Abstract
Metastatic pulmonary calcification (MPC) is characterized by deposition of calcium in the normal lung parenchyma secondary to elevation of serum calcium. Most patients are asymptomatic and routine chest radiograph is not sensitive to make the diagnosis. Further imaging is needed such as computed tomography (CT) which typically shows small centrilobular nodules in the upper lobes. We report a case of a 30‐year‐old woman with end stage kidney disease who was diagnosed with pulmonary tuberculosis which was then revised to metastatic pulmonary calcification. The CT thorax feature for this patient was atypical for metastatic pulmonary calcification where it demonstrated tree‐in‐bud nodules suggestive of infection. The final diagnosis was made based on bronchoalveolar lavage which was culture‐negative for Mycobacterium and transbronchial lung biopsy demonstrating calcium deposition in the interstitium.
Keywords: metastatic pulmonary calcification, MPC, pulmonary tuberculosis
We report a case of a 30‐year‐old woman with end stage kidney disease who was diagnosed with pulmonary tuberculosis, which was then revised to metastatic pulmonary calcification. The CT thorax feature for this patient was atypical for metastatic pulmonary calcification where it demonstrated tree‐in‐bud nodules suggestive of infection. The final diagnosis was made based on bronchoalveolar lavage, which was culture‐negative for Mycobacterium and transbronchial lung biopsy demonstrating calcium deposition in the interstitium.

INTRODUCTION
Metastatic pulmonary calcification (MPC) is a metabolic lung disorder characterized by deposition of calcium in the lung parenchyma secondary to chronic elevation of serum calcium. The most common cause of MPC is chronic kidney disease, although it is also associated with hypercalcaemia due to other benign or malignant causes. The typical CT thorax features of MPC include upper lobes centrilobular nodules with or without calcification. Ground glass attenuation and less commonly, dense consolidation can also be present.
Here we report a case of a 30‐year‐old woman with end stage kidney disease who was diagnosed with MPC but having atypical features on chest imaging, mimicking pulmonary tuberculosis.
CASE REPORT
A 30‐year‐old woman with end stage kidney disease of unknown aetiology on regular peritoneal dialysis presented with left inner thigh swelling and pain for 3 days. On examination, there was a bluish‐black lesion measuring 5 × 5 cm with blister formation over the medial aspect of the left thigh. Radiograph of the left thigh showed calcified blood vessels. Blood investigation revealed elevated serum calcium of 2.74 mmol/L, serum phosphate of 3.3 mmol/L and serum iPTH of 151 pmol/L (normal range 1.96–8.49 pmol/L). Blood urea and creatinine were also significantly deranged at 36.8 mmol/L and 1250 μmol/L, respectively. Based on these findings, she was diagnosed to have calcific uremic arteriolopathy. Admission chest radiograph showed diffuse small granular shadows in bilateral lung fields (Figure 1). She did not have any respiratory or constitutional symptoms. There was no known previous contact with tuberculosis. On examination, she was not in respiratory distress and oxygen saturation was 98% on air. There was percussion dullness and reduced breath sounds over the lung bases. Computed tomography (CT) of the thorax showed bilateral pulmonary nodules with tree‐in‐bud appearance mainly in the upper lobes (Figure 2A,B).
FIGURE 1.
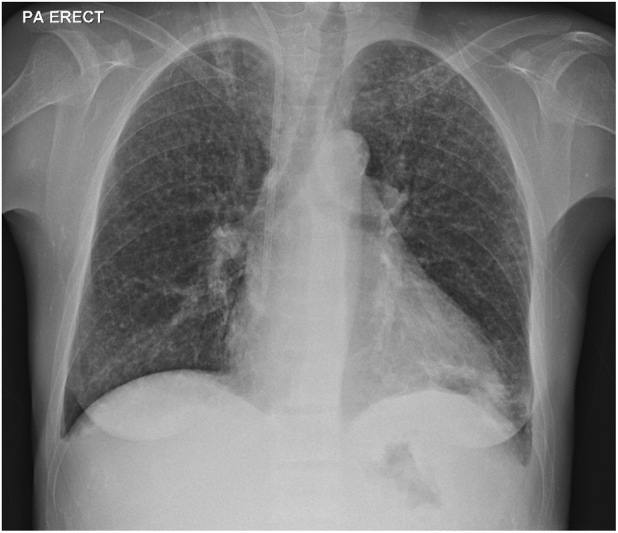
Chest radiograph (CXR) showing diffuse small granular shadows especially in the upper lobes
FIGURE 2.

(A) Lung window of coronal view of CT thorax showing tree‐in‐bud appearance predominantly in the upper lobes. (B) Lung window of axial view of CT thorax showing tree‐in‐bud appearance in the upper lobes and superior segments of the lower lobes with bilateral pleural effusions
The patient was unable to produce sputum specimens for acid‐fast bacilli and mycobacterium culture despite attempts at sputum induction. Interferon gamma release assay (IGRA) was not performed as it cannot differentiate an active versus latent TB infection especially in a high prevalence countries such as Malaysia. Subsequently, the patient underwent bronchoscopy during which bronchoalveolar lavage (BAL) and transbronchial lung biopsy were performed. The BAL specimen was direct smear‐negative for acid‐fast bacilli and culture‐negative for Mycobacterium tuberculosis. It was also culture‐negative for bacteria and fungi. Histology of the lung biopsy showed deposits in the interstitium which were von Kossa stain‐positive, consistent with calcification (Figure 3A,B).
FIGURE 3.

(A) Dense basophilic deposit in the interstitium on haematoxylin and eosin staining. (B) Positivity of von Kossa stain (dark brown to black) demonstrating calcium deposition
She was referred for total parathyroidectomy to treat the tertiary hyperparathyroidism and its complications. Her renal replacement therapy regimen was also optimized with the change of peritoneal dialysis to haemodialysis.
DISCUSSION
MPC is characterized by diffuse calcium deposits in the otherwise normal lung parenchyma. It occurs in conditions which directly or indirectly cause elevation in serum calcium and calcium‐phosphate products. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 It is associated with both benign and malignant conditions such as chronic kidney disease, hyperparathyroidism, hypervitaminosis D, milk‐alkali syndrome, multiple myeloma, parathyroid carcinoma and osteolysis from metastatic cancers. 2 , 3 , 6 , 8 Of these, the most common cause is chronic kidney disease. MPC is reported to be present in autopsy findings of 60%–75% of patients on haemodialysis. 2 , 3 , 5 , 6 , 8
MPC can develop over both a short or long period of time. The majority of patients with MPC are asymptomatic but a small proportion may develop restrictive ventilatory defect and potentially life‐threatening respiratory failure. 4 , 5 Common presenting symptoms include dyspnoea and chronic non‐productive cough. 6 , 8 However, the degree of respiratory symptoms may not corelate with the extent of lung involvement. Patients with extensive lung involvement may be asymptomatic whereas those with mild radiological changes may have severe respiratory failure. 6 , 7
Many patients are remained undiagnosed as routine chest radiograph is not sensitive or specific to detect MPC. In many cases, the chest radiograph maybe normal or demonstrates patchy or nodular opacities involving the upper lobes more than the lower lobes. 2 The opacities are ill‐defined with or without calcification. 3 In moderate calcification, there is reticulation with pattern of interstitial disease. 6 , 9 In this patient, the chest radiograph showed atypical findings of diffuse small nodules.
CT of the thorax is a better imaging modality in detecting MPC as it can detect minor changes compared to the chest radiograph. Common features of MPC on CT include centrilobular nodules measuring 3–10 mm, which may or may not calcify. 1 , 2 , 3 , 6 , 7 , 8 Calcifications can be punctate, or ring‐like within the lung nodule or involve the entire nodule. 3 , 6 , 8 Patchy ground glass attenuation and less commonly dense lobar consolidation can also be seen. 4 , 6 , 7 , 8 Calcification of other structures, such as superior vena cava, pulmonary arteries, chest wall vessels and bronchial wall, can also be observed. 2 , 4 , 8 The lung parenchyma changes are usually peripheral and upper lobe in distribution where the higher ventilation‐perfusion ratio with a lower pCO2 and a higher pH environment is conducive for calcium deposition. 1 , 2 , 3 , 4 , 5 , 6
Centrilobular nodules, a common finding on CT thorax, can generally be divided into two patterns: centrilobular nodules with tree‐in‐bud appearance and ill‐defined centrilobular nodules with ground glass attenuation. 10 Centrilobular nodules with tree‐in‐bud appearance are commonly observed in pneumonias due to Mycobacterium tuberculosis, Mycoplasma pneumoniae, non‐tuberculous Mycobacterium (NTM) (commonly, Mycobacterium avium intracellulare and Mycobacterium kansasii), fungal and human T‐lymphotropic virus 1 infections. 10 , 11 This pattern is also observed in diffuse pan‐bronchiolitis and allergic bronchopulmonary aspergillosis (ABPA). In contrast, ill‐defined centrilobular nodules with ground glass attenuation are observed in metastatic calcification, hypersensitivity pneumonitis, respiratory bronchiolitis‐interstitial lung disease (RB‐ILD), lymphoid interstitial pneumonia, eosinophilic granulomatosis with polyangiitis and systemic lupus erythematosus. 10 , 11 Upper lobe distribution is commonly seen in pulmonary tuberculosis (PTB), NTM infection, ABPA, metastatic calcification, hypersensitivity pneumonitis and RB‐ILD. Tree‐in‐bud appearance is a result of mucus impaction in the respiratory bronchioles 10 , 11 and is most commonly associated with infections, 12 whereas the calcium product deposits in MPC are mainly at the alveolar wall at the terminal bronchioles. Hence, although upper lobe predominant tree‐in‐bud appearance as in this patient is seen in PTB, it is not expected in MPC. 6 , 10
In MPC, calcification is commonly observed at the alveolar septa. The deposits may be found along the alveolar epithelial basement membrane and capillary endothelial walls. 3 , 5 , 6 On haematoxylin and eosin staining, MPC appears as basophilic materials. 6 It will also demonstrate positivity on von Kossa and Alizarin red staining which indicate calcium deposition in the tissue. 6 , 7 In more severe cases, fibrosis activity may be seen in the interstitium. 6 , 7
The mainstay of treatment for MPC is normalization of serum calcium and phosphate. 2 , 6 , 7 In the case of end stage kidney disease, this can be achieved with treatment such as bisphosphonate and parathyroidectomy. 2 Optimizing dialysis plays a role to control serum phosphate. 6 The evidence for discontinuation of vitamin D supplementation is still unclear; while it can potentially worsen hyperparathyroidism, some demonstrated benefit in treating MPC. 6 Spontaneous resolution has also been reported in some cases. 2 , 6
In conclusion, MPC is a metabolic lung disease caused by chronic elevation of serum calcium and is common in patients with chronic kidney disease. It is rarely diagnosed antemortem because most patients are asymptomatic and routine chest radiograph is not sensitive to detect MPC. Diagnosis of MPC can be made based on the combination of radiological features and histological findings. It is important to investigate for other causes such as infections based on certain geographical areas in combination with suggestive signs and symptoms. Bronchoscopy, BAL and lung biopsy may be needed to ascertain the diagnosis. The mainstay of management includes treatment of the underlying diseases causing hypercalcaemia, and normalization of calcium and phosphate levels.
AUTHOR CONTRIBUTION
All authors contributed significantly in the data collection, drafting and writing of the manuscript. All authors had read and approved the final manuscript. Thian Chee Loh: Data collection and main author (clinical and radiological). Jiunn Liang Tan: Data collection and co‐author (clinical and radiological). Man Fong Chew: Data collection and co‐author (pathology). Chong Kin Liam: Supervisor. Yong Kek Pang: Supervisor.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Loh TC, Pang YK, Liam CK, Chew MF, Tan JL. Metastatic pulmonary calcification mimicking pulmonary tuberculosis: A case report. Respirology Case Reports. 2022;10:e01030. 10.1002/rcr2.1030
Associate Editor: Kazuhisa Takahashi
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jarjou'i A, Bogot N, Kalak G, Chen‐Shuali C, Rokach A, Izbicki G, et al. Diffuse pulmonary calcifications: a case series and review of literature. Respirol Case Rep. 2021;9(10):e0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thurley PD, Duerden R, Roe S, Pointon K. Rapidly progressive metastatic pulmonary calcification: evolution of changes on CT. Br J Radiol. 2009;82(980):e155–9. [DOI] [PubMed] [Google Scholar]
- 3. Hochhegger B, Marchiori E, Soares Souza Jr A, Soares Souza L, Palermo L. MRI and CT findings of metastatic pulmonary calcification. Br J Radiol. 2012;85(1011):e69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madhusudhan KS, Shad PS, Sharma S, Goel A, Mahajan H. Metastatic pulmonary calcification in chronic renal failure. Int Urol Nephrol. 2012;44(4):1285–7. [DOI] [PubMed] [Google Scholar]
- 5. Murris‐Espin M, Lacassagne L, Didier A, Voigt JJ, Cisterne JM, Giron J, et al. Metastatic pulmonary calcification after renal transplantation. Eur Respir J. 1997;10(8):1925–7. [DOI] [PubMed] [Google Scholar]
- 6. Belém LC, Zanetti G, Souza AS Jr, Hochhegger B, Guimaraes MD, Nobre LF, et al. Metastatic pulmonary calcification: state‐of‐the‐art review focused on imaging findings. Respir Med. 2014;108(5):668–76. [DOI] [PubMed] [Google Scholar]
- 7. Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165(12):1654–69. [DOI] [PubMed] [Google Scholar]
- 8. Belém LC, Souza CA, Souza AS, Escuissato DL, Hochhegger B, Nobre LF, et al. Metastatic pulmonary calcification: high‐resolution computed tomography findings in 23 cases. Radiol Bras. 2017;10(50):231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown K, Mund DF, Aberle DR, Batra P, Young DA. Intrathoracic calcifications: radiographic features and differential diagnoses. Radiographics. 1994;14(6):1247–61. [DOI] [PubMed] [Google Scholar]
- 10. Okada F, Ando Y, Yoshitake S, Ono A, Tanoue S, Matsumoto S, et al. Clinical/pathologic correlations in 553 patients with primary centrilobular findings on high‐resolution CT scan of the thorax. Chest. 2007;132(6):1939–48. [DOI] [PubMed] [Google Scholar]
- 11. Raoof S, Amchentsev A, Vlahos I, Goud A, Naidich DP. Pictorial essay: multinodular disease. Chest. 2006;129(3):805–15. [DOI] [PubMed] [Google Scholar]
- 12. Miller WT Jr, Panosian JS. Causes and imaging patterns of tree‐in‐bud opacities. Chest. 2013;144(6):1883–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


