site by enhancing responses to pleasant when compared with more neutral touch similar to what has been found in the visual and auditory modality (Schirmer et al., 2005; Stolarova et al., 2006).
Methods
The methods and hypotheses of Experiment 1 were pre-registered with the Open Science Framework (OSF), the details of which can be reviewed here: https://osf.io/fy6ta. This site also provides access to our data and analysis scripts. Given the exploratory nature of Experiment 1, we replicated key findings in another independent sample (Supplementary Materials) and, after successful replication, conducted a second experiment targeting glabrous, rather than hairy, skin. These latter efforts were not pre-registered as they immediately derived from the findings of Experiment 1. The study protocol was approved by the Clinical Research Ethics Committee of Hong Kong.
Participants
We recruited 37 participants for Experiment 1, seven of whom were excluded from statistical analysis because noise in the EEG signal resulted in less than 30 epochs in one or more conditions when epoching was done using a 4-s window. However, six participants had sufficient data when epoching was done using a 2-s window, so their data were subsequently included in the replication of Experiment 1 (Supplementary Materials), which required only 2-s-long epochs. The final sample of Experiment 1 comprised 15 men and 15 women with a mean age of 21 years (s.d. 2.98). For Experiment 2, we recruited and analyzed 30 participants (half female) with a mean age of 20.1 years (s.d. 2.09). All participants reported being right-handed.
Stimuli and apparatus
The tactile stimuli were delivered using a custom-built cable-driven robot capable of 3D motion (for a video demonstration please refer to our OSF archive). We opted for robotically controlled rather than human skin-to-skin touch as to maximize the accuracy and consistency of stroking and to facilitate comparison with prior research that also employed robotic touch (Löken et al., 2009; Essick et al., 2010; Ackerley et al., 2013, 2014; Luong et al., 2017; Croy et al., 2021; Sailer et al., 2020). The robot used here was operated via MATLAB and entailed eight motors that could move a touch stimulator in any direction with high spatial and temporal precision and accuracy. In keeping with previous research, the touch stimulus in this study was a soft cosmetic brush with a tip size of about half a centimeter. To enable temporal alignment between touch onset and the EEG, we weaved soft copper wires into the brush that connected with ESP32 Capacitive Touch Sensor pins. When the brush contacted skin, these pins sent a signal to the EEG data acquisition computer. The touch sensing pins also facilitated calibrating the touch device for a given participant. They enabled position read-outs for a planned stroking trajectory, allowing us to adjust this trajectory to the surface curvature of the target skin area and ensuring a consistent brush force.
The touch stimulus in this study was low force (∼0.3 N) stroking of the left forearm. In Experiment 1, the brush stroked the participant’s dorsal forearm, whereas in Experiment 2, the brush stroked her/his palm. Although most studies in the field have probed linear trajectories, recent evidence suggests oval trajectories are more pleasant (Shirato et al., 2018) and more representative of actual touch interactions (Lo et al., 2021). We, therefore, opted for an oval rather than a linear trajectory. The set points for this trajectory were a ∼15 cm circumference, a minor radius of ∼1 cm and a major radius of ∼3.22 cm. Small deviations from these set points were necessary due to variation in skin area curvature across participants. Strokes were delivered at five velocities including 0.5, 1, 3, 10 and 20 cm/s for a duration of 2.5 s. Because different velocities necessarily covered different distances across the skin, we adopted a number of control measures. Specifically, we adjusted the starting position of strokes such that motion along the oval was balanced across trials for a given velocity within participants. Thus, all velocities completed the full oval at least once across trials (for further details see Supplementary Materials).
Please note that velocity manipulations are inherently confounded, forcing experimenters to accept condition differences in either travel distance or stimulus duration. Here, we opted for the former because the latter creates issues for the interpretation of ERPs as both the onset and offset of a stimulus elicit an ERP (Luck, 2014). For the velocities tested here, the onset response would have always coincided. However, the offset response would have occurred within less than a second for the fastest and outside our analysis window for the slowest condition, making it impossible to compare their ERPs.
Procedure
After completing an informed consent procedure, the participant was seated and the experimenter prepared her/him for the EEG recording. The participant then placed her/his left forearm onto a comfortable arm rest under the touch stimulator. In Experiment 1, the arm was placed with a supine position, whereas in Experiment 2, it was placed with a prone position. As the prone position was slightly more effortful to maintain for the duration of the experiment, we attached a couple of soft elastic straps to assist the participant. A curtain precluded the participant from seeing the forearm and the touch device.
Next, the participant received instructions via a computer monitor placed in front of her/him. The participant was asked to insert noise-canceling earphones into the ears, which presented a soft white noise meant to block out any remaining noise from the movement of the touch device. Then, the experimenter operated the device and re-adjusted the white noise volume until the participant no longer heard the touch device. After ensuring the participants’ comfort, the experimenter started the experiment.
The experiment comprised 300 trials across which the five stimulus velocities were presented with equal probability in pseudo-random order such that the same velocity would not be presented consecutively. This resulted in 60 trials per condition—a number that falls within the range of published research. Indeed, published haptic research has been conducted with ∼25 trials per condition (Forschack et al., 2017), while the original work on affective touch recorded as many as 240 trials (Ackerley et al., 2013). Although in general, a larger trial number has been linked with a better signal-to-noise ratio, this link is nonlinear and tapers off with more trials (Luck, 2014). Hence, using more trials is not necessarily better because it must be weighed against other design features such as experiment length, stimulus habituation and participant fatigue. We, therefore, piloted this paradigm with only 60 trials per condition and found this to be sufficient to establish the expected touch effects.
A trial began with a fixation cross lasting for 0.4–0.55 s, coinciding with the downward motion of the touch stimulator. After the stimulator contacted the skin, it began moving along the oval trajectory for 2.5 s. During this time and the following one second, the fixation cross remained on the computer screen and was then replaced by a pleasantness rating scale. The participants now used their right arm to operate a mouse and to move a cursor to a position on a continuous scale that reflected the pleasantness associated with the touch. The scale endpoints were marked with very unpleasant on the left and with very pleasant on the right and scores coded within a range of −100 to 100. Following the participant’s response, there was a short inter-trial interval during which the screen remained blank. The interval lasted for 1, 1.5 or 2 s, drawn from a uniform distribution. The experiment was divided into four blocks of 75 trials. Participants had a short break after every 30 trials and a 5-min break between blocks. Trials lasted about 6.5 s and an experimental session lasted about 50 min. The procedures are summarized in Figure 1.
Fig. 1.
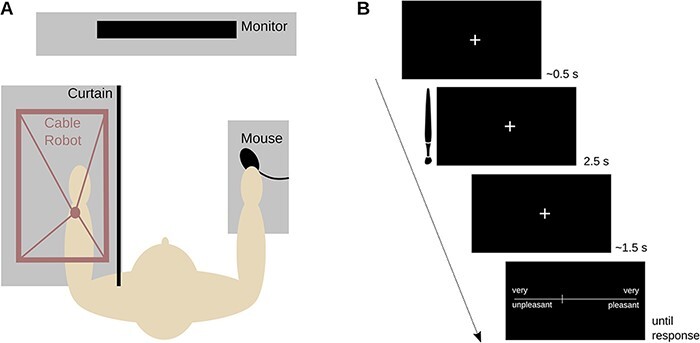
Study procedures. A) The experimental set-up. B) The events making up a touch trial.
Electrophysiological recording and analysis
The EEG was recorded using 64 Ag/AgCl electrodes, which were located according to the extended 10–20 system of the American Clinical Neurophysiology Society (Acharya et al., 2016). CPz was used as the online reference. Electrode impedance was below 20 kΩ. The data were recorded at 500 Hz with an ANT EEGo system. Only an anti-aliasing filter was applied during data acquisition (i.e. sinc filter with a half-power cut-off at half the sampling rate).
EEG data were pre-processed with EEGLAB v14.1.1 (Delorme and Makeig, 2004) implemented in MATLAB. The data were down-sampled to 250 Hz, low-pass filtered at 30 Hz (7.5 Hz transition bandwidth, −6 dB cut-off) and high-pass filtered at 0.1 Hz (0.1 Hz transition bandwidth, −6 dB cut-off). Then the data were re-referenced to the channel average and epoched with a window from −1 to 3 s around each stimulus onset (Experiment 1) or from −1 to 1 s around each stimulus onset (Experiments 1 and 2). Afterward, the data were subjected to manual inspection where channels and epochs with non-typical artifacts caused, for example, by muscle movements or drifting were interpolated or rejected, respectively. The cleaned data were then high-pass filtered at 1 Hz and subsequently entered in an adaptive mixture independent component analysis (Palmer et al., 2011). The resulting independent component structure was applied to the original data with the 0.1–30 Hz filter setting. Components reflecting typical artifacts (i.e. horizontal and vertical eye movements and eye blinks) were removed and the data were back-projected from component space into EEG channel space. The data were subjected to another round of visual inspection during which residual artifacts were removed. A current source density transformation was applied using the CSD Toolbox (Kayser and Tenke, 2015). This served to enhance spatial separation of temporally overlapping signal components and to facilitate the detection of independent cortical sources (Kamarajan et al., 2015).
For the ERP analysis, we then conducted a baseline correction using mean voltages within a window between −200 and 0 ms from stimulus onset. Subsequently, trial data were averaged within subjects and conditions. For the time–frequency analysis, we subjected epochs ranging from −1 to 1 s to a continuous wavelet transformation with cycles ranging from 3 to 7 for frequencies from 5 to 28 Hz in steps of 1 Hz. This returned 153 time points ranging from −663 and 667 ms around stimulus onset. The wavelet transforms were then baseline corrected using a window from −500 to −100 ms and their power was obtained and averaged for each participant, condition, time point and frequency. For statistical analysis, we divided Rolandic rhythms into α‐1 (8–9.9 Hz), α‐2 (10–11.9 Hz), β‐1 (12–17.9 Hz), β‐2 (18–20.9 Hz) and β‐3 (21–28 Hz) in line with earlier research (Ritter et al., 2009). This enabled us to detect potential frequency specific effects of touch on the power of somatosensory processes.
Ultimate trial numbers per condition averaged across participants ranged from 50 to 53 for the long epochs in Experiment 1 (participant-wise min = 31 and max = 68). They ranged from 55 to 57 for the short epochs in Experiment 1 (participant-wise min = 37 and max = 74) and from 54 to 57 for the short epochs in Experiment 2 (participant-wise min = 38 and max = 71).
Statistical analysis
In line with past research, we subjected our measures of interest to separate second-order polynomial regression analyses with the common logarithm of velocity as the independent variable (Löken et al., 2009). To facilitate the interpretation of linear and quadratic terms in the model, we normalized dependent and independent variables. Thus, beta values expressed change in terms of standard deviations. Moreover, the sign of the linear term (i.e. ±) could be interpreted as showing a positive or negative relationship, while the sign of the quadratic term could be interpreted as showing a convex (u-shaped) or concave (inverted u-shaped) relationship. Note that these modifications were strictly cosmetic and had no impact on the actual significance of linear and quadratic terms. To account for the repeated measures nature of the velocity variable, we added a random effects term to the regression that specified slopes and intercepts for the rating analysis and intercepts only for the ERP analysis. As ERPs are necessarily an averaged measure, they afforded no trial data to estimate slopes. F-statistics were obtained using the Satterthwaite approximation for degrees of freedom.
In addition to the analyses reported below, we also visually examined individual participants and conducted a bootstrap analysis to test the robustness of the reported effects. Moreover, we conducted a study aimed at replicating the results of Experiment 1 and report all this in the Supplementary Materials.
Results of Experiment 1—stroking of hairy skin
ERPs
In line with a previous report (Ackerley et al., 2013), visual inspection of the 4-s ERP epochs at Fz revealed a positive deflection starting around 700 ms after touch onset. This modulation lasted for about 300 ms after which there were no further visible effects (Figure 2). Because long ERP epochs are associated with lower trial numbers following artifact rejection, we re-analyzed our EEG data with shorter epochs as described in the methods. We then conducted our statistical analysis on Fz voltages in keeping with an earlier study (Ackerley et al., 2013).
Fig. 2.
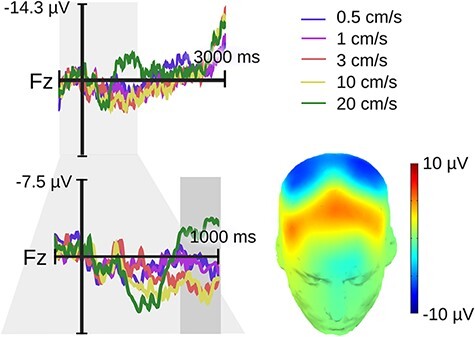
Experiment 1 (hairy skin) late positive velocity effect over a frontal midline region. The upper electrode shows the original epochs lasting up to 3 s following touch onset. The lower electrode shows the re-epoched data lasting up to 1 s following touch onset. The gray rectangle highlights our window for statistical analysis ranging from 0.7 to 1 s. No modulation was seen after this time window. The topography of the velocity effect (3 cm/s minus 20 cm/s) is printed on a 3D head.
Analysis of Fz mean voltages from 700 to 1000 ms returned a significant velocity effect (F[2,118] = 4.86, P = 0.009). The linear term was non-significant (P > 0.25), but the quadratic term pointed to the expected concave relationship (ß = −1.91, SE = 0.65, P = 0.004).
Apart from the Fz effect, we also observed an interesting negative deflection over somatosensory cortex contra-laterally to the stimulation, which we refer to as a somatosensory N400 (hereafter sN400). Like the Fz effect, the sN400 showed a velocity-dependent negative quadratic pattern but emerged significantly earlier between 300 and 500 ms following touch onset. For analysis, we computed mean voltages within this time window and across a subset of right centro-medial electrodes (Cz, C2 and CP2). Figure 3 illustrates the dipole of this effect and guided electrode selection (the negativity peaked centrally and reversed polarity laterally). A polynomial mixed effects model was again significant (F[2,118] = 14.11, P < 0.001). Its linear term was non-significant (P > 0.25), while the quadratic term indexed a convex relationship (ß = 3.37, SE = 0.65, P < 0.0001). Because the sN400 is a negative deflection, however, this convex effect highlights a concave relationship between velocity and component amplitude.
Fig. 3.
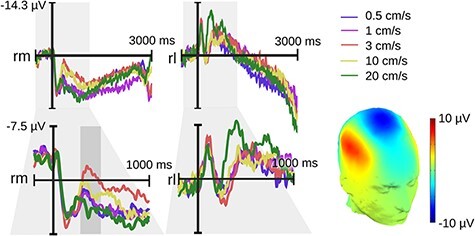
Experiment 1 (hairy skin) velocity sN400 effect over right medial electrodes (rm: Cz, C2, CP2; rl: C6, T8, CP6). The upper electrode shows the original epochs lasting up to 3 s following touch onset. The lower electrode shows the re-epoched data lasting up to 1 s following touch onset. The gray rectangle highlights our window for statistical analysis ranging from 0.3 to 0.5 s. No other modulation occurred subsequently. The topography of the velocity effect (3 cm/s minus 20 cm/s) is printed on a 3D head.
Rolandic rhythms over contra-lateral somatosensory cortex
Given the novelty of the observed sN400 effect, we examined the power of Rolandic rhythms (Figure 4) to determine whether activity in the somatosensory cortex increased with increasing velocity as one would expect for Aß input (Löken et al., 2009; Case et al., 2016). Like the ERP, mean power between 300 and 500 ms following touch onset in the alpha and beta bands at right centro-medial electrodes was subjected to a polynomial mixed effects model with velocity, frequency band and their interaction as the fixed effects. This revealed a significant effect of velocity (F[2,706] = 36.97, P < 0.001). A significant linear term indicated that power declined with increasing velocity (ß = −5.88, SE = 0.7, P < 0.0001). The quadratic term was only marginally significant (ß = −1.23, SE = 0.7, P = 0.078). The interaction of velocity and frequency band was non-significant (P = 0.832).
Fig. 4.
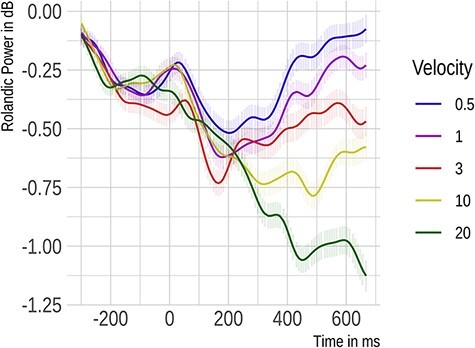
Rolandic rhythms recorded from electrodes Cz, C2 and CP2 located over the right somatosensory cortex in Experiment 1 (hairy skin). Here, we show the time course of power changes evoked by tactile stimuli averaged across alpha and beta bands.
Pleasantness ratings
Pleasantness ratings are illustrated in Figure 5. Their analysis revealed a significant velocity effect (F[2,29] = 3.47, P = 0.044). A significant linear term indicated that pleasantness decreased as velocity increased (ß = −19.26, SE = 9.04, P = 0.042), while a significant quadratic term corroborated the expected concave relationship (ß = −9.4, SE = 3.75, P = 0.018). We followed up these results with one-sample t-tests on original, non-normalized data corrected for multiple comparisons. This served to probe whether ratings in each of the five velocity conditions differed from 0. Results showed that ratings were positive for 0.5 (t(29) = 2.8, PFDR = 0.023), 1 (t(29) = 2.98, PFDR = 0.023) and 3 cm/s (t(29) = 2.03, PFDR = 0.086), whereas they failed to differ from 0 for 10 and 20 cm/s (PFDR values > 0.125).
Fig. 5.
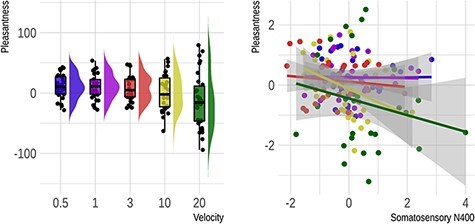
Pleasantness rating results from Experiment 1 (hairy skin). On the left is a rain cloud plot illustrating the change in subjective pleasantness as a function of stimulus velocity. This plot shows raw rating scores. On the right is a scatter plot with regression lines showing the significant relationship between subjective pleasantness and sN400 amplitude at the subject level. Here, values for both measures have been normalized to a mean of 0 and a standard deviation of 1.
Lastly, we examined which of the above-mentioned brain measures predict pleasantness ratings using a linear regression approach. Mean pleasantness served as the dependent variable, a given brain measure served as the fixed effect, velocity slopes and the participants’ intercepts served as random effects. Velocity was included simply to reduce type 1 error and was of no interest. For the Fz ERP, the result was non-significant (P = 0.26). However, we observed a significant relationship for the sN400 (F[1,123] = 5.96, P = 0.016), indicating that more negative ERPs were associated with greater pleasantness. The power of Rolandic rhythms over the somatosensory region was unrelated to pleasantness (P = 0.661).
Results of Experiment 2—stroking of glabrous skin
ERP. Again, we first explored a late frontal midline modulation between 700 and 1000 ms (Figure 6A). As for hairy skin, our statistical analysis returned a significant effect of velocity on the mean ERP amplitude (F[2,118] = 5.04, P = 0.008). While the linear term was non-significant (P > 0.25), the quadratic term revealed a concave relationship (ß = −2.1, SE = 0.66, P = 0.002). Mid-range velocities elicited a more positive ERP deflection than the slowest and fastest velocities.
Fig. 6.
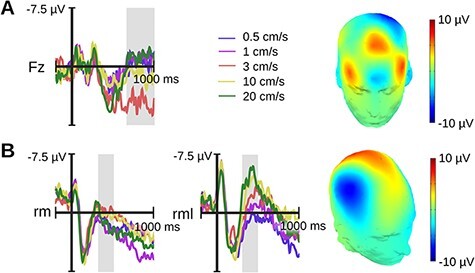
Velocity ERP effects for Experiment 2 (glabrous skin). A) Frontal velocity effect shown for Fz. B) sN400 velocity effect over the right medial region explored in Experiment 1 (rm; Cz, C2, CP2) and a slightly more lateral region adapted for the cortical representation of the palm (rml; C2, C4, CP4). Gray rectangles highlight windows for statistical analysis ranging from 0.7 to 1 s for the Fz effect and from 0.3 to 0.5 s for the sN400. The topography of each velocity effect (Fz/rm—3 cm/s minus 20 cm/s; rml—20 cm/s minus 0.5 cm/s) is printed on a 3D head.
Next, we examined the sN400 as identified in Experiment 1. The result was non-significant (P = 0.131). Because the hand of the somatosensory homunculus sits more laterally than the forearm, we also examined a more lateral region composed of C2, C4 and CP4. Here, a statistical analysis was significant (F[2,118] = 26.76, P < 0.001). A significant linear term indicated that the sN400 was larger for faster velocities (ß = −4.88, SE = 0.67, P < 0.0001; Figure 6B). The quadratic term was non-significant (P > 0.25).
As these results differed drastically from those obtained in Experiment 1, we performed an additional analysis comparing the two datasets. Specifically, we tested a model with velocity, experiment and their interaction as fixed effects. We found the interaction was significant (F[2,236] = 3.7, P = 0.026), corroborating differences between the stroking of hairy and glabrous skin. Inspection of linear and quadratic effects showed that the interaction was non-significant for the former (P = 0.808) and significant for the latter (ß = −1.71, SE = 0.63, P = 0.007). Thus, the significant quadratic term in Experiment 1 differed significantly from the non-significant quadratic term in Experiment 2.
Rolandic rhythms over contra-lateral somatosensory cortex
As for the ERP, we examined the power of Rolandic rhythms for the original somatosensory region identified in Experiment 1 and for the more lateral one described above. Over the original region, illustrated in Figure 7, the velocity main effect was significant (F[2,706] = 12.58, P < 0.001). A significant linear term indicated that with increasing velocity, Rolandic power declined (ß = −3.43, SE = 0.71, P < 0.0001). The quadratic term was non-significant (P = 0.142). The interaction of velocity and frequency band was non-significant (P = 0.938). These results replicated over the more lateral region (velocity: F[2,706] = 9.8, P < 0.001; linear term: ß = −2.93, SE = 0.7, P < 0.0001, quadratic term: P = 0.159; velocity by frequency band: P = 0.994).
Fig. 7.
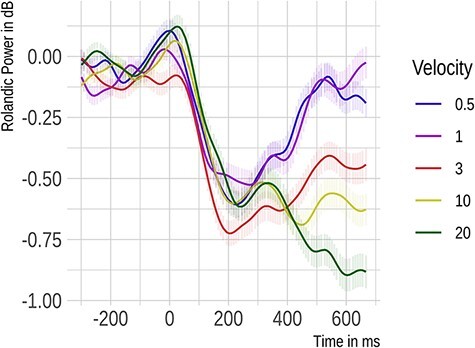
Rolandic rhythms recorded from electrodes Cz, C2 and CP2 located over the right somatosensory cortex in Experiment 2 (glabrous skin). Power changes evoked by tactile stimuli averaged across alpha and beta bands are presented relative to touch onset.
Pleasantness ratings
Analysis of rating data returned a main effect of velocity (F[2,29] = 9.94, P < 0.001). While the linear term identified a positive association between velocity and pleasantness (ß = 19.77, SE = 9.26, P = 0.041), the quadratic term implied that this relationship was also concave (ß = −12.47, SE = 4.86, P = 0.016). Again, we followed up these results with one-sample t-tests on original, non-normalized data. This showed that ratings were negative for 0.5 cm/s (t(29) = −2.42, PFDR = 0.05), failed to differ from 0 for 1 and 20 cm/s (PFDR values > 0.27) and were positive for 3 (t(29) = 2.28, PFDR = 0.05) and 10 cm/s (t(29) = 4, PFDR = 0.002).
Again, we probed whether the relationship between velocity and pleasantness differed between Experiments 1 and 2. Because a model run on trial data failed to converge, we computed the mean pleasantness for each condition and participant and subjected those means to a model with velocity, experiment and their interaction as fixed effects and the participant intercepts as the random effect. This returned a significant interaction of velocity and experiment (F[2,356] = 15.04, P < 0.001). Inspection of linear and quadratic effects showed that the interaction was significant for the former (ß = 2.85, SE = 0.53, P < 0.0001) and non-significant for the latter (P = 0.378). This implies that the association between stroking velocity and pleasantness was significantly smaller or more negative for hairy when compared with glabrous skin.
Next, we tested whether, as in Experiment 1, ERP and Rolandic rhythms could predict participants’ pleasantness ratings. There was no effect for the frontal midline ERP (P = 0.24). The sN400 over the original right medial region (F[1,73] = 2.89, P = 0.093; Figure 8) and the more lateral somatosensory region (F[1,89] = 2.39, P = 0.125) were non-significant. Likewise, Rolandic power over the original and the more lateral somatosensory region was unrelated to pleasantness (P values > 0.338).
Fig. 8.
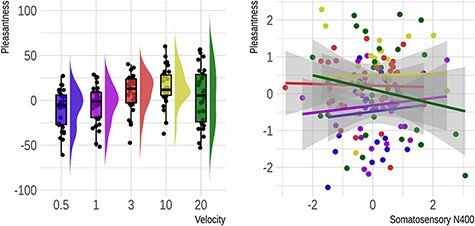
Pleasantness rating results from Experiment 2 (glabrous skin). On the left is a rain cloud plot illustrating the change in subjective pleasantness as a function of stimulus velocity. Original rating scores are shown. On the right is a scatter plot with regression lines showing the non-significant relationship between subjective pleasantness and the right medial sN400 amplitude (Cz, C2 and CP2) at the subject level. Here, values for both measures have been normalized to a mean of 0 and a standard deviation of 1.
Lastly, we probed whether the relationship between sN400 and pleasantness differed statistically between Experiments 1 and 2. For the original and the more lateral region, a model with mean ERPs, experiment and their interaction as fixed effects indicated that the interaction was non-significant (P values > 0.225).
Discussion
Here we sought to examine the neural signatures of tactile affect. Following up on earlier work, we pursued a frontal LPP, marking the late confluence of bottom-up and top-down processing, and probed whether its amplitude predicts pleasantness ratings and depends on the activity and/or presence of CTs. Additionally, we asked whether tactile affect shapes early sensory representations in a bottom-up manner in line with evidence from other modalities. In the following discussion, we first reflect on the pleasantness ratings recorded in this and previous studies and then consider the functional significance of both the frontal LPP and the novel somatosensory ERP response identified here.
Stroking velocity modulates tactile affect
It has been well-established that the pleasantness of soft brush strokes varies as a function of velocity. Across participants, intermediate velocities have been rated as more pleasant than faster or slower velocities (Essick et al., 1999; Löken et al., 2009; McGlone et al., 2012; Luong et al., 2017).
The present study replicated these results. The pleasantness of being stroked on hairy skin in both Experiment 1 and its replication (Supplementary Materials) was characterized by a significant quadratic term indexing the inverted u-shaped pattern reported previously. Experiment 1, albeit not its replication, also produced a significant linear term, indicating that faster speeds tended to be less pleasant than slower speeds. We reason that the complexity of rating scores and interindividual differences account for cross-experimental variation in rating results. As regards complexity, affective ratings depend on various types of information (Barrett, 2017). For example, at the sensory level they depend on the activity of a number of different skin receptors apart from CTs (McGlone et al., 2012; Luong et al., 2017); at a more conceptual level they depend on an individual’s past tactile experiences and current attitudes toward touch (Croy et al., 2021; Sorokowska et al., 2021). In line with this, research has highlighted that the prototypical inverted u-shaped pattern does not show consistently at an individual level. Indeed, there is substantial interindividual variation in the preference for slower/calming vs faster/stimulating touch (Croy et al., 2021) such that the overall results of a study may be subject to sampling biases. Importantly, however, the present stroking stimuli, especially those of intermediate velocities, were rated above 0 and thus as affectively positive.
As with the stroking of hairy skin, the stroking of glabrous skin can be pleasurable. Past work comparing the two failed to find differences (Guest et al., 2011; Löken et al., 2011; McGlone et al., 2012; Schirmer and Gunter, 2017), implying that both may be characterized by an inverted u-shaped relationship between velocity and subjective pleasantness (Guest et al., 2011; Luong et al., 2017). The present study appears to corroborate this. In line with previous work, it produced an inverted u-shaped response for hairy and glabrous touch. Additionally, however, there was a linear association of stroking velocity and pleasantness that differed between skin sites. Whereas it was negative or non-significant (see Supplementary Materials) for hairy touch, it was strongly positive for glabrous touch. As previous research typically focused on only quadratic effects, we do not know the extent to which the present results converge or diverge from existing data. However, they do highlight a potentially interesting parallel between the affective response to glabrous stimulation and the firing of Aβ receptors, which densely innervate glabrous skin.
Stroking velocity modulates a frontal LPP similarly for hairy and glabrous skin
In an early attempt to examine the neural correlates of affective touch, Ackerley and colleagues (2013) recorded the ERP to gentle, CT-targeted brush strokes. Specifically, strokes of 3 cm/s were occasionally interrupted by strokes of 6 cm/s and participants were asked to verbally report these oddballs. Strokes at 3 cm/s elicited a frontal positivity that emerged about 700 ms following stimulus onset and declined about 700 ms following stimulus offset. Based on the observed response delay, the authors speculated that CT signals underpin this effect. Here we sought to extend this research by adding both faster and slower velocities and by testing both hairy and glabrous skin. Thus, we hoped to effectively modulate tactile affect and clarify a dependence on CTs.
Converging with previous results, we observed a frontal component of positive polarity about 700 ms following touch onset that was larger for intermediate stroking (1–10 cm/s) when compared with slower and faster velocities. Moreover, this component showed across three different samples and hence seems fairly robust. Notably, however, aspects of the present positivity diverged from what was reported previously, including that it was smaller and declined before stimulus offset. We reason that methodological choices account for this. First, unlike the rotatory tactile stimulator used by Ackerley and colleagues, the tactile stimulator used here applied pressure evenly across the skin and enabled the dissociation of velocity from stimulus duration. Moreover, affect rather than velocity/stimulus duration were task relevant. Therefore, we speculate that past and present LPP results overlap in that both reflect a higher-order tactile effect, resulting from a combination of bottom-up and top-down processes. However, past results may additionally reflect a tracking of stimulus onsets and offsets and/or probabilities, which are known to modulate the ERP (Luck, 2014).
Importantly, the present study identified a clear relation between the frontal tactile LPP and stroking velocity. As one would expect if CTs were to underpin this effect, CT optimal velocities elicited larger amplitudes than CT sub-optimal velocities. Yet, this velocity effect showed independently of whether stroking occurred on hairy or glabrous skin and failed to predict pleasantness ratings. Thus, we speculate that, instead of being dependent on CTs, the frontal LPP reflects a more general, fiber-independent cortical representation of tactile input that at least partially dissociates from affect. This conclusion agrees with past research demonstrating an overlap in higher-order perceptual processes for hairy and glabrous touch (Luong et al., 2017; Pawling et al., 2017). It also converges with findings showing that apart from pleasure, other psychological properties prefer intermediate velocities (Sailer et al., 2020) including, for example, how human a touch feels (Wijaya et al., 2020).
Stroking velocity modulates the sN400 differently for hairy and glabrous skin
Based on evidence from the auditory and visual modalities, we speculated that tactile affect would modulate bottom-up sensory processes prior to the LPP. In line with this, stroking of hairy skin elicited a negative deflection peaking around 400 ms following stimulus onset over somatosensory cortex contra-lateral to the stimulation site. The amplitude of this sN400 component was larger for CT optimal when compared with slower and faster stroking velocities and significantly predicted pleasantness ratings. Notably, these results replicated in a separate sample marking them as robust. Indeed, a supplementary bootstrap analysis drawing from both datasets (N = 60) implied that, with only 10 participants, one has a 94% chance of observing an inverted u-shaped relation between sN400 amplitude and stroking velocity.
What might seem odd is that ERP and Rolandic rhythms dissociate in their response to the velocity of stroking hairy skin. The inverted u-shaped sN400 was accompanied by a negatively linear change in alpha and beta power as recorded from the same electrodes. This difference arises from the manner in which post-synaptic potentials contribute to event-related and oscillatory measures (Luck, 2014). Whereas the former amplify voltage changes time-locked to stimulus onset reducing or eliminating voltage changes that are temporally inconsistent, the latter (as examined here) are unaffected by variation phase delay. Thus, ERPs help identify temporally fixed processing steps, whereas oscillatory power reveals broader state changes that previous research has linked to the activity level of somatosensory cortex (Ritter et al., 2009; Singh et al., 2014). As Aß receptors fire more strongly with faster stroking velocities (Löken et al., 2009) and because these receptors project to somatosensory cortex, this cortex is generally more active for faster when compared with the slower stroking (Case et al., 2016). The present findings align with this.
Existing neuroimaging research is equivocal over the role of primary and secondary somatosensory cortex in tactile affect. On the one hand, there is evidence that pleasantness modulates other brain regions including the orbitofrontal cortex and posterior insula (Olausson et al., 2002; Rolls et al., 2003; Case et al., 2016). Indeed, inhibiting the somatosensory cortex with transcranial magnetic stimulation was found to alter perceived touch intensity but not pleasantness (Case et al., 2016). On the other hand, work contrasting more with less pleasant touch reported activity in primary somatosensory cortex (Shirato et al., 2018). Similarly, a recent meta-analysis implicated secondary somatosensory cortex as an area in which affective and discriminative tactile processes converge (Morrison, 2016).
The present data further corroborate an involvement of the somatosensory cortex in tactile affect. Additionally, however, they suggest that the manner in which the somatosensory cortex contributes to affective representations dissociates for input from hairy and glabrous skin. Unlike the sN400 elicited to stroking of hairy skin, the sN400 elicited to stroking of glabrous skin behaved antiparallel to Rolandic power. That is, it increased linearly with increasing velocity and, like Rolandic power, failed to significantly predict touch pleasantness. Thus, whereas hairy skin stimulation emphasized CT-like engagement of the somatosensory cortex, glabrous skin stimulation emphasized Aβ-like engagement of the somatosensory cortex. Possibly, the latter reflects involvement of discriminate processes associated with object manipulation and identification, which are thought to be central to the functional significance of glabrous but not hairy touch (McGlone et al., 2014).
Together, the present timing and skin type effects allow us to speculate about the mechanisms reflected by the sN400 and their dependence on both unmyelinated C and myelinated Aß fibers. While the sN400 occurred too early to be dependent strictly on C-fiber projections (<2 m/s; Vallbo et al., 1993; Watkins et al., 2017), its inverted u-shaped response to the stroking of hairy but not glabrous skin suggests that CTs might have been nevertheless relevant. Recent work in rodents suggests how. It revealed fiber-type interactions in the dorsal horn that are enabled by interneurons, which connect first-order C low-threshold mechanoreceptors onto second-order Aß projections, and that can support the fast delivery of original C-fiber input to the brain (70 m/s; Susuki, 2010).
If a similar mechanism exists in humans, it could account for the present sN400 effects. With an average distance of 57 cm (N = 10) between the skin stroking area and the spinal cord, CT input could have started to arrive there at about 285 ms following stimulus onset and would then have reached the brain within 6 ms coinciding with the onset of the sN400. This volley of CT signals might then have modulated ongoing somatosensory processes, informed by the earlier arrival of pure Aß information and amplified emerging brain representations as a function of CT-specific affective relevance. When stroking targeted the palm, Aß-driven somatosensory processes unfolded independently of CT-specific input and thus amplified faster over slower speeds. Such pure Aß processing may have also been affectively relevant as it paralleled a linear effect of velocity on pleasantness ratings. However, in the absence of a significant correlation between the ERP and rating data, a role for the glabrous sN400 in subjective tactile pleasure may be small and requires further research.
Open questions and future directions
Although the present study offers new and interesting insights into the neural mechanisms underpinning the affective processing of touch, it also raises a number of questions. Importantly, these questions converge onto key issues in the literature that are currently attracting debate.
One such issue concerns the question of how to define affective touch. From a traditional perspective, which we adopted here, affective touch depends on the stimulation of CTs whose firing characteristics seem uniquely suited to the enjoyment of physical contact with conspecifics (Olausson et al., 2002; McGlone et al., 2014). Yet, here and elsewhere (Löken et al., 2009; Guest et al., 2011; Luong et al., 2017; Schirmer and Gunter, 2017) stroking has been rated as positive irrespective of CT activation. In light of this, we reason that there may be different forms of tactile affect associated with hairy and glabrous touch, with the former being more passive and interoceptive and the latter being more active and exteroceptive (Björnsdotter et al., 2010; McGlone et al., 2012). The different velocity-dependent pleasantness ratings and ERP responses support this possibility and offer new avenues for contrasting hairy and glabrous touch.
A second question concerns the usefulness of pleasantness ratings in the study of affective touch. Because such ratings have dominated prior research, they were also implemented in the present experiments. Specifically, they served to link our results to existing microneurography and psychophysical data (e.g. Löken et al., 2009; Ackerley et al., 2014; Luong et al., 2017; Croy et al., 2021). However, the use of affective self-reports has been challenged repeatedly as being subject to biases related, for example, to memory or demand characteristics (Russell, 1994; Mauss and Robinson, 2009; Kassam and Mendes, 2013). Thus, pleasantness ratings, although frequently used, may afford limited insights into the affect of touch. In line with this, the present ERP measures differentiated hairy and glabrous touch more clearly than the rating results did. Moreover, although the sN400 predicted the participants’ reported pleasantness for hairy but not glabrous skin, both effects failed to statistically differ. Thus, we reason that the rating data were more complex and/or noisy, obscuring potentially important skin site effects. Future research could tackle this issue by considering alternative measures of affect such as recordings of facial muscle activity, heart rate or skin conductance.
Finally, an important obstacle to current research is its dependence on evidence from microneurography. This technique is available to only a few laboratories and requires both special expertise and significant time commitment. To date, there exist just a handful of published reports relying on small samples and thus limited statistical power (Vallbo et al., 1993, 1999; Löken et al., 2009; Ackerley et al., 2014). What is needed, hence, is a more accessible tool to examine peripheral nerve fiber responding and projection of these nerve fibers to the brain. The present sN400 response might be such a tool. Its clear dissociation between glabrous and hairy skin hints that it might be differently sensitive to first-order CT and Aß activation. Yet, more evidence is needed linking the sN400 to these somatosensory fibers. Apart from manipulating hairy and glabrous touch within participants, relevant future studies may combine EEG with microneurography, examine patient groups lacking myelinated or unmyelinated fibers (Olausson et al., 2002; Morrison et al., 2011) or attempt blocking C and Aß fiber transmission chemically (Kankel et al., 2012). Convergent evidence from these different approaches could establish the sN400 as a relatively cheap and convenient option for the study of affective touch and its dependence on different mechanosensory pathways.
Conclusions
Here we pursued the neural signatures of tactile affect as they unfold in time. We observed a late frontal ERP component that was more positive for intermediate—CT directed—when compared with faster and slower stroking velocities. Its independence from skin type and dissociation from affect imply a role in the higher-order conceptual processing of touch (e.g. humanness). We also identified a novel ERP response referred to as sN400 that dissociates for hairy and glabrous skin stimulation. For hairy skin only, the sN400 tracks velocity in an inverted u-shaped manner and shows a significantly linear relation with touch pleasantness. Together, these data present original evidence for a role of the somatosensory cortex in tactile affect that may arise from a convergence of CT with faster Aß signaling in the spinal cord and thus reflect the importance of CT stimulation for an individual’s well-being (Dagnino-Subiabre, 2022; Van Puyvelde and Mairesse, 2022; Wigley et al., 2022). Taken together, both the frontal LPP and the sN400 promise to be useful as we further investigate the different mental representations arising from tactile input to glabrous skin, with which we typically reach out to touch, and hairy skin, with which we typically receive touch.
Supplementary Material
Acknowledgements
The authors would like to thank Trevor Penney for helpful feedback and comments.
Contributor Information
Annett Schirmer, Department of Psychology, The Chinese University of Hong Kong, Shatin, Hong Kong SAR; The Brain and Mind Institute, The Chinese University of Hong Kong, Shatin, Hong Kong SAR.
Oscar Lai, Department of Psychology, The Chinese University of Hong Kong, Shatin, Hong Kong SAR.
Francis McGlone, School of Natural Sciences & Psychology, Liverpool John Moores University, Liverpool L3 3AF, UK; Institute of Psychology, Health & Society, University of Liverpool, Liverpool L3 3AF, UK.
Clare Cham, Department of Psychology, The Chinese University of Hong Kong, Shatin, Hong Kong SAR.
Darwin Lau, Department of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Shatin, Hong Kong SAR.
Funding
This research was supported by the Humanities and Social Sciences Prestigious Fellowship Scheme (34000219) awarded to A.S. by the Research Grants Council of Hong Kong.
Conflict of interest
The authors declare no conflicts of interest.
Supplementary data
Supplementary data is available at SCAN online.
References
- Abraira V.E., Kuehn E.D., Chirila A.M., et al. (2017). The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell, 168, 295–310.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya J.N., Hani A., Cheek J., Thirumala P., Tsuchida T.N. (2016). American clinical neurophysiology society guideline 2: guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 33, 308–11. [DOI] [PubMed] [Google Scholar]
- Ackerley R., Eriksson E., Wessberg J. (2013). Ultra-late EEG potential evoked by preferential activation of unmyelinated tactile afferents in human hairy skin. Neuroscience Letters, 535, 62–6. [DOI] [PubMed] [Google Scholar]
- Ackerley R., Wasling H.B., Liljencrantz J., Olausson H., Johnson R.D., Wessberg J. (2014). Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34, 2879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley R. (2022). C-tactile (CT) afferents: evidence of their function from microneurography studies in humans. Current Opinion in Behavioral Sciences, 43, 95–100. [Google Scholar]
- Adler J., Gillmeister H. (2019). Bodily self-relatedness in vicarious touch is reflected at early cortical processing stages. Psychophysiology, 56, e13465. [DOI] [PubMed] [Google Scholar]
- Barrett L.F. (2017). The theory of constructed emotion: an active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsdotter M., Morrison I., Olausson H. (2010). Feeling good: on the role of C fiber mediated touch in interoception. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale, 207, 149–55. [DOI] [PubMed] [Google Scholar]
- Brown S., van Steenbergen H., Band G., de Rover M., Nieuwenhuis S. (2012). Functional significance of the emotion-related late positive potential. Frontiers in Human Neuroscience, 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L.K., Laubacher C.M., Olausson H., Wang B., Spagnolo P.A., Bushnell M.C. (2016). Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 36, 5850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I., Bierling A., Sailer U., Ackerley R. (2021). Individual variability of pleasantness ratings to stroking touch over different velocities. Neuroscience, 464, 33–43. [DOI] [PubMed] [Google Scholar]
- Cruciani G., Zanini L., Russo V., Boccardi E., Spitoni G.F. (2021). Pleasantness ratings in response to affective touch across hairy and glabrous skin: a meta-analysis. Neuroscience and Biobehavioral Reviews, 131, 88–95. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A. (2022). Resilience to stress and social touch. Current Opinion in Behavioral Sciences, 43, 75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Essick G.K., James A., McGlone F.P. (1999). Psychophysical assessment of the affective components of non-painful touch. Neuroreport, 10, 2083–7. [DOI] [PubMed] [Google Scholar]
- Essick G.K., McGlone F., Dancer C., et al. (2010). Quantitative assessment of pleasant touch. Neuroscience and Biobehavioral Reviews, 34, 192–203. [DOI] [PubMed] [Google Scholar]
- Forschack N., Nierhaus T., Müller M.M., Villringer A. (2017). Alpha-band brain oscillations shape the processing of perceptible as well as imperceptible somatosensory stimuli during selective attention. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37, 6983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean D., Sander D., Scherer K.R. (2008). Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Consciousness and Cognition, 17, 484–95. [DOI] [PubMed] [Google Scholar]
- Guest S., Dessirier J.M., Mehrabyan A., et al. (2011). The development and validation of sensory and emotional scales of touch perception. Attention, Perception & Psychophysics, 73, 531–50. [DOI] [PubMed] [Google Scholar]
- Hagberg E., Ackerley R., Lundqvist D., Schneiderman J., Jousmäki V., Wessberg J. (2019). Spatio-temporal profile of brain activity during gentle touch investigated with magnetoencephalography. NeuroImage, 201, 116024. [DOI] [PubMed] [Google Scholar]
- Haggarty C.J., Malinowski P., McGlone F.P., Walker S.C. (2020). Autistic traits modulate cortical responses to affective but not discriminative touch. European Journal of Neuroscience, 51, 1844–55. [DOI] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35, 129–55. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Nieuwenhuis S. (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience, 6, 291–7. [DOI] [PubMed] [Google Scholar]
- Jiang X., Pell M.D. (2015). On how the brain decodes vocal cues about speaker confidence. Cortex, 66, 9–34. [DOI] [PubMed] [Google Scholar]
- Kamarajan C., Pandey A.K., Chorlian D.B., Porjesz B. (2015). The use of current source density as electrophysiological correlates in neuropsychiatric disorders: a review of human studies. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 97, 310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankel J., Obreja O., Kleggetveit I.P., et al. (2012). Differential effects of low dose lidocaine on C-fiber classes in humans. The Journal of Pain: Official Journal of the American Pain Society, 13, 1232–41. [DOI] [PubMed] [Google Scholar]
- Kassam K.S., Mendes W.B. (2013). The effects of measuring emotion: physiological reactions to emotional situations depend on whether someone is asking. PLoS One, 8, e64959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J., Tenke C.E. (2015). On the benefits of using surface Laplacian (Current Source Density) methodology in electrophysiology. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 97, 171–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C., Chu S.T., Penney T.B., Schirmer A. (2021). 3D hand-motion tracking and bottom-up classification sheds light on the physical properties of gentle stroking. Neuroscience, 464, 90–104. [DOI] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience, 12, 547–8. [DOI] [PubMed] [Google Scholar]
- Löken L.S., Evert M., Wessberg J. (2011). Pleasantness of touch in human glabrous and hairy skin: order effects on affective ratings. Brain Research, 1417, 9–15. [DOI] [PubMed] [Google Scholar]
- Luck S. (2014). An Introduction to the Event-Related Potential Technique, 2nd edn, London, England: MIT Press. [Google Scholar]
- Luong A., Bendas J., Etzi R., Olausson H., Croy I. (2017). The individual preferred velocity of stroking touch as a stable measurement. Physiology & Behavior, 177, 129–34. [DOI] [PubMed] [Google Scholar]
- Marshall A.G., Sharma M.L., Marley K., Olausson H., McGlone F.P. (2019). Spinal signalling of C-fiber mediated pleasant touch in humans. eLife, 8, e51642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss I.B., Robinson M.D. (2009). Measures of emotion: a review. Cognition & Emotion, 23, 209–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F., Olausson H., Boyle J.A., et al. (2012). Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. The European Journal of Neuroscience, 35, 1782–8. [DOI] [PubMed] [Google Scholar]
- McGlone F., Wessberg J., Olausson H. (2014). Discriminative and affective touch: sensing and feeling. Neuron, 82, 737–55. [DOI] [PubMed] [Google Scholar]
- Moran T.P., Jendrusina A.A., Moser J.S. (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. [DOI] [PubMed] [Google Scholar]
- Morrison I., Löken L.S., Minde J., et al. (2011). Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain, 134, 1116–26. [DOI] [PubMed] [Google Scholar]
- Morrison I. (2016). ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch. Human Brain Mapping, 37, 1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus T., Forschack N., Piper S.K., et al. (2015). Imperceptible somatosensory stimulation alters sensorimotor background rhythm and connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35, 5917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., et al. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5, 900–4. [DOI] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo Å.B. (2010). The neurophysiology of unmyelinated tactile afferents. Neuroscience and Biobehavioral Reviews, 34, 185–91. [DOI] [PubMed] [Google Scholar]
- Palmer J.A., Kreutz-Delgado K., Makeig S. (2011). AMICA: an adaptive mixture of independent component analyzers with shared components.
- Paulmann S., Kotz S.A. (2008). Early emotional prosody perception based on different speaker voices. Neuroreport, 19, 209–13. [DOI] [PubMed] [Google Scholar]
- Pawling R., Cannon P.R., McGlone F.P., Walker S.C., Tremblay F. (2017). C-tactile afferent stimulating touch carries a positive affective value. PLoS One, 12, e0173457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled-Avron L., Levy-Gigi E., Richter-Levin G., Korem N., Shamay-Tsoory S.G. (2016). The role of empathy in the neural responses to observed human social touch. Cognitive, Affective & Behavioral Neuroscience, 16, 802–13. [DOI] [PubMed] [Google Scholar]
- Perri R.L., Rossani F., Di Russo F. (2019). Neuroelectric evidences of top-down hypnotic modulation associated with somatosensory processing of sensory and limbic regions. NeuroImage, 202, 116104. [DOI] [PubMed] [Google Scholar]
- Polich J. (2007). Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaja N., Harjunen V., Ahmed I., Jacucci G., Spapé M.M. (2017). Feeling touched: emotional modulation of somatosensory potentials to interpersonal touch. Scientific Reports, 7, 40504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P., Moosmann M., Villringer A. (2009). Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping, 30, 1168–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., O’Doherty J., Kringelbach M.L., Francis S., Bowtell R., McGlone F. (2003). Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cerebral Cortex (New York, N.Y.: 1991), 13, 308–17. [DOI] [PubMed] [Google Scholar]
- Russell J.A. (1994). Is there universal recognition of emotion from facial expression? A review of the cross-cultural studies. Psychological Bulletin, 115, 102–41. [DOI] [PubMed] [Google Scholar]
- Sailer U., Hausmann M., Croy I. (2020). Pleasantness only? Experimental Psychology, 67, 224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A., Striano T., Friederici A.D. (2005). Sex differences in the preattentive processing of vocal emotional expressions. Neuroreport, 16, 635–9. [DOI] [PubMed] [Google Scholar]
- Schirmer A., Escoffier N. (2010). Emotional MMN: anxiety and heart rate correlate with the ERP signature for auditory change detection. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 121, 53–9. [DOI] [PubMed] [Google Scholar]
- Schirmer A., Gunter T.C. (2017). The right touch: stroking of CT-innervated skin promotes vocal emotion processing. Cognitive, Affective & Behavioral Neuroscience, 17, 1129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A., Kotz S.A. (2006). Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends in Cognitive Sciences, 10, 24–30. [DOI] [PubMed] [Google Scholar]
- Schirmer A., McGlone F. (2019). A touching sight: EEG/ERP correlates for the vicarious processing of affectionate touch. Cortex, 111, 1–15. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37, 257–61. [PubMed] [Google Scholar]
- Shirato M., Kikuchi Y., Machida A., Inoue T., Noriuchi M. (2018). Gentle touch opens the gate to the primary somatosensory cortex. Neuropsychiatry, 8, 1696–707. [Google Scholar]
- Singh H., Bauer M., Chowanski W., et al. (2014). The brain’s response to pleasant touch: an EEG investigation of tactile caressing. Frontiers in Human Neuroscience, 8, 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B. (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23, 2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokowska A., Saluja S., Sorokowski P., et al. (2021). Affective interpersonal touch in close relationships: a cross-cultural perspective. Personality & Social Psychology Bulletin, 47, 1705–21. [DOI] [PubMed] [Google Scholar]
- Spapé M.M., Harjunen V., Ravaja N. (2017). Effects of touch on emotional face processing: a study of event-related potentials, facial EMG and cardiac activity. Biological Psychology, 124, 1–10. [DOI] [PubMed] [Google Scholar]
- Stolarova M., Keil A., Moratti S. (2006). Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cerebral Cortex (New York, N.Y.: 1991), 16, 876–87. [DOI] [PubMed] [Google Scholar]
- Susuki K. (2010). Myelin: a specialized membrane for cell communication. Nature Education, 3, 59. [Google Scholar]
- Vallbo Å.B., Olausson H., Wessberg J., Norrsell U. (1993). A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Research, 628, 301–4. [DOI] [PubMed] [Google Scholar]
- Vallbo Å.B., Olausson H., Wessberg J. (1999). Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. Journal of Neurophysiology, 81, 2753–63. [DOI] [PubMed] [Google Scholar]
- Van Puyvelde M., Mairesse O. (2022). Do C-tactile afferents go to sleep? A potential role for somatosensory stimulation in sleep regulation. Current Opinion in Behavioral Sciences, 43, 62–8. [Google Scholar]
- Watkins R.H., Wessberg J., Backlund Wasling H., et al. (2017). Optimal delineation of single C-tactile and C-nociceptive afferents in humans by latency slowing. Journal of Neurophysiology, 117, 1608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Hajcak G. (2011). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience, 23, 2994–3007. [DOI] [PubMed] [Google Scholar]
- Wigley I.L.C.M., Mascheroni E., Bonichini S., Rosario M. (2022). Epigenetic protection: maternal touch and DNA-methylation in early life. Current Opinion in Behavioral Sciences, 111–7. [Google Scholar]
- Wijaya M., Lau D., Horrocks S., McGlone F., Ling H., Schirmer A. (2020). The human “feel” of touch contributes to its perceived pleasantness. Journal of Experimental Psychology. Human Perception and Performance, 46, 155–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


