Abstract
STUDY QUESTION
What is the association between menopausal hormone therapy (MHT) and cause-specific mortality?
SUMMARY ANSWER
Self-reported MHT use following early natural menopause, surgical menopause or premenopausal hysterectomy is associated with a lower risk of breast cancer mortality and is not consistently associated with the risk of mortality from cardiovascular disease or other causes.
WHAT IS KNOWN ALREADY
Evidence from the Women’s Health Initiative randomized controlled trials showed that the use of estrogen alone is not associated with the risk of cardiovascular mortality and is associated with a lower risk of breast cancer mortality, but evidence from the Million Women Study showed that use of estrogen alone is associated with a higher risk of breast cancer mortality.
STUDY DESIGN, SIZE, DURATION
Cohort study (the UK Biobank), 178 379 women, recruited in 2006–2010.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Postmenopausal women who had reported age at menopause (natural or surgical) or hysterectomy, and information on MHT and cause-specific mortality. Age at natural menopause, age at surgical menopause, age at hysterectomy and MHT were exposures of interest. Natural menopause was defined as spontaneous cessation of menstruation for 12 months with no previous hysterectomy or oophorectomy. Surgical menopause was defined as the removal of both ovaries prior to natural menopause. Hysterectomy was defined as removal of the uterus before natural menopause without bilateral oophorectomy. The study outcome was cause-specific mortality.
MAIN RESULTS AND THE ROLE OF CHANCE
Among the 178 379 women included, 136 790 had natural menopause, 17 569 had surgical menopause and 24 020 had hysterectomy alone. Compared with women with natural menopause at the age of 50–52 years, women with natural menopause before 40 years (hazard ratio (HR): 2.38, 95% CI: 1.64, 3.45) or hysterectomy before 40 years (HR: 1.60, 95% CI: 1.23, 2.07) had a higher risk of cardiovascular mortality but not cancer mortality. MHT use was associated with a lower risk of breast cancer mortality following surgical menopause before 45 years (HR: 0.17, 95% CI: 0.08, 0.36), at 45–49 years (HR: 0.15, 95% CI: 0.07, 0.35) or at ≥50 years (HR: 0.28, 95% CI: 0.13, 0.63), and the association between MHT use and the risk of breast cancer mortality did not differ by MHT use duration (<6 or 6–20 years). MHT use was also associated with a lower risk of breast cancer mortality following natural menopause before 45 years (HR: 0.59, 95% CI: 0.36, 0.95) or hysterectomy before 45 years (HR: 0.49, 95% CI: 0.32, 0.74).
LIMITATIONS, REASONS FOR CAUTION
Self-reported data on age at natural menopause, age at surgical menopause, age at hysterectomy and MHT.
WIDER IMPLICATIONS OF THE FINDINGS
The current international guidelines recommend women with early menopause to use MHT until the average age at menopause. Our findings support this recommendation.
STUDY FUNDING/COMPETING INTEREST(S)
This project is funded by the Australian National Health and Medical Research Council (NHMRC) (grant numbers APP1027196 and APP1153420). G.D.M. is supported by NHMRC Principal Research Fellowship (APP1121844), and M.H. is supported by an NHMRC Investigator Grant (APP1193838). There are no competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: natural menopause, surgical menopause, hysterectomy, menopausal hormone therapy, mortality
Introduction
Menopause, the cessation of menstruation, marks the end of women’s reproductive life (Nelson, 2008). Age at menopause may be a harbinger of long-term health problems and early menopause (before the age of 45 years) has been consistently associated with increased risks of morbidity and mortality (Kingsberg et al., 2020). For women with early menopause, the International Menopause Society guideline on women’s midlife health and menopausal hormone therapy (MHT) recommends MHT until the average age at menopause (approximately age 50) (Baber et al., 2016). However, the health benefits of this are poorly defined (Zhu et al., 2020). Specifically, it is unknown whether taking MHT reduces the elevated mortality risk associated with early menopause.
Surgical menopause, defined as bilateral oophorectomy performed prior to natural menopause, removes the risk of ovarian cancer but is associated with higher risks of adverse long-term health outcomes (e.g. cardiovascular disease (Zhu et al., 2020)), compared with natural menopause. Changes in circulating sex steroids are more rapid following surgical menopause compared to natural menopause. Hence, it is essential to separately understand the effect of MHT use on mortality following natural menopause compared with surgical menopause (Kaunitz et al., 2021).
Hysterectomy is the removal of the uterus. Globally, hysterectomy is one of the most common surgical procedures in women (Whiteman et al., 2008; Wilson et al., 2017). Although normal ovaries are generally conserved at the time of hysterectomy in premenopausal women, women who undergo this procedure are at an increased risk of ovarian failure compared with women with intact uteri (Moorman et al., 2011). Hysterectomy may reduce the age at menopause by around four years compared to women who retain their uterus (Farquhar et al., 2005). Hence, we wished to examine the effect of MHT on mortality in three groups of women: natural menopause, surgical menopause (bilateral oophorectomy) or premenopausal hysterectomy (hysterectomy without bilateral oophorectomy).
This study used individual participant data from the UK Biobank, mainly aiming to examine if MHT is associated with the risk of cause-specific mortality following natural menopause, surgical menopause or premenopausal hysterectomy at different ages. This information will inform clinical practice including decision making about hysterectomy and oophorectomy and recommendations about use of MHT for the prevention of chronic disease.
Materials and methods
Exposures
The present study analyzed data from postmenopausal women in the UK Biobank (Sudlow et al., 2015). Prior to examining the association between MHT use and cause-specific mortality in women with menopause or premenopausal hysterectomy at different ages, we first assessed the associations of age at menopause and age at hysterectomy with cause-specific mortality. Hence, the exposure variables were natural menopause, surgical menopause, hysterectomy, age at menopause, age at hysterectomy and MHT use. Natural menopause was defined as spontaneous cessation of menstruation for 12 months with no previous hysterectomy or oophorectomy. Surgical menopause was defined as removal of both ovaries prior to natural menopause. Hysterectomy was defined as removal of the uterus before natural menopause without bilateral oophorectomy. In the UK Biobank, women were asked ‘Have you had both ovaries removed?’ and ‘Have you had a hysterectomy (womb removed)?’. Participants were not asked about unilateral oophorectomy. Hence, the ‘hysterectomy’ group included hysterectomy alone and hysterectomy plus unilateral oophorectomy, as we could not distinguish between these groups. Those with hysterectomy or bilateral oophorectomy after natural menopause were included in the ‘natural menopause’ group.
For women with natural menopause, age at natural menopause was used as their age at menopause. For women with surgical menopause, age at bilateral oophorectomy (prior to natural menopause) was used as their age at menopause. For women in the hysterectomy group, age at hysterectomy was used. Age at menopause and age at hysterectomy were categorized as <40, 40–44, 45–49, 50–52 and ≥53 years. MHT use was self-reported. There were two MHT-related variables analyzed in our study: status of MHT use (yes or no) and duration of MHT use. The duration of MHT use was calculated as age at last MHT use minus age at first MHT use and categorized as <6 or 6–20 years.
Outcomes
Age at death (from specific causes) or end of the study period, 31 December 2019, was the outcome variable. In examining the associations of age at menopause and age at hysterectomy with cause-specific mortality, we selected deaths from cardiovascular diseases (coronary heart disease or stroke), selected cancers (breast, cervical, ovarian, endometrial, lung and colorectal cancers) and all other causes using the International Classification of Disease version 10 (ICD-10). The specific codes used were: coronary heart disease (ICD 10: I20–I25), stroke (ICD-10: I60–I69), breast cancer (ICD-10: C50), cervical cancer (ICD-10: C53), ovarian cancer (ICD-10: C56), endometrial cancer (ICD-10: C541), lung cancer (ICD-10: C33, C34) and colorectal cancer (ICD-10: C18, C19, C20). Deaths from breast, cervical, ovarian, endometrial, lung or colorectal cancer are called as ‘cancer mortality’ unless otherwise stated. As the association between MHT use and cancer mortality may vary across different types of cancer (Zhang et al., 2021), in assessing the association between MHT use and cause-specific mortality, specific causes were considered separately (coronary heart disease or stroke, breast cancer, lung cancer and colorectal cancer and all other causes).
Covariates
The following factors were included in the models as covariates: baseline information on race/ethnicity, year of birth, BMI, smoking status, years of education, age at menarche, parity, family history of cardiovascular disease, family history of breast cancer, family history of lung cancer and family history of colorectal cancer (Zhu et al., 2020). Race/ethnicity was categorized into four groups: Caucasian, Asian, Black or others. Year of birth was categorized into <1940, 1940–1949, 1950–1959 and ≥1960. BMI was categorized into <18.5, 18.5–24.9, 25–29.9 and ≥30 kg/m2 according to the World Health Organization criteria. Smoking status was categorized into three groups: never smokers, former smokers and current smokers. Years of education were categorized as ≤10, 11–12 and >12 years. Age at menarche was categorized as ≤11, 12, 13, 14 and ≥15 years. Parity was categorized as 0, 1, 2 and ≥3 live births.
Family histories of cardiovascular disease, breast cancer, lung cancer and colorectal cancer were all binary variables (yes or no). Women with a family history of breast cancer, lung cancer or colorectal cancer were considered having a ‘family history of cancer’. In the analyses examining the associations of age at menopause and age at hysterectomy with cancer mortality, the binary variable ‘family history of cancer’ was included as a covariate. In the analyses assessing the association between MHT use and cause-specific mortality, family history of each cancer was included as a covariate only in the model for mortality from that cancer.
Exclusion criteria
Exclusion criteria included: women who had missing information on hysterectomy status or bilateral oophorectomy status (n = 4647); women who had reported hysterectomy or oophorectomy before the age of 30 years (n = 1440); women who reported hysterectomy or oophorectomy after the age of 55 years but did not report age at menopause (n = 5429); and women with pre- or peri- or unknown menopausal status (n = 80 036). We also excluded women with coronary heart disease, stroke, breast cancer, cervical cancer, ovarian cancer, endometrial cancer, lung cancer or colorectal cancer prior to menopause or hysterectomy (n = 2330). A total of 178 379 women were included in the final analysis.
Ethical approval
The UK Biobank study was approved by the National Health Service’s National Research Ethics Service. No ethics approval was acquired for the analysis using publicly available data.
Statistical analysis
We used Cox proportional hazards models to examine the associations of age at menopause, age at hysterectomy and MHT use with time, in years, from birth to death or the end of follow-up. The proportional hazards assumption was evaluated by visual inspection of the Schoenfeld residual plots, which did not suggest violation of the assumption. We treated the time between birth and natural or surgical menopause or hysterectomy as an unexposed period and natural or surgical menopause or hysterectomy as a time-dependent variable to avoid immortal time bias. There were two steps in the data analysis: Step I, first examined the associations of age at menopause and age at hysterectomy with cause-specific mortality; Step II, then examined the association between MHT use and cause-specific mortality in women with natural menopause, surgical menopause or hysterectomy at different ages.
Step I: to examine the associations of age at menopause and age at hysterectomy with time to cause-specific mortality, we created a 15-category variable: natural menopause, surgical menopause or hysterectomy at <40, 40–44, 45–49, 50–52 or ≥53 years. Two models were fitted with step-by-step adjustment of covariates: (i) Model I only included age at menopause or hysterectomy (with natural menopause at 50–52 years as the reference group) and (ii) Model II included adjustments for race/ethnicity, year of birth, BMI, smoking, years of education, age at menarche, parity, family history of cardiovascular disease (for the model for cardiovascular mortality) and family history of cancer (for the model for cancer mortality). Results from Model II were presented as the main results in this article. Further analyses were conducted to assess if mortality risk decreased linearly with the increase of age at menopause or hysterectomy (Cheng et al., 2018).
Step II: to investigate if MHT use was associated with the risk of cause-specific mortality following natural menopause, surgical menopause or hysterectomy, we fitted separate models for natural menopause, surgical menopause and hysterectomy. With MHT non-users as the reference group, we modeled the association between MHT use and cause-specific mortality stratified by age at menopause or hysterectomy. In the step II analyses, we included the covariates: race/ethnicity, year of birth, BMI, smoking, years of education, age at menarche, parity, family history of cardiovascular disease (for the model for cardiovascular mortality), family history of breast cancer (for the model for breast cancer mortality), family history of lung cancer (for the model for lung cancer mortality) and family history of colorectal cancer (for the model for colorectal cancer mortality). As the current guidelines recommend that women with early menopause use MHT until about age 50, we re-grouped age at menopause or hysterectomy into three groups: <45, 45–49 and ≥50 years. As Step II analyses suggested that MHT use was associated with a lower risk of breast cancer mortality, we further examined if the association between MHT use and the risk of breast cancer mortality differed by the duration of MHT use.
Sensitivity analysis
Two sensitivity analyses were conducted. First, to assess if there was bias from differential competing risks of death, we fitted Fine and Gray competing risk models (Fine and Gray, 1999) to analyze the associations of age at menopause or hysterectomy with cause-specific mortality. Second, there were 8883 women with missing information on at least one covariate. We performed multiple imputation (with 10 imputations) for missing covariates to test if the associations of age at menopause or hysterectomy with cause-specific mortality were robust to missing data.
The descriptive analyses, the Cox survival analyses, the Fine and Gray competing risk analyses and the multiple imputations were performed using SAS (version 9.4, SAS Institute Inc, Cary, NC, USA). The analyses to assess if mortality risk decreased linearly with the increase of age at menopause were conducted in R (version 3.5.3) and the figures were plotted using the ‘ggplot2’ and ‘ggthemes’ packages in R.
Results
Population characteristics
The mean age at baseline of the 178 379 women included was 60.0 years, and the average follow-up time was 10.3 years. Among these women, 136 790 (76.69%) had natural menopause, 17 569 (9.85%) had surgical menopause and 24 020 (13.47%) had premenopausal hysterectomy. There were 1125 cardiovascular deaths (deaths from coronary heart disease or stroke), 2915 deaths from cancer and 4573 deaths from all other causes. Specifically, there were 1085 deaths from breast cancer, 1185 deaths from lung cancer and 547 deaths from colorectal cancer. Information on year of birth, race/ethnicity, BMI, smoking status, years of education, age at menarche, parity, family history of cardiovascular disease, family history of breast cancer, family history of lung cancer and family history of colorectal cancer is presented in Table I. The three groups of women (natural menopause, surgical menopause, hysterectomy) differed in all baseline characteristics except for family history of cardiovascular disease. For instance, women with surgical menopause or hysterectomy were more likely to be obese and have ≤10 years of education, compared with women with natural menopause. MHT users and MHT non-users also differed in all baseline characteristics (Table II). Compared with MHT non-users, MHT users were more likely to be overweight or obese, be past or present smokers, have ≤10 years of education, have menarche at ≤11 years and have a family history of cardiovascular disease or lung cancer or colorectal cancer. However, MHT users were less likely to have a family history of breast cancer than MHT non-users.
Table I.
Baseline characteristics of women with natural or surgical menopause or hysterectomy.
| Natural menopause | Surgical menopause | Hysterectomy | Chi-squared value† | |
|---|---|---|---|---|
| (n = 136 790) | (n = 17 569) | (n = 24 020) | ||
| Year of birth | ||||
| <1940 | 5677 (4.2%) | 646 (3.7%) | 1214 (5.1%) | χ2 = 2717.7, df = 6 |
| 1940–1949 | 76 524 (55.9%) | 9424 (53.6%) | 13 413 (55.8%) | |
| 1950–1959 | 50 661 (37.0%) | 6136 (34.9%) | 7259 (30.2%) | |
| ≥1960 | 3928 (2.9%) | 1363 (7.8%) | 2134 (8.9%) | |
| Race/ethnicity | ||||
| Caucasian | 131 423 (96.1%) | 16 768 (95.4%) | 22 918 (95.4%) | χ2 = 221.1, df = 6 |
| Asian | 2632 (1.9%) | 307 (1.8%) | 351 (1.5%) | |
| Black | 1758 (1.3%) | 350 (2.0%) | 570 (2.4%) | |
| Others | 977 (0.7%) | 144 (0.8%) | 181 (0.8%) | |
| BMI (kg/m2) | ||||
| Underweight, <18.5 | 1084 (0.8%) | 76 (0.4%) | 118 (0.5%) | χ2 = 1435.2, df = 6 |
| Normal, 18.5–24.9 | 53 174 (39.0%) | 5394 (30.8%) | 7206 (30.1%) | |
| Overweight, 25.0–29.9 | 51 605 (37.9%) | 6782 (38.8%) | 9540 (40.0%) | |
| Obese, ≥30 | 30 349 (22.3%) | 5239 (30.0%) | 7057 (29.5%) | |
| Smoking status | ||||
| Never | 79 577 (58.4%) | 10 042 (57.4%) | 13 822 (57.8%) | χ2 = 48.6, df = 4 |
| Past | 45 675 (33.5%) | 5841 (33.4%) | 7894 (33.0%) | |
| Present | 11 076 (8.1%) | 1616 (9.2%) | 2193 (9.2%) | |
| Years of education | ||||
| ≤10 years | 69 460 (51.0%) | 10 182 (58.2%) | 14 543 (60.8%) | χ2 = 1098.8, df = 4 |
| 11–12 years | 16 103 (11.8%) | 1968 (11.3%) | 2692 (11.3%) | |
| >12 years | 50 724 (37.2%) | 5343 (30.5%) | 6686 (28.0%) | |
| Age at menarche | ||||
| ≤11 | 26 349 (19.7%) | 4151 (24.2%) | 5705 (24.3%) | χ2 = 437.8, df = 8 |
| 12 | 25 491 (19.1%) | 3285 (19.1%) | 4402 (18.7%) | |
| 13 | 32 809 (24.5%) | 3830 (22.3%) | 4248 (22.4%) | |
| 14 | 26 895 (20.1%) | 3101 (18.1%) | 4270 (18.2%) | |
| ≥15 | 22 276 (16.7%) | 2801 (16.3%) | 3859 (16.4%) | |
| Parity | ||||
| 0 | 23 534 (17.2%) | 3159 (18.0%) | 2652 (11.1%) | χ2 = 901.2, df = 6 |
| 1 | 17 522 (12.8%) | 2333 (13.3%) | 2625 (10.9%) | |
| 2 | 61 912 (45.3%) | 7799 (44.4%) | 11 323 (47.2%) | |
| ≥3 | 33 733 (24.7%) | 4266 (24.3%) | 7407 (30.9%) | |
| Family history of CVD # | ||||
| Yes | 82 297 (61.2%) | 10 663 (61.9%) | 14 512 (61.7%) | χ2 = 4.8, df = 2 |
| No | 52 201 (38.8%) | 6560 (38.1%) | 9017 (38.3%) | |
| Family history of breast cancer | ||||
| Yes | 10 428 (7.8%) | 1487 (8.7%) | 1889 (8.1%) | χ2 = 18.0, df = 2 |
| No | 122 851 (92.2%) | 15 546 (91.3%) | 21 378 (91.9%) | |
| Family history of lung cancer | ||||
| Yes | 162 555 (1.3%) | 2332 (13.5%) | 3207 (13.6%) | χ2 = 46.8, df = 2 |
| No | 117 943 (87.7%) | 14 891 (86.5%) | 20 322 (86.4%) | |
| Family history of colorectal cancer | ||||
| Yes | 13 433 (10.0%) | 1894 (11.0%) | 2457 (10.4%) | χ2 = 19.6, df = 2 |
| No | 121 065 (90.0%) | 15 329 (89.0%) | 21 072 (89.6%) |
All P-values were <0.001 except for family history of CVD.
Cardiovascular diseases, including coronary heart disease and stroke.
Table II.
Baseline characteristics of women in menopausal hormone therapy (MHT) users and MHT non-users.
| MHT users | MHT non-users | Chi-squared value† | |
|---|---|---|---|
| (n = 86 980) | (n = 91 024) | ||
| Year of birth | |||
| <1940 | 3845 (4.4%) | 3669 (4.0%) | χ2 = 6892.8, df = 3 |
| 1940–1949 | 56 788 (65.3%) | 42 389 (46.6%) | |
| 1950–1959 | 23 820 (27.4%) | 40 099 (44.1%) | |
| ≥1960 | 2527 (2.9%) | 4867 (5.4%) | |
| Race/ethnicity | |||
| Caucasian | 84 715 (97.4%) | 86 095 (94.6%) | χ2 = 962.9, df = 3 |
| Asian | 987 (1.1%) | 2263 (2.5%) | |
| Black | 751 (0.9%) | 1900 (2.1%) | |
| Others | 527 (0.6%) | 766 (0.8%) | |
| BMI (kg/m2) | |||
| Underweight, <18.5 | 495 (0.6%) | 781 (0.9%) | χ2 = 219.0, df = 3 |
| Normal, 18.5–24.9 | 30 942 (35.7%) | 34 718 (38.3%) | |
| Overweight, 25.0–29.9 | 34 346 (39.6%) | 33 456 (36.9%) | |
| Obese, ≥30 | 20 853 (24.1%) | 21 663 (23.9%) | |
| Smoking status | |||
| Never | 46 620 (53.8%) | 56 583 (62.4%) | χ2 = 1342.7, df = 2 |
| Past | 32 176 (37.1%) | 27 131 (29.9%) | |
| Present | 7838 (9.1%) | 7015 (7.7%) | |
| Years of education | |||
| ≤10 years | 48 603 (56.1%) | 45 368 (50.0%) | χ2 = 653.5, df = 2 |
| 11–12 years | 9475 (10.9%) | 11 252 (12.4%) | |
| >12 years | 28 573 (33.0%) | 34 062 (37.6%) | |
| Age at menarche | |||
| ≤11 | 18 492 (21.7%) | 17 640 (19.9%) | χ2 = 115.8, df = 4 |
| 12 | 15 982 (18.7%) | 17 127 (19.3%) | |
| 13 | 19 876 (23.3%) | 21 948 (24.7%) | |
| 14 | 16 662 (19.5%) | 17 537 (19.7%) | |
| ≥15 | 14 280 (16.7%) | 14 585 (16.4%) | |
| Parity | |||
| 0 | 13 102 (15.1%) | 16 187 (17.8%) | χ2 = 241.8, df = 3 |
| 1 | 11 100 (12.8%) | 11 333 (12.5%) | |
| 2 | 40 324 (46.4%) | 40 565 (44.6%) | |
| ≥3 | 22 405 (25.8%) | 22 877 (25.2%) | |
| Family history of CVD # | |||
| Yes | 53 802 (63.0%) | 53 454 (59.7%) | χ2 = 201.7, df = 1 |
| No | 31 569 (37.0%) | 36 065 (40.3%) | |
| Family history of breast cancer | |||
| Yes | 6050 (7.2%) | 7731 (8.7%) | χ2 = 144.2, df = 1 |
| No | 78 498 (92.8%) | 80 953 (91.3%) | |
| Family history of lung cancer | |||
| Yes | 11 287 (13.2%) | 10 762 (12.0%) | χ2 = 57.0, df = 1 |
| No | 74 084 (86.8%) | 78 757 (88.0%) | |
| Family history of colorectal cancer | |||
| Yes | 9002 (10.5%) | 8757 (9.8%) | χ2 = 27.8, df = 1 |
| No | 76 369 (89.5%) | 80 762 (90.2%) |
All P-values were <0.001.
Cardiovascular diseases, including coronary heart disease and stroke.
Age at menopause, age at hysterectomy and cause-specific mortality
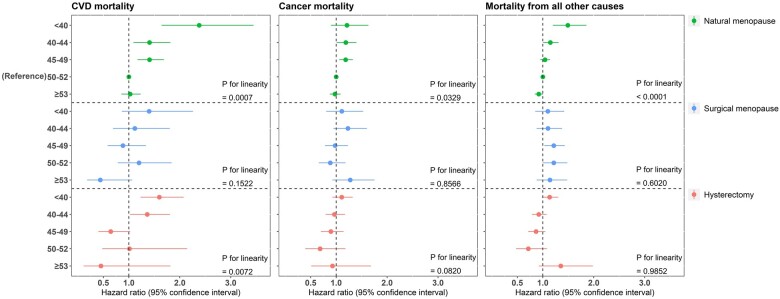
Compared with natural menopause at age 50–52 years, natural menopause or hysterectomy before the age of 40 years was associated with a greater risk of cardiovascular mortality (hazard ratio (HR): 2.38, 95% CI: 1.64, 3.45 for natural menopause; HR: 1.60, 95% CI: 1.23, 2.07 for hysterectomy), but this was not statistically significant for cancer mortality (HR: 1.21, 95% CI: 0.90, 1.63 for natural menopause; HR: 1.11, 95% CI: 0.93, 1.32 for hysterectomy). Natural menopause before the age of 40 years was also associated with mortality from all other causes (HR: 1.49, 95% CI: 1.20, 1.86 for natural menopause) (Fig. 1). Older age at natural menopause was associated with decreased risks of cardiovascular mortality, cancer mortality and mortality from all other causes (Fig. 1). The estimates for the associations of age at menopause and age at hysterectomy with cause-specific mortality are presented in Supplementary Tables SI, SII and SIII.
Figure 1.
The associations of age at menopause and age at hysterectomy with cause-specific mortality. CVD mortality, cardiovascular mortality.
MHT use and cause-specific mortality
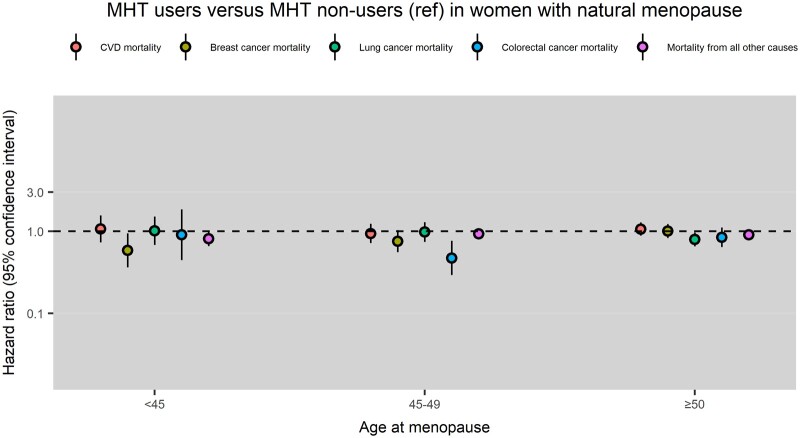
Compared with MHT non-users, MHT users had a lower risk of breast cancer mortality following natural menopause at <45 years (HR: 0.59, 95% CI: 0.36, 0.95) and a lower risk of colorectal cancer mortality following natural menopause at 45–49 years (HR: 0.47, 95% CI: 0.29, 0.76) (Fig. 2).
Figure 2.
Menopausal hormone therapy (MHT) use and cause-specific mortality in women with natural menopause at different ages. CVD mortality, cardiovascular mortality; Ref, reference group.
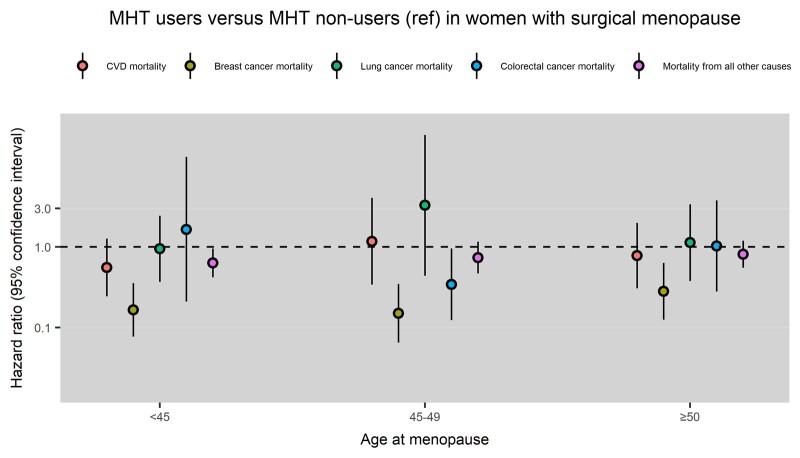
Compared with MHT non-users, MHT users had a lower risk of breast cancer mortality following surgical menopause at <45 years (HR: 0.17, 95% CI: 0.08, 0.36), 45–49 years (HR: 0.15, 95% CI: 0.07, 0.35) or ≥50 years (HR: 0.28, 95% CI: 0.13, 0.63) (Fig. 3). The association between MHT use and the risk of breast cancer mortality following surgical menopause did not differ between women using MHT for <6 years and those using MHT for 6–20 years (Supplementary Fig. S1, MHT non-users as the reference group).
Figure 3.
Menopausal hormone therapy (MHT) use and cause-specific mortality in women with surgical menopause at different ages. CVD mortality, cardiovascular mortality; Ref, reference group.
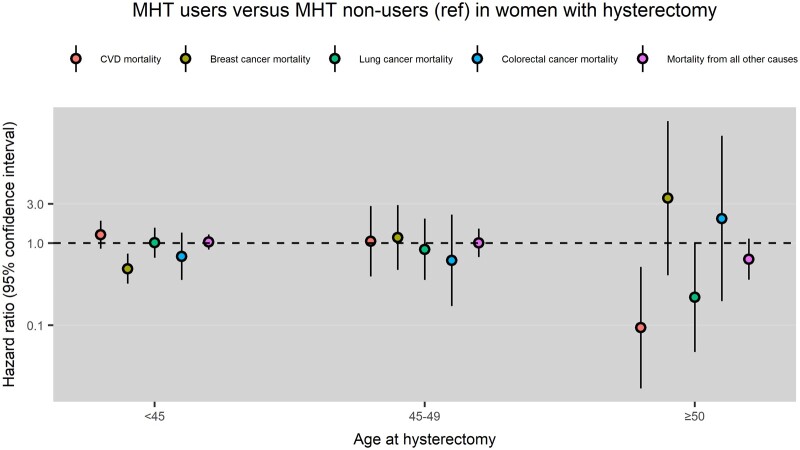
Compared with MHT non-users, MHT users had a lower risk of breast cancer mortality following hysterectomy at <45 years (HR: 0.49, 95% CI: 0.32, 0.74) and MHT users had a lower risk of cardiovascular mortality following hysterectomy at ≥50 years (HR: 0.09, 95% CI: 0.02, 0.51) (Fig. 4).
Figure 4.
Menopausal hormone therapy (MHT) use and cause-specific mortality in women with hysterectomy at different ages. CVD mortality, cardiovascular mortality; Ref, reference group.
The estimates for the association between MHT use and cause-specific mortality following natural menopause, surgical menopause or hysterectomy are presented in Supplementary Tables SIV, SV and SVI.
Sensitivity analysis results
Sensitivity analysis showed that the associations of age at menopause and age at hysterectomy with cause-specific mortality remained robust in the models incorporating death as a competing risk (Supplementary Table SVII). The inclusion of imputed covariates also did not change the conclusions (Supplementary Table SVIII).
Discussion
This study has yielded several novel and clinically significant findings: (i) self-reported MHT use following early natural menopause, surgical menopause or premenopausal hysterectomy was associated with a lower risk of breast cancer mortality but not consistently associated with the risk of mortality from cardiovascular disease or other causes. Also, the association between self-reported MHT use and the risk of breast cancer mortality following surgical menopause did not differ by the duration of MHT use; (ii) older age at natural menopause was associated with lower risks of cardiovascular mortality, cancer mortality and mortality from all other causes; and (iii) natural menopause or hysterectomy before age 40 years was associated with a higher risk of cardiovascular mortality, compared with natural menopause at 50–52 years.
Following early surgical or natural menopause, international guidelines advise the use of MHT until around age 50 years for indications including the prevention of cardiovascular disease (Baber et al., 2016). However, the evidence to support this advice is inconsistent (Duan et al., 2012; Li et al., 2013). In women without coronary heart disease, stroke and selected cancers prior to menopause or hysterectomy, we did not find a consistent association between MHT use and the risk of cardiovascular mortality, which generally accords with recent findings from a long-term follow-up study of the Women’s Health Initiative (WHI) randomized controlled trials (Manson et al., 2017). It was documented that observational studies assessing the association between MHT use and risk of cardiovascular events could be subject to ‘healthy user bias’ (van der Schouw and Grobbee, 2005) because in some studies MHT users were more healthy than MHT non-users (Matthews et al., 1996; Wharton et al., 2009). However, we found in the present study that, compared with MHT non-users, MHT users were more likely to be overweight or obese, be past or present smokers, have ≤10 years of education and have a family history of cardiovascular disease. Previous studies in France (the French E3N cohort) (Morois et al., 2012) and the USA (the California Teachers Study cohort) (Clague et al., 2014) also observed that MHT users were more likely to be past or present smokers than MHT non-users and another study in Denmark (the Diet, Cancer and Health cohort) (Holm et al., 2018) found that women who used MHT before the baseline survey were more likely to be overweight, be past or present smokers and have ≤7 years of education, than MHT non-users. Findings from these studies and our study suggest that MHT users may not always possess fewer pre-existing cardiovascular risk factors than do MHT non-users. Although we did not observe an association between MHT use and risk of cardiovascular mortality following natural or surgical menopause, we did notice that MHT use following hysterectomy at ≥50 years was associated with a lower risk of cardiovascular mortality (HR = 0.09, 95% CI: 0.02, 0.51). The Australian Longitudinal Study on Women’s Health also found that MHT use following hysterectomy or oophorectomy was associated with a lower risk of cardiovascular mortality (HR = 0.14, 95% CI: 0.02, 0.98) (Chen et al., 2017). Although this finding might be explained by the cardioprotective effect of estrogen (Zhu et al., 2019), it needs to be interpreted with caution because we did not find an association between MHT use and risk of cardiovascular mortality in women with hysterectomy before the age of 50 years.
In this study, we observed that self-reported MHT use was associated with a lower risk of breast cancer mortality following early natural menopause, surgical menopause at any age or hysterectomy before 45 years of age. This finding is clinically important. Whilst clinical guidelines commonly advise use of MHT in women with early menopause, uptake is suboptimal and concerns about breast cancer are a major barrier to MHT use (Kingsberg et al., 2020). The existing evidence on the association between MHT use and breast cancer mortality from randomized controlled trials or observational studies is scarce (Zhang et al., 2021). The Million Women Study found that use of estrogen only or combined MHT was associated with an increased risk of breast cancer mortality in 907 162 women free from breast cancer at the recruitment (Beral et al., 2019). A long-term follow-up study of the WHI randomized controlled trials found that use of estrogen alone (conjugated equine estrogen, CEE) following hysterectomy was associated with a lower risk of breast cancer mortality but that combined MHT (CEE plus medroxyprogesterone acetate) was not associated with the risk of breast cancer mortality (Chlebowski et al., 2020). Whilst we were unable to determine exactly what type of MHT was used by women in our study, it is likely that following hysterectomy alone or bilateral oophorectomy (concurrent with hysterectomy for almost all women in our study) that estrogen-only MHT would have been used. Hence, our findings are consistent with those from WHI that estrogen-only MHT was associated with a lower risk of breast cancer mortality.
We also observed that MHT use following early natural menopause (likely to be estrogen combined with progestin) was associated with a lower risk of breast cancer, which is inconsistent with the previous findings in the Million Women Study or the WHI randomized controlled trials (Beral et al., 2019). The existing evidence on the association between combined MHT use and breast cancer mortality is controversial and warrants further investigation (Zhang et al., 2021).
The association between age at menopause with mortality has been widely reported (Gong et al., 2016; Muka et al., 2016), but previous studies either focused on age at natural menopause (Mondul et al., 2005; Li et al., 2013; Wu et al., 2014; Gong et al., 2016; Roman Lay et al., 2018) or were unable to conduct separate analysis for natural menopause and surgical menopause (Malek et al., 2019; Zhang et al., 2019; Shen et al., 2020), so whether the association varied by type of menopause (i.e., natural or surgical) remained largely unknown (Ossewaarde et al., 2005; Amagai et al., 2006). Our observation that older age at natural menopause is associated with lower risks of cardiovascular and cancer mortality is consistent with the large Prostate, Lung, Colorectal, and Ovarian cohort study (Zhang et al., 2019), but inconsistent with other studies from Brazil (Roman Lay et al., 2018) and Japan (Amagai et al., 2006; Cui et al., 2006). A study conducted among African American women reported that all-cause mortality risk increased with the decrease of age at menopause, but this pattern was not observed for cardiovascular mortality (Li et al., 2013). Following surgical menopause, we did not observe a consistent decreasing risk of cardiovascular mortality or cancer mortality with the increase of age at menopause, which is different from the pattern observed for natural menopause.
Several previous studies have reported that premature menopause (before age 40 years) is associated with an increased risk of cardiovascular disease (Honigberg et al., 2019; Zhu et al., 2019). However, these have been limited by small numbers of women with premature menopause (Amagai et al., 2006; Li et al., 2013). To our knowledge, this is the largest prospective study examining the association between premature menopause and cause-specific mortality. Our findings confirm that premature natural menopause is associated with an increased risk of cardiovascular mortality compared with women with natural menopause at 50–52 years. We observed a weak association between cardiovascular mortality and surgical menopause before the age of 40 (HR: 1.40, 95% CI: 0.86, 2.26), compared with natural menopause at 50–52 years. A prior study in Japan also found similar results on all-cause mortality and surgical menopause before the age of 40 (HR: 1.58, 95% CI: 0.46, 5.39), although the sample size was small (seven deaths among women with premature surgical menopause) (Amagai et al., 2006).
A major strength of our study is the large sample size which allowed us to quantify the associations of age at menopause, age at hysterectomy and MHT with the risk of cause-specific mortality. Several limitations of our study need to be acknowledged. First, our study used self-reported information on age at menopause, age at hysterectomy, hysterectomy, oophorectomy and MHT use. However, previous studies have reported good validity and reproducibility of self-reported age at menopause (den Tonkelaar, 1997) and moderate concordance between self-reported oophorectomy and oophorectomy identified from surgical records (Phipps and Buist, 2009). Second, we only examined the effects of self-reported MHT use status and duration on mortality in our study. We acknowledged that the type, route and dose of MHT use may impact differently on mortality (Lobo, 2017), but we were unable to take these factors into account due to data unavailability. Third, although we included family history of breast cancer and other factors such as BMI, we could not rule out the possibility that women at a higher risk of breast cancer were less likely to take MHT, and this may partially explain the association between MHT use and a lower risk of breast cancer mortality that we observed. Fourth, we also could not rule out the possibility that MHT users may be more likely to use breast cancer screening than MHT non-users (Cook et al., 2009), which could reduce their risk of breast cancer mortality (IUPoBC Screening, 2012). Fifth, there was no information on the indications for hysterectomy or oophorectomy. As the indications for these surgeries in young women (e.g. <40 years) and older women (e.g. ≥53 years) are likely to differ and may have changed over time (e.g. before and after WHI announced that the study of estrogen-progestin therapy was being halted prematurely in July 2002), this limited our ability to demonstrate whether the associations of surgical menopause and hysterectomy with mortality varied according to the indication for surgery. However, we excluded women with cervical cancer, ovarian cancer or endometrial cancer prior to menopause or hysterectomy as cancers were one of the main reasons for hysterectomy/oophorectomy.
Conclusions
Self-reported MHT use following early natural menopause, surgical menopause or premenopausal hysterectomy is associated with a lower risk of breast cancer mortality and is not consistently associated with the risk of mortality from cardiovascular disease or other causes.
Supplementary Material
Contributor Information
Zhiwei Xu, The University of Queensland, School of Public Health, NHMRC Centre for Research Excellence on Women and Non-communicable Diseases (CRE WaND), Brisbane, Australia.
Hsin-Fang Chung, The University of Queensland, School of Public Health, Brisbane, Australia.
Annette J Dobson, The University of Queensland, School of Public Health, Brisbane, Australia.
Louise F Wilson, The University of Queensland, School of Public Health, NHMRC Centre for Research Excellence on Women and Non-communicable Diseases (CRE WaND), Brisbane, Australia.
Martha Hickey, Department of Obstetrics and Gynaecology, University of Melbourne and the Royal Women's Hospital, Melbourne, Australia.
Gita D Mishra, The University of Queensland, School of Public Health, NHMRC Centre for Research Excellence on Women and Non-communicable Diseases (CRE WaND), Brisbane, Australia.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data Availability
Data from UK Biobank are available on application at https://www.ukbiobank.ac.uk/enable-your-research/register
Authors’ roles
ZX conducted the literature review, statistical analyses and interpretation of the results and drafted the manuscript. HC harmonized the data and contributed to the interpretation of the results. AJD contributed to the statistical analyses and interpretation of the results. GDM conceived the study and contributed to the interpretation of the results. ZX, HC, AJD, LFW, MH and GDM all contributed to the critical revision of the manuscript.
Funding
This research is supported by the Australian National Health and Medical Research Council (NHMRC) (grant numbers APP1027196 and APP1153420). ZX and LFW are supported by NHMRC Centre for Research Excellence (APP1153420) and GDM is supported by NHMRC Principal Research Fellowship (APP1121844). MH is supported by an NHMRC Investigator Grant (APP1193838). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors have declared that no competing interests exist.
References
- Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E.. Age at menopause and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol 2006;16:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016;19:109–150. [DOI] [PubMed] [Google Scholar]
- Beral V, Peto R, Pirie K, Reeves G.. Menopausal hormone therapy and 20-year breast cancer mortality. Lancet 2019;394:1139. [DOI] [PubMed] [Google Scholar]
- Chen L, Mishra GD, Dobson AJ, Wilson LF, Jones MA.. Protective effect of hormone therapy among women with hysterectomy/oophorectomy. Hum Reprod 2017;32:885–892. [DOI] [PubMed] [Google Scholar]
- Cheng J, Xu Z, Bambrick H, Su H, Tong S, Hu W.. Heatwave and elderly mortality: an evaluation of death burden and health costs considering short-term mortality displacement. Environ Int 2018;115:334–342. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, Barrington W, Kuller LH, Simon MS, Lane D. et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020;324:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague J, Reynolds P, Henderson KD, Sullivan-Halley J, Ma H, Lacey JV Jr, Chang S, Delclos GL, Du XL, Forman MR. et al. Menopausal hormone therapy and lung cancer-specific mortality following diagnosis: the California Teachers Study. PLoS One 2014;9:e103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Rosner BA, Hankinson SE, Colditz GA.. Mammographic screening and risk factors for breast cancer. Am J Epidemiol 2009;170:1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y. et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol 2006;16:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–123. [DOI] [PubMed] [Google Scholar]
- Duan L, Xu X, Koebnick C, Lacey JV Jr, Sullivan-Halley J, Templeman C, Marshall SF, Neuhausen SL, Ursin G, Bernstein L. et al. Bilateral oophorectomy is not associated with increased mortality: the California Teachers Study. Fertil Steril 2012;97:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar CM, Sadler L, Harvey SA, Stewart AW.. The association of hysterectomy and menopause: a prospective cohort study. BJOG 2005;112:956–962. [DOI] [PubMed] [Google Scholar]
- Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- Gong D, Sun J, Zhou Y, Zou C, Fan Y.. Early age at natural menopause and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective observational studies. Int J Cardiol 2016;203:115–119. [DOI] [PubMed] [Google Scholar]
- Holm M, Olsen A, Kyrø C, Overvad K, Kroman N, Tjønneland A.. The influence of menopausal hormone therapy and potential lifestyle interactions in female cancer development—a population-based prospective study. Horm Cancer 2018;9:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL Jr, Scott NS, Natarajan P.. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 2019;322:2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPoBC Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778–1786. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM, Kapoor E, Faubion S.. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA 2021;326:1429–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsberg SA, Larkin LC, Liu JH.. Clinical effects of early or surgical menopause. Obstet Gynecol 2020;135:853–868. [DOI] [PubMed] [Google Scholar]
- Li S, Rosenberg L, Wise LA, Boggs DA, LaValley M, Palmer JR.. Age at natural menopause in relation to all-cause and cause-specific mortality in a follow-up study of US black women. Maturitas 2013;75:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 2017;13:220–231. [DOI] [PubMed] [Google Scholar]
- Malek AM, Vladutiu CJ, Meyer ML, Cushman M, Newman R, Lisabeth LD, Kleindorfer D, Lakkur S, Howard VJ.. The association of age at menopause and all-cause and cause-specific mortality by race, postmenopausal hormone use, and smoking status. Prev Med Rep 2019;15:100955–100955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Chlebowski RT, Howard BV, Thomson CA, Margolis KL. et al. ; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative Randomized Trials. JAMA 2017;318:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P.. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol 1996;143:971–978. [DOI] [PubMed] [Google Scholar]
- Mondul AM, Rodriguez C, Jacobs EJ, Calle EE.. Age at natural menopause and cause-specific mortality. Am J Epidemiol 2005;162:1089–1097. [DOI] [PubMed] [Google Scholar]
- Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N.. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol 2011;118:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morois S, Fournier A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault M-C.. Menopausal hormone therapy and risks of colorectal adenomas and cancers in the French E3N prospective cohort: true associations or bias? Eur J Epidemiol 2012;27:439–452. [DOI] [PubMed] [Google Scholar]
- Muka T, Oliver-Williams C, Kunutsor S, Laven JSE, Fauser BCJM, Chowdhury R, Kavousi M, Franco OH.. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- Nelson HD. Menopause. Lancet 2008;371:760–770. [DOI] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek ALM, Peeters PHM, van der Graaf Y, Grobbee DE, van der Schouw YT.. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–562. [DOI] [PubMed] [Google Scholar]
- Phipps AI, Buist DSM.. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause 2009;16:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman Lay AA, do Nascimento CF, de Oliveira Duarte YA, Porto Chiavegatto Filho AD.. Age at natural menopause and mortality: a survival analysis of elderly residents of São Paulo, Brazil. Maturitas 2018;117:29–33. [DOI] [PubMed] [Google Scholar]
- Shen TY, Strong C, Yu T.. Age at menopause and mortality in Taiwan: a cohort analysis. Maturitas 2020;136:42–48. [DOI] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schouw YT, Grobbee DE.. Menopausal complaints, oestrogens, and heart disease risk: an explanation for discrepant findings on the benefits of post-menopausal hormone therapy. Eur Heart J 2005;26:1358–1361. [DOI] [PubMed] [Google Scholar]
- Wharton W, Dowling M, Khosropour CM, Carlsson C, Asthana S, Gleason CE.. Cognitive benefits of hormone therapy: cardiovascular factors and healthy-user bias. Maturitas 2009;64:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, Marchbanks PA.. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol 2008;198:34.e31–e37. [DOI] [PubMed] [Google Scholar]
- Wilson LF, Pandeya N, Mishra GD.. Hysterectomy trends in Australia, 2000–2001 to 2013–2014: joinpoint regression analysis. Acta Obstet Gynecol Scand 2017;96:1170–1179. [DOI] [PubMed] [Google Scholar]
- Wu X, Cai H, Kallianpur A, Gao YT, Yang G, Chow WH, Li HL, Zheng W, Shu XO.. Age at menarche and natural menopause and number of reproductive years in association with mortality: results from a median follow-up of 11.2 years among 31,955 naturally menopausal Chinese women. PLoS One 2014;9:e103673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GQ, Chen JL, Luo Y, Mathur MB, Anagnostis P, Nurmatov U, Talibov M, Zhang J, Hawrylowicz CM, Lumsden MA. et al. Menopausal hormone therapy and women’s health: an umbrella review. PLoS Med 2021;18:e1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu L, Song F, Song Y, Dai H.. Ages at menarche and menopause, and mortality among postmenopausal women. Maturitas 2019;130:50–56. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Dobson AJ, Pandeya N, Brunner EJ, Kuh D, Greenwood DC, Hardy R, Cade JE, Giles GG. et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies. Hum Reprod 2020;35:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Chung H-F, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE. et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from UK Biobank are available on application at https://www.ukbiobank.ac.uk/enable-your-research/register