Abstract
Breast cancer (BC) is the most common life-threatening malignancy amongst women with high incidence worldwide. In Egypt, it is the most known malignancy amongst females. Epithelial-mesenchymal transition (EMT) participates in breast tumors’ invasiveness, and metastasis, but the process is poorly understood. The involvement of voltage-gated calcium channels signaling in EMT has not yet been fully explored. Therefore, the aim of this study was to investigate the possible role of T-type calcium channels in metastasis and EMT among breast cancer patients. The study was carried out on 48 female breast cancer patients who were divided into two groups; metastatic and non-metastatic. qRT-PCR was employed to measure the expression of EMT marker genes (N- cadherin, E-cadherin, Snail, Vimentin and T-type VGCCs genes (CACNA1G, CACNA1H, and CACNA1I). The results of the present study revealed differential expression of the EMT marker genes in blood and tissue of non-metastatic and metastatic breast cancer patients, with a clear tendency for the mesenchymal markers to be significantly elevated in metastatic patients as well as malignant tissues taken from non-metastatic patients as compared to their paired tumor adjacent normal (TAN) tissue. Both CACNA1H and CACNA1I (T-type VGCCs oncogenes) were significantly elevated in blood of metastatic patients when compared to non-metastatic ones. In contrast, CACNA1G (tumor suppressor) exhibited a significant decrease in metastatic patients. The strong correlation between the expression of T-type VGCCs and mesenchymal marker genes in metastatic breast cancer patients casts light on the role of T-type VGCCs in metastasis and their involved in tumor invasiveness.
Keywords: Breast cancer, Metastasis, EMT, T-type VGCCs, CACNA1H, CACNA1I, CACNA1G Ncadherin, E- cadherin
Breast cancer; Metastasis; EMT; T-type VGCCs; CACNA1H; CACNA1I; CACNA1G Ncadherin; E- cadherin.
1. Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and the leading cause of cancer-related death in women worldwide [1]. The etiology of breast cancer involves multiple genetic predispositions, combined with environmental factors; such as sedentary lifestyle, alcohol consumption, and excess body weight [2].
Despite the recent advances in diagnosis and treatment of breast cancer, metastasis of BC still very common; involving bone, liver, lung, and brain. In this regard, more than half a million women worldwide suffer from metastatic breast cancer annually, and 90% of the deaths could be attributed to metastasis [3]. Contributing to metastatic potential, Epithelial–mesenchymal transition (EMT) is a process in which epithelial cells are converted to a mesenchymal-like phenotype and is associated with the loss of cell contacts and production of type-III intermediate filament protein, vimentin resulting in enhanced cell migration and invasion. Also, EMT is a key event in developmental and physiological processes including embryogenesis and wound healing [4].
The loss of cell-to-cell adhesion, the acquisition of motility, the ability to degrade the surrounding extracellular matrix (ECM) and to survive stresses; such as these induced by entry into the circulation are all features of metastatic cancer cells. It is therefore not surprising that it is believed that as cells leave the primary tumor, they may undergo processes similar to EMT [5]. To do this transformation, cells express specific transcription factors; such as SNAIL and Twist, and also express mesenchymal markers; such as vimentin and N-cadherin, and lose epithelial markers, E-cadherin for example [6].
Referring to calcium role in cancer metastasis as a universal cellular messenger, calcium regulates a plethora of physiological events including gene transcription, cell survival and proliferation [7]. In addition, it was reported that changes in calcium signals affect metastatic potential, cell proliferation and apoptosis [8]. Amongst the different calcium channels that are implicated in cancer, T-type calcium channels (TTCCs) have been linked to tumor initiation and progression [7]. It has been lately well-recognized that alterations in calcium signaling are crucial for the sustenance of proliferation and migration during tumor progression [9]. For this reason, TTCCs were suggested as promising therapeutic targets in many cancer types [10]. However, there are a lot of gaps in the case of breast cancer owing to disparate reports of TTCCs activity and expression in different breast cancer subtypes; there is no definitive answer to the causal/consequential association of TTCCs with breast cancer yet [11]. Moreover, an aspect, which is yet unexplored is the role of T-type in epithelial to mesenchymal transition (EMT), and whether T-type channels are implicated in regulating basal EMT markers [11]. Therefore, the aim of this study was to assess the possible association between T-type calcium channels genes and EMT marker genes along with exploring the correlation of both gene types to metastasis in breast cancer patients. In addition, we sought to correlate gene expression profiles to patients’ clinicopathological characteristics.
1.1. Patients and methods
This study was conducted on 48 breast cancer patients (23 metastatic and 25 non-metastatic cases) who were admitted to Cancer Management and Research Department, Medical Research Institute, University of Alexandria and Ayadi El mostaqbal hospital through the period from December 2018 to Octrober 2019. The study was reviewed and approved by the institutional review board at the Medical Research Institute (code: IORG 0008812), and a signed consent was obtained from all participants prior to enrollment. The demographic and clinicopathological data; such as age, menstrual status, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), tumor histological grade and clinical stage were collected from the patients’ medical records. Patients with confirmed histopathological diagnosis of breast cancer were included in the study, whilst these who were receiving chemo- or radio-therapy and/or immunosuppressive drugs were excluded from participation. Twenty cancer-free apparently healthy women were used as control group. All patients have undergone mammography and fine needle aspiration biopsy (FNAC). A blood sample was collected form all participants; control subjects, the non-metastatic group before surgery, and the metastatic group. During surgical operation, biopsies from the malignant breast tissue and its corresponding adjacent microscopically normal breast tissues (TAN) were taken from all participants in the non-metastatic group (the cold ischemic time was ∼30–45 min), the biopsies collected from the malignant tissues were then divided into two portions; one was placed directly into 37% formalin for histopathological examination, which was done within 24 h of biopsy collection and fixation. The other portion of the biopsy was stored with blood samples immediately at -80 ͦ C until further investigations where the other biopsy was used to extract RNA for qRT-PCR.
1.2. Quantitative reverse transcriptase polymerase chain reaction (q-RT-PCR)
The q-RT-PCR reactions in the present study employed an intercalating dye (SYBR Green), which binds indiscriminately to all double stranded DNA products. A melt-curve analysis indicates the presence of nonspecific products or primer dimmers.
Briefly, total RNA was isolated from blood using Easy-REDTM Total RNA Extraction Kit (# 17063, iNtRON Biotechnology Inc., South Korea) according to the manufacturer instructions. The extracted RNA pellet was then dried and dissolved in 50 μl of RNase free water. Total tissue RNA was isolated using easy-spinTM (DNA free) total RNA extraction kit (# 17221, iNtRON Biotechnology Inc., South Korea) according to the manufacturer instructions. In this procedure, the chaotropic salt in lysis buffer immediately inactivates RNase to ensure isolation of intact RNA. The RNA purity and concentration was determined by measuring absorbance at 230, 260 and 280 nm by Genova nano UV-visible spectrophotometer (JENWAY, Staffordshire, UK).
1.3. cDNA synthesis & amplification
Full-length cDNA from a total or poly (A)+ RNA samples were synthesized using the HiSenScript™ RH (-) cDNA Synthesis Kit (# 25087, iNtRON Biotechnology Inc., South Korea) according to the manufacturer protocol. The starting RNA material could be as low as 1 pg to 5 μg per reaction and is applicable for the synthesis of full-length first strand cDNA. In the real time PCR, the reaction is carried out in a real-time PCR machine that watches the reaction as it occurs and detects a signal that is proportional to the product. SYBR Green dye binds the DNA double helix. This alters the structure of the dye and causes it to fluoresce more. As very simply as the PCR creates more DNA, more dye can bind and more fluorescence is generated. For 20 μl reaction, 2 μl cDNA, 1.25 μl of the forward and reverse primers (10pMol/μl), 10 μl PCR master mix and 5.5 μl RNase-free water were dispensed into PCR plates. Samples were mixed gently and tubes were capped and placed in the thermal cycler. The amplification profile was as follow: Initial denaturation at 95 °C for 10 min, followed by 55 cycles of: denaturation at 95 °C for 15 s, annealing at 56 °C for 15 s, extension at 72 °C for 30 Sec. Data were collected during the extension step using PCR 7500 Applied Biosystems. The relative expression of target genes was quantified relative to the expression of the reference gene (GAPDH) in the same sample. The 2−ΔΔCt method was used to calculate the relative gene expression of target genes (ΔΔCt = ΔCt sample - ΔCt control; and ΔCt = Ct target gene – Ct reference gene (GAPDH)). The levels of gene expression in the blood samples of patients groups are estimated relative to the levels of these genes in the in the control group, then the relative gene expression between groups was calculated by diving the 2−ΔΔCt value of the metastatic group/2−ΔΔCt value of the non-metastatic group. With respect to the expression levels in the malignant tissues, the expression was estimated relative to the expression levels in the adjacent normal tissues. Primer sets are provided in the supplementary file 1 (table S1).
1.4. Statistical analysis
Data were tested for Gaussian distribution and parametric data were presented as mean ± S.D and non-parametric data were presented as median and interquartile range. Chi square test was used to compare categorical variables and Man Whitney U test and Wilcoxon signed-rank test were used to compare non-parametric independent and dependent variables, respectively. Pearson and spearman's correlation were performed to test the association between continuous and ordinal variables in order. Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) package (version 22). P value ≤ 0.05 was considered statistically significant.
2. Results
2.1. Age and clinicopathological characteristics of patients
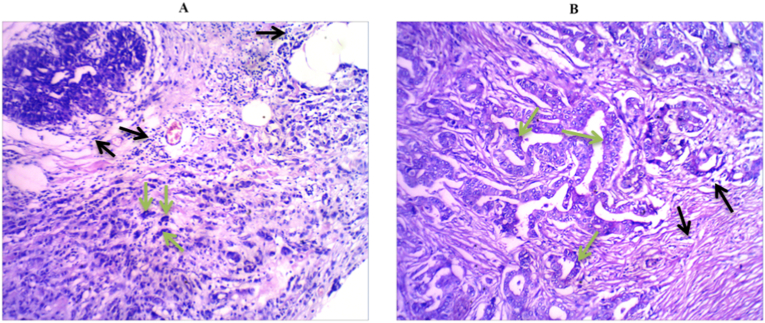
The mean age and menstrual status of metastatic and non-metastatic breast cancer patients along with clinicopathological characteristics including; histopathological subtype of breast tumors, histological grade, clinical stage, number of total as well as infiltrated axillary lymph nodes are summarized in Table 1. There was no significant difference between the two groups regarding the mean age and the menstrual status (p = 0.535 and 0.790, respectively). The difference was also not significant between the two groups regarding tumor size (p = 0.250) and histological grade (p = 0.613), total and infiltrated axillary lymph nodes (0.422 and 0.699, respectively). With respect to the histological subtypes of breast carcinoma, the difference between metastatic and non-metastatic patients was statistically significant (p = 0.001). Figure 1 shows representative samples of histopathology micrographs of tumors.
Table 1.
Age and clinicopathological characteristics of patients.
| Non-metastatic (n = 25) |
Metastatic (n = 23) |
Test of sig. | P- value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Age (years) | ||||||
| Min. – Max. | 30.0–68.0 | 26.0–62.0 | t = 0.626 | 0.535 | ||
| Mean ± SD. | 47.96 ± 11.19 | 46.0 ± 10.45 | ||||
| Menstrual status | ||||||
| Post-menopause | 11 | 44.0 | 11 | 47.8 | c2= 0.071 |
0.790 |
| Pre-menopause | 14 | 56.0 | 12 | 52.2 | ||
| Histopathological subtypes | ||||||
| Invasive Ductal C | 24 | 96.0 | 22 | 95.7 | c2= 13.057 |
MCp= 0.001 |
| Mucoid cancer | 1 | 4.0 | 0 | 0.0 | ||
| Invasive lobular C | 0 | 0.0 | 1 | 4.3 | ||
| Tumor size (cm). | ||||||
| Min. – Max. | 2.0–8.0 | 1.50–10.0 | t= 1.166 |
0.250 | ||
| Mean ± SD. | 3.84 ± 1.81 | 4.55 ± 2.41 | ||||
| Total axillary lymph nodes | ||||||
| Min. – Max. | 4.0–31.0 | 8.0–26.0 | U= 158.50 |
0.422 | ||
| Median (IQR) | 13.0 | 14 | ||||
| Infiltrated axillary lymph nodes | ||||||
| Min. – Max. | 0.0–27.0 | 0.0–8.0 | U= 269.0 |
0.699 | ||
| Median (IQR) | 2.0 (0.0–2.0) | 3.0 (9.0–5.0) | ||||
| Histological Grade | ||||||
| II | 18 | 72.0 | 15 | 65.2 | c2= 0.257 |
0.613 |
| III | 7 | 28.0 | 8 | 34.8 | ||
| Clinical Stage | ||||||
| II | 12 | 48.0 | 0 | 0.0 | c2 = 48.0∗ | <0.001∗ |
| III | 13 | 52.0 | 0 | 0.0 | ||
| IV | 0 | 0.0 | 23 | 100.0 | ||
χ2: Chi square test MC: Monte Carlo U: Mann Whitney test t: Student t-test.
Figure 1.
Representative histopathology micrographs of patients' tumors. A represents Invasive lobular carcinoma (ILC) and (B) represents Invasive ductal carcinoma (IDC); Hematoxylin and Eosin stain (100x magnifications). Black arrows show infiltrated inflammatory cells and green arrow points to a malignant cell with apparent high nuclear/cytoplasmic ratio.
2.2. Statistical analysis of genes expression profiles in blood of non-metastatic and metastatic BC patients
2.2.1. EMT genes
The expression of E-cadherin gene was significantly increased in metastatic group when compared to the non-metastatic patients (P = 0.022) with 5.22 fold increase. N-cadherin gene expression showed a significant increase in metastatic group when compared to that in the non-metastatic group (P = 0.038) with 3.32 fold increase. Also, Snail gene expression was significantly higher in the metastatic patients than in the non-metastatic cases (5.43 fold increase; P = 0.045). Likewise, Vimentin gene expression was significantly higher in the metastatic than the non-metastatic group (1.22 fold increase; P = 0.028).
2.2.2. T-type VGCCs genes
CACNA1G gene expression showed a significant decrease in the metastatic group when compared to the non-metastatic patients (P = 0.044) with 0.77 fold decrease. Whilst, CACNA1H and CACNA1I expression was significantly higher in metastatic patients than the non-metastatic cases (P = 0.002 and 0.001, respectively) with 3830 and 2212 fold increase, respectively. The median relative expression values of both EMT and T-type VGCCs and the fold change (metastatic vs. non-metastatic) are summarized in Table 2.
Table 2.
Statistical analysis of EMT and T-type VGCCs gene expression profiles in blood of non-metastatic and metastatic BC patients.
| Genes | Non-metastatic N = 25 |
Metastatic N = 23 |
Fold change groups (RGE metastatic/RGE non-metastatic) | U | P- value |
|---|---|---|---|---|---|
| E-Cadherin | |||||
| Min. – Max. | 8.0–77859.0 | 130.0–6000.0 | 5.22 | 137.000∗ | 0.022∗ |
| Median (IQR) | 318.0 (86.0–1222.5) | 1660.5 (376.3–4000.0) | |||
| N-Cadherin | |||||
| Min. – Max. | 6.0–447.0 | 14.0–1153406.0 | 3.32 | 168.500∗ | 0.038∗ |
| Median (IQR) | 129.53 (39.50–185.0) | 429.0 (42.0–62084.0) | |||
| Snail | |||||
| Min. – Max. | 0.12–123.95 | 0.29–4893.6 | 5.43 | 156.0∗ | 0.045∗ |
| Median (IQR) | 4.60 (1.69–12.96) | 25.03 (2.57 5311.48) | |||
| Vimentin | |||||
| Min. – Max. | 59.37–366.8 | 152.7–1315.9 | 1.22 | 126∗ | 0.028∗ |
| Median (IQR) | 236.3 (191.7–275.7) | 290.1 (222.8–360.4) | |||
| CACNA1G | |||||
| Min. – Max. | 0.1386–207.1 | 0.01–0.5 | 0.77 | 111.000∗ | 0.044∗ |
| Median (IQR) | 0.44 (0.26–1.14) | 0.34 (0.1–0.46) | |||
| CACNA1H | |||||
| Min. – Max. | 0.0–161.0 | 0.00009–176.7 | 3830 | 135.500∗ | 0.002∗ |
| Median (IQR) | 0.0064 (0.0021–0.0094) | 24.8 (0.0003–29.0) | |||
| CACNA1I | |||||
| Min. – Max. | 0.0002–81.84 | 0.0105–105.2 | 2212 | 89.0∗ | <0.001∗ |
| Median (IQR) | 0.028 (0.004–1.016) | 62.15 (0.0905–106) | |||
U: Mann Whitney test.
RGE = Relative gene expression.
= significant difference.
2.3. Statistical analysis of EMT and T-type VGCCs gene expression profiles in TAN and malignant tissue in non-metastatic BC patients
2.3.1. EMT genes
The expression of E-cadherin gene was significantly decreased in malignant tissues when compared to the expression level in TAN (P = 0.042). Both N-cadherin and Vimentin showed significantly increased expression in malignant tissues when compared to TAN (P = 0.015 and 0.021, respectively). Whereas, Snail gene expression in malignant tissues was not significantly different than that in TAN (P = 0.568).
2.3.2. T-type VGCCs genes
With respect to T-type VGCCs genes, CACNA1G expression was significantly lowered in malignant tissues when compared to TAN (P = < 0.001). Whereas, CACNA1H expression showed significant increase in malignant tissues than that in TAN (P = 0.04). On the other hand, CACNA1I expression was not significantly different between malignant tissues and TAN (P = 0.549). Comparison between malignant tissues and TAN regarding EMT and T-type VGCCs genes is summarized in Table 3.
Table 3.
Statistical analysis of EMT and T-type VGCCs gene expression profiles in TAN and malignant tissue in non-metastatic BC patients.
| Genes | TAN N = 25 |
Malignant N = 25 |
Fold change groups (RGE metastatic/RGE non-metastatic) | Z | p- value |
|---|---|---|---|---|---|
| E-Cadherin | |||||
| Min. – Max. | 0.0–2.50 | 0.0–1.65 | 0.75 | 2.029∗ | 0.042∗ |
| Median (IQR) | 1.04 (0.82–1.35) | 0.78 (0.72–1.10) | |||
| N-Cadherin | |||||
| Min. – Max. | 0.13–2.51 | 0.47–102.39 | 1.15 | 2.442∗ | 0.015∗ |
| Median (IQR) | 1.08 (0.56–1.30) | 1.24 (1.2–2.8) | |||
| Snail | |||||
| Min. – Max. | 0.002–0.052 | 0.002–0.223 | 0.75 | 0.571 | 0.568 |
| Median (IQR) | 0.008 (0.004–0.010) | 0.006 (0.004–0.109) | |||
| Vimentin | |||||
| Min. – Max. | 0.15–3.49 | 0.06–147.95 | 1.17 | 2.315∗ | 0.021∗ |
| Median (IQR) | 1.06 (0.63–2.02) | 1.24 (0.9–6.3) | |||
| CACNA1G | |||||
| Min. – Max. | 0.0–605.09 | 0.0–0.98 | 0.42 | 3.632∗ | <0.001∗ |
| Median (IQR) | 1.16 (0.85–3.07) | 0.49 (0.41–0.76) | |||
| CACNA1H | |||||
| Min. – Max. | 0.0–2.59 | 0.0–89.64 | 1.12 | 2.052∗ | 0.040∗ |
| Median (IQR) | 1.22 (0.79–1.68) | 1.37 (1.2–3.7) | |||
| CACNA1I | |||||
| Min. – Max. | 0.01–3.43 | 0.18–58.51 | 0.92 | 0.600 | 0.549 |
| Median (IQR) | 0.92 (0.42–1.50) | 0.85 (0.28–0.79) | |||
Z: Wilcoxon signed ranks test.
RGE = Relative gene expression.
= significant difference.
2.4. Correlation between the expression of studied genes in blood of non-metastatic and metastatic BC patients and clinicopathological characteristics
E- Cadherin and N-Cadherin showed significant positive correlation with infiltrated lymph nodes and tumor size (r = 0.459 P = 0.036; and r = 0.497, P = 0.011, respectively). On the other hand, non-significant correlation was observed between tumor size, total and infiltrated axillary lymph nodes and the other genes in the non-metastatic group. In addition, in the metastatic patients, there was no significant correlation between any of the studied genes and tumor size, total and infiltrated axillary lymph nodes (P > 0.05) for all (Table 4).
Table 4.
Correlation between the expression of studied genes in blood of non-metastatic and metastatic BC patients and clinicopathological characteristics.
| E- cadherin | N- cadherin | Snail | Vimentin | CACNA1G | CACNA1H | CACNA1I | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-metastatic | |||||||||
| Tumor size (cm) | r | 0.224 | 0.497 | -0.054 | -0.001 | 0.198 | -0.229 | -0.146 | |
| p | 0.329 | 0.011∗ | 0.806 | 0.995 | 0.403 | 0.272 | 0.485 | ||
| Total axillary lymph nodes | rs | 0.195 | 0.352 | 0.406 | -0.132 | -0.090 | -0.140 | 0.223 | |
| p | 0.396 | 0.085 | 0.055 | 0.568 | 0.707 | 0.505 | 0.284 | ||
| Infiltrated axillary lymph nodes | rs | 0.459∗ | 0.151 | 0.231 | -0.391 | 0.361 | -0.106 | 0.142 | |
| p | 0.036∗ | 0.470 | 0.288 | 0.079 | 0.118 | 0.613 | 0.499 | ||
| Metastatic | |||||||||
| Tumor size (cm) | r | -0.021 | 0.149 | 0.010 | 0.336 | 0.053 | 0.023 | -0.182 | |
| p | 0.927 | 0.521 | 0.967 | 0.148 | 0.836 | 0.916 | 0.417 | ||
| Total axillary lymph nodes | rs | 0.009 | 0.256 | 0.035 | -0.363 | -0.059 | 0.110 | -0.307 | |
| p | 0.976 | 0.398 | 0.905 | 0.201 | 0.863 | 0.696 | 0.266 | ||
| Infiltrated axillary lymph nodes | rs | 0.012 | -0.213 | -0.049 | 0.015 | 0.051 | 0.200 | 0.232 | |
| p | 0.958 | 0.354 | 0.832 | 0.949 | 0.840 | 0.361 | 0.299 | ||
r: Pearson coefficient.
rs: Spearman coefficient.
p: p value.
Statistically significant at p ≤ 0.05.
2.5. Correlation between the expression of studied genes in blood of non-metastatic and metastatic BC patients
In the non-metastatic group, there was no significant correlation between any of the studied genes with each other (P > 0.05). Of note there was negative, but not yet significant correlation between CACNA1G and vimentin (r = - 0.488, P = 0.055), and between E-cadherin and vimentin (r = - 0.468, P = 0.058). Besides, a positive, but also non-significant correlation was between E-cadherin and CACNA1G (r = 0.449, P = 0.071). In the metastatic group, E-cadherin expression was positively correlated significantly with snail (r = 0.504, P = 0.023). Likewise, N. cadherin was significantly correlated in a positive manner with snail, CACNA1G and CACNA1H (r = 0.816, P = 0.001; r = 0.565, P = 0.023; and r = 0.549, P = 0.010, respectively). Whilst on the other hand, vimentin expression was not correlated with any of the other genes. Also, CACNA1H was positively correlated with snail (r = 0.491, p = 0.024); whereas, CACNA1I expression was not correlated to the expression profile of all other genes (Table 5).
Table 5.
Correlation between the expression of studied genes in blood of non-metastatic and metastatic BC patients.
| E- cadherin | N- cadherin | Snail | Vimentin | CACNA1G | CACNA1H | ||
|---|---|---|---|---|---|---|---|
| Non-metastatic | |||||||
| E- cadherin | rs | ||||||
| p | |||||||
| N- cadherin | rs | -0.161 | |||||
| p | 0.486 | ||||||
| Snail | rs | -0.126 | 0.198 | ||||
| p | 0.596 | 0.365 | |||||
| Vimentin | rs | -0.468 | -0.150 | 0.109 | |||
| p | 0.058 | 0.515 | 0.658 | ||||
| CACNA1G | rs | 0.449 | -0.085 | 0.172 | -0.488 | ||
| p | 0.071 | 0.723 | 0.494 | 0.055 | |||
| CACNA1H | rs | -0.354 | -0.204 | 0.059 | 0.090 | -0.026 | |
| p | 0.115 | 0.329 | 0.790 | 0.699 | 0.912 | ||
| CACNA1I | rs | 0.231 | 0.165 | 0.137 | -0.292 | 0.426 | -0.143 |
| p | 0.313 | 0.431 | 0.533 | 0.199 | 0.061 | 0.494 | |
| Metastatic | |||||||
| E. cadherin | rs | ||||||
| p | |||||||
| N. cadherin | rs | 0.382 | |||||
| p | 0.087 | ||||||
| Snail | rs | 0.504∗ | 0.816∗ | ||||
| p | 0.023∗ | <0.001∗ | |||||
| Vimentin | rs | 0.306 | -0.092 | 0.282 | |||
| p | 0.203 | 0.717 | 0.257 | ||||
| CACNA1G | rs | 0.307 | 0.565∗ | 0.397 | -0.109 | ||
| p | 0.231 | 0.023∗ | 0.128 | 0.688 | |||
| CACNA1H | rs | 0.403 | 0.549∗ | 0.491∗ | -0.154 | 0.422 | |
| p | 0.063 | 0.010∗ | 0.024∗ | 0.517 | 0.081 | ||
| CACNA1I | rs | 0.127 | -0.137 | 0.053 | 0.322 | -0.287 | 0.013 |
| p | 0.582 | 0.565 | 0.825 | 0.178 | 0.264 | 0.953 | |
rs: Spearman coefficient.
p: p value.
Statistically significant at p ≤ 0.05.
2.6. Correlation between expression of EMT and T-type VGCCs genes in tissues of non-metastatic BC patients
The results showed that E-cadherin was not significantly correlated with any of the other genes. However, N-cadherin was positively correlated with vimentin (r = 0.629, P = 0.012), CACNA1H (r = 0.643, P = 0.01) and CACNA1I (r = 0.819, P = 0.001). On the other hand, snail was not correlated with any of the other genes; whereas, vimentin was positively correlated with CACNA1H (r = 0.647, P = 0.005) and CACNA1I (r = 0.588, P = 0.005), and this relationship was statistically significant. Also, CACNA1G was not in a significant correlation with any of the other genes; Meanwhile, CACNA1I was positively correlated significantly with N-cadherin (r = 0.819, P = 0.001), vimentin (r = 0.588, P = 0.005) and CACNA1H (r = 0.484, P = 0.031) (Table 6).
Table 6.
Correlation between expression of EMT and T-type VGCCs genes in tissues of non-metastatic BC patients.
| E- cadherin | N- cadherin | Snail | Vimentin | CACNA1G | CACNA1H | ||
|---|---|---|---|---|---|---|---|
| E- cadherin | rs | ||||||
| p | |||||||
| N- cadherin | rs | -0.143 | |||||
| p | 0.597 | ||||||
| Snail | rs | 0.075 | 0.391 | ||||
| p | 0.735 | 0.134 | |||||
| Vimentin | rs | -0.176 | 0.629∗ | 0.078 | |||
| p | 0.457 | 0.012∗ | 0.743 | ||||
| CACNA1G | rs | 0.181 | 0.132 | 0.051 | 0.206 | ||
| p | 0.420 | 0.638 | 0.816 | 0.398 | |||
| CACNA1H | rs | -0.289 | 0.643∗ | 0.402 | 0.647∗ | 0.156 | |
| p | 0.230 | 0.010∗ | 0.088 | 0.005∗ | 0.536 | ||
| CACNA1I | rs | -0.169 | 0.819∗ | 0.356 | 0.588∗ | 0.305 | 0.484∗ |
| p | 0.429 | <0.001∗ | 0.088 | 0.005∗ | 0.158 | 0.031∗ |
rs: Spearman coefficient.
p: p value.
Statistically significant at p ≤ 0.05.
2.7. Correlation between the expression status of each gene in blood and tissue in non-metastatic BC patients
With regard to the assessment of the relationship between blood and tissue levels of the studied genes, there was no significant correlation between blood and tissue levels for N-cadherin, snail, CACNA1G, CACNA1H and CACNA1I (P > 0.05). A positive, but not yet significant correlation was found for E-cadherin (r = 0.401, P = 0.08) and vimentin (r = 0.436, P = 0.08) (Table 7).
Table 7.
Correlation between the expression status of each gene in blood and tissue in non-metastatic BC patients.
| Blood vs. Tissue | rs | P |
|---|---|---|
| E. cadherin | 0.401 | 0.080 |
| N. cadherin | 0.171 | 0.511 |
| Snail | 0.280 | 0.208 |
| Vimentin | 0.436 | 0.080 |
| CACNA1G | 0.009 | 0.971 |
| CACNA1H | 0.074 | 0.757 |
| CACNA1I | 0.035 | 0.869 |
rs: Spearman coefficient.
p: p value.
Correlation between EMT and T-type VGCCs target genes for the molecular subtypes of breast cancer patients are presented in the supplementary file 2 (Tables-S1-S7) for both metastatic and non-metastatic groups.
3. Discussion & conclusion
Breast cancer is the most common female cancer worldwide. According to the World Cancer Research Fund International (WCRFI), it represents quarter of all cancers [12]. In Egypt, the incidence rate of breast cancer is 29.9/100,000 population making the second-highest number of new cases (23.081) and deaths (9.254) of breast cancer in 2018 [1]. The reports of Alexandria Cancer Registry, Department of Bioinformatics and Medical Statistics, Medical Research Institute, University of Alexandria in 2017 revealed that BC counted for 49 % of all cancers among females in Alexandria City [13]. Therefore, there is a continuous need to oversee and understand cellular mechanisms of disease progression; such as metastasis, which is one of the hallmarks of advanced cancer, and is usually associated with poor patients’ outcomes. Metastasis is facilitated by four essential steps: detachment, migration, invasion, and adhesion; this cascade is termed “invasion metastatic cascade” [14].
In the context, epithelial-mesenchymal transition (EMT) is a biological process that allows a polarized epithelial cell, which normally interacts with basement membrane via its basal surface, to undergo multiple biochemical changes enabling it to take a mesenchymal cell phenotype; this includes enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and greatly increased production of ECM components [4]. These properties are critical for invasion and metastasis [14]. Therefore, the main objective of the current study was to explore the profile of EMT-related genes in conjunction with t-type VGCCs genes to estimate the involvement of calcium channel genes in the process of ETM.
In cancer, calcium signaling is a mechanism for rapid translation of signals from the tumor microenvironment into cellular responses [15]. Davis and colleagues [16] indicated that calcium signaling involvement in EMT of MDA-MB-231 BC cells is mediated through both epidermal growth factor (EGF) and hypoxia-inducible factor (HIF), thus increasing m-RNA level of N-cadherin and vimentin. They also explored the identity of calcium channels associated with EMT induction revealing that the calcium-permeable ion channel TRRM7 regulates EGF-STAT-3 phosphorylation. Similarly, Liu and colleagues [17] stated that TRPM7 silencing and chelation of Ca2+ in ovarian cancer samples significantly mitigated EGF and insulin-like growth factor (IGF)- stimulated migration, invasion and EMT by decreasing the level of intracellular Ca2+. In this regard, it was reported that altered genes in the VGCCs family are associated with 19 different cancers as breast, kidney, lung, and brain [10]. To the best of our knowledge, no previous studies were performed to clarify the role of T-type VGCCs in EMT and metastasis in breast cancer patients, since most of the studies were performed either on cell line models or human cancers other than breast cancer.
The findings of this study showed that EMT associated genes were differentially expressed between breast cancer tissue and their adjacent normal tissue, as well as in blood of metastatic and non-metastatic breast cancer patients. Collectively, there was a tendency for the mesenchymal genes to be elevated in blood of metastatic patients as compared to non-metastatic cases as well as in malignant tissues of non-metastatic patients when compared to their adjacent normal tissue. This could confirm the role of EMT in metastasis in breast cancer.
In this context, a change in the expression of E-cadherin is a typical epithelial cell marker of EMT [18]. Suppression of E-cadherin function or expression leads to mesenchymal morphology and increased cell migration and invasion, as well as metastasis [19, 20]. This may explain our results that revealed decreased expression of E cadherin in malignant tissues; where in agreement with our results, it was found that, in breast cancer, partial or total loss of E-cadherin expression correlates with loss of differentiation characteristics, acquisition of invasiveness, increased tumor grade, metastatic behavior and poor prognosis [21]. Having said that, it was found that the level of E-cadherin in blood of metastatic group is higher than that in the non-metastatic group; which seems to be in contradiction with the default knowledge that decreased E-cadherin promotes metastasis. From our point of view, this contradiction could be explained by the possible presence of clustered circulating tumor cells that secrete E-cadherin mRNA into patients’ blood. In this regard, it has been reported that these tumor cell clusters are more potent in secreting adherens being affected by humoral interaction from non-tumor cells [22, 23]. In addition, E-cadherin expression was reported in blood vessels of metastatic breast cancer patients that are CD-31-positive; a marker of tumor vascularization [24]. Also, in a similar context, we would like to denote that heterogeneity between studies was reported regarding the prognostic value of E-cadherin in inflammatory luminal and ductal carcinomas of the breast [25, 26, 27].
Regarding N- cadherin expression, it was up-regulated at the level of both blood and malignant tissues, where in blood of metastatic patients there was 3.3 fold increase in its level when compared to the non-metastatic patients. Likewise, there was a significant increase of N- cadherin in malignant tissues in comparison to their TAN tissue. In this aspect, Hazan and colleagues [28] indicated that N-cadherin may mediate a less stable and more dynamic form of cell-cell adhesion, which may allow both attachment and detachment of individual cells from the primary tumor. The decreased expression of membranous E-cadherin accompanied by a simultaneous increase in the expression of N-cadherin (termed the ‘cadherin switch’) has been recognized to be associated with lymph node metastasis in head and neck squamous cell carcinoma, and disease recurrence in laryngeal squamous cell carcinoma [29].
In addition, the results of our study revealed an increased expression of snail in metastatic patients when compared to non-metastatic ones. Elevated expression of Snail, not only enhances cell motility and invasiveness by down-regulating epithelial markers, but also up-regulates mesenchymal phenotype markers. Therefore, Snail promotes metastasis of breast cancer cells and is a biomarker of poor clinical outcomes in breast cancer patients [30]. Furthermore, it has been reported that Snail is sufficient to promote mammary tumor recurrence in vivo and that high levels of Snail predict decreased relapse-free survival in women with breast cancer. Therefore, blocking Snail function has a great potential to prevent metastasis [30].
According to previous data, a negative correlation exists between E-cadherin and Snail in blood or tissue of non-metastatic patients. However, on the contrary there was a strong positive correlation between them in the blood of metastatic patients in the current study. Hence, we couldn't provide any conclusive correlation due to the limited number of cases and the diversity of histological and molecular subtypes of BC in our study.
Regarding vimentin, it was shown that the gene was highly expressed in tongue squamous cell carcinoma tissues as compared to their paired TAN tissues [31]. Running with this result, our finding showed that vimentin expression was significantly up-regulated by 1.23 fold in blood of metastatic as compared to non-metastatic patients, besides it was significantly elevated in malignant tissues when compared to their TAN tissue. In line with this, E-cadherin expression showed non-significant negative correlation with vimentin in blood of non-metastatic patients; whereas, a strong positive correlation was reported between N- cadherin and vimentin in malignant tissues. Of note, the strongest correlation between mesenchymal transformation markers was between N- cadherin and Snail in blood of metastatic patients confirming their complementary role in metastasis.
When correlating the level of gene expression with clinicopathological characteristics, the tumor size was positively correlated with genes involved in proliferation and metastasis as N- cadherin, vimentin, CACNA1H, and CACNA1I. On the other hand, tumor size was negatively correlated with the tumor suppressor genes, E-cadherin and CACNA1G. In concordance with our results, N- cadherin was found to be closely related to neoplastic events; such as transformation, adhesion, apoptosis and angiogenesis [32]. On the other hand the loss of E-cadherin's function as a tumor suppressor protein correlates with increased invasiveness and metastatic potential so it is referred to as tumor invasive suppressor gene [33]. In accordance with this, our results revealed a significant inverse correlation between E-cadherin expression and tumor size in tissues of non-metastatic patients.
Coming to the role of T-type VGCCs in EMT; the function of VGCCs remains controversial. However, it was found that T-type VGCCs was significantly overexpressed in undifferentiated retinoblastoma when compared to the differentiated cells, suggesting an association with malignant phenotype. It was then hypothesized that T-type VGCCs participate in propagating stemness in cancer; and perhaps, supporting the development of cancer stem cells [34]. It was also reported that T-type VGCCs have a role in cell cycle regulation being overexpressed during S-phase and beginning of the M-phase [35]. In harmony with this, inhibition of T-type Ca2+ channel in a nude mice model suppressed tumor development and reduced tumor mass. In concordance with the above mentioned information, mibefradil, a nonselective calcium channel blocker caused a concentration-dependent inhibition of cell motility and invasion of HT1080 fibrosarcoma cell line [36]. Similarly, the drug evoked a significant reduction in cell migration in U87 glioblastoma cells [37]. In addition, endostatin, a C-terminal proteolytic fragment of collagen, which inhibits T-type Ca2+ channel function, resulted in a significant reduction in cell migration [37]. Having said that, CACNA1G silencing enhanced proliferation of MCF-7 cells as compared to CACNA1H silencing [38]. The study also indicated that calcium influx through Cav3.1 channel supported apoptosis, providing evidences of CACNA1G being a candidate tumor suppressor gene. This is consistent with our results that revealed that CACNA1G was significantly down-regulated in blood of metastatic patients when compared to non-metastatic ones with 0.7 fold decrease. Our findings also showed that the gene was significantly attenuated in malignant tissues as compared to TAN tissue. In addition, our results indicated that both CACNA1H and CACNA1I were significantly increased in blood of metastatic patients than non-metastatic cases with remarkable high fold changes of 3875 and 2219, respectively. Also, malignant tissues showed significantly elevated expression of CACNA1H as compared to its TAN tissue. On the other hand, no significant difference was found for CACNA1I gene. In this regard and in the same line with our findings, CACNA1H gene was previously found to be hypomethylated in T-cell leukemia when compared to peripheral blood mononuclear cells, suggesting that its role as an oncogene is regulated by epigenetic methylation [39]. In the present study, a remarkable high expression of CACNA1H gene was observed (Table 2), which could be attributed to a specific disease phenotype and should be further evaluated.
Of note, in the current study, a non-significant positive correlation was found between blood levels of CACNA1G and E-cadherin. In tissues of non-metastatic BC patients, a positive significant correlation was found between CACNA1H and N- cadherin and vimentin. In addition, CACNA1I showed a significant positive correlation with N- cadherin, Vimentin and CACNA1H. Regarding metastatic patients, a strong positive correlation was found between blood levels of CACNA1H and N- cadherin and Snail.
It is also noteworthy to highlight the relationship between CACN1A gene family and macrophages, where the expression of this voltage gated calcium channel genes in macrophages is known to mediated their migration towards the site of inflammation in response to activation and expression of chemokines. In this context, overexpression of CACNA1G in macrophages along with cadherins and selectins promoted macrophages interaction with chemokines at the site of inflammation [40]. However, CACNA1G was downregulted in the metastatic group in the present study. In this respect, the apparent infiltration of inflammatory cells that can be observed in Figure 1 could be also associated with overexpression of CACNA1I gene, and this should be further investigated.
In summary, the current study casted a beam of light on the possible association between EMT marker genes and the pro-oncogenic members of VGCCs genes in malignant tissues and in blood of metastatic breast cancer patients suggests that both sets of genes might coordinates with each other to support transformation processes contributing to metastasis and overall worse prognosis in breast cancer. Therefore, targeting these genes altogether may provide a double-edge therapy of metastatic breast cancer.
3.1. Recommendations
We would recommend building up on the basis of the present study findings and further exploring the role of both classes of the discussed genes using syngenic animal models. The replication of the present study would help to better understand the role of EMT marker genes and VGCCs genes in molecular subtypes of breast cancer including triple negative breast cancer, Her 2+ and luminal breast cancers. Furthermore, using knockdown/out breast cancer animal model and implementation of epigenetic modulation of gene function should be considered in future studies to support the current findings and reinforce the present study's conclusion.
Declarations
Author contribution statement
Fawziya A.E. Ragab Ibrahim: Conceived and designed the experiments; Analyzed and interpreted the data.
Amany I. Yousef, Nadia Ahmed Abd El Moneim: Conceived and designed the experiments.
Zain Ulabdeen Naser Hussein, Amr Mahmoud Hussein, Ayman Farouk Mohammad Ahmed, Noha Mohamed Ragab: Performed the experiments.
O. Al-Masry: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wang S., Ding Z. Fibroblast growth factor receptors in breast cancer. Tumour Biol. 2017;39(5) doi: 10.1177/1010428317698370. 1010428317698370. [DOI] [PubMed] [Google Scholar]
- 3.Soni A., et al. Breast cancer subtypes predispose the site of distant metastases. Am. J. Clin. Pathol. 2015;143(4):471–478. doi: 10.1309/AJCPYO5FSV3UPEXS. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heerboth S., et al. EMT and tumor metastasis. Clin. Transl. Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai J.H., Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornaro L., et al. Prognostic relevance of a T-type calcium channels gene signature in solid tumours: a correlation ready for clinical validation. PLoS One. 2017;12(8):e0182818. doi: 10.1371/journal.pone.0182818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziegielewska B., Gray L.S., Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflügers Archiv. 2014;466(4):801–810. doi: 10.1007/s00424-014-1444-z. [DOI] [PubMed] [Google Scholar]
- 9.Azimi I., Roberts-Thomson S.J., Monteith G.R. Calcium influx pathways in breast cancer: opportunities for pharmacological intervention. Br. J. Pharmacol. 2014;171(4):945–960. doi: 10.1111/bph.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan N.N., et al. Voltage-gated calcium channels: novel targets for cancer therapy. Oncol. Lett. 2017;14(2):2059–2074. doi: 10.3892/ol.2017.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhargava A., Saha S. T-Type voltage gated calcium channels: a target in breast cancer? Breast Cancer Res. Treat. 2019;173(1):11–21. doi: 10.1007/s10549-018-4970-0. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A., Shridhar K., Dhillon P.K. A review of breast cancer awareness among women in India: cancer literate or awareness deficit? Eur. J. Cancer. 2015;51(14):2058–2066. doi: 10.1016/j.ejca.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Bioinformatics and Medical Statistics . Medical Research Institute, University of Alexandria Egypt; 2017. Annual Report. Alexandria Cancer Registry. [Google Scholar]
- 14.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm. Sin. B. 2015;5(5):402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteith G.R., Davis F.M., Roberts-Thomson S.J. Calcium channels and pumps in cancer: changes and consequences. J. Biol. Chem. 2012;287(38):31666–31673. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis F.M., et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33(18):2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J. Exp. Clin. Cancer Res. 2019;38(1):106. doi: 10.1186/s13046-019-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay E.D., Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am. J. Kidney Dis. 1995;26(4):678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- 19.Canel M., et al. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 2013;126(Pt 2):393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 20.Perl A.K., et al. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 21.Heimann R., et al. Separating favorable from unfavorable prognostic markers in breast cancer: the role of E-cadherin. Cancer Res. 2000;60(2):298–304. [PubMed] [Google Scholar]
- 22.Aceto N., et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung K.J., et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. U. S. A. 2016;113(7):1. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha B., et al. Overexpression of E-cadherin protein in metastatic breast cancer cells in bone. Anticancer Res. 2007;27(6B):3903–3908. [PubMed] [Google Scholar]
- 25.Borcherding N., et al. Re-evaluating E-cadherin and β-catenin: a pan-cancer proteomic approach with an emphasis on breast cancer. Am. J. Pathol. 2018;188(8):1910–1920. doi: 10.1016/j.ajpath.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould Rothberg B.E., Bracken M.B. E-Cadherin immunohistochemical expression as a prognostic factor in infiltrating ductal carcinoma of the breast: a systematic review and meta-analysis. Breast Cancer Res. Treat. 2006;100(2):139–148. doi: 10.1007/s10549-006-9248-2. [DOI] [PubMed] [Google Scholar]
- 27.Venhuizen J.H., et al. P120 and E-cadherin: double-edged swords in tumor metastasis. Semin. Cancer Biol. 2020;60:107–120. doi: 10.1016/j.semcancer.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Hazan R.B., et al. Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 29.Cappellesso R., et al. The prognostic role of the epithelial-mesenchymal transition markers E-cadherin and Slug in laryngeal squamous cell carcinoma. Histopathology. 2015;67(4):491–500. doi: 10.1111/his.12668. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., et al. The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets. 2013;13(9):963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P.F., et al. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial-mesenchymal transition-related proteins. PLoS One. 2017;12(6):e0178581. doi: 10.1371/journal.pone.0178581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Tillo E., et al. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc. Natl. Acad. Sci. U. S. A. 2011;108(48):19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vleminckx K., et al. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66(1):107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 34.Hirooka K., et al. T-Type calcium channel alpha1G and alpha1H subunits in human retinoblastoma cells and their loss after differentiation. J. Neurophysiol. 2002;88(1):196–205. doi: 10.1152/jn.2002.88.1.196. [DOI] [PubMed] [Google Scholar]
- 35.Antal L., Martin-Caraballo M. T-Type calcium channels in cancer. Cancers. 2019;11(2):E134. doi: 10.3390/cancers11020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J.B., et al. Identification of channels promoting calcium spikes and waves in HT1080 tumor cells: their apparent roles in cell motility and invasion. Cancer Res. 2004;64(7):2482–2489. doi: 10.1158/0008-5472.can-03-3501. [DOI] [PubMed] [Google Scholar]
- 37.Jacquemet G., et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat. Commun. 2016;7 doi: 10.1038/ncomms13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkubo T., Yamazaki J. T-type voltage-activated calcium channel Cav 3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int. J. Oncol. 2012;41(1):267–275. doi: 10.3892/ijo.2012.1422. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M., et al. Aberrant expression of the MEL1S gene identified in association with hypomethylation in adult T-cell leukemia cells. Blood. 2004;103(7):2753–2760. doi: 10.1182/blood-2003-07-2482. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y., et al. Macrophage transcriptional profile identifies lipid catabolic pathways that can Be therapeutically targeted after spinal cord injury. J. Neurosci. : Off. J. Soc. Neurosci. 2017;37(9):2362–2376. doi: 10.1523/JNEUROSCI.2751-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.