Abstract
Objective
Respiratory-gated Auricular Vagal Afferent Nerve stimulation (RAVANS) is a safe nonpharmacological approach to managing chronic pain. The purpose of the current study was to examine (1) the feasibility and acceptability of RAVANS, combined with mindful meditation (MM) for chronic low back pain (CLBP), (2) the potential synergy of MM+RAVANS on improving pain, and (3) possible moderators of the influence of MM+RAVANS on pain.
Design
Pilot feasibility and acceptability study.
Setting
Pain management center at large academic medical center.
Subjects
Nineteen adults with CLBP and previous MM training.
Methods
Participants attended two sessions during which they completed quantitative sensory testing (QST), rated pain severity, and completed a MM+stimulation session. Participants received RAVANS during one visit and sham stimulation during the other, randomized in order. Following intervention, participants repeated QST.
Results
MM+RAVANS was well tolerated, acceptable, and feasible to provide relief for CLBP. Both MM+stimulation sessions resulted in improved back pain severity, punctate pain ratings, and pressure pain threshold. Individuals with greater negative affect showed greater back pain improvement from MM+RAVANS while those with greater mindfulness showed greater back pain improvement from MM+sham.
Conclusions
Results suggest that for CLBP patients with prior MM training, the analgesic effects of MM may have overshadowed effects of RAVANS given the brief single session MM+RAVANS intervention. However, those with greater negative affect may benefit from combined MM+RAVANS.
Introduction
Chronic low back pain (CLBP) is highly prevalent impacting 13% of adults in the United States [1]. This multifactorial condition is consistently responsible for the largest number of years lived with disability among the US population [2] and is associated with upwards of $102 billion in costs annually [3]. Despite the multitude of possible treatments, as well as frequent health care utilization by patients with CLBP, pain often persists [4]. One potential reason for this lack of treatment response is that patients may have central augmentation of pain processing [5]. Indeed, research suggests patients with CLBP experience hyperalgesia (e.g., increased pain in response to normally painful stimuli) and alterations in the neural processing of pain [6, 7]. This central sensitization coupled with the current opioid crisis has led to considerable interest in the development of nonpharmacologic treatments for CLBP.
Mindfulness meditation-based interventions (MM) are a set of nonpharmacologic, cognitive-affective approaches that has been shown to improve pain and function across a wide range of chronic pain conditions, including CLBP [8–12]. Although the mechanisms of MM are still being studied [13], there is evidence for top-down inhibition of ascending nociceptive information [14]. For example, brief MM led to a 40% reduction in perceived heat pain intensity and was associated with greater activation of subgenual anterior cingulate (sgACC), orbitofrontal (OFC), and anterior insula cortices, areas of the brain associated with affective control of pain, contextual evaluation of sensory events, and modulation of afferent nociceptive processing [15, 16].
Conversely, Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS) is a form of transcutaneous auricular vagus nerve stimulation (taVNS) therapy that targets the auricular branch of the vagus nerve and may thus reduce pain via bottom-up mechanisms. Previous studies have suggested that vagus nerve stimulation (VNS) raises pain thresholds (i.e., reduces sensitivity) for pressure and heat pain as well as mitigating temporal summation of pain for mechanical stimulation [17, 18]. Moreover, noninvasive taVNS approaches have also been demonstrated to increase mechanical and pressure pain thresholds and inhibit temporal summation of heat pain among healthy, pain free individuals [19, 20]. Furthermore, gating taVNS stimulation to the exhalation phase of respiration (RAVANS) has been shown to augment brainstem response [21–23], and reduce evoked pain intensity and temporal summation of mechanical pain in patients suffering from chronic pelvic pain [24].
As CLBP is a multifactorial condition, synergistic effects of combined top-down and bottom-up therapies may prove superior to non-pharmacological monotherapies. Our study sought to [1] examine the feasibility and acceptability of RAVANS for CLBP and [2] examine potential synergy of RAVANS and MM by comparing the effects of MM paired with real versus sham RAVANS on pain sensitivity, temporal summation of pain, and pain severity among patients with CLBP who had prior MM training. Moreover, because both MM and RAVANS have been shown to improve negative affect (e.g., depression, anxiety, pain catastrophizing), and there is evidence that RAVANS improves chronic by modulating depression and anxiety [25] we explored these variables and trait-level mindfulness as possible moderators of the influence of MM and RAVANS on short-term (i.e., single session) pain outcomes.
Methods
Participants
Participants included 19 adults, age 21 to 70 years, who endorsed chronic low back pain as their primary complaint for greater than six months with an average pain rating of at least 3 on a 0–10 point numerical rating scale (NRS) and were maintained on at least 15 milligrams of morphine equivalents (MME) [26]. As our design evaluated single-session effects, to ensure participants were already familiar with MM, only individuals who had previously completed 8 sessions of group-based MM intervention for CLBP within the Brigham and Women’s Pain Management Center [27] were included in the study. Participants were excluded if there was evidence of cognitive impairment preventing completion of the study, history of cardiac disease (due to risk of bradycardia [28]), history of neurological disease, peripheral neuropathy, pregnancy, permanent hearing aid in the left ear, damage to the left ear interfering with auricular electrode fit (e.g., perichondrial hematoma), or Raynaud’s syndrome.
Study Design
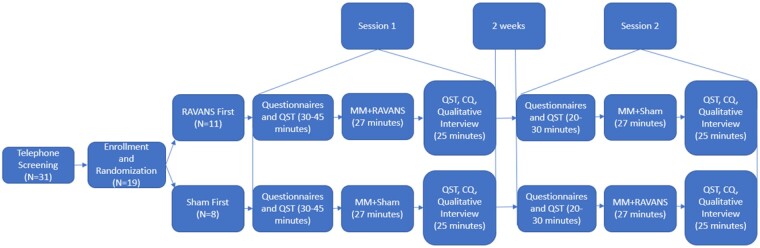
This study was a mixed-methods pilot crossover study. All participants attended two in-person sessions at the Brigham and Women’s Hospital Pain Research Lab allowing for assessment of feasibility, acceptability, and preliminary efficacy for RAVANS paired with audio-guided MM. The study was approved by the BWH Institutional Review Board and is registered on ClinicalTrials.gov (NCT04053127). Participants were first screened by study staff via telephone and scheduled for their first laboratory visit. Participants were asked to refrain from taking any analgesic medication, including opioid medication, in the 2 hours prior to their laboratory visits. Upon arrival to the laboratory and following informed consent, participants were randomized to receive either RAVANS or sham stimulation during the first session. During each session, participants first completed psychosocial and pain-related questionnaires followed by a brief, baseline QST session. Participants then underwent either RAVANS or sham stimulation paired with MM. Following the stimulation, participants immediately underwent another round of QST, completed the Credibility Questionnaire (CQ), and a brief qualitative interview. After a 2-week washout period, participants returned to complete the same procedures and received the alternate stimulation condition. All participants were blinded to RAVANS versus sham stimulation allocation. See Figure 1 for study schematic.
Figure 1.
Study schematic for study flow.
Respiratory-Gated Auricular Vagus Afferent Nerve Stimulation (RAVANS)
During the combined intervention, participants were seated in a reclining chair. They were asked to remove any earrings and glasses and were instructed that they may sit upright or recline. Signa-gel was applied to two custom-built, ergonomically shaped auricular electrodes to ensure good electrical contact (Bionik Medical Devices, Bucaramanga, Colombia), which were then placed into the cymba concha of the left ear—the auricular region rich in vagal innervation [29]. Electrical stimulation to the electrodes was provided by a current-constant stimulator (Urostim, Schwa-Medico GmbH, Ehringshausen, Germany). Simulation was gated, with a 0.1-second delay, after peak inhalation (i.e., during exhalation). Respiratory gating required real-time evaluation of the respiratory cycle. To do so, we placed a pneumatic belt around the participant’s lower thorax. Low-compliance tubing connected the belt to a pressure transducer (PX138-03D5V, Omegadyne, Inc., Sunbury, OH, USA) thereby producing voltage output that corresponded to changes in respiratory volume. The voltage signal from the transducer was acquired by a laptop-controlled device (National Instruments DAQ USB-6009, 14 bit i/o, with Labview 7.0 data acquisition software, Austin, TX, USA). Detection of peak inspiration and expiration occurred in real time and a TTL signal was send to a miniature high-frequency relay (G6Z-1P-DC5, Omron Electronics Components, Schaumburg, IL) to open the gate and allow electrical stimulation to pass to the subject (see Figure 2).
Figure 2.
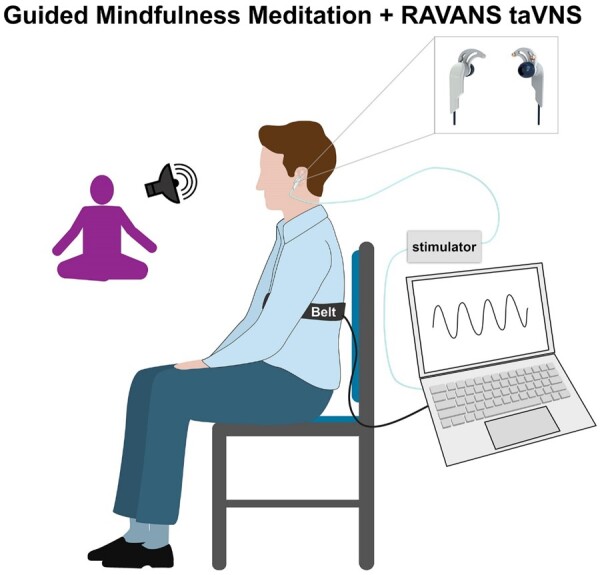
Schematic of RAVANS taVNS combined with audio-guided mindfulness meditation setup.
Verum Condition
Once electrodes were set up, participants were asked to rate the (non-noxious) stimulation intensity on a 0 to 10 NRS where 0 indicates no sensation and 10 indicates on the verge of being a painful stimulation. Stimulation intensity was set to achieve moderate-to-strong, but not painful, sensation (NRS target of 5/10).
Sham Condition
During the sham condition, electrodes were placed in the ear, but the stimulator was NOT operational. Instead, subjects were instructed that during this trial, stimulation was set to a predetermined level and that they may or may not feel the stimulation but that they should indicate if the stimulus is painful. This procedure was used to ensure patients remained blinded to condition.
Mindfulness Meditation
During the stimulation sessions, participants were instructed to listen to and follow along with a 27-minute guided MM audio recording that encouraged mindfulness of their breath as well as the sensations they were experiencing from the stimulation device. Participants were encouraged to remain awake during this meditation but to remain still and not to talk until the intervention was complete.
Quantitative Sensory Testing
We assessed mechanical pain using three weighted pinprick stimulators (64, 128, and 256 mN; MRC Systems GmbH, Heidelberg, Germany). Each stimulator was applied for one second to the skin on the dorsum of the third phalange between the second and third knuckle. Participants were asked to rate pain of each stimulator application using a 0–100 NRS ranging from no pain to worst pain imaginable. These pain ratings were averaged to determine the mean pain intensity rating for each probe [6]. After a 60 second washout period, the lowest-force stimulator that produced a sensation of pain (at least 10/100 on the NRS) was used to apply a train of 10 stimuli at a rate of one per second. Participants rated the painfulness of the first, fifth, and tenth stimulus using the same 0–100 NRS [30]. Temporal summation was calculated by subtracting the pain rating of the first stimulus from that of the tenth.
We assessed pressure pain thresholds by applying mechanical force to the metacarpophalangeal joint of the thumb. Using a pressure algometer with a 0.5-cm2 probe, pressure was applied and increased at a steady rate of 30 kPa/s until the participant indicated the pressure was first perceived as painful [31]. This was repeated twice and averaged across trials.
Self-Report Questionnaire Measures
The Pain Catastrophizing Scale (PCS) is a 13-item self-report measure of rumination, magnification, and helplessness about pain [32]. Participants were instructed to rate how frequently they endorsed various thoughts and experiences related to pain ranging from 0 (not at all) to 4 (all the time). Scores on the PCS range from 0 to 52 with higher scores indicative of greater pain catastrophizing. The PCS has strong reliability and validity [33].
The Freiberg Mindfulness Inventory (FMI) is a 14-item self-report measure of trait-level mindfulness [34]. Participants were instructed to rate how frequently they engage in mindful experiences from 1 (rarely) to 4 (almost always). Scores range from 14 to 56 with higher scores indicating greater mindfulness.
The 29-item Patient Reported Outcomes Measurement Information System (PROMIS-29) is a self-reported measure of health-related quality of life [35]. It includes four items across each of seven domains (i.e., depression, anxiety, physical functioning, fatigue, sleep disturbance, social functioning, and pain interference) rated on five-point scales. Additionally, there is a single 11-point NRS for pain intensity (o, no pain, to 10, worst imaginable pain). Scores for each of the domains are summed and then converted to t-scores with greater numbers indicative of greater amounts of the construct. The Pain intensity rating is reported without t-score transformation. The PROMIS-29 has been shown to be reliable and valid among patients with chronic pain [36].
The Credibility Questionnaire (CQ) is a five -item self-report measure of treatment credibility and expectancy for use in clinical outcome studies [37]. Participants were asked to rate their perceptions of the credibility of the treatment (e.g., its ability to treat and relieve back pain) from 0 (definitely) to 5 (definitely not) across four items and to rate the expectations for the treatment’s success on a four-point scale (1 = very successful to 4 = not at all successful). Scores were averaged across all five items.
Back pain severity was assessed at the onset of each round of QST. Participants were asked to rate the current severity of their back pain using a 0 (not at all painful) to 100 (worst pain imaginable) NRS.
Qualitative Interviews
Each participant was interviewed briefly following both RAVANS and sham stimulation. During the interviews, researchers asked a series of structured but open-ended questions assessing participants’ perceptions of the stimulation experience. Participants were asked their general impressions of the device, what they liked and did not like about the device, and how likely they would be to use the device in the future to relieve their pain. In addition to the structured questions, researchers were encouraged to follow up for clarification or to obtain more detailed responses from participants. All responses were recorded verbatim.
Data Analysis
Descriptive statistics were computed to characterize the sample and to determine feasibility and acceptability. To compare credibility of the RAVANS and sham stimulation trials, we conducted a repeated measures t-test comparing CQ scores between conditions. In order to evaluate acceptability, we also reviewed transcripts of qualitative interviews to identify both positive and negative feedback following both RAVANS and sham sessions.
The relationship between stimulation condition (i.e., RAVANS vs. sham) and time (i.e., pre-stimulation vs. post-stimulation) was examined for each QST outcome as well as for back pain severity ratings using two (Stimulation) × two (Time) repeated measures ANOVAs (RMANOVAs). Both main and interaction effects were examined. Significance levels were set at P < .05.
To examine potential moderators of the differences in improvement across pain and QST variables, we used the MEMORE SPSS macro with 5,000 bootstrapped samples and 95% confidence intervals (CI) [38]. The MEMORE macro assesses moderation in repeated measures designs by calculating the difference scores between the within-subjects variables (e.g., stimulation condition) and regressing them onto the between-subjects variable (e.g., back pain severity and QST outcomes) allowing for probing the interaction in repeated measures designs. Moderation is indicated when the moderator predicts the difference score between the instances of the within-subjects variable. We included depression, anxiety, pain catastrophizing, and mindfulness as potential moderators as these variables have been shown to be related to treatment-related changes in pain outcomes [39].
Results
Of the 31 individuals screened via telephone for this study, 25 were eligible. Individuals were ineligible due to having non-removable hearing aids (N = 1), severe cardiac disease (N = 2), epilepsy (N = 1), and ear damage precluding electrode placement (N = 2). An additional six participants did not attend their first scheduled laboratory session. A total of 19 participants attended and were enrolled in the study. Of these, all 19 completed both sessions of the study (i.e., zero dropouts). Participants were mostly White (N = 13), non-Hispanic (N = 15), and female (N = 12). Their mean age was 54 years (SD = 16; see Table 1). All participants had completed a high school education, with more than half graduating college or technical school (N = 10). Only three participants were employed, and more than two thirds were receiving disability compensation (N = 13). Approximately half of the sample (N = 10) reported a household income under $22,500. Nearly half of participants had undergone at least one spine surgery (N = 8). All but one reported their back pain had lasted 5 years or more. All participants were taking opioid medications with a mean daily milligram morphine equivalence of 117 (SD = 152; range 9–600). Participants completed two study visits spaced approximately 14 days apart (M = 18.9 days; range =13–35 days).
Table 1.
Participant characteristics
| N (%) or M (SD) | |
|---|---|
| Age | 54 (16) |
| Sex | 7 (37) |
| Male | 7 (37) |
| Female | 12 (63) |
| Race | |
| White | 13 (68) |
| Black | 1 (5.3) |
| Other/Prefer not to answer | 5 (26) |
| Ethnicity | |
| Hispanic | 1 (5) |
| Non-Hispanic | 15 (79) |
| Prefer not to answer | 3 (16) |
| Education | |
| High school graduate/GED | 9 (47) |
| College/technical school graduate | 10 (53) |
| Employment | |
| Employed | 3 (16) |
| Unemployed | 16 (84) |
| Disability | |
| Disability compensation | 6 (32) |
| No disability compensation | 13 (68) |
| Income | |
| <$22,500 | 10 (53) |
| $22,501–45,000 | 2 (10) |
| $45,001–100,000 | 3 (16) |
| >$100,000 | 3 (16) |
| Prefer not to answer | 1 (5) |
| Spine surgeries | |
| None | 11 (58) |
| One or more | 8 (42) |
| Back pain duration | |
| <5 years | 1(5) |
| 5+ years | 18 (95) |
| Depression | 58.1 (8.2) |
| Anxiety | 58.4 (11.1) |
| Pain interference | 63.3 (3.7) |
| Pain intensity | 6.0 (1.5) |
| Catastrophizing | 17.8 (11.8) |
| Mindfulness | 10.1 (7.1) |
| Opioid dose (mg morphine equivalent; MME) | 116.5 (152.3) |
All participants tolerated the RAVANS and QST procedures. The average electrical current intensity used for RAVANS stimulation (M = 7.03 mA; SD = 1.01) produced a moderate sensation. Results of a paired-samples t-test comparing credibility for RAVANS versus sham indicated no significant difference [t (18) = −0.264; P = .80) between stimulation conditions. Average credibility (CQ) scores, ranging from 0 (definitely) to 4 (definitely not), for both groups neared 2, indicating “probable credibility”, for both RAVANS (M = 1.96, SD = 0.94) and sham (M = 1.99, SD = 1.06). Of the 19 participants, 15 participants rated the RAVANS device positively (CQ <3), three were unsure (CQ = 3), and one did not believe in the ability of the device to improve back pain. Similarly, 14 participants rated the sham device positively, four were unsure, and one did not believe in its ability to improve back pain.
Participants also provided qualitative feedback regarding both the RAVANS and sham sessions. Regarding the RAVANS session, most participants (N = 16) were quite happy with the results. They indicated that (P#1) “It’s a cool device,” (P#13) “I found it relaxing…I liked how it reduced my pain” (P#15) “It was so different and I couldn’t believe it actually worked. It totally alleviated the pain,” (P#2) “Initially I didn’t think it would work but I’m happily surprised how well it works.” However, a couple participants (N = 2) indicated that it was uncomfortable [e.g., (P#24) “It was annoying, I wanted to rip it out,”) and one indicated that it did not improve pain but denied any discomfort during use. Regarding the sham session, a few participants noted they did not feel any stimulation (N = 3; two underwent the sham session first, one underwent sham session second) while most others indicated their satisfaction (N = 15): (P#2) “It significantly reduced back pain; I’m still pain free,” (P#4) “It was relaxing; it reduces the time you feel pain, takes your mind off the pain,” (P#20) “It really calmed my mind and pain and breathing, emotionally, mentally and physically.” Two participants also expressed discomfort with the sham stimulation, e.g., (P#5) “The more I relaxed the more uncomfortable it got, the stimulation increased and was uncomfortable.”
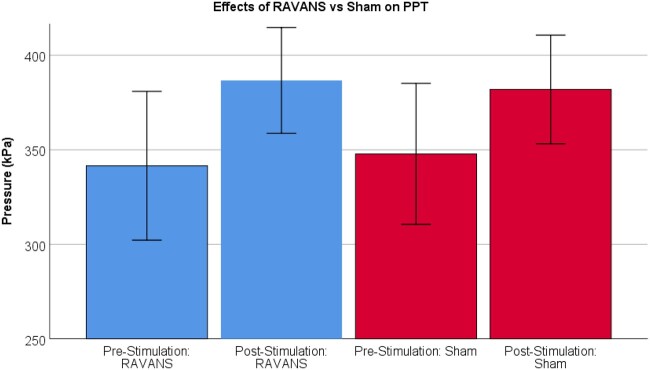
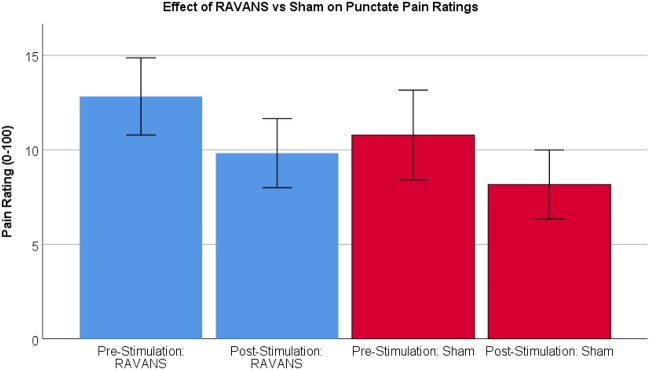
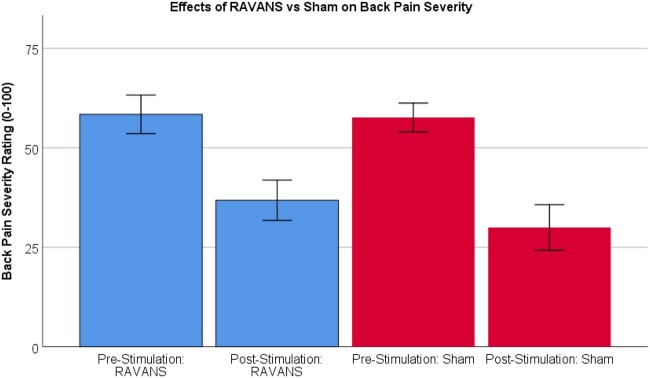
Results of RMANOVAs varied across outcomes (Tables 2–5). For pressure pain threshold (PPT) on the thumb, there was a significant effect of Time such that PPT was higher post-intervention (M = 384.30, SE = 26.45; see Figure 3) compared to pre-intervention (M = 344.68, SE = 36.47), regardless of Stimulation condition (F = 9.48, P = .01). There were no differences between RAVANS and sham conditions (F < 0.01, P = .97) nor was there a Time × Stimulation interaction (F = 0.30, P = .59). For mean punctate pain ratings, there was a significant main effect of Time such that participants rated probes as less painful following intervention (M = 9.47, SE = 1.96) compared to pre-intervention (M = 11.33, SE = 1.86), regardless of Stimulation condition (F = 7.39, P = .01; see Figure 4). There were no effects of Stimulation (F = 1.05, P = .32) nor an interaction (F = 0.05, P = .83) for mean punctate pain. For temporal summation of punctate pain, there were no main effects of Time or Stimulation (F = 0.62, P = .44; F = 0.14, P = .72; respectively). There were also no interaction effects (F = 0.43, P = .52). For back pain severity ratings, there was a significant main effect of Time such that all participants showed a lower back pain rating following the intervention (M = 33.42, SE = 4.96) compared to pre-intervention (M = 58.03, SE = 3.69), regardless of Stimulation condition (F = 44.62, P < .01; see Figure 5). There were no effects of Stimulation (F = 1.26, P = .28) nor any interaction effects (F = 1.29, P = .27).
Table 2.
Results of stimulation X time repeated measures ANOVA for pressure pain threshold
| Effect | M (SE) | F | P | Eta2 |
|---|---|---|---|---|
| Time | 9.48 | .01 | 0.35 | |
| Pre-stim | 344.68 (36.47) | |||
| Post-stim | 384.30 (26.45) | |||
| Stimulation | <0.01 | .97 | <0.01 | |
| RAVANS | 364.11 (32.81) | |||
| Sham | 364.88 (32.65) | |||
| Time × Stimulation | 0.30 | .59 | 0.02 |
Table 3.
Results of stimulation × time repeated measures ANOVA for mean punctate pain rating
| Effect | M (SE) | F | P | Eta2 |
|---|---|---|---|---|
| Time | 7.39 | .01 | 0.29 | |
| Pre-stim | 11.80 (1.91) | |||
| Post-stim | 9.00 (1.59) | |||
| Stimulation | 1.05 | .32 | 0.06 | |
| RAVANS | 11.33 (1.86) | |||
| Sham | 9.47 (1.96) | |||
| Time × Stimulation | 0.05 | .83 | <0.01 |
Table 4.
Results of stimulation X time repeated measures ANOVA for temporal summation of punctate pain
| Effect | M (SE) | F | P | Eta2 |
|---|---|---|---|---|
| Time | 0.62 | .44 | 0.03 | |
| Pre-stim | 16.57 (2.33) | |||
| Post-stim | 18.71 (3.30) | |||
| Stimulation | 0.14 | .42 | 0.01 | |
| RAVANS | 17.17 (2.31) | |||
| Sham | 18.11 (3.23) | |||
| Time × Stimulation | 0.43 | .52 | 0.02 |
Table 5.
Results of stimulation X time repeated measures ANOVA for back pain severity
| Effect | M (SE) | F | P | Eta2 |
|---|---|---|---|---|
| Time | 44.62 | <.01 | 0.71 | |
| Pre-stim | 58.03 (3.69) | |||
| Post-stim | 33.42 (4.96) | |||
| Stimulation | 1.26 | .28 | 0.07 | |
| RAVANS | 47.63 (4.65) | |||
| Sham | 43.82 (3.96) | |||
| Time × Stimulation | 1.29 | .27 | 0.07 |
Figure 3.
Results of 2 (Time) × 2 (Stimulation) ANOVA demonstrating main effect of time such that MM+Stimulation resulted in greater Pressure Pain Thresholds (PPT), regardless of stimulation condition (P < .01).
Figure 4.
Results of 2 (Time) × 2 (Stimulation) ANOVA demonstrating main effect of time such that MM+Stimulation resulted in lower Mean Punctate Pain ratings, regardless of stimulation condition.
Figure 5.
Results of 2 (Time) × 2 (Stimulation) ANOVA demonstrating main effect of time such that MM+Stimulation resulted in lower Back Pain Severity ratings, regardless of stimulation condition (P < .01).
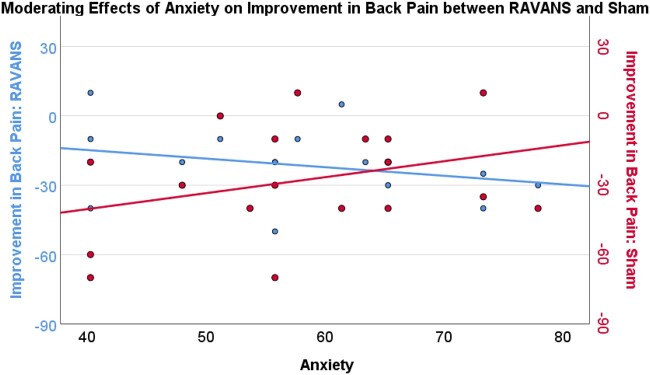
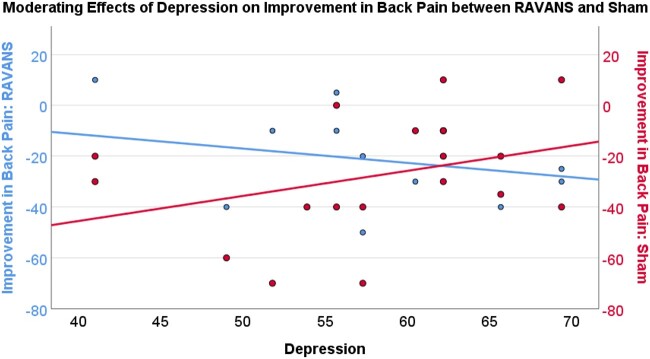
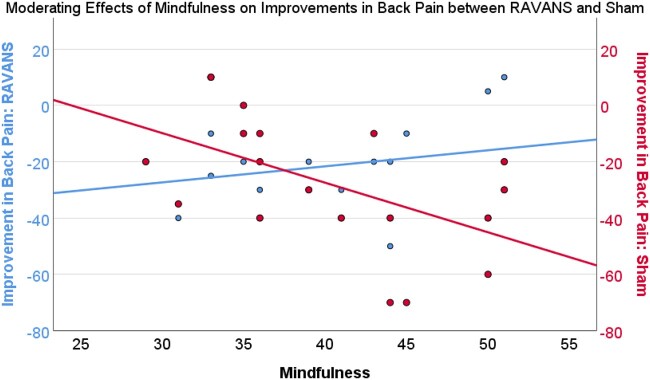
Results of moderation analyses indicated that depression severity (PROMIS-depression) moderated the difference between improvement in back pain in the RAVANS and sham conditions. That is, greater depression severity was associated with greater improvement in back pain severity in the RAVANS condition and less improvement in the sham condition (effect = −1.55, SE = 0.58, P = .02, 95% CI = −2.76 to −0.32; see Figure 6). Similarly, greater anxiety severity (PROMIS-anxiety) moderated the difference between improvement in back pain in the RAVANS and sham conditions such that greater anxiety severity was associated with greater improvement in back pain severity in the RAVANS condition and less improvement in the sham condition (effect=−1.06, SE = 0.43, P = .03, 95% CI= −1.97 to −0.14; see Figure 7). On the other hand, mindfulness significantly moderated the difference between improvement in back pain severity in the RAVANS and sham conditions such that greater trait mindfulness, as measured by the Freiberg Mindfulness Inventory, was associated with greater improvement in back pain severity in the sham condition and less improvement in the RAVANS condition (effect = 2.33, SE = 0.55, P < .01, 95% CI = 1.16–3.50; See Figure 8). There was no moderating effect of catastrophizing on the difference between improvement in back pain in the RAVANS and sham conditions, though the direction of the statistical trend was similar to that for the moderating effects of depression and anxiety (effect=−0.89, SE = 0.44, P = .06, 95% CI = −1.83 to 0.04). There were no significant moderators of the difference between change in pressure pain thresholds, average punctate pain ratings, or temporal summation of pain in the RAVANS and sham conditions (P > .05).
Figure 6.
Anxiety (PROMIS-29) moderates improvement in Back Pain Severity between RAVANS and Sham Stimulation such that those with greater anxiety showed a greater improvement in back pain severity from MM+RAVANS compared to MM+Sham stimulation (P < .05).
Figure 7.
Depression (PROMIS-29) moderates improvement in Back Pain Severity between RAVANS and Sham Stimulation such that those with greater depression showed a greater improvement in back pain severity from MM+RAVANS compared to MM+Sham stimulation (P < .05).
Figure 8.
Trait Mindfulness (Freiberg) moderates improvement in Back Pain Severity between RAVANS and Sham Stimulation such that those with greater trait mindfulness showed a greater improvement in back pain severity from MM+Sham compared to MM+RAVANS stimulation (P < .01).
Discussion
Results of this mixed-methods, pilot acceptability and feasibility trial indicate that participants not only tolerated the RAVANS device well, but generally rated the device positively and reported finding it relaxing and helpful in alleviating pain. Although there were no differences in pain outcomes between mindfulness supported RAVANS and sham conditions, participants demonstrated strongly reduced back pain severity and pain sensitivity after only a single session of both MM+RAVANS and MM+sham stimulation. The 42% reduction in back pain intensity in this group of patients on long-term opioid therapy for moderate-to-severe chronic back pain is likely to be quite clinically significant, as supported by the positive descriptions of the treatment during qualitative interviews with the participants. Furthermore, we found that those with greater levels of negative affect (i.e., depression and anxiety) showed greater improvement in back pain severity from mindfulness-supported verum RAVANS compared to sham stimulation.
In this pilot study we sought to assess the acceptability and feasibility of the RAVANS device both quantitatively and qualitatively. Not only did all participants tolerate the RAVANS device, but most described it positively indicating that they “found it relaxing” and that it “alleviated the pain.” Similar descriptions were provided during the sham sessions. Moreover, there were no differences in the credibility ratings provided between the verum RAVANS and sham stimulation. Together, this suggests it is not only feasible to continue studying RAVANS among patients with chronic pain but that clinically, this treatment is acceptable to patients.
Results of this study indicated that, regardless of RAVANS condition, back pain severity decreased and pressure pain thresholds increased following a single MM + “stimulation” session. Although this is contrary to our hypothesis about a synergistic effect of MM and RAVANS, it is possible that the analgesic effect of MM may have overshadowed any effect of RAVANS. This may have occurred for several reasons. First, given the brief single session design of this study, it is possible the RAVANS stimulation was not potent enough to produce a significant effect after a single stimulation session. Indeed, other tVNS studies have used a longer course of treatment over multiple weeks. For example, in a study examining electrical stimulation of the auricular branch of the vagal nerve for CLBP and cervical pain, participants received stimulation for 48 hours, once per week for 6 weeks [40, 41]. Similarly, in a study of people with migraine, participants received tVNS for 4 hours per day over 3 months [42]. Given the substantial benefits for current back pain intensity produced by a single treatment session in the present study (i.e., approximately 42% reduction in current back pain), it is exciting to contemplate that longer-term MM+RAVANS treatment may provide enhanced and durable improvements. Future studies should examine longer courses of MM+RAVANS treatment to further elucidate possible synergistic effects. It is also possible that the concomitant use of opioids by this sample may have blunted the potential synergistic effects of MM+RAVANS. It is possible that the effects of these combined therapies may have a greater effect in patients who are not using opioid medications to manage their pain. Thus, future studies should examine the possible synergistic effects of MM+RAVANS among patients not on long-term opioid therapy.
Another possibility for the lack of difference between RAVANS and sham conditions is that there was a ceiling effect of MM. Because participants had experienced 8 weeks of prior mindfulness training and, in our study, performed MM regardless of stimulation condition, they may have received so much benefit from the MM that they could not have experienced additional analgesia from RAVANS (e.g., a ceiling effect). This hypothesis is indeed supported by our moderation findings indicating that individuals with higher levels of mindfulness demonstrated less improvement in back pain in the RAVANS condition and greater improvement in the sham condition. It is possible that those participants who benefitted most from prior MM training (i.e., demonstrated the greatest trait-level mindfulness) may have paradoxically been distracted by the sensation of RAVANS and therefore found less benefit relative to the sham condition (in which presumably they would be less distracted by stimulation). However, it is also possible that those with high levels of trait mindfulness prefer other types of mindfulness meditation (e.g., body scan, loving kindness) rather than mindful breathing, and this preference impacted our results. Thus, RAVANS may prove to be more facilitatory during the learning process of mindfulness training, rather than after the training is completed.
We also found that patients with higher levels of negative affect (i.e., depression and anxiety) demonstrated greater benefit from the RAVANS stimulation while those with lower levels of negative affect had a greater benefit in the sham condition for back pain severity. In fact, vagal nerve stimulation, including TVNS has been shown to improve depressive and anxiety symptoms for psychiatric and chronic pain disorders [24, 43]. As negative affect is a strong predictor of back pain [44, 45], it is possible that high levels of negative affect contributed to back pain severity in our sample. Although the moderating effect of catastrophizing, a pattern of negative thoughts common among individuals with CLBP, depression and anxiety [46–48], did not reach significance, we observed a trend in the same direction as that of depression and anxiety. Thus, MM+RAVANS may synergistically be helpful in improving negative affect and back pain. Importantly, anxiety and depression may make MM practice more difficult with increased room for benefit from parasympathetic modulation [49], so adjunctive parasympathetic tone provided by taVNS may help these patients tolerate the physiologic aspects of negative affect. In addition, depression can impact attention and cognitive control [50], so that the alignment of the sensations related to stimulation during exhalation may help those with difficulty with attention be able to achieve greater focus during mindfulness of breathing at the end of each exhalation cycle. However, future studies should examine possible mediating effects of changes in negative affect in driving the effects of MM+RAVANS on back pain severity, over a full course of therapy. We should note that high levels of negative affect are a robust risk factor for treatment non-response and for deleterious long-term outcomes in patients with chronic pain [39, 45]. The development and refinement of interventions that produce substantial benefits in these high-risk patients is of particular importance to the field, as this group does not seem to be optimally managed by current pharmacologic treatments.
Results of this study should be interpreted in the context of several limitations. First, this is a small, pilot single-session stimulation study of participants with previous MM training. A post-hoc power analysis indicated a sample of 30 participants would have been necessary to identify the interaction between time and stimulation. While we were underpowered, we believe it is multisession, longitudinal studies that are necessary to understand the potential synergistic effects of MM + RAVANS. Thus, not only should future studies aim to replicate these findings in larger, more diverse samples, but should examine the long-term outcomes of multiple RAVANS sessions using longitudinal designs. Moreover, all participants received MM while participating in this study, thereby limiting our ability to draw conclusions about the efficacy of the MM component of the intervention. It is also important to note that many of our participants were using opioid medications. While opioid use the day of the session may have impacted the effects of RAVANS and/or MM, this is a crossover study and patients were reportedly on a stable dose of medication, thus the effects of timing of opioid use should be negligible. Moreover, had participants withheld their opioid medications for more than two hours prior to the session, they would have been more likely to experience withdrawal symptoms. Additionally, though participants rated the sham condition as equally credible as the verum condition, we did not ask participants to guess the condition order. Lastly, we only included participants with CLBP. It is important to assess these nonpharmacologic interventions across various pain disorders, as the intervention may indeed show disease specificity.
Taken together, our results demonstrate that RAVANS-assisted MM is well tolerated and an acceptable and feasible non-pharmacologic intervention for CLBP. Although there were no differences between the RAVANS and sham conditions, MM + “stimulation” sessions resulted in improved back pain severity and pressure pain threshold demonstrating the powerful effect of mindfulness on pain outcomes. Moreover, individuals with greater negative affect showed greater improvement in back pain severity from MM+RAVANS while those with greater trait-level mindfulness show less benefit from MM+RAVANS, suggesting that MM+RAVANS may be especially beneficial for chronic pain patients suffering from high negative affect, anxiety or depression. Our study suggests that research needs not only to identify novel nonpharmacologic therapeutic interventions for CLBP but also to move toward precision and personalized medicine and identify characteristics of patients for whom these treatments work best.
Authors’ Contributions
S.M., V.N., and R.E. designed the study protocol with input from R.G., M.C., M.D., G.S., and G.D. S.M. and M.C. collected data. S.M., V.N., and R.E. carried out the analysis, interpretation of the data, and drafted the manuscript. All authors critically revised the manuscript for intellectual content and read and approved the final manuscript.
Acknowledgments
We would like to thank Lauren Papianou and Kathleen Dorado for their assistance with participant recruitment.
Contributor Information
Samantha M Meints, Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Chestnut Hill, Massachusetts, USA.
Ronald G Garcia, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Zev Schuman-Olivier, Center for Mindfulness and Compassion, Department of Psychiatry, Cambridge Health Alliance, Harvard Medical School, Cambridge, Massachusetts, USA.
Michael Datko, Center for Mindfulness and Compassion, Department of Psychiatry, Cambridge Health Alliance, Harvard Medical School, Cambridge, Massachusetts, USA; Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts, USA.
Gaelle Desbordes, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts, USA.
Marise Cornelius, Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Chestnut Hill, Massachusetts, USA.
Robert R Edwards, Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Chestnut Hill, Massachusetts, USA.
Vitaly Napadow, Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Chestnut Hill, Massachusetts, USA; Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts, USA.
Funding sources: United States National Institutes for Health (NIH), Office of the Director (OT2-OD023867 to V.N.); National Center for Complementary and Integrative Health (NCCIH), NIH (P01-AT009965, R61/R33-AT009306 to V.N.); and National Institute for Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIH (R01-AR064367 to R.R.E. and V.N.).
Conflicts of interest: Dr. Vitaly Napadow has a financial interest in Cala Health, a company developing wearable neuromodulation therapies to deliver individualized peripheral nerve stimulation. Dr. Napadow’s interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict of interest policies.
References
- 1. Shmagel A, Foley R, Ibrahim H.. Epidemiology of chronic low back pain in US adults: Data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res 2016;68(11):1688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mokdad AH, Ballestros K, Echko M, US Burden of Disease Collaborators, et al. The state of US health, 1990-2016: Burden of diseases, injuries, and risk factors among US states. JAMA 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299(6):656–64. [DOI] [PubMed] [Google Scholar]
- 4. Qaseem A, Wilt TJ, McLean RM, et al. ; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166(7):514–30. [DOI] [PubMed] [Google Scholar]
- 5. McPhee ME, Vaegter HB, Graven-Nielsen T.. Alterations in pronociceptive and antinociceptive mechanisms in patients with low back pain: A systematic review with meta-analysis. Pain 2020;161(3):464–75. [DOI] [PubMed] [Google Scholar]
- 6. Meints SM, Mawla I, Napadow V, et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain 2019;160(4):833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HJ, Lee JI, Kang KT, et al. Influence of pain sensitivity on surgical outcomes after lumbar spine surgery in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 2015;40(3):193–200. [DOI] [PubMed] [Google Scholar]
- 8. Davis MC, Zautra AJ.. An online mindfulness intervention targeting socioemotional regulation in fibromyalgia: Results of a randomized controlled trial. Ann Behav Med 2013;46(3):273–84. [DOI] [PubMed] [Google Scholar]
- 9. Wells RE, Burch R, Paulsen RH, Wayne PM, Houle TT, Loder E.. Meditation for migraines: A pilot randomized controlled trial. Headache 2014;54(9):1484–95. [DOI] [PubMed] [Google Scholar]
- 10. Fox SD, Flynn E, Allen RH.. Mindfulness meditation for women with chronic pelvic pain: A pilot study. J Reprod Med 2011;56(3-4):158–62. [PubMed] [Google Scholar]
- 11. Cherkin DC, Anderson ML, Sherman KJ, et al. Two-year follow-up of a randomized clinical trial of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care for chronic low back pain. JAMA 2017;317(6):642–4. [DOI] [PubMed] [Google Scholar]
- 12. Morone NE, Greco CM, Moore CG, et al. A mind-body program for older adults with chronic low back pain: A randomized clinical trial. JAMA Internal Medicine 2016;176(3):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuman-Olivier Z, Trombka M, Lovas DA, et al. Mindfulness and behavior change. Harvard Rev Psychiatry 2020;28(6):371–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeidan F, Grant J, Brown C, McHaffie J, Coghill R.. Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 2012;520(2):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeidan F, Emerson NM, Farris SR, et al. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J Neurosci 2015;35(46):15307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC.. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 2011;31(14):5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirchner A, Birklein F, Stefan H, Handwerker H.. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology 2000;55(8):1167–71. [DOI] [PubMed] [Google Scholar]
- 18. Kirchner A, Stefan H, Bastian K, Birklein F.. Vagus nerve stimulation suppresses pain but has limited effects on neurogenic inflammation in humans. Eur J Pain 2006;10(5):449–55. [DOI] [PubMed] [Google Scholar]
- 19. Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P.. The effect of transcutaneous vagus nerve stimulation on pain perception—an experimental study. Brain Stimul 2013;6(2):202–9. [DOI] [PubMed] [Google Scholar]
- 20. Johnson M, Hajela V, Ashton C, Thompson J.. The effects of auricular transcutaneous electrical nerve stimulation (TENS) on experimental pain threshold and autonomic function in healthy subjects. Pain 1991;46(3):337–42. [DOI] [PubMed] [Google Scholar]
- 21. Garcia RG, Lin RL, Lee J, et al. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) in migraine patients. Pain 2017;158(8):1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sclocco R, Garcia RG, Kettner NW, et al. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimul 2019;12(4):911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sclocco R, Garcia RG, Kettner NW, Fisher HP, et al. Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimul 2020;13(4):970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Napadow V, Edwards RR, Cahalan CM, et al. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med 2012;13(6):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frangos E, Richards EA, Bushnell MC.. Do the psychological effects of vagus nerve stimulation partially mediate vagal pain modulation? Neurobiol Pain 2017;1:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dworkin RH, Turk DC, Peirce-Sandner S, et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain 2012;153(6):1148–58. [DOI] [PubMed] [Google Scholar]
- 27. Zgierska AE, Burzinski CA, Cox J, et al. Mindfulness meditation and cognitive behavioral therapy intervention reduces pain severity and sensitivity in opioid-treated chronic low back pain: Pilot findings from a randomized controlled trial. Pain Med 2016;17(10):1865–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krahl SE, Clark KB.. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg Neurol Int 2012;3(suppl 4):S255–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peuker ET, Filler TJ.. The nerve supply of the human auricle. Clin Anatomy 2002;15(1):35–7. [DOI] [PubMed] [Google Scholar]
- 30. Martel MO, Petersen K, Cornelius M, Arendt-Nielsen L, Edwards R.. Endogenous pain modulation profiles among individuals with chronic pain: Relation to opioid use. J Pain 2019;20(4):462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN.. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain 2011;12(9):953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sullivan MJ, Bishop SR, Pivik J.. The pain catastrophizing scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 33. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'neill E.. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997;20(6):589–605. [DOI] [PubMed] [Google Scholar]
- 34. Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S.. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI). Personal Individ Diff 2006;40(8):1543–55. [Google Scholar]
- 35. Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khutok K, Janwantanakul P, Jensen MP, Kanlayanaphotporn R.. Responsiveness of the PROMIS-29 scales in individuals with chronic low back pain. Spine 2021;46(2):107–13. [DOI] [PubMed] [Google Scholar]
- 37. Borkovec TD, Nau SD.. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry 1972;3(4):257–60. [Google Scholar]
- 38. Montoya AK. Moderation analysis in two-instance repeated measures designs: Probing methods and multiple moderator models. Behav Res Methods 2019;51(1):61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meints S, Edwards R.. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuro-Psychopharmacol Biol Psychiatry 2018;87(Pt B):168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sator-Katzenschlager SM, Scharbert G, Kozek-Langenecker SA, et al. The short-and long-term benefit in chronic low back pain through adjuvant electrical versus manual auricular acupuncture. Anesth Analg 2004;98(5):1359–64. [DOI] [PubMed] [Google Scholar]
- 41. Sator-Katzenschlager SM, Szeles JC, Scharbert G, et al. Electrical stimulation of auricular acupuncture points is more effective than conventional manual auricular acupuncture in chronic cervical pain: A pilot study. Anesth Analg 2003;97(5):1469–73. [DOI] [PubMed] [Google Scholar]
- 42. Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R.. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): A randomized, monocentric clinical trial. J Headache Pain 2015;16(1):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Groves DA, Brown VJ.. Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev 2005;29(3):493–500. [DOI] [PubMed] [Google Scholar]
- 44. Jamison RN, Edwards RR, Liu X, et al. Relationship of negative affect and outcome of an opioid therapy trial among low back pain patients. Pain Pract 2013;13(3):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards R, Dolman A, Michna E, et al. Changes in pain sensitivity and pain modulation during oral opioid treatment: The impact of negative affect. Pain Med 2016;17(10):1882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Granot M, Ferber SG.. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain 2005;21(5):439–45. [DOI] [PubMed] [Google Scholar]
- 47. Meints SM, Edwards RR, Gilligan C, Schreiber KL.. Behavioral, psychological, neurophysiological, and neuroanatomic determinants of pain. JBJS 2020;102(Suppl 1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Edwards RR, Cahalan C, Calahan C, Mensing G, Smith M, Haythornthwaite JA.. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7(4):216–25. [DOI] [PubMed] [Google Scholar]
- 49. Mankus AM, Aldao A, Kerns C, Mayville EW, Mennin DS.. Mindfulness and heart rate variability in individuals with high and low generalized anxiety symptoms. Behav Res Ther 2013;51(7):386–91. [DOI] [PubMed] [Google Scholar]
- 50. Grahek I, Shenhav A, Musslick S, Krebs RM, Koster EHW. Motivation and Cognitive Control in Depression. bioRxiv2018:500561. [DOI] [PMC free article] [PubMed]