Abstract
Background
RATIONALE 302 (NCT03430843) an open-label, phase III study of second-line treatment of advanced/metastatic esophageal squamous cell carcinoma (ESCC), reported that tislelizumab, relative to investigator-chosen chemotherapy (ICC), was associated with improvements in overall survival and a favorable safety profile. This study assessed the health-related quality of life (HRQoL) and ESCC-related symptoms of patients in RATIONALE 302.
Methods
Adults with advanced/metastatic ESCC whose disease progressed following prior systemic therapy were randomized 1 : 1 to receive either tislelizumab or ICC (paclitaxel, docetaxel, or irinotecan). HRQoL was measured using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items (EORTC QLQ-C30), the EORTC Quality of Life Questionnaire Oesophageal Cancer Module 18 items (QLQ-OES18), and the EuroQoL Five-Dimensions Five-Levels (EQ-5D-5L) visual analogue scale. Mixed effect modeling for repeated measurements examined changes from baseline to weeks 12 and 18. The Kaplan–Meier method was used to examine time to deterioration.
Results
Overall, 512 patients were randomized to tislelizumab (n = 256) or ICC (n = 256). The tislelizumab arm maintained QLQ-C30 global health status/quality whereas the ICC arm worsened at week 12 {difference in least square (LS) mean change: 5.8 [95% confidence interval (CI): 2.0-9.5], P = 0.0028} and week 18 [difference in LS mean change: 8.1 (95% CI: 3.4-12.8), P = 0.0008]. Physical functioning (week 18) and fatigue (weeks 12 and 18) worsened less in the tislelizumab compared with the ICC arm. The tislelizumab arm improved in reflux symptoms, whereas the ICC worsened at week 12 [difference in LS mean change: −4.1 (95% CI: −7.6 to −0.6), P = 0.0229]. The visual analogue scale remained consistent in the tislelizumab arm whereas it worsened in the ICC arm. The hazard of time to deterioration was lower in tislelizumab patients compared with ICC for physical functioning and reflux.
Conclusions
HRQoL, including fatigue symptoms and physical functioning, was maintained in patients with advanced or metastatic ESCC receiving tislelizumab compared with ICC-treated patients. These results provide additional support for the benefits of tislelizumab in this patient population.
Key words: esophageal squamous cell carcinoma, PD-1/PD-L1 inhibitors, health-related quality of life
Highlights
-
•
Global health status and HRQoL remained consistent in the tislelizumab arm whereas the ICC arm experienced worsening.
-
•
Fatigue and physical functioning worsened in both arms; however, the worsening was greater in the ICC arm.
-
•
The tislelizumab arm was at lower risk of reaching the threshold for worsening in physical functioning and reflux.
Introduction
Individuals with esophageal squamous cell carcinoma (ESCC), worldwide the most common histological subtype of esophageal cancer, typically experience a severe symptom burden at diagnosis and associated reductions in health-related quality of life (HRQoL) due to esophageal obstruction throughout the course of their disease.1, 2, 3 In addition, self-reported health status and ESCC-specific symptoms associated with HRQoL are predictive of overall survival (OS) in patients with potentially curable and advanced esophagogastric esophageal cancer, further underscoring the importance of these domains.4,5 A better understanding of ESCC-specific symptoms, functioning, and associated HRQoL are especially needed for patients with advanced or metastatic ESCC given that second-line chemotherapy has limited efficacy with marginal antitumor activity, poor long-term survival, and significant toxicities.6, 7, 8, 9, 10
Recent clinical trials of immuno-oncological therapies targeting the programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) pathway, collectively referred to as PD-(L)1, have demonstrated prolonged survival and safety benefits with anti-PD-1 antibodies versus chemotherapy in patients with advanced or metastatic ESCC whose disease progressed after first-line systemic therapy.8, 9, 10 These trials have also reported maintenance (reduced risk of deterioration) as well as improvements in HRQoL and symptom burden in esophageal cancer patients treated with a PD-(L)1 versus chemotherapy.8,9,11,12
Tislelizumab is a humanized immunoglobulin G4 variant monoclonal antibody against PD-1. Tislelizumab was specifically engineered to minimize binding to Fcγ receptors on macrophages in order to abrogate antibody-dependent phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy.13,14 Clinical trials have previously demonstrated the clinical benefits and safety of first-line tislelizumab with chemotherapy combination therapy versus chemotherapy alone in patients with advanced squamous and nonsquamous non-small cell lung cancer.15,16 Tislelizumab plus chemotherapy was also associated with improvements or maintenance in patients’ HRQoL and disease-specific symptoms compared with chemotherapy alone.17
RATIONALE 302 (NCT03430843), a global, open-label, randomized, phase III study, investigated tislelizumab compared with investigator-chosen chemotherapy (ICC) as second-line treatment of patients with advanced or metastatic ESCC whose disease progressed after first-line systemic therapy. Compared with ICC, tislelizumab was found to prolong OS [median of 8.6 versus 6.3 months; hazard ratio (HR) 0.70, 95% confidence interval (CI) 0.57-0.85, P = 0.0001] and was associated with a higher objective response rate (20.3% versus 9.8%).18 Tislelizumab was also found to have a more durable antitumor response compared with ICC (median of 7.1 versus 4.0 months). Furthermore, fewer patients experienced grade ≥3 treatment-related adverse events (18.8% versus 55.8%) with tislelizumab compared with ICC.18
HRQoL and ESCC symptoms, assessed using patient-reported outcome (PRO) questionnaires, were included as secondary endpoints in RATIONALE 302. The objective of the current analysis was to determine whether tislelizumab could improve HRQoL, reduce symptom burden, and delay the time to deterioration (TTD) compared with chemotherapy alone in patients with advanced or metastatic ESCC.
Materials and methods
Study design, population, and treatment
Eligible patients from RATIONALE 302 were randomized (1 : 1) to receive tislelizumab or ICC (one of the following single-agent chemotherapies: paclitaxel, docetaxel, or irinotecan). The study design and primary efficacy and safety data have been published elsewhere.18 Tislelizumab was administered intravenously (i.v.) 200 mg every 3 weeks (Q3W). Paclitaxel was administered as 135-175 mg/m2 i.v. Q3W, or in doses of 80-100 mg/m2 weekly as per regional guidelines. In Japan, paclitaxel was administered as 100 mg/m2 i.v. in cycles consisting of weekly dosing for 6 weeks, followed by 1 week of rest. Docetaxel was administered as 75 mg/m2 i.v. Q3W (70 mg/m2 i.v. Q3W in Japan). Irinotecan 125 mg/m2 i.v. was administered on days 1 and 8, every 21 days. Randomization was stratified by region [Asia (excluding Japan) versus Japan versus Europe/North America], Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1), and ICC (paclitaxel versus docetaxel versus irinotecan).
Eligible patients were adults (≥18 years of age) with histologically confirmed ESCC who had advanced or metastatic disease which progressed during or after first-line systemic treatment. Patients who had tumor progression during or within 6 months after definitive chemoradiotherapy, neo-adjuvant, or adjuvant therapy were also eligible. Patients were required to have an ECOG performance status of 0 or 1, at least one measurable/evaluable lesion by RECIST v1.1, and adequate hematological, hepatic, renal, and coagulation function. Patients who had received prior therapies targeting PD-1 or PD-L1, active brain or leptomeningeal metastasis, active autoimmune disease, or other prior malignancies active within 2 years before randomization were ineligible. The study was carried out in accordance with the International Conference on Harmonization Good Clinical Practice Guideline, the principles of the Declaration of Helsinki, and local laws and regulations. All patients provided written informed consent before participation.
HRQoL measures
HRQoL and ESCC symptoms were assessed via two validated patient-reported outcome (PRO) instruments administered via the paper versions: the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 items (QLQ-C30)19,20 and the EORTC Quality of Life Questionnaire Oesophageal Cancer Module 18 items (QLQ-OES18).20,21 The EuroQoL Five-Dimensions Five-Levels (EQ-5D-5L) was also included.22 Specific PRO endpoints were selected from the QLQ-EOS18 based on the most prevalent ESCC symptoms of dysphagia, eating, reflux, and pain (single items), as well as the full QLQ-EOS18 symptom index score; the QLQ-C30’s global health status/quality of life (GHS/QoL) scale, physical functioning scale, and fatigue symptom scale were also selected as they prominently measure the disease impact. The criteria for selection of these specific endpoints were based on the ESCC clinical trials and previous publications of ECC clinical trials.8,12,23 The key clinical cycles were cycle 4 and 6 and were selected to represent times during (week 12) and after (week 18) chemotherapy treatment to minimize data loss due to disease progression or death. Cycle 4 and cycle 6, which was the end of chemotherapy for most patients, were selected to measure the longer-term effects of treatment while >50% remained in trial.
For the QLQ-C30 and QLQ-OES18 assessment at each visit, the raw scores for functional and symptom scales and items were transformed from 0 to 100, with higher scores representing better outcomes on the GHS/QoL scale and physical functioning scale and worse outcomes on the symptom scales.24
Statistical analyses
The PRO analyses included all randomized patients who completed baseline, received at least one dose of study drug, and completed at least one HRQoL assessment at a future cycle. Completion rates were defined as the number of patients who completed all the questionnaires divided by the total number of patients in the relevant treatment arm. Adjusted completion rates were defined as the proportion of patients who completed all the questions in a questionnaire divided by the total number of patients in the study at the relevant visit in the relevant treatment arm.
Evaluation of least square (LS) mean change from baseline to week 12 and week 18 in the PRO instrument scores was based on a mixed-effect model for repeated measurements, with the PRO score as the response variable. The covariates included treatment, study visit, treatment by study visit interaction, baseline score, and randomization stratification factors [ECOG performance status (0 versus 1) and ICC option (paclitaxel versus docetaxel versus irinotecan)]. The models were based on the missing at random assumption. Between-group comparisons were reported as differences in the LS mean change from baseline with the 95% CI.
TTD was examined using the GHS/QoL scale and physical functioning scale scores of the QLQ-C30, as well as the dysphagia, eating, reflux, and pain symptom item scores from the QLQ-OES18. TTD was defined as time to first onset of ≥10 points25 towards a worsening direction (e.g. increase score in symptoms) from baseline with confirmation by a worsening in the subsequent cycle. The Kaplan–Meier method was used to estimate the deterioration curve in each group; a stratified Cox model with Efron’s method of tie handling was used to assess between-group differences. Stratification factors included ECOG performance status (0 versus 1) and ICC option (paclitaxel versus docetaxel versus irinotecan). Descriptive analysis for the EQ-5D-5L’s visual analogue scale (VAS) was also conducted. All calculations and analyses were conducted using SAS version 9.4 or higher.
Results
Overall, 512 patients were randomized to either the tislelizumab arm (n = 256) or the ICC arm (n = 256). The demographics and clinical characteristics were generally balanced across the two treatment arms and were representative of the target patient population (Table 1). The data cut-off date for the current analysis was 1 December 2020.
Table 1.
Patient demographics and baseline characteristics
| Tislelizumab (n = 256) | ICC (n = 256) | |
|---|---|---|
| Age, median (range), years | 62.0 (40-86) | 63.0 (35-81) |
| <65 years, n (%) | 157 (61.3) | 161 (62.9) |
| ≥65 years, n (%) | 99 (38.7) | 95 (37.1) |
| Sex, n (%) | ||
| Male | 217 (84.8) | 215 (84.0) |
| Female | 39 (15.2) | 41 (16.0) |
| Geographic region,an (%) | ||
| Asia | 201 (78.5) | 203 (79.3) |
| Europe/North America | 55 (21.5) | 53 (20.7) |
| ECOG performance status, n (%) | ||
| 0 | 66 (25.8) | 60 (23.4) |
| 1 | 190 (74.2) | 196 (76.6) |
| PD-L1 expression, n (%) | ||
| TAP ≥10% | 89 (34.8) | 68 (26.6) |
| TAP <10% | 116 (45.3) | 140 (54.7) |
| Unknown | 51 (19.9) | 48 (18.8) |
| Smoking status, n (%) | ||
| Never smoker | 68 (26.6) | 63 (24.6) |
| Current/former smoker | 188 (73.4) | 192 (75.0) |
| Missing | 0 (0.0) | 1 (0.4) |
| Previous therapies, n (%) | ||
| Surgery | 94 (36.7) | 99 (38.7) |
| Radiotherapy | 169 (66.0) | 163 (63.7) |
| Platinum-based chemotherapy | 249 (97.3) | 252 (98.4) |
| Disease stage at study entry, n (%) | ||
| Locally advanced | 5 (2.0) | 20 (7.8) |
| Metastatic | 251 (98.0) | 236 (92.2) |
ECOG, Eastern Cooperative Oncology Group; ICC, investigator-chosen chemotherapy; PD-L1, programmed death-ligand 1; TAP, tumor abnormal protein.
There were 50 patients from Japan: 25 patients in the tislelizumab arm and 25 patients in the chemotherapy arm.
Completion rates
For the QLQ-C30, QLQ-OES18, and EQ-5D-5L, the adjusted completion rate at baseline was ≥93.8% across all assessments (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100517). At week 12, the completion rate for all PRO instruments dropped to 57% in the tislelizumab arm and 30% in the ICC arm. At week 18, the completion rate for all PRO instruments continued to decline to 39% in the tislelizumab arm and 15% in the ICC arm. The adjusted completion rates remained >90% for both arms at week 12 and week 18.
EORTC QLQ-C30
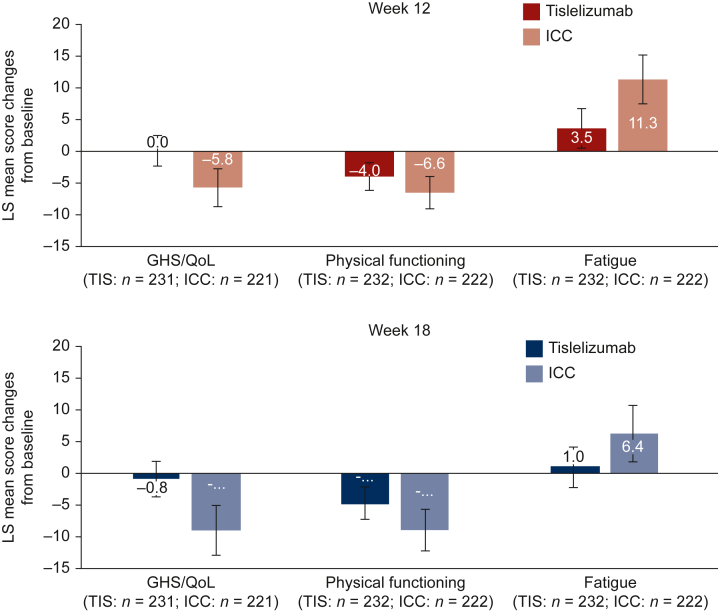
The observed means and change from baseline for each of the QLQ-C30 scales are provided in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100517. Results from the mixed-effect model for repeated measurements indicated that the tislelizumab-treated patients maintained QLQ-C30 GHS/QoL scale scores (Figure 1) at both week 12 [LS mean change: 0.0 (95% CI: −2.5 to 2.4)] and week 18 [LS mean change: −0.8 (95% CI: −3.5 to 2.0)], whereas the ICC arm experienced worsening at both week 12 [LS mean change: −5.8 (95% CI: −8.8 to −2.8)] and week 18 [LS mean change: −8.9 (95% CI: −12.8 to −4.9)]. There was a difference in change from baseline between the two arms at week 12 [difference in LS mean change: 5.8 (95% CI: 2.0-9.5), P = 0.0028] and week 18 [difference in LS mean change: 8.1 (95% CI: 3.4-12.8), P = 0.0008].
Figure 1.
Change from baseline for EORTC QLQ-C30 scores at week 12 and week 18. EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items; GHS, global health status; ICC, investigator-chosen chemotherapy; LS, least square; n, patients with baseline and at least one post-baseline measurement; QoL, quality of life; TIS, tislelizumab.
There were no differences in change from baseline between the arms at week 12 in physical functioning [difference in LS mean change: 2.6 (95% CI: −0.07 to 6.0), P = 0.1266]; however, at week 18 the reduction in physical functioning from baseline was less in the tislelizumab arm [LS mean change: −4.6 (95% CI: −7.1 to −2.1)] compared with the ICC arm [LS mean change: −8.9 (95% CI: −12.1 to −5.6)] and there was a difference in decline [difference in LS mean change: 4.2 (95% CI: 0.4-8.1), P = 0.0327].
Finally, fatigue symptoms worsened at week 12 for both the tislelizumab arm [LS mean change: 3.5 (95% CI: 0.4-6.6)] and the ICC arm [LS mean change: 11.3 (95% CI: 7.5-15.1)] and remained consistent at week 18 for the tislelizumab arm [LS mean change: 1.0 (95% CI: −2.1 to 4.2)] while continuing to increase for the ICC arm [LS mean change: 6.4 (95% CI: 2.0-10.9)]. The worsening of fatigue was less in the tislelizumab arm at week 12 [difference in LS mean change: −7.8 (95% CI: −12.6 to −3.1), P = 0.0014] and week 18 [difference in LS mean change: −5.4 (95% CI: −10.5 to −0.3), P = 0.0379].
EORTC QLQ-OES18
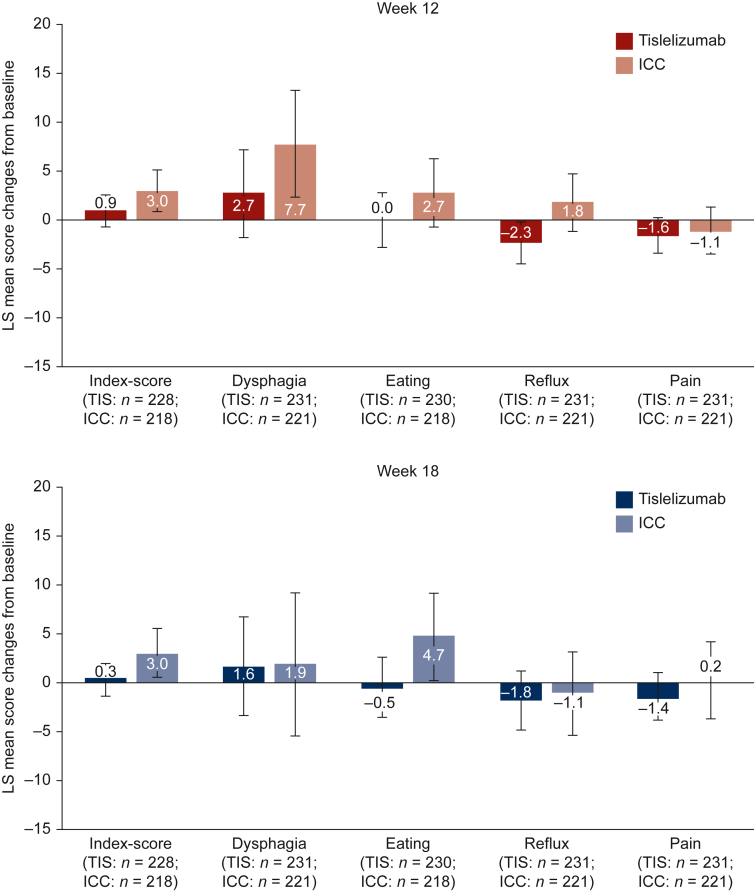
The observed means and change from baseline for each of the QLQ-OES18 scales are provided in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100517. Results from the mixed-effect model for repeated measurements indicated that the QLQ-OES18 symptom index scale scores (Figure 2) in the tislelizumab arm were maintained at both week 12 [LS mean change: 0.9 (95% CI: −0.7 to 2.5)] and week 18 [LS mean change: 0.3 (95% CI: −1.4 to 2.0)], whereas the ICC arm experienced worsening at both week 12 [LS mean change: 3.0 (95% CI: 1.0-5.1)] and week 18 [LS mean change: 3.0 (95% CI: 0.6-5.5)]. There was no difference in change from baseline, however, between the two arms at either week 12 [difference in LS mean change: −2.1 (95% CI: −4.6 to 0.4), P = 0.0929] or week 18 [difference in LS mean change: −2.7 (95% CI: −5.6 to 0.2), P = 0.0678].
Figure 2.
Change from baseline for EORTCQLQ-OES18scores at week 12 and week 18. EORTC QLQ-OES18, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Oesophageal Cancer Module 18 items; ICC, investigator-chosen chemotherapy; LS, least square; n, patients with baseline and at least one post-baseline measurement; TIS, tislelizumab.
Dysphagia symptoms at week 12 worsened less in the tislelizumab arm [LS mean change: 2.7 (95% CI: −1.7 to 7.1)] than in the ICC arm [LS mean change: 7.7 (95% CI: 2.2-13.2)]; however, there was no difference between the two arms [difference in LS mean change: −4.9 (95% CI: −11.8 to 1.9), P = 0.1581]. At week 18, the tislelizumab arm [LS mean change: 1.6 (95% CI: −3.5 to 6.6)] and the ICC arm (LS mean change: 1.9 [95% CI: −5.5 to 9.2] also experienced similar changes from baseline in dysphagia symptoms, and there was no difference between the arms [difference in LS mean change: −0.3 (95% CI: −9.1 to 8.5), P = 0.9528].
With regards to eating symptoms, patients in the tislelizumab arm maintained their scores at week 12 [LS mean change: 0.0 (95% CI: −2.8 to 2.8)], whereas the ICC arm experienced worsening in problems with eating [LS mean change: 2.7 (95% CI: −0.8 to 6.2)]; however, there was no difference between the arms [difference in LS mean change: −2.7 (95% CI: −7.0 to 1.6), P = 0.2218]. At week 18, there was a difference change from baseline in eating problems [difference in LS mean change −5.2 (95% CI: −10.3 to 0.0), P = 0.0487], with the tislelizumab arm experiencing fewer eating problems than at baseline [LS mean change: −0.5 (95% CI: −3.6 to 2.6)] compared with the ICC arm [LS mean change: 4.7 (95% CI: 0.3-9.1].
For reflux symptoms at week 12, there was a difference in change from baseline [difference in LS mean change: −4.1 (95% CI: −7.6 to −0.6), P = 0.0229] with the tislelizumab arm experiencing fewer reflux symptoms than at baseline [LS mean change: −2.3 (95% CI: −4.6 to −0.1)] compared with the ICC arm which experienced a worsening in reflux symptoms [LS mean change: 1.8 (95% CI: −1.1 to 4.7)]. At week 18, both arms experienced similar slight reduction from baseline in reflux symptoms, but the differences in change between the two arms was not significant [difference in LS mean change: −0.6 (95% CI: −5.7 to 4.5), P = 0.8034].
Finally, for pain symptoms, tislelizumab-treated patients consistently maintained their scores at both week 12 [LS mean change: −1.6 (95% CI: −3.4 to 0.2)] and week 18 [LS mean change: −1.4 (95% CI: −3.9 to 1.0)] as did the chemotherapy-treated patients at both week 12 [LS mean change: −1.1 (95% CI: −3.6 to 1.3)] and week 18 [LS mean change: 0.2 (95% CI: −3.6 to 4.1)]. There were no differences, however, in change from baseline between the two arms at either week 12 [difference in LS mean change: −0.5 (95% CI: −3.4 to 2.5), P = 0.7660] or week 18 [difference in LS mean change: −1.7 (95% CI: −6.1 to 2.8), P = 0.4573].
EQ-5D-5L
According to the EQ-5D-5L at week 12, the tislelizumab arm experienced less of a decrease in health status according to the VAS score of the EQ-5D-5L {mean change: −0.2 [standard deviation (SD): 10.91]} compared with the ICC arm [mean change: −1.8 (SD: 14.17); Table 2]. At week 18, the tislelizumab arm continued to experience less decline in health status [mean change: −0.6 (SD: 4.81)] compared with the ICC arm [mean change: −5.9 (SD: 16.34)].
Table 2.
Change from baseline for EQ-5D-5L VAS scores at week 12 and week 18
| Tislelizumab (n = 256) | ICC (n = 256) | |
|---|---|---|
| Baseline, mean (SD) | 73.7 (17.05) | 72.5 (18.13) |
| Week 12 | ||
| Change from baseline, mean (SD) | -0.2 (10.91) | -1.8 (14.17) |
| Week 18 | ||
| Change from baseline, mean (SD) | -0.6 (14.81) | -5.9 (16.34) |
EQ-5D-5L, EuroQoL Five-Dimensions Five-Levels; SD, standard deviation; VAS, visual analogue scale.
TTD
The stratified HR (95% CI) showed that risk of experiencing a deterioration event was lower for the patients in the tislelizumab arm in comparison with the ICC arm for physical functioning [Table 3; 0.67 (95% CI: 0.45-1.00), P = 0.0239] and reflux [0.50 (95% CI: 0.32-0.80), P = 0.0014]. There were no differences in the risk of deterioration for the GHS/QoL scale for the dysphagia, eating, and pain symptoms between the two arms.
Table 3.
Time to deterioration for EORTC QLQ-C30 and QLQ-OES18
| Tislelizumab (n = 256) | ICC (n = 256) | ||
|---|---|---|---|
|
QLQ-C30 GHS/QoL scale |
Patients with event, n (%) | 59 (23.0) | 47 (18.4) |
| Median time to deterioration, months (95% CI) | NR (NE-NE) | NR (NE-NE) | |
| Stratifieda HR (95% CI) | 0.96 (0.65-1.41) 0.4156 |
||
| Stratifieda log-rank test P value | |||
| Physical functioning scale | Patients with event, n (%) | 47 (18.4) | 52 (20.3) |
| Median time to deterioration, months (95% CI) | NR (NE-NE) | 10.0 (4.5-NE) | |
| Stratifieda HR (95% CI) | 0.67 (0.45-1.00) 0.0239 |
||
| Stratifieda log-rank test P value | |||
|
QLQ-OES18 Dysphagia |
Patients with event, n (%) | 63 (24.6) | 63 (24.6) |
| Median time to deterioration, months (95% CI) | NR (NE-NE) | NR (3.7-NE) | |
| Stratifieda HR (95% CI) | 0.76 (0.53-1.07) 0.0562 |
||
| Stratifieda log-rank test P value | |||
| Eating | Patients with event, n (%) | 35 (13.7) | 27 (10.5) |
| Median time to deterioration, months (95% CI) | NR (NE-NE) | NR (NE-NE) | |
| Stratifieda HR (95% CI) | 1.06 (0.64-1.75) 0.4158 |
||
| Stratifieda log-rank test P value | |||
| Reflux | Patients with event, n (%) | 32 (12.5) | 45 (17.6) |
| Median time to deterioration, months (95% CI) | NR (15.1-NE) | NR (NE-NE) | |
| Stratifieda HR (95% CI) | 0.50 (0.32-0.80) 0.0014 |
||
| Stratifieda log-rank test P value | |||
| Pain | Patients with event, n (%) | 49 (19.1) | 44 (17.2) |
| Median time to deterioration, months (95% CI) | NR (NE-NE) | NR (NE-NE) | |
| Stratifieda HR (95% CI) | 0.89 (0.59-1.35) 0.2969 |
||
| Stratifieda log-rank test P value | |||
CI, confidence interval; EORTC, European Organization for Research and Treatment of Cancer; GHS, global health status; HR, hazard ratio; NE, not estimated; NR, not reached; QLC-C30, Quality of Life Questionnaire Core 30 items; QLC-EOS18, Quality of Life Questionnaire Oesophageal Cancer Module 18 items; QoL, quality of life.
Stratification factors included Eastern Cooperative Oncology Group performance status (0 versus 1) and investigator-chosen chemotherapy option (paclitaxel versus docetaxel versus irinotecan cells).
Discussion
In the RATIONALE 302 clinical trial, patients treated with tislelizumab as a second-line treatment of advanced or metastatic ESCC generally experienced more favorable HRQoL outcomes than patients receiving ICC. Analysis further indicated that through the course of treatment, patients in the tislelizumab arm were at lower risk of reaching the threshold for worsening in physical functioning and reflux symptoms.
This study adds to the growing literature regarding the benefit of anti-PD-(L)1 therapies on HRQoL and other PROs. Consistent with our results, the phase 3III KEYNOTE-181 study reported maintenance in QLQ-C30 and QLQ-OES18 endpoints among patients treated with pembrolizumab versus chemotherapy.12 In KEYNOTE-181, however, the prespecified analysis only examined changes from baseline to week 9 whereas the current study design extends the follow-up period to week 18. Maintenance in the EQ-5D-5L VAS score for pembrolizumab-treated patients and declines for chemotherapy-treated patients were also reported in KEYNOTE-181,12 whereas the phase III ATTRACTION-3 study reported improvement in the EQ-5D-5L VAS score associated with nivolumab.9 Similar to our results, the phase III ESCORT study reported significant differences in GHS and fatigue symptomatology from baseline to week 8 in patients treated with camrelizumab compared with patients treated with chemotherapy.8 With respect to TTD, no significant differences were found in KEYNOTE-181,12 whereas our study found that tislelizumab was associated with a lower risk of reaching the threshold for worsening in physical functioning and reflux symptoms.
Although the results of this study are encouraging, they should be considered alongside the following limitations. The current study used an open-label design so patients were aware of the study treatment which may have influenced responses to the PROs. Second, the completion rates of the QLQ-C30, QLQ-OES18, and EQ-5D-5L at week 12 and week 18 were markedly lower than at baseline; although, the adjusted completion rates remained high in both arms at each assessment period. This reduction in sample size was, in part, due to disease progression. Third, several ESCC-related symptoms from the QLQ-OES18 were assessed as single items, which may have limited their discriminative ability. Additional research is needed to determine the extent to which these results translate to clinical benefit in real-world practice. Finally, the current study did not examine the longer-term effect of tislelizumab on HRQoL. It is possible that some later worsening was not captured or the failure to find differences between arms could be due to the shorter-term follow-up.
Conclusions
Overall, HRQoL, including fatigue symptoms and physical functioning, was maintained or improved in patients with advanced or metastatic ESCC receiving tislelizumab compared with patients receiving ICC. Specific self-reported ESCC symptom endpoints were more variable, perhaps related to their assessment as single items. These HRQoL data, together with the efficacy and safety results from the RATIONALE 302 trial,18 support the favorable risk–benefit ratio for second-line tislelizumab in advanced or metastatic ESCC.
Acknowledgements
The authors acknowledge the investigative centers’ study staff and study patients and to recognize those from BeiGene, Ltd who have substantially contributed to the development of this manuscript. Editorial assistance was provided by Jason C. Allaire, PhD (Generativity - Health Economics and Outcomes Research, Durham, NC). This assistance was funded by BeiGene, Ltd.
Funding
This work was supported by BeiGene, Ltd (no grant number).
Disclosure
VC reports receiving grants or contracts from the following: Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, Servier. VC also reported receiving consulting fees from Array, Astellas, AstraZeneca, Bayer, BeiGene, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Daiichi, Halozyme, GlaxoSmithKline, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Pierre Fabre, Roche, Servier, Sirtex, and Taiho. KK reports receiving grants or contracts from the following: ONO, Bristol Myers Squibb, Merck Sharp & Dohme, Shionogi, BeiGene, Chugai, AstraZeneca, Bayer, and Oncolys Biopharma. LS reports advisory board membership for BeiGene. TX, ND, LZ, and GB, are employees of BeiGene. SBK reports receiving research funding from Novartis, Sanofi-Aventis, and DongKook Pharm Co. SBK also reports receiving consulting fees from and participating in advisory boards for Novartis, AstraZeneca, Lilly, DAE HWA Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi Sankyo. SBK also reports owning stocks in Genopeaks and NeogeneTC. JA has declared no conflicts of interest.
Supplementary data
References
- 1.Sunde B., Lindblad M., Malmström M., Hedberg J., Lagergren P., Nilsson M. Health-related quality of life one year after the diagnosis of oesophageal cancer: a population-based study from the Swedish National Registry for Oesophageal and Gastric Cancer. BMC Cancer. 2021;21(1):1277. doi: 10.1186/s12885-021-09007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis L.E., Gupta V., Allen-Ayodabo C., et al. Patient-reported symptoms following diagnosis in esophagus cancer patients treated with palliative intent. Dis Esophagus. 2020;33(8):doz108. doi: 10.1093/dote/doz108. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q.L., Xie S.H., Wahlin K., Lagergren J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol. 2018;10:717–728. doi: 10.2147/CLEP.S166078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kleef J.J., Dijksterhuis W.P.M., van den Boorn H.G., et al. Prognostic value of patient-reported quality of life for survival in oesophagogastric cancer: analysis from the population-based POCOP study. Gastric Cancer. 2021;24(6):1203–1212. doi: 10.1007/s10120-021-01209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ter Veer, Evan Kleef J.J., Schokker S., et al. Prognostic and predictive factors for overall survival in metastatic oesophagogastric cancer: a systematic review and meta-analysis. Eur J Cancer. 2018;103:214–226. doi: 10.1016/j.ejca.2018.07.132. [DOI] [PubMed] [Google Scholar]
- 6.Assersohn L., Brown G., Cunningham D., et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004;15(1):64–69. doi: 10.1093/annonc/mdh007. [DOI] [PubMed] [Google Scholar]
- 7.Ford H.E., Marshall A., Bridgewater J.A., et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 8.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 9.Kato K., Cho K.C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 10.Kojima T., Shah M.A., Muro K., et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M., Kato K., Okada M., et al. Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3) Esophagus. 2021;18(1):90–99. doi: 10.1007/s10388-020-00794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adenis A., Kulkarni A.S., Girotto G.C., et al. Impact of pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer on health-related quality of life in KEYNOTE-181. J Clin Oncol. 2021;40:382–391. doi: 10.1200/JCO.21.00601. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T., Song X., Xu J., et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahan R., Sega E., Engelhardt J., et al. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28(3):285–295. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Lu S., Wang J., Yu X., et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512–1522. doi: 10.1016/j.jtho.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Lu S., Yu X., et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709–717. doi: 10.1001/jamaoncol.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Yu X., Barnes G., Leaw S., Bao Y., Tang B. The effects of tislelizumab plus chemotherapy as first-line treatment on health-related quality of life of patients with advanced squamous non-small cell lung cancer: results from a phase 3 randomized clinical trial. Cancer Treat Res Commun. 2021;30 doi: 10.1016/j.ctarc.2021.100501. [DOI] [PubMed] [Google Scholar]
- 18.Shen L., Kato K., Kim S.B., et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302): a randomized phase 3 study. J Clin Oncol. 2022 doi: 10.1200/JCO.21.01926. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 20.EORTC Quality of Life. https://qol.eortc.org/questionnaire/eortc-qlq-c30/ Available at.
- 21.Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39(10):1384–1394. doi: 10.1016/s0959-8049(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 22.Pickard A.S., De Leon M.C., Kohlmann T., et al. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care. 2007;45(3):259–263. doi: 10.1097/01.mlr.0000254515.63841.81. [DOI] [PubMed] [Google Scholar]
- 23.Luo H., Lu J., Bai T., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. J Am Med Assoc. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fayers P.M., Aaronson N.K., Bjordal K., et al. 3rd ed. European Organisation for Research and Treatment of Cancer; Brussels: 2001. The EORTC QLQ-C30 Scoring Manual. [Google Scholar]
- 25.Osoba D., Rodrigues G., Myles J., et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.