Abstract
Monitoring immune responses to SARS-CoV-2 vaccination and its clinical efficacy over time in Multiple Sclerosis (MS) patients treated with disease-modifying therapies (DMTs) help to establish the optimal strategies to ensure adequate COVID-19 protection without compromising disease control offered by DMTs. Following our previous observations on the humoral response one month after two doses of BNT162b2 vaccine (T1) in MS patients differently treated, here we present a cross-sectional and longitudinal follow-up analysis six months following vaccination (T2, n=662) and one month following the first booster (T3, n=185). Consistent with results at T1, humoral responses were decreased in MS patients treated with fingolimod and anti-CD20 therapies compared with untreated patients also at the time points considered here (T2 and T3). Interestingly, a strong upregulation one month after the booster was observed in patients under every DMTs analyzed, including those treated with fingolimod and anti-CD20 therapies. Although patients taking these latter therapies had a higher rate of COVID-19 infection five months after the first booster, only mild symptoms that did not require hospitalization were reported for all the DMTs analyzed here. Based on these findings we anticipate that additional vaccine booster shots will likely further improve immune responses and COVID-19 protection in MS patients treated with any DMT.
Keywords: SARS-CoV-2, multiple sclerosis, humoral immunity, disease-modifying therapy (DMT), vaccine efficacy and effectiveness, BNT162b2 messenger RNA (mRNA), COVID-19 vaccination, COVID-19
Introduction
General population data on SARS-CoV-2 vaccination support its effectiveness in preventing COVID-19 infection (1). Still, the magnitude of protection it offers to Multiple Sclerosis (MS) patients receiving certain disease-modifying therapies (DMTs) is not completely clear.
Several evidence has already demonstrated that the humoral and cellular response after SARS-CoV-2 vaccination was strongly affected by the treatment with certain DMTs used to ameliorate MS symptoms (2–4). Notably, azathioprine (AZA), fingolimod (FTY) and anti-CD20 treatments, including ocrelizumab (OCR) and rituximab (RTX), negatively influences the humoral response after SARS-CoV-2 vaccination and likely affect the level of protection against COVID-19 (2–5). Additionally, older age, male sex and active smoking were significantly associated with lower antibody titers against SARS-CoV-2 vaccine in MS patients (2, 6).
Based on these results, it has been suggested that the immune response after SARS-CoV-2 vaccination can be improved through additional booster shots (7, 8) and by optimally adjusting vaccination timing based on the timing of immune cell repopulation after the last administration of specific immunosuppressive DMTs (9). Specifically, in contrast to the initial international recommendation for timing DMTs (msfi.org) of a 3-month waiting time after the last dose of immune-suppressive therapies, we and others have observed that a 6-month waiting time would be optimal (2, 10). It is now important to further monitor, over time and after booster doses, the immune responses and clinical efficacy of SARS-CoV-2 vaccination in MS patients. This will provide new insight to define the most effective strategies that will ensure optimal treatment of MS providing at the same time the most effective prevention from COVID-19 and especially its more forms.
Following our initial observations of humoral response one month after two doses of BNT162b2 vaccine in MS patients treated with different DMTs or untreated (2), here we present a cross-sectional and longitudinal follow-up analysis on the humoral responses to BNT162b2 vaccination 6 months after the second dose, and a month after the third dose (booster) in a Sardinian MS cohort. The effect of previous or concomitant detectable SARS-CoV-2 infection, as well as age, sex, and active smoking was also considered. Reciprocally, we provide preliminary evidence of the impact of vaccination on the incidence of SARS-CoV-2 infection and its clinical severity 5 months after the third dose of vaccine. These findings further help in defining an appropriate immunization strategy in MS patients in relation to DMTs and other factors influencing humoral immunity.
Methods
Study participants
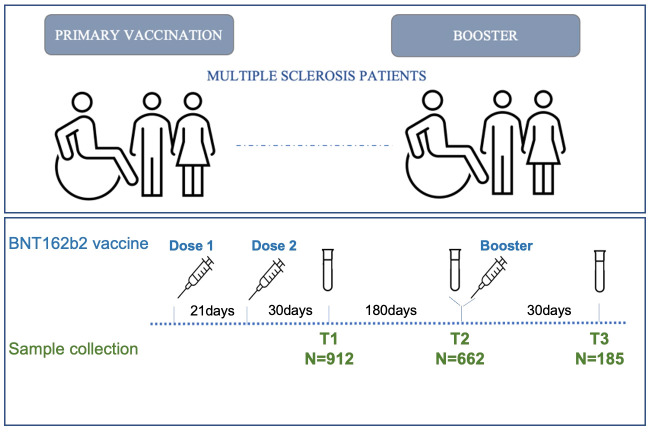
847 MS patients who had received two BNT162b2 vaccine injections, 21 days apart, followed by a booster 6 month later were enrolled between October 2021 and January 2022 from the MS clinical centers of Cagliari and Sassari, Sardinia (Italy). Each injection contained 30μg of BNT162b2 (0.3ml volume per dose).
662 MS patients were analyzed 6 months after the second dose (T2) and 185 MS patients a month after the booster (third) dose (T3). Data from 912 MS patients at 4 weeks after the second dose (T1) were also used (2) ( Figure 1 ).
Figure 1.
Timeline of MS patients’ enrollment. Schematic of the vaccination (blue) and sample collection (green) timeline in MS patients.
In summary we analyzed 1,944 serum samples of 1,307 unique MS patients. Of these, only 94 were recruited at each time point (T1, T2 and T3). The overlap between samples was 31.8% between T2 and T1, 22.8% between T2 and T3, and 13.9% between T3 and T1.
All MS patients, diagnosed according to the McDonald criteria, were contacted using different communication methods. They were questioned concerning previous SARS-CoV-2 infection and the manifestation of any adverse events following vaccination. Clinical and demographic information were collected for each patient, including age, sex, disability score, disease subcategory and disease-modifying treatment. Disease-modifying treatment with average years on treatment in the Sardinia MS cohort analyzed are: alemtuzumab (5.5 years), azathioprine (16.0 years), cladribine (2.2 years), dimethyl fumarate (4.7 years), fingolimod (7.9 years), glatiramer acetate (4.7 years), interferon (9.0 years), natalizumab (6.6 years), ocrelizumab (3.2 years), rituximab (5.4 years), teriflunomide (7.4 years). For pulsed treatments the average delay between treatment and booster vaccination (T3) is 3 years for ALEM and 120 days for OCR. All the data collected have been summarized in Table 1 .
Table 1.
Clinical and demographic characteristics of multiple sclerosis patients at T1, T2 and T3.
| Colonna1 | T1, pwMS (n=912) (2) | T2, pwMS, (n=662) | T3, pwMS, (n=185) |
|---|---|---|---|
| Female, n (%) | 658 (73.1) | 488 (73.6) | 137 (74.1) |
| Age, median (IQR 25-75) | 48.8 (38.8 - 57.8) | 47.39 (38.4 - 55.7) | 48.07 (38.9 – 55) |
| Disease duration, median (IQR 25-75) | 13.6 (7.6 - 21.6) | 13.58 (7.57 – 21) | 13.58 (8.57 - 19) |
| EDSS, median (IQR 25-75) | 2 (1 - 4.5) | 2 (1 - 4) | 2 (1 - 4) |
| RRMS, n (%) | 750 (82.7) | 562 (84.9) | 166 (89.7) |
| PPMS, n (%) | 16 (1.8) | 10 (1.51) | 1 (0.54) |
| SPMS, n (%) | 142 (15.6) | 90 (13.6) | 18 (9.7) |
| Positivity for N antibodies | 38 (4.16) | 23 (3.47) | 9 (4.86) |
| Previosly Covid-19 positive record | 25 | 16 | 8 |
| DMTs, n - Age median (IQR 25-75) | |||
| ALEM | 17 - 41.3 (36.5 - 45) | 10 - 39.1 (34.0 - 44.1) | 3 - 44.18 (38.84 - 48.05) |
| AZA | 28 - 58.9 (55.2 - 64.1) | 20 - 59.5 (48.7 - 63.8) | 6 - 47.5 (41.56 - 58.00) |
| CLA | 6 - 46.8 (37.8 - 48) | 15 - 36.8 (29.9 - 44.1) | 1 - 47.0 |
| DMF | 161 - 44.6 (36.3 - 51.8) | 133 - 44.9 (37.1 - 53.1) | 39 - 49.17 (42.40 - 57.45) |
| FTY | 75 - 45 (37.8 - 52.1) | 112 - 47.7 (39.4 - 53.1) | 45 - 45.3 (39.08 - 52.38) |
| GA | 96 - 53.6 (42.8 - 59.4) | 40 - 52.6 (43.7 - 58.1) | 5 - 48.5 (47.40 - 60.99) |
| IFN | 135 - 47.9 (36.7 - 54.4) | 57 - 49.9 (37.1 - 56.2) | 11 - 38.8 (31.00 - 53.72) |
| NAT | 75 - 36.3 (31.2 - 46.2) | 55 - 37.2 (32.0 - 47.0) | 24 - 38.2 (33.90 - 53.25) |
| OCR | 42 - 43.4 (35.4 - 52.4) | 80 - 41.2 (34.2 - 49.3) | 19 - 42.6 (34.54 - 51.46) |
| RTX | 13 - 53.9 (41.5 - 56.8) | 15 - 48.6 (42.5 - 55.3) | 1 |
| TER | 56 - 51.5 (45.6 - 56.5) | 52 - 52.5 (47.0 - 59.5) | 18 - 54.4 (50.82 - 60.36) |
| MET | 2 - 61.4 | ||
| BTK | 1 | 1 | |
| UNT | 205 - 59 (48.3 - 65.6) | 73 - 59.8 (49.4 - 68.2) | 13 - 54.10 (40.10 - 59.46) |
pwMS, multiple sclerosis patients; RRMS, relapsing-remitting multiple sclerosis; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; EDSS, Expanded Disability Status Scale; IQR, interquartile range DMT,disease modifying therapy; UNT,untreated; ALEM,alemtuzumab; IFN,interferon; GA,glatiramer acetate; DMF,dimethyl fumarate; NAT,natalizumab; CLA,cladribine; TER,teriflunomide; AZA,azathioprine; FTY,fingolimod; RTX,rituximab; OCR,ocrelizumab; MET, methotrexate; BTK, Bruton Tyrosine Kinase trial; IQR, interquartile range.
Detection of SARS-CoV-2 IgG antibodies
Blood samples were collected in vacutainer tubes containing clot activator and gel separator.
Samples were processed within two hours after blood collection to avoid time-dependent artifacts, and subsequently serum was stored at -80°C until use. Quantification of SARS-CoV-2 antibodies direct against the proteins Spike (S) or Nucleocapsid (N) in human serum was performed using the electrochemiluminescence immunoassays Elecsys® Anti-SARS- CoV-S and Elecsys® Anti-SARS-CoV-N (Roche). Anti-S and Anti-N results are expressed as units per ml (U/ml). The anti-N reactive index cutoff (COI) used is > 1.
Statistical analysis
Absolute number and percentage were considered for all categorical variables, and median and interquartile range (IQR) for quantitative variables. Differences between patient groups - stratified according to therapy and selected for Anti-N antibody negativity - were assessed with a negative binomial generalized linear mixed-effects model, which takes into account the nature of the outcome variable (anti-S, non-negative count data); in addition to therapy, the models also considers the contribution of other variables such as age, smoke, sex, Expanded Disability Status Scale (EDSS), disease duration, and clinical sampling center. Results are presented as Incidence Rate Ratio, calculated as the exponential of the regression coefficient (11). Differences between medians were tested with the Mann-Whitney test. Differences between groups in longitudinal data were tested using the non-parametric Friedman test for repeated measures, followed by a post-hoc pairwise comparison using paired Wilcoxon signed-rank test. All statistical analyses were performed using R v.4.0.3 software with the following CRAN libraries: ggplot2, dplyr, readxl, MASS, kableExtra, rstatix. P values <0.05 were considered statistically significant.
Ethics and data collection
The study was reviewed and approved by the Ethical Review Boards ATS Sardegna - Prot. N° 2492/CE. Patient data and samples were coded anonymously to ensure confidentiality during sample processing and data analysis. The patients/participants provided their written informed consent to participate in this study.
Results
Description of the studied MS cohort
A total of 1,944 sera were analyzed at three different time points following BNT162b2 vaccination. Results from 912 MS patients obtained 4 weeks after the second dose (T1) of vaccine have already been published (2) and have been used here as baseline for the two subsequent time points. 662 MS patients were analyzed 6 months after the second dose (T2) and 185 MS patients 4 weeks after the booster dose (T3) ( Figure 1 ).
The T1 cohort has been previously described (2). The T2 MS cohort included 448 (73.6%) female and 214 (26.4%) male patients of whom 84.9% had relapsing-remitting MS (RR), 1.51% had primary progressive MS (PP), and 13.6% had secondary progressive MS (SP). Finally, the T3 MS cohort included 137 (74.1%) female and 48 (25.9%) male patients of whom 89.7% had RR, 0.54% had PP and 9.7% had SP ( Table 1 ).
Untreated MS patients were 73 (11%) at T2 and 13 (7%) at T3. The remaining 589 (88.9%) at T2 and 172 (92.9%) at T3 were treated with different DMTs. The most common treatments were dimethyl fumarate (DMF) at T2 and FTY at T3. DMTs used less frequently included cladribine (CLA) and RTX, and only one patient included in a clinical trial with a Bruton’s tyrosine kinase (BTK) inhibitor. No patients treated with methotrexate (MET) were present either at T2 or T3. A detailed description of the specific DMTs received by the MS patients along with their demographic characteristics at T1, T2 and T3 are summarized in Table 1 .
Disease-modifying therapies impact humoral responses to BNT162b2 vaccine
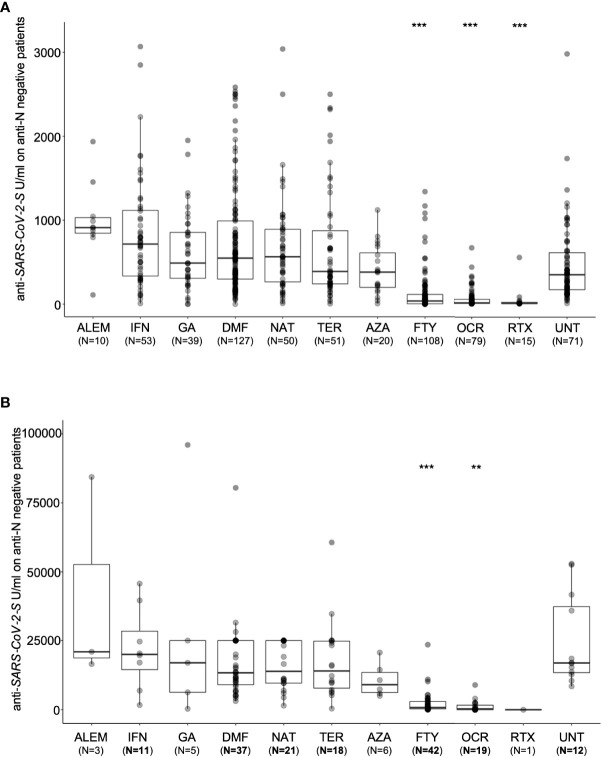
To evaluate the effects of different DMTs on humoral responses to BNT162b2 vaccine, we applied a negative binomial generalized linear mixed-effects model in patients negative for anti-N antibodies production for both T2 and T3 time points separately. Only treatments with data available for at least 10 patients were considered in this analysis.
In line with previously reported humoral responses to vaccine one month after the second dose (T1) (2, 10), after six months (T2) we observed a significant difference in anti-S antibody levels between patients untreated (UNT) and those treated with FTY (IRR = 0.17, p = 1.82x10-21), OCR (IRR = 0.20, p = 4.54x10-42) and RTX (IRR = 0.33, p = 1.35x10-17). No significant difference was observed for the other DMTs ( Table 2 and Figure 2A ). Similarly, one month after the booster (T3) we observed a significantly lower level of anti-S antibodies in MS patients treated with FTY (IRR = 0.37, p = 1.52x10-11) and OCR (IRR = 0.34, p = 1.20x10-14) compared to untreated patients. Other treatments did not show significant results compared to UNT. ( Table 3 and Figure 2B ).
Table 2.
Negative binomial generalized linear mixed-effects model of anti-S-Ab levels in untreated and treated MS patients (anti-N negative) 6 months after the second dose (T2) of BNT162b2 vaccine.
| DMTs | N | Median anti-S in U/ml (IQR 25-75) | IRR (IQR 25-75) | SE | p value |
|---|---|---|---|---|---|
| IFN | 53 | 735 (342.3-1137) | 1.08 (0.70-1.69) | 0.22 | 0.73 |
| DMF | 127 | 554 (299.75-1010.5) | 1.07 (0.73-1.57) | 0.19 | 0.71 |
| ALEM | 10 | 912 (845.1-1030.9) | 1.00 (0.48-2.34) | 0.39 | 0.99 |
| TER | 51 | 389 (242-875.15) | 1.00 (0.64-1.57) | 0.22 | 0.99 |
| GA | 39 | 489 (308.65-854.8) | 0.91 (0.57-1.48) | 0.24 | 0.69 |
| NAT | 50 | 564 (265.55-892.27) | 0.70 (0.43-1.15) | 0.24 | 0.14 |
| AZA | 20 | 382 (200.07-611.3) | 0.68 (0.38-1.27) | 0.30 | 0.19 |
| UNT | 71 | 357 (170.6-625.7) | Reference | – | – |
| FTY | 108 | 37 (4.55-115.77) | 0.17 (0.11-0.24) | 0.19 | 1.82E-21 |
| OCR | 79 | 9 (4-52.815) | 0.07 (0.04-0.10) | 0.20 | 4.54E-42 |
| RTX | 15 | 4 (4-16.63) | 0.06 (0.03-0.12) | 0.33 | 1.35E-17 |
DMT, disease modifying therapy; UNT, untreated; ALEM, alemtuzumab; IFN, interferon; GA, glatiramer acetate; DMF, dimethyl fumarate; NAT, natalizumab; CLA, cladribine; TER, teriflunomide; AZA, azathioprine; FTY, fingolimod; RTX, rituximab; OCR, ocrelizumab; MET, methotrexate; BTK, Bruton Tyrosine Kinase trial; IQR, interquartile range; SE, standard error; IRR, Incidence Rate Ratio calculated as exp(coefficient) (11). Bold value indicate the Reference sample.
Figure 2.
Post-vaccination SARS-CoV-2 anti-S antibody response by disease- modifying therapy (DMT) in MS patients negative for anti-N antibodies. (A) Antibody response to SARS-CoV-2 vaccination by DMT in MS patients six month after SARS-CoV-2 vaccination (T2). (B) Antibody response one month after booster (T3) by DMT in MS patients. Results are reported as boxplots, showing the median value (in bold) and the quartiles as box limits; whiskers at the top and bottom sides represent the overall maximum value and the overall minimum value, respectively. Data outside boxes and whiskers represent the outliers of the distribution. (**P<0.01; ***<0.001). (UNT, untreated; ALEM, alemtuzumab; IFN, interferon; GA, glatiramer acetate; DMF, dimethyl fumarate; NAT, natalizumab; CLA, cladribine; TER,terifluomide; AZA, azathioprine; FTY, fingolimod; RTX, rituximab; OCR, ocrelizumab).
Table 3.
Negative binomial generalized linear mixed-effects model of anti-S-Ab levels in untreated and treated MS patients (anti-N negative) one month after the BNT162b2 booster (T3).
| DMTs | N | Median anti-S in U/ml (IQR 25-75) | IRR (IQR 25-75) | SE | p value |
|---|---|---|---|---|---|
| IFN | 11 | 24639 (18339-89010) | 1.95 (0.75-5.08) | 0.48 | 0.16 |
| TER | 18 | 14008 (7791-24802) | 0.90 (0.36-2.15) | 0.43 | 0.80 |
| NAT | 21 | 16540 (9608-25000) | 0.76 (0.30-1.82) | 0.43 | 0.52 |
| DMF | 37 | 13346 (9031-25000) | 0.74 (0.32-1.60) | 0.39 | 0.44 |
| UNT | 12 | 16989 (12475-37407) | Reference | - | - |
| FTY | 42 | 764 (315-2944) | 0.08 (0.04-0.17) | 0.37 | 1.52e-11 |
| OCR | 19 | 324 (8-1668) | 0.04 (0.01-0.09) | 0.43 | 1.20e-14 |
DMT, disease modifying therapy; UNT, untreated; IFN, interferon; DMF, dimethyl fumarate; NAT, natalizumab; TER, teriflunomide; FTY, fingolimod; RTX, rituximab; OCR, ocrelizumab; IQR, interquartile range; SE, standard error; IRR, Incidence Rate Ratio calculated as exp(coefficient) (11).
The nucleocapsid protein antigens analysis, used to discriminate the immune response generated by the vaccination from immune response generated by natural SARS-CoV-2 infection, identified 23 anti-N positive patients at T2 (3.5% of 662 patients) and 9 at T3 (4.9% of 185 patients) ( Table 1 ). Prior natural SARS-CoV-2 infection impact the humoral responses to BNT162b2 vaccine (2). Indeed, in MS patients with evidence of a natural exposure to SARS-CoV-2 virus, postvaccination anti-S antibodies levels were significantly higher than in patients who did not experience SARS-CoV-2 infection at T2 (medians 2,500 vs. 303.4 U/ml, Mann-Whitney test p = 2.36x10-11). We observed a comparable trend at T3 (medians 10,965 vs. 9,600 U/ml, Mann-Whitney test p = 0.4816). However, the number of MS patients positive for anti-N proteins was small, especially at T3, and additional data are required to properly evaluate the impact of SARS- CoV-2 infection on the immune response to BNT162b2 vaccine.
The influence of disability status, sex, age, and smoking on anti-S production after BNT162b2 vaccine
We also tested at T2 and T3 the impact of additional factors that had been shown to influence anti-S production after BNT162b2 vaccine at T1 (2). In contrast with findings at T1, at T2 our statistical model did not reveal additional significant effects for sex, while age and the Expanded Disability Status Scale (EDSS) continue to show consistent effects. Specifically, we observed reduced postvaccination levels of anti-SARS-CoV-2 antibodies directed against the S protein in older patients (IRR = 0.98, p = 4.459x10-7) as well as in patients with increased EDSS (IRR = 0.95, p = 0.022). Patients with greater disability showed reduced antibody response to vaccine. At T3 only the EDSS showed a significant effect (IRR = 0.89, p = 0.01) on anti-SARS-CoV-2 antibodies production.
Due to the impact of smoking on antibodies production in healthy and unhealthy cohorts of smokers compared to non-smokers (2, 12), we examined the effects of active cigarette smoking on humoral response to SARS-CoV-2 vaccine in a subset of MS patients negative for anti-N antibodies production for whom smoking status was available (T2 = 510, T3 = 157). Our analyses showed that active cigarettes smoking reduced anti-S antibodies production (median = 128.0 U/ml) compared to non-smokers (median = 349.6 U/ml) in response to BNT162b2 vaccine (Mann-Whitney test p = 4.9x10-4) at T2. No differences were observed at T3 (Mann-Whitney test p = 0.3). The discrepancy between the three time points analyzed could be due to the effect of vaccination over time or the sample size, significantly smaller at T3 than T2.
Cross sectional and longitudinal study on anti-S and anti-N SARS-CoV-2 antibody levels
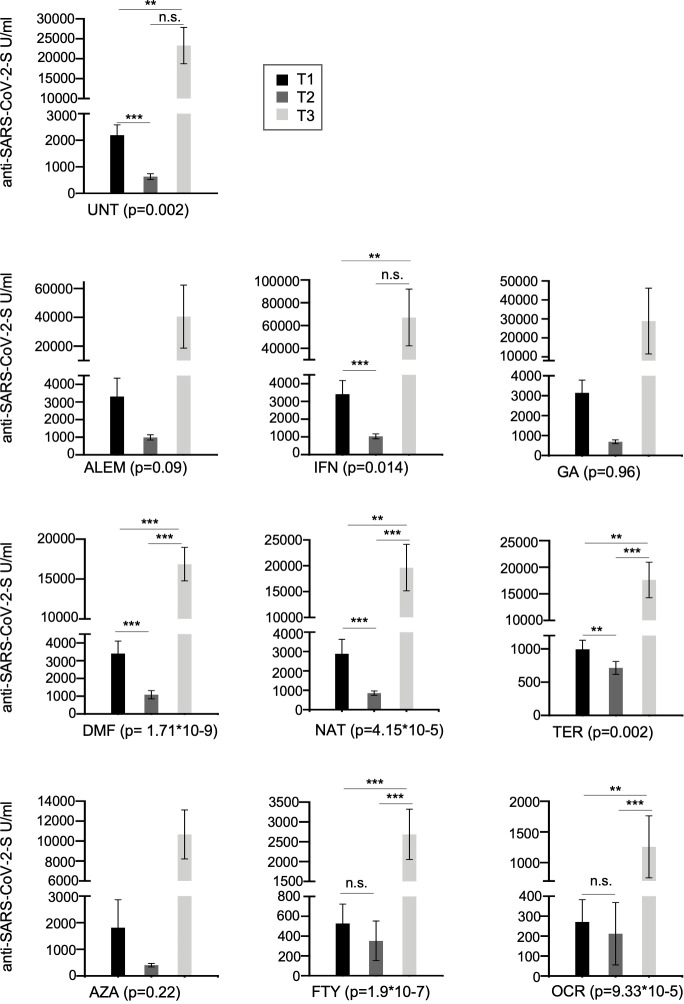
Overall, evaluating the median of anti-S antibody levels at each timepoint (T1-T2-T3) ( Figure 1 ) for all the patients collected, we found that 6 months after the second dose of BNT162b2 vaccination (T2), the levels of anti-S decrease 3-fold compared to T1 (median T1 = 962.2 U/ml and T2 = 323.7 U/ml). Furthermore, in line with previous reports (13), a strong upregulation in serum anti-S antibody levels was observed a month after the BNT162b2 booster (median T3 = 9,758 U/ml). The anti-S antibody median at T3 is ~30-fold higher than T2 and ~10-fold higher than T1. After stratification for each DMTs analyzed in this study, we observed that antibody levels significantly decrease when comparing T1 with T2 for the following treatments: interferon (IFN), DMF, natalizumab (NAT) and teriflunomide (TER) ( Figure 3 ). Furthermore, the booster significantly increased anti-S production for DMF, NAT, TER, FTY and OCR. Interestingly, the increase in anti-S antibodies due to the booster was also observed for FTY and OCR although with a lower impact ( Figure 3 ).
Figure 3.
Cross-sectional humoral response over time (T1, T2 and T3) in all the MS patients treated with different DMTs or untreated. Post-vaccination anti-S antibody response by disease- modifying therapy (DMT) in MS patients. Results are represented as histogram showing the median value. Significance was tested using Mann-Whitney test. (**P<0.01; ***<0.001). (UNT, untreated; ALEM, alemtuzumab; IFN, interferon; GA, glatiramer acetate; DMF, dimethyl fumarate; NAT, natalizumab; CLA,cladribine; TER, terifluomide; AZA, azathioprine; FTY, fingolimod; RTX, rituximab; OCR, ocrelizumab). n.s. indicate not significant.
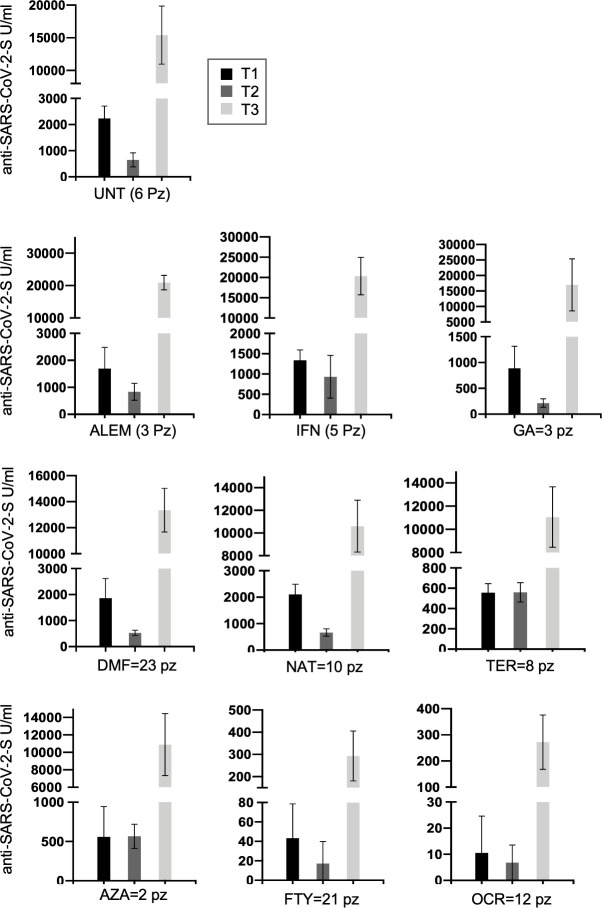
Next a longitudinal analysis was performed on serum levels for the 94 patients analyzed in all the assessed time points (T1, T2, T3). Upregulation of SARS-CoV-2 anti-S antibodies at 4 weeks after the booster (T3) was confirmed in our analysis and highlight the importance of the booster especially for MS patients under anti-CD20 and FTY (Median T1 = 888 U/ml, T2 = 562 U/ml and T3 = 13,346 U/ml) ( Table 4 and Figure 4 ).
Table 4.
Median of anti-S SARS-CoV-2 antibody levels in 94 MS patients enrolled at every time point (T1, T2 and T3) and stratified by DMTs and used for longitudinal analysis.
| DMTs | N | Median anti-S in U/ml (IQR 25-75) | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| ALEM | 3 | 1696 (1204-2297) | 833 (472-871) | 20942 (18717-52661) |
| AZA | 2 | 559 (368-750) | 565 (490-641) | 10894 (9132-12656) |
| DMF | 23 | 1859 (1065-3200) | 530 (293-766) | 13346 (8705-22508) |
| FTY | 21 | 45 (8-113) | 18 (4-156) | 555 (144-3937) |
| GA | 3 | 888 (466-2221) | 214 (132-685) | 16962 (8623-56476) |
| IFN | 5 | 1341 (862-1502) | 934 (500-2849) | 20335 (19660-132280) |
| NAT | 10 | 1700 (853-2787) | 619 (503-746) | 13577 (9596-23535) |
| OCR | 12 | 10 (8-405) | 6 (0.4-99) | 272 (8-978) |
| RTX | 1 | 8 (8-8) | 0.4 (0.4-0.4) | 8 (8-8) |
| TER | 8 | 557 (389-664) | 559 (176-661) | 11060 (6156-17912) |
| UNT | 6 | 2230 (1420-2835) | 647 (342-895) | 15406 (13028-31248) |
UNT, untreated; ALEM, alemtuzumab; IFN, interferon; GA, glatiramer acetate; DMF, dimethyl fumarate; NAT, natalizumab; CLA, cladribine; TER, teriflunomide; AZA, azathioprine; FTY, fingolimod; RTX, rituximab; OCR, ocrelizumab; IQR, interquartile range.
Figure 4.
Longitudinal humoral response over time (T1, T2 and T3) in 94 MS patients treated with different DMTs or untreated and present at each time point. Post-vaccination anti-S antibody response by disease- modifying therapy (DMT) in MS patients is represented longitudinally. Results are represented as histogram showing the median value. (UNT, untreated; ALEM, alemtuzumab; IFN, interferon; GA, glatiramer acetate; DMF, dimethyl fumarate; NAT, natalizumab; CLA, cladribine; TER, terifluomide; AZA, azathioprine; FTY, fingolimod; RTX, rituximab; OCR, ocrelizumab; pz, patients).
Longitudinal analysis of anti-N antibodies also revealed a progressive decline in anti-N proteins from T1 to T3. Indeed, all 13 MS patients positive for SARS-CoV-2 infection at T1 (as determined by anti-N positivity), and with data for at least another time point, were anti-N negative ~7-9 month later demonstrating a consistent loss of circulating humoral response against N antigens over time (data not shown). It is important to take this into account when testing samples without information on COVID-19 disease status. Indeed, if exposure occurred well before the time of antibody testing, it may be impossible to detect individuals with natural humoral immunity against the virus.
Impact of different DMTs on COVID-19 risk and severity
To evaluate the risk of COVID-19 in patients receiving anti-CD20 (OCR and RTX) and FTY therapies, which are associated with reduced humoral responses to vaccine, we assessed the number of MS patients who became positive for COVID-19 five months after the booster. The presence of symptomatic infection and its severity was evaluated. A subgroup of 142 MS patients, who agreed to provide feedback on the occurrence of Sars-Cov-2 infection and eventually its clinical severity was interviewed 5 months after the booster (T3). In these 142 patients examined after the third dose 51 (37%) were treated with anti-CD20 or FTY, while 91 (62.9%) received other therapies or were untreated. Overall, 29 MS patients (20%) of the 142 examined reported occurrence of COVID-19, ascertained by molecular swab or antigenic test. Among them, COVID-19 infection was more common (hazard ratio = 1.95, 95% C.I. = 1.01 to 3.77, p = 0.046) in patients receiving anti-CD20 and FTY therapies (15 of 36 patients) than in those receiving other therapies or untreated (14 of 77 patients). The higher incidence of disease in the group treated with anti-CD20 and FTY compared with the other therapies is consistent with the lower antibody titers observed in these categories of DMT. However, the number of individuals considered here is small; thus, it will be important to consider evidence from larger case series. Interestingly, regardless of the DMT received, all patients reported mild symptoms that did not require hospitalization, but again the exact risk of severe COVID-19 in patients undergoing specific DMTs should be verified in larger data sets.
Discussion
Following previous observations on humoral responses to the BNT162b2 vaccine one month after the second dose of vaccine (T1) in MS patients treated with different DMTs or untreated (2), here we performed a cross-sectional and longitudinal follow-up study to analyze the humoral response in MS patients 6 months (T2) after the second dose and 1 month after the first booster dose (T3) of BNT162b2 vaccine.
In line with early results obtained at T1 (2), we show that, compared to untreated patients, humoral responses in patients treated with FTY, OCR and RTX were impaired at T2 while in general they were not significantly different in patients treated with the other DMTs including alemtuzumab (ALEM), IFN, glatiramer acetate (GA), DMF, NAT, TER and AZA ( Table 2 ).
Regarding the effects of the first booster dose of vaccine, at T3 we observed a strong upregulation of the humoral response in both treated and untreated patients, although the humoral responses observed in patients treated with anti-CD20 (OCR) and FTY remained significantly lower than those observed in untreated patients ( Table 3 and 4 ). Yet, the first booster dose significantly increased anti-S levels even in most patients taking these immunosuppressive therapies, in line with other studies (14). For example, in anti-N negative patients under OCR and FTY, median anti-S antibody levels were, respectively, 40.5 and 28.6-fold higher at T3 than at T1. Interestingly, the data showed the same trend whether we analyzed all the patients collected at each time point (cross-sectional analysis, Figure 3 ) or only the 94 patients analyzed at all time points (longitudinal analysis, Figure 4 and Table 4 ).
Likewise, prior natural SARS-CoV-2 infection, documented through the presence of anti-N antibodies, also strongly potentiated the humoral responses to BNT162b2 vaccine by inducing a strong increase in levels of anti-S antibodies at both T2 and T3 regardless of the DMTs analyzed and including, albeit to a lesser extent, patients treated with anti-CD20 or FTY.
Anti-N antibody analysis also provided additional data on the rate of decline in anti-N seropositivity which is essential for identifying patients with asymptomatic SARS-CoV-2 infection. We found that anti-N antibody levels declined rapidly over time until they were no longer detectable, at least with the assay used in this study, 7 to 9 months after infection. Thus, it is possible that natural asymptomatic SARS-CoV-2 infections during the early phase of the pandemic were not detected in some of the MS patients considered here, and that these early infections still had a residual but not ascertainable effect on anti-S antibody levels produced after vaccination. This is in agreement with a cross-sectional study by Alfego and colleagues that showed a reduction rate of anti-N responses of 68.2% after only 9.7 months following infection, whereas anti-S levels maintained a positivity rate of 87.8% 10 months later (15).
In agreement with previous result at T1 (2), we confirmed that both age and EDSS also influence antibody response to vaccine, in addition to DMTs and prior SARS-CoV-2 infection. Anti-S antibodies levels, however, were significantly lower in older patients at T2 but not a T3. The discrepancy between T1, T2 and T3 may be due to the smaller sample size we analyzed at T3. Similar findings have been reported in a general population (16) where older age was associated with lower seroconversion rates. By contrast, Cohen and colleague have reported associations between older age and higher immune responses to natural infection, including IgG neutralizing antibody and memory B-cell levels (17).
We also evaluated the relationship between antibodies titers in response to BNT162b2 vaccine and active smoking status in a subgroup of MS patients. Our data showed a significant downregulation in anti-S antibodies in MS patients who were active smokers, in line with the association of smoking with immune system dysfunctions (18).
A preliminary observation of this study is the approximately two-fold higher risk of COVID-19 disease observed in the group treated with anti-CD20 and FTY compared with other therapies; a result which is somewhat expected given the lower antibody levels observed in those DMT categories. However, despite the higher incidence of COVID-19, all these patients reported mild symptoms that did not require hospitalization, like those treated with other DMTs. This suggests that vaccination, and in particular booster doses of the vaccine, may still provide additional protection against severe COVID-19 disease most likely by enhancing immune responses like those mediated by T cells, that are not directly affected by these DMTs, and by further stimulating residual B-cell and humoral responses. However, the sample size considered in this study, especially for patients treated with some DMTs, is small to draw firm conclusions. Our observations should be thus expanded to larger case series and further followed over time, including consideration of the immune and clinical impact of additional vaccine booster shots.
Another relevant question to be addressed is whether and which subpopulations of lymphocytes contribute to immune responses to COVID-19 vaccine in the presence of different DMTs. Some interesting studies have already been published. For example, in MS patients treated with anti-CD20 (OCR) has been observed a specific T-cell responses comparable to those of healthy controls after vaccination (19). In contrast, it was reported the absence of SARS-CoV-2 memory T-cell and B-cell immune responses in patients treated with FTY even 3 months after the booster (20). A comprehensive flow cytometry analysis of B-cell and T-cell subpopulations will be fundamental to properly monitor the behavior of these cell populations in MS patients after COVI-19 vaccination undergoing different regime therapies with attention to the ones reducing humoral immune response.
Concluding, the results presented here refer to the evaluation of the effects of the initial anti-Sars-Cov2 vaccination followed by a single booster dose of the vaccine completed several months ago and should therefore be placed in a more current context. Initial reports suggest that an additional booster - already approved by many regulatory agencies for the elderly and immunocompromised individuals - improves protection without affecting safety (21). Given these findings and the improved immune responses to vaccine after prior infection and/or the first booster dose of vaccine, it is likely that the use of further booster shots –with existing mRNA vaccines and possible new generation of mRNA vaccines designed on emerging SARS-CoV-2 variants of concern – will further improve immune responses against SARS-CoV-2 of MS patients under any DMT, and thus their safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was reviewed and approved by the Ethical Review Boards ATS Sardegna - Prot. N° 2492/CE. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MLI, MP, RZ, and ECo organized and participated to sample collection. VL, AL, JF, MZ, FV, GD, MGM, MMi, LL, MFr, DC, ECa, SP, PCa, PCh, and GF contributed to sample collection. MMa and ML provided technical support. SU, EF, PS, and MD provided expertise and critical feedback. MD, MS, and MFl performed statistical analysis. MP, MLI, RZ, ECo, and FC conceived the study. MP and MLI wrote the first draft of the manuscript. MP, MLI, MFl, MD, MS, RZ, ECo, and FC revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The study was supported by the Italian Foundation for Multiple Sclerosis- FISM (Grant N. 22021/Special/002 and 2021/C19-R-Single/010).
Acknowledgments
We thank all the patients and volunteers who generously participated in this study. We would also like to thank all the medical doctors, nurses, and students who have collaborated to the realization of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Multiple sclerosis (MS), disease-modifying therapies (DMTs), severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2), Coronavirus disease 2019 (COVID-19), Antibody (Ab), Spike (S), Nucleocapsid (N).
References
- 1. Pritchard E, Matthews PC, Stoesser N, Eyre DW, Gethings O, Vihta K-D, et al. Impact of vaccination on new SARS-CoV-2 infections in the united kingdom. Nat Med (2021) 27:1370–8. doi: 10.1038/s41591-021-01410-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pitzalis M, Idda ML, Lodde V, Loizedda A, Lobina M, Zoledziewska M, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in sardinian multiple sclerosis patients. Front Immunol (2021) 12:781843. doi: 10.3389/fimmu.2021.781843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Achiron A, Dolev M, Menascu S, Zohar D-N, Dreyer-Alster S, Miron S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler (2021) 27:864–70. doi: 10.1177/13524585211003476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med (2021) 27:1990—2001. doi: 10.1038/s41591-021-01507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zabalza A, Arrambide G, Otero-Romero S, Pappolla A, Tagliani P, López-Maza S, et al. Is humoral and cellular response to SARS-CoV-2 vaccine modified by DMT in patients with multiple sclerosis and other autoimmune diseases? Mult Scler (2022) 28(7). doi: 10.1177/13524585221089540 [DOI] [PubMed] [Google Scholar]
- 6. Moncunill G, Aguilar R, Ribes M, Ortega N, Rubio R, Salmerón G, et al. Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naïve healthcare workers. eBioMedicine (2022) 75:103805. doi: 10.1016/j.ebiom.2021.103805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milo R, Staun-Ram E, Karussis D, Karni A, Hellmann MA, Bar-Haim E, et al. Israeli Neuroimmunology study group on COVID-19 vaccination in multiple sclerosis. humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: An Israeli multi-center experience following 3 vaccine doses. Front Immunol (2022) 13:868915. doi: 10.3389/fimmu.2022.868915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jakimovski D, Zakalik K, Awan S, Kavak KS, Pennington P, Hojnacki D, et al. COVID-19 vaccination in multiple sclerosis and inflammatory diseases: Effects from disease-modifying therapy, long-term seroprevalence and breakthrough infections. Vaccines (2022) 38:e3465. doi: 10.3390/vaccines10050695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Otero-Romero S, Ascherio A, Lebrun-Frénay C. Vaccinations in multiple sclerosis patients receiving disease-modifying drugs. Curr Opin Neurol (2021) 34:322–8. doi: 10.1097/WCO.0000000000000929 [DOI] [PubMed] [Google Scholar]
- 10. Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine (2021) 72:103581. doi: 10.1016/j.ebiom.2021.103581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hilbe J. Negative binomial regression. Cambridge; New York: Cambridge University Press; (2011). [Google Scholar]
- 12. Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev (2021). doi: 10.1002/dmrr.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dreyer-Alster S, Menascu S, Mandel M, Shirbint E, Magalashvili D, Dolev M, et al. COVID-19 vaccination in patients with multiple sclerosis: Safety and humoral efficacy of the third booster dose. J Neurol Sci (2022) 434:120155. doi: 10.1016/j.jns.2022.120155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Capuano R, Altieri M, Conte M, Bisecco A, d'Ambrosio A, Donnarumma G, et al. Humoral response and safety of the third booster dose of BNT162b2 mRNA. COVID-19 vaccine in patients with multiple sclerosis treated with ocrelizumab or fingolimod. J Neurol (2022) 1–8. doi: 10.1007/s00415-022-11296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alfego D, Sullivan A, Poirier B, Williams J, Grover A, Gillim L, et al. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the united states. EClinicalMedicine (2021) 36:100902. doi: 10.1016/j.eclinm.2021.100902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei J, Matthews PC, Stoesser N, Maddox T, Lorenzi L, Studley R, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat Commun (2021) 12:6250. doi: 10.1038/s41467-021-26479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory b and T cells. Cell Rep Med (2021) 2:100354. doi: 10.1016/j.xcrm.2021.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. 23andMe Research Team, HUNT All-In Psychiatry. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brill L, Rechtman A, Zveik O, Haham N, Oiknine-Djian E, Wolf DG, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol (2021) 78:1510–14. doi: 10.1001/jamaneurol.2021.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Achiron A, Mandel M, Gurevich M, Dreyer-Alster S, Magalashvili D, Sonis P, et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J Neurol (2022) 269:2286–92. doi: 10.1007/s00415-022-11030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arbel R, Sergienko R, Friger M, Peretz A, Beckenstein T, Yaron S, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med (2022) 28:1486–90. doi: 10.1038/s41591-022-01832-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.