Abstract
The VirB proteins of Agrobacterium tumefaciens form a transport pore to transfer DNA from bacteria to plants. The assembly of the transport pore will require interaction among the constituent proteins. The identification of proteins that interact with one another can provide clues to the assembly of the transport pore. We studied interaction among four putative transport pore proteins, VirB7, VirB8, VirB9 and VirB10. Using the yeast two-hybrid assay, we observed that VirB8, VirB9, and VirB10 interact with one another. In vitro studies using protein fusions demonstrated that VirB10 interacts with VirB9 and itself. These results suggest that the outer membrane VirB7-VirB9 complex interacts with the inner membrane proteins VirB8 and VirB10 for the assembly of the transport pore. Fusions that contain small, defined segments of the proteins were used to define the interaction domains of VirB8 and VirB9. All interaction domains of both proteins mapped to the N-terminal half of the proteins. Two separate domains at the N- and C-terminal ends of VirB9 are involved in its homotypic interaction, suggesting that VirB9 forms a higher oligomer. We observed that the alteration of serine at position 87 of VirB8 to leucine abolished its DNA transfer function. Studies on the interaction of the mutant protein with the other VirB proteins showed that the VirB8S87L mutant is defective in interaction with VirB9. The mutant, however, interacted efficiently with VirB8 and VirB10, suggesting that the VirB8-VirB9 interaction is essential for DNA transfer.
Agrobacterium tumefaciens transforms plants by donating a segment of its tumor-inducing plasmid (Ti-plasmid) DNA into the plant cell. The transferred DNA (T-DNA) is integrated into the plant nuclear genome and is stably maintained in the transformed plant. The function encoded in the virulence (vir) region of the Ti-plasmid is essential for DNA transfer (reviewed in references 10 and 32). The products of the virB, virD, and virE loci catalyze the processing, transfer, and integration of the T-DNA. The T-DNA is processed by VirD1 and VirD2 to yield a single-stranded T-strand DNA, the intermediate in DNA transfer (25, 29, 31). DNA transfer requires VirD4 and the VirB proteins (5, 30).
The virB operon encodes 11 proteins that are integral membrane proteins or are associated with the membranes (18, 23, 24, 26). These proteins are postulated to form a transport pore structure that allows the T-strand DNA to travel across the bacterial membranes (reviewed in reference 8). Genetic studies using gene fusions with the Escherichia coli phoA gene indicated that several VirB proteins, VirB1, VirB5, VirB6, VirB7, VirB8, VirB9, and VirB10, contain large periplasmic domains (12, 13). Expression of the vir genes leads to the formation of a T pilus that is primarily constituted by VirB2 (17, 19). To facilitate DNA transfer across the bacterial membranes, the transport pore and the T pilus may function in concert.
Several pathogenic and nonpathogenic bacteria contain homologs of the Agrobacterium VirB proteins (reviewed in reference 9). Conjugal plasmids of E. coli and the human pathogens Bordetella pertussis, Helicobacter pylori, Legionella pneumophila, and Rickettsia prowazekii contain several VirB homologs. These systems presumably function in macromolecule transport, suggesting that many bacteria use a common mechanism for the export of biologically active macromolecules. These systems, collectively known as the type IV transport system, allow the transfer of DNA, protein, and other unidentified molecules across the bacterial membranes.
The constituents of a transport pore have not been identified. Assembly of the pore structure will require interactions among the constituent proteins. The identification of these interactions can therefore lead to the identity of the constituent proteins. Two general approaches, chemical cross-linking and immunoprecipitation, have been used to identify proteins involved in homotypic and heterotypic interactions. The use of the chemical cross-linker bis(sulfosuccinimidyl) suberate led to the identification of homo-oligomers of VirB10 (28). Further studies indicated that VirB9 is essential for VirB10 oligomerization, suggesting that interaction of VirB9 and VirB10 is required for oligomerization (4). Using another chemical cross-linker, dimethyl 3,3′-dithiobispropionimidate, we identified the first heteromeric VirB protein complex, a disulfide-linked protein complex of VirB7 and VirB9 (1). The formation of this complex was observed by immunoprecipitation and by the yeast two-hybrid assay (3, 11, 22).
We hypothesized that VirB6, VirB7, VirB8, VirB9, and VirB10 are the constituents of the transport pore (12). VirB6, VirB8, and VirB10 form a complex at the inner membrane while VirB7 and VirB9 form a complex at the outer membrane. VirB7, a lipoprotein, is anchored to the outer membrane by a covalent interaction (13). VirB5 may be a part of the pore complex. A prediction of this hypothesis is that these proteins should interact with one another. To test this hypothesis we used genetic and biochemical methods to study interactions among the putative transport pore proteins. The yeast two-hybrid assay and protein affinity chromatography were used to identify the interacting proteins (20). Our results indicate that the pTiA6 VirB8, VirB9, and VirB10 proteins interact with one another and that interaction of VirB8 with VirB9 is essential for DNA transfer.
MATERIALS AND METHODS
Strains and plasmids.
Strains and plasmids used in this study are listed in Table 1. Plasmids pJK202 and pJG4-5 were used as vectors for the construction of fusions used in the yeast two-hybrid assay (16). Plasmid pJK202 is a derivative of pEG202 that contains a nuclear localization signal sequence. Fusions of the virB proteins were constructed by molecular cloning of an EcoRI fragment containing the appropriate sequences (2). The DNA fragments were generated by PCR-mediated amplification of target sequences using Vent DNA polymerase (New England Biolabs, Inc.). The PCR primers contained a sequence of 18 to 20 homologous nucleotides and were 28 to 30 residues in length. The additional 10 nonhomologous nucleotides at the 5′ end were GGGG (or CCCC), followed by the six-base restriction endonuclease recognition sequence. A generic primer with an EcoRI restriction endonuclease site has the sequence 5′GGGGGAATTCn18–20, where n18–20 is the homologous sequence. The VirB-coding region (and the corresponding residues, numbered according to Ward et al. [26]) present in an individual clone is listed in Table 1 and is indicated by a single-letter amino acid code followed by its position. Where a single residue is identified, the entire coding region, starting from the identified residue, is present. For example, plasmid pAD1483 contains an activator-VirB7 fusion at position 11 of VirB7, where alanine was fused to the activator. The sequence of the junction region of all plasmids were confirmed by DNA sequence analysis using the dideoxy chain termination method with double-stranded DNA template and Sequenase (U.S. Biochemical Corporation) (21).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA relA1 λ− φ80d/lacZΔM15 (ΔlacZYA-argF)U169 | Laboratory stock |
| A. tumefaciens | ||
| A136 | C58 heat cured of pTiC58 | Laboratory stock |
| A348 | A136 containing pTiA6 | Laboratory stock |
| A348ΔB8 | A348 containing a deletion in pTiA6 virB8 | 5 |
| Saccharomyces cerevisiae | ||
| EGY48 | ura3 his3 trp1 LexAop-leu2 | 16 |
| AD842 | EGY48 containing pSH18-34 | 11 |
| Plasmids | ||
| pEG202 | Cloning vector for the construction of LexA fusions | 16 |
| pJK202 | Derivative of pEG202 with a nuclear localization signal | A. Peterson |
| pJG4-5 | Cloning vector for the construction of activator fusions | 16 |
| pSH18-34 | A plasmid containing Gal1-LexAop-lacZ, a reporter gene in yeast | 16 |
| pAD1483 | 0.25-kb EcoRI fragment containing virB7 obtained by PCR amplification in pEG202 (activator-VirB7 A11 fusion; bp 6261–6509 of Ward et al. [26]) | This study |
| pAD1522 | 1.0-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB10 I47 fusion; bp 8111–9108) | This study |
| pAD1523 | 0.6-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB8 T60 fusion; bp 6562–7197) | This study |
| pAD1663 | 0.6-kb EcoRI-XhoI fragment of virB8S87L obtained by PCR amplification in pJK202 (LexA-VirB8S87L fusion at T60) | This study |
| pAD1526 | 0.9-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB9 T17 fusion; bp 7143–8019) | This study |
| pAD1447 | 0.25-kb EcoRI fragment containing virB7 obtained by PCR amplification of pAD1399 in pJG4-5 (activator-VirB7 A11 fusion) | 11 |
| pAD1493 | 1.0-kb EcoRI fragment obtained by PCR amplification in pJG4-5 (activator-VirB10 I47 fusion; bp 8111–9108) | This study |
| pAD1516 | 0.6-kb EcoRI-XhoI fragment obtained by PCR amplification in pJG4-5 (activator-VirB8 T60 fusion; bp 6562–7197) | This study |
| pAD1517 | 0.9-kb EcoRI fragment obtained by PCR amplification in pJG4-5 (activator-VirB9 T17 fusion; bp 7143–8019) | This study |
| pAD1529 | 0.35-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB8 T60-T172 fusion; bp 6562–6900) | This study |
| pAD1530 | 0.33-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB8 P145-P237 fusion; bp 6817–7098) | This study |
| pAD1531 | 0.33-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB9 T17-H122 fusion; bp 7143–7460) | This study |
| pAD1533 | 0.45-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB9 D75-P220 fusion; bp 7317–7754) | This study |
| pAD1492 | 0.4-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB9 L173-293 fusion; bp 7611–8019) | This study |
| pAD1528 | 0.7-kb EcoRI-SalI fragment obtained by PCR amplification in pJK202 (LexA-VirB10 I47-V277 fusion; bp 8111–8803) | This study |
| pAD1534 | 0.65-kb EcoRI fragment obtained by PCR amplification in pJK202 (LexA-VirB10 R161-377 fusion; bp 8453–9108) | This study |
| pAD1598 | 1.0-kb EcoRI fragment of pAD1493 in pRsetB (His-VirB10 fusion) | This study |
| pAD1599 | 1.0-kb EcoRI fragment of pAD1493 in pGEX1λT (GST-VirB10 fusion) | This study |
| pAD1601 | 0.6-kb EcoRI fragment of pAD1523 in pGEX1λT (GST-VirB8 fusion) | This study |
| pAD1606 | 0.9-kb EcoRI fragment of pAD1517 in pGEX1λT (GST-VirB9 fusion) | This study |
The appropriate EcoRI fragments were cloned into the EcoRI site of plasmids pRsetB (Invitrogen Corp.) and pGEX1λT (Pharmacia) to construct histidine-tagged proteins and fusions with glutathione S-transferase (GST), respectively. Plasmid pAD1274 expresses a trpE-virB10 fusion protein and was constructed by cloning a 1.1-kb BamHI fragment that encodes the entire VirB10 coding region except for the first 17 residues into the BamHI site of plasmid pATH3.
Two-hybrid assay.
Two-hybrid assay with yeast was performed essentially as described earlier (11). The LexA and activator fusion plasmids were introduced into yeast AD842 by transformation (16) and tested for interaction by colony color phenotype on plates containing the chromogenic substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and by growth in leucine-free medium. For control experiments, the second vector plasmid was introduced into yeast AD842 containing the LexA-activator fusion and tested for the lacZ phenotype. The method for monitoring β-galactosidase activity has been described previously (11).
Protein binding assay.
In vitro analysis of protein-protein interaction was monitored by a “GST-pulldown assay” (2). An E. coli strain containing a GST-VirB fusion or a His-VirB fusion was grown overnight in Luria-Bertani medium containing ampicillin (final concentration, 100 μg/ml). Cells were diluted 1:100 in fresh medium, and 400-ml cultures were grown to an A600 of 0.6 to 0.8. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 0.1 mM, and the culture was grown for an additional 1 h. Bacteria were collected by centrifugation, washed with cold 0.8% NaCl, and stored frozen in four aliquots. Frozen cell pellet (from 100 ml of culture) was thawed and resuspended in 5 ml of lysis buffer (10% sucrose, 40 mM Tris-HCl [pH 7.5], 50 mM NaCl, 0.2 mM EDTA). After addition of lysozyme (final concentration, 0.4 mg/ml) and phenylmethylsulfonyl fluoride (final concentration, 1 mM), the mixture was incubated for 20 min on ice. Bacteria were lysed in a French pressure cell, and the lysate was centrifuged at 12,000 × g for 15 min. The supernatant was collected and stored frozen in 1-ml aliquots.
GST-Sepharose beads (packed volume, 0.4 ml) was washed four times with 1× binding buffer (25 mM Tris-HCl [pH 7.6], 50 mM NaCl, 0.05% Triton X-100). The washed beads were mixed with 1 ml of cell lysate, and the mixture was placed on a rotator for 1 h at 4°C. The mixture was centrifuged at 1,000 × g for 1 min to pellet the beads. After three washings with 1× binding buffer, beads were stored at 4°C until use.
To study interactions, the GST-VirB fusion bound to glutathione-Sepharose (10 to 50 μl) was preincubated with 2.5% bovine serum albumin in 1× binding buffer for 30 min at 4°C. The reaction mixture was centrifuged for 1 min, and the supernatant was discarded. Purified His-VirB10 (200 μl) and bovine serum albumin (final concentration, 2.5%) was added to the pellet. The mixture was incubated for 1 h at 4°C on a rotator. The beads were collected by centrifugation, washed four times with 1× binding buffer, and resuspended in 20 μl of 2× sodium dodecyl sulfate (SDS) denaturation buffer. Samples were electrophoresed on an SDS–12.5% polyacrylamide gel and analyzed by Western blot assay using purified anti-VirB10 antibodies (1). Anti-VirB10 antibodies were raised in rabbits by immunizing them against the TrpE-VirB10 fusion protein. Antibodies were purified by affinity chromatography on solid support containing immobilized TrpE-VirB10.
VirB8 mutant.
The virB8S87L mutant was constructed by site-specific mutagenesis of virB8 using single-stranded pAD1420 DNA as a template (1). Plasmid pAD1420 contains virB7 and virB8 under the control of the virD promoter and was constructed by cloning a 1.66-kb blunt-ended AlwNI-SphI fragment (bp 6131 to 7197) into plasmid pAD1416. Plasmid pAD1416 is a pUC118 derivative containing a 391-bp virD promoter fragment (1). Plasmids pAD1420 and pAD1420S87L were fused to the broad-host-range vector pTJS75 to construct pAD1433 and pAD1433S87L, respectively. The two plasmids were introduced into Agrobacterium sp. strain A348ΔB8 (5) for complementation studies.
RESULTS
Interactions of VirB7, VirB8, VirB9, and VirB10.
We used the yeast two-hybrid assay to study interactions among four putative transport pore proteins, VirB7, VirB8, VirB9, and VirB10. Biochemical and genetic studies suggest that these proteins are likely to interact with one another (1, 3, 4, 15, 22, 27). The cellular location of the four proteins will support interactions between them since they either have a large periplasmic domain or reside in the periplasm (12). To study protein-protein interactions by the two-hybrid assay, each gene was cloned into the appropriate vector to construct a fusion with either the LexA DNA binding domain or the acidic activator (16). The hydrophilic regions of the proteins were used for fusion construction to avoid potential complications, if any, from the endogenous hydrophobic sequences.
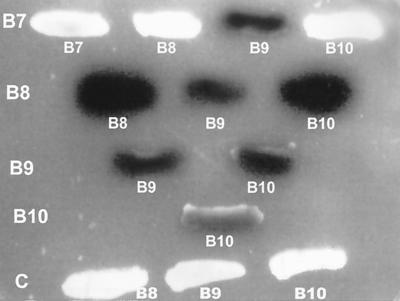
Transcription activation of a lacZ reporter gene was used to monitor protein-protein interactions. A yeast strain harboring both the LexA fusion and the activator fusion was plated on solid medium containing the chromogenic substrate X-Gal. A lacZ-positive strain exhibits a blue colony color phenotype. Studies on interactions of the VirB proteins indicated that VirB7 interacts with VirB9 (Fig. 1). It does not interact with either VirB8 or VirB10 (row 1). A weak interaction of VirB7 with itself was observed. The failure of the VirB7 fusion to interact with VirB8 and VirB10 demonstrates that this fusion does not self-activate. VirB8 interacts with VirB9, VirB10, and itself (row 2). VirB9 interacts with VirB8, VirB10, and itself (rows 2 and 3). VirB10 interacts with VirB8, VirB9, and itself (rows 2 to 4). In control experiments, VirB8, VirB9, and VirB10 showed no interaction with the activator protein (row 5). These results indicate that three proteins, VirB8, VirB9, and VirB10, interact with one another and that VirB7 interacts with VirB9. In addition, all four proteins participate in homotypic interactions. Both homotypic and heterotypic interactions of VirB7 and VirB9 have previously been reported (1, 3, 11, 22).
FIG. 1.
Interactions of VirB7, VirB8, VirB9, and VirB10. Interaction of VirB proteins was studied by the yeast two-hybrid assay (16). Yeast strains harboring the appropriate plasmids were streaked on solid medium containing X-Gal. A positive interaction is manifested by the blue colony color phenotype. The interactions of VirB7 (B7), VirB8 (B8), VirB9 (B9), and VirB10 (B10) with one another were investigated. One interacting protein is indicated on the left column and the partner proteins are indicated under the colony. For control (C) experiments, the activator vector was introduced into a strain containing the LexA-VirB8, -VirB9, or -VirB10 fusion.
VirB10 interacts with the other VirB proteins in vitro.
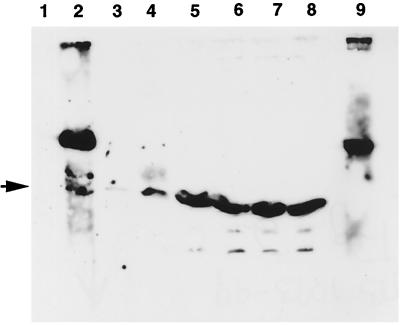
Studies with the yeast two-hybrid assays indicated that VirB8, VirB9, and VirB10 interact with one another. To confirm these interactions, we used a biochemical method that involved affinity chromatography. We analyzed interaction of VirB10 with fusions of VirB8, VirB9, and VirB10 by the GST pulldown assay (2). The periplasmic domains of VirB8, VirB9, and VirB10 were fused to GST, and the fusion proteins were immobilized on glutathione-Sepharose beads. Following incubation with a histidine-tagged VirB10 fusion protein, proteins bound to glutathione-Sepharose were identified by Western blot assays following SDS-polyacrylamide gel electrophoresis (Fig. 2). The VirB10 fusion bound to both the GST-VirB10 and GST-VirB9 fusions (lanes 2 and 4). A very low level of binding of VirB10 to GST-VirB8 was also observed (lane 3). In control experiments, no binding of VirB10 to GST-Sepharose was observed (lane 1). These results indicate that VirB10 interacts with VirB9 and VirB10 and interacts weakly with VirB8.
FIG. 2.
In vitro analysis of the interactions of VirB10. Interaction of VirB10 with VirB8, VirB9, and VirB10 was monitored by the GST-pulldown assay (2). Purified histidine-tagged VirB10 was incubated with GST or a GST fusion protein immobilized on glutathione-Sepharose. Binding of His-VirB10 was determined by analysis of bound proteins by SDS-polyacrylamide gel electrophoresis, followed by Western blot analysis using anti-VirB10 antibodies. Lanes 1 to 4, protein bound to immobilized GST, GST-VirB10, GST-VirB8, and GST-VirB9, respectively; lanes 5 to 8, the corresponding unbound proteins; lane 9, immobilized GST-VirB10 incubated with buffer only. The higher-molecular-weight band in lane 2 is the GST-VirB10 fusion protein. The His-VirB10 protein is indicated by the arrowhead.
The N-terminal domain of VirB8 contains two interaction domains.
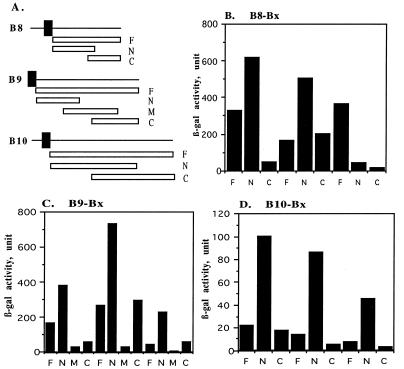
To identify sequences of VirB8 involved in its interactions with the VirB proteins, we constructed two new fusions where amino acids 60 to 172 (N-terminal fragment) or amino acids 145 to 237 (end) (C-terminal fragment) of VirB8 were fused to the DNA binding domain of LexA (Fig. 3A). The two fusions were introduced into a yeast strain containing the activator-VirB8, -VirB9, or -VirB10 fusion protein and tested for interaction by monitoring β-galactosidase activity in liquid assays (Fig. 3B). The N-terminal domain fusion was active in interactions with both VirB8 and VirB9, indicating that the 113-residue N-terminal segment contains both interaction domains. This fusion did not interact with VirB10. The failure of the N-terminal fusion to interact with VirB10 serves as a control demonstrating that the fusion does not interact nonspecifically. The C-terminal fusion did not interact with VirB8 and VirB10 but interacted with VirB9. These results demonstrate that both the N- and C-terminal domains, which share an overlapping region (residues 145 to 172), contain a VirB9 interaction domain(s). Either the overlapping sequences contain the interaction domain or there are two different VirB8 domains that are proficient in interaction with VirB9. In VirB8-VirB8 interaction only, the N-terminal fusion is active, indicating that this region contains the VirB8 interaction domain. The failure of both smaller fusions to interact with VirB10 suggests that the VirB10 interaction domain was probably destroyed during fusion construction. This domain either lies near the middle of the protein or is composed of sequences from both regions. Both fusions were active in interaction with other proteins, indicating that the lack of interaction with VirB10 is not due to instability or inappropriate localization of the fusion protein.
FIG. 3.
Delineation of interaction domains of VirB8, VirB9, and VirB10. Interactions of VirB8 (B), VirB9 (C), VirB10 (D), and their derivatives with VirB8, VirB9, and VirB10 were investigated by the two-hybrid assay. (A) A linear representation of the VirB proteins and their derivatives used for fusion construction. Fusions containing the periplasmic domain (F), an N-terminal fragment (N), a central fragment (M), and a C-terminal fragment (C) were tested for interaction with the three proteins. The solid box indicates a hydrophobic region.
Two distinct domains participate in VirB9-VirB9 interaction.
To identify the interaction domains of VirB9, we constructed three fusions in which the LexA DNA binding domain was fused to residues 17 to 122, 75 to 220, and 173 to 293 of VirB9 (Fig. 3A). The fusions were introduced into the appropriate yeast strains to study the interaction with the VirB proteins. The N-terminal fusion (residues 17 to 122) interacted with all three proteins, indicating that this region contains the VirB8, VirB9, and VirB10 interaction domains (Fig. 3C). In control experiments, the fusion did not interact with the unrelated Drosophila homeotic proteins Scm and Esc (data not shown). The central fragment interacted with none of the proteins. The C-terminal domain fusion interacted with VirB9 and not with VirB8 or VirB10. A fusion containing either the N-terminal or C-terminal region was proficient in VirB9-VirB9 interaction. Since the two fusions share no common sequence, two separate domains must be involved in VirB9-VirB9 interactions. One of the domains may participate in dimerization while the other may be involved in forming a higher oligomer. In a previous study, we demonstrated that the C-terminal domain of VirB9 contains a second interaction domain, the VirB7 interaction domain (11). Therefore, both the N-terminal and C-terminal regions of VirB9 contain multiple interaction domains.
Studies on the VirB9-VirB10 interaction showed that the VirB9 N-terminal fusion interacted efficiently with VirB10, indicating that this domain contains the VirB10 interaction domain (Fig. 3C). The fusion containing the entire periplasmic domain of VirB9 had a very low level of β-galactosidase activity, indicating that the larger protein is extremely inefficient in interaction with VirB10 (bar 9). However, the N-terminal fusion was highly active in interaction with VirB10 (bar 10). A possible explanation for these observations is that VirB9 can interact with VirB10 only when it is in a protein complex. In the fusion proteins, the smaller fragment can adopt an interaction proficient conformation more efficiently than the full-length protein can.
Interaction domains of VirB10.
Two VirB10 fusions that contained residues 47 to 277 or 167 to 377 fused to the LexA DNA binding domain were analyzed to identify the VirB10 interaction domains. The N-terminal domain fusion interacted with VirB8, VirB9, and VirB10, indicating that this fragment contains all three interaction domains (Fig. 3D). The C-terminal fragment interacted with none of the proteins. Since the two fragments have a long overlap (111 amino acids) and the C-terminal fusion is inactive in all interactions, all the interaction domains must be contained within the N-terminal half of the protein. Interestingly, the LexA-VirB10 fusion that contained the entire periplasmic domain (residues 47 to 377) failed to interact with all three proteins (bars 1, 4, and 7). However, two sets of the reciprocal fusions (VirB8-LexA– VirB10-activator and VirB9-LexA–VirB10-activator) were functional (Fig. 3B and C). These results suggest that the VirB10-LexA fusion is inactive or has another deficiency. The LexA-VirB10 N-terminal domain fusion, however, was active in interaction with all three proteins, indicating that VirB10 can interact with the three VirB proteins. The larger fusion may contain an autoinhibitory domain or the truncated fusion can more easily adopt a conformation essential for interaction with the other proteins.
Interaction of VirB8 with VirB9 is essential for T-DNA transfer.
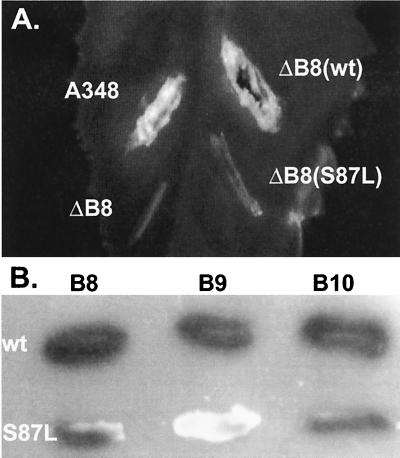
In the course of a separate study aimed at defining the functional domains of VirB8 (to be described elsewhere), we constructed a mutant, virB8S87L, that failed to complement a virB8 deletion mutant in tumor formation assays (Fig. 4A). virB8S87L has a C→T substitution at residue 6644 that resulted in the substitution of leucine for serine at position 87. Analysis of the protein by Western blot assays indicated that the mutation did not affect protein stability (data not shown). To study whether it has an effect on the interaction of VirB8 with the other VirB proteins, we used the yeast two-hybrid assay. VirB8S87L interacted efficiently with VirB10 and itself, but failed to interact with VirB9 (Fig. 4B). These results indicate that interaction between VirB8 and VirB9 is essential for DNA transfer and confirms the finding (Fig. 2B) that the N-terminal domain of VirB8 contains the VirB9 interaction domain.
FIG. 4.
Phenotype of a virB8 mutant. The effect of alteration of VirB8 serine 87 to leucine on DNA transfer was monitored by complementation assays (A). Agrobacterium sp. strain A348ΔB8 (ΔB8) harboring plasmid pAD1433 containing wild-type (wt) virB8 or its derivative pAD1433S87L containing virB8S87L (S87L) was used to infect Kalanchöe leaves. Tumor formation was monitored 3 weeks after infection. A348, Agrobacterium sp. strain A348 that harbors the octopine Ti-plasmid pTiA6. (B) Interaction of VirB8S87L with VirB8, VirB9, and VirB10 was studied by the yeast two-hybrid assays. A blue colony color phenotype indicates a positive interaction. Row 1, VirB8; row 2, VirB8S87L.
DISCUSSION
The Agrobacterium VirB proteins presumably form a transport pore to allow DNA transfer to plants. The identities of the transport pore constituent proteins are not known. Since assembly of the transport pore will require interactions among the constituent proteins, analysis of interactions of the VirB proteins will greatly facilitate the identification of the transport pore constituents. Two VirB proteins, VirB7 and VirB9, interact with each other (1, 22). The VirB7-VirB9 complex is anchored to the outer membrane by a covalent interaction of VirB7 (13). Other proteins that are part of the transport pore will interact with VirB7 and/or VirB9. The present study shows that VirB9 interacts with two other VirB proteins, VirB8 and VirB10. VirB8 and VirB10 interact with each other. VirB7, on the other hand, does not interact with either VirB8 or VirB10. VirB8 and VirB10 are integral membrane proteins anchored to the inner membrane. Interaction of two proteins with VirB9, a protein in an outer membrane complex, supports our proposed model of the transport pore that suggested that the VirB7-VirB9 complex interacts directly with an inner membrane protein complex of VirB6, VirB8, and VirB10 (12).
The present study identified three new intermolecular complexes, the VirB8-VirB9, VirB8-VirB10, and VirB9-VirB10 complexes. The N-terminal half of the periplasmic domain of VirB8 contains the VirB9 and self-interacting domains. The N-terminal third of VirB9 contains domains required for its interaction with VirB8, VirB10, and itself. The C-terminal third contains the VirB7 interaction domain and a second VirB9 interaction domain. The discovery of the second VirB9 interaction domain suggests that transport pore assembly may require the formation of a higher oligomer of VirB9. These results also suggest that the delineation of sequences involved in an interaction is necessary to unmask all interaction domains.
The yeast two-hybrid assay is a powerful genetic tool to study protein-protein interactions (7, 14). A positive result is indicative of an interaction while a negative result is not conclusive. In studies with VirB10, we observed that a LexA-VirB10 fusion failed to interact with the VirB8-activator, VirB9-activator, and VirB10-activator fusions (Fig. 3D). However, an activator-VirB10 fusion was positive in interaction with both LexA-VirB8 and LexA-VirB9 fusions (Fig. 3B and C). These results indicate that it is necessary to analyze both sets of fusions to avoid a false negative result. Another interesting observation we made is that in a few cases, a smaller fusion was more efficient in an interaction than a larger fusion. In VirB9-VirB10 interaction, a fusion that contained the N-terminal segments of both VirB9 and VirB10 were much more efficient than the fusion with the entire periplasmic domain (Fig. 3). There are several explanations for this observation. First, the expression, stability, and/or nuclear localization of the smaller fusion are higher. Second, the smaller fusion can more easily adopt an interaction-proficient conformation. This is likely to be the case if a conformational change is required for an interaction. An example is the interaction of a protein with a protein complex. The partner protein in the protein complex can undergo a conformational change due to its interaction with the other protein in the complex. The modified conformation is essential for the new interaction. In the large fusion, the active conformation cannot be attained because of the absence of the third protein. The smaller fusion can better mimic the active conformation due to the lack of one or more domains. Therefore, the use of smaller fragments can unveil an interaction between a protein and a protein complex. Third, results with the small fragments may be artifact; however, this is unlikely because a false positive is not highly prevalent in this assay (7).
What is the biological significance of the interactions of the VirB proteins? The isolation of the avirulent virB8 mutant, virB8S87L, that failed to interact with VirB9 but was active in interaction with VirB8 and VirB10 suggests that the interaction of VirB8 and VirB9 is essential for DNA transfer. VirB10 forms homo-o-ligomers (28). The requirement of VirB9 in the oligomerization of VirB10 suggests that VirB9 interacts with VirB10 (6). The present study provides direct evidence for this interaction. Several virB9 mutants that are defective in DNA transfer are also defective in VirB10 oligomerization, indicating that interaction of VirB9 and VirB10 is essential for DNA transfer (4). The role of the other interactions is presently under investigation.
Interaction of VirB9 with the two integral membrane proteins VirB8 and VirB10 suggests that the VirB7-VirB9 complex can interact directly with the inner membrane complex. Interaction of VirB8 with VirB10 suggests that these two proteins are components of a complex at the inner membrane. We hypothesize that VirB6, a protein with multiple transmembrane domains, is a third component of this complex. This complex interacts with the VirB7-VirB9 complex to form the transport pore (Fig. 5). This model suggests that interactions of VirB6, VirB7, VirB8, VirB9, and VirB10 lead to the formation of a unit complex. Oligomerization of the unit complex will form the transport pore. Homotypic interactions of the VirB proteins observed here and in previous studies can play an important role in oligomerization. Our results that demonstrated the interaction of VirB9 with VirB8 and VirB10 support this model and rule out the possibility that another protein (e.g., VirB5) functions as a linker between the two complexes. In the latter case, VirB9 should not interact with VirB8 and VirB10. The transport pore complex spans the inner membrane and the interior face of the outer membrane. To exit the outer membrane, a pore through this membrane is essential. Either the T pilus or a chromosomal porin performs this function.
FIG. 5.
A model for the T-DNA transport pore. Individual VirB proteins are identified by numbers. The T pilus composed of VirB2 can form a channel through the outer membrane.
ACKNOWLEDGMENTS
We thank Roger Brent for the plasmids and strains for the yeast two-hybrid assays, Peter Christie for the Agrobacterium sp. strain A348ΔB8 mutant, and Aidan Peterson for the Drosophila clones and advice on in vitro interaction studies.
This work was supported in part by grants from the University of Minnesota Agricultural Experiment Station and the University of Minnesota Graduate School.
REFERENCES
- 1.Anderson L B, Hertzel A V, Das A. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc Natl Acad Sci USA. 1996;93:8889–8894. doi: 10.1073/pnas.93.17.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene and Wiley-Interscience; 1993. [Google Scholar]
- 3.Baron C, Thorstenson Y R, Zambryski P C. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaupre C E, Bohne J, Dale E M, Binns A N. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1997;179:78–89. doi: 10.1128/jb.179.1.78-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beijersbergen A, Dulk-Ras A D, Schilperoort R A, Hooykaas P J. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 6.Berger B, Christie P. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brent R, Finley R L J. Understanding gene and allele function with two-hybrid methods. Annu Rev Genet. 1997;31:663–704. doi: 10.1146/annurev.genet.31.1.663. [DOI] [PubMed] [Google Scholar]
- 8.Christie P. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 10.Das A. DNA transfer from Agrobacterium to plant cells in crown gall tumor disease. In: Biswas B B, Das H K, editors. Subcellular biochemistry: plant microbe interactions. London, England: Plenum Publishing Corp.; 1998. pp. 343–363. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Anderson L, Xie Y-H. Delineation of the interaction domains of Agrobacterium tumefaciens VirB7 and VirB9 by use of the yeast two-hybrid assay. J Bacteriol. 1997;179:3404–3409. doi: 10.1128/jb.179.11.3404-3409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. doi: 10.1046/j.1365-2958.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez D, Dang T A, Spudich G, Zhou X-R, Berger B, Christie P. The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T-complex transport apparatus, is a membrane-associated lipoprotein exposed at the periplasmic surface. J Bacteriol. 1996;178:3156–3167. doi: 10.1128/jb.178.11.3156-3167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 15.Finberg K E, Muth T R, Young S P, Maken J B, Heitritter S M, Binns A N, Banta L M. Interactions of VirB9, -10, and -11 with the membrane fraction of Agrobacterium tumefaciens: solubility studies provide evidence for tight associations. J Bacteriol. 1995;177:4881–4889. doi: 10.1128/jb.177.17.4881-4889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finley R, Brent R. Interaction trap cloning in yeast. In: Hames B, Glover D, editors. DNA cloning, expression systems—a practical approach. Oxford, England: Oxford University Press; 1995. pp. 169–203. [Google Scholar]
- 17.Fullner K, Lara J C, Nester E. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1107–1109. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- 18.Kuldau G A, De Vos G, Owen J, McCaffrey G, Zambryski P. The virB operon of Agrobacterium tumefaciens pTiC58 encodes 11 open reading frames. Mol Gen Genet. 1990;221:256–266. doi: 10.1007/BF00261729. [DOI] [PubMed] [Google Scholar]
- 19.Lai E M, Kado C I. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol. 1997;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phizicky E M, Fields S. Protein-protein interactions: methods for detection and analysis. Microbiol Rev. 1995;59:94–123. doi: 10.1128/mr.59.1.94-123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spudich G, Fernandez D, Zhou X-R, Christie P. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport pore. Proc Natl Acad Sci USA. 1996;93:7512–7517. doi: 10.1073/pnas.93.15.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson D V, Melchers L S, Idler K B, Schilperoort R A, Hooykaas P J. Analysis of the complete nucleotide sequence of the Agrobacterium tumefaciens virB operon. Nucleic Acids Res. 1988;16:4621–4636. doi: 10.1093/nar/16.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorstenson Y R, Kuldau G A, Zambryski P C. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J Bacteriol. 1993;175:5233–5241. doi: 10.1128/jb.175.16.5233-5241.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinland B, Hohn B, Puchta H. Agrobacterium tumefaciens transfers single-stranded transferred DNA (T-DNA) into the plant cell nucleus. Proc Natl Acad Sci USA. 1994;91:8000–8004. doi: 10.1073/pnas.91.17.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward J E, Akiyoshi D E, Regier D, Datta A, Gordon M P, Nester E. Characterization of the virB operon from Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988;263:5804–5814. . (Author's correction, 265:4768, 1990). [PubMed] [Google Scholar]
- 27.Ward J E, Dale E M, Binns A N. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc Natl Acad Sci USA. 1991;88:9350–9354. doi: 10.1073/pnas.88.20.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward J E, Jr, Dale E M, Nester E W, Binns A N. Identification of a VirB10 protein aggregate in the inner membrane of Agrobacterium tumefaciens. J Bacteriol. 1990;172:5200–5210. doi: 10.1128/jb.172.9.5200-5210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanofsky M F, Porter S G, Young C, Albright L M, Gordon M P, Nester E W. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell. 1986;47:471–477. doi: 10.1016/0092-8674(86)90604-5. [DOI] [PubMed] [Google Scholar]
- 30.Yoshioka Y, Takahashi Y, Matsumoto S, Kojima S, Matsuoka K, Nakamura K, Ohshima K, Okada N, Machida Y. Mechanisms of T-DNA transfer and integration into plant chromosomes: role of virB, virD4 and virE2 and a short interspersed repetitive element (SINE) from tobacco. In: Kado C, Crosa J, editors. Molecular mechanisms of bacterial virulence. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 231–248. [Google Scholar]
- 31.Yusibov V, Steck T, Gupta V, Gelvin S. Association of single-stranded transferred DNA from Agrobacterium tumefaciens with tobacco cells. Proc Natl Acad Sci USA. 1994;91:2994–2998. doi: 10.1073/pnas.91.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zupan J, Zambryski P. Transfer of T-DNA from Agrobacterium to the plant cell. Plant Physiol. 1995;107:1041–1047. doi: 10.1104/pp.107.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]