Highlights
-
•
Invasive group B streptococcal (GBS) infections can occur in any age group, not only neonates.
-
•
Most of the patients are elderly or have severe concomitant diseases.
-
•
Screening-based intrapartum antibiotic prophylaxis (based on maternal risk factors) significantly decreases very early-onset infections.
Key words: group B streptococcus, invasive infection, age difference, prognosis
Abbreviations: GBS, Group B streptococcus (Streptococcus agalactiae); CSF, cerebrospinal fluid
Abstract
Objectives
This was a retropective study of invasive group B streptococcal (GBS) infections isolated from blood, cerebrospinal fluid (CSF), synovial fluid, peritoneal fluid, pleural fluid, pericardial fluid and corpus vitreum in a defined region in southwest Sweden over a 14-year period.
Design
Information on all invasive GBS infections was obtained from all four bacteriological laboratories in the region, with data obtained from individual patient records.
Results
GBS was isolated from normally sterile body fluids in 1244 samples (579 from females and 665 from males) from 1101 patients. Of these patients, 196 were neonates. The incidence in neonates (0–27 days) was 7.3 per 100 000 live births per year, but there was a significant decrease from 2012 when risk-factor-based intrapartum antibibiotic prophylaxis was implemented. The great majority of neonatal infections were very early-onset infections. The incidence rates in children (28 days to 17 years), adults (18–64 years) and elderly patients (≥ 65 years) were 1.3, 3.6, and 12.9 per 100 000 per year, respectively. The majority of children and adults had severe underlying diseases, but severe infections were also seen in individuals with no risk factors.
Conclusions
GBS is an important pathogen in all age groups. Intrapartum antibiotic prophylaxis significantly decreases very early-onset infections.
Introduction
Streptococcus agalactiae (a group B streptococcus (GBS)) was initially well known within veterinary medicine as a cause of mastitis in cows (Rosa-Fraile et al, 2017; Anthony and Okada 1977; Schuchat, 1998). During the 1970s it emerged as a human pathogen, in particular among neonates (McCracken, 1973; Rosa-Fraile and Spellerberg, 1973; Freedman et al., 1981) and soon became the most common cause of neonatal sepsis (Franciosi et al., 1973; McCracken, 1973; Anthony and Okada, 1977; Tessin et al., 1990; Johansson Gudjónsdóttir et al., 2019). Later, GBS was also shown to be an important pathogen in the elderly (≥ 65 years) and in children and younger adults with severe underlying diseases (Hussain et al., 1995; Farley et al., 1993; Tyrrell et al., 2000; Dahl et al., 2003).
The aim of this study was to evaluate the total burden of invasive GBS infections in terms of incidence, hospitalizations, manifestations, risk factors, and short-term mortality. A second aim was to investigate whether the incidence of neonatal very early-onset infections (0–2 days of age) decreased after 2012 when selective intrapartum antibiotic prohylaxis was implemented.
Patients and Methods
This was a retrospective study of invasive GBS infections in all age groups in the region of Västra Götaland, southwest Sweden, during 2003–2016. The mean population in the region was 1 267 512 during the 14-year study period. A request was sent to the four clinical bacteriology laboratories that cover all hospitals and outpatient clinics in the whole region. The laboratories were asked to identify all patients from whom GBS was isolated in blood, cerebrospinal fluid (CSF), synovial fluid, pleural fluid, peritoneal fluid, pericardial fluid, and corpus vitreum during 2003–2016. All laboratories responded and, in total, 1134 patients with 1277 infectious episodes were identified. Twenty-eight patients living outside of the area were excluded, as were five neonates from neighbouring regions, who had been sent to the neonatal intensive care unit at Sahlgrenska University Hospital in Gothenburg, which is the largest city in the Västra Götaland region. The patients were divided into four groups according to age: neonates (0–27 days after birth), children (28 days to 17 years of age), adults (18–64 years of age), and elderly (≥ 65 years of age). The neonates were divided into three groups: very early onset (0–2 days after birth), early onset (3–6 days after birth), and late onset (7–27 days after birth). A fourth group of neonates with very late-onset infections was defined as those with onset of symptoms between 28 and 89 days after birth. For analysis purposes, these were included among children and not neonates.
A risk-based approach for identifying candidates for intrapartum antibiotic prophylaxis was recommended in 2008 in Sweden (Swedish National Board of Health and Welfare, 2008). The risk factors prompting intrapartum antibiotic prophylaxis are intrapartum fever (≥ 38°C), preterm birth (gestational age < 37 weeks), premature rupture of the membranes (≥ 18 hours before birth), previous delivery of an infant with invasive GBS infection, and GBS bacteriuria in the current pregnancy (http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/8836/2008-130-7_20081307.pdf). If one of those risk factors is present, a high dose of intravenous penicillin is given. Even though the recommendations were issued in 2008, they were not implemented until the end of 2011, because many gynecologists did not approve of them. There was widespread concern that increased use of penicillin would change the bacterial flora of both mothers and neonates, and create a risk of development of resistance against both penicillins and other beta-lactam anibiotics. The largest gynecological clinic in the region studied here — in Gothenburg — did not implement the recocommendations until the end of 2011.
Clinical data for the patients, with regard to age, sex, concomitant diseases, and other risk factors (e.g. smoking habits), as well as medical data from the GBS infectious episodes, were retrieved from medical records. Information on the yearly population per age group was obtained from the Swedish Social Board of Health and Welfare (http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/).
Statistics
Changes in incidence over time were analyzed using with the chi-squared test for trends, and confirmed with logistic regression. Proportions were compared using Fisher's exact test with two-sided intervals.
Ethics
The study was approved by the Ethics Committee, Gothenburg University, Gothenburg, Sweden (reference number 524-03).
Results
GBS was isolated from normally sterile body fluids in 1244 samples (579 in females and 665 in males) from 1101 patients. Two separate infections were seen in 133 patients, with intervals between 4 and 167 months (median 78 months). Five patients had three episodes of GBS infection with intervals between 6 and 128 months (median 35 months). GBS was isolated from blood in 1026 cases, from blood and CSF in two cases, from CSF in 35 cases, from synovial fluid in 155 cases, from peritoneal fluid in 16 cases, from pleural fluid in nine cases, and from corpus vitreum in one case. GBS was also isolated from nonsterile sites in many cases — blood + urine in patients with urinary tract infection and blood + pus or wound secretions in patients with abscess or wound infection. Altogether the patients occupied 6452 hospital beds (including convalescent homes) in the region, which meant that 461 hospital beds per year were occupied by patients with GBS infections.
Table 1 shows clinical manifestations for the different age-groups. Sepsis of unknown focus was by far the most common manifestation in all age groups (n = 599), followed by meningitis among neonates and children (n = 21).
Table 1.
Clinical manifestations of GBS infections in different age groups
| Neonates (N = 196) | Children (N = 60) | Adults (N = 468) | Elderly (N = 520) | |
|---|---|---|---|---|
| Sepsis with unknown focus | 168 | 41 | 169 | 220 |
| Pneumonia1 | 9 | 2 | 11 | 9 |
| Meningitis2 | 14 | 7 | 8 | 7 |
| Arthritis, bursitis5 | 2 | 3 | 78 | 73 |
| Appendicitis1 | 1 | |||
| Abscess1 | 1 | 19 | 17 | |
| Urinary tract infection1 | 1 | 3 | 33 | 54 |
| Spondylitis1 | 2 | 12 | 12 | |
| Erysipelas1 | 1 | 63 | 60 | |
| Sudden infant death4 | 1 | |||
| Endocarditis1 | 24 | 14 | ||
| Wound infection1 | 17 | 23 | ||
| Symphysitis1 | 4 | |||
| Chorioamnionitis1 | 4 | |||
| Cervisitis1 | 1 | |||
| Cholecystitis, cholangitis1 | 1 | 2 | ||
| Endometritis1 | 3 | 1 | ||
| Peritonitis6 | 6 | 11 | ||
| Pleuritis7 | 3 | 5 | ||
| Vulvitis1 | 2 | |||
| Pancreatitis1 | 2 | |||
| Osteomyelitis1 | 3 | 5 | ||
| Orchitis1 | 1 | |||
| Necrotizing fascitis1 | 1 | |||
| Oophoritis1 | 1 | |||
| Sacroiliitis1 | 1 | |||
| Keratitis3 | 1 | |||
| Colitis1 | 2 | |||
| Mediastinitis1 | 1 | 2 | ||
| Exacerbation of chronic obstructive pulmonary disease | 1 | |||
| Aortitis1 | 1 |
GBS isolated from blood
GBS isolated from CSF
GBS isolated from corpus vitreum
GBS isolated from heart blood post mortem
GBS isolated from synovial fluId
GBS isolated from peritoneal fluid
GBS isolated from pleural fluid
GBS was isolated in the blood + urine in all patients with urinary tract infections and from blood + pus or wound secretions in all patients with abscesses and wounds. GBS was also isolated from blood in all patients with erysipelas, spondylitis, symphysitis, chorioamnionitis, cervisitis, cholecystitis, endometritis, mediastinitis, peritonitis, pneumonia, vulvitis, pancreatitis, osteomyelitis, orchitis, necrotizing fascitis, ovarian infection, and sacroiliitis.
Neonates
There were 196 neonates, giving a mean incidence of 7.3 cases/10 000 live births/year. Of these, 156 were very early onset (0–2 days after birth; mean incidence 6.1/10 000 live births/year), 10 were early onset (3–6 days after birth) and 30 were late onset (7–27 days after birth).
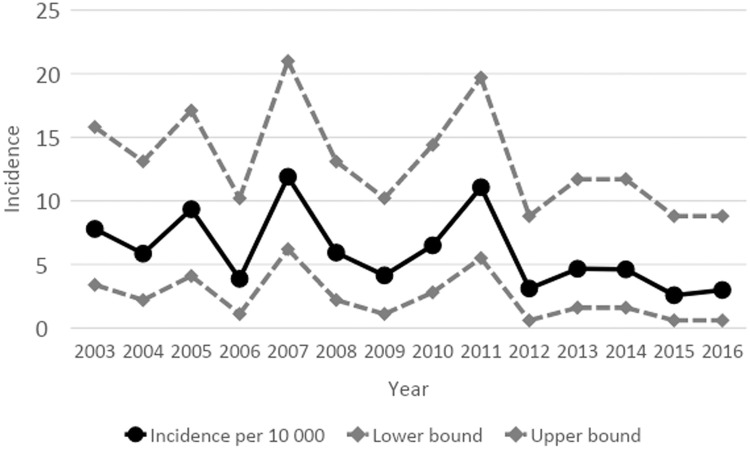
The overall incidence among neonates was 6.0/100 000 live births/year, but there was a significant decrease from the year 2012, when intrapartum antibiotic prophylaxis was fully implemented. Before 2012, the incidence was 7.3/10 000 live births/year, while between 2012 and 2016 it was 3.4/10 000 live births/year (p = 0.002, chi-squared test for trend; p = 0.003, logistic regression) (Figure 1).
Fig. 1.
The decrease in neonates was confined to a decrease in very early-onset infections (Figure 1). The median time spent in hospital for all neonates was 10 days (range 0–128).
Sixteen neonates died. Four of these had very low gestational age (22–26 weeks), two were operated for duodenal atresia, one had Down's syndrome, and one had an aorta stenosis. For the remaining deaths no obvious risk factor was known. In two of the fatal cases the mother had received intrapartum antibiotic prophylaxis because of preterm delivery, in both cases after year 2011. Only two neonates had persistent sequelae, involving cerebral paresis and epilepsy.
Children
There were 60 GBS infections in children aged 28 days to 17 years (median age 1.3 years). The incidence was 1.31 cases/100 000 population/year, with no changes over the time period. One child, who died via sudden infant death, had GBS isolated from blood post mortem.
Twenty-eight cases were very late-onset infections. All of them had complicated births and neonatal periods due to low gestational age (< 30 weeks) involving asphyxia during birth or congenital malformations. They had not left the hospital when they were infected. All infections were considered as hospital acquired.
The median time spent in hospital was 8 days (range 0–150).
Thirteen children died, in most cases due to the infection itself without known contributing factors. Sixteen children needed assisted ventilation in the intensive care unit. Four children were later diagnosed with intellectual disability, one had a subarachnoidal hemorrhage, and one with arthritis required a hip prosthesis.
Adults
There were 468 invasive infections in adults (18–64 years; median age 52 years). This corresponded to an incidence of 3.57 cases/100 000 population/year, with no changes over the time period.
Many of the adults had other factors that might have contributed to the infection. Ten women were pregnant and 13 were newly delivered, either by vaginal delivery or cesarean section. Of the patients with arthritis, 15 had known severe arthrosis and 13 had protheses in the knee or hip. Other important concomitant diseases were cardiovascular disease (previous stroke or myocardial infarction, current angina pectoris, or peripheral vascular diseases) (119 patients), diabetes mellitus (105 patients), malignant diseases (74 patients), and alcohol or drug abuse (52 patients). Other less common concomitant diseases were liver cirrhosis (alcohol or hepatitits B induced), renal diseases, and various immunological diseases.
The median time spent in hospital was 8 days (0–346).
Fifty-three patients needed treatment in the intensive care unit, with 29 patients requiring assisted ventilation. Fifty patients died, usually due to a combination of the infection and a severe underlying disease. In 52 cases long-lasting or chronic sequlae occurred. These included long-lasting pain in the affected joint or wound (13 patients), amputation of the lower leg (three patients), amputation of an infected toe (one patient), limpness (three patients), fetal death in an infected pregnant woman (two women), installation or exchange of a prosthesis in the hip or knee (five patients), operation of a cardiac valve (five patients), epilepsy and other neurological symptoms (four patients), impaired vision (two patients), impaired mobility of the knee (two patients), renal failure (one patient), chronic wound infection (one patient), esophageal perforation (one patient), chronic paresthesia (one patient), recurrent venous thromboses (one patient).
Elderly
GBS was isolated from normally sterile body fluids on 520 occasions in patients ≥ 65 years (median age 78.5 years, range 65–105 years). The mean incidence was 12.9/100 000/year with no changes over the time period.
Forty patients were admitted to an intensive care unit and seventeen patients needed a ventilator. The median time spent in hospital was 9 days (range 0–358).
There were 78 deaths among the elderly, with a significant difference in deaths below and above 80 years of age. 28 of 271 patients aged 65–79 years and 51 of 232 patients ≥ 80 years died (p = 0.0005). Thirty-four elderly patients had chronic sequelae. Twelve patients required an amputation. Six patients had chronic pain in the affected joint or wound. Three patients became blind in one eye. Four patient aquired chronic cardiac problems or needed a replacement of a heart-valve. One patient required prosthesis replacement. One patient acquired tetraparesis and one patient acquired aphasia. One patient needed a hip prosthesis and one required a pyelostomy catheter.
Discussion
Our study supported the results of several previous studies — that GBS is an important pathogen in neonates. Together with Escherichia coli, it is the most common cause of sepsis and meningitis in this age group (Gudjónsdóttir M et al., 2019; Vergnano et al., 2011; Cohen-Wolkowiez et al., 2009; Weston et al., 2011; Braye, 2019; Fjalstad et al., 2016; Tessin et al., 1990; Persson et al., 2002). There are two different approaches to decreasing the incidence in neonates. One is to vaccinate pregnant women with a conjugated polysaccharide vaccine against the most common serotypes in neonates. Two polysaccharide–protein conjugated hexavalent vaccines are currently undergoing clinical trials (Buurman et al., 2019; Absalon et al., 2021). Since they are directed against all important GBS serotypes (Ia, Ib, II, III, IV, V) they should be suitable for pregnant women, elderly people, and immunodeficient patients. Vaccines based on GBS surface proteins and pili are also under development (Johnson and Ferrieri, 1984; Madoff et al., 1991; Lauer et al., 2005; Carreras at al., 2020). The advantage of such vaccines would be that they could cover more strains than the serotype-specific conjugated polysaccharide vaccines. However, many women are reluctant to undergo vaccination during pregnancy. The World Health Organization lists antivaccination movements as among the 10 top threats against global health [https//www.who.int/newsroom/spotlight/ten-threats-to-global-health-in-2019].
The other method used to decrease very early-onset infections in neonates is intrapartum antibiotic prophylaxis, which can be risk based (as in our study) or screening based, when penicillin is given to mothers during delivery if GBS is isolated from vaginal or rectal cultures (Schrag et al., 2002; Schrag and Verani, 2013; Di Renzo et al., 2015). Our results showed that risk factor-based intrapartum antibiotic prophylaxis significantly decreases very early-onset infections. A recent Dutch metaanalysis showed that a screening-based policy was superior to risk factor-based policy in preventing early-onset GBS infection (Hasperhoven et al., 2020).
Whilst not denying the important role of GBS as a cause of invasive infections in neonates, our study showed that only 196 of 1244 invasive infections occurred in neonates (16%). Our results, and those of some previous studies, confirmed that the organism is an important pathogen in all age groups (Farley et al., 2001; Björnsdóttir et al., 2016; Dahl et al., 2003; Tyrrell et al., 2000; High et al., 2005). The infection is particularly prevalent in elderly people and in individuals with concurrent diseases that may predispose to severe infection. In our study, as many as 519/1244 (42%) of all infections occurred in patients ≥ 65 years, resulting in the highest incidence among the groups studied (12.9/100 000 population/year). A remarkable finding was that none of the patients in our study had splenectomy or asplenia as a risk factor, even though these conditions are known to increase the risk of invasive infections with other bacteria with a polysaccharide capsule, such as pneumococci and menigococci (Tahir et al., 2020; Sinwar et al., 2014).
Sepsis of unknown origin was by far the most common manifestation in all age groups. The typical GBS infection in neonates was a very early-onset infection of sepsis of unknown focus in a preterm neonate. The second most common manifestation among adults and elderly patients was arthritis and bursitis. This was also shown in a previous study from the same region (Dahl et al., 2003) and has been demonstrated in a recent study from Thailand (Ruksasakul et al., 2019). Aside from these two main manifestations, there was a large number of manifestations associated with different organs.
The leading underlying diseases in adult and elderly patients were cardiovascular disease, diabetes, malignant diseases, and alcohol and drug abuse. However, GBS can cause invasive infections in children and adults with no concurrent disease. Among the children and adults < 65 years studied, 19/60 (32%) and 120/469 (26%), respectively, had no known concurrent disease. The very high incidence of invasive GBS infections in elderly patients suggests the need to consider vaccination against GBS in this age group, in line with recommendations for pneumococcal vaccination among elderly people issued by the US Centers for Disease Control (www.cdc.gov/vaccines/vpd pneumo/hcp/PCV13-adults.html) as well as the Swedish Board of Health and Welfare (folkhalsomyndigheten.se). It would be important for such a vaccine to contain GBS serotype V, the most common serotype found in nonpregnant adults and elderly patients (High et al., 2005; Johansson Gudjónsdóttir et al., 2015).
Conclusion
GBS is an important pathogen in all age groups, particularly in elderly patients and in those with severe underlying diseases. Furthermore, intrapartum antibiotic prophylaxis significantly decreases the incidence of very early-onset infections (Table 2).
Table 2.
Most common concomitant diseases
| Neonates (N = 196) | Children (N = 60) | Adults (N = 468 | Elderly (N = 519) | |
|---|---|---|---|---|
| Preterm | 68 | |||
| PROM | 15 | |||
| Febrile mother at birth | 10 | |||
| Down's syndrome | 3 | |||
| Congenital malformation | 2 | 1 | ||
| Very late onset with complicated neonatal period | 9 | |||
| Asphyxia at birth | 5 | |||
| Trauma | 1 | |||
| Pregnant | 11 | |||
| Recently delivered | 13 | |||
| Arthrosis | 4 | 14 | ||
| Prosthesis | 13 | 16 | ||
| Cardiovascular disease | 31 | 119 | 219 | |
| Diabetes | 105 | 116 | ||
| Malignant disease | 4 | 73 | 89 | |
| Alcohol or drug abuse | 1 | 52 | 15 | |
| Lung disease | 22 | 292 | 472 | |
| Immunological disease | 2 | 25 | 30 | |
| Immunosuppressive treatment | 9 | 28 | ||
| Transplantation | 2 | 3 | ||
| Hepatic disease | 25 | 14 | ||
| Renal disease | 47 | 57 | ||
| Peritoneal dialysis | 1 | 13 | 3 | |
| No concomitant disease | 102 | 35 | 120 | 107 |
Most often congenital valvular disease
In children, asthma bronchiale; in adults and elderly patients, chronic pulmonary disease or lung fibrosis
Declaration of Competing Interest
None of the authors has any conflicts of interest.
Funding
Financial support was obtained from the Healthcare Board, Region Västra Götaland, SE 462 80 Vänersborg, Sweden.
References
- Absalon JA, Segall N, Block SL, Center KJ, Scully IL, Giardina PC, et al. Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: a phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect Dis. 2021;21:263–274. doi: 10.1016/S1473-3099(20)30478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony BF, Okada DM. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- Björnsdóttir ES, Martins ER, Erlendsdóttir H, Haraldsson G.J., Melo-Cristino J, Kristinsson KG, et al. Changing epidemiology of group B streptococcal infections among adults in Iceland: 1975–2014. Clin Microbiol Infect. 2016;22:379. doi: 10.1016/j.cmi.2015.11.020. e9–379.e16. [DOI] [PubMed] [Google Scholar]
- Braye K, Foureur M, de Waal K, Jones M, Putt E, Ferguson J, East CE. Epidemiology of neonatal early-onset sepsis in a geographically diverse Australian health district 2006–2016. PLOS One. 2019;14 doi: 10.1371/journal.pone.0214298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buurman ET., Timofeyeva Y, Gu J, Kim J-R, Kodali S, Liu Y, et al. A. Novel hexavalent capsular polysaccharide conjugate vaccine (GBS6) for the prevention of neonatal group B streptococcal infections by maternal immunization. J Infect Dis. 2019;220:105–115. doi: 10.1093/infdis/jiz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Abad C, Ramkhelawon L, Heath PT, Le Doare K. A vaccine against group B Streptococcus; recent advances. Infect Drug Resist. 2020;13:1263–1272. doi: 10.2147/IDR.S203454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Wolkowiez KM, Moran MC, Benjamin HD, Cotten KC, Clark BR, Benjamin BD, Smith BP. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052–1056. doi: 10.1097/INF.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MS, Tessin I, Trollfors B. Invasive group B streptococcal infections in Sweden: incidence, predisposing factors and prognosis. Int J Infect Dis. 2003;7:113–119. doi: 10.1016/s1201-9712(03)90006-3. [DOI] [PubMed] [Google Scholar]
- Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, Hakansson S, et al. Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med. 2015;28:766–782. doi: 10.3109/14767058.2014.934804. [DOI] [PubMed] [Google Scholar]
- Farley MM, Harvey RC, Stull T, Smith JD, Schuchat, Wenger JD, Stephens DS. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N Engl J Med. 1993;328:1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- Farley MM. Group B streptococcal disease in nonpregnant adults. Clinical Infectious Diseases. 2001;33:556. doi: 10.1016/j.cmi.2015.11.020. –1. [DOI] [PubMed] [Google Scholar]
- Fjalstad WJ, Stensvold JH, Bergseng SH, Simonsen EG, Salvesen EB, Rønnestad EA. Klingenberg EC. Early-onset sepsis and antibiotic exposure in term infants: a nationwide population-based study in Norway. Pediatr Infect Dis J. 2016;35:1–6. doi: 10.1097/INF.0000000000000906. [DOI] [PubMed] [Google Scholar]
- Freedman RM, Ingram DL, Gross I, Ehrenkranz RA, Warshaw JB, Baltimore RS. A half century of neonatal sepsis at Yale, 1929 to 1978. Am J Dis Child. 1981;135:140–144. doi: 10.1001/archpedi.1981.02130260032010. [DOI] [PubMed] [Google Scholar]
- Hasperhoven GF, Al-Nasiry S, Bekker V, Villamor E, Kramer BWW. Universal screening versus risk-based protocols for antibiotic prophylaxis during childbirth to prevent early-onset group B streptococcal disease: a systematic review and meta-analysis. BJOG. 2020;127:880–891. doi: 10.1111/1471-0528.16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Edwards MS, Carol J., Baker CJ. Group B Streptococcal Infections in Elderly Adults. Clinical Infectious Diseases. 2005;41:839–847. doi: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- Hussain SM, Luedtke GS, Baker CJ, Schlievert PM, Leggiadro RJ. Invasive group B streptococcal disease beyond early infancy. Pediatr Infect Dis J. 1995;14:278–281. doi: 10.1097/00006454-199504000-00006. [DOI] [PubMed] [Google Scholar]
- Johansson Gudjónsdóttir M, Hentz E, Berg S, Backhaus E, Elfvin A, Kawash S, Trollfors B. Serotypes of group B streptococci in western Sweden and comparison with serotypes in two previous studies starting from 1988. BMC Infect Dis. 2015;15:507. doi: 10.1186/s12879-015-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson Gudjónsdóttir M, Elfvin A, Hentz E, Adlerberth I, Tessin I, Trollfors B. Changes in incidence and etiology of early-onset neonatal infections 1997–2017 — a retrospective cohort study in western Sweden. BMC Pediatrics. 2019;19(1):490. doi: 10.1186/s12887-019-1866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FD, Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984;19:506. doi: 10.1128/jcm.19.4.506-i510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer P., Rinaudo CD, Soriani M, Margarit I, Maione D, Rosini R, et al. Genome analysis reveals pili in group B Streptococcus. Science. 2005;309:105. doi: 10.1126/science.1111563. [DOI] [PubMed] [Google Scholar]
- Madoff LC, Hori S, Michel JL, Baker C. Kasper. Phenotypic diversity in the alpha C protein of group B streptococci. Infect Immun. 1991;59:2638. doi: 10.1126/science.1111563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken GH., Jr. Group B streptococci; the new challenge in neonatal infections. J Pediatr. 1973;82:703–706. doi: 10.1016/s0022-3476(73)80603-1. [DOI] [PubMed] [Google Scholar]
- Persson E, Trollfors B, Brandberg LL, Tessin I. Septicaemia and meningitis in neonates and during early infancy in the Göteborg area of Sweden. Acta Paediatr. 2002;91:1087–1092. doi: 10.1111/j.1651-2227.2002.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Rosa-Frail M, Spellerberg C. Reliable detection of group B Streptoccus in the clinical laboratory. J Clin Microbiol. 2017;55:2590–2598. doi: 10.1128/JCM00582-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruksasakul R, Narongroeknawin P, Assavatanabodee P, Chaiamnuay S. Group B streptococcus is the most common pathogen for septic arthritis with unique clinical characteristics: data from 12 years retrospective cohort study. BMC Rheumatol. 2019;3:38. doi: 10.1186/s41927-019-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002;225:233–239. doi: 10.1056/NEJMoa020205. [DOI] [PubMed] [Google Scholar]
- Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31(Suppl 4):D20–D26. doi: 10.1016/j.vaccine.2012.11.056. [DOI] [PubMed] [Google Scholar]
- Schuchat A. Epidemiology of Group B Streptococcal Disease in the United States: Shifting Paradigms. Clinical Microbiology Reviews. 1998;11(3):497–513. doi: 10.1128/CMR.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinwar PD. Overwhelming post splenectomy infection syndrome — review study. Int J Surg. 2014;12:1314–1316. doi: 10.1016/j.ijsu.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Tahir F, Ahmed J, Malik F. Post-splenectomy sepsis: a review of the literature. Cureus. 2020;12:e6898. doi: 10.7759/cureus.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessin I, Trollfors B, Thiringer K. Incidence and etiology of neonatal septicaemia and meningitis in western Sweden 1975–1986. Acta Paediatr Scand. 1990;79:1023–1030. doi: 10.1111/j.1651-2227.1990.tb11378.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell GJ, Senzilet LD, Spika JS, Kertesz DA, Alagaratnam M, Lovgren M, Talbot JA. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study 1996. J Infect Dis. 2000;182:168–173. doi: 10.1086/315699. [DOI] [PubMed] [Google Scholar]
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, Robinson MJ, Collinson A, Heath PT. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F9–F14. doi: 10.1136/adc.2009.178798. [DOI] [PubMed] [Google Scholar]
- Weston JE, Pondo MT, Lewis IM, Martell-Cleary JP, Morin LC, Jewell JB, Daily JP, Apostol JM, Petit JS, Farley JM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]