Abstract
The mechanisms by which Legionella pneumophila, a facultative intracellular parasite and the agent of Legionnaires' disease, acquires iron are largely unexplained. Several earlier studies indicated that L. pneumophila does not elaborate siderophores. However, we now present evidence that supernatants from L. pneumophila cultures can contain a nonproteinaceous, high-affinity iron chelator. More specifically, when aerobically grown in a low-iron, chemically defined medium (CDM), L. pneumophila secretes a substance that is reactive in the chrome azurol S (CAS) assay. Importantly, the siderophore-like activity was only observed when the CDM cultures were inoculated to relatively high density with bacteria that had been grown overnight to log or early stationary phase in CDM or buffered yeast extract. Inocula derived from late-stationary-phase cultures, despite ultimately growing, consistently failed to result in the elaboration of siderophore-like activity. The Legionella CAS reactivity was detected in the culture supernatants of the serogroup 1 strains 130b and Philadelphia-1, as well as those from representatives of other serogroups and other Legionella species. The CAS-reactive substance was resistant to boiling and protease treatment and was associated with the <1-kDa supernatant fraction. As would also be expected for a siderophore, the addition of 0.5 or 2.0 μM iron to the cultures repressed the expression of the CAS-reactive substance. Interestingly, the supernatants were negative in the Arnow, Csáky, and Rioux assays, indicating that the Legionella siderophore was not a classic catecholate or hydroxamate and, hence, might have a novel structure. We have designated the L. pneumophila siderophore legiobactin.
The bacterium Legionella pneumophila is a ubiquitous inhabitant of natural and man-made aquatic environments, surviving both free, in biofilms, and as an intracellular parasite of protozoa (1, 8, 26, 49, 50). Yet, this gram-negative microbe is best known for being the principal etiologic agent of Legionnaires' disease, a potentially fatal form of human pneumonia (59, 95). Within the lung, L. pneumophila flourishes as an intracellular parasite of the alveolar macrophages and perhaps the epithelium (1, 8, 13, 41, 63, 84). A variety of studies indicate that iron is a key requirement for L. pneumophila extracellular replication, intracellular infection, and virulence (7, 9, 10, 25, 31, 39, 40, 44, 45, 60, 65, 71, 72, 76, 77, 78, 86, 89, 91). Despite this, the molecular basis of Legionella iron acquisition, particularly its earliest stages, is relatively unclear.
Among all of the considerations regarding Legionella iron acquisition, it is the issue of siderophore production by L. pneumophila that has been the most controversial. In the early 1980s, shortly after the discovery of the Legionella genus, it was reported that L. pneumophila does not produce siderophores (79). This conclusion was based upon the negative results that were obtained from a standard bioassay, as well as the Arnow and Csáky assays, the customary methods for detecting what were then the two known classes of siderophores, i.e., catecholates and hydroxamates. Some 10 years later, the question of Legionella siderophores was revisited using the chrome azurol S (CAS) assay, a procedure which had recently been developed and detects siderophores independently of their structure (83). This study indicated that L. pneumophila supernatants had CAS reactivity, suggesting the existence of a noncatecholate, nonhydroxamate siderophore (34). Curiously, the CAS reactivity was only appreciable in static, as opposed to standard shaking, cultures (34). Our later work, however, determined that the CAS reactivity in the static cultures was simply the medium's cysteine, which was somehow maintained by the slow-growing legionellae (54). When the CAS assay was repeated using supernatants from fully aerated cultures generated with cysteine-free, defined media, no siderophore activity was detected (54). Using a chemostat, a later study also failed to identify any CAS-, Arnow-, or Csáky-reactive substances in iron-starved L. pneumophila cultures (43). These data, along with the belief that L. pneumophila survives much of the time as an intracellular parasite, have promoted the broadly held notion that Legionella does not (need to) produce siderophores (8, 35, 92, 93, 95, 97).
Recently, we identified an iron-regulated L. pneumophila gene that is predicted to encode a protein with striking similarity to hydroxamate synthetases, raising, once again, the possibility that the legionellae elaborate siderophores (40). Interestingly, a mutation in that gene rendered L. pneumophila defective for intracellular but not extracellular growth, suggesting that the putative siderophore functions only within host cells (40). In other studies, we isolated L. pneumophila mutants that are impaired for growth under low-iron extracellular conditions (53, 76, 90). However, while preparing to characterize our various mutants, we discovered that wild-type strains of L. pneumophila could elaborate a bona fide siderophore activity. Here, we describe this ironic turn of events and provide an initial biochemical characterization of the Legionella iron chelator.
(Portions of this work were presented previously [55].)
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
Siderophore activity was first assayed for using the well-studied L. pneumophila serogroup 1 strains 130b and Philadelphia-1 (24, 59). The three serogroup 1 mutants, NU229, JR32, and JR32-1, that were later examined were also described before (40, 96). Other wild-type legionellae that were tested for the production of iron chelators are listed in Table 1. Bacteria were maintained on buffered charcoal yeast extract agar for 3 days at 37°C (23). In preparation for examination of their behavior in low-iron media, bacteria were grown in buffered yeast extract (BYE) broth, the standard liquid medium used to culture L. pneumophila. In order to assess the production of siderophores, legionellae were grown in a chemically defined medium (CDM) that lacked its iron component (79). Deferrated BYE could not be reliably used for this purpose, since it, like other rich media, interfered with the siderophore (i.e., the CAS) assay (83). Briefly, CDM ordinarily consists of the 20 amino acids, nine trace metals in addition to iron, pyruvate, glutathione, α-ketogluturate, morpholinepropanesulfonic acid (MOPS) buffer, KH2PO4, and NaCl (79). All chemicals were purchased from Sigma Chemical Co., St. Louis, Mo.
TABLE 1.
Strains of Legionellaa used in this study
| Species | Serogroup | Strain, source |
|---|---|---|
| L. pneumophila | 1 | 130b, Wadsworth |
| 1 | Philadelphia-1, ATCC 33217 | |
| 1 | Oxford-4032E, ATCC 43110 | |
| 2 | Togus-1, ATCC 33154 | |
| 3 | Bloomington-2, ATCC 33155 | |
| 4 | Los Angeles-1, ATCC 33156 | |
| 5 | Dallas-1E, ATCC 33216 | |
| 6 | Chicago-2, ATCC 33215 | |
| 7 | Chicago-8, ATCC 33823 | |
| 8 | Concord-3, ATCC 35096 | |
| 13 | B2A3105, ATCC 43736 | |
| 14 | 1169-MN-H, ATCC 43703 | |
| L. birminghamensis | 1 | 1407-AL-H, ATCC 43702 |
| L. bozemanii | WIGA, ATCC 33217 | |
| L. brunensis | 441-1, ATCC 43878 | |
| L. micdadei | Tatlock, ATCC 33218 | |
| L. parisiensis | PF-209C-C2, ATCC 35299 | |
| L. wadsworthii | 81-716A, ATCC 33877 |
Siderophore assays.
Supernatants from Legionella cultures to be tested for siderophore activity were typically prepared in the following manner. First, bacteria from buffered charcoal yeast extract agar were suspended in 25 ml of BYE broth, contained within 125-ml flasks, to an optical density at 660 nm (OD660) of approximately 0.1. The cultures were then incubated for different periods of time in an air incubator shaker set at 250 rpm and 37°C, and bacterial growth was assessed by recording the OD660. Next, the cultures were centrifuged for 10 min in a Beckman GPR tabletop centrifuge set at 3,000 rpm (1,620 × g) and room temperature. After removal of the supernatants, the bacteria were suspended in 15 ml of the CDM base buffer (50 mM MOPS [pH 6.5], 2 mM KH2PO4, 50 mM NaCl) and then centrifuged as before. Following an additional wash and centrifugation, the bacteria were finally inoculated to different levels into 20 ml volumes of CDM contained within 125-ml flasks that either lacked an iron supplement or contained various amounts of ferric pyrophosphate. The CDM cultures were incubated in the same manner as the BYE cultures. At different times, 2.2-ml volumes of the cultures were removed, with the first 1 ml being examined in the spectrophotometer and under the microscope to gauge the culture's state of growth and purity, respectively. The remaining 1.2 ml was microcentrifuged at 16,000 × g for 5 min, and then 1.0 ml of the resultant supernatant was carefully removed and tested for the presence of siderophores. Iron-chelating activity was detected within the supernatants (see below) whether or not the samples were passed through 0.2 μm-pore-size sterilizing filters. In a few experiments, the bacteria were grown in CDM broth prior to final inoculation into the iron-depleted CDM assay medium. In those cases, the bacteria were adapted to liquid growth using BYE broth prior to culturing in the defined medium. All supernatants were stored in the refrigerator in polypropylene tubes, with the siderophore activity appearing to be stable for at least 3 months.
To observe siderophore activity in Legionella supernatants, we used the universal CAS assay as previously described (54, 68, 74, 83). To detect certain types of siderophores, the Arnow, Csáky, and Rioux assays were performed (4, 17, 74, 81). For these latter procedures, we increased the size of our cultures and assayed ≥50 ml of supernatant. Desferrioxamine (DFX) was used as the standard for the CAS and Csáky assays (34, 43, 54), while 2,3-dihydroxybenzoic acid served as the standard for the Arnow and Rioux procedures (34, 79, 81). The degrees of iron-chelating activity associated with cysteine, sodium citrate, phosphate (KH2PO4 or NaH2PO4), and salicylate were confirmed using the CAS assay (54, 83, 85). Incidentally, although α-ketoglutarate and pyruvate are in CDM, we have confirmed that these keto acids do not have iron-chelating activity (20, 54).
The approximate size of the CAS-reactive substance that was associated with the L. pneumophila cultures was determined by dialysis. More specifically, supernatants were placed within cellulose ester membrane bags (Spectrum, Houston, Tex.) that had 1-, 3-, and 10-kDa molecular size cutoffs and then dialyzed at 15°C in 2 liters of water that was replaced twice over a 24-h period. To assess the heat and protease susceptibility of the Legionella CAS reactivity, the supernatants were either boiled for 5 to 20 min or exposed to 1 mg of proteinase K per ml for 3 h at 37°C. Finally, to help ascertain the nature of the L. pneumophila siderophore, the supernatants were extracted with ethyl acetate, dichloromethane, or butanol and then reassessed for CAS reactivity (68, 74).
Miscellaneous biochemical analyses.
The concentration of cysteine within L. pneumophila cultures was ascertained using the ninhydrin assay (29, 54), while the level of citrate was determined by the citrate lyase assay (64). A colorimetric detection kit from Sigma Chemical Co. was used to assess the presence of salicylate in the bacterial cultures. Fluorescence associated with L. pneumophila cultures was observed by examining filter-sterilized supernatants with a hand-held UV lamp.
RESULTS
Discovery of CAS reactivity in L. pneumophila supernatants.
While preparing to define the character of putative iron acquisition mutants, we observed that the growth of wild-type serogroup 1 strains in iron-depleted CDM was not consistent. In some experiments, as had been observed previously (54), the bacteria displayed a prolonged lag phase, with peak growth occurring after 2 to 3 days of incubation. In others instances, they replicated with relatively little delay and achieved maximal levels within 1 day. The more rapid growth in low-iron media suggested that the strains might, under certain circumstances, be producing a siderophore. Thus, we tested the supernatants from iron-limited CDM cultures for the presence of a substance that reacts in the CAS assay. Both strains 130b and Philadelphia-1 appeared to produce high levels of CAS reactivity when grown through log phase in shaking, iron-depleted medium (see below). Before embarking upon a biochemical characterization of the L. pneumophila CAS reactivity, we sought to refine our protocol such that the siderophore-like activity was both consistently observed and optimally produced. In the process, we also hoped to reconcile our positive results with past negative reports regarding siderophore production by strains 130b and Philadelphia-1 and others (34, 43, 54, 79).
Effect of inoculum on L. pneumophila CAS reactivity.
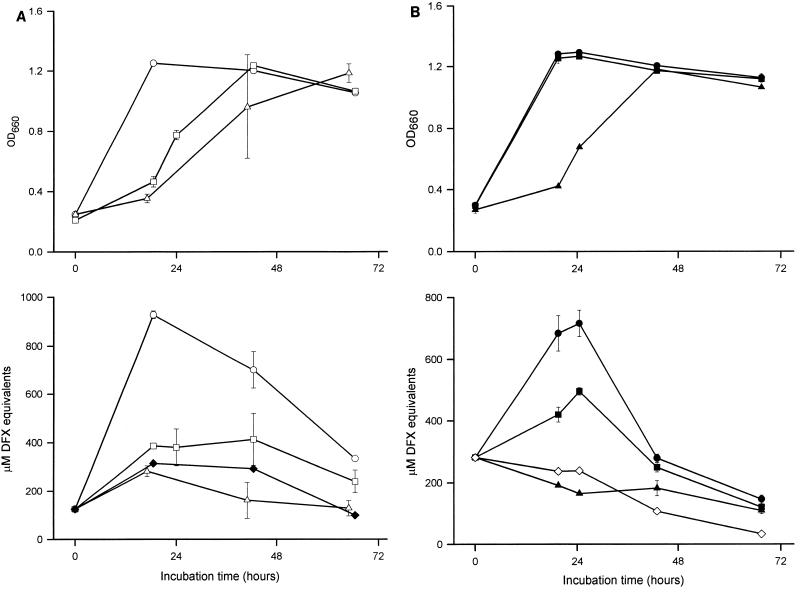
Since all of our recent experiments had used comparably sized inocula derived from BYE cultures, we first determined whether the stage of growth of the inoculum influences CAS reactivity. Thus, we inoculated CDM with bacteria that had been grown for different amounts of time in standard BYE and then, at various times, assayed for multiplication and iron-chelating activity. Strain 130b inocula that were derived from late log to early stationary phase grew well in the CDM and displayed a strong CAS reactivity that was coincident with peak growth (Fig. 1A). In contrast, inocula that were obtained from late-stationary-phase BYE cultures replicated more slowly and, despite ultimately reaching a level of growth that was comparable to that of the other CDM cultures, never exhibited a CAS reactivity that was above that of the medium control (Fig. 1A). In subsequent experiments, we observed that 130b inocula originating from early- to mid-log BYE cultures also elicited a positive CAS reaction (Fig. 1B). A strikingly similar result was obtained with strain Philadelphia-1 (data not shown). In all cases, the amount of CAS-reactive substance in the cultures, for some unknown reason, declined over time (Fig. 1, and below). The inability of the late-stationary-phase inocula to grow within the first 24 h was not unexpected; i.e., they probably contained fewer viable bacteria than did the inocula taken from earlier stages of growth. Since the ultimate production of a CAS-reactive substance could have been tightly linked to the overall viability of the starting culture, we examined CDM cultures that had been inoculated with increasing amounts of late-stationary-phase bacteria. As anticipated, rapid growth was achieved in those cultures that had received twice or three times the inoculum level used in the earlier experiments. However, these same cultures did not display any CAS reactivity (data not shown). To determine whether the inability of late-stationary-phase inocula to yield CAS-positive cultures was reversible, late-stationary-phase bacteria were inoculated into BYE, and then, after the culture reached log phase, a portion of it was used to inoculate iron-depleted CDM. In this instance, CAS reactivity was evident in the resultant log-phase CDM culture, suggesting that the late stationary phase does not select for mutant legionellae that are defective for siderophore production but rather induces a phenotypic state that is not immediately conducive to the elaboration of an iron chelator. Taken together, these data confirm that L. pneumophila strains produce high levels of CAS-reactive material when grown in an iron-depleted defined medium. However, the production of that siderophore-like activity was highly dependent upon the stage of growth of the bacteria used to inoculate the medium.
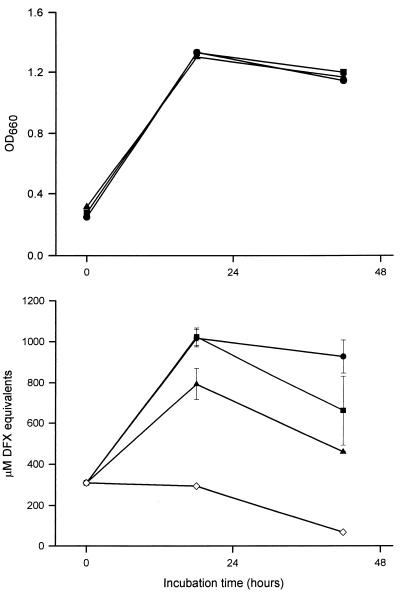
FIG. 1.
Growth in CDM and CAS reactivity of L. pneumophila strain 130b. (A) Bacteria were grown in BYE to an OD660 of either 1.3 (○), 1.7 (□), or 1.9 (▵); washed; and then inoculated into iron-depleted CDM to an OD660 of 0.3. Over the next 3 days, the growth of the CDM cultures was monitored spectrophotometrically (top), and the CAS reactivity of culture supernatants, reported as DFX equivalents, was examined (bottom). (B) Bacteria were grown in BYE to an OD660 of either 0.7 (●), 1.1 (■), or >2.0 (▴); introduced into iron-depleted CDM at an OD660 of 0.3; and then assayed for growth and CAS reactivity. The CAS reactivities contained within CDM blanks are also depicted (⧫) in A, ◊ in B). The values presented represent the means and standard deviations from duplicate cultures.
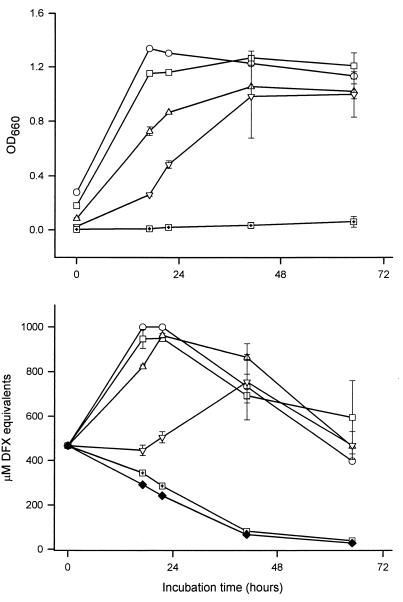
The above-described experiments utilized relatively high numbers of bacteria to inoculate the assay medium; i.e., the initial OD660 values of the CDM cultures were ≥0.3. In contrast, the starting OD660 for earlier studies, when it was noted, was at least threefold lower (34, 54, 79). Therefore, to determine whether the production of CAS-reactive material was also dependent upon the size of the inoculum, we inoculated iron-depleted CDM with decreasing amounts of bacteria and then monitored growth and iron-chelating activity (Fig. 2). To optimize the chances of detecting a CAS reaction, the assay media were inoculated with 130b and Philadelphia-1 bacteria that had been grown to mid- or late-log phase. Cultures initiated at an OD660 of 0.2 had growth and CAS reactivity patterns that were similar to those of cultures started at an OD660 of 0.3. Inocula of 0.1 yielded slower growth but comparable levels of CAS reactivity, whereas inocula of 0.05 were associated with a prolonged lag phase and delayed and diminished chelating activity. Finally, those cultures started at an OD660 of 0.01 either never grew or did so very slowly, yielding very little, if any, CAS-reactive substance. Thus, inoculum size influenced the ability of L. pneumophila to grow within iron-depleted CDM and to ultimately elaborate siderophore-like activity.
FIG. 2.
Effect of inoculum size on L. pneumophila CDM growth and CAS reactivity. Strain 130b bacteria were grown in BYE to late log phase (i.e., an OD660 of 1.3), washed, and then introduced into iron-depleted CDM at an OD660 of either 0.3 (○), 0.2 (□), 0.1 (▵), 0.05 (▿), or 0.01 (□+). Over the next 3 days, the growth of the CDM cultures was monitored spectrophotometrically (top) and the CAS reactivity of culture supernatants was examined (bottom). The CAS reactivity contained within the CDM blank is also depicted (⧫). The values presented represent the means and standard deviations from duplicate cultures. Similar results were obtained with strain Philadelphia-1 (data not shown). Incidentally, the higher starting CAS reactivity of the CDM observed here was not unique to this experiment. Although we do not know its precise basis, it is linked to the age of the medium.
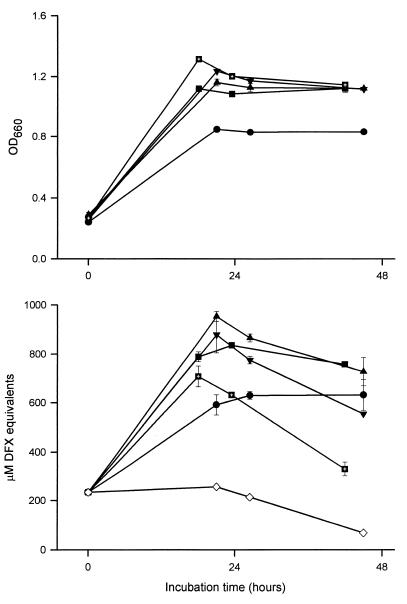
Whereas inocula in previous studies had been grown in either CDM, iron-depleted CDM, or iron- and other-metal-deficient CDM (34, 43, 54, 79), we used inocula derived from standard BYE cultures. Since it seemed possible that medium constituents could affect ultimate CAS reactivity (74), we repeated the basic experiment utilizing inocula that were derived from either BYE or CDM. To control for the amount of iron in the medium, the CDM was initially supplemented with ferric pyrophosphate, as is done for BYE. Strain 130b and Philadelphia-1 cultures started with a CDM inoculum produced just as much CAS reactivity as those initiated with a BYE inoculum. As had been seen with BYE inocula, the ability of CDM inocula to elicit rapid growth and CAS positivity was dependent upon their stage of growth; i.e., late-stationary-phase inocula never yielded a CAS-reactive substance (data not shown). To determine the effect of iron in the inoculum on final CAS reactivity, we started the assay culture using bacteria that had been grown in CDM containing decreasing amounts of ferric pyrophosphate (Fig. 3). As seen earlier, inocula derived from CDM supplemented with 335 μM iron grew very well in the iron-depleted defined medium and yielded a strong reaction in the CAS assay. Cultures that had been initiated with bacteria from CDM containing ca. 100, 40, and 20 μM iron grew slightly slower but had marginally higher CAS reactivity. Finally, inocula taken from iron-depleted CDM promoted the least vigorous replication but still elicited CAS reactivity. These data indicate that the production of a siderophore-like activity by L. pneumophila is not strictly dependent upon the medium used to derive the inoculum but is influenced, to a relatively minor degree, by the amount of iron in that medium.
FIG. 3.
Growth and CAS reactivity of L. pneumophila cultures inoculated with bacteria grown in CDM containing differing amounts of iron. Strain 130b bacteria were grown to late log phase (i.e., an OD660 of 1.0 to 1.1) in CDM supplemented with either 0 (●), 20 (■), 40 (▴), 100 (▾), or 335 ( ) μM ferric pyrophosphate; washed; and then inoculated into iron-depleted CDM to an OD660 of ca. 0.3. Over the next 2 days, the growth of the iron-limited CDM cultures was observed spectrophotometrically (top) and the CAS reactivity of culture supernatants was recorded (bottom). For reference, the CAS reactivity of the CDM blank is included (◊). The values presented represent the means and standard deviations from duplicate cultures.
) μM ferric pyrophosphate; washed; and then inoculated into iron-depleted CDM to an OD660 of ca. 0.3. Over the next 2 days, the growth of the iron-limited CDM cultures was observed spectrophotometrically (top) and the CAS reactivity of culture supernatants was recorded (bottom). For reference, the CAS reactivity of the CDM blank is included (◊). The values presented represent the means and standard deviations from duplicate cultures.
The CAS reactivity in L. pneumophila supernatants behaves like a siderophore.
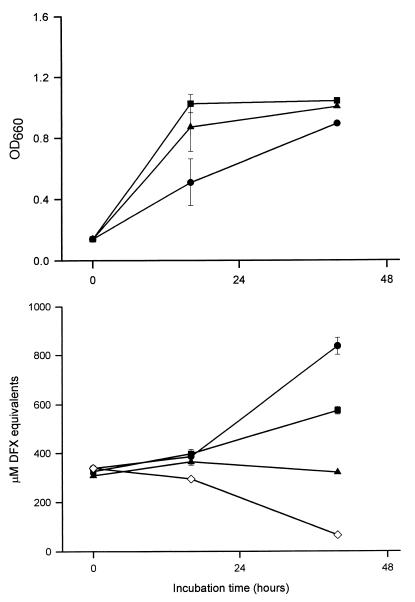
Since the Legionella CAS reactivity correlated with the extent of growth of iron-starved cultures, it appeared to represent a microbial siderophore. To gain support for this hypothesis, we first sought to determine whether the production of the CAS-reactive material, like that of siderophores, is repressed by increased amounts of iron in the assay medium (68, 74). The inclusion of micromolar amounts of the metal diminished the CAS reactivity of Legionella supernatants (Fig. 4). Whereas cultures receiving 0.5 μM iron produced about one-half of the level of CAS reactivity of the 0 μM iron control, those cultures supplemented with 2.0 μM iron did not show any increase in CAS reactivity. To be sure that these losses of reactivity were not due to the ability of added iron to directly reverse or inhibit the reaction between a chelator and the CAS dye, ferric pyrophosphate was added to supernatants that had been derived from iron-depleted cultures and then CAS reactions were performed as usual. In these cases, ≥4.0 μM iron was needed before there was even the slightest reduction in the CAS reactivity of supernatants that initially contained 400 μM or more DFX equivalents. Thus, the L. pneumophila CAS-reactive substance was iron regulated. To roughly ascertain the size of this material, 130b and Philadelphia-1 supernatants were dialyzed against membranes with various exclusion sizes and the retentate was tested for loss of iron-chelating activity. As is often the case for siderophores (68), the Legionella CAS-reactive substance was less than 1 kDa in size. Finally, we observed that the CAS reactivity in 130b and Philadelphia-1 cultures was not diminished by heat or protease treatment, as is commonly seen with siderophores. Taken together, these data indicated that L. pneumophila CAS reactivity is due to an iron-repressed, low-molecular-weight, nonprotein substance.
FIG. 4.
Effect of iron on production of CAS-reactive material. Strain 130b was grown in BYE to an OD660 of 1.0, washed, and inoculated at an OD660 of 0.1 into CDM containing 0 (●), 0.5 (■), or 2.0 (▴) μM ferric pyrophosphate. Over the next 2 days, the growth (top) and CAS reactivity (bottom) of the cultures were monitored. The CAS reactivity pattern is the same for CDM controls containing 0, 0.5, or 2.0 μM added iron. Thus, for simplicity, we have only depicted the CAS reactivity for the CDM blank with 0 μM added iron (◊). The values presented represent the means and standard deviations from duplicate cultures. The delay in peak growth and CAS reactivity for the culture with 0 μM iron was due to the small size of the inoculum used (Fig. 2). Another experiment determined that the CAS reactivity of Philadelphia-1 was also abolished by the inclusion of 2.0 μM iron in the assay medium (data not shown).
Further considerations implied that this substance was not cysteine, phosphate, citrate, or tyrosine-based pigment, four factors that are not traditionally viewed as siderophores but which can facilitate the CAS reaction and/or bind iron (36, 53, 54, 83, 94). First, the concentration of cysteine within the shaking 130b and Philadelphia-1 CDM cultures, although initially high, declined over time to a level that is CAS negative. Second, CAS activity was also observed after growth in CDM in which the cysteine was replaced with cystine (data not shown) (54). Third, since CDM only contains 2 mM phosphate (79), the legionellae would have to have produced >98 mM phosphate to account for any of the CAS reactivity that was observed (83). Our own standard curve for phosphate further indicated that a >498 mM concentration of the molecule would have been needed to provide the ca. 1,000 μM DFX equivalents that was commonly seen in the L. pneumophila cultures (data not shown). Fourth, CDM does not contain any citrate (79), and although only 5 mM citrate corresponds to 800 μM DFX equivalents, specific assays failed to detect the compound within the CAS-positive bacterial cultures (data not shown). Fifth, a Philadelphia-1 derivative that is defective for production of brown pigment, a heterogeneous polymer of homogentisic acid, was not impaired in CAS reactivity (Fig. 5) (96). Sixth, L. pneumophila CAS reactivity, like most siderophore activities, peaked during late log to early stationary phase (Fig. 1), further indicating that it is not a secondary metabolite or late-stationary-phase pigment, like the homogentisic acid polymer, with low iron-chelating activity (74, 83). It should be added that since the Legionella supernatants had a nearly neutral pH, their CAS reactivity is not simply a pH effect (83). In summary, our initial biochemical characterization of the CAS reactivity in 130b and Philadelphia-1 supernatants indicated that L. pneumophila strains do, indeed, produce siderophores.
FIG. 5.
CAS reactivity of an L. pneumophila serogroup 1 pigment mutant. Strains Philadelphia-1 (●); JR32, a restriction mutant of Philadelphia-1 (■); and JR32-1, a pigment mutant of JR32 (▴), were grown in BYE to an OD660 of 1.1, washed, and then inoculated into iron-depleted CDM. Over the next 2 days, the growth (top) and CAS reactivity (bottom) of the cultures were recorded. For reference, the CAS reactivity of the CDM blank is included (◊) in the lower panel. The values presented represent the means and standard deviations from duplicate cultures. The slightly reduced level of CAS reactivity seen in the JR32-1 culture was not observed in either of the two additional experiments that were done.
Initial investigation into the structural nature of the L. pneumophila siderophore.
For two reasons, we initially suspected that the Legionella siderophore was catecholate in nature. First, the supernatants promoted a very fast CAS reaction, a characteristic that is commonly associated with catecholates (68). Second, the CAS reactivity within the cultures declined with prolonged incubation, and the catecholates, being more susceptible to oxidation, are less stable than hydroxamates. Thus, we determined if Philadelphia-1 and 130b supernatants contain a substance that is recognized in the Arnow assay (4, 68, 74). Using supernatants that contained as much as 1,000 μM DFX equivalents, the Arnow test yielded negative results, showing that the Legionella siderophore was not a catecholate. To confirm this notion, the supernatants were examined using the protocol of Rioux et al. (81), a method that is seven times more sensitive than the Arnow assay and detects catechols substituted at position 3 or 4. Again, the L. pneumophila samples gave a negative reaction. Since the Legionella CAS reactivity was clearly not associated with a catecholate molecule, 130b and Philadelphia-1 supernatants were next tested for the ability to react in the Csáky assay, the standard protocol for detection of hydroxamate siderophores (17, 68, 74). The fact that we had found an iron-regulated, aerobactin synthetase-like gene (frgA) in strain 130b also suggested that L. pneumophila might elaborate a hydroxamate siderophore (40). However, the CAS-positive fractions were also nonreactive in the Csáky assay and our 130b frgA mutant, strain NU229, produced a level of CAS reactivity that was comparable to that of the wild-type strains (data not shown). Taken together, the classic structure-based assays imply that the L. pneumophila siderophore is not a typical catecholate or hydroxamate. To explore this further, we extracted the 130b and Philadelphia-1 supernatants with several organic solvents and then assayed for loss of CAS reactivity. The siderophore activity was not extracted by either ethyl acetate or dichloromethane, further indicating that it is not a classic catecholate or phenolate (5, 12, 15, 37, 68, 74, 85). The reactivity was also not extractable with butanol, confirming that it is not a typical alcohol-soluble hydroxamate (68, 74). Because some atypical siderophores, such as pyoverdin and salicylate, are fluorescent (15, 48, 85), we examined the L. pneumophila supernatants under UV light. Interestingly, the log-phase and early-stationary-phase CAS-positive samples exhibited a blue fluorescence. Such fluorescence is reminiscent of salicylate, an Arnow- and Csáky-negative phenolate made by strains of Pseudomonas and Burkholderia (18, 61, 85). However, 130b and Philadelphia-1 supernatants were found not to contain significant levels of salicylic acid.
Production of CAS reactivity by other L. pneumophila serogroups and other Legionella species.
The L. pneumophila species consists of 14 serogroups, all of which have been represented by both clinical and environmental isolates (95). In addition to L. pneumophila, the Legionella genus includes 42 other species of aquatic bacteria, of which 19 have also been associated with human disease (57, 70). Thus, we determined whether CAS reactivity was produced by strains of nine other L. pneumophila serogroups and six other Legionella species. Bacteria representing these strains (Table 1) were grown in BYE to either log or early stationary phase, inoculated into shaking, iron-depleted CDM at an OD660 of 0.3, and then monitored for 3 days. All L. pneumophila strains had a pattern of siderophore production that was comparable to that of strains 130b and Philadelphia-1 (data not shown). Strains representing five of the other Legionella species had a CAS reactivity profile that was similar to the L. pneumophila pattern (data not shown). Interestingly, the L. micdadei strain did not exhibit CAS reactivity, despite showing good growth in the CDM. Overall, these data suggest that siderophores are produced by many, but not all, legionellae.
DISCUSSION
We have discovered a substance within L. pneumophila supernatants that is reactive in the CAS assay. For a number of reasons, we strongly believe that this CAS-reactive substance represents a bona fide siderophore. First, the CAS assay has reliably identified and characterized a range of siderophores encompassing the iron chelators of over 20 bacterial genera (2, 3, 5, 12, 18, 20, 21, 27, 33, 36, 37, 38, 47, 48, 51, 58, 66, 68, 74, 80, 82, 85, 98). Second, the presence of CAS-reactive material correlated with enhanced aerobic growth in an iron-depleted defined medium (68). Third, the peak in reactivity occurred during the late log to the early stationary phase of growth (52, 74, 83). Fourth, the chelating activity was repressed by as little as 0.5 μM iron, a process that we suspect is mediated, at least in part, by Fur (16, 39). Fifth, it was <1 kDa in size and was resistant to heat and proteases (68). Sixth, it promoted a rapid CAS reaction; i.e., the color change began within 2 min and achieved a maximum in 30 to 60 min (83). Seventh, the Legionella CAS reaction was intense, with cultures routinely containing ca. 1,000 μM DFX equivalents. Eighth, it was not cysteine, the only CDM component that is CAS reactive (54). Ninth, it was not phosphate or citrate, the only other nonsiderophores that are commonly associated with bacterial cultures and are known to be CAS reactive (68, 83). Finally, a strong CAS reaction was consistently observed in the supernatants of two distinct strains of L. pneumophila. Hence, we designated the Arnow-negative, Csáky-negative, CAS-positive material produced by strains 130b and Philadelphia-1 legiobactin. Incidentally, the discovery of legiobactin and its promotion of growth in CDM lacking added iron also indicate that the L. pneumophila requirement for iron is not as great as has been intimated and may even be <1 μM (6, 25, 45, 60, 71, 78).
With the identification of legiobactin, Legionella is added to the list of 40 or so other bacterial genera that are known to secrete siderophores (94, 97). This list has grown appreciably in recent years, largely due to the availability of the CAS assay. There are a number of other bacterial siderophores that, like legiobactin, do not possess the classic catecholate or hydroxamate structure and thus were or would have been overlooked by the Arnow and Csáky assays (12, 15, 18, 33, 37, 61, 68, 85, 94). In some ways, it is not surprising that L. pneumophila produces a siderophore. Indeed, a number of other aquatic bacteria elaborate siderophores; e.g., species of Aeromonas, Alcaligenes, Alteromonas, Pseudomonas, and Vibrio all utilize this type of iron scavenger (94). Similarly, a variety of other human pathogens are siderophore producers, i.e., species of Aeromonas, Bordetella, Brucella, Burkholderia, Citrobacter, Corynebacterium, Enterobacter, Escherichia, Klebsiella, Mycobacterium, Proteus, Pseudomonas, Salmonella, Serratia, Shigella, Staphylococcus, Vibrio, and Yersinia (5, 18, 19, 28, 35, 38, 56, 62, 97). For some of these pathogens, including intraphagosomal, intracellular parasites, the siderophore is known to be critical for disease (19, 28, 38, 62, 67, 97). Additional work is clearly needed to determine how legiobactin promotes L. pneumophila survival and if it fosters disease production. However, at this time, three basic scenarios can be presented. First, the siderophore only enhances extracellular replication and/or persistence. Support for this hypothesis comes from an increasing realization that L. pneumophila does grow extracellularly in the environment, particularly in biofilms (49, 50). Second, legiobactin only promotes intracellular growth, be it in a protozoan or in a macrophage. Since the Legionella phagosome is now recognized as an iron-stressed environment, this hypothesis also has merit (9, 10, 14, 40, 76). Finally, it is, of course, conceivable that legiobactin enhances both intra- and extracellular survival.
In addition to possessing a potentially novel structure, legiobactin appeared to be regulated in a unique manner. Curiously, whether an iron-starved culture produced legiobactin was heavily influenced by the nature of its initiating dose, with ultimate CAS reactivity being most affected by the stage of growth of the inoculum. Numerous experiments, using a variety of legionellae, indicated that log-phase or early-stationary-phase, but not late-stationary-phase, bacteria initiated a growth pattern that yielded legiobactin. We suspect that this is a reason why others and we did not observe legiobactin previously. For example, we had derived inocula from overnight BYE cultures that were undoubtedly in late stationary phase and others had inoculated with bacteria taken from 4-day-old CDM cultures (34, 54). Incidentally, the fact that log-phase or early-stationary-phase inocula are needed for the robust growth of L. pneumophila in iron-deficient media may have also contributed to the past overestimates of the bacterium's iron requirement. To our knowledge, the dramatic influence of growth phase status on subsequent siderophore elaboration has not been documented before, for any type of bacteria. On the one hand, earlier studies were much concerned with ensuring that bacteria were sufficiently iron depleted before their introduction into the assay medium (68, 74). Indeed, we also found that the iron content of the L. pneumophila inoculum influenced final siderophore output, albeit to a relatively minor degree. On the other hand, many studies have focused on the parameters that are present at the time that the siderophore is actually being produced and measured (22, 32, 42, 52, 74, 88). In addition to the effects of iron, aeration, and growth phase that were noted earlier, carbon sources, other metals (e.g., cobalt, copper, magnesium, manganese, nickel, and zinc), cell density (i.e., quorum sensing), temperature, and pH all influence the ongoing production of siderophores. We, too, saw that the density of the initial CDM culture influenced the ultimate level of CAS reactivity within the iron-starved Legionella cultures. However, the reduced ability of small inocula to elicit legiobactin may have been an indirect effect of an inability to initiate appropriate growth. Thus, it will be of most interest to see if the inoculum growth phase effect that we observed has relevance in other microbial systems.
The molecular basis and biological significance of the novel aspects of legiobactin production remain to be determined; however, the following hypothesis seems plausible. It appears that as long as the legionellae are replicating, they are positioned to begin making legiobactin should iron levels decline. Yet, once the bacteria (completely) cease multiplying in late stationary phase, they apparently commit to a form of existence that is incompatible with and/or does not require subsequent siderophore production, even if iron becomes or remains scarce. Although other scenarios exist, it seems most logical that the siderophore normally promotes both extra- and intracellular replication, but then environmental alterations (akin to those seen in late-stationary-phase broth) temporarily convert the legionellae into a (less metabolically active?) form that does not need the iron chelator. Interestingly, data are accumulating which indicate that alterations in both extra- and intracellular environments cause L. pneumophila to undergo major shifts in physiology and structure (11, 30, 73, 87).
The identification of legiobactin has increased significantly our appreciation of L. pneumophila iron acquisition. Furthermore, it led to the realization that other Legionella species can produce siderophore-like activity. We suspect, but clearly need to prove, that the CAS-reactive substances that are secreted by the other legionellae are legiobactin. Also, additional strains and growth conditions need to be tested before it can be concluded that L. micdadei does not excrete siderophores. However, L. micdadei is known to lack a number of key activities that are expressed by L. pneumophila (46, 69). The discovery of legiobactin also confirms that the legionellae, like many other microbes, have multiple mechanisms for iron assimilation. As alluded to earlier, recent genetic data suggest the existence of a second L. pneumophila siderophore, which, unlike legiobactin, appears to be a hydroxamate (40). We also believe the legionellae use heme acquisition, iron-loaded peptides, cytoplasmic and periplasmic ferric reductases, transferrin-degrading proteases, an iron-containing pigment, and ABC transporters (43, 45, 53, 71, 75, 89, 90; U. Prasad, S. Kurtz, V. Viswanathan, and N. P. Cianciotto, unpublished data). In addition, we are intrigued by those experiments in which strains of L. pneumophila and L. micdadei achieved high levels of growth in iron-depleted CDM without producing a siderophore activity. It will be interesting to determine what types of iron uptake were operating under these conditions.
ACKNOWLEDGMENTS
We thank V. K. Viswanathan, Virginia Aragon, Ombeline Rossier, Sherry Kurtz, Pamela Sokol, and Gunther Winkelmann for helpful discussions and Jörg Hacker for providing the legiolysin mutant and its parent.
This work was supported by NIH grant AI34937 awarded to N.P.C.
REFERENCES
- 1.Abu Kwaik Y, Gao L-Y, Stone B J, Harb O S. Invasion of mammalian and protozoan cells by Legionella pneumophila. Bull Inst Pasteur. 1998;96:237–247. [Google Scholar]
- 2.Agiato L-A, Dyer D W. Siderophore production and membrane alterations by Bordetella pertussis in response to iron starvation. Infect Immun. 1992;60:117–123. doi: 10.1128/iai.60.1.117-123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankenbauer R G, Cox C D. Isolation and characterization of Pseudomonas aeruginosa mutants requiring salicylic acid for pyochelin biosynthesis. J Bacteriol. 1988;170:5364–5367. doi: 10.1128/jb.170.11.5364-5367.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnow L E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine tyrosine mixtures. J Biol Chem. 1937;118:531–537. [Google Scholar]
- 5.Barghouthi S, Payne S M, Arceneaux J E L, Byers B R. Cloning, mutagenesis and nucleotide sequence of a siderophore biosynthetic gene (amoA) from Aeromonas hydrophila. J Bacteriol. 1991;173:5121–5128. doi: 10.1128/jb.173.16.5121-5128.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker J, Farrell I D, Hutchison J G P. Factors affecting growth of Legionella pneumophila in liquid media. J Med Microbiol. 1986;22:97–100. doi: 10.1099/00222615-22-2-97. [DOI] [PubMed] [Google Scholar]
- 7.Bortner C A, Arnold R R, Miller R D. Bactericidal effect of lactoferrin on Legionella pneumophila: effect of the physiological state of the organism. Can J Microbiol. 1989;35:1048–1051. doi: 10.1139/m89-174. [DOI] [PubMed] [Google Scholar]
- 8.Brand B C, Hacker J. The biology of Legionella infection. In: Kaufmann S H E, editor. Host response to intracellular pathogens. R. G. Austin, Tex: Landes; 1996. pp. 291–312. [Google Scholar]
- 9.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Investig. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd T F, Horwitz M A. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Investig. 1991;88:1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers C E, McIntyre D D, Mouck M, Sokol P A. Physical and structural characterization of yersiniophore, a siderophore produced by clinical isolates of Yersinia enterocolitica. Biometals. 1996;9:157–167. doi: 10.1007/BF00144621. [DOI] [PubMed] [Google Scholar]
- 13.Cianciotto N P, Kim Stamos J, Kamp D W. Infectivity of Legionella pneumophila mip mutant for alveolar epithelial cells. Curr Microbiol. 1995;30:247–250. doi: 10.1007/BF00293641. [DOI] [PubMed] [Google Scholar]
- 14.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox C D, Rinehart K L, Jr, Moore M L, Cook J C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1981;78:4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csáky T Z. On the estimation of bound hydroxylamines in biological materials. Acta Chem Scad. 1948;2:450–454. [Google Scholar]
- 18.Darling P, Chan M, Cox A D, Sokol P A. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect Immun. 1998;66:874–877. doi: 10.1128/iai.66.2.874-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Voss J J, Rutter K, Schroeder B G, Barry C F., III Iron acquisition and metabolism by mycobacteria. J Bacteriol. 1999;181:4443–4451. doi: 10.1128/jb.181.15.4443-4451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drechsel H, Freund S, Nicholson G, Haag H, Jung O, Zähner H, Jung G. Purification and chemical characterization of staphyloferrin B, a hydrophilic siderophore from staphylococci. Biometals. 1993;6:185–192. doi: 10.1007/BF00205858. [DOI] [PubMed] [Google Scholar]
- 21.Drechsel H, Thieken A, Reissbrodt R, Jung G, Winkelmann G. α-Keto acids are novel siderophores in the genera Proteus, Providencia, and Morganella and are produced by amino acid deaminases. J Bacteriol. 1993;175:2727–2733. doi: 10.1128/jb.175.9.2727-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy B K, Défago G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol. 1999;65:2429–2438. doi: 10.1128/aem.65.6.2429-2438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engleberg N C, Drutz D J, Eisenstein B I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984;44:222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feeley J C, Gorman G W, Weaver R E, Mackel D C, Smith H W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978;8:320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 27.Fiss E H, Yu S, Jacobs W R., Jr Identification of genes involved in the sequestration of iron in mycobacteria: the ferric exochelin biosynthetic and uptake pathways. Mol Microbiol. 1994;14:557–569. doi: 10.1111/j.1365-2958.1994.tb02189.x. [DOI] [PubMed] [Google Scholar]
- 28.Furman M, Fica A, Saxena M, di Fabio J L, Cabello F C. Salmonella typhi uptake mutants are attenuated in mice. Infect Immun. 1994;62:4091–4094. doi: 10.1128/iai.62.9.4091-4094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaitonde M K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967;104:627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garduno R A, Hoffman P S. Abstracts of the 99th General Meeting of the American Society for Microbiology 1999. Washington, D.C.: American Society for Microbiology; 1999. Legionella pneumophila displays a developmental cycle, abstr. D/B-69; pp. 222–223. [Google Scholar]
- 31.Gebran S J, Newton C, Yamamoto Y, Widen R, Klein T W, Friedman H. Macrophage permissiveness for Legionella pneumophila growth modulated by iron. Infect Immun. 1994;62:564–568. doi: 10.1128/iai.62.2.564-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giardina P C, Foster L-A, Musser J M, Akerley B J, Miller J F, Dyer D W. bvg repression of alcaligin synthesis in Bordetella bronchoseptica is associated with phylogenetic lineage. J Bacteriol. 1995;177:6058–6063. doi: 10.1128/jb.177.21.6058-6063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilis A, Khan M A, Cornelis P, Meyer J M, Mergeay M, van der Lelie D. Siderophore-mediated iron uptake in Alcaligenes eutrophus CH34 and identification of aleB encoding the ferric iron-alcaligin E receptor. J Bacteriol. 1996;178:5499–5507. doi: 10.1128/jb.178.18.5499-5507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldoni P, Visca P, Pastoris M C, Valenti P, Orsi N. Growth of Legionella spp. under conditions of iron restriction. J Med Microbiol. 1991;34:113–118. doi: 10.1099/00222615-34-2-113. [DOI] [PubMed] [Google Scholar]
- 35.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 36.Guerinot M L, Meidl E J, Plessner O. Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol. 1990;172:3298–3303. doi: 10.1128/jb.172.6.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haag H, Hantke K, Drechsel H, Stojiljkovic I, Jung G, Zahner H. Purification of yersiniabactin: a siderophore and possible virulence factor of Yersinia enterocolitica. J Gen Microbiol. 1993;139:2159–2165. doi: 10.1099/00221287-139-9-2159. [DOI] [PubMed] [Google Scholar]
- 38.Heesemann J, Hantke K, Vocke T, Saken E, Rakin A, Stojiljkovic I, Berner R. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane polypeptide of 65,000 Da and pesticin sensitivity. Mol Microbiol. 1993;8:397–408. doi: 10.1111/j.1365-2958.1993.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 39.Hickey E K, Cianciotto N P. Cloning and sequencing of the Legionella pneumophila fur gene. Gene. 1994;143:117–121. doi: 10.1016/0378-1119(94)90615-7. [DOI] [PubMed] [Google Scholar]
- 40.Hickey E K, Cianciotto N P. An iron- and Fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetases. Infect Immun. 1997;65:133–143. doi: 10.1128/iai.65.1.133-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitz M A. Interactions between macrophages and Legionella pneumophila. Curr Top Microbiol Immunol. 1992;181:265–282. doi: 10.1007/978-3-642-77377-8_10. [DOI] [PubMed] [Google Scholar]
- 42.Huyer M, Page W J. Zn2+ increases siderophore production in Azotobacter vinelandii. Appl Environ Microbiol. 1988;54:2625–2631. doi: 10.1128/aem.54.11.2625-2631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James B W, Mauchline W S, Dennis P J, Keevil C W. A study of iron acquisition mechanisms of Legionella pneumophila grown in chemostat culture. Curr Microbiol. 1997;34:238–243. doi: 10.1007/s002849900176. [DOI] [PubMed] [Google Scholar]
- 44.James B W, Mauchline W S, Fitzgeorge R B, Dennis P J, Keevil C M. Influence of iron-limited continuous culture on physiology and virulence of Legionella pneumophila. Infect Immun. 1995;63:4224–4230. doi: 10.1128/iai.63.11.4224-4230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson W, Varner L, Poch M. Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect Immun. 1991;59:2376–2381. doi: 10.1128/iai.59.7.2376-2381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi A D, Swanson M S. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect Immun. 1999;67:4134–4142. doi: 10.1128/iai.67.8.4134-4142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang H Y, Brickman T J, Beaumont F C, Armstrong S K. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J Bacteriol. 1996;178:4877–4884. doi: 10.1128/jb.178.16.4877-4884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koedam N, Wittouck E, Gaballa A, Gillis A, Hofte M, Cornelis P. Detection and differentiation of microbial siderophores by isoelectric focusing and chrome azurol S overlay. Biometals. 1994;7:287–291. doi: 10.1007/BF00144123. [DOI] [PubMed] [Google Scholar]
- 49.Kramer M H, Ford T E. Legionellosis: ecological factors of an environmentally ‘new’ disease. Zentbl Hyg. 1994;195:470–482. [PubMed] [Google Scholar]
- 50.Lee J V, West A A. Survival and growth of Legionella species in the environment. J Appl Bacteriol. 1991;20:121S–129S. [PubMed] [Google Scholar]
- 51.Lemos M L, Salinas P, Toranzo A E, Barja J L, Crosa J H. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988;170:1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liles M R. Ph.D. thesis. Evanston, Ill: Northwestern University; 1998. [Google Scholar]
- 54.Liles M R, Cianciotto N P. Absence of siderophore-like activity in Legionella pneumophila supernatants. Infect Immun. 1996;64:1873–1875. doi: 10.1128/iai.64.5.1873-1875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liles M R, Cianciotto N P. Abstracts of the 99th General Meeting of the American Society for Microbiology 1999. Washington, D.C.: American Society for Microbiology; 1999. Identification of a Legionella pneumophila siderophore, abstr. B/D-201; p. 68. [Google Scholar]
- 56.Litwin C M, Rayback T W, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996;64:2834–2838. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo Presti F, Riffard S, Meugnier H, Reyrolle M, Lasne Y, Grimont P A, Grimont F, Vandenesch F, Etienne J, Fleurette J, Freney J. Legionella taurinensis sp. nov., a new species antigenically similar to Legionella spiritensis. Int J Syst Bacteriol. 1999;49:397–403. doi: 10.1099/00207713-49-2-397. [DOI] [PubMed] [Google Scholar]
- 58.Mahé B, Masclaux C, Rauscher L, Enard C, Expert D. Differential expression of two siderophore-dependent iron-acquisition pathways in Erwinia chrysanthemi 3937: characterization of a novel ferrisiderophore permease of the ABC transporter family. Mol Microbiol. 1995;18:33–43. doi: 10.1111/j.1365-2958.1995.mmi_18010033.x. [DOI] [PubMed] [Google Scholar]
- 59.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R The Laboratory Investigation Team. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 60.Mengaud J M, Horwitz M A. The major iron-containing protein of Legionella pneumophila is an aconitase homologous with the human iron-responsive element-binding protein. J Bacteriol. 1993;175:5666–5676. doi: 10.1128/jb.175.17.5666-5676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer J M, Azelvandre P, Georges C. Iron metabolism in Pseudomonas: salicylic acid, a siderophore of Pseudomonas fluorescens CHAO. Biofactors. 1992;4:23–27. [PubMed] [Google Scholar]
- 62.Meyer J M, Neely A, Stintzi A, Georges C, Holder I A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mody C H, Paine R, Shahrabadi M S, Simon R H, Pearlman E, Eisenstein B I, Toews G B. Legionella pneumophila replicates within rat alveolar epithelial cells. J Infect Dis. 1993;167:1138–1145. doi: 10.1093/infdis/167.5.1138. [DOI] [PubMed] [Google Scholar]
- 64.Moellering H, Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966;17:369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- 65.Muller D, Edwards M L, Smith D W. Changes in iron and transferrin levels and body temperature in experimental airborne legionellosis. J Infect Dis. 1983;147:302–307. doi: 10.1093/infdis/147.2.302. [DOI] [PubMed] [Google Scholar]
- 66.Münzinger M, Taraz K, Budzikiewicz H, Drechsel H, Heymann P, Winkelmann G, Meyer J-M. S,S-rhizoferrin (enantio-rhizoferrin)—a siderophore of Ralstonia (Pseudomonas) pickettii DSM 6297—the optical antipode of R,R-rhizoferrin isolated from fungi. Biometals. 1999;12:189–193. [Google Scholar]
- 67.Nassif X, Mazert M-C, Mounier J, Sansonetti P J. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect Immun. 1987;55:1963–1969. doi: 10.1128/iai.55.9.1963-1969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neilands J B. Siderophore systems of bacteria and fungi. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley & Sons, Inc.; 1989. pp. 141–163. [Google Scholar]
- 69.O'Connell W A, Bangsborg J M, Cianciotto N P. Characterization of a Legionella micdadei mip mutant. Infect Immun. 1995;63:2840–2845. doi: 10.1128/iai.63.8.2840-2845.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connell W A, Dhand L, Cianciotto N P. Infection of macrophage-like cells by Legionella species that have not been associated with disease. Infect Immun. 1996;64:4381–4384. doi: 10.1128/iai.64.10.4381-4384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Connell W A, Hickey E K, Cianciotto N P. A Legionella pneumophila gene that promotes hemin binding. Infect Immun. 1996;64:842–848. doi: 10.1128/iai.64.3.842-848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Owens W E, Rolfe R D, Finegold S M. Abstracts of the 82nd General Meeting of the American Society for Microbiology 1982. Washington, D.C.: American Society for Microbiology; 1982. The effect of iron on the virulence of Legionella pneumophila, abstr. CC I, II 13; p. 89. [Google Scholar]
- 73.Paszko-Kolva C, Shahamat M, Colwell R R. Long-term survival of Legionella pneumophila serogroup 1 under low-nutrient conditions and associated morphological changes. FEMS Microbiol Ecol. 1992;102:45–55. [Google Scholar]
- 74.Payne S M. Detection, isolation, and characterization of siderophores. Methods Enzymol. 1994;235:329–344. doi: 10.1016/0076-6879(94)35151-1. [DOI] [PubMed] [Google Scholar]
- 75.Poch M T, Johnson W. Ferric reductases of Legionella pneumophila. Biometals. 1993;6:107–114. doi: 10.1007/BF00140111. [DOI] [PubMed] [Google Scholar]
- 76.Pope C D, O'Connell W A, Cianciotto N P. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect Immun. 1996;64:629–636. doi: 10.1128/iai.64.2.629-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quinn F D, Weinberg E D. Killing of Legionella pneumophila by human serum and iron-binding agents. Curr Microbiol. 1988;17:111–116. [Google Scholar]
- 78.Reeves M W, Pine L, Hutner S H, George J R, Harrell W K. Metal requirements of Legionella pneumophila. J Clin Microbiol. 1981;13:688–695. doi: 10.1128/jcm.13.4.688-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reeves M W, Pine L, Neilands J B, Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983;154:324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reigh G, O'Connell M. Siderophore-mediated iron transport correlates with the presence of specific iron-regulated proteins in the outer membrane of Rhizobium meliloti. J Bacteriol. 1993;175:94–102. doi: 10.1128/jb.175.1.94-102.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rioux C, Jordan D C, Rattray J B M. Colorimetric determination of catechol siderophores in microbial cultures. Anal Biochem. 1983;133:163–169. doi: 10.1016/0003-2697(83)90238-5. [DOI] [PubMed] [Google Scholar]
- 82.Schmitt M P, Payne S M. Genetics and regulation of enterobactin genes in Shigella flexneri. J Bacteriol. 1988;170:5579–5587. doi: 10.1128/jb.170.12.5579-5587.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 84.Shuman H A, Purcell M, Segal G, Hales L, Waiter L A. Intracellular multiplication of Legionella pneumophila: human pathogen or accidental tourist? Curr Top Microbiol Immunol. 1998;225:99–112. doi: 10.1007/978-3-642-80451-9_6. [DOI] [PubMed] [Google Scholar]
- 85.Sokol P A, Lewis C J, Dennis J J. Isolation of a novel siderophore from Pseudomonas cepacia. J Med Microbiol. 1992;36:184–189. doi: 10.1099/00222615-36-3-184. [DOI] [PubMed] [Google Scholar]
- 86.States S J, Conley L F, Ceraso M, Stephenson T E, Wolford R S, Wadowsky R M, McNamara A M, Yee R B. Effects of metals on Legionella pneumophila growth in drinking water plumbing systems. Appl Environ Microbiol. 1985;50:1149–1154. doi: 10.1128/aem.50.5.1149-1154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinert M, Emody L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Visca P, Colotti G, Serino L, Verzili D, Orsi N, Chiancone E. Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Appl Environ Microbiol. 1992;58:2886–2893. doi: 10.1128/aem.58.9.2886-2893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viswanathan, V. K., P. H. Edelstein, C. D. Pope, and N. P. Cianciotto. The Legionella pneumophila iraAB locus is required for iron assimilation, intracellular infection, and virulence. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 90.Viswanathan V K, Krcmarik K, Liles M R, Prasad U, Cianciotto N P. Abstracts of the 99th General Meeting of the American Society for Microbiology 1999. Washington, D.C.: American Society for Microbiology; 1999. Genetic and phenotypic characterization of mutants defective for iron uptake and infection in Legionella pneumophila, abstr. B/D-200; p. 68. [Google Scholar]
- 91.Warren W J, Miller R D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979;10:50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinberg E D. Acquisition of iron and other nutrients in vivo. In: Roth J A, et al., editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 79–93. [Google Scholar]
- 93.Weinberg E D. Iron loading and disease surveillance. Emerging Infect Dis. 1999;5:346–352. doi: 10.3201/eid0503.990305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winkelmann G, Drechsel H. Microbial siderophores. In: Rehm H-J, Reed G, editors. Biotechnology. Weinheim, Germany: VCH; 1997. pp. 200–246. [Google Scholar]
- 95.Winn W C., Jr Legionnaires disease: historical perspective. Clin Microbiol Rev. 1988;1:60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wintermeyer E, Flügel M, Ott M, Steinert M, Rdest U, Mann K-H, Hacker J. Sequence determination and mutational analysis of the lly locus of Legionella pneumophila. Infect Immun. 1994;62:1109–1117. doi: 10.1128/iai.62.3.1109-1117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 98.Wyckoff E E, Stoebner J A, Reed K E, Payne S M. Cloning of a Vibrio cholerae vibriobactin gene cluster: identification of genes required for early steps in siderophore biosynthesis. J Bacteriol. 1997;179:7055–7062. doi: 10.1128/jb.179.22.7055-7062.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]