Abstract
In recent decades, researchers have conducted in-depth studies of the design and synthesis of colorimetric/fluorometric probes and the application of such probes to biological and practical samples. The multifunctional colorimetric and fluorescent azo benzene-based probe (4′-hydroxyl-2,4-diaminoazobenzene, MP) was designed to detect Al3+, Fe3+, Cu2+ and F¯. Based on the distinct redshift of the absorption band and a significant color change (yellow → purple), MP was utilized for both naked-eyed and quantitative detection of Al3+ and Fe3+ after formation of the 1:1 complex. Test paper coated with MP and used in conjunction with a cell phone was used for colorimetric detection of Al3+ and Fe3+ ions (20 μM–2.0 mM) in water samples through naked-eye and digital image colorimetry. The “MP-Fe3+” coordination shift that occurs in the presence of the competitive ligand F¯ was used in the colorimetric measurement of F¯ in toothpaste. In the presence of Cu2+ ion, the non-emissive MP has transformed into fluorescent benzotriazole product PMP (Φ = 0.53) through the bimolecular rate-limiting step, and the second-order rate constant k is calculated as 31 ± 2 M−1 s−1. MP exhibits a “turn-on” fluorescence response in the presence of Cu2+ that is greater than its response in the presence of competitive species such as Fe3+, Al3+, Co2+, Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, Ni2+ and Ag+. MP was shown to have low toxicity to living HeLa cells and to present good imaging characteristics for tracking of Cu2+ in vivo.
Keywords: Fluorometric, Colorimetric, Multi-analytes, Ortho-amino azobenzene
Fluorometric; Colorimetric; Multi-analytes; Ortho-amino azobenzene.
1. Introduction
Metal cations and anions are vitally necessary in many physiological processes, in daily life, and in industrial production, and the levels at which they are present may have a profound impact on normal events [1, 2]. In the past two decades, numerous chemosensors that possess the merits of simplicity, low cost, high sensitivity, selectivity, and applicability to on-site analysis through spectral technology have been designed for the detection of metal cations and anions [3, 4, 5, 6, 7, 8]. From the perspective of practical application, the detection of multiple analyses with a single chemosensor would be more effective and economical than the use of many individual analysis procedures [9, 10], and the studies on recognition of multiple target species, including tri- or tetra-ions, are still urgently needed. Aluminum ion (Al3+) as the third most abundant element in the Earth's crust are found everywhere in our daily life, however, the high concentration of Al3+ in the environment is toxic, and this causes many health problems, such as Alzheimer's disease and Parkinson's disease. Moreover, iron ions (Fe3+) and copper ions (Cu2+) play the irreplaceable role in many fundamental physiological processes in organisms. In fact, more than 2/3 of the body's iron can be found in blood and muscle cells in the forms of hemoglobin and myoglobin. Cu2+ ions are involved in the process of iron absorption in the body, and maintenance of an appropriate concentration of Cu2+ is useful in maintaining normal bodily functions. The fluorine anion (F¯), as a typical halogen ion, also has a close association with human health. It is widely added to drinking water at concentrations ranging from 2–4 mg‧L−1 to help avoid osteoporosis and dental fluorosis [11]. To date, a certain number of chemosensors that can be used to monitor one or two of these four ions (Al3+, Fe3+, Cu2+, and F¯) have been developed; however, no studies have reported the selective detection of the abovementioned four ions using a single chemosensor.
Recently, considerable effort has been focused on designing fluorescent and colorimetric probes for Cu2+ based on azo dyes. These dyes have advantages that include their low cost as industrial colorants, their simple synthesis, and the rich colours they produce [12]. Ortho-substituted azobenzenes, a subgroup of classical azo dyes, can distinguish Cu2+ through reversible coordination reaction and through irreversible oxidation process [13, 14, 15, 16, 17, 18]. In the development of chemosensors belonging to the ortho-amino azobenzene family, three different strategies have been applied. The first strategy is the introduction of electron-donating groups, such as alkyl or amine group onto the ortho substituted azobenzene, which can accelerate the oxidative cyclization reaction by trace Cu2+ ions in neutral water solution [13]. The second strategy is related to inner ligand method, which served as a powerful tool in activation of C–H bonds for constructing C–C bonds [19, 20]. A third strategy is introduction of multifunctional groups in o-(phenylazo)aniline based molecular probes, such as Si–O groups in which the Si–O bond is broken due to the effect of F¯; a bifunctional probe for Cu2+ and F¯ was designed using this method [21]. Furthermore, chemical equilibrium has been applied to quantitative analysis, for example, through the use of S2- [22,23], the introduction of which alters the spectral and electrical signals produced by coordination compounds.
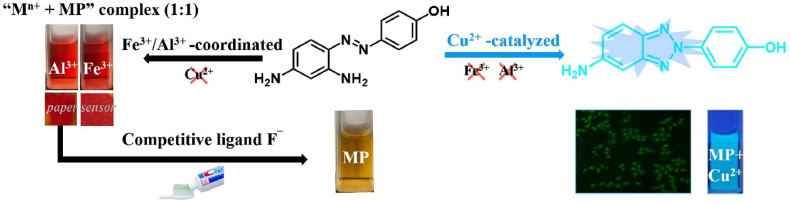
Inspired by the above three strategies, which can be used to design chemosensors based on the o-(phenylazo)aniline platform and on the principle of chemical equilibrium, a multifunctional azobenzene probe (4′-hydroxyl-2,4-diaminoazobenzene, MP) for Fe3+, Al3+, Cu2+ and F¯ is studied in this paper. The work presented here focuses on the spectral characteristics, mechanisms, and applications of MP. The hydroxyl and amino groups in MP act as internal ligands and influence the density of the electron cloud in the benzene ring. The interaction of MP with Fe3+, Al3+ ions based the coordination reaction indicating both changes of naked eye color and UV/Vis spectra. The determination of F¯ has been achieved by the movement of chemical equilibrium, in which F¯ was severed as the competitive ligand of “MP-Fe3+” complex. High selectivity and sensitivity of MP towards Cu2+ was achieved by synthesis of the highly fluorescent product 2-(4-hydroxylphenyl)-2H-benzo[d] [1, 2, 3]triazol-5-amine (PMP) during the Cu2+-catalytic process. Test paper coated with MP can be used in a pure water system for field determination of the levels of Fe3+ and Al3+ ions. The “MP-Fe3+” complex could also be a reliable probe for the quantitative determination of F¯ in toothpaste. MP has been studied in HeLa cells by confocal fluorescence microscopy and shown to allow fluorescence imaging and successful tracking of Cu2+ levels in vivo. The spectral characterization, mechanism of action, and application of MP in three different modes are summarized in Scheme 1.
Scheme 1.
Multi-functional probe MP based on different modes for visual colorimetric and fluorometric sensoring multiple analytes (Al3+, Fe3+, Cu2+ and F¯).
2. Results and discussion
2.1. Colorimetric analysis of Al3+ and Fe3+ using MP
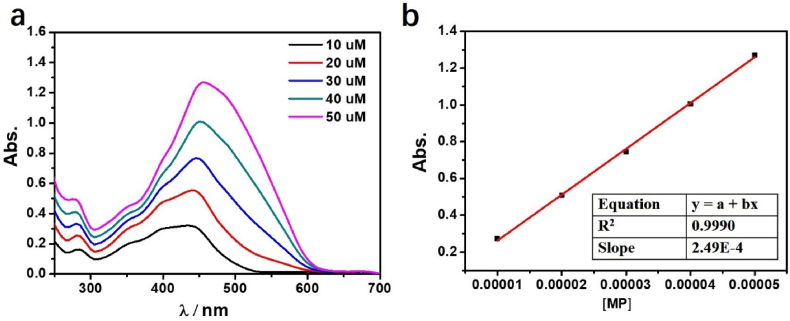
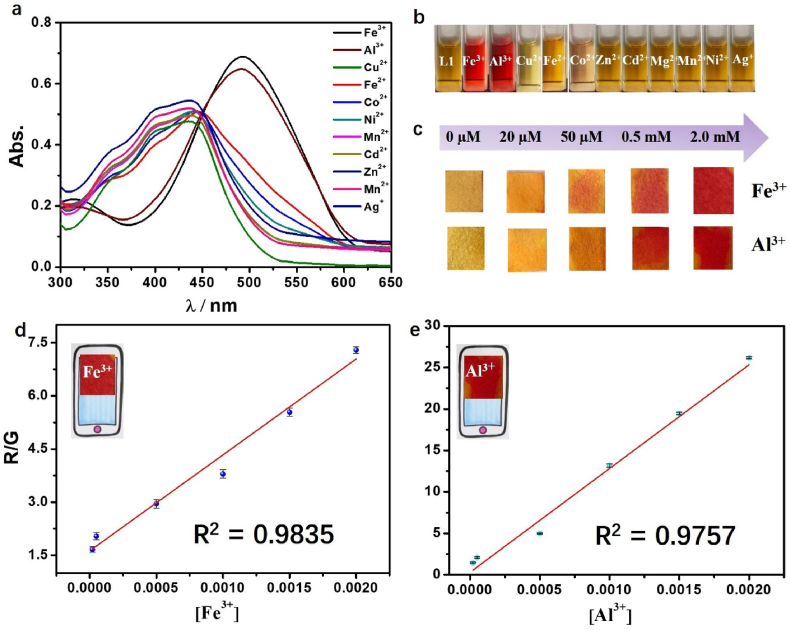
The colorimetric abilities of MP with different metal ions were investigated by UV/Vis spectroscopy. The free MP shows the strong absorption band at 439 nm, which can attribute to the azo chromophore with molar absorption coefficient ε439 nm = 2.49 × 104 L mol−1·cm−1 (R2 = 0.9990) (Figure 1). Addition of Fe3+ and Al3+ induced an apparent spectral change, whereas other ions like Co2+, Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, Ni2+, Ag+ and Cu2+ did not cause any obvious variations. Accompanied by the addition of Fe3+, Al3+ ions to the MP solution with molar ratio 1:1, the color of mixture changed from yellow to light-purple rapidly, and the purple solution can be stable over 20 days in atmosphere. The Fe3+, Al3+ ion recognition behaviors of MP were also investigated by UV/Vis spectroscopy with the addition of various cations concentrations to MP solution, and the ratio metric decrease of the band focused at ∼439 nm and increase at ∼487–489 nm (Fe3+ 487 nm, Al3+ 489 nm) with the isosbestic point at ∼447 nm. The formation of “Mn+ + MP” complex (Mn+ = Fe3+, Al3+) were proved by the red shift and good defined isosbestic point. As shown in the UV/Vis spectra, the large red shift for MP may be due to extensive delocalization of electrons with addition of metal ions that decreased the required energy for transition.
Figure 1.
(a) The UV absorbance spectra of different concentrations of MP in EtOH solution at room temperature; (b) The function relationship between Abs vs different concentrations of MP at 439 nm.
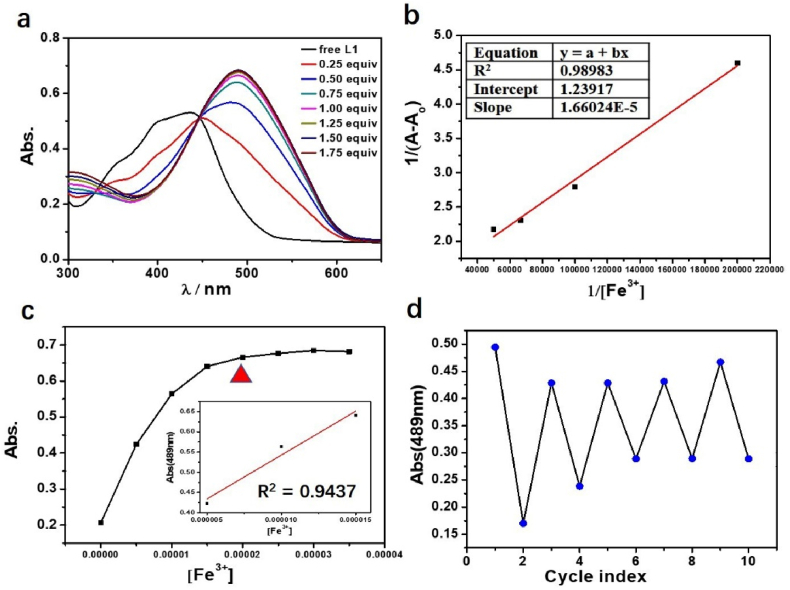
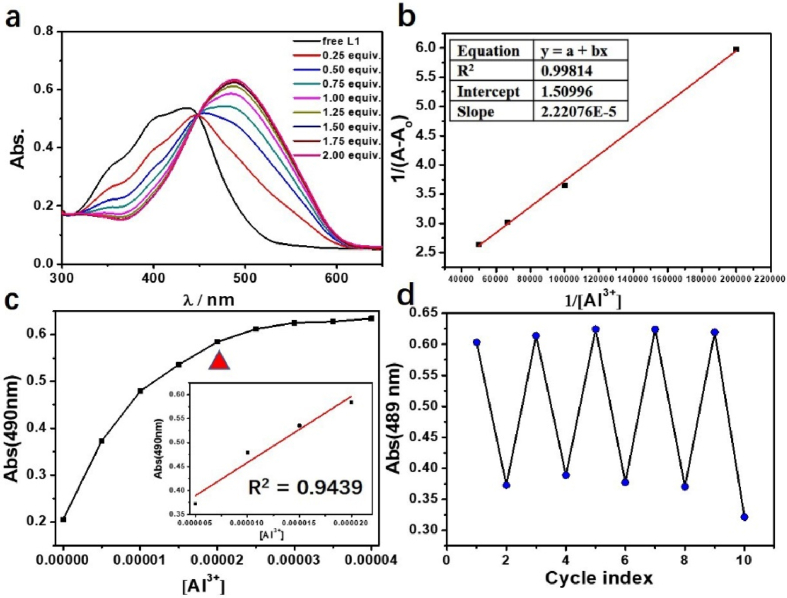
As the plot of absorbance MP vs Fe3+, Al3+ concentration (5–15 μM for Fe3+, 5–20 μM for Al3+) showed a good linear relation, the quantitative detection of Fe3+, Al3+ through UV/Vis spectra is possible (Figures 2 and 3), and the limit of detection (LOD) is 2.36 μM for Al3+, and 2.68 μM for Fe3+. The patterns of UV/Vis spectra remain largely unchanged with addition of more than 1.0 eq of metal ions. The Benesi–Hildebrand equation are introduced to calculate the binding constant k of “Mn+ + MP” complex. The functional relationship between the 1/(A-A0) and 1/[Mn+] has been established with the perfect linear relationship, providing evidence of 1:1 stoichiometry between MP and Fe3+ with k of 7.46×104 M−1, and k for Al3+ is 6.80×104 M−1. The value of binding constant k has indicated that Fe3+, Al3+ and MP combinate to a large extent, and the concentration of “Mn+ + MP” complex in the solution is dominant compared with Al3+, Fe3+ and free MP (Fig 4a-b).
Figure 2.
(a) UV/Vis spectra titrations of MP(20 μM) with gradually increasing of Fe3+ ion; (b) The function relationship between 1/(A-A0) and 1/[Fe3+]; (c) The relationship of Abs. vs different concentrations of the Fe3+ ion, and the red triangle represents that the quantitative relationship between Fe3+ and MP is 1:1; Insert figure: the good linear relationship between the Fe3+ ion concentration (5–15 μM) and Abs. at 489 nm; (d) UV/Vis absorption of MP (20 μM) solution circulated with addition of EDTA and Fe3+ by turns.
Figure 3.
(a) UV/Vis spectra titrations of MP (20 μM) with gradually increasing of Al3+ ion; (b) The function relationship between 1/(A-A0) and 1/[Fe3+]; (c) The relationship of Abs. vs different concentrations of the Al3+ ion, and the red triangle represents that the quantitative relationship between Al3+ and MP is 1:1; Insert figure: the good linear relationship between the Al3+ ion concentration (5–20 μM) and Abs. at 490 nm; (d) UV/Vis absorption of MP (20 μM) solution circulated with addition of EDTA and Al3+ by turns.
Figure 4.
(a) UV absorption spectra of MP (20 μM) in the presence of 20 μM different cations (Fe3+, Al3+, Co2+ Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, Ni2+, Ag+ and Cu2+); (b)The colour change of MP with 11 kinds of cations. (c) Yellow paper treated with MP (5.0 mM) turn into purple paper in the exist of Fe3+, Al3+ ion (20 μM–2.0 mM). (d–e) Digital image colorimetry for Fe3+, Al3+ detection based on MP test paper using Redmi Note 10 cellphone.
To give insight of the response ability between MP and Fe3+, Al3+, the addition of EDTA to “Mn+ + MP” (Mn+ = Fe3+, Al3+) system indicated that the sensing process of MP with Fe3+, Al3+ was reversible in the present of EDTA (Figures 2 and 3), reflecting the coordination interaction mechanism between ions with MP [24, 25, 26]. Combined with the results of UV/Vis spectra titrations, EDTA cycle experiment, and calculation of binding constant k, the coordination mechanism and mode between MP and Fe3+, Al3+ ions are proved sufficiently.
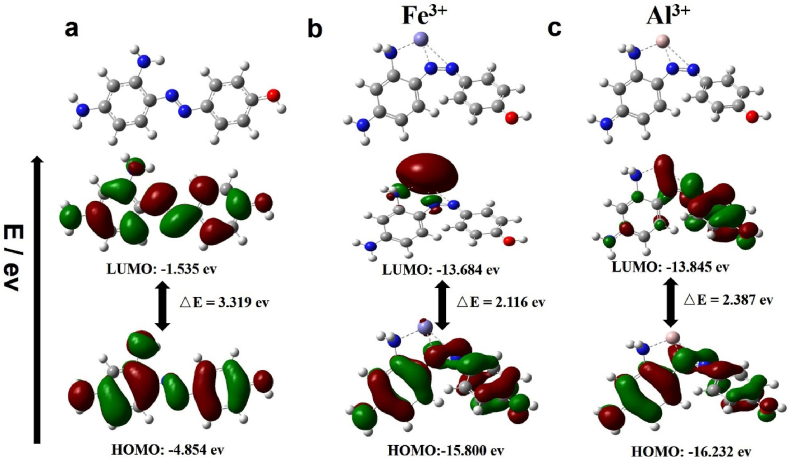
The energy-optimized structures of MP and the “Mn+ + MP” complex were studied using the Gaussian 16 program (Mn+ = Fe3+, Al3+). Geometry optimization calculations for C, H, O and N were performed by using the B3LYP DFT method. The basis set used for the C, H, O and N atoms was 6–311++G (d,p), and the LANL2DZ pseudopotential basis set was employed for Fe and Al atom. Frequency calculations under the same level were performed to determine the nature of a stationary point. The electron distributions and orbital energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of chemosensor MP and “Mn+ + MP” complex has been given in Figure 5. From the point of energy, the energy gap between the HOMO (−15.800 eV) and LUMO (−13.684 eV) of “Fe3+ + MP” complex was calculated to be 2.116 eV, and the energy gap for Al3+ are 2.387 eV, indicating the formation of complex has reduced energy of mixture and stabilized the system corresponding the red shift in UV/Vis spectroscopy. The azo and amino group served as the coordinate sites in constructing the “Mn+ + MP” complex, afforded information on the coordination behavior between MP and metal ions.
Figure 5.
Energy-optimized structures and energy diagram of HOMO and LUMO orbitals for (a) MP; (b) “Fe3+ + MP” (c) “Al3+ + MP”.
In order to expand the scope of application, the colorimetric test paper experiment was carried out with MP [27]. The filter papers coated with MP (5.0 mM) could detect Fe3+, Al3+ with minimum concentration of 20 μM together with color change from yellow to purple and distinguish them from other metals. With increasing the concentration of Fe3+ and Al3+ ions (20 μM–2.0 mM), the color of the test paper strips changes by degrees (Figure 4c). The paper sensor treaded with MP can be used in 100% aqueous solution for rapid visual detection of Fe3+, Al3+, serving as an ideal naked-eye sensor for on-site assays.
For Fe3+ and Al3+ ion exist widely in the environment, a convenient, low cost and fast method for Fe3+ and Al3+detection in water quality control is highly necessary. Nowadays, cellphone provides portability and accessibility, and there is no demand on the skills of user. Integrating the colorimetric test paper with the cellphone will prompt the quantitative sensing of Fe3+ and Al3+ based on the RGB color values through color analyzing APP. In detail, we take photos of the colorimetric test paper immersed with a series of concentration (20 μM–2.0 mM) of Fe3+ and Al3+ which have been added in the tap water in advance. Then the colors of test paper are analyzed, the linear relationship of R/G (red/green) ratios and ion concentrations are built with satisfactory R2 value (Figure 4d,e).
2.2. Colorimetric studies of the interaction of “MP + Mn+” complex with F¯ ions
During the past decade, a great deal of probes for detecting F¯ ion have been reported based on diverse mechanism such as Lewis's acid-base combination, hydrogen bonding and so on. For example, desilication reaction has been introduced to detect F¯ ion with high selectivity, and many probes have been designed to detect F¯ ion due to the fracture of C–Si, O–Si and O–P bond [28, 29, 30]. Moreover, the sulfonamide-conjugated poly-(phenylacetylene)s have been prepared to afford a novel colorimetric probe for F¯ ion through Lewis acid-base interaction [31]. Nevertheless, most of the F¯ probes reported works in organic solution system and the source of F¯ is tetrabutylammonium fluoride (TBAF) [32]. In short, detecting F¯ using NaF as sources in pure water sample still face challenging for the small atomic radius and high electronegativity of fluorine.
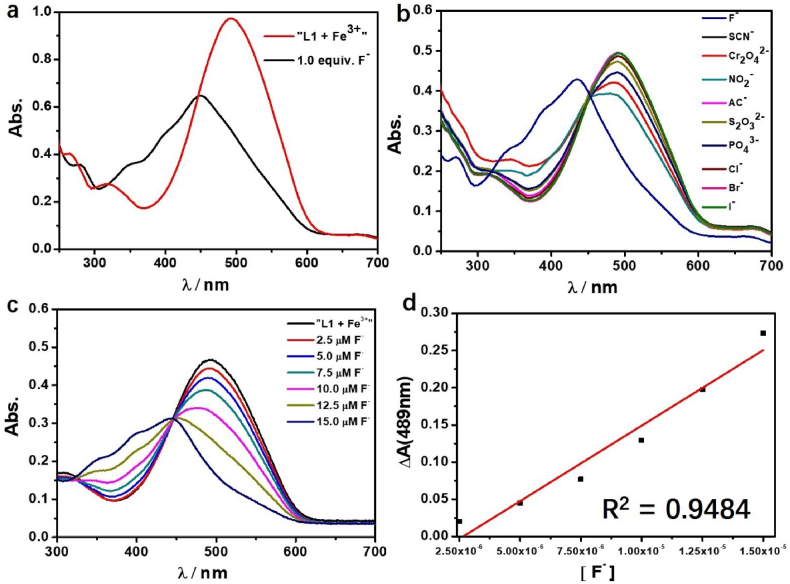
The coordinate interaction between fluoride ions and hard metal ions is strong according to hard and soft acid base theory (such as [FeF6]3- and [AlF6]3-), and it has been proved that the interactions between hard metal ions and F− would break the metal-ligand coordination bond [33]. To study the binding properties of “Mn+-MP” (Mn+ = Fe3+, Al3+) with F¯, UV/Vis spectra titration was performed (Figures 6 and 7). With the molecular ratio of F− and Mn+ is equal to 1:1, the absorbance increased at 439–449 nm and decreased at ∼555 and ∼487 nm, which indicates the recovered to original position of ligand from the reaction of “Mn+-MP” with F− along with the color change from purple to yellow. This dissociation can be explained by the stronger binding affinity between F¯ and Fe3+, Al3+ than the metal-MP interaction (Kfθ[AlF6]3- = 6.9 × 1019, Kf1θ[FeF]2+ = 7.1 × 106 and Kf2θ[FeF2]+ = 3.8 × 1011), which is much bigger than binding constant k with MP (k for Fe3+ is 7.46×104 M−1, and k for Al3+ is 6.80×104 M−1). In briefly, the reaction between “Mn+-MP” with F− can be attributed to the ligand substitution reaction, in which the F− coordinate with Fe3+, Al3+ instead of MP ligand.
Figure 6.
(a) The UV/Vis spectra of “MP + Fe3+” complex (Molar ratio = 1:1) with addition of F¯. (b) Absorption of “MP + Fe3+” complex adding with a series of anions (F¯, Cl¯, Br¯, I¯, CrO4¯, PO43-, NO2¯, S2O32-, SCN¯ and AC¯). (c) Absorption of “MP + Fe3+” complex with the addition of F¯. (d) Working curve of F¯ (2.5–15.0 μM) at 489 nm.
Figure 7.
The UV absorbance spectra of “MP + Al3+” complex (Molar ratio = 1:1) with addition of F¯.
Moreover, we focused the studies on the complex “MP-Fe3+”, the inhibition test of “MP-Fe3+” towards F¯ was conducted with a number of anions (Cl¯, Br¯, I¯, CrO4¯, PO43-, NO2¯, S2O32-, SCN¯ and AC¯) (Figure 6b). With the UV/Vis spectra titration, the complex “Fe3+-MP” can detect F¯, showing the good linear relationship in a certain concentration range (2.5–15.0 μM). By introducing competitive coordination ions to affect the equilibrium of “MP-Fe3+”, the detection working curse for F¯ ion was established successfully. To prove whether the “MP-Fe3+” complex could determine fluoride content in authentic specimen, we compared the F¯ content in Crest® brand toothpaste with detected value, as calculated from weight of toothpaste, respectively. The results shown that “MP-Fe3+” complex can be used to determine F¯ in the toothpaste. Tagged and found F¯ concentration represented recovery percentage in range of 104.1–116.4% (Table 1). The F¯ ion concentration in practical samples such as mouthwash, tap water and groundwater from different areas could be analyzed by the method established by “MP-Fe3+” complex toward F¯ ion [34].
Table 1.
Determination of F¯ in toothpaste samples.
| Sample | F¯ tagged (μM) | F¯ found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 5.80 | 5.85 | 104.1% |
| 2 | 11.6 | 13.5 | 116.4% |
| 3 | 17.4 | 18.8 | 108.0% |
2.3. Fluorescence spectral response of MP to Cu2+ ion
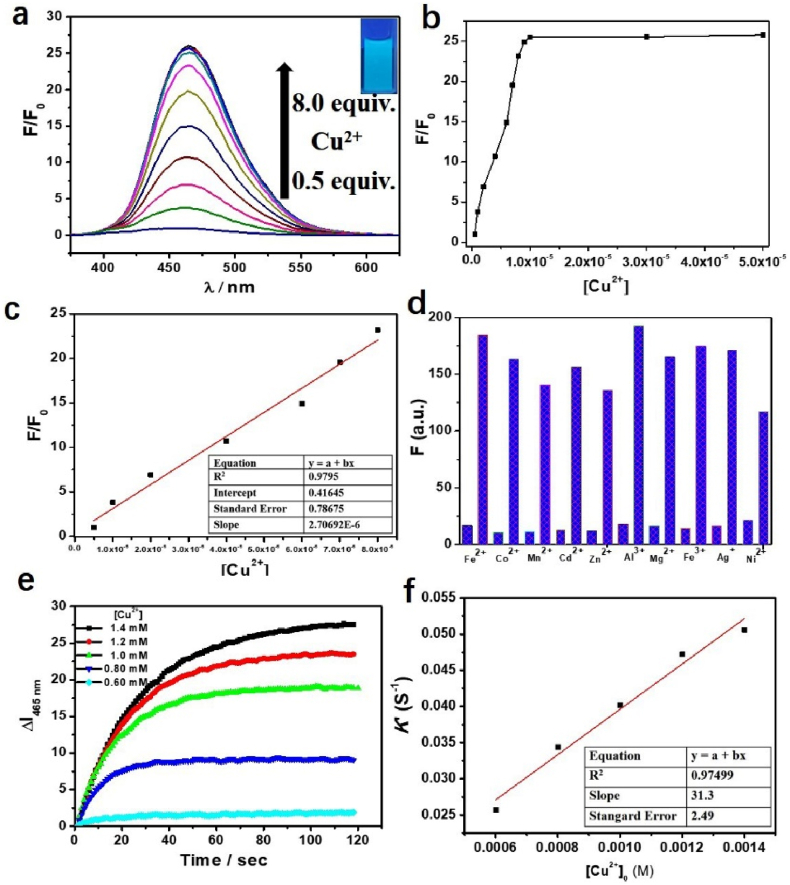
Far different from Fe3+ and Al3+, Cu2+ ion has not immediately caused the color change of MP solution, but the color of the solution began to lighten after 10 min, and the solution faded to colorless within 24h, while the non-emissive MP solution has shown strong blue-green light under UV lamp, hence we have carried out the research on fluorescence properties of MP with Cu2+ (Figure 8). When 0.50 to 8.0 equivalents of Cu2+ ion were added to MP solution, an obvious bright blue fluorescence at about 460 nm induced, which is far different from of initial MP solution. The fluorescence emission intensity of MP exhibited a good linear relationship with the concentration of Cu2+ (0.50–8.0 μM) and LOD of MP for Cu2+ ion was determined to be 0.959 μM through 3σ/k method, which is far less than the maximum allowable concentration 31.5 μM of Cu2+ in potable water as set by the WHO [10]. The selectivity of the MP was investigated by preparing a series of metal ion solutions (Fe3+, Al3+, Co2+, Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, Ni2+and Ag+), and then added separately to the MP (1.0 μM) solution. It is observed that only addition of Cu2+ (1.0 μM) caused the apparent fluorescence enhancement of MP solution, while other metal ions cause no significant changes. In order to prove the anti-interference of probe, a competing experiment was performed by the addition of Cu2+ (1.0 μM) in the presence of other species with molecular ratio as 1:1. For MP, the metal ions Fe3+, Al3+, Co2+ Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, and Ni2+ give no disturbance with Cu2+. For the merits of high selectivity, anti-interference and rapid response, the application of MP could determine Cu2+ instantly in environmental and biological systems.
Figure 8.
(a) Fluorescence spectra of MP (1.0 μM) and Cu2+ (0.5–8.0 equiv). Insert: the mixture of MP and Cu2+ under UV lamp. (b) The fluorescence intensity vs Cu2+ ion concentrations at ∼460 nm. (c) The linear relationship of fluorescence intensity vs Cu2+ ion (0.5–8.0 μM). (d) Fluorescence intensity changes of the MP (1.0 μM) to Cu2+ (1.0 μM) with interfering ions (1.0 μM) at ∼465 nm (Lower: Interfering ion; Higher: Interfering ion + Cu2+ ion). (e) At 465 nm, time-dependent changes in the fluorescence intensity observed for the reaction between MP (2.0 μM) and Cu2+ (0.60, 0.80, 1.0, 1.2, and 1.4 mM; from bottom to top traces) at room temperature. (f) The linear relationship of k’ (= k[Cu2+]0) vs [Cu2+]0 to gain the second-order rate constant k = 31 ± 2 M−1 s−1.
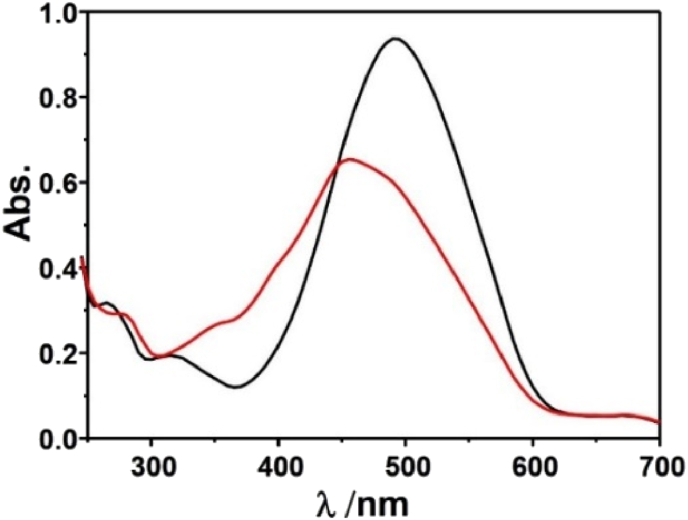
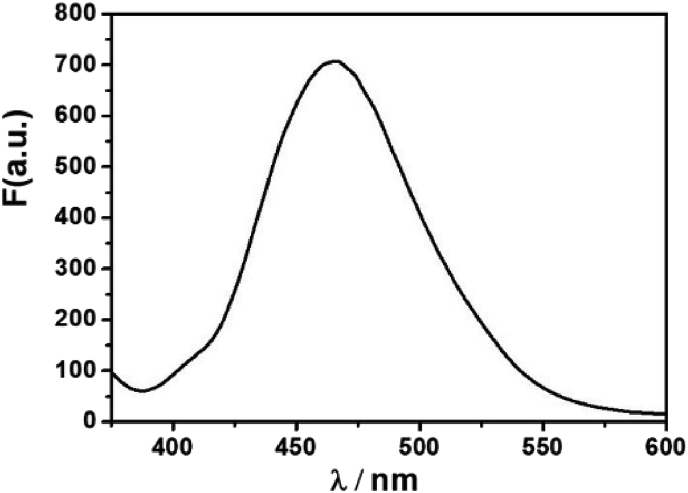
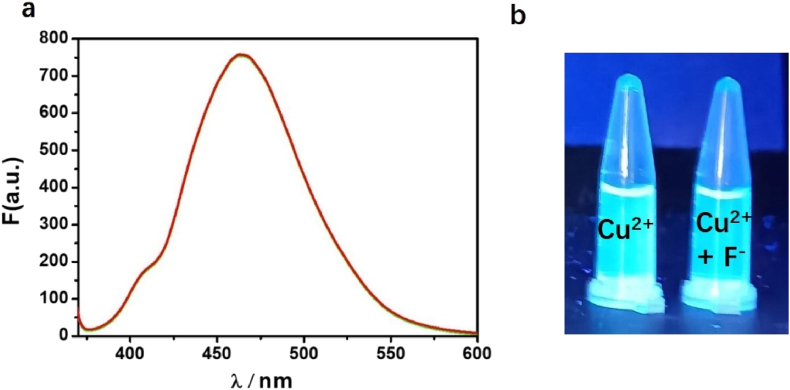
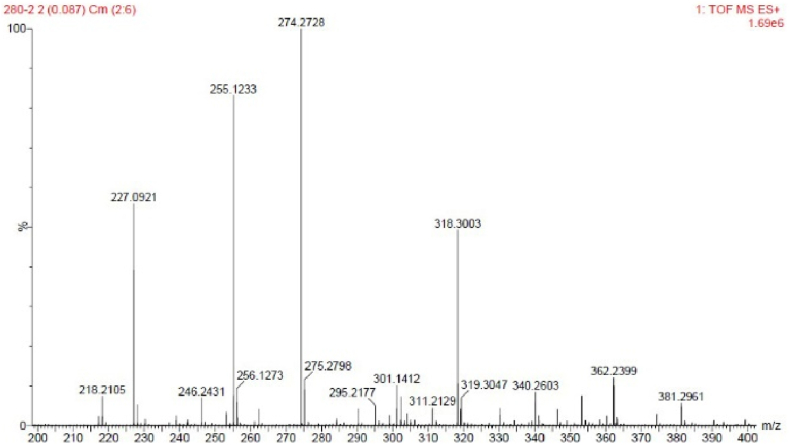
The fluorescent probes including two basic mechanisms for achieving specificity has been designed: (1) Cu2+-coordinated reaction sensing and (2) Cu2+-promoted reaction sensing [35]. In both types, a fluorescent chemosensor consists of a molecule incorporating an ion-binding site and fluorophore, and the sensing process can be concluded as the target metal analyte attached to the fluorophore affect the change of fluorescence. The coordinating-based type depends on a chelating ligand that reversibly binds the targeted metal and induces a fluorescence response through mechanisms containing internal charge transfer (ICT), photoinduced electron transfer (PET), and Förster resonance energy transfer (FRET). The fluorescence recognition process is usually reversible by adding other chelating reagents (EDTA or critic acid [36]) For the other type, the activity-based sensing trend to utilize the specific transformations induced by specific metal, the Cu2+-promoted reaction sensing involved the irreversible chemical reactions in which the non-emissive reactants transform to fluorescent products. The Cu2+ ion probes based on azobenzene can served both as Cu2+-coordinated and Cu2+-promoted reaction sensing [15, 17]. In this paper, N=N isomerization of MP were prevented in the present of Cu2+, and the Cu2+-catalyzed oxidation mechanism is recommended (Scheme 1). To prove MP as Cu2+-promoted reaction sensing, the fluorescent product (PMP) was prepared directly and fully charactered. The emission peak of PMP is around 460 nm, which coincides with the position of “MP + Cu2+” in fluorescent spectra (Figure 9). Using quinine sulfate as reference, the fluorescence quantum yields Φ of PMP have been measured (Φ = 0.53). Owing to the irreversible Cu2+ catalysis process, the addition EDTA to “MP + Cu2+” mixture has not changed the fluorescence emission as predicted (Figure 10a). With the molecular ratio of F− and Cu2+ is equal to 1:1, the fluorescence properties of “MP + Cu2+” mixture has remained the same under the UV lamp (Figure 10b). The mixture solution of “MP + Cu2+” was analyzed by HR-Mass, a peak of 227.09 (m/z) was suggestive to be [PMP]+ [calcd. 229.24]. The strong peaks of reactants PMP demonstrated that MP has been converted into PMP completely, the MP as the Cu2+-promoted reaction sensing has been fully proved (Figure 11).
Figure 9.
The fluorescence spectra of PMP in ethanol solution.
Figure 10.
(a) The fluorescence emission spectra of solution of “MP + Cu2+” adding with EDTA with same concentration of MP. Red line: MP + Cu2+; green lines: MP + Cu2++ EDTA. (b) Left: The MP (4.0 μM) ethanol solution (0.5 mL) added with Cu2+(4.0 μM); Right: The initial solution of “MP + Cu2+” added with F¯ (4.0 μM).
Figure 11.
Mass spectra of solution of “MP + Cu2+”, which has demonstrated the formation of PMP for the peak at 227.09.
The systematic kinetic studies between reactants MP and Cu2+ have been carried out. We concluded by the evidence that the reaction between the MP and Cu2+ proceeds at room temperature to provide a highly fluorescent product PMP. We set that Cu2+ reactant was greatly excessive (≥300 equiv of [Cu2+] with respect to MP), and its consumption in the reaction was negligible relative to MP. The generation of the product PMP was monitored by time-dependent enhancement in the fluorescence intensity at 465 nm under pseudo-first-order reaction condition. Using Eq. (1), the exponential increase in ΔI465 nm as a function of time was fitted to calculate the pseudo-first-order rate constant k′:
| eq 1 |
Furtherment, the linear dependence of k′ (= k [Cu2+]o; eq 1) on [Cu2+]o established that the reaction proceeds via bimolecular rate-limiting step with first-order in both Cu(II) ion and MP, and the second-order rate constant is calculated as k = 31 ± 2 M−1 s−1, which is slightly bigger than the azobenzene-based probes reported [13]. It is also shown that the accelerating effect of inner ligand effect on cyclization reaction [20] (Figure 8).
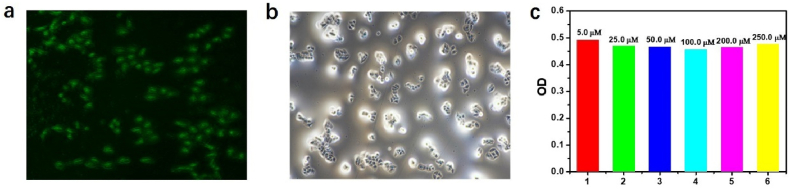
Given that the probe MP senses Cu2+ with fluorescence enhancement, we further used it for the cellular imaging. The Hela cells were alive with high OD value (92.6%) with 250 μM MP incubated in cells for 24 h according to the CCK8 method, suggesting that MP has no toxicity to cells. Subsequently, Hela cells incubated with the probe (50 μM, aqueous solution) for 30 min, and then were washed with PBS solution for three times. The cells were further incubated with Cu2+ (5 mM). The strong fluorescence can be seen after Hela cells pretreated by MP. By contract, Hela cell remained non-emissive only in the presence of Cu2+ or MP. During the reaction process, the morphology of cells was not damaged which is consistent with the results of CCK8. The results indicated that the MP offered a reliable detection method to intracellular Cu2+ by fluorescence imaging (Figure 12).
Figure 12.
(a) Fluorescence image in green channel of Hela cells preloaded with Cu2+ (5 mM) for additional 0.5h. (b) Bright-field image of Hela cells preloaded with probe MP (50 μM) in PBS (0.01 M, pH 7.4) for 30 min at 37 °C. (c) The effects of different concentrations of MP on the Hela cells by cck8 method for 24 h.
2.4. Comparison of MP with other chemosensors
In this paper, MP served as a chemosensor for multiple targets. The MP coordinated with Fe3+ and Al3+ ions, resulting in changes in colour visible to the naked eye and changes in the UV/Vis spectra. The coordination equilibrium of “MP-Fe3+” can shift due to binding of the competitive ligand F¯. Although MP is nonfluorescent, a fluorophore can be released via Cu2+-promoted oxidative cyclization to the benzotriazole product PMP. A comparison of MP with other reported chemosensors is given in Table 2. Although fluorescence detection is more sensitive than UV/Vis detection, the LODs of Fe3+, Al3+ and F¯ for MP are close to or better than those of fluorescent probes [37, 38, 39, 40]. The LOD of Cu2+ for MP reaches a level that is above average among chemosensors based on small organic molecules [22, 23, 38, 39], allowing it to be used to test conformity to the standard for drinking water established by WHO (≤31.5 μM). Moreover, EtOH, the solvent used in the MP sensing system, has relatively lower toxicity than other solvents. Thus, MP has expanded the scope of applications to water quality detection, daily chemical analysis, and biology.
Table 2.
The comparison of MP with other reported chemosensors.
| Methods | Target ions | Mechanism types | LOD/μM | Solvent | Application |
|---|---|---|---|---|---|
| This paper | Fe3+ Al3+ F¯ Cu2+ |
Colorimetric; Colorimetric; Colorimetric; Fluorescence. |
2.68 2.36 2.5 0.959 |
EtOH | Paper strip for practical water sample; F¯ in toothpaste; Hela cells. |
| [37] | Al3+ F¯ |
Fluorescence; Fluorescence. | 0.19 1.9 |
Bis–Tris buffer solution (pH = 7.0) | Real water sample, Hela cells; Real water sample. |
| [38] | Al3+ Cu2+ |
Colorimetric; Colorimetric. |
1.07 1.6 |
EtOH/H2O | Human lymphocyte cells. |
| [39] | Cu2+ F¯ |
Fluorescence; Fluorescence. | 2.0 2.8 |
DMF/bis-tris buffer | --- |
| [22] | Cu2+ | Colorimetric | 7.69 | EtOH | Paper strip for practical water sample. |
| [23] | Cu2+ | Fluorescence | 0.0767 | HEPES buffer | RKO cells. |
| [40] | Fe3+ | Fluorescence | 360 | Methanol/H2O | Hela cells. |
3. Conclusion
The azobenzene-based chemosensor MP was developed for effective use in colorimetric recognition of Al3+ and Fe3+ through a coordination mechanism and sequential sensing of F¯ through an equilibrium-shift mechanism. For Cu2+ ion recognition, efficient chemical transformation was obtained using azo dye transfer to the fluorescent benzotriazole PMP. Through combined UV/Vis spectra titration, analysis of fluorescence spectra, and HR-MS and EDTA chelation studies, we have provided insight into the multianalyte sensing process. MP is the first example in which a chemosensor based on an azo dye can simultaneously sense Al3+, Fe3+, Cu2+ and F¯. Test paper coated with MP can be used to detect Al3+ and Fe3+ over a wide concentration range through color change (yellow→ purple), and this can be combined with a cell phone system to allow quantitative sensing. The “MP-Fe3+” complex was able to detect F¯ in a toothpaste sample. Furthermore, MP efficiently and selectively recognized Cu2+ over other metal ions with high selectivity and sensitivity (LOD = 0.959 μM), as indicated by significant fluorescence enhancement at 460 nm. The successful preparation of test paper and the determination of F¯ in toothpaste and Cu2+ in HeLa cells using imaging based on MP has paved the way for the use of azo dyes in sensing applications related to environmental and biological monitoring and daily life. It is clear that a single probe that can simultaneously detect these four ions (Al3+, Fe3+, Cu2+, and F¯), all of which are closely related to daily life and to human health, will be both attractive and practical. The results indicate that MP represents a model for the design of a single chemosensor for multianalytes that can be used in both colorimetric and fluorescent modes.
4. Experimental section
4.1. General information
Most chemicals in this paper were purchased from commercial companies at analytic grade and used without further purification. Fresh double distilled water was used throughout the experiment. UV/Vis spectra were recorded on a Cary 60 spectrophotometer of Agilent Technologies using a 10 mm path length quartz cuvette. Infrared spectra were measured on a Nicolet Impact 410 spectrometer between 400 and 4000 cm−1, using the KBr pellet method. Mass spectra were collected on a Waters Xevo G2-XS QTof spectrometer. The NMR spectra were obtained on a Bruker-400 spectrometer, and the chemical shifts are expressed in δ ppm using TMS as an internal standard. The fluorescence data were determined on a PerkinElmer LS 45 luminescence spectrometer. For all luminescence measurement, excitation and emission slit widths of 10 nm were used. The wave number of excited light for UV lamp is 365 nm. The cell experiment was carried out with Nikon eclipse Ti microscope.
4.2. Preparation of MP and PMP
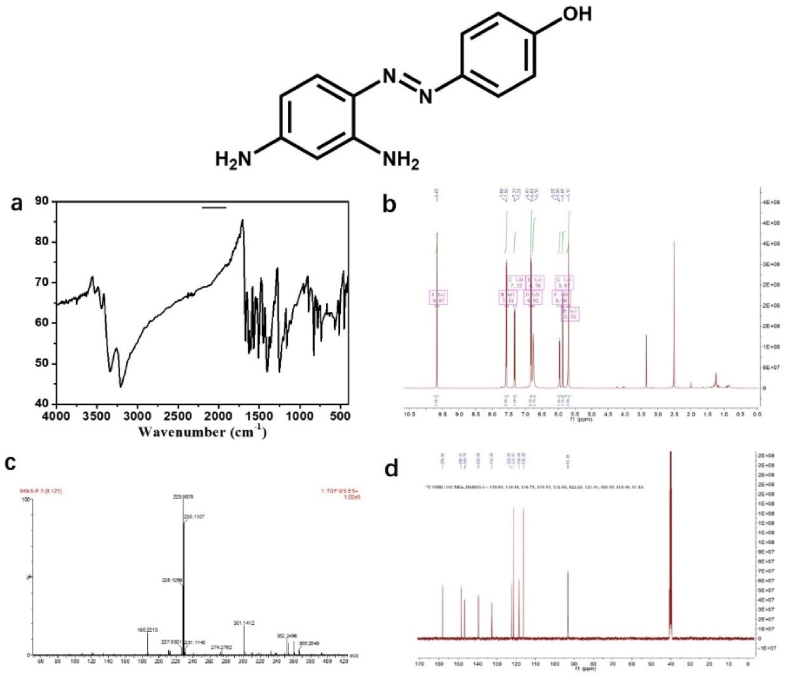
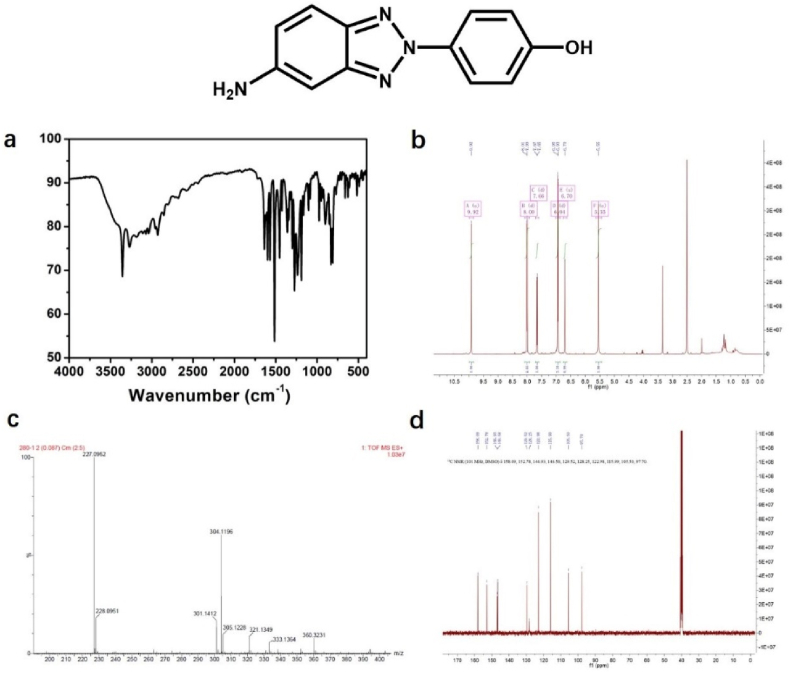
The MP was prepared using 4-aminophenol and 1,3-phenyldiamine as reactants through classical diazo coupling reaction (Figure 13). Using MP as raw material, the PMP was prepared under the oxidation of Cu2+. The chemical structure of MP, PMP were confirmed by 1H NMR, 13C NMR, Mass and IR spectra (Figures 14 and 15). 1H NMR (400 MHz, DMSO-d6) of MP: δ 9.67 (s, 1H), 7.57 (d, J = 8.6 Hz, 2H), 7.32 (d, J = 8.7 Hz, 1H), 6.82 (d, J = 8.7 Hz, 2H), 6.76 (s, 1H), 5.96 (d, J = 6.8 Hz, 1H), 5.87 (s, 1H), 5.70 (s, 2H). 13C NMR (101 MHz, DMSO-d6) of MP: δ 158.00, 148.41, 146.75, 139.59, 132.68, 122.26, 121.41, 118.50, 116.40, 93.18. MS (ES-API) for MP: calcd. C12N4OH13 [M + H]+: 229.26; found 229.09. For IR spectra, the peaks at 3341 and 3210 cm−1 are corresponding to the hydroxyl group. MP Yield: ∼85%. 1H NMR (400 MHz, DMSO-d6) of PMP: δ 9.92 (s, 1H), 8.00 (d, J = 8.9 Hz, 2H), 7.66 (d, J = 9.1 Hz, 1H), 6.94 (d, J = 8.9 Hz, 3H), 6.70 (s, 1H), 5.55 (s, 2H). 13C NMR (101 MHz, DMSO-d6) of PMP: δ 158.09, 152.78, 146.93, 146.58, 129.52, 128.25, 122.98, 115.99, 105.50, 97.70. MS (ES-API) for PMP: calcd. C12N4OH11 [M + H]+: 227.24; found 227.09. IR spectra for PMP, the peak around 1644–1424 cm−1 is attributed to benzotriazole ring in products [41]. PMP Yield: ∼60%.
Figure 13.
Schematic diagram of synthetic MP.
Figure 14.
(a) IR (b) 1H NMR (c) HR-Mass and (d) 13C NMR of MP.
Figure 15.
(a) IR (b) 1H NMR (c) HR-Mass and (d) 13C NMR of PMP.
4.3. General procedure for UV/Vis spectroscopy
4.3.1. Preparation of MP solution for UV/Vis spectroscopy
Add 10, 20, 30, 40 and 50 μL MP solution (2.0 mmol L−1) to ethanol solution to obtain the molar absorption coefficient from C-A relationship, and the total volume of ethanol solution was kept as 2.0 mL.
4.3.2. UV/Vis spectra titrations
The initial MP ethanol solution (20 μM, 2.0 mL) was titrated by addition of Fe3+, Al3+ solution (0, 0.25, 0.50, 0.75,1.0, 1.25, 1.50, 1.75, 2.0 equivalents). For other metal salts (Co2+, Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, Ni2+, Ag+ and Cu2+), the MP solution was added by salt solution (5.0 mM) with 1.0 equivalents once time.
4.3.3. The EDTA chelation experiment
The formation of “Mn+-MP” complex in ethanol solution can be gained by mixing the Fe3+, Al3+ salt (5 mM, 8 μL) and MP (2.0 mM, 20 μL) solution. Then the “Mn+-MP” complex solution was added by EDTA and Fe3+, Al3+ salt solution (5 mM), alternately.
4.4. Determination of F¯ content in toothpaste
4.4.1. Sample pretreatment of toothpaste
The Crest® toothpaste containing 0.11wt% F¯ as labeled was selected. Firstly, the 200 mg toothpaste samples were dried for 10h in oven at 80 °C, then the sample was dissolved in 10 mL water. The above solutions were sonicated for 1 h, balanced for another 24 h, centrifuged at 3000 r/min for 10 min and filtered to get clear solution for measuring.
4.4.2. The F¯ titrations experiment
The complex “Fe3+ + MP” can be gained by mixing the Fe3+ salt (5.0 mM, 8 μL) and MP (2.0 mM, 20 μL) in ethanol system. Then the “Fe3+ + MP” solution was added by F¯ sample (1.16 mM) step by step which was prepared from toothpaste. The NaF solution (0.50 mM) is used as the standard working curve to calculate the F¯ content.
4.5. Colorimetric test paper
The filter paper was immersed with MP (∼5.0 mM) ethanol solution, then was dried in air. A series of ions (Fe3+, Al3+, Co2+, Fe2+, Zn2+, Cd2+, Mg2+, Mn2+, Ni2+, Ag+ and Cu2+) were dissolved in distilled water to furnish 2.0 mM stock solution. The test papers prepared in advance were dipped into in metal ions solution.
4.6. General procedure for fluorescence spectroscopy
All the fluorescence spectroscopy of MP were recorded upon the addition of metal salts in ethanol system. Using quinine sulfate as the standard reference, fluorescence quantum yields of MP were measured. The quantum yield was determined according to the following equation:
| eq 2 |
Where Φ is the quantum yield, and the Yu and Ys represent the fluorescence quantum yield of standard and sample, respectively. Fu and FS denote the integral fluorescence intensity of the substance to be tested and the reference substance, while Au and As represent the absorbance of the substance to be tested and the reference substance at certain wavelength.
4.7. Cell culture and confocal imaging of Hela cells
In humidified atmosphere of CO2/air (5: 95%) at 37 °C, Hela cells were cultured in modified Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS). Then, the cells (3×105/well) were seeded in a 12-well plate for 24 h, and washed with PBS buffer (pH = 7.4). The cells in logarithmic growth stage were detected by CCK8 method, the cell viability was measured according to the MP concentration gradient from 5–250 μM after 24 h of cultivation.
Declarations
Author contribution statement
Hong Ren: Conceived and designed the experiments; Wrote the paper.
Fei Li: Performed the experiments.
Shihua Yu: Contributed reagents, materials, analysis tools or data.
Ping Wu: Analyzed and interpreted the data.
Funding statement
This work was supported by the scientific research funds of Jilin Institute of Chemical Technology, the Natural Science Foundation of China (NSFC, No. 22106051), and the education department of Jilin Province (No. JJKH20210240KJ).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to two graduates LIU Fang and QIU Zhuo for experiment on spectroscopy.
Contributor Information
Hong Ren, Email: renhong@jlict.edu.cn.
Ping Wu, Email: wuping@jlict.edu.cn.
References
- 1.Wu D., Sedgwick A.C., Gunnlaugsson T., Akkaya E.U., Yoon J., James T.D. Fluorescent chemosensors: the past, present and future. Chem. Soc. Rev. 2017;46:7105–7123. doi: 10.1039/c7cs00240h. [DOI] [PubMed] [Google Scholar]
- 2.Lippard S.L., Berg J.M. University Science Books; New York: 1994. Principles of Bioinorganic Chemistry. Chapter 6. [Google Scholar]
- 3.Dujols V., Ford F., Czarnik A.W. A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J. Am. Chem. Soc. 1997;119:7386–7387. [Google Scholar]
- 4.Udhayakumari D., Naha S., Velmathi S. Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013-15. Anal. Methods. 2017;9:552–578. [Google Scholar]
- 5.Liu S., Wang Y.M., Han J. Fluorescent chemosensors for copper(II) ion: structure, mechanism and application. J. Photoch. Photobio. C. 2017;32:78–103. [Google Scholar]
- 6.Liu G.C., Li Y., Chi J., Xu N., Wang X.L., Lin H.Y., Chen Y.Q. Multi-functional fluorescent responses of cobalt complexes derived from functionalized amide-bridged ligand. Dyes Pigments. 2020;174 [Google Scholar]
- 7.Carneiro L.M., Bartoloni F.H., Angolini C.F.F., Keppler A.F. Solvent-free synthesis of nitrone-containing template as a chemosensor for selective detection of Cu(II) in water. Spectrochim. Acta A. 2022;267 doi: 10.1016/j.saa.2021.120473. [DOI] [PubMed] [Google Scholar]
- 8.Luo W.F., Zhang S., Ye J., Jiang B.H., Meng Q.H., Zhang G.H., Li J.Y., Tang Y.P. A multimodal fluorescent probe for portable colorimetric detection of pH and it’s application in mitochondrial bioimaging. Spectrochim. Acta A. 2022;267 doi: 10.1016/j.saa.2021.120554. [DOI] [PubMed] [Google Scholar]
- 9.Liu H.Y., Zhang B.B., Tan C.Y., Liu F., Cao J.K., Tan Y., Jiang Y.Y. Simultaneous bioimaging recognition of Al3+ and Cu2+ in living-cell, and further detection of F¯ and S2¯ by a simple fluorogenic benzimidazole-based chemosensor. Talanta. 2016;161:309–319. doi: 10.1016/j.talanta.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 10.Tarai A., Li Y., Liu B., Zhang D., Li J., Yan W., Zhang J.F., Qua J.L., Yang Z.G. A review on recognition of tri-/tetra-analyte by using simple organic colorimetric and fluorometric probes. Coord. Chem. Rev. 2021;445 [Google Scholar]
- 11.Zhou Y., Zhang J.F., Yoon J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014;114:5511–5571. doi: 10.1021/cr400352m. [DOI] [PubMed] [Google Scholar]
- 12.Sareen D., Kaur P., Singh K. Strategies in detection of metal ions using dyes. Coord. Chem. Rev. 2014;265:125–154. [Google Scholar]
- 13.Jo J.Y., Lee H.Y., Liu W.J., Olasz A., Chen C.H., Lee D.W. Reactivity-based detection of copper(II) ion in water: oxidative cyclization of azoaromatics as fluorescence turn-on signaling mechanism. J. Am. Chem. Soc. 2012;134:16000–16007. doi: 10.1021/ja307316s. [DOI] [PubMed] [Google Scholar]
- 14.Jung J.Y., Dinescu A., Kukrek A. Synthesis and comparative kinetic study of reaction-based copper(II) probes to visualize aromatic substituent effects on reactivity. J. Chem. Educ. 2020;97:533–537. [Google Scholar]
- 15.Wang Q.M., Wu S.S., Tan Y.Z., Yan Y.L., Guo L., Tang X.H. A highly selective, fast-response and fluorescent turn on chemosensor for the detection of Cu2+ ions and its potential applications. J. Photochem. Photobiol., A. 2018;357:149–155. [Google Scholar]
- 16.Sun X.L., Xu Y.F., Zhu W.P., He C.S., Xu L., Yang Y.J., Qian X.H. Copper-promoted probe for nitric oxide based on o-phenylenediamine: large blue-shift in absorption and fluorescence enhancement. Anal. Methods. 2012;4:919–922. [Google Scholar]
- 17.Ren H., Wu P., Li F., Jin L., Lou D.W. Visual colorimetric and fluorescence turn-on probe for Cu(II) ion based on coordination and catalyzed oxidative cyclization of ortho amino azobenzene. Inorg. Chim. Acta. 2019;487:234–239. [Google Scholar]
- 18.Ren H., Wu P., Li F., Jin L., Yu S.H., Lou D.W. Development of a colorimetric and fluorescent Cu2+ ion probe based on 2′-hydroxy-2,4-diaminoazobenzene and its application in real water sample and living cells. Inorg. Chim. Acta. 2020;507 [Google Scholar]
- 19.Dai L.X. Ullmann reaction, a centennial memory and recent renaissance-related formation of Carbon-Heteroatom bond. Prog. Chem. 2018;30(9):1257–1297. [Google Scholar]
- 20.Rej S., Ano Y., Chatani N. Bidentate directing groups: an efficient tool in C-H bond functionalization chemistry for the expedient construction of C-C bonds. Chem. Rev. 2020;120(3):1788–1887. doi: 10.1021/acs.chemrev.9b00495. [DOI] [PubMed] [Google Scholar]
- 21.Gupta M., Balamurugan A., Lee H. Azoaniline-based rapid and selective dual sensor for copper and fluoride ions with two distinct output modes of detection. Sens. Actuator. B-Chem. 2015;211:531–536. [Google Scholar]
- 22.Wu P., Ren H., Han D.D., Jin L., Yan L.N., Cui X.T. Effects of chemical equilibrium on Cu2+ colorimetric probe based on azobenzene with ortho amino and sulfonamide group. J. Mol. Struct. 2021;1244 [Google Scholar]
- 23.Wang P., Sun L.Y., Wu J., Yang X.P., Lin P.C., Wang M. A dual-functional colorimetric and fluorescent peptide-based probe for sequential detection of Cu2+ and S2- in 100% aqueous buffered solutions and living cells. J. Hazard Mater. 2021;407 doi: 10.1016/j.jhazmat.2020.124388. [DOI] [PubMed] [Google Scholar]
- 24.Kaur B., Kaur N., Kumar S. Colorimetric metal ion sensors - a comprehensive review of the years 2011-2016. Coord. Chem. Rev. 2018;358:13–69. [Google Scholar]
- 25.Zhang J., Yan Z., Wang S., She M., Zhang Z., Cai W., Liu P., Li J. Water soluble chemosensor for Ca2+ based on aggregation-induced emission characteristics and its fluorescence imaging in living cells. Dyes Pigments. 2018;150:112–120. [Google Scholar]
- 26.Wei T., Liang G., Chen X., Qi J., Lin Q. A functional applied material on recognition of metal ion zinc based on the double azine compound. Tetrahedron. 2017;73:2938–2942. doi: 10.1016/j.tet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan K.Q., Wang X.B., Yang H.R., Gao L.J., Han G.L., Zhou L.M., Fang S.M. Semiquantitative naked-eye detection of Cu(II) with a standard colorimetric card via a hydrogel-coated paper sensor. Anal. Methods. 2020;12:1561–1566. [Google Scholar]
- 28.Muwal P.K., Nayal A., Jaiswal M.K., Pandey P.S. A dipyrromethane based receptor as a dual colorimetric sensor for F¯ and Cu2+ ions. Tetrahedron. 2018;59:29–32. [Google Scholar]
- 29.Gale P.A., Caltagirone C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018;354:2–27. [Google Scholar]
- 30.Liu Y., Wang S.Q., Zhao B.X. A novel pyrazoline-based fluorescent probe for detecting fluoride ion in water and its application on real samples. RSC Adv. 2015;5:32962–32966. [Google Scholar]
- 31.Sakai R., Barasa E.B., Sakai N., Sato S., Satoh T., Kakuchi T. Colorimetric detection of anions in aqueous solution using poly(phenylacetylene) with sulfonamide receptors activated by electron withdrawing group. Macromolecules. 2012;45:8221–8227. [Google Scholar]
- 32.Kim G., Suh B., Kim C. A naked-eye sulfonamide-based colorimetric and fluorescent “turn-on” chemosensor for detecting fluoride. J. Mol. Struct. 2022;1254 [Google Scholar]
- 33.Ren Y.P., Han J., Wang Y., Tang X., Wang L., Ni L. An OFF-ON-OFF type fluorescent probe based on a naphthalene derivative for Al3+ and F¯ ions and its biological application. Luminescence. 2018;33:15–21. doi: 10.1002/bio.3366. [DOI] [PubMed] [Google Scholar]
- 34.Yadav P., Laddha H., Agarwal M., Kushwaha H.S., Gupta R. Studies on 1,8-naphthalimide derivative as a robust multi-responsive receptor for an array of low cost microanalytical techniques for selective prompt and on-site recognition of duplicitous fluoride in semi-aqueous medium. J. Fluor. Chem. 2021;249 [Google Scholar]
- 35.Iovan D.A., Jia S., Chang C.J. Inorganic chemistry approaches to activity-based sensing: from metal sensors to bioorthogonal metal chemistry. Inorg. Chem. 2019;58:13546–13560. doi: 10.1021/acs.inorgchem.9b01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chi Z., Ran X., Shi L.L., Lou J., Kuang Y.M., Guo L.J. Molecular characteristics of a fluorescent chemosensor for the recognition of ferric ion based on photoresponsive azobenzene derivative. Spectrochim. Acta A. 2017;171:25–30. doi: 10.1016/j.saa.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 37.Hwang S.M., Kim M.S., Lee M., Lim M.H., Kim C. Single fluorescent chemosensor for multiple targets: sequential detection of Al3+ and pyrophosphate and selective detection of F¯ in near-perfect aqueous solution. New J. Chem. 2017;41 [Google Scholar]
- 38.Mabhai S., Dolai M., Dey S.K., Dhara A., Choudhury S.M., Das B., Dey S., Jana A. Rhodamine-azobenzene based single molecular probe for multiple ions sensing: Cu2+, Al3+, Cr3+ and its imaging in human lymphocyte cells. Spectrochim. Acta: Mol. Biomol. Spectrosc. 2019;219:319. doi: 10.1016/j.saa.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 39.Beneto A.J., Siva A. A phenanthroimidazole based effective colorimetric chemosensor for Copper(II) and Fluoride ions. Actuators B: Chem. 2017;247:526–531. [Google Scholar]
- 40.Qu X.Y., Bian Y.J., Bai Y., Shen Z. A visible BODIPY probe for “Naked-Eye” detection of pH value and intracellular Fe3+ Chin. J. Inorg. Chem. 2019;4:649–657. [Google Scholar]
- 41.Lu Z.J., Luo W.Z., Huang X.N., Yu H.X., Li Z.W., Liu G.C., Liu L.W., Chen X.X. Highly-stable cobalt metal organic framework with sheet-like structure for ultra-efficient water oxidation at high current density. J. Colloid Interface Sci. 2022;611:599–608. doi: 10.1016/j.jcis.2021.12.132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.