Highlights
-
•
We assessed how a fatiguing task alters motor activation in multiple sclerosis.
-
•
In the recovery phase after a fatiguing task, motor activity increased in prefrontal cortex and putamen.
-
•
Activity of premotor cortex during recovery phase predicted fatigue during everyday life.
-
•
Stronger premotor activity in the recovery phase was associated with less motor fatigue.
Keywords: Motor fatigability, Multiple sclerosis, Functional magnetic resonance imaging, Grip-force task, Putamen, Premotor cortex
Abbreviations: FMS, Fatigued multiple sclerosis; FSMCMOTOR, Fatigue Scale for Motor and Cognitive Functions, motor subscale; NFMS, Non-fatigued multiple sclerosis
Abstract
Background
Motor fatigue is common in multiple sclerosis (MS), but its pathophysiology is still poorly understood. Here we used functional magnetic resonance imaging (fMRI) to delineate how the acute induction of motor fatigue alters functional activity of the motor system and how these activity changes are related to motor fatigue.
Method
Forty-four right-handed mildly disabled patients with relapsing-remitting MS and 25 healthy controls performed a maximal tonic precision grip with their right hand until they developed motor fatigue. Before and after the fatiguing task, participants performed a non-fatiguing tonic grip force task, producing 15–20% of their maximum grip force based on visual feedback. Task related brain activity was mapped with blood-oxygen level dependent fMRI at 3 T. Statistical parametric mapping was used to identify relative changes in task-related activation from the pre-fatigue to the recovery MRI session.
Results
Following fatigue induction, task performance was perturbed in both groups, and task-related activation increased in the right (ipsilateral) primary motor hand area. In patients with MS, task-related activity increased bilaterally during the recovery phase in the ventrolateral portion of the middle putamen and lateral prefrontal cortex relative to controls. The more patients increased task-related activity in left dorsal premotor cortex after the fatiguing task, the less they experienced motor fatigue during daily life.
Conclusion
Patients with MS show enhanced functional engagement of the associative cortico-basal ganglia loop following acute induction of motor fatigue in the contralateral hand. This may reflect increased mental effort to generate movements in the recovery phase after fatigue induction. The ability to recruit the contralateral dorsal premotor cortex after fatigue induction may constitute a protective mechanism against experiencing motor fatigue in everyday life.
1. Introduction
Fatigue and fatigability are among the most common symptoms in multiple sclerosis (MS), affecting physical (motor fatigue) and mental (cognitive fatigue) activities (Fox et al., 2015, Kluger et al., 2013, Minden et al., 2006, Simpson et al., 2016). While fatigue is an increased feeling of tiredness, exhaustion and lack of energy that is already present in the absence of activity (Kluger et al., 2013, Krupp, 2006), excessive fatigability refers to a rapid emergence of a feeling of exhaustion and fatigue during motor or mental activities (Kluger et al., 2013, Zijdewind et al., 2016). The pathophysiology of fatigue and fatigability is complex and still poorly understood and treatment remains a challenge (Khan et al., 2014). Multiple processes are potentially involved, including structural and functional brain damage as well as immunological and endocrinological factors (Induruwa et al., 2012, Patejdl et al., 2016). Together, these changes are thought to produce dysfunction at the brain-circuit level that ultimately triggers the experience of excessive fatigue and fatigability (Induruwa et al., 2012, Patejdl et al., 2016). To advance our understanding of the mechanism behind central fatigue and fatigability, functional neural measures that capture this dysfunction at the network level are needed.
Functional magnetic resonance imaging (fMRI) has been successfully employed to link dynamic changes in functional neuronal activity in the motor network to the subjective experience of motor fatigue and fatigability in mildly disabled patients with MS (Chalah et al., 2015). Rocca et al. 2007 (Rocca et al., 2007) reported increases in task-related activation in left premotor cortex, left primary sensorimotor cortex, right thalamus and right basal ganglia nuclei in MS patients in whom interferon beta-1a (IFNβ-1a) induced fatigue compared to patients in whom it did not. Specogna et al. (2012) found that the performance of a fatiguing thumb-to-finger opposition task was associated with stronger task-related activity in the right middle frontal gyrus (MFG), premotor cortex and putamen in MS patients with fatigue (FMS) compared to MS patients without fatigue (NFMS). Another study by Rocca et al. (2016) found altered time adaption in the brain activity in FMS patients compared to healthy controls and NFMS patients in the precentral motor hand area and motor putamen. This study applied a finger flexion task that did not induce fatigue. In the healthy controls the task-related activity gradually increased across task blocks in the right and left putamen and left pre-central cortex, whereas the change in these areas was more limited in FMS patients (Rocca et al., 2016). We recently employed whole-brain fMRI to trace changes in task-related activity, while patients with relapsing-remitting MS or healthy controls performed a non-fatiguing precision grip task. In healthy controls, linear increases in activity across consecutive task blocks scaled positively with the experienced level of motor fatigue in everyday life (Svolgaard et al., 2018). Conversely, the more patients suffered from motor fatigue during everyday life, the more they were impaired at up scaling task-related activity in premotor and dorsomedial prefrontal areas (Svolgaard et al., 2018). Taken together, these studies link task-related activity in the putamen and in frontal motor and premotor cortex to the emergence of MS-related motor fatigue and fatigability.
The premotor cortex is involved in motor preparation and particularly the dorsal premotor cortex is involved in visuo-motor mapping, higher-order cognitive control of movement and is well connected to the prefrontal cortex (Boussaoud, 2001, Hanakawa et al., 2006, Picard and Strick, 2001, Ward et al., 2010). Transcranial magnetic stimulation (TMS) of the motor cortex has revealed that the premotor cortical drive to the primary motor cortex (M1) is altered in FMS patients (Morgante et al., 2011, Russo et al., 2015). TMS during a reaction time task showed that FMS patients lacked pre-movement facilitation in the non-fatigued and fatigued state (Morgante et al., 2011, Russo et al., 2015). In the non-fatigue state, the lack of pre-movement facilitation correlated with the amount of self-reported fatigue (Morgante et al., 2011). Furthermore, in a former study including a subgroup of subjects participating in the current study, we applied dual site TMS over the ipsilateral dorsal premotor cortex (PMd) and the M1 and found that the self-experienced fatigue scaled positively with an attenuation of premotor-to-motor functional connectivity (Ruiu et al., 2020).
The present study extends previous neuroimaging work on motor fatigue in MS by investigating task relevant BOLD-fMRI changes before and after a fatigue inducing manual motor task. We transiently induced motor fatigue in the right hand by letting participants perform a tonic maximal grip force task in-between the task-related fMRI sessions. Given the functional role of the PMd and the link between dynamic changes in task-related activity and fatigue (Morgante et al., 2011, Rocca et al., 2007, Rocca et al., 2016, Russo et al., 2015, Specogna et al., 2012), we hypothesized that the amount of fatigue experienced during everyday life is associated with an inability to activate the premotor cortex in the recovery phase after fatiguing task.
2. Material and methods
2.1. Participants
Fifty patients with relapsing-remitting MS were consecutively included mainly from the Danish Multiple Sclerosis Center at Rigshospitalet, Copenhagen, Denmark (Svolgaard et al., 2018). The inclusion criteria were (i) no clinical or radiological evidence of disease activity during three months before study initiation, (ii) a relapsing-remitting disease course, (iii) an Expanded Disability Status Scale (EDSS) score of ≤ 3.5 (Kurtzke, 1983), (iv) age 18 – 55 years, and (v) being right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Patients received no pharmaceutical treatment of fatigue and women were not pregnant at the time of study. All patients had no changes in MS-related medication for three months and did not have other medical or psychiatric comorbidities. They reported no previous infections affecting the central nervous system, no sleeping problems, no drug or alcohol abuse, and had no contraindications for MRI. Additionally, 25 age- and sex-matched healthy individuals were included. The ethics committee of the Capital Region of Denmark (Protocol H-4–2013-182) approved the study and all participants gave informed consent.
2.2. Clinical assessment
Fatigue was evaluated with the Fatigue Scale for Motor and Cognitive Functions (FSMC) (Penner et al., 2009), and the FSMCMOTOR sub-scores were used as index of the motor fatigue level experienced by the participants during everyday life. Neurological disability was scored with the EDSS (Kurtzke, 1983) and skilled hand function with the Nine-Hole Peg Test (9-HPT) (Lamers et al., 2014) and the Jebsen-Taylor Hand Function Test (JTHFT) (Jebsen et al., 1969). The Beck Depression Inventory II (BDI-II) (Goldman, 2005, Minden et al., 2014), Epworth Sleepiness Scale (ESS) (Johns, 1991, Popp et al., 2017), Pittsburgh Quality of sleep index (PQSI) (Buysse et al., 1988), Symbol Digit Modality Test (SDMT) (Lopez-Gongora et al., 2015), and Paced Auditory Serial Addition Test (PASAT) (Possa, 2010) were performed to assess mood, sleep quality and cognitive impairment.
2.3. Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed with a Philips Achieva 3.0 T scanner and a 32-channel receive head coil (Philips, Best, The Netherlands). During the precision grip task, whole-brain fMRI scans were acquired with an Echo Planar Imaging (EPI) sequence with repetition time (TR) = 2500 ms, echo time (TE) = 30 ms, and flip-angle of 80˚. Each brain volume consisted of 42 axial slices acquired in interleaved order with a slice thickness of 3 mm, resulting in a 3x3x3 mm voxel resolution and a field-of-view (FOV) of 192x192x126 mm. For quantifying lesion load and overall brain atrophy, structural MRI including T1- and T2-weighted and Fluid Attenuated Inversion Recovery (FLAIR) images were obtained. The T1-weighted image was acquired with a sagittal magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR = 6 ms, TE = 2.70 ms, flip-angle = 8°, 0.85 mm isotropic voxel size and a FOV of 245x245x208 mm). The T2-weighted image was acquired with a turbo spin echo sequence (TR = 2500 ms, TE = 270 ms, flip-angle = 90°, 0.85 mm isotropic voxel size and a FOV of 245x245x190 mm). The FLAIR image was acquired with a TR = 4800 ms, TE = 327 ms and an isotropic voxel size of 1 mm3 resulting in a FOV of 256 × 256 × 202 mm.
2.4. Precision grip task during fMRI
All subjects received verbal and written instruction and trained the task before entering the scanner. In addition, maximal precision grip force of the right hand was measured to individually adjust the target force level in the fMRI experiment. During fMRI, participants performed a precision grip task on a force device and continuously received visual feedback. The task had a pre-fatigue, fatiguing and a recovery phase (Fig. 1). The results obtained during the pre-fatigue fMRI run have been reported in a previous publication (Svolgaard et al., 2018). The pre-fatigue and recovery phases were identical and included 24 blocks of each 20 s duration; 12 resting blocks and 12 active blocks, alternating. During the active blocks the participants performed a tonic precision-grip, continuously pressing on a force sensor with their right index finger and thumb. The participants had to continuously produce 20 % of individual maximal grip force and received concurrent visual feedback. Participants were required to match the diameter of a blue circle to the size of a ring presented on a screen. The blue circle corresponded to the actual grip force produced by the participant, while the ring corresponded to the target force level (i.e., 20 % of maximal grip force). During the fatiguing phase, participants produced a tonic grip force that had to be above 75 % of maximal individual grip force, until subjects became fatigued and could no longer maintain such a high force level. Before and after the fatiguing phase of the precision grip task, the participants rated their fatigue on a scale from 0 to 10 (10 the highest degree of fatigue) displayed on the screen in the scanner. This was done using the force device. The performance of the precision grip task was monitored on-line and the data were visually inspected to evaluate if each individual subject had performed adequately. Behavioural force data was scaled to represent the force in newton and the mean and standard deviation was extracted for each task block with Matlab (The Mathworks Inc., USA, https://se.mathworks.com/products/matlab.html).
Fig. 1.
Fatiguing precision grip task. (A) The task had a pre-fatigue, fatiguing and recovery phase (“Post”) (Mucke et al., 2015). The pre-fatigue and recovery phase were identical and included 12 blocks with 20 s compressions at 20 % of the individual subject’s maximal voluntary contraction (MVC) and 12 blocks of 20 s with rest. During the active blocks the subject had to expand a blue circle by applying a precision grip on force-measuring device. The fatiguing phase was a long tonic contraction of between 75 % and 100 % of MVC, which lasted until the subjects were fatigued. (B) The precision grip force device. (C) Task-related activity was analysed using a General Linear Model to find the constant main effect of task (main effect) as well as linear modulation of task-related activity (linear time effect), the latter is not shown in the figure. The first block (red line) was separately modelled and treated as effect of no interest. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. Pre-processing and analysis of MRI data
Cortical reconstruction and volumetric segmentation of the structural MRI data were performed using FreeSurfer software (version 5.3.0; https://surfer.nmr.mgh.harvard.edu). We employed the same processing pipeline, which has been described in detail (Dale et al., 1999, Fischl et al., 1999). Briefly, the data processing includes intensity normalization to Montreal Neurological Institute (MNI) space, skull stripping, filtering, segmentation, and surface deformation. A trained researcher reviewed the quality of the skull stripping and accuracy of grey and white matter outer boundaries. The estimated total intracranial volume (eTIV), white matter volume (WMV), grey matter volume (GMV) and white matter hyperintensity volume (WMHV) were extracted using specialized tools for automated parcellation of grey and white matter in FreeSurfer (Desikan et al., 2006). Finally, the extracted volume measures were converted to z-scores for further analyses. Hyperintense lesions were delineated on FLAIR images by a trained blinded observer, using a semi-automatically local thresholding technique (Jim 6.0 Xinapse System, Leicester, UK, https://www.xinapse.com/). T1- and T2-weighted images were used for lesion clarification.
The fMRI data were analysed with SPM 12 software (Welcome Department of Imaging Neuroscience, London, UK, https://www.fil.ion.ucl.ac.uk/spm/software/) and Matlab. The functional images were realigned to the mean EPI image using a six-parameter, rigid-body transformation and spatial normalized to the MNI ICMB European brain template using the parameters from the normalisation of the mean image. The images were resampled to 2 mm isotropic resolution in MNI space and spatially smoothed with a 6 mm full-width at half-maximum isotropic Gaussian kernel. A general linear model (GLM) was set up to estimate the voxel-wise Blood Oxygen Level Dependent (Rocca et al., 2009) signal response to the active blocks within each subject. The fatiguing phase was not included in the analysis. The regressor modelling the main effect of task was defined by convolving the block-stimulus function with the canonical hemodynamic response functions in SPM. To estimate the change across the blocks within the two phases, a first-order time modulation regressor was added, which estimated linear changes in BOLD amplitude across the task blocks. In addition, a 24 parameter Volterra expansion of the realignment parameters was added to reduce residual head movement artefacts. The 24 regressors constituted the 6 realignment parameters from the realignment procedure, their finite difference approximated temporal derivative, and all of them squared (Friston et al., 1996). The first task block, the pre-fatigue phase, was modelled as a separate regressor of no interest because subjects needed the first task block to reach steady-state performance. The change in BOLD contrast due to motor fatigue was estimated by contrasting the parameter estimates from the non-fatiguing grip force task in the “pre-fatigue” phase and the “recovery” phase after the fatiguing grip force task. We additionally contrasted the time-modulation estimates from the two phases to test for differences in linear time-effect between the two phases. These two contrast maps were used in the group modelling.
2.6. Statistical analyses
Random effects inference models were used to test for task-related BOLD response differences within and between groups for the main effect as well as the time effect of task. The within-group models contained age and hand function, measured with the JTHFT, as nuisance regressors. Between-group comparisons were additionally controlled for duration of the fatiguing phase, as there was a group difference in duration. Additionally, models were set up containing the FSMCMOTOR scores as effect of interest, and age and hand function as nuisance regressors, to test for the correlation of fatigue with main and time effects. Post-hoc, the beta values from the identified clusters in the FSMCMOTOR model were extracted and correlated with the online subjective fatigue induced by the fatiguing task.
Small volume correction (SVC) was performed for PMd using a left-hemispheric PMd mask as the task was performed with right hand (King et al., 2014, Kuhtz-Buschbeck et al., 2001, Kuhtz-Buschbeck et al., 2008). The PMd mask was constructed by converting the Human Motor Area Template (HMAT) from Talairach to MNI space (Mayka et al., 2006, m2html©, 2005).
For all voxels within the ROI, the cluster-forming threshold was set to puncorrected = 0.001 and findings were considered significant at family-wise error (FWE) corrected p < 0.05 on cluster-level, while findings with a cluster-level FWE corrected p-value ≤ 0.1 and number of voxels ≥ 10 were defined as trends. In addition, we performed a whole-brain analysis with the same criteria to define statistical significance. The only difference was that whole-brain analysis corrected for all voxels within the brain mask.
Clinical, behavioural, and structural MRI data were analysed with SPSS software (version 22, IBM Corp., Armonk, New York, USA), using t-tests, repeated measures ANOVA, and Pearson or Spearman correlation when applicable. Significance threshold was set at p < 0.05.
3. Results
3.1. Clinical and grip force data
Forty-nine patients with relapsing-remitting MS and 25 healthy controls participated in all examinations. Two patients had to be excluded from analysis because of movement artefacts in the fMRI data, and another patient because of inability to perform the task as instructed. Two additional MS patients were excluded because their BDI-II score indicated the possibility of depression, although they had no clinical diagnosis of depression. Table 1 summarizes the clinical characteristics and the structural MRI data. There was no difference between controls and patients in age and gender distributions. The patients had higher total, motor, and cognitive FSMC scores as well as higher BDI-II, PSQI, and JTHFT scores relative to healthy controls. In the MS group, the FSMC scores correlated positively with BDI-II scores (r = 0.53, p < 0.001) and EDSS scores (r = 0.47, p = 0.001). Patients did not differ from healthy controls with respect to task performance. In the pre-fatigue phase, the mean grip force produced by the participants during the task blocks was 16.2 N (SD 4.7 N) in healthy controls and 16.2 N (SD 2.4 N) in the MS group. In the recovery phase, mean grip force was 16.0 N (SD 4.8 N) in healthy controls and 15.9 N (SD 2.4 N) in MS patients. Mean force level was slightly lower in the recovery phase compared to the pre-fatigue phase (F (1, 67) = 9.73, p = 0.003), but there was no group difference or group by time interaction. There was an increase in force output variability from the pre-fatigue to recovery phase (F (1, 67) = 4.87, p = 0.031) and an overall difference in force variability among the blocks within the pre-fatigue and recovery phase (F (2.48, 166.27) = 2.92, p = 0.045), but no difference between groups and no group-by-time interaction. The duration of the fatiguing phase was significantly longer in healthy controls (308 sec) compared to MS patients (227 sec) (p = 0.015). The duration of the fatiguing phase did not correlate with the FSMCMOTOR score.
Table 1.
Clinical characteristics of MS patients and healthy controls.
| MS n = 44 | HC n = 25 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | p | ||
| Age | 35.9 | (22–53) | 8.8 | 35.8 | (19–55) | 10.6 | 0.979 | |
| Gender (M:F) | 14:30 | 32 %:68 % | 9:16 | 36 %:64 % | 0.723 | |||
| Median EDSS | 2.5 | (0–3.5) | 1.0 | |||||
| DiseaseDURATION | 6.3 | (0–28) | 5.2 | |||||
| On MSTREATMENT | 40MS | 90.9 % | ||||||
| Clinical scores | ||||||||
| FSMCTOTAL* | 59.3 | (20–92) | 21.3 | 28.0 | (20–46) | 8.2 | 0.000 | |
| FSMCMOTOR* | 28.8 | (10–45) | 10.6 | 12.9 | (10–23) | 3.2 | 0.000 | |
| FSMCCOGNITIVE* | 30.5 | (10–48) | 11.9 | 15.0 | (10–28) | 5.6 | 0.000 | |
| BDI - II* | 7.2 | (0–22) | 6.0 | 1.6 | (0–11) | 2.8 | 0.000 | |
| PSQI* | 5.2 | (1–18) | 3.7 | 3.4 | (1–5) | 1.4 | 0.005 | |
| ESS | 8.2 | (2–17) | 3.9 | 6.4 | (0–14) | 4.0 | 0.080 | |
| PASAT | 50.1 | (33–60) | 7.5 | 51.1 | (43–59) | 5.0 | 0.506 | |
| SDMT | 54.2 | (35–89) | 10.5 | 56.5 | (41–70) | 6.7 | 0.280 | |
| JTHFTRIGHT HAND* | 37.7 | (30–53) | 4.2 | 35.4 | (29–41) | 3.5 | 0.026 | |
| 9-HPTRIGHT HAND | 15.9 | (13–24) | 2.0 | 15.7 | (13–19) | 1.8 | 0.628 | |
| Structural MRI metrics | ||||||||
| Mean TIV | 1561.3 | 141.4 | 1594.9 | 154.7 | 0.362 | |||
| Mean WM | 482.7 | 59.9 | 500.2 | 56.9 | 0.135 | |||
| Mean GMV | 637.5 | 47.9 | 657.2 | 47.9 | 0.170 | |||
| Mean WMHV | 5.9 | (0.3–30.7) | 6.5 | |||||
Table 1. * = p – value < 0.05 Abbreviations: Age = Age in years, BDI - II = Beck depression inventory version II, Disease duration = Years since diagnose, EDSS = Expanded disability status score, ESS = Epworth sleepiness scale, FMS = MS patients with fatigue, FSMCCOGNITIVE = FSMC cognitive score, FSMCMOTOR = FSMC motor score, FSMCTOTAL = Fatigue scale for motor and cognitive functions total score, Gender (M: F) = Male: female ratio, HC = Healthy controls, GMV = Grey matter volume in millilitre, JTHFT = Jebsen-Taylor hand function test, MS = Multiple sclerosis, NFMS = MS patients without fatigue, WMHV = White matter hyperintensities volume (i.e. white matter lesions, in millilitre), 9-HPT = Nine hole peg test, p = P – value, PASAT = Paced auditory serial addition test, PSQI = Pittsburgh sleep quality index, SD = Standard deviation, SDMT = Symbol digit modalities test, TIV = Total intracranial (volume in millilitre), Treatment = In treatment with multiple sclerosis disease modifying drugs, WMV = White matter volume in millilitre.
3.2. Task-related brain activity
3.2.1. Dorsal premotor cortex
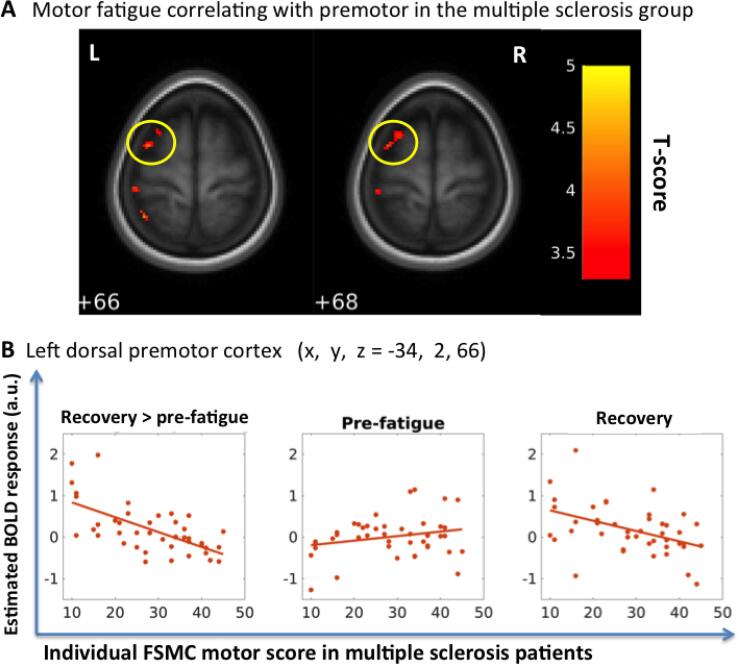
The MS group showed a negative linear relationship between changes in task-related activation in the rostral part of left PMd and FSMCMOTOR scores (Table 2, Fig. 2). The higher the MS patients’ individual FSMCMOTOR score, the smaller was the activation increase in the rostral PMd from the pre-fatigue to the recovery phase. In other words, those patients who showed an increase in task related PMd activation in the recovery phase were less affected by motor fatigue during their everyday lives.
Table 2.
Group results of fMRI data.
|
MNI coordinates |
T |
Cluster |
Cluster |
|||||
|---|---|---|---|---|---|---|---|---|
| Contrast | Region | Side | x | y | z | Value | P value | Size |
| MS | ||||||||
| Recovery > pre | Middle temporal gyrus | L | −62 | −20 | −6 | 5.86 | < 0.001 | 500 |
| Inferior frontal gyrus (pars triangularis) | L | −34 | 26 | 20 | 5.76 | < 0.001 | 341 | |
| Superior frontal gyrus | R | 22 | 38 | 52 | 5.26 | 0.02 | 146 | |
| Middle cingulum gyrus | L | −2 | −10 | 44 | 4.97 | < 0.001 | 297 | |
| Paracentral lobule | R | 2 | –32 | 70 | 4.95 | < 0.001 | 420 | |
| Precentral gyrus | R | 32 | −26 | 74 | 4.91 | 0.038 | 126 | |
| Middle frontal gyrus (pars orbital) | L | −34 | 46 | −6 | 4.91 | 0.001 | 239 | |
| Hippocampus | L | −12 | −36 | 6 | 4.9 | 0.008 | 175 | |
| Inferior frontal gyrus (pars orbitalis) | L | −40 | 34 | −6 | 4.82 | 0.015 | 155 | |
| Cerbellum (Crus2) | R | 34 | −78 | −40 | 4.51 | 0.001 | 244 | |
| Precuneus | L | −2 | −68 | 46 | 4.4 | < 0.001 | 341 | |
| Medial superior frontal gyrus | L | 0 | 56 | 2 | 4.34 | 0.006 | 187 | |
| Recovery > pre FSMCmotor | PMd | L | −34 | 2 | 66 | 4.11 | 0.03SVC | 32 |
| L | −40 | 2 | 62 | 3.85 | ||||
| L | −48 | 10 | 54 | 3.87 | 0.073SVC* | 16 | ||
| L | −26 | 10 | 68 | 3.72 | 0.069SVC* | 17 | ||
| HC | ||||||||
| Recovery > pre | Primary motor cortex | R | 42 | −20 | 56 | 5.4 | 0.011 | 175 |
| Middle temporal gyrus | R | 62 | −4 | −14 | 5.46 | 0.056* | 119 | |
| Pre- & postcentral gyrus | R | 62 | −6 | 34 | 4.62 | 0.082* | 107 | |
| Recovery > pre FSMCmotor | – | |||||||
| MS > HC | ||||||||
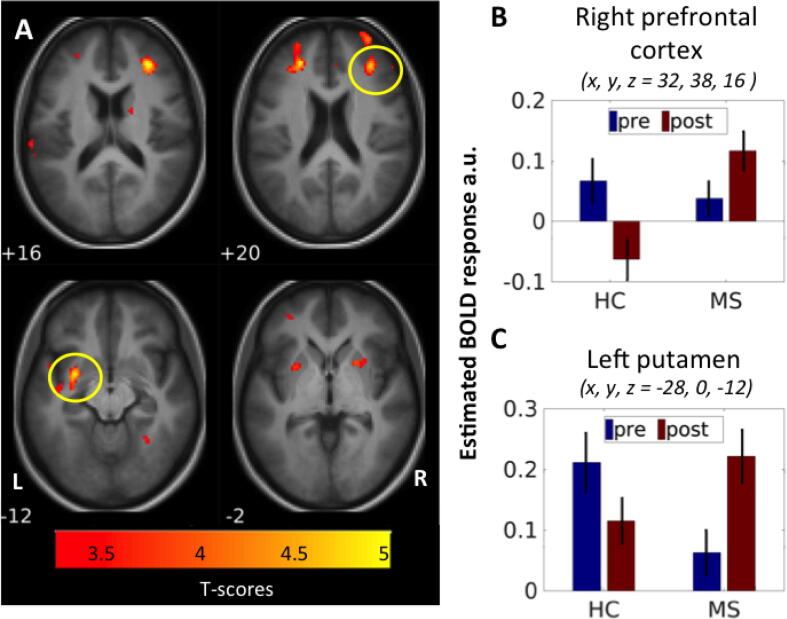
| Recovery > pre | Middle frontal gyrus | R | 32 | 38 | 16 | 5.26 | 0.034 | 149 |
| Middle frontal gyrus | L | −24 | 40 | 20 | 4.94 | 0.051* | 134 | |
| Putamen | L | −28 | 0 | −12 | 4.9 | 0.012 | 189 | |
| Putamen | R | 22 | 10 | −2 | 3.92 | 0.061* | 127 | |
| Recovery > pre FSMCmotor | – | |||||||
Table 2. The table lists clusters in the brain showing a change in task related activation after acute induction of motor fatigue compared to pre-fatigue activation at baseline (recovery > pre-fatigue) or a change in the relationship between the individual FSMCmotor scores and task related activation as triggered by acute induction of motor fatigue (recovery > pre FSMCmotor). The cluster-forming threshold was set to an uncorrected p-value ≤ 0.001. Findings were considered significant at family-wise error (FWE) corrected p < 0.05 at the cluster level.
* Trend findings showing a p-value ≤ 0.1 (FWE, cluster level) and a cluster extent of ≥ 10 voxels.
SVC: Significant results with small volume correction, p < 0.05 (FWE, cluster-level). Small volume corrected was performed with whole PMd L as mask (HMAT 10).
SVC*: Trend findings showing a p-value < 0.1 (FWE, cluster-level) and a cluster extent of ≥ 10 voxels.
Within-group analyses of MS patients were corrected for age and JTHF. Within-group analyses of healthy controls were corrected for age and JTHF. Between MS patients and healthy controls group analysis: corrected for age, JTHF and duration. Cluster size = number of voxels.
Abbreviations: FSMCMOTOR = Fatigue Scale for Motor and Cognitive Functions, motor subscale, HC = Healthy controls, MS = Multiple sclerosis, PMd = Dorsal premotor cortex, Pre = Pre-fatigue phase, R = Right hemisphere, t = T-value.
Fig. 2.
Fatigue-induced premotor activity correlated with self-reported motor fatigue in the multiple sclerosis patients. (A) In the MS group, pre-fatigue to recovery (post-fatigue) changes in the BOLD activity in wide area in rostral part of left PMd correlated with the subjects’ individual FSMCMOTOR scores. (B) The higher the MS patients’ individual FSMCMOTOR scores were, the lower was the PMd activation change from pre-fatigue to recovery (post-fatigue). The graphically visualization shows that there was no significant relation between the self-reported fatigue and the estimate BOLD response in the pre-fatigue phase, however that a negative correlation between the self-reported fatigue and the estimate BOLD response emerges in the recovery phase (post-fatigue) after the performance of the fatiguing phase.
3.2.2. Whole brain analyses
Whole-brain analysis identified a stronger activation of the ipsilateral primary sensorimotor area in the recovery phase in both the MS group and the healthy controls (Table 2, Fig. 3). Moreover, the MS group showed an increased task-related activity in additional brain areas, comprising clusters in left superior medial, middle and inferior frontal gyrus, middle temporal gyrus, pre- and post-central gyrus, paracentral lobule, precuneus, hippocampus and right cerebellum. In contrast, enhanced task-related recruitment was limited to right pre- and post-central gyrus in the control group. No brain region showed a significant reduction in task-related activity in either of the groups despite lower force production in recovery phase. A direct contrast of fatigue-induced activity changes between groups identified two clusters, a cluster in right dlPFC and another cluster in left middle putamen, where MS patients showed a stronger increase in task-related activity from the pre-fatigue phase to the recovery phase relative to healthy controls (Fig. 4, Table 2). The same pattern was present in the left dlPFC and right putamen, however only trend-wise. In these areas, task-related activation increased in MS patients, whereas activity levels decreased in healthy controls (Fig. 4).
Fig. 3.
Fatigability-induced activation increase in the right primary motor cortex. After the fatiguing phase both groups had a higher activity in the ipsilateral primary sensory-motor areas. (A) Showing the fatigue-induced change in all participants, (B) showing the change in the patients with relapsing-remitting MS and (C) showing the change in the healthy controls.
Fig. 4.
Fatigability-induced increased activation in MS patients compared to healthy controls. (A) The MS patients had a significant higher activation increase from pre-fatigue to recovery (Mucke et al., 2015) compared to healthy controls in right dorsolateral prefrontal cortex (dlPFC) and in left putamen, and trend-wise in left dlPFC and right putamen. The bar-plots showing the relative activation increased in the MS patients compared to the decrease in the healthy controls group in (B) right prefrontal cortex and (C) left putamen.
Whole-brain analysis also identified two areas where the change in activation from the pre-fatigue to the recovery phase scaled with individual FSMCMOTOR scores in patients with MS, namely the left mesial prefrontal cortex (MNI-coordinates at peak: x, y, z = -2, 36, 46, cluster-level puncorrected = 0.028, t-value = 4.31) and left inferior parietal lobule ((MNI-coordinates x, y, z = -50, -54, 44), cluster-level puncorrected = 0.032, t-value = 3.84). Yet these relationships between the fatigue-induced activity changes and experienced fatigue during everyday life did not survive whole-brain FWE-correction, and thus are not considered further. We also conducted between-group analyses to test whether the fatiguing motor task altered the time modulation of task-related activity in the recovery state. We found no group differences regarding the effect of the fatiguing task on the time modulation of task-related activity or its relation to the FSMCMOTOR scores.
4. Discussion
In this study, we “challenged” the motor system by acutely introducing a state of motor fatigue asking participants to perform a fatiguing grip force task. At the behavioural level, patients with MS showed a higher degree of fatigability than healthy controls, because they experienced motor fatigue faster than healthy controls. Conversely, the fatiguing tonic grip at maximal force levels had a comparable “perturbing” effect on the task performance during recovery. Both groups, MS patients and healthy controls, showed a slight reduction in force levels in the fMRI session after provoking fatigue, along with a higher variability of the required force output.
At the neural level, our fatigue-inducing perturbation approach yielded three main findings. Firstly, after the fatiguing phase of the precision grip task, the neural activity in motor related areas generally increased in both groups, supporting that the fatiguing phase of the precision grip task was indeed fatiguing and the motor system was indeed challenged in both groups.
Secondly, we identified a rostral premotor cluster in the dominant left hemisphere where dynamic changes in task-related activity reflected the severity of motor fatigue experienced by the patients during their everyday lives. In agreement with our hypothesis, patients in who left rostral PMd became more engaged in the task during recovery reported less motor fatigue during their everyday lives. Secondly, we found that patients with MS showed a stronger increase in task-related engagement of right dlPFC and left middle putamen from the pre-fatigue to recovery state compared to healthy controls with similar trend-wise changes in the other hemisphere. In these regions, the increased functional engagement after the fatiguing grip force task was unrelated to self-experienced motor fatigue.
4.1. Dynamic change in task-related premotor activity and self-perceived fatigue
In the left rostral PMd, the fatiguing task triggered a relative change in activation that reflected self-perceived daily-life fatigue. Patients, who already showed relatively high rostral PMd activation at baseline but failed to increase its engagement in a recovery state, were affected more strongly by motor fatigue in their everyday life, resulting in higher FSMCMOTOR scores. Conversely, those patients who initially showed little or no task-related activity of left rostral PMD but scaled up their functional engagement in the recovery state reported no or little fatigue in everyday life.
The relationship between task related PMd activation and self-reported fatigue during everyday life suggests that the activation dynamics of the left rostral PMd may determine the individual expression of motor fatigue during everyday life. If a non-fatiguing task already triggers a “compensatory” recruitment of the left rostral PMD, no further recruitment is possible when being challenged by a fatigue-inducing task. Whether this would be the same for other neurodegenerative diseases suffering from fatigue is unknown. However, the notion that a limited capability to dynamically recruit a key area plays an important role in determining functional compensation is in accordance with explicit models of functional compensation and functional reserve in fMRI studies of preserved cognitive function and neurodegenerative diseases (Gregory et al., 2017).
The PMd is a key motor area which critically contributes to visuomotor guidance of manual activities (Hanakawa et al., 2006, Ward et al., 2010) with the caudal “motor” PMd being involved in movement execution and the rostral “frontal” PMd taking part in more cognitive aspects of motor control (Boussaoud, 2001, Picard and Strick, 2001). Our results therefore suggest that the ability to recruit the rostral PMd of the dominant hemisphere during the “aftermath” of a fatiguing motor task may reflect the compensatory potential of the PMd to protect patients with MS against experiencing motor fatigue in everyday life. Our results also support the notion that self-perceived motor fatigue during everyday life may be linked to over-recruitment of frontal motor areas that take part in higher-order cognitive control of actions already during non-fatiguing everyday activities.
Several previous fMRI studies have used prolonged motor tasks requiring the continuous production of repetitive movement, which may have resulted in motor fatigue to varying degrees (Filippi et al., 2002, Pardini et al., 2013, Rocca et al., 2016, Specogna et al., 2012). Similar to our study, Specogna et al. 2012 (Specogna et al., 2012) found that self-reported (cognitive and motor) fatigue correlated positively with motor activity in the prefrontal and premotor cortices, albeit in the right hemisphere. The findings of other studies have been more diverse. In mildly disabled MS patients, self-reported (cognitive and motor) fatigue correlated negatively with task-related motor activity in the contralateral thalamus, bilaterally in the rolandic operculum (Filippi et al., 2002) or positively in the right cerebellar hemisphere, left orbitofrontal cortex (Pardini et al., 2013) or in the right dlPFC (Rocca et al., 2016) during the performance of simple right-handed motor tasks. Only one study focused on motor fatigue component in isolation (Rocca et al., 2016). That study found a negative correlation between motor fatigue in everyday life and task-related activity in the right supplementary motor area (SMA), right thalamus and left middle temporal gyrus (Rocca et al., 2016). The lack of consistency across previous studies may be explained by the fact that the motor activity during fMRI varied in their fatigue-provoking effects and how this engaged higher-order cognitive control.
4.2. Motor activity in the aftermath of fatigue induction
In the MS patients, the associative cortico-basal ganglia loops became more activated bilaterally during recovery after the fatiguing unilateral motor task, potentially reflecting an increasing mental effort contributing to fatigue. Given the diverse role of the putamen and the dlPFC in motor learning (DeLong and Wichmann, 2009, Doyon et al., 2009) and executive functions such as working memory and attentional selection (Abe and Hanakawa, 2009), the observed group difference could be related to cognitive impairment, performance dysfunction or learning. Nonetheless, the task was simple, the participants were trained before entering the scanner and there was no group difference in task performance in neither in the pre-fatigue nor in the recovery phase. In addition, the MS group performed equally well in the clinical hand function tests, and the group fMRI analyses were controlled for hand function measured by the JTHFT. Furthermore, we have previously shown that there were no group differences in the prefrontal cortex or in the putamen during the pre-fatigue phase (Svolgaard et al., 2018), indicating that the differences between the pre-fatigue phase and recovery phase were induced by the fatiguing task.
In line with our findings, the prefrontal-striato-thalamo-cortical loops, and particularly the basal ganglia, have been highlighted as crucial areas in the pathophysiology of fatigue in neurological diseases in general by several authors (Dobryakova et al., 2013, Chaudhuri and Behan, 2000). In MS, several studies using different neuroimaging modalities have found fatigue-related changes in the prefrontal cortex and in the basal ganglia (for review see (Chalah et al., 2015)). This includes fMRI studies using right-hand motor tasks exploring fatigue in mildly disabled MS patients.
To the best of our knowledge, only a few studies have used tasks that were designed to induce fatigue, and most of these studies have used cognitive tasks to induce fatigue. Among these, Deluca et al. 2008 (Deluca et al., 2008), found increased activation in the prefrontal cortex and in the basal ganglia in MS patients during the performance of a cognitively fatiguing task. Furthermore, Tartaglia et al. 2008 (Tartaglia et al., 2008) found increased activation in the right MFG and in the left post-central gyrus, and bilaterally in the cingulate gyrus in MS patients during the performance of a motor task after having been cognitively fatigued. Focusing on cognitive fatigue, (Chen et al., 2020) reported a relationship between task-related rostral premotor activation during a cognitive task and self-reported fatigue in MS patients that depended on cognitive task load (Chen et al., 2020). As fatigue ratings increased during task execution the rostral PMd showed a bilateral increase in task-related activation in the high-load and a decrease in activation in the low-load task condition (Chen et al., 2020).
Thus, most fMRI studies performed this far were not designed to induce fatigability a priori, but explored the neural underpinnings of fatigue by comparing healthy controls with MS patients or FMS patients with NFMS patients, and by correlating the brain activation pattern with the self-reported fatigue. These studies did not distinguish between fatigability and the overall sensation of fatigue. Specogna et al. (2012) found a stronger fatigue-associated task-related activity in the right MFG, premotor cortex and putamen in FMS patients compared to NFMS patients.Rocca et al. (2007) found a motor task-related activation increase in the frontal cortex and thalamus in MS patients with IFNβ-1a-induced fatigue. Finally, the study by Rocca et al. (2016) found an altered time adaption in FMS patients compared to healthy controls and NFMS patients in the premotor cortex and putamen, and a positive fatigue correlation in the dlPFC. The divergent findings across studies investigating the neural underpinnings of fatigue in MS may be explained by the heterogeneous nature of MS and the multidimensionality of fatigue. Furthermore, patient-related factors and methodological differences might also account for some of the differences between studies.
4.3. Methodological considerations
Within the MRI scanner, it is impossible to use a task that truly mimics the fatiguing challenges in everyday life. Whether the neural correlates of fatigue during a hand-motor task are applicable to other forms of motor fatigue, such as gait fatigability, is unknown. In the present study, we used the FSMCMOTOR scores to quantify self-perceived motor fatigue during daily life, why the findings might not be generalizable to cognitive fatigue. Furthermore, the findings might not be generalizability to patients with EDSS greater than 3.5 as they were excluded from the study. On-line fatigue severity, in other words the state of being fatigued (Enoka and Duchateau, 2016), was measured during the precision-grip task using a VAS score. However, it is unclear how much these two measures of fatigue are related to each other (Enoka and Duchateau, 2016, Zijdewind et al., 2016). Another challenge is that the level of manual motor impairment may increase the tendency to experience motor fatigue and fatigability in patients with MS. Therefore; our analyses controlled for inter-individual differences in hand function.
Given the subjective nature of fatigue, future fMRI studies on the neural correlates of motor fatigue may consider the dynamical properties of task related functional recruitment of brain networks. To dissociate brain activity related to trait fatigue and fatigability, the fMRI design should capture brain activity during non-fatiguing and fatiguing motor tasks. Along with including large well-characterized patient samples, this might increase the consistency across studies.
5. Conclusions
By inducing acute motor fatigue during a fMRI experiment, we show that dynamic task-related activity changes in the PMd and in the associative prefrontal – basal ganglia loop may contribute to self-perceived fatigue and fatigability. The ability to recruit these areas in the recovery phase after a fatiguing task state may protect against experiencing motor fatigue in everyday life. While there is no clear relationship between subjective (trait) fatigue and fatigability at the clinical level (Kluger et al., 2013, Mosso, 1904), our results point to a relationship at the neural level. We found a relation between the individual FSMC scores and the change in neural activation levels in the left rostral PMd provoked by a fatigue-inducing task. We hypothesize that the ability to recruit the rostral PMd of the dominant hemisphere during the “aftermath” of a fatiguing motor task may reflect a compensatory reserve in the PMd that protects patients with MS against experiencing motor fatigue in everyday life.
Funding
This work has been supported by research grants from the Danish Multiple Sclerosis Society (Grant number: R308-A19386, R367-A25015, R399-A27956, R431-A29804, year 2012-2015), Foundation of the Capital Region, Consultant Torben Fogs and Erik Triers foundation, the Jascha Foundation (Grant number: 5588) and Biogen Idec (Grant number GDRC-002-2014). Finn Sellebjerg holds a professorship at the Faculty of Health and Medical Sciences, University of Copenhagen, which is sponsored by the Danish Multiple Sclerosis Society. Hartwig R. Siebner received financial support from the Lundbeck Foundation (Grant of Excellence “Mapping, Modulation and Modeling the Control of Actions”; Grant number R59-A5399) and holds a 5-year professorship in precision medicine at the Faculty of Health and Medical Sciences, University of Copenhagen, which is sponsored by the Lundbeck Foundation (Grant Nr. R186-2015-2138).
Credit authorship contribution statement
Olivia Svolgaard: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Kasper Winther Andersen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Christian Bauer: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Kristoffer Hougaard Madsen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Morten Blinkenberg: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Finn Sellebjerg: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Hartwig Roman Siebner: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Olivia Svolgaard has received travel support from Biogen Idec and works for Bristol-Myers Squibb. Morten Blinkenberg has served on scientific advisory boards for Genzyme, Roche, Biogen Idec, Merck Serono, Novartis and Teva, received speaker honoraria from Genzyme, Biogen Idec, Merck Serono, Bayer Schering, Novartis, Teva and Roche, received consulting honoraria from the Danish Multiple Sclerosis Society, Teva, Biogen Idec, and Merck Serono, received funding for travel from Genzyme, Roche, Biogen Idec and Merck Serono. Finn Sellebjerg has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria, and received research support for his laboratory from Biogen Idec, EMD Serono, Genzyme, Merck, Novartis, Roche, Sanofi and Teva. Hartwig R. Siebner has received honoraria as speaker from Sanofi Genzyme, Denmark and Novartis, Denmark, as consultant from Sanofi Genzyme, Denmark and Lundbeck AS, Denmark, and as editor-in-chief (Neuroimage Clinical) and senior editor (NeuroImage) from Elsevier Publishers, Amsterdam, the Netherlands. He has received royalties as book editor from Springer Publishers, Stuttgart, Germany and from Gyldendal Publishers, Copenhagen, Denmark. The remaining authors have no conflict of interest.
Acknowledgements
We thank all the patients and volunteers who participated in the study. We also thank Sasha Gude, DRCMR Reader Center, for her contribution to the delineation of lesions in the MS patients, Sussi Larsen, DRCMR Research Radiographer for help with scanning the subjects and Silas H. Nielsen for his contribution to analysis of the structural MRI data.
Contributor Information
Olivia Svolgaard, Email: oliviasvolgaard@gmail.com.
Hartwig Roman Siebner, Email: h.siebner@drcmr.dk.
References
- Zijdewind I., Prak R.F., Wolkorte R. Fatigue and Fatigability in Persons With Multiple Sclerosis. Exerc. Sport Sci. Rev. 2016;44(4):123–128. doi: 10.1249/JES.0000000000000088. [DOI] [PubMed] [Google Scholar]
- Abe M., Hanakawa T. Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behav. Brain Res. 2009;198(1):13–23. doi: 10.1016/j.bbr.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Boussaoud D. Attention versus Intention in the Primate Premotor Cortex. NeuroImage. 2001;14:40–45. doi: 10.1006/nimg.2001.0816. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburg Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1988;12(28):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chalah M.A., Riachi N., Ahdab R., Creange A., Lefaucheur J.P., Ayache S.S. Fatigue in Multiple Sclerosis: Neural Correlates and the Role of Non-Invasive Brain Stimulation. Front. Cell. Neurosci. 2015;9:460. doi: 10.3389/fncel.2015.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.H., DeLuca J., Genova H.M., Yao B., Wylie G. Cognitive fatigue is associated with altered functional connectivity in interoceptive and reward pathways in multiple sclerosis. Diagnostics (Basel) 2020;10(11) doi: 10.3390/diagnostics10110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Related Disorders. 2009;Dec;15 Suppl 3::S237–40. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca J., Genova H., Hillary F., Wylie G. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J. Neurol. Sci. 2008;270(1–2):28–39. doi: 10.1016/j.jns.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dobryakova E., DeLuca J., Genova H.M., Wylie G.R. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. JINS. 2013;19(8):849–853. doi: 10.1017/S1355617713000684. [DOI] [PubMed] [Google Scholar]
- Doyon J., Bellec P., Amsel R., Penhune V., Monchi O., Carrier J., et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Enoka R.M., Duchateau J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016;48(11):2228–2238. doi: 10.1249/MSS.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A., Colombo B., Falini A., Codella M., Scotti G., et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage. 2002;15(3):559–567. doi: 10.1006/nimg.2001.1011. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fox R.J., Bacon T.E., Chamot E., Salter A.R., Cutter G.R., Kalina J.T., et al. Prevalence of multiple sclerosis symptoms across lifespan: data from the NARCOMS Registry. Neurodegenerative disease management. 2015;5(6 Suppl):3–10. doi: 10.2217/nmt.15.55. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Goldman C.G. The Goldman Consensus statement on depression in multiple sclerosis. Mult Scler. 2005;11(3):328–337. doi: 10.1191/1352458505ms1162oa. [DOI] [PubMed] [Google Scholar]
- Gregory S., Long J.D., Tabrizi S.J., Rees G. Measuring Compensation in Neurodegeneration using MRI. Curr Opin Neurol. 2017;30(4):380–387. doi: 10.1191/1352458505ms1162oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T., Honda M., Zito G., Dimyan M.A., Hallett M. Brain activity during visuomotor behavior triggered by arbitrary and spatially constrained cues: an fMRI study in humans. Exp Brain Res. 2006;172(2):275–282. doi: 10.1007/s00221-005-0336-z. [DOI] [PubMed] [Google Scholar]
- Induruwa I., Constantinescu C.S., Gran B. Fatigue in multiple sclerosis - a brief review. J. Neurol. Sci. 2012;323(1–2):9–15. doi: 10.1016/j.jns.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Jebsen R.H., Taylor N., Trieschmann R.B., Trotter M.J., Howard L.A. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50(6):311–319. [PubMed] [Google Scholar]
- Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Khan F., Amatya B., Galea M. Management of fatigue in persons with multiple sclerosis. Front. Neurol. 2014;5:177. doi: 10.3389/fneur.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M., Rauch H.G., Stein D.J., Brooks S.J. The handyman’s brain: a neuroimaging meta-analysis describing the similarities and differences between grip type and pattern in humans. NeuroImage. 2014;15(102 Pt 2):923–937. doi: 10.1016/j.neuroimage.2014.05.064. [DOI] [PubMed] [Google Scholar]
- Kluger B.M., Krupp L.B., Enoka R.M. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80(4):409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367–368. doi: 10.1191/135248506ms1373ed. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck J.P., Ehrsson H.H., Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. The European journal of neuroscience. 2001;14(2):382–390. doi: 10.1046/j.0953-816x.2001.01639.x. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck J.P., Gilster R., Wolff S., Ulmer S., Siebner H., Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. NeuroImage. 2008;40(4):1469–1481. doi: 10.1016/j.neuroimage.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lamers I., Kelchtermans S., Baert I., Feys P. Upper limb assessment in multiple sclerosis: a systematic review of outcome measures and their psychometric properties. Arch. Phys. Med. Rehabil. 2014;95(6):1184–1200. doi: 10.1016/j.apmr.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Lopez-Gongora M., Querol L., Escartin A. A one-year follow-up study of the Symbol Digit Modalities Test (SDMT) and the Paced Auditory Serial Addition Test (PASAT) in relapsing-remitting multiple sclerosis: an appraisal of comparative longitudinal sensitivity. BMC Neurol. 2015;15:40. doi: 10.1186/s12883-015-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue and basal ganglia. J. Neurolog. Sci. 2000 Oct 1;179(S 1-2):34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- m2html©2003. tal2mni 2005 [cited 2017 10.07]. Available from: http://eeg.sourceforge.net/doc_m2html/bioelectromagnetism/tal2mni.html.
- Mayka M.A., Corcos D.M., Leurgans S.E., Vaillancourt D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. NeuroImage. 2006;31(4):1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden S.L., Feinstein A., Kalb R.C., Miller D., Mohr D.C., Patten S.B., Bever C., Schiffer R.B., Gronseth G.S., Narayanaswami P. Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(2):174–181. doi: 10.1212/WNL.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden S.L., Frankel D., Hadden L., Perloffp J., Srinath K.P., Hoaglin D.C. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24–38. doi: 10.1212/WNL.0000000000000013. [DOI] [PubMed] [Google Scholar]
- Morgante F., Dattola V., Crupi D., Russo M., Rizzo V., Ghilardi M.F., Terranova C., Girlanda P., Quartarone A. Is central fatigue in multiple sclerosis a disorder of movement preparation? J. Neurol. 2011;258(2):263–272. doi: 10.1007/s00415-010-5742-x. [DOI] [PubMed] [Google Scholar]
- Mosso A. Fatigue. Swan Sonnenschein & Co. Ltd.; London: 1904. [Google Scholar]
- Mucke M, Mochamat, Cuhls H, Peuckmann-Post V, Minton O, Stone P, et al. Pharmacological treatments for fatigue associated with palliative care. The Cochrane database of systematic reviews. 2015;(5):):CD006788. doi: 10.1002/14651858.CD006788.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pardini M., Bonzano L., Roccatagliata L., Mancardi G.L., Bove M. The fatigue-motor performance paradox in multiple sclerosis. Sci. Rep. 2013;3:2001. doi: 10.1038/srep02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patejdl R., Penner I.K., Noack T.K., Zettl U.K. Multiple sclerosis and fatigue: A review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun. Rev. 2016;15(3):210–220. doi: 10.1016/j.autrev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Penner I.K., Raselli C., Stocklin M., Opwis K., Kappos L., Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler. 2009;15(12):1509–1517. doi: 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- Picard N., Strick P.L. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11(6):663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Popp R.F.J., Fierlbeck A.K., Knüttel H., König N., Rupprecht R., Weissert R., Wetter T.C. Daytime sleepiness versus fatigue in patients with multiple sclerosis: A systematic review on the Epworth sleepiness scale as an assessment tool. Sleep Med. Rev. 2017;32:95–108. doi: 10.1016/j.smrv.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Possa M.F. Neuropsychological measures in clinical practice. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2010;31(Suppl 2):S219–22. doi: 10.1007/s10072-010-0374-6. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Agosta F., Colombo B., Mezzapesa D.M., Falini A., Comi G., et al. fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum. Brain Mapp. 2007;28(5):373–382. doi: 10.1002/hbm.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Gatti R., Agosta F., Broglia P., Rossi P., Riboldi E., Corti M., Comi G., Filippi M. Influence of task complexity during coordinated hand and foot movements in MS patients with and without fatigue. A kinematic and functional MRI study. J. Neurol. 2009;256(3):470–482. doi: 10.1007/s00415-009-0116-y. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Meani A., Riccitelli G.C., Colombo B., Rodegher M., Falini A., Comi G., Filippi M. Abnormal adaptation over time of motor network recruitment in multiple sclerosis patients with fatigue. Mult Scler. 2016;22(9):1144–1153. doi: 10.1177/1352458515614407. [DOI] [PubMed] [Google Scholar]
- Ruiu E., Dubbioso R., Madesen K.H., Svolgaard O., Raffin E., Andersen K.W., et al. Probing context-dependent modulations of ipsilateral premotor-motor connectivity in relapsing-remitting multiple sclerosis. Front. Neurol. 2020;(11):1–10. doi: 10.3389/fneur.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M., Crupi D., Naro A., Avanzino L., Buccafusca M., Dattola V., Terranova C., Sottile F., Rizzo V., Ghilardi M.F., Girlanda P., Bove M., Quartarone A. Fatigue in patients with multiple sclerosis: from movement preparation to motor execution. J. Neurol. Sci. 2015;351(1–2):52–57. doi: 10.1016/j.jns.2015.02.031. [DOI] [PubMed] [Google Scholar]
- Simpson S., Jr., Tan H., Otahal P., Taylor B., Ponsonby A.L., Lucas R.M., et al. Anxiety, depression and fatigue at 5-year review following CNS demyelination. Acta Neurol. Scand. 2016 doi: 10.1111/ane.12554. [DOI] [PubMed] [Google Scholar]
- Specogna I., Casagrande F., Lorusso A., Catalan M., Gorian A., Zugna L., Longo R., Zorzon M., Naccarato M., Pizzolato G., Ukmar M., Cova M.A. Functional MRI during the execution of a motor task in patients with multiple sclerosis and fatigueRM-f durante un compito motorio in pazienti con sclerosi multipla e fatica. Radiol. Med. (Torino) 2012;117(8):1398–1407. doi: 10.1007/s11547-012-0845-3. [DOI] [PubMed] [Google Scholar]
- Svolgaard O., Andersen K.W., Bauer C., Madsen K.H., Blinkenberg M., Selleberg F., Siebner H.R., Paul F. Cerebellar and premotor activity during a non-fatiguing grip task reflects motor fatigue in relapsing-remitting multiple sclerosis. PLoS ONE. 2018;13(10):e0201162. doi: 10.1371/journal.pone.0201162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M.C., Narayanan S., Arnold D.L. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2008;15(4):413–419. doi: 10.1111/j.1468-1331.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- Ward N.S., Bestmann S., Hartwigsen G., Weiss M.M., Christensen L.O.D., Frackowiak R.S.J., Rothwell J.C., Siebner H.R. Low-frequency transcranial magnetic stimulation over left dorsal premotor cortex improves the dynamic control of visuospatially cued actions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(27):9216–9223. doi: 10.1523/JNEUROSCI.4499-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]