Summary
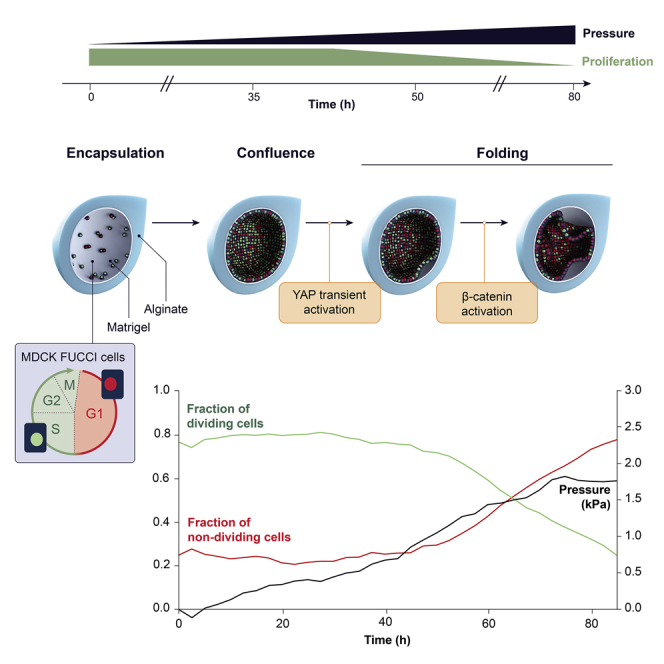
Morphogenesis requires spatiotemporal regulation of proliferation, both by biochemical and mechanical cues. In epithelia, this regulation is called contact inhibition of proliferation, but disentangling biochemical from mechanical cues remains challenging. Here, we show that epithelia growing under confinement accumulate pressure that inhibits proliferation above a threshold value. During growth, epithelia spontaneously buckle, and cell proliferation is transiently reactivated within the fold. Reactivation of proliferation within folds correlated with the local reactivation of the mechano-sensing YAP/TAZ pathway. At late time points, when the pressure is highest, β-catenin activity increases. The threshold pressure increases when β-catenin is overactivated and decreases when β-catenin is inhibited. Altogether, our results suggest that different mechanical cues resulting from pressure inhibition of proliferation are at play through different mechano-sensing pathways: the β-catenin pathway sustains cell division under high pressure, and the YAP pathway senses local curvature.
Keywords: contact inhibition, pressure inhibition, cell-cycle control, 3D cell culture, threshold pressure, epithelium mechanics
Graphical abstract
Highlights
-
•
Encapsulation of MDCK cells enables quantification of growth-induced pressure
-
•
Confined epithelia reach a threshold pressure that inhibits cell-cycle progression
-
•
Overactivation of β-catenin activity sustains cell division under high pressure
Di Meglio et al. grow epithelial cells inside spherical elastic shells to measure the lateral pressure that accumulates from growth of confined tissues. They show that pressure eventually reaches a threshold value that inhibits proliferation, pointing to a crosstalk between mechanical forces and cell-cycle regulation in growing tissues.
Introduction
Cell proliferation control—where and when cells divide—is essential for tissue formation and maintenance. Physical cues like substrate stiffness or compressive stress participate in proliferation control (Delarue et al., 2014; Gudipaty et al., 2017; Huang et al., 1998; Kim and Asthagiri, 2011; Streichan et al., 2014). Cell-cycle regulation is controlled by these cues to maintain the functional integrity of tissues, and loss of this regulation leads to overgrowth, a hallmark of many diseases and development failures. In epithelia, contact inhibition englobes mechanisms by which a decrease in cell area, concomitant to increased cell-cell contacts, leads to proliferation arrest. Extensive evidence suggests that increased cell-cell contacts are important but not sufficient for proliferation inhibition (Aragona et al., 2013; Puliafito et al., 2012), yet we still lack a full picture of how mechanical cues control proliferation in tissues. This is in part because mechanical cues are sensed via adhesion protein complexes that link force and adhesion sensing at the molecular level. For instance, the α/β-catenin pathway effector β-catenin, normally present at adherens junctions (AJs), shuttles to the nucleus after mechanical activation and promotes transcription of cell-cycle genes (Torre et al., 2011). Another signaling protein, the Hippo pathway effector Yes-associated protein (YAP), is involved in contact inhibition (Aragona et al., 2013; Zhao et al., 2007) and is a general mechano-sensing pathway (Aragona et al., 2013; Benham-Pyle et al., 2015). While β-catenin promotes S-phase progression, YAP nuclear translocation promotes re-entry into mitosis (Dong et al., 2007). Importantly, recent work showed that mechanical stretch transiently activates YAP within 1 h, whereas activation of β-catenin occurs 6 h after, and is maintained over 16 h (Benham-Pyle et al., 2015).
Many studies propose that cell area represents a readout of cell mechanical state (Chen et al., 1997; Nelson et al., 2005; Puliafito et al., 2012; Shraiman, 2005; Uroz et al., 2018). Consistently, studies using two-dimensional (2D) epithelial models proposed that an increase in cell density generates compressive stresses within the tissue to inhibit proliferation (Aragona et al., 2013; Puliafito et al., 2012; Streichan et al., 2014). However, it is hard to test this hypothesis because measuring pressure in tissues is difficult. It is, however, established that proliferation is trigged by increased mechanical tension (Benham-Pyle et al., 2015; Gudipaty et al., 2017; Huang et al., 1998; Nelson et al., 2005; Uroz et al., 2018) and inhibited by compression (Alessandri et al., 2013; Delarue et al., 2014; Dolega et al., 2017). But how contact inhibition balances adhesive and compressive forces to regulate proliferation is not well understood.
Another process coupled to proliferation control during morphogenesis is tissue curvature. During formation of Drosophila wing veins, for instance, areas with higher proliferation rates bulge out (Tozluoglu et al., 2019); proliferative veins accumulate compressive stress and buckle due to confinement from surrounding, less proliferative areas. Overall, proliferation under confinement and tissue buckling are proposed to generate many curved shapes observed during morphogenesis (Diaz de la Loza and Thompson, 2017; Savin et al., 2011; Shyer et al., 2013). While measuring stresses in real tissues is challenging (Campas, 2016; Hofemeier et al., 2021; Mongera et al., 2018), we showed that proliferation of an epithelium growing under spherical confinement in vitro generates compressive stress that drives folding through buckling (Trushko et al., 2020). However, how fold formation regulates proliferation is still poorly studied. Interestingly, the Hippo pathway could be regulated by curvature via YAP nuclear localization changes (Luciano et al., 2020).
Here, we investigate whether compressive stress regulates cell-cycle progression. To this end, we encapsulate and grow an epithelial monolayer inside hollow hydrogel microspheres (Alessandri et al., 2013). We determine the relation between cell-cycle progression using cells stably expressing fluorescent ubiquitination-based cell-cycle indicator (FUCCI) and tissue pressure by measuring deformation of the elastic hydrogel shells.
Results
Cell encapsulation provides a system to study epithelial proliferation under spherical confinement
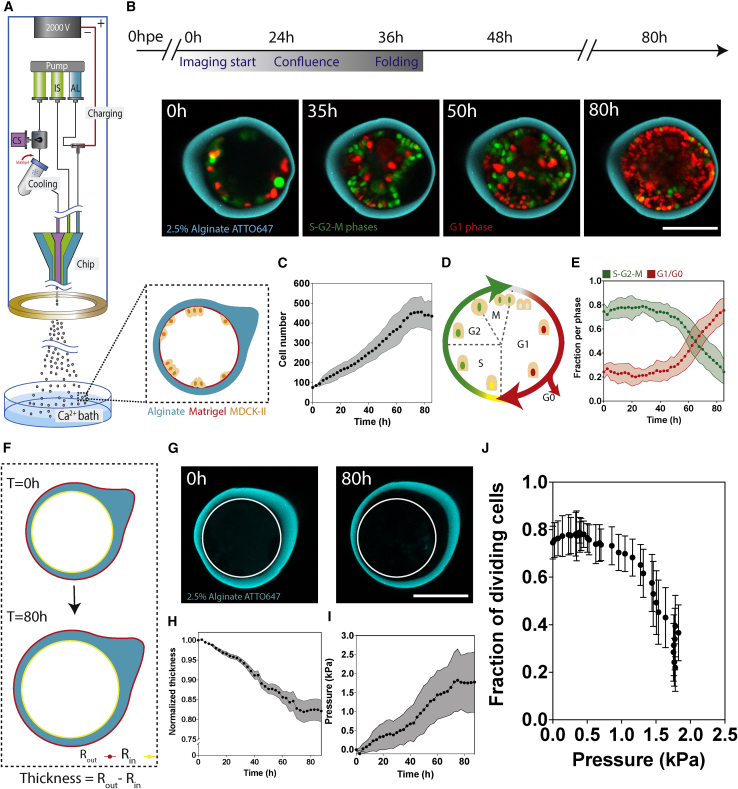
To investigate the link between mechanical forces and cell-cycle dynamics, we monitored the growth of an epithelial layer under spherical confinement. We encapsulated Madin-Darby canine kidney II (MDCK-II) cells inside hollow alginate microspheres (hereafter capsules) using a microfluidic device (Figure 1A; Alessandri et al., 2016). Capsules consist of an outer layer (AL) of alginate, an inner layer (CS) with MDCK cells and Matrigel, and an intermediate sorbitol solution (IS) to avoid mixing of AL and CS (Figure 1A; STAR Methods). Upon capsule formation, Matrigel forms a 3- to 4-μm-thick layer on the capsule inner surface onto which cells grow (Alessandri et al., 2016; Trushko et al., 2020).
Figure 1.
Cell-cycle progression is regulated by pressure
(A) Schematic of experimental setup (left) and capsule midplane at encapsulation time (bottom right) (STAR Methods).
(B) Timeline of monolayer growth (top) and confocal midplane images of FUCCI cells in 2.5% capsule (bottom).
(C) Cell number over time for FUCCI cells encapsulated in 2.5% capsules. See Figure S3 for cell density over time.
(D) Schematic of FUCCI cell-cycle marker.
(E) Mean cell fraction in S-G2-M (green) and G1/G0 (red) phases over time in 2.5% alginate capsules. See Figure S3 for G1 and S-G2-M cell numbers.
(F) Schematic of capsule expansion and wall thinning with inner (yellow) and outer (red) boundaries used to measure pressure.
(G) Confocal midplane image of capsule showing capsule expansion over time; white circular contour shows inner perimeter of the capsules at T = 0 h.
(H) Mean normalized thickness of 2.5% capsules over time.
(I) Mean pressure (kPa) over time for 2.5% capsules.
(J) Mean FDCs over mean pressure (kPa).
For (C)–(J), 3 experiments, n = 20 capsules. Scale bars, 100 μm.
To assess monolayer growth dynamics, we used cells stably expressing the FUCCI reporter (STAR Methods) in 2.5% alginate capsules starting at ∼24 h post-encapsulation (hpe) for a duration of 60 to 80 h (times given after imaging starts [AIS]; Figure 1B). Cells attach onto the capsule inner layer, grow to form a confluent monolayer, fold, and eventually fill the available space within capsules (Figure 1B; Video S1). As we showed in our recent publication (Trushko et al., 2020), proliferation generates compressive stress that drives folding through buckling. Even after folding, monolayers maintain epithelial polarity and organization (Figure S1).
Red channel is Cdt1-mKO2 (Kusabira Orange), green channel is geminin-mAG (Azami Green) and cyan is Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
Cell number per capsule increased linearly up to 60 h AIS (Figure 1C), supporting that neither confluence (at 20–30 h AIS) nor folding (30–40 h AIS) limit proliferation. This linear growth rate is in accordance with MDCK growth dynamics (Soderberg et al., 1983). Cell number plateaued at 450 cells (9 ± 1 cells/1,000 μm2; see Figure S2) between 70 and 80 h AIS (Figure 1C). As also proposed by other studies (Aragona et al., 2013; Puliafito et al., 2012; Streichan et al., 2014), since the cell-division rate decreases ∼2–3 days post-confluence, this supports the notion that cell-cell contact alone is not sufficient to inhibit proliferation. We next sought to investigate quantitatively how mechanical cues associated with confinement affect cell-cycle dynamics.
Cell-cycle progression is halted past a threshold pressure
To probe how confinement affects the cell cycle, we used the FUCCI reporter (STAR Methods), which allowed us to monitor and quantify cell-cycle dynamics. In G1/G0, nuclei express mKO2-Cdt1 and are red; at the onset of S phase, nuclei express mAG-Geminin and become green; and nuclei remain green throughout S, G2, and M phases until cells divide (Figure 1D). Importantly, red nuclei indicate cell-cycle arrest at G1-S or cell-cycle exit at G0 (Sakaue-Sawano et al., 2008). We followed FUCCI cells over ∼80 h (Figures 1C–1E and S2) and observed that cells initially actively cycle, with most cells in S-G2-M (Figure 1B). As the monolayer grows and folds, we observe a shift to cells that are mostly red, in G1/G0, which suggests that confinement constrains cells to remain in G1/G0 (Figure 1B).
We quantified the fraction of dividing cells (FDCs), the ratio of S-G2-M cells over total cell number (Figure 1E). For the first 30 h, the FDCs remains constant and much higher than the G1/G0 fraction, as most are actively cycling. At 40–50 h, the FDCs begins to decrease, while the G1/G0 fraction increases (Figure 1E). Interestingly, the FDCs remained constant even past confluence, which was reached at 18 ± 6 h (mean ± SD, N = 25). The delay between confluence and decrease in the FDCs was over ∼20 h, which confirms that confinement limits cell growth but that cell-cell contact alone is not sufficient to inhibit proliferation. At ∼50 h, shortly before cell density plateaus (Figure 1C), the FDCs begins to decrease to almost undetectable levels at >80 h (Figure 1E). This decrease in the FDCs, accompanied by capsule radial expansion, suggests that cells continue to divide and generate pressure that results in capsule deformation (Figures 1F and 1G; Video S2). In line with this, enzymatic degradation of capsules reactivated cell-cycle progression, showing that a release of pressure restores cell proliferation (Figure S3).
Cyan is Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
To assess how pressure affects cell-cycle progression, we measured pressure accumulation from capsule deformation (STAR Methods; Alessandri et al., 2013; Trushko et al., 2020; Figures 1F and 1G). Changes in capsule radius and wall thickness were computed from time-lapse images of the shell (Figures 1G and 1H; STAR Methods; Trushko et al., 2020), and pressure was calculated over time (Figure 1I; STAR Methods). We observe that pressure accumulates linearly, reaching a plateau at 1.8 ± 0.7 kPa between 75 and 80 h (Figure 1J). Analysis of the FDCs as a function of pressure reveals that as growth-induced pressure within capsules accumulates, the FDCs decreases asymptotically toward a threshold pressure of ∼1.8 kPa, above which cells no longer progress into the cell cycle (Figure 1J). We conclude that a confined epithelial tissue accumulates compressive stress that impedes cell-cycle progression past a threshold pressure of ∼1.8 kPa.
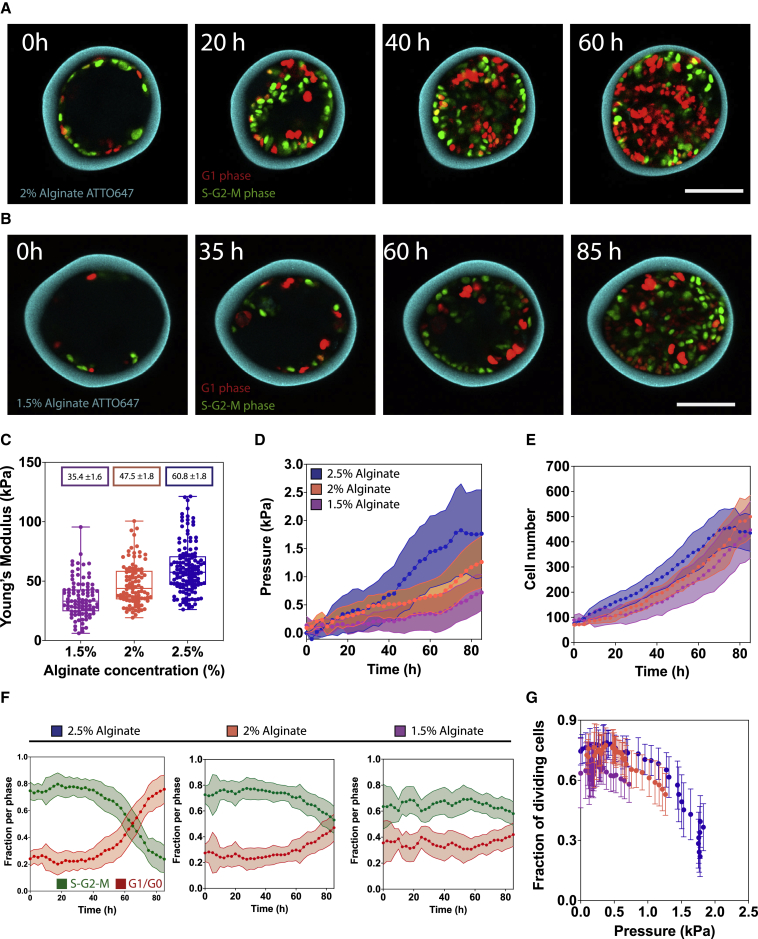
The effect of pressure on cell-cycle progression is independent of capsule rigidity
We next encapsulated cells in softer shells to further assess pressure as the parameter regulating the cell cycle. Substrate stiffness is known to regulate cell proliferation (Aragona et al., 2013), so we sought to assess cell-cycle progression when modulating capsule rigidity. By changing alginate percentage, the Young’s modulus (E) of capsules can be tuned (Trushko et al., 2020). Time-lapse images of 2% and 1.5% capsules illustrate MDCK monolayer growth and cell-cycle dynamics over 3–4 days (Figures 2A and 2B). The measured Young’s moduli for 2.5%, 2%, and 1.5% alginate capsules are 61 ± 2, 48 ± 2, and 35 ± 2 kPa, respectively (Figure 2C). Pressure accumulation clearly exhibited a delay for reduced stiffnesses (Figure 2D): within the same period, 2.5% alginate capsules reached a maximum pressure of 1.8 ± 0.7 kPa, 2% capsules a pressure of 1.3 ± 0.5 kPa, and 1.5% capsules a pressure of only 0.7 ± 0.3 kPa. Interestingly, despite the delay in pressure accumulation of 1.5% capsules, the rate of cell-density increase was similar for all alginate concentrations (Figure 2E). In contrast, quantification of cell-cycle fractions revealed different dynamics, which are thus dependent on capsule rigidity (Figure 2F). For 2% alginate capsules, the FDCs increased slightly up to ∼40 h and began to decrease like in 2.5% alginate capsules but only reached a minimum of 0.5 ± 0.1 kPa, whereas the G1-G0-phase cells remained low and only increased slightly between 60 and 80 h (Figure 2F). In 1.5% alginate capsules, the initial FDCs is lower compared with both 2.5% and 2% alginate capsules and remained at around 60% throughout (Figure 2F). The FDCs exhibited a slow decrease as a function of increasing pressure but no drop for either 2% or 1.5% alginate capsules Figure 2G), as the pressure never reached the threshold pressure of ∼1.8 kPa. However, in all cases, the curves of the FDCs with pressure followed the same path, albeit the lower value in 1.5% alginate (Figure 2G). Altogether, this confirms that pressure, but not capsule rigidity (within these range), regulates cell-cycle progression.
Figure 2.
Pressure regulates cell-cycle progression independently of capsule rigidity
(A) Confocal midplane image of MDCK FUCCI cells in 2% capsules.
(B) Confocal midplane image of MDCK FUCCI cells in 1.5% capsules.
(C) Young’s modulus (kPa) for each alginate concentration measured using AFM. Respective Young’s moduli are 1.5%, 35.4 ± 1.6 kPa (N = 94); 2%, 47.5 ± 1.8 kPa (N = 98); and 2.5%, 60.8 ± 1.8 kPa (N = 137).
(D) Mean pressure (kPa) over time for 1.5%, 2%, and 2.5% capsules.
(E) Mean MDCK FUCCI cell number over time for 1.5%, 2%, and 2.5% capsules. Differences between means not statistically significant (ns) with two-tailed p values of 0.660 (1.5% versus 2%), 0.053 (1.5% versus 2.5%), and 0.304 (2% versus 2.5%). See Figure S3 for cell density over time.
(F) Mean fraction of S-G2-M (FDCs, green) and G1/G0 phase (red) cells over time for 2.5%, 2%, and 1.5% capsules.
(G) Mean FDCs as a function of mean pressure (kPa) for 2.5%, 2%, and 1.5% capsules.
For (C)–(F), statistical significance was measured using one-way ANOVA and Tukey’s multiple comparisons test; at p < 0.05, samples were assumed to be different. For (D)–(G), 1.5% alginate n = 17; 2% alginate n = 15; 2.5% alginate n = 20. Scale bars, 100 μm.
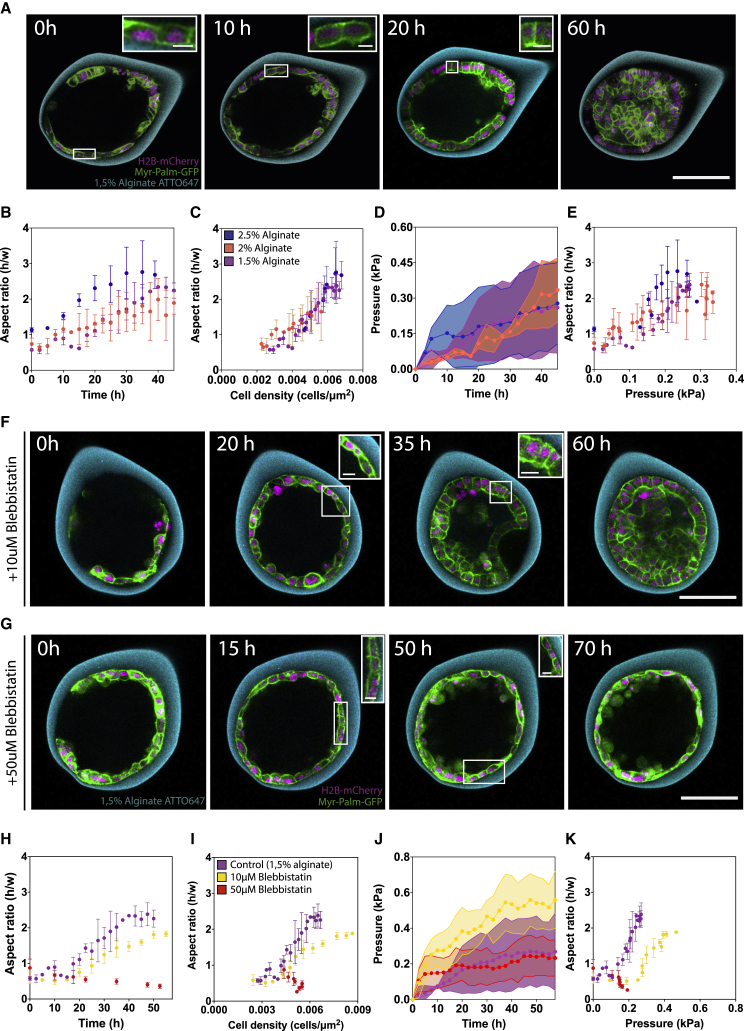
Changes in cell aspect ratio do not depend on pressure
Previous studies proposed that cell aspect ratio (AR)—the ratio between cell height and cell width—would be a readout of pressure within epithelia (Legoff et al., 2013; Nestor-Bergmann et al., 2018). Unlike cuboidal cells (AR = 1), compressed cells would be columnar (AR > 1) and stretched cells squamous (AR < 1). To test whether cells in capsules accommodate lateral forces through AR variations, we measured AR over time, as a function of pressure, and different capsule rigidities. To estimate the cell AR more accurately, we manually measured cell AR using the MDCK Myr-Palm-GFP H2B-mCherry cell line (STAR Methods; Figure 3A). Prior to monolayer confluence, cells are mostly squamous (Figures 3A and 3B). As the monolayer reached confluence (4–5 cells/1,000 μm2), cells became cuboidal, independently of rigidity (Figure 3B). Past confluence, the AR kept increasing for all rigidities (Figure 3B). However, AR change with time depended on capsule rigidity, as cells in 2.5% capsule changed their AR faster and more abruptly than for 2% and 1.5%, the latter being slower and displaying a more continuous AR change. We wondered whether the effect on AR dynamics could originate from capsule rigidity itself or from the lower pressure accumulation in softer capsules.
Figure 3.
Changes in cell aspect ratio depend on capsule rigidity and cell contractility
(A) Confocal midplane images of MDCK Myr-Palm-GFP H2B-mCherry cells in 1.5% alginate capsules.
(B) Mean aspect ratio (AR; cell height/width) over time for different alginate concentrations. Differences between means for 1.5% and 2% are ns with a p value of 0.99.
(C) Mean AR over cell density (cells/um2) for different alginate concentrations.
(D) Mean pressure over time for different alginate concentrations. Differences between means are ns with a p value of 0.99.
(E) Mean AR over mean pressure for different alginate concentrations.
(F and G) Confocal midplane image of MDCK cells in 1.5% capsules treated with 10 (F) or 50 μM (G) Blebbistatin.
(H) Mean AR over time for control or 10 or 50 μM Blebbistatin-treated 1.5% capsules. Difference between means for control and 10μM Blebbistatin not statistically significant (ns) with p value 0.26.
(I) Mean AR over cell density (cells/um2) for control and Blebbistatin-treated.
(J) Mean pressure (kPa) over time for control or 10 or 50 μM Blebbistatin-treated 1.5% capsules. Differences between means for control and 50 μM Blebbistatin treatment are ns with a p value of 0.2699.
(K) Mean AR over mean pressure (kPa) for control and Blebbistatin-treated cells.
For (A)–(K), cell line used was MDCK Myr-Palm-GFP H2B-mCherry cells. For (B)–(E), 1.5% alginate n = 12; 2% alginate n = 18; 2.5% alginate n = 15. For (H)–(J), control 1.5% alginate n = 12; 10 μM Blebbistatin n = 5; 50 μM Blebbistatin n = 9. Statistical significance was measured using one-way ANOVA with (B–E) Tukey’s multiple comparisons test or (H–J) Dunnet’s test; at p < 0.05, samples were assumed to be different. Scale bars,100 μm; insets,10 μm.
First, as expected from purely geometrical considerations, we observed that cell AR increased linearly with cell density Figure 3C). Importantly, this linear dependence was independent from capsule rigidity, as the curves AR versus cell density perfectly collapsed for all three alginate conditions. We thus postulated that pressure-accumulation differences in capsules of different rigidities may account for differences of cell AR dynamics (Figure 3B). The Myr-Palm-GFP/H2B-mCherry cell line accumulated pressure rather slowly compared with the FUCCI and H2B-mCherry cell lines used before (Trushko et al., 2020; Figure 3D), explaining why pressure accumulation was rather similar in all capsules, regardless of alginate concentration (Figure 3D). Consequently, when plotting AR versus pressure, differences in AR dynamics are observed for different stiffnesses: the larger the capsule rigidity is, the faster the AR changes (Figure 3E). Also, AR reached higher values, and the transition to larger values of AR were more abrupt for stiffer capsules (Figure 1E).
These results point to an internal regulation of AR dynamics independent of pressure but dependent on rigidity. Actomyosin contractility, organized into an apical belt in MDCK cells, participates in AR determination and depends on substrate stiffness (Huang et al., 1998; Wada et al., 2011; Wu et al., 2014). We thus wondered how contractility could influence AR dynamics. For this, we reduced contractility in capsules using Blebbistatin at two concentrations, 10 (Figure 3F; Video S3) and 50 μM (Figure 3G; Video S4). At 10 μM, while AR increased more progressively than control cells, cells only reached a moderate columnar shape (Figures 3F and 3H). At 50 μM, AR decreased with time, being more squamous at 70 than at 0 h (Figures 3G and 3H). Overall, reducing contractility strongly affected AR dynamics (Figures 3H and 3I). Interestingly, pressure accumulated faster in capsules treated with 10 μM Blebbistatin than in control capsules, suggesting that contractility limits pressure accumulation by promoting cell AR variation (Figure 3J). However, when contractility is abolished (50 μM Blebbistatin), capsules accumulated almost no pressure, probably because cell division was also affected at this drug concentration. Altogether, these results suggest that contractility is the primary parameter controlling AR and that pressure has little influence on AR dynamics. Capsule rigidity varies the dynamics of AR more than pressure, consistent with cell contractility depending on substrate stiffness (Aragona et al., 2013).
Green is Myr-Palm-GFP, magenta is H2B-mCherry, cyan is 1.5% Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
Green is Myr-Palm-GFP, magenta is H2B-mCherry, cyan is 1.5% Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
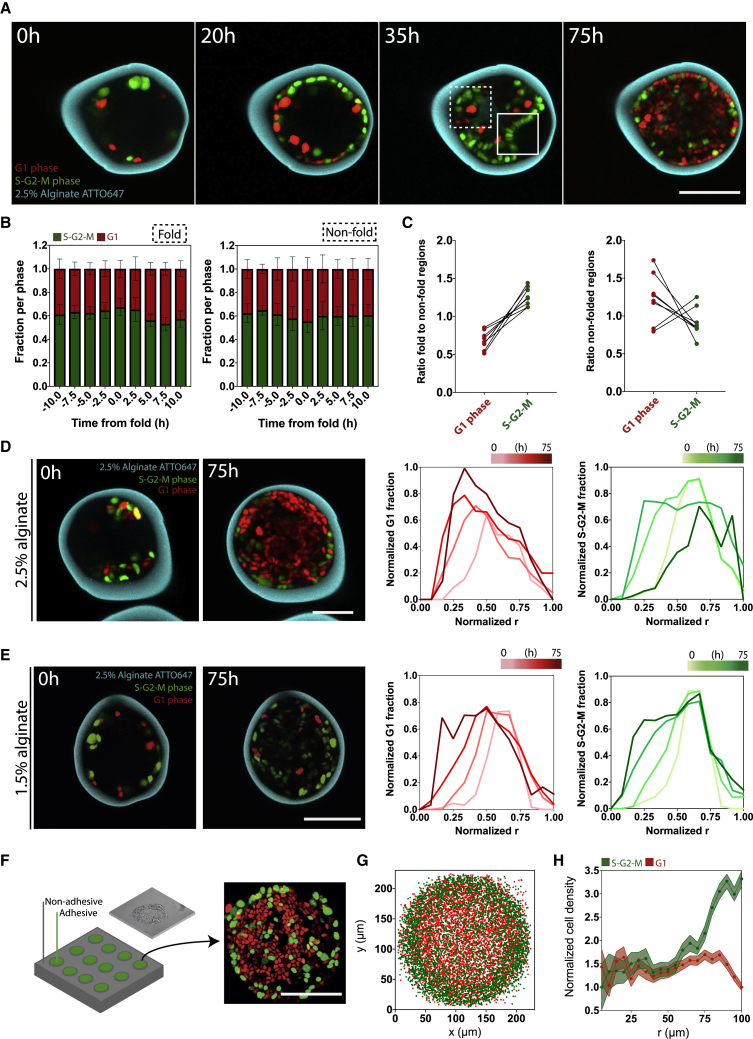
Spatial distribution changes in cell-cycle progression
We have previously shown that epithelia growing in alginate capsules fold by buckling (Trushko et al., 2020). Curvature induction is associated with cell-density and -shape changes and could be associated with local stress relaxation, at least within folds. We hypothesized that these morphological changes may also affect cell-cycle dynamics (see Figure 1).
To assess how cell-cycle temporal dynamics are coupled to epithelial curvature, we assessed spatial distributions of G1-G0 phase and S-G2-M phase MDCK FUCCI cells within folds versus non-folded regions (Figure 4). Folds often featured mainly dividing cells (Figure 4A). Quantifying G1-G0 and S-G2-M cell number in folds and outside folds with time, we observe an increase in the FDCs in folds at the time of folding (Figure 4B, left), whereas regions outside the fold have a non-significant decrease of the FDCs at the time of folding (Figure 4B, right). Therefore, at the time of folding, the FDCs within folds is significantly higher than in surrounding non-folded regions, a difference not observed when comparing non-folded regions of the same capsule (Figure 4C). This is consistent with the notion that curvature induction leads to mechanical changes that transiently trigger cell division.
Figure 4.
Cell-cycle progression is spatially and temporally regulated within capsules
(A) Representative confocal midplanes of FUCCI cells in 2.5% alginate capsules. Insets show folded compared with non-folded region used to compare spatial distribution of FUCCI cells in (B) and (C).
(B) Fraction of cells in G1 or S-G2-M phases within fold (left) or in a non-folded region (right). Time is aligned to folding time (t = 0 h).
(C) Ratio of G1 phase in a folded over non-folded region versus S-G2-M-phase cells in a folded over non-folded region (left) compared with ratios in a non-folded region over another non-folded region (right).
(D and E) Representative confocal midplane image of FUCCI cells in 2.5% (D) and 1.5% (E) capsules showing quantification of normalized fraction of cells in G1 (red, left) and S-G2-M (green, right) phases (normalized to max. fraction) as a function of radial distance from capsule center (normalized to max. radius) in time.
(F–H) Schematic of 2D adhesive circular patterns with representative confocal max projection of FUCCI cells at over-confluence (about 4 days after seeding) on circular pattern (F), position of the G1-phase cells (red) and S-G2-M-phase (green) cells on circular patterns at over-confluence (G), and mean normalized cell density (to min. cell density) of G1- and S-G2-M-phase cells as a function of the radius from the pattern center (r = 0 μm) to periphery (r = 100 μm) of the pattern (H). See also Figure S4.
For (B) and (C), n = 8 capsule folded positions and n = 8 randomly chosen non-folded positions. For (D) and (E), 3 experiments, n = 15 capsules. For (G) and (H), n = 78 patterns. Scale bars, 100 μm.
To further study whether transient cell-cycle activation during folding could impact the overall distribution of dividing cells, we followed the radial distribution of dividing and resting cells in time. We quantified the average radial distribution of cells by first normalizing cell positions by the final inner capsule radius and averaged distributions over 15–20 capsules per time point (Figure 4D). Because capsules expand with time, cells are initially positioned at r < 1. In 2.5% alginate capsules, the peak of resting cell distribution progressively moved toward the capsule center, whereas the peak of dividing cell distribution moved first toward the capsule center upon folding and then back to the boundaries after folding (Figure 4D). This suggests that transient activation of cell cycle in folds impacts the overall distribution of cell divisions in capsules.
At long times, the striking persistence of dividing cells at the 2.5% capsule boundary is reminiscent of previous observations in spheroids growing under osmotic pressure (Delarue et al., 2014; Dolega et al., 2017). This may be linked to a pressure gradient within the forming tissue, with lower pressure allowing for cell division at the periphery. To test this, we used 1.5% alginate capsules, where pressure only reaches ∼0.2 kPa, and found that both dividing and resting cells slowly moved inward with no accumulation of dividing cells at the periphery (Figure 4E), unlike 2.5% capsules (compare Figures 4D and 4E).
While maintenance of dividing cells at the periphery may be due to a pressure gradient, it could also be due to cell-adhesion differences or nutrient diffusion, since only cells at the periphery are adhering to Matrigel and are directly exposed to nutrients and growth factors diffusing through alginate.
To test these possibilities, we grew MDCK FUCCI cells on 2D adhesive patterns. In this case, all cells adhere to the substrate and are uniformly exposed to nutrients and growth factors. Cells were seeded on circular fibronectin-coated patterns (Figure 4F, left; STAR Methods) and grown for ∼72 h until over-confluence (Figure 4F, right). Superimposing nuclei positions in patterns showed that dividing cells are prominent at the periphery (Figure 4G). Quantification of radial cell distributions revealed most resting cells in the center, whereas dividing cells were predominantly at the periphery (Figure 4H). Thus, neither detachment nor limited access to nutrients could cause the increased FDCs at the capsule surface since the same effect was observed on 2D patterns. It also suggests that the lower pressure experienced by cells at the boundary may favor cell division. Indeed, cells at the center of patterns also have smaller nuclear area (Figure S4). This is in line with other previous studies of cells grown on circular patterns, where cells at the periphery proliferate but cells at the center are under higher compressive stress and do not proliferate (Aragona et al., 2013; Nelson et al., 2005).
De-regulation of β-catenin activity alters how cell-cycle dynamics respond to pressure
Finally, we sought to assess how pressure and curvature sensing may be transduced via mechano-sensing pathways to regulate the cell cycle. Two evident candidates are YAP and β-catenin (Benham-Pyle et al., 2015, 2016; Fernandez-Sanchez et al., 2015). The former can be quantified via nucleo-cytoplasmic shuttling of YAP isoform1 (YAP1) (Piccolo et al., 2014) and levels of phosphorylated YAP within cells; the latter can be assessed by measuring levels of the β-catenin/TCF/LEF-targeted gene c-Myc.
We first assessed YAP/TAZ pathway activation through nuclear localization of YAP over time. Encapsulated cells were fixed and immunostained for YAP at pre-monolayer stage, time of confluence, and late stages of folding (STAR Methods). Initially, YAP nuclear localization was evident, but upon reaching confluence, most nuclear YAP disappeared and then stayed low on average (Figure 5A). These findings are consistent with YAP establishing contact inhibition of proliferation (CIP) upon confluence and epithelium polarization (Martin-Belmonte and Perez-Moreno, 2011; Zhao et al., 2007) but also suggest that YAP is not involved in inhibition of cell-cycle progression upon reaching the threshold pressure.
Figure 5.
Inhibition and over-activation of β-catenin transcriptional activity alters pressure response
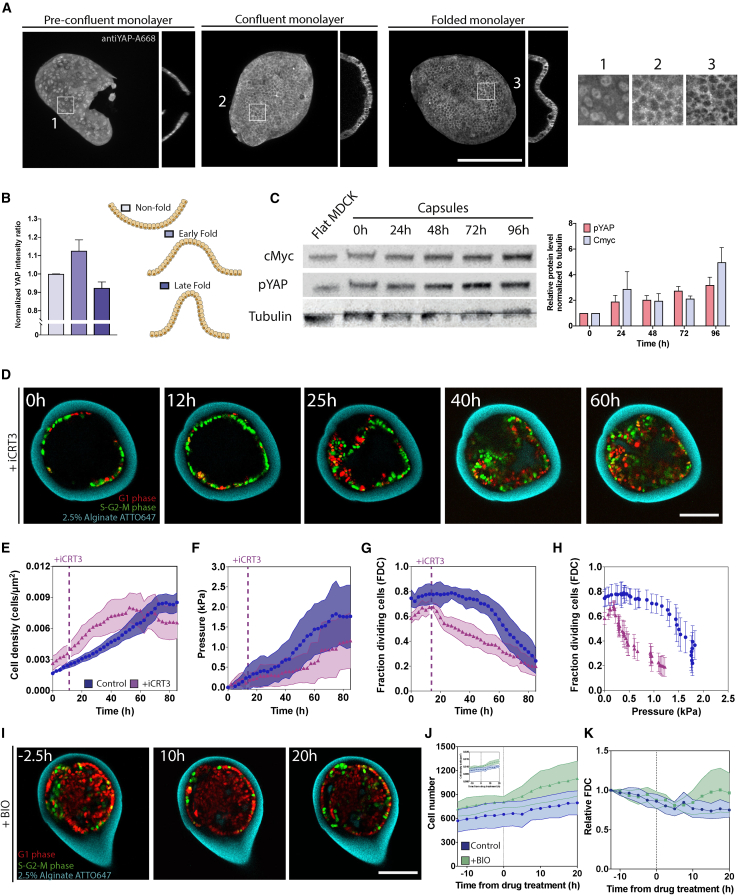
(A) Max z projections and orthogonal view of pre-confluent, confluent, and folded (left to right) fixed MDCK monolayers immunostained with anti-YAP-A568.
(B) Normalized YAP intensity ratio (nuclear YAP intensity normalized with mean nuclear YAP intensity in non-folded regions) of non-folded and early and late folded capsules (see schematic). See also Figure S4.
(C) Total cell lysates from flat versus MDCK cells in 2.5% alginate capsules were analyzed by western blotting. Tubulin was used as loading control.
(D–H) Representative confocal midplane image of FUCCI cells in 2.5% alginate capsules treated with 25 μM iCRT3 (D) and quantification of mean cell density (cells/um2) over time (E), mean pressure (kPa) over time (F), mean FDCs over time (G), and mean FDCs over mean pressure (kPa) (H) for control (circle, blue) and iCRT3-treated (triangle, purple) FUCCI cells within 2.5% capsules.
(I–K) Representative confocal equatorial plane images of FUCCI cells in 2.5% capsules from −10 to 20 h from 100 nM BIO treatment (I) with quantification of mean cell number (J) and mean relative FDCs (K) for control (circle, blue) and BIO-treated (triangle, green) FUCCI cells in 2.5% capsules. Inset in (J) shows mean cell density (cells/um2) over time. Relative FDCs refers to FDCs normalized to FDCs at 12.5 h prior to drug treatment. Differences between means in (K) are ns with a p value of 0.40. For (B), N = 3 capsules and n < 120 cells per condition, error bars are SEM. For (E)–(H), control n = 20 and iCRT3-treated n = 12; statistical significance measured using t test with Welch’s correction p < 0.05. For (J), control N = 8 capsules, BIO-treated capsules N = 4; statistical significance was measured using one-way ANOVA with Dunnet’s test, at p < 0.05, samples were assumed to be different. Scale bars, 100 μm.
Since YAP seemed involved in early mechanoregulation of our system, we wondered if YAP could be involved in transient re-activation of the cell cycle during folding. We analyzed fixed capsules with folds and compared YAP fluorescent intensity in nuclei found in both early and late folds with outside the fold (Figures 5B and S5). We observed a small but significant increase of nuclear YAP in early folds, compared with non-folded parts, but older folds rather showed a small but significant decrease of nuclear YAP (Figure 5B). These findings suggest that YAP is involved in triggering entry into mitosis of folding cells. These results also match the average movements of dividing cells in the radial direction during folding (Figures 4D and 4E).
Given that YAP regulation occurs early while pressure accumulates over several days, we posited that inhibition of cell-cycle progression at long times scales may be mediated by β-catenin (Benham-Pyle et al., 2015, 2016). In fact, we found that YAP phosphorylation increases over time, indicating YAP inactivation over time, while levels of c-Myc also increase in time (Figure 5C), indicating that β-catenin is activated later. To test this functionally, we encapsulated cells in 2.5% capsules (Figure 5D) and treated them with iCRT3, an inhibitor of β-catenin/TCF interactions. Treatment with iCRT3 did not slow down growth immediately compared with control monolayers (Figure 5E), but growth reached steady state much earlier (50 instead of 70–80 h). Therefore, at steady state, cell density was lower compared with non-treated capsules. This result suggests that inhibition of β-catenin/TCF lowers the threshold pressure at which cells stop dividing. We, however, observed that in iCRT3 treatment, E-cadherin staining was slightly disrupted, probably interfering with junctional mechano-sensing (Figure S5).
To test this further, we quantified pressure accumulation (Figure 5F) and the FDCs with time (Figure 5G). Consistent with proliferation reaching steady state faster, pressure accumulation was much slower in iCRT3-treated capsules Figure 5F). Accordingly, the FDCs initially increased like in control conditions until iCRT3 treatment, after which the FDCs showed a steeper, but limited, decrease (Figure 5G). After this iCRT3-induced initial decrease, the FDCs decreased continuously and faster than control conditions, consistent with the slower pressure accumulation (Figure 5F). When plotting the FDCs as a function of pressure, a dramatic difference is observed compared with non-treated cells, with the lowest values of the FDCs not even reaching 1.4 kPa (Figure 5H). This supports that β-catenin transcriptional activity is required to set the threshold pressure.
We next sought to investigate whether promoting β-catenin activity at threshold pressure could affect the pressure accumulation response. We encapsulated FUCCI cells in 2.5% capsules and let them grow for 60–70 h cells, when most cells are in G1-G0 phases and capsules have reached threshold pressure. We then treated them with 6-bromoindirubin-3′-oxime (BIO), a GSK3 inhibitor that blocks β-catenin degradation (Figure 5I), and checked that BIO transiently activated the β-catenin pathway 1 h after treatment, as seen from the increase in c-Myc (Figure S5). We monitored monolayer growth (Figure 5J) and found that both cell number and the FDCs increased upon BIO treatment. Altogether, these findings support that increased β-catenin transcriptional activity is required to sustain cell-cycle progression under high pressure.
Discussion
While the link between mechanical tension and compression with cell division is clear (Assoian and Klein, 2008; Huang and Ingber, 1999; Uroz et al., 2018), it is hard to quantify this link. In this study, we show that cell-cycle progression is regulated by compressive stresses arising from tissue growth under confinement. We find that cell-cycle progression halts past a threshold pressure of ∼1.8 kPa. This is surprising considering that previous studies on cells with no contact inhibition—such as cancer cells—showed that even if pressure lowers proliferation, it never completely inhibits it (Alessandri et al., 2013; Dolega et al., 2017). Our work suggests that cells sense pressure and control proliferation through “pressure inhibition of proliferation” rather than only upon contact. This response is directly altered if we inhibit or over-activate β-catenin (Benham-Pyle et al., 2015, 2016).
In fact, previous studies showed that mechanical stretch activates mitosis via β-catenin-mediated transcription (Benham-Pyle et al., 2016). This could explain why we observe a reactivation of proliferation upon transient over-activation of β-catenin. Furthermore, inhibition of β-catenin-mediated transcription with iCRT3 blocked the activation of mitosis upon stretch, consistent with our data that iCRT3 reduces the threshold pressure. These results are consistent with the notion that growth pressure sensing occurs via the β-catenin pathway.
Another essential pathway for CIP is the YAP/TAZ pathway. In agreement with previous findings (Aragona et al., 2013; Dupont et al., 2011; Furukawa et al., 2017; Zhao et al., 2007), we find that YAP nuclear localization changes upon confluency, at a time when cells change their AR and polarization.
We also find a spatial regulation of cell-cycle dynamics within folds and across the tissue. Within epithelial folds, the FDCs transiently increases. In our system, however, it is hard to postulate whether convex curvature promotes cell-cycle progression or if it is rather a compressive stress release that transiently re-activates cell-cycle progression, but these two scenarios are also not mutually exclusive. That lower compressive stress may maintain cell-cycle progression is also supported by the observation that dividing cells are only found at tissue boundaries in late stages of growth within capsules, an observation also made for confined cancer cells (Delarue et al., 2014; Dolega et al., 2017). However, the striking correlation we observed between transient cell-cycle re-activation and the YAP/TAZ pathway in forming folds suggests that β-catenin may not be involved here but rather that YAP/TAZ intervenes in sensing local, rapid mechanical changes in cells.
In contrast to cell-cycle progression, cell AR dynamics depend on subtle changes of substrate rigidity. More precisely, cell AR is higher at higher pressures, but stiffer substrates allow cells to change their ARs to higher values and faster compared with softer substrates. This change strongly depends on actomyosin contractility.
Thus, cells may first reduce pressure accumulation via AR changes (through contractility), this transition being under YAP/TAZ control. The balance between contractility (cell AR) and substrate adhesion will determine if the tissue adapts to confinement. When proliferation continues under confinement, this balance determines whether folding or detachment of the tissue occurs (Trushko et al., 2020). When pressure becomes too high, the cell AR cannot adapt further, and proliferation is only sustained by β-catenin activity. Thus, β-catenin would be involved in long-term, global sensing of mechanical forces, while YAP/TAZ would be involved in rapid, local changes of the mechanical environment. Overall, we propose that epithelia growing under confinement can adapt to increasing pressure by initially changing their AR through contractility, then folding if detachment from the substrate is allowed, and, finally, inhibiting proliferation for pressure values >1.8 kPa, as proposed recently (Delarue et al., 2014).
Limitations of the study
Using encapsulated epithelial tissues, we quantify lateral compressive stresses arising from tissue growth. We conclude that pressure regulates cell-cycle progression independently of capsule rigidity, yet we can only conclude that this is independent at rigidities between approximately 30 and 60 kPa. It would therefore be interesting to expand this to a larger range of physiologically relevant substrate rigidities. Similarly, a deeper understanding of the role of YAP in our system would require assessing its temporal dynamics live. Importantly, higher time resolution would also provide more insight into the role of YAP before, during, and after folding from buckling. Finally, it would also be interesting to assess pressure inhibition of proliferation using other epithelial model systems.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-YAP | Cell Signalling | Cat#14074S, RRID:AB_2650491 |
| Anti-E-cadherin | BD Biosciences | Cat# 610181, RRID:AB_39758 |
| Donkey anti-mouse, AlexaFluor 647 | Thermo Fisher Scientific | Cat# A32787; RRID:AB_2762830 |
| Donkey anti-rabbit, AlexaFluor 647 | Thermo Fisher Scientific | Cat# A32795; RRID:AB_2762835 |
| MEM | Thermo Fisher Scientific | Cat# 51200087) |
| Anti-GAPDH | Thermo Fisher Scientific | Cat# MA5-15738; RRID:AB_10977387 |
| anti-c-Myc | Santa Cruz Biotechnology | Cat# sc-40; RRID:AB_627268 |
| anti-Phospho-YAP (Ser127) | Cell Signaling Technology | Cat# 4911; RRID:AB_2218913 |
| Sheep anti-mouse IgG, HRP secondary antibody | Thermo Fisher Scientific | Cat# 10106134; RRID:AB_772193 |
| Donkey anti-rabbit IgG, HRP secondary antibody | Thermo Fisher Scientific | Cat# 10710965;RRID:AB_772191 |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM | Invitrogen | Cat# 10566016 |
| FBS | Thermo Fisher Scientific | Cat# 10270106) |
| Penicillin-Streptomycin | Gibco | Cat# 15070063 |
| Non-Essential Amino Acids (NEAA) 100X | Invitrogen | Cat# 11140050 |
| ATTO647N-amine | ATTO-TEC | Cat# AD647N-95 |
| Alginate | FMS BioPolymer | Protanal LF200FTS, |
| DMSO (anhydrous) | Invitrogen | Cat# D12345 |
| sulfo-NHS | Sigma-Aldrich | Cat# 56485 |
| EDC | Sigma-Aldrich | Cat# 03449 |
| SDS | Sigma-Aldrich | Cat# 428018 |
| Paraformaldehyde (PFA) | Sigma-Aldrich | F8775 |
| Glycine | Sigma-Aldrich | G8898 |
| Triton X-100 | Applichem | Ref. A1388 |
| Phalloidin488 | Thermo Fisher Scientific | A12379 |
| Hoechst 33342 | Invitrogen | H3570 |
| EDTA | Sigma-Aldrich | E7889 |
| Halt phosphatase inhibitor cocktail | Life Technologies | 78428 |
| Halt protease inhibitor cocktail | Life Technologies | 78425 |
| WesternBright ECL | Advansta | K-12045 |
| Blebbistatin | Sigma-Aldrich | Cat# B0560 |
| iCRT3 | Sigma-Aldrich | SML0211 |
| GSK3 inhibitor 6-bromoindirubin-3-oxime (BIO) | Sigma-Aldrich | Cat# B1686 |
| Low-melting agarose | Sigma-Aldrich | 49414 |
| Alginate lyase | Sigma-Aldrich | A1603 |
| PDMS (polydimethylsiloxane) | Dow Corning | Sylgard 184 silicone elastomer kit |
| Fibronectin | Gibco | Cat# 33016015 |
| Fish Gelatin | Sigma-Aldrich | Cat# G7041 |
| PBS | Gibco | Cat# 18912014 |
| Matrigel matrix | Corning | 354230 |
| Deposited data | ||
| Raw and analysed data | Mendeley Data | https://doi.org/10.17632/r2fb355wks.1 |
| Experimental models: Cell lines | ||
| Madin-Darby Canine Kidney II (MDCK-II) | ECACC | Cat# 00062107; RRID:CVCL_0424 |
| MDCK FUCCI | A gift from Prof. Lars Hufnagel (EMBL Heidelberg, Germany). | N/A |
| MDCK Myr-Palm-GFP H2B-mCherry | Trushko et al., 2020 | N/A |
| Software and algorithms | ||
| Imaris 8.4.2 | Bitplane | RRID:SCR_007370 |
| Matlab | MathWorks | RRID:SCR_001622 |
| Prism 7 | GraphPad Prism | RRID:SCR_002798 |
| Fiji | Fiji | RRID:SCR_002285) |
| Solidworks | Dassault Systèmes | N/A |
| Illustrator CS6 | Adobe | N/A |
| PRIMO | Alveole | N/A |
| Other | ||
| 3D printer Micro Hi-Res Plus | EnvisionTEC | N/A |
| 3D printer resin | EnvisionTEC | HTM140V2 |
| Loctite EA M-31 CL | Henkel | N/A |
| Dremler 8000 | Dremler | F0138000JF |
| Dremler Workstation (220) | Dremler | 26150220AA |
| Pumps neMESYS (low pressure module V2) | Cetoni | N/A |
| Copper ring, 21 mm OD | RadioSpare (RS) | Cat# 367-6177 |
| High Voltage DC Power Supply | Stanford Research | PS350 |
| Slide-A-Lyzer™ Dialysis cassette 10K 12-30mL capacity | Thermo Fisher | Cat# 66456 |
| Acrodisc Syringe Filter with Glass Fiber – 1um | Pall | AP-4529 |
| Microscope LSM780 | Carl Zeiss | N/A |
| Objective Plan-Apochromat 20x/0.8 M27 | Carl Zeiss | 420650-9902-000 |
| Microscope LSM710 | Carl Zeiss | N/A |
| Objective W Plan-Apochromat 20x/1.0 DIC M27 75mm | Carl Zeiss | 421452-9800-000 |
| Nikon Eclipse Ti-2 | Nikon | N/A |
| 4-20% polyacrylamide gel | Eurogentec | Ref. ID-PA4201 |
| Glass-bottom dishes | Mattek | Cat# P35G-1.0-14-C |
| Syringe needles | Terumo | AN-1925R1 |
| Nitrocellulose membrane | Life Technologies | ref. IB23001 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Aurélien Roux (aurelien.roux@unige.ch).
Materials availability
This study did not generate any new unique reagents.
Experimental model and subject details
Cell culture and stable cell line generation
Madin-Darby Canine Kidney II (MDCK-II) (ECACC, Cat. No. 00062107), MDCK FUCCI, and MDCK Myr-Palm-GFP cells were maintained in DMEM (Invitrogen, cat. no. 10566016) supplemented with 10% (vol/vol) FBS (Thermo Fisher, Cat. No. 10270106), 1% (vol/vol) Penicillin-Streptomycin (Gibco BRL), and 1% (vol/vol) non-essential amino acids (NEAA) 100X (Invitrogen, ref. 11140050) in cell culture flasks (Falcon) at 37°C and 5% CO2. Encapsulated MDCK monolayers were maintained under the same conditions as above and for live imaging transferred to MEM (Thermo Fisher, cat. no. 51200087) supplemented with 10% (vol/vol) FBS (Thermo Fisher, Cat. No. 10270106), 1% (vol/vol) Penicillin-Streptomycin (Gibco BRL), and 1% (vol/vol) non-essential amino acids (NEAA) 100X (Invitrogen, ref. 11140050) for image acquisition. Cell lines were regularly tested negative for contamination with mycoplasma.
Method details
Microfluidic device fabrication
The microfluidic device (MD) was printed with the 3D printer EnvisionTEC Micro Hi-Res Plus, using the resin HTM140V2 (EnvisionTEC), with expected Z resolution of 25μm. The following printing parameters (set automatically based on the resin) were used: burn-in range thickness 400 μm, base plate of 300 μm, and exposure time 3000 ms. The printer light intensity
is 225 mW/cm. The printed device was washed using ethanol and air dried using an air gun. A thin layer of PDMS (polydimethylsiloxane) at a ratio of 1:10 (curing agent: elastomer) was put on the cone of the chip with the help of a syringe needle and baked at 70°C for 30 min and subsequently baked using a UV chamber for 10 min. To ensure hydrophobicity and reduce the diameter of the device tip, a glass capillary (100 μm ID) was added to the tip of the chip. The capillary was pulled and cut to obtain a tip of around 200-300 μm in length and 100 μm in inner diameter and glued on the tip of the microfluidic device with epoxyglue EA M-31CL (Loctite) and left to solidify for 1h at RT. To make the inlets, three 19-gauge stainless steel needles (Terumo, ref.AN-1925R1) were cut into segments 1.5cm long and polished to avoid sharp edges. A small droplet of glue EA M-31CL was spread at the edge of the needles and they were inserted into the inlets of the devices, the glue was then left to solidify for 24 h at RT.
Microfluidic device operation and cell encapsulations
The working principle of the microfluidic device is explained in detail in [27]. In brief, the system is composed of three syringes connected to a pump (Nemesys) for flow rate control, a Matrigel cooling part, the AL-charging part system and the microfluidic device. The MD consists of three coaxial cones inside which three different solutions are injected from the syringes. The outermost cone contains the alginate solution (AL), the intermediate cone contains 300mM sorbitol solution (IS) and the innermost cone contains cells/Matrigel/sorbitol solution (CS) in a ratio of 2:1:2 (v/v), with a cell number in the range of 5x10. The AL and IS
solutions are loaded into two syringes controlled by the pumps for injection into the MD. The CS is injected into a cooling part to maintain Matrigel liquid, and this part is connected to a third syringe containing sorbitol that pushes out CS into the MD. The volume of Matrigel (Corning, Ref. 354230) used was optimized to a total protein concentration of 0.2mg (optimized to ensure formation of a 3- 4μm thick layer of Matrigel). The flow rates are set to 45 mL/h, 40 mL/h and 35 mL/h for AL, IS and CS, respectively, ensuring droplet formation upon exiting the MD. Once connected to the pumps the MD is positioned 50-60 cm above the petri dish with a 100mM CaCl2 solution for collection of capsules. The alginate charging part and copper ring, both connected to a high voltage (2000V) generator, are used to improve capsule shape and monodispersity of size. The alginate charging part is a glass T connector that on opposite sides of the T has a high voltage wire (coming from the generator) and a tubing containing AL that flows down the T. The HV wire is coupled to a silver wire (OD 1 mm) that crosses the T such that it is in contact with the alginate and charges the solution, after which the charged AL then flows into the MD. The copper ring is held below the tip of the MD at a distance of about 1cm and centered with respect to the MD tip. The charged formed droplets passing through the copper ring under electrical tension get deflected as they cross the ring, creating a shower-like jet that prevents capsule merging. After 30 min in the calcium bath, capsules are washed and transferred to cell culture medium.
Preparation of alginate solutions
Labelling of 1% alginate solution with ATTO647N-amine (ATTO-TEC, ref. AD647N-95): 0.25 g Alginate (Protanal LF200FTS, FMS BioPolymer) was dissolved in 25mL 0.1M MES pH 6.0. Next, 5mg ATTO647N-amine dissolved in 200μL DMSO (anhydrous), was added into the tube and mixed, rotating for 30 min. Next, 21.5 mg sulfo-NHS (Sigma, ref. 56485) dissolved in 200μL of 0.1 M MES pH 6.0 was added and let mix for 30min. Finally, 24mg EDC (Sigma, ref.03449) dissolved in 200ul of 0.1M MES pH 6.0 was added and let mix and react overnight at RT. The labeled alginate solution was transferred to a Slide-A- Lyzer™cassette 10K 12-20 mL capacity and let dialyze in miliQ for 2 h, changing milliQ after the 2h and let to dialyze overnight. After dialysis, the labeled alginate was filtered (Acrodisc 25mm Syringe filter with 1um glass finer media, Pall, Life Science). The final concentration of the ATTO647N-labelled alginate was 0.55%, the solution was stored at 4°C. For preparation of 2.5%, 2%, or 1.5% ATTO647N-labeled alginate solutions, 0.27g, 0.165g, 0.22g of alginate powder is mixed with 10 mL milliQ water, respectively. Then, 1mL 0.5% ATTO647N alginate and 10 μl SDS 20% solution (Sigma, ref. 428018) are added and the mixture is left to rotate overnight at RT. Before use the solutions were spun down at 19,000 rpm for 30 min at 20°C, after which alginate was filtered with a glass fiber filter before use.
Micro-patterningMicropatterns (200μm in diameter) were generated using the system PRIMO (Alvéole), mounted on an inverted microscope Nikon Eclipse Ti-2, via a UV-activated mPEG-scission reaction as described in [47]. After rinsing with PBS, the PEG-free PLL motifs were coated with Fibronectin (Sigma) at 50ug/mL for 5 min and then excess fibronectin was washed out. PBS was replaced with medium and MDCK cells were added. Samples were kept at 37°C and 5% CO2. After 10-15 min, non-adherent MDCK FUCCI cells were washed out. Cells were left to grow to over-confluence for about 4–5 days before fixation.
Immunofluorescence
Cell monolayers inside capsules were fixed with 4% (v/v) paraformaldehyde (PFA) (Sigma, ref. F8775) in MEM (not PBS, to avoid dissolving alginate capsule) for 30 min at RT, then permeabilized and dissolved in 100 mM Glycine (Sigma, ref. G8898), 0.1% Triton X-100 (Applichem, ref. A1388) and 1% Gelatin (Sigma, ref. G7765) in 1X PBS for 30 min at RT. Cells were then incubated with primary antibody diluted in 1X PBS with 1% Gelatin overnight at 4°C, then washed, and further incubated with secondary antibodies (1:1000) diluted in 1X PBS with 1% Gelatin for 1 h at RT. Primary antibodies used for immunofluorescence staining were: rabbit anti-YAP (Cell Signalling, ref. 14074S) and mouse anti-E-Cadherin (BD, ref. 610181). When necessary, monolayers were also counterstained for f-actin and nuclei using Phalloidin488 (1:40, AlexaFluor488, Thermo Fisher, ref. A12379) and Hoechst 33342 (1:1000, Invitrogen, ref. H3570), respectively. Samples were rinsed 3X with 1X PBS.
Western blots
Western blotting was implemented following standard procedures. Capsules were first dissolved in 1X PBS with 0.5mM EDTA (Sigma-Aldrich, ref. E7889) for 5 minutes rotating at 4°C and centrifuged at 100xg for 5 minutes to collect cells. Cells in the pellet were lysed using RIPA buffer + Halt phosphatase inhibitor cocktail (Life Technologies, ref. 78428) and protease inhibitor cocktail (Life Technologies, ref. 78425). Samples were denatured and then lysates were loaded on 4-20% polyacrylamide gels (Eurogentec, Ref. ID-PA4201) and transferred onto a nitrocellulose membrane (Life Technologies, ref. IB23001). After blocking, membranes were incubated with primary antibody at 4°C overnight and then for 45 minutes at RT with horseradish-peroxidase (HRP)-conjugated secondary antibody. Western blot analysis was performed using WesternBright ECL (Advansta, ref. K-12045). The intensity of protein bands was analyzed using Fiji. Primary antibodies used were monoclonal mouse anti-α-tubulin (Sigma-Aldrich, ref. T9026), monoclonal mouse anti-GAPDH (Thermo Fisher, ref. MA5-15738), mouse monoclonal anti-c-Myc (Santa Cruz, ref. sc-40), rabbit polyclonal against phosphorylated (Ser127) YAP (Cell Signaling, Ref. 4911S).
Inhibitor studies
For contractility inhibition experiments, Blebbistatin (Sigma. ref. B0560) was used at a final concentration of either 10 μM or 50 μM (always freshly thawed from −20°C). Capsules were selected and pre-treated for 1 h with Blebbistatin at 37°C and 5% CO2, after which they were embedded and imaged. For β-catenin-inhibition experiments, iCRT3 (Sigma-Aldrich, SML0211) was used to inhibit β-catenin/TCF interactions and transcriptional activity. iCRT3 was added 15 h after imaging start (AIS) at a final concentration of 25μM and fresh inhibitor added every 24 h until the end of the experiment. For activation of β-catenin activity, 100nM of the GSK3 inhibitor 6-bromoindirubin-3-oxime (BIO) (Sigma-Aldrich, B1686) was added at approximately 60h after imaging start (AIS) (equivalent to 84hpe).
Image acquisition
Capsules were first selected 24 hours post-encapsulation and embedded in 0.4% low-melting agarose (0.04 g in 10 mL) (Sigma, 49414) in a 35 mm glass-bottom dish (Mattek, Part No. P35G-1.0-14-C) to maintain them in the same position for several days of time-lapse imaging. The agarose was left to solidify for 15 min and 2-3mL of medium were added for imaging.
For live fluorescence imaging, confocal images of samples were obtained using an inverted LSM780 NLO microscope (Carl Zeiss) using air objective 20× W Plan-APOCHROMAT 20x/1.0 DIC (UV) VIS-IR (Zeiss, 421452-9800). During imaging, capsules were maintained at 37 with 5% CO2. For each capsule, 3D confocal Z-stacks were acquired with 1μm intervals, and each capsule was imaged every 2.5-3 h for 25-40 cycles using definite focus (autofocus). For fluorescence imaging of fixed samples, images were acquired using the upright microscope LSM710 NLO 2-photon using an air objective 20X Plan-Apochromat 20x/0.8 M27 (Zeiss, 420650-9902-000).
Capsule dissolution with alginate lyase
For experiments assessing FUCCI response to capsule dissolution, FUCCI cell monolayers in capsules were embedded in 0.4% agarose for imaging in a 35 mm glass-bottom dish. The Mattek dish was manually modified by punching a hole on the side of the dish with a 19G hot needle to introduce teflon tubing into the dish sterilely for alginate lyase (Sigma-Aldrich, ref. A1603) injection. Once the Mattek dish was positioned onto the inverted LSM780 NLO microscope (Carl Zeiss) (section Imaging), 100 μL PBS alginate lyase solution (1 unit per 10 μL of PBS alginate lyase) was added both after 1min of imaging. The imaging was performed was in section Imaging. The imaging of capsules was performed with time intervals of 30 min for 8 h and with definite focus (autofocus).
Capsule stiffness (Young modulus, E) measurements
Indentation measurements for empty (without encapsulated cells) alginate capsules of concentrations 1.5%, 2% and 2.5% were performed as described in (Trushko et al., 2020).
Quantification and statistical analysis
Capsule pressure calculated from capsule deformations To measure pressure accumulation, confocal images of ATTO647-labelled alginate capsules were converted into binary black-white image masks using thresholding on Fiji (ImageJ). These masks were used to quantify pressure accumulation for encapsulated MDCK FUCCI cells as already described in (Trushko et al., 2020).
Image segmentation and processing
The Imaris software (Bitplane) was used for 3D image visualization and nuclei segmentation from time-lapse confocal images. For quantification of MDCK FUCCI cell number and of S-G2-M and G1/G0 phase cells, Imaris software was used to segment nuclei and quantify intensity per channel (red and green individually). A custom-written MATLAB script was used to obtain cell number per phase and fraction of cells in either S-G2-M and G1 phase cells over time. In case a nucleus was identified in both channels (at the G1-S transition), it was automatically assigned to S phase. For each capsule, we then set t0 to time at which cell density was ∼2 cells/1000μm2 (Figure S2), a value consistent with proliferating MDCK epithelia (Streichan et al., 2014; Uroz et al., 2018). For radial distribution analysis, a custom-written MATLAB script was used to define capsule centre and distance of nuclei from centre. For Yap1 analysis, nuclear segmentation was performed with Imaris software using Hoechst staining followed by pixel intensity calculation for each nucleus in the Yap1 channel. Using this method, cytoplasmic and plasma membrane staining were excluded, and the obtained nuclear pixel intensity of Yap1 was used to calculate a ratio of folds over non-folded regions. For cell aspect ratio measurements, the width and height of 5–20 cells were measured from fluorescence images of the equatorial plane of 10–20 capsules per time point and per condition.
Statistical analysis
All statistical analyses for experimental data were performed using Prism 7 (GraphPad Software). Statistics comparing two populations used two-tailed Student’s t-test with Welch’s correction and with analysis of variance (ANOVA) tests when more cases were analyzed. Results were considered statistically significant for p values below 0.05. Data are represented as mean ± SD (Standard Deviation) unless stated otherwise. Sample sizes and differences that were not statistically significant (ns) are specified in figure legends.
Acknowledgments
The authors thank Karsten Kruse for his useful insights into the project. A.R. acknowledges funding from Human Frontier Science Program Young Investigator grant RGY0076/2009-C, the Swiss National Fund for Research grants N°31003A_149975, N°31003A_173087, N°310030_200793, and N°CRSII5_189996, and the European Research Council Consolidator grant N° 311536. I.D.M. and A.R. acknowledge funding from Secrétariat d’Etat à la Recherche et à l’Innovation grant agreement REF-1131-52107. I.D.M. and A.R. acknowledge funding from the EU Horizon2020 Marie Sklodowska-Curie ITN “BIOPOL” (grant agreement no. 641639). P.G. acknowledges support from the Human Frontiers of Science Program (grant number LT-000793/2018-C).
Author contributions
I.D.M. and A.R. designed the project; I.D.M. performed all experiments and image analyses, except micropatterning experiments; A.T. helped with cell encapsulations, 4D confocal imaging, and image analysis; P.G. performed 2D adhesive pattern experiments and analysis; C.B.-M. helped with image analysis; S.A. performed atomic force microscopy (AFM) measurements of capsules’ Young’s moduli. I.D.M. and A.R. analyzed results and wrote the paper, with editions from other co-authors.
Declaration of interests
No competing interests stated.
Published: August 23, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111227.
Supplemental information
Data and code availability
-
•
All raw data related to each figure have been deposited at Mendeley data (https://doi.org/10.17632/r2fb355wks.1) and will be publicly available as of the date of publication.
-
•
This paper does not report original code
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request
References
- Alessandri K., Feyeux M., Gurchenkov B., Delgado C., Trushko A., Krause K.H., Vignjevic D., Nassoy P., Roux A. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC) Lab Chip. 2016;16:1593–1604. doi: 10.1039/c6lc00133e. [DOI] [PubMed] [Google Scholar]
- Alessandri K., Sarangi B.R., Gurchenkov V.V., Sinha B., Kiessling T.R., Fetler L., Rico F., Scheuring S., Lamaze C., Simon A., et al. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. USA. 2013;110:14843–14848. doi: 10.1073/pnas.1309482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Assoian R.K., Klein E.A. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle B.W., Pruitt B.L., Nelson W.J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle B.W., Sim J.Y., Hart K.C., Pruitt B.L., Nelson W.J. Increasing beta-catenin/Wnt3A activity levels drive mechanical strain-induced cell cycle progression through mitosis. Elife. 2016;5 doi: 10.7554/eLife.19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campas O. A toolbox to explore the mechanics of living embryonic tissues. Semin. Cell Dev. Biol. 2016;55:119–130. doi: 10.1016/j.semcdb.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.S., Mrksich M., Huang S., Whitesides G.M., Ingber D.E. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Delarue M., Montel F., Vignjevic D., Prost J., Joanny J.F., Cappello G. Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys. J. 2014;107:1821–1828. doi: 10.1016/j.bpj.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz de la Loza M.C., Thompson B.J. Forces shaping the Drosophila wing. Mech. Dev. 2017;144:23–32. doi: 10.1016/j.mod.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Dolega M.E., Delarue M., Ingremeau F., Prost J., Delon A., Cappello G. Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression. Nat. Commun. 2017;8:14056. doi: 10.1038/ncomms14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S.A., Gayyed M.F., Anders R.A., Maitra A., Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez M.E., Barbier S., Whitehead J., Bealle G., Michel A., Latorre-Ossa H., Rey C., Fouassier L., Claperon A., Brulle L., et al. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature. 2015;523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- Furukawa K.T., Yamashita K., Sakurai N., Ohno S. The epithelial circumferential actin belt regulates YAP/TAZ through nucleocytoplasmic shuttling of merlin. Cell Rep. 2017;20:1435–1447. doi: 10.1016/j.celrep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Gudipaty S.A., Lindblom J., Loftus P.D., Redd M.J., Edes K., Davey C.F., Krishnegowda V., Rosenblatt J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543:118–121. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofemeier A.D., Limon T., Muenker T.M., Wallmeyer B., Jurado A., Afshar M.E., Ebrahimi M., Tsukanov R., Oleksiievets N., Enderlein J., et al. Global and local tension measurements in biomimetic skeletal muscle tissues reveals early mechanical homeostasis. Elife. 2021;10 doi: 10.7554/eLife.60145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Chen C.S., Ingber D.E. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Ingber D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Asthagiri A.R. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J. Cell Sci. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoff L., Rouault H., Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- Luciano M., Xue S.-L., De Vos W.H., Morata L.R., Surin M., Lafont F., Hannezo E., Gabriele S. Large-scale curvature sensing by epithelial monolayers depends on active cell mechanics and nuclear mechanoadaptation. bioRxiv. 2020;2007:187468. doi: 10.1101/2020.07.04.187468. Preprint at. [DOI] [Google Scholar]
- Martin-Belmonte F., Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer. 2011;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- Mongera A., Rowghanian P., Gustafson H.J., Shelton E., Kealhofer D.A., Carn E.K., Serwane F., Lucio A.A., Giammona J., Campas O. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature. 2018;561:401–405. doi: 10.1038/s41586-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.M., Jean R.P., Tan J.L., Liu W.F., Sniadecki N.J., Spector A.A., Chen C.S. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor-Bergmann A., Goddard G., Woolner S., Jensen O.E. Relating cell shape and mechanical stress in a spatially disordered epithelium using a vertex-based model. Math. Med. Biol. 2018;35:1–27. doi: 10.1093/imammb/dqx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S., Dupont S., Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- Puliafito A., Hufnagel L., Neveu P., Streichan S., Sigal A., Fygenson D.K., Shraiman B.I. Collective and single cell behavior in epithelial contact inhibition. Proc. Natl. Acad. Sci. U. S. A. 2012;109:739–744. doi: 10.1073/pnas.1007809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A., Kurokawa H., Morimura T., Hanyu A., Hama H., Osawa H., Kashiwagi S., Fukami K., Miyata T., Miyoshi H., et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Savin T., Kurpios N.A., Shyer A.E., Florescu P., Liang H., Mahadevan L., Tabin C.J. On the growth and form of the gut. Nature. 2011;476:57–62. doi: 10.1038/nature10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraiman B.I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyer A.E., Tallinen T., Nerurkar N.L., Wei Z., Gil E.S., Kaplan D.L., Tabin C.J., Mahadevan L. Villification: how the gut gets its villi. Science. 2013;342:212–218. doi: 10.1126/science.1238842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg K., Rossi B., Lazdunski M., Louvard D. Characterization of ouabain-resistant mutants of a canine kidney cell line, MDCK. J. Biol. Chem. 1983;258:12300–12307. [PubMed] [Google Scholar]
- Streichan S.J., Hoerner C.R., Schneidt T., Holzer D., Hufnagel L. Spatial constraints control cell proliferation in tissues. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5586–5591. doi: 10.1073/pnas.1323016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre C., Perret C., Colnot S. Transcription dynamics in a physiological process: beta-catenin signaling directs liver metabolic zonation. Int. J. Biochem. Cell Biol. 2011;43:271–278. doi: 10.1016/j.biocel.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Tozluoglu M., Duda M., Kirkland N.J., Barrientos R., Burden J.J., Munoz J.J., Mao Y. Planar differential growth rates initiate precise fold positions in complex epithelia. Dev. Cell. 2019;51:299–312.e294. doi: 10.1016/j.devcel.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushko A., Di Meglio I., Merzouki A., Blanch-Mercader C., Abuhattum S., Guck J., Alessandri K., Nassoy P., Kruse K., Chopard B., et al. Buckling of an epithelium growing under spherical confinement. Dev. Cell. 2020;54:655–668. doi: 10.1016/j.devcel.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uroz M., Wistorf S., Serra-Picamal X., Conte V., Sales-Pardo M., Roca-Cusachs P., Guimera R., Trepat X. Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat. Cell Biol. 2018;20:646–654. doi: 10.1038/s41556-018-0107-2. [DOI] [PubMed] [Google Scholar]
- Wada K., Itoga K., Okano T., Yonemura S., Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Wu S.K., Gomez G.A., Michael M., Verma S., Cox H.L., Lefevre J.G., Parton R.G., Hamilton N.A., Neufeld Z., Yap A.S. Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat. Cell Biol. 2014;16:167–178. doi: 10.1038/ncb2900. [DOI] [PubMed] [Google Scholar]
- Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red channel is Cdt1-mKO2 (Kusabira Orange), green channel is geminin-mAG (Azami Green) and cyan is Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
Cyan is Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
Green is Myr-Palm-GFP, magenta is H2B-mCherry, cyan is 1.5% Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
Green is Myr-Palm-GFP, magenta is H2B-mCherry, cyan is 1.5% Alginate labelled with ATTO647. Time-lapse is 2.5 hours. Scale bar, 100 μm.
Data Availability Statement
-
•
All raw data related to each figure have been deposited at Mendeley data (https://doi.org/10.17632/r2fb355wks.1) and will be publicly available as of the date of publication.
-
•
This paper does not report original code
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request