Abstract
Salmonella pathogenicity island 2 (SPI-2) encodes a putative, two-component regulatory system, SsrA-SsrB, which regulates a type III secretion system needed for replication inside macrophages and systemic infection in mice. The sensor and regulator homologs, ssrAB (spiR), and genes within the secretion system, including the structural gene ssaH, are transcribed after Salmonella enters host cells. We have studied the transcriptional regulation of ssrAB and the secretion system by using gfp fusions to the ssrA and ssaH promoters. We found that early transcription of ssrA, after entry into macrophages, is most efficient in the presence of OmpR. An ompR mutant strain does not exhibit replication within cultured macrophages. Furthermore, footprint analysis shows that purified OmpR protein binds directly to the ssrA promoter region. We also show that minimal medium, pH 4.5, induces SPI-2 gene expression in wild-type but not ompR mutant strains. We conclude that the type III secretion system of SPI-2 is regulated by OmpR, which activates expression of ssrA soon after Salmonella enters the macrophage.
Salmonella enterica serovar Typhimurium is a facultative intracellular bacterium that generally causes gastroenteritis in humans and a typhoid fever-like disease in mice. During the course of infection in mice, serovar Typhimurium colonizes many different organs, including the Peyer's patches of the small intestine, mesenteric lymph nodes, spleen, and liver, where it is found in both extracellular and intracellular locations (23, 45, 71). The ability of Salmonella to replicate inside cultured cell lines has been linked to virulence in mice (22, 49). Salmonella flourishes inside host macrophages in specialized vacuolar compartments and resists oxygen radicals, defensins, and cationic antimicrobial peptides. Salmonella-containing vacuoles (also called phagolysosomes) have an acidic pH and low concentrations of Fe2+ and Mg2+ (28, 29, 69, 70). Once inside the host cell, Salmonella induces expression of over 30 proteins as determined by two-dimensional gel electrophoresis (1, 9). In vitro studies that mimic the vacuolar environment reveal that Salmonella has a network of coordinately regulated genes to counter the hostile intracellular environment (4, 34, 55).
Pleiotropic regulators of Salmonella virulence genes include rpoS, phoP, and ompR; mutations in any of these render Salmonella avirulent (14, 19, 21, 57). rpoS encodes an alternative sigma factor that regulates genes expressed during stationary phase in response to nutrient deprivation (53, 65). RpoS regulates the spv genes carried on a plasmid essential for Salmonella virulence in mice (21, 47, 63). However, Salmonella rpoS mutants are reported to replicate normally inside macrophages (10, 62). Both phoP and ompR encode elements of bacterial two-component regulatory systems (also called sensor-kinase systems), PhoP-PhoQ and OmpR-EnvZ, respectively. PhoP-PhoQ regulates over 40 genes in Salmonella; only a subset of these is involved in virulence (30, 56, 58). The PhoP-PhoQ system responds to environmental Mg2+ concentrations (29). A phoP mutant strain of Salmonella is defective for survival inside macrophages (9, 32, 57). OmpR-EnvZ has been studied primarily in Escherichia coli, where it was first discovered to respond to changes in osmolarity and to regulate expression of the porin proteins OmpF and OmpC (2, 68). Acidified medium also affects transcription of ompC and ompF, independent of osmolarity (38, 77). Salmonella also has the ompR and envZ operon (50, 51). In Salmonella, the OmpR-EnvZ system regulates ompC and ompF, as well as the genes encoding tripeptide permease (tppB), Salmonella-induced filaments (sifA) in HeLa cells, and 2-acylglycerolphosphoethanolamine acyltransferase (aas), which is induced under acidic conditions and inside macrophages (31, 60, 80, 81). Mutations in ompF, ompC, tppB, or aas have no effect on virulence, whereas ompF ompC double mutants or sif mutants are partially attenuated for virulence in mice (11, 19, 76; R. H. Valdivia, M. Rathman, and S. Falkow, unpublished data). In addition, a Salmonella ompR mutant does not kill macrophages in vitro (52).
Serovar Typhimurium has two pathogenicity islands (SPI-1 and SPI-2) that code for type III secretion systems (25, 59, 64, 74). (For reviews of type III secretion systems, see references 43 and 54.) SPI-1 mutants are defective for virulence in mice when the bacteria are administered orally, not intraperitoneally, suggesting that SPI-1 has a role early in infection (26). SPI-1 promotes bacterial invasion of the M cells in the small intestine and is essential for apoptosis (13, 46, 59, 61). InvF and HilA are transcriptional regulators of SPI-1 genes that are encoded within SPI-1; hilA itself is regulated by several factors, including PhoP-PhoQ, SirA, HilC, and HilD (3–5, 20, 37, 44, 66, 72).
SPI-2 genes are required for systemic infection, since SPI-2 mutants are avirulent in mice infected orally, intraperitoneally, or intravenously (35, 64, 74). SPI-2 has been characterized by several groups as essential for replication inside macrophages (12, 36, 64). It has been suggested that changes in Mg2+ concentration and pH in growth media also affect Spi2 gene expression (6, 16). Little is known about the genetic regulation of SPI-2 except that expression of genes encoding the type III secretion system of SPI-2 is dependent on a sensor-kinase system, SsrA-SsrB (also called SpiR) (12, 16, 36, 64). The ssrAB operon is also encoded within SPI-2 (64, 74). ssrA is the first gene of the operon, and its product is a sensor homolog belonging to the BvgS family of cytoplasmic-membrane sensor proteins (79). ssrB is a response regulator with a receiver domain and a helix-turn-helix DNA binding domain (16).
Plasmid-encoded promoter fusions to the gene for green fluorescent protein (GFP) have shown that transcription of SPI-2 genes, including ssrA and ssaH, is induced inside macrophages (12). ssaH encodes a structural gene of the type III secretion system (81). Transcription from the ssaH promoter, but not the ssrA promoter, is abolished in an ssrA mutant strain after entry into cultured macrophages (12). The activation of the ssrA promoter in an ssrA mutant strain suggests that the two-component regulatory system of the type III secretion system itself is induced by a different regulator. We report here that OmpR positively regulates the SsrA-SsrB two-component regulatory system by directly binding to the ssrA promoter region. These studies show that in Salmonella, the global regulator, OmpR, plays a major role in coordinating gene expression upon entry into the host cell.
MATERIALS AND METHODS
Growth conditions of bacterial strains and cells.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown standing at 37°C overnight in Luria-Bertani (LB) broth (optical density at 600 nm [OD600], ∼0.4) unless otherwise stated. The LB broth and M9 (without CaCl2) recipes have been published elsewhere (2a). M9 minimal medium was supplemented with 0.004% histidine and 0.01 M glucose; the pH was adjusted with concentrated HCl. Antibiotics were used as needed at the following concentrations: ampicillin, 50 μg/ml; streptomycin, 200 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 30 μg/ml. Plasmids were moved into serovar Typhimurium by electroporation or P22-HT-mediated transduction.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| Serovar Typhimurium | ||
| SL1344 | xyl hisG rpsL | 40 |
| P3F4 | SL1344 ssrA::miniTn5 | 35 |
| PhoP | SL1344 phoP::Tn10 | 70 |
| SL1344K | SL1344 rpoS::kan | 14 |
| CJD359 | SL1344 ompR1009::Tn10 | 19 |
| P3F4/CJD359 | SL1344 ssrA::miniTn5 ompR1009::Tn10 | This work |
| BRD454 | SL1344 ompC396::Tn10 | 19 |
| BRD456 | SL1344 ompF1006::Tn10 | 19 |
| CJD372 | SL1344 ompC396::Tn10 ompF1006::Mu dl-8 | 11 |
| CJD408 | SL1344 tppB83::MudJ | 11 |
| Plasmids | ||
| pFPV25 | colE1 mob bla gfp; promoterless gfp; no GFP expression | 80 |
| pFPV25.1 | pFPV25 with promoter region for rpsM, a gene encoding the ribosomal protein S13; constitutive GFP expression | 80 |
| pMIC-10C32 | 2,273-bp fragment spanning promoter region of ssrA and part of the ssaBCD operon cloned into pFPV25 | 12 |
| pFMI10 | Promoter fragment of ssaH cloned in pFPV25 | 81 |
| pFMI10-cam | pFMI10 with chloramphenicol gene cloned into EcoRV site | This work |
| pANT30.2-cam | pANT30.2 with chloramphenicol gene cloned into EcoRV site | This work |
| pANT30.1 | 645-bp fragment spanning promoter of ssrA and part of ssaB cloned into pFPV25 | This work |
| pANT30.2 | 335-bp fragment spanning promoter of ssrA cloned into pFPV25 | This work |
| pANT30.3 | 192-bp fragment upstream of ssrA ATG site cloned into pFPV25 | This work |
RAW264.7 cells were routinely maintained in Dulbecco modified Eagle medium containing sodium pyruvate and 10% fetal bovine serum (Gibco) at 37°C and 5% CO2.
Construction of pANT30 plasmids.
Standard molecular cloning techniques were used to clone fragments of the ssrA promoter region upstream of gfp in plasmid pFPV25 (Table 1). The following oligonucleotides with restriction sites were generated: reverse primer 3ssrA (5′ATTAGGTACCGGATCCGCCTGATTACTAAAGATGTTTGC3′) includes KpnI and BamHI sites; forward primer 5ssrA.1 (5′ATACGGATCCGAATTCGTCGACGGCAAGACAAGGCTTAGGTAAGC3′) includes BamHI, EcoRI, and SalI sites and was used to clone pANT30.1; forward primer 5ssrA.2 (5′ATACGGATCCGAATTCGTAGTCATCGACTGGG3′), with BamHI and EcoRI sites, was used to clone pANT30.2; and forward primer 5ssrA.3 (5′ATACGGATCCGAATTCGTAGTCATCGACTGGG3′), with BamHI and EcoRI sites, was used to clone pANT30.3. Standard PCR and molecular biology protocols were used to amplify these fragments from SL1344, and fragments were cloned directionally into plasmid pFPV25 in either E. coli DH5α or DH10β before the plasmids were moved into serovar Typhimurium.
Gentamicin protection assay.
RAW264.7 cells were seeded at 2.5 × 105 per well in a 24-well tissue culture plate. Salmonellae were grown in LB broth on a rotating wheel and opsonized with 50% normal mouse serum for 15 min at 37°C. The cultures were diluted in phosphate-buffered saline (PBS) to allow a multiplicity of infection of 10. Infected cells were centrifuged at 1,800 × g for 5 min and incubated for 1 h at 37°C and 5% CO2. The cells were washed two times with medium and incubated with 100 μg of gentamicin/ml for 90 min. The cells were lysed with 1% Triton X-100, and intracellular bacteria were counted as CFU, or the cells were allowed to incubate an additional 20 h with 10 μg of gentamicin/ml, and intracellular counts were determined as stated above.
Assays for induction of gene expression inside macrophages.
RAW264.7 cells were seeded to confluency in 24-well tissue culture plates. Bacteria were grown standing at 37°C overnight and diluted for a multiplicity of infection of ∼50, and the cells were infected without centrifugation. After incubation for 30 min (37°C; 5% CO2), the medium was replaced and the bacteria were allowed to incubate for various times. Supernatant containing extracellular bacteria was set aside for analysis. The cells were washed three times with medium and lysed with Triton X-100 to release intracellular bacteria. A total of 10,000 intracellular or extracellular bacterium-size particles were measured with the FACscalibur (Becton Dickinson) cytometer for fluorescence intensity as previously described (12, 80, 81). All induction experiments were repeated at least twice.
In vitro gene induction.
Bacteria were grown standing in standard LB broth at neutral pH overnight at 37°C, and 1 ml of liquid culture was pelleted and washed two times in PBS. The bacteria were incubated for 3 h in the following media: LB broth, pH 7.0; LB broth, pH 4.5; M9 minimal medium, pH 7.0, with 1, 10, or 100 μM or 1 mM MgSO4; or M9 minimal medium, pH 4.5, with 1, 10, or 100 μM or 1 mM MgSO4. When appropriate, 0.5 M NaCl or 20% sucrose was added to the M9 medium. The bacteria were also tested in 1% ethanol, 0.5% sodium dodecyl sulfate, or 0.1 μg of polymyxin B/μl for 2 h. Subcultured bacteria were diluted in PBS and analyzed by flow cytometry.
Flow cytometry.
Flow cytometry experiments were performed with a FACScalibur (Becton Dickinson) cytometer. The bacteria were detected as described previously (80). Briefly, 10,000 bacterium-size particles were collected for each experiment, and the fluorescence intensity of each particle was detected by the FACScalibur machine. CELLQUEST (Becton Dickinson) software was used for data analyses and histogram production. The area under the curve of the histograms represents the total number of detected particles. The fluorescence intensity for each particle is reported on the x axis. A peak representing background machine noise is always present in the negative fluorescent population and cannot be avoided because of the small size of bacteria. Peak fluorescence, the fluorescence value of the bacterial majority, was determined with CELLQUEST software and is reported where appropriate. Induction ratios (IR) were calculated by dividing the peak fluorescence induction of bacteria in the inducing condition (i.e., inside macrophages or in M9 medium) by the peak fluorescence induction of bacteria in noninducing conditions (i.e., outside macrophages or in LB broth). GFP fluorescence for induced and uninduced bacteria was compared within each individual experiment, not between experiments, because the nature of flow cytometry analysis only allows data interpretation relative to internal positive and negative controls on a per-experiment basis. Each flow cytometry experiment included a negative control, SL1344 carrying plasmid pFPV25 (promoterless GFP), and a positive control, SL1344 carrying plasmid pFPV25.1 (constitutive GFP expression), to calibrate the relative fluorescence for each experiment. The negative and positive controls do not change fluorescence in response to the macrophage environment or to changes in the media tested (reference 80 and data not shown).
DNase protection assays.
DNase protection assays were carried out as described previously (41) with the following modifications. The forward PCR oligonucleotide, 5ssrA.2, was labeled with [γ-32P]ATP by T4 polynucleotide kinase forward reaction (New England Biolabs). The radioactive oligonucleotide and a nonradioactive reverse primer, 3ssrA, were used in a PCR to generate radioactive target DNA for subsequent binding and sequencing reactions. Excess radioactive label from the kinase reaction and unincorporated deoxynucleoside triphosphates from the PCR were removed by using Qiaqik (Qiagen) columns according to the manufacturer's instructions. Target DNA equivalent to 2.3 × 105 cpm was bound to 1.76 μg of purified OmpR protein or 2.5 μg of MBP-EnvZ in the appropriate buffers and conditions as described previously (41). DNase I reactions were carried out by the method of Huang and Igo, as was the parallel A+G Maxam-Gilbert sequencing reaction (41). Samples were separated by electrophoresis on a 7.5 M urea–6% polyacrylamide gel, and detected by autoradiography.
RESULTS
OmpR is important for inducing ssrA transcription.
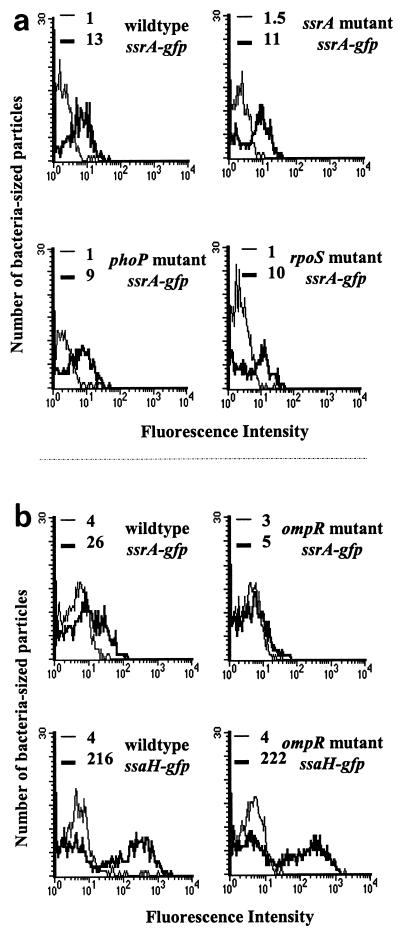
ssrA transcription is induced inside macrophages, presumably in response to the vacuolar environment (12). Global regulators often modulate bacterial gene expression in response to environmental changes, so we chose to examine the effects of mutations in three known global regulators, phoP, rpoS, and ompR, on ssrA gene expression because mutations in these genes are known to decrease Salmonella virulence (14, 19, 21, 57). We tested the abilities of strains with mutations in these genes to induce the ssrA promoter inside RAW264.7 murine macrophage-like cells. Plasmid pMIC-10C32, which contains a transcriptional fusion of the ssrA promoter region to gfp, was used to measure transcriptional activity of the promoter. RAW264.7 macrophages were infected with either SL1344 (wild type), P3F4 (ssrA mutant), PhoP (phoP mutant), SL1344K (rpoS mutant), or CJD359 (ompR mutant) carrying plasmid pMIC-10C32 (Table 1 and Fig. 1). The bacteria were released from the cells 2 h postinfection, and GFP expression from extracellular and intracellular bacteria was compared as described in Materials and Methods. The IR was calculated by dividing the peak fluorescence of intracellular bacteria by the peak fluorescence of extracellular bacteria (Fig. 1). Intracellular wild-type and ssrA mutant bacteria harboring the plasmid reporter construct induced GFP expression (IR, 13 and 11, respectively), as did phoP (IR = 9) and rpoS (IR = 10) strains (Fig. 1a). In contrast, gfp expression was reduced fourfold for intracellular bacteria in an ompR mutant background (IR = 1.6) compared to the wild type (IR = 6.5) (Fig. 1b). This result suggested that either OmpR or an OmpR-dependent event induced ssrA transcription.
FIG. 1.
ssrA gene expression is reduced significantly in an ompR mutant background. RAW264.7 cells were infected for 2 h with strains bearing reporter plasmids. The histograms show GFP expression from extracellular bacteria (thin lines) and intracellular bacteria (thick lines). Peak fluorescence intensity is indicated for each sample. (a) Macrophages were infected with the following strains: SL1344 (wild type) with pMIC-10C32 (ssrA-gfp), P3F4 (ssrA mutant) with pMIC-10C32, PhoP (phoP mutant) with pMIC-10C32, and SL1344K (rpoS mutant) with pMIC-10C32. (b) Macrophages were infected with SL1344 with pMIC-10C32, CJD359 (ompR mutant) with pMIC-10C32, SL1344 with pFMI10 (ssaH gfp), and CJD359 with pFMI10. The experiments shown in panels a and b were performed on different days.
We also tested the transcription of ssaH in an ompR mutant strain. Surprisingly, we found that the ompR mutant carrying plasmid pFMI10 (ssaH promoter fusion to gfp) still induced inside macrophages (IR = 55.5) (Fig. 1b). This result suggested that some SsrA might be present at 2 h postinfection, since ssaH transcription requires SsrA (81), and might be sufficient for induction of ssaH. We addressed this phenomenon by comparing the kinetics of ssrA and ssaH transcription in wild-type and ompR mutant backgrounds.
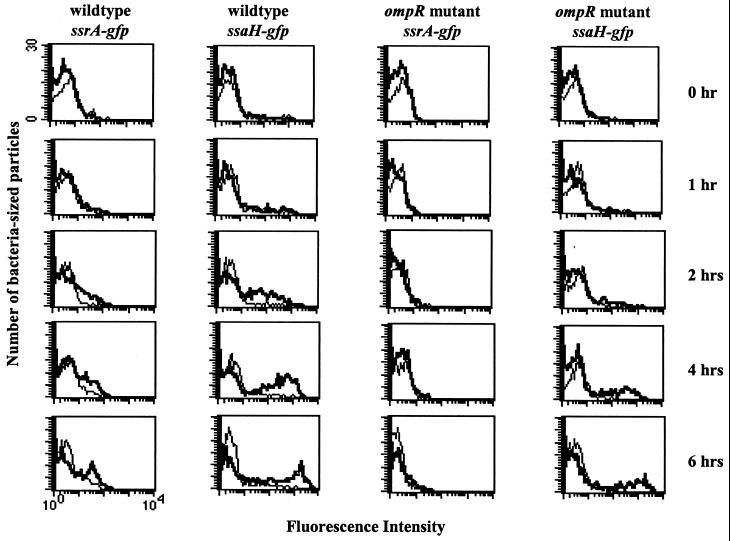
We measured the relative amounts of GFP expression from strains carrying plasmid pMIC-10C32 (ssrA-gfp) or pFMI10 (ssaH-gfp) over time to determine when ssrA and ssaH were transcribed in the absence of OmpR inside macrophages. For this time course, we monitored transcription of either ssrA or ssaH by watching for the appearance of an inducing population of intracellular bacteria over time. In a wild-type background, ssrA transcription was evident by 2 h and strong by 6 h (Fig. 2, column 1). In an ompR mutant background, ssrA induction was barely detectable even at 6 h postinfection, indicating that ompR is important for ssrA induction (Fig. 2, column 3). The absence of OmpR delayed but did not eliminate induction of the ssaH reporter (Fig. 2, columns 2 and 4). Therefore, ssrA expression may not be completely abolished in the ompR mutant, and a small but undetectable (by our assay) level of SsrA probably accumulates in the ompR mutant. We conclude that OmpR is important for early transcription of ssrA inside macrophages.
FIG. 2.
Kinetics of induction for ssrA- and ssaH-gfp fusions inside macrophages. RAW264.7 macrophages were infected with the following bacteria: SL1344 (wild type) with pMIC-10C32 (ssrA-gfp), SL1344 with pFMI10 (ssaH-gfp), CJD359 (ompR mutant) with pMIC-10C32, or CJD359 with pFMI10. The histograms show GFP expression from extracellular bacteria (thin lines) and intracellular bacteria (thick lines) at each time point postinfection. The axes for all histograms are identical.
SPI-2 induction is independent of OmpC and OmpF porin defects.
It was possible that the reduced ssrA expression in an ompR mutant strain was an indirect effect of defects from other OmpR-regulated genes, specifically, ompC and ompF. In vitro, OmpR regulates the proportion of OmpF and OmpC in an osmolarity-dependent fashion, and the amount of OmpC and OmpF is reduced in an ompR mutant of Salmonella (51). Is the ompR mutant strain defective for SPI-2 expression because the absence of porin expression prevents Salmonella from sensing an environmental cue inside macrophages? We tested the induction of ssrA and ssaH promoter-gfp fusions in ompF, ompC and ompC ompF mutant backgrounds and compared the induction inside macrophages to that of wild-type bacteria. A chloramphenicol gene was cloned into plasmid pANT30.2 to make pANT30.2-cam and into pFMI10 to make pFMI10-cam for these experiments. Wild-type, ompC, ompF, and ompC ompF strains all induced ssrA and ssaH transcription to the same level (IR, ∼5 and ∼60, respectively) inside macrophages. We also tested plasmids pANT30.2-cam and pFMI10-cam in a tppB mutant strain; this strain also induced ssrA and ssaH transcription at wild-type levels inside macrophages. These results indicate that ssrAB is controlled independently of the OmpR-regulated genes, ompC, ompF, and tppB.
An ompR mutant strain of Salmonella does not survive inside macrophages.
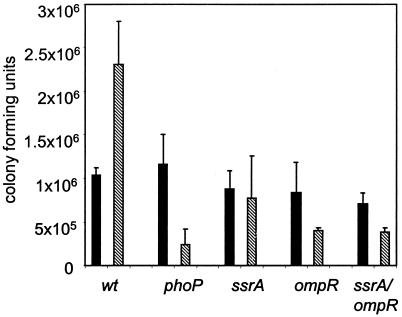
In wild-type serovar Typhimurium, the type III secretion system in SPI-2 and its two-component regulatory system, SsrA-SsrB, are essential for replication inside macrophages (12, 36, 64, 73). We established that OmpR has a marked effect on induction of ssrA and next investigated whether ompR mutants, like ssrA mutants, fail to replicate inside macrophages by using a gentamicin protection assay. Wild-type Salmonella replicated while a phoP mutant negative control did not survive inside macrophages (Fig. 3). Strain P3F4 (ssrA mutant) did not replicate after 20 h inside macrophages, as expected (12, 36). We found that serovar Typhimurium carrying an ompR::Tn10 insertion did not replicate inside macrophages, in contrast to what was reported previously (52). While strain differences might account for this discrepancy, it is most likely due to the differences in the way Salmonella was grown. Lindgren et al. used bacterial cultures grown with reduced aeration, which optimizes for bacterial invasion and bacterium-induced host cell apoptosis (48, 61). Thus, in their experiments, wild-type bacteria would cause apoptosis by 2 to 6 h postinfection, releasing bacteria from the macrophages into gentamicin-containing medium. This would result in an underrepresentation of CFU for wild-type bacteria, which may obscure the difference in the actual number of intracellular bacteria between wild-type and ompR mutant Salmonella. We circumvented the apoptosis effect by growing the bacteria to stationary phase with aeration and using complement-mediated phagocytosis. Bacteria grown in this manner do not induce apoptosis (61), allowing differences in intracellular replication to be detected.
FIG. 3.
Comparison of survival and replication in RAW264.7 macrophages. RAW264.7 macrophages were infected with either SL1344 (wild type), PhoP (phoP), P3F4 (ssrA), CJD359 (ompR), or P3F4-CJD359 double mutant (ssrA/ompR) for 1 h before the addition of gentamicin. Gentamicin-protected CFU were determined at 3 (solid bars) and 23 (hatched bars) h postinfection.
It has been proposed that the SPI-2 secretion system is needed for bacterial replication, but not survival, in vivo (73). Our data support this view, since the CFU for the ssrA mutant remain similar at both time points postinfection, in contrast to the phoP mutant, which dies inside macrophages (Fig. 3). Finally, a strain with mutations in both ssrA and ompR also did not replicate inside macrophages.
OmpR protein binds directly to the ssrA promoter region.
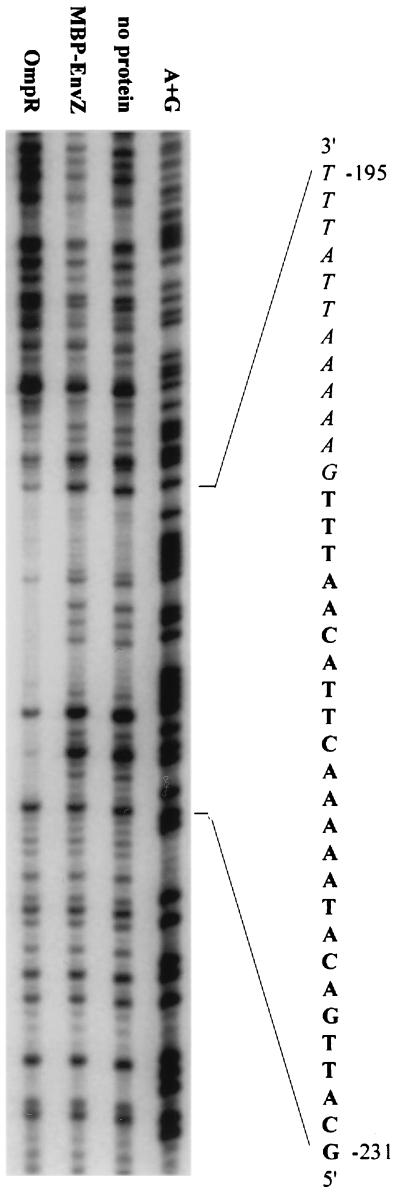
We asked if OmpR interacts directly with the ssrA promoter region to activate ssrA gene expression. Studies of E. coli have not revealed a clear consensus for an OmpR binding sequence; however, a common feature of OmpR binding regions is the abundance of adenines and thymines (24, 41, 67). The sequence of the region upstream of the translational start for the ssrA gene is rich in adenines and thymines, suggesting a possible binding site for OmpR protein.
A DNase I protection assay was used to determine if OmpR binds directly to the ssrA promoter region. E. coli OmpR protein is 99% identical at the amino acid level to Salmonella OmpR protein. MBP-EnvZ, the sensor component for OmpR and a protein that does not bind DNA, was included as a negative control. Purified OmpR from E. coli was incubated with a 335-bp DNA fragment that included 51 bp downstream of the ATG start codon and 284 bp upstream of the ATG. The samples were treated with DNase I and separated on a 6% acrylamide gel. OmpR protein binds between −207 and −231 from the ATG start codon (Fig. 4). Based on the OmpF studies, a single OmpR binding site is 18 bp (41). Approximately 24 bp are protected here, so we conclude that there are at least one and a half, probably two, binding sites for OmpR. An additional 12-bp area from −195 to −206 may also be bound by OmpR protein, but that cannot be determined by this assay because DNase I enzyme does not cleave efficiently in this region. The sequence of the ssrA promoter region that is protected by OmpR is rich in adenines and thymines but has no exact homology with published OmpR binding sites for the OmpR-regulated genes ompC and ompF (24, 41, 67).
FIG. 4.
OmpR protein binds the ssrA promoter region. A 335-bp fragment spanning the ssrA promoter region was the target in a DNase protection assay. Purified OmpR protein binds the region −207 through −231 from the ATG of the ssrA gene. Each lane contained target DNA plus the indicated proteins. The exact sequence of the binding region is indicated, with protected sequences in boldface. The italicized letters indicate a potential OmpR binding half-site that cannot be determined from this experiment.
Deletion analysis of ssrA promoter region.
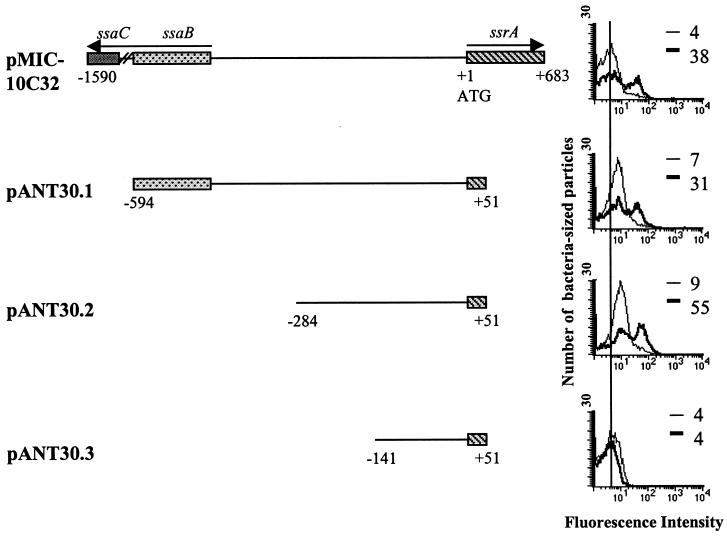
We analyzed the intergenic region between ssrA and ssaB (spiC) to define the promoter region of ssrA. In accordance with the universal nomenclature for type III secretion systems, we will refer to spiC and spiA genes as ssaB and ssaC (8, 36). The pMIC-10C32 plasmid carries a 2.3-kb fragment that includes much of the coding region for ssaB and ssaC (12, 64) as well as a 683-bp coding region of ssrA (Fig. 5). In plasmid pANT30.1, a 645-bp fragment cloned upstream of promoterless gfp in plasmid pFPV25 contains 194 bp of the ssaB coding region and 51 bp of the ssrA coding region. This fragment induces GFP inside macrophages as well as the original pMIC-C32 clone. A 335-bp fragment cloned in plasmid pANT30.2 also induces GFP inside macrophages. However, an additional 143-bp deletion in plasmid pANT30.3 does not induce GFP inside macrophages. These analyses show that the region between −141 and −284 is necessary for transcription of ssrA. The induction profiles inside macrophages for pANT30.1 and pANT30.2 in strains CJD359 (ompR mutant) and P3F4 (ssrA mutant) were similar to that of plasmid pMIC-10C32, while pANT30.3 was never induced in any strain.
FIG. 5.
Effects of ssrA promoter region deletions on expression inside macrophages. Different fragments of the ssrA promoter region cloned upstream of gfp in plasmid pFPV25 are shown. RAW264.7 cells were infected with wild-type bacteria harboring the indicated plasmids. The histograms show GFP expression from extracellular bacteria (thin lines) and intracellular bacteria (thick lines). The vertical line drawn through the histograms shows the peak fluorescence of extracellular bacteria from constructs pMIC-10C32 and pANT30.3. Peak fluorescences are indicated for each sample. Maps are not drawn to scale.
While serovar Typhimurium carrying plasmids pANT30.1 and pANT30.2 induced GFP inside macrophages, the peak fluorescence of extracellular bacteria increased for both deletions (Fig. 5). This shift was consistently reproduced in several independent experiments. The increase in extracellular GFP expression was not observed in pANT30.3, which contains only 141 bp upstream of the ssrA ATG, and also does not induce ssrA inside macrophages. These results imply that additional regulatory mechanisms which repress ssrA expression exist and exert their effect in the intergenic region between ssaB and ssrA or the region downstream of +51. Our induction experiments cannot determine if there is direct binding from a repressor protein or if repression is due to DNA conformational changes. We addressed the first possibility by creating a random MudJ insertional library in which each clone carried the reporter plasmid pMIC-10C32. We failed to generate any clones that resulted in constitutive GFP expression, although such a mutation could be lethal to Salmonella grown on LB agar.
ssrA and ssaH are induced in minimal medium, pH 4.5.
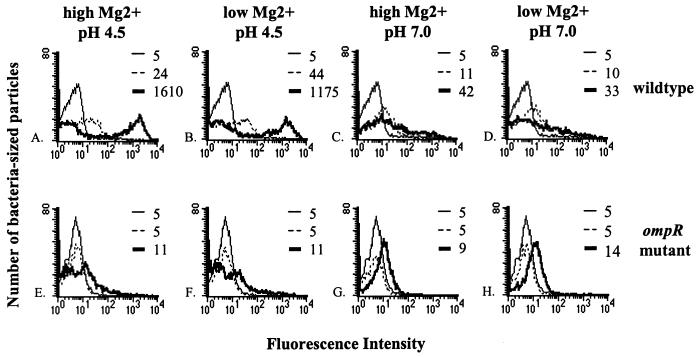
We tested several in vitro growth conditions for induction of ssrA and ssaH transcription to learn what Salmonella might be detecting inside the host cell. The wild-type and the ssrA and ompR mutant strains carrying pMIC-10C32 (ssrA-gfp) or pFMI10 (ssaH-gfp) plasmids were grown standing overnight in LB broth at 37°C (OD600, ∼0.4) and then subcultured for 3 h in either LB broth at pH 7.0, LB broth at pH 4.5, or minimal media at pH 7.0 and 4.5 and high (1 mM) or low (1 μM) Mg2+. Fluorescence profiles in all media tested remained unchanged for SL1344 carrying plasmid pFPV25 or pFPV25.1 (GFP-negative and -positive control reporters, respectively, included in all experiments) (reference 80 and data not shown). Only subcultures in minimal medium, pH 4.5, with either high or low Mg2+, induced ssrA (IR = 4.8) and ssaH (IR = 180) transcription in a wild-type strain (Fig. 6A to D). Consistent with our macrophage data, ssrA and ssaH induction was reduced significantly in an ompR mutant background at low pH with either high or low Mg2+ (Fig. 6E to H). We observed slight induction in minimal medium at pH 7.0 (IR = 2.2 for ssrA and 8.4 for ssaH) but not in LB broth at pH 7.0 (IR = 1) or 4.5 (IR = 1), indicating that SPI-2 genes are transcribed to some extent in response to other signals in minimal medium itself, independent of acidity. We also titrated the pH of minimal medium from pH 7.0 to 4.5 at 0.5-unit intervals and tested the induction of our reporter genes. We found that induction of ssrA and ssaH increased as acidity increased; maximum induction occurred from pH 5.5 to 4.5 (data not shown). We were able to detect the putative effector proteins, SseA and SseD, by immunoblot analysis from cultures induced in minimal medium at pH 4.5, indicating that components of the SPI-2 type III secretion system are made during our assays.
FIG. 6.
Minimal medium at low pH is optimal for in vitro induction of ssrA and ssaH transcription. Salmonella cells were grown in LB broth overnight and subcultured in the indicated minimal media for 3 h. The histograms show GFP expression from bacteria carrying plasmids pFMI10 (ssaH-gfp) subcultured in LB broth (thin lines), pMIC-10C32 (ssrA-gfp) subcultured in minimal medium (dotted lines), or pFMI10 subcultured in minimal medium (thick lines). Peak fluorescences are indicated for each population. The histograms for bacteria with pMIC-10C32 subcultured in LB broth are superimposed on histograms from bacteria carrying plasmid pFMI10 subcultured in LB broth (data not shown).
Our results agree with the recent report that acidic medium induces SPI-2 expression (6) but are in contrast to the report that SPI-2 genes are induced by Mg2+ deprivation, not acidity (16). The differences in our results might be attributed to differences in the growth phase of the bacteria (Deiwick et al. used bacteria grown to stationary phase with aeration, while we used bacteria grown with reduced aeration [OD600, ∼0.4]). Deiwick et al. also reported that SPI-2 gene expression is induced during Mg2+ deprivation and that this induction is dependent on the PhoP-PhoQ two-component regulatory system. We tested the induction of ssaH-promoter fusions in wild-type and phoP mutant strains in minimal medium over 10-fold dilutions of Mg2+ concentration (1 mM through 1 μM) and at pH 7.0 or 4.5. Consistent with the results presented in Fig. 6, wild-type bacteria induced high levels of ssrA and ssaH transcription at low pH, regardless of Mg2+ concentration (data not shown). In contrast, a phoP mutant strain showed reduced ssaH induction at 1 and 10 μM Mg2+ but wild-type levels of induction at 100 μM and 1 mM Mg2+ at pH 4.5. We conclude that PhoP-PhoQ has an effect on SPI-2 induction in minimal medium only when the combination of low pH and an Mg2+ concentration below 10 μM is present. However, we also found that a phoP mutant strain inside macrophages is capable of inducing ssrA (Fig. 1) and ssaH (data not shown and reference 81) expression to the levels of wild-type bacteria. It is possible that inside a macrophage vacuole, the concentration of Mg2+ is above 10 μM, allowing induction of SPI-2 in the absence of phoP (28). An alternative explanation is that minimal-medium systems do not accurately mimic the conditions inside the phagosome.
Increasing osmolarity of minimal medium, pH 4.5, represses SPI-2 expression.
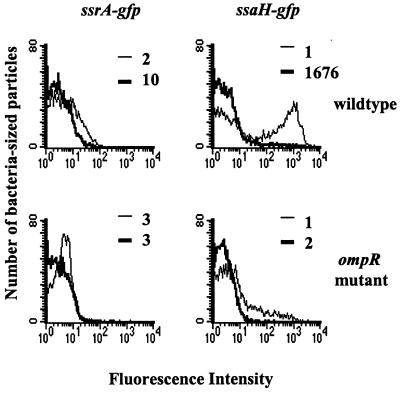
OmpR-EnvZ is thought to sense differences in osmolarity; therefore, we hypothesized that changing the osmolarity of our in vitro inducing conditions might affect SPI-2 expression. Figure 7 shows that increasing the osmolarity of our in vitro inducing medium, minimal medium at pH 4.5, had a marked effect on SPI-2 expression. We added either 0.5 M NaCl (final concentration of Na2+, 1.0 M) or 20% sucrose (data not shown) to minimal medium at pH 4.5. We then compared the inductions of ssrA-gfp and ssaH-gfp fusions in the original minimal medium at pH 4.5 or in the minimal medium at pH 4.5 with raised osmolarity (Fig. 7). In a wild-type background, ssrA and ssaH transcription is induced in minimal medium at pH 4.5 (peak fluorescence, 10 and 1,676, respectively). However, when the osmolarity is increased, wild-type strains have reduced ssrA (peak fluorescence, 2) and ssaH (peak fluorescence, 1) transcription. Fluorescence profiles for the GFP control reporters, pFPV25 and pFPV25.1, remained the same under all conditions. Consistent with the results shown in Fig. 6, induction is abrogated in an ompR mutant background. We conclude from these data that high osmolarity represses, whereas low pH induces, SPI-2 expression and that OmpR is required for responding to both conditions.
FIG. 7.
Increasing the osmolarity of minimal medium at pH 4.5 in an OmpR+ strain represses ssrA and ssaH expression. Salmonella was grown in LB broth overnight and subcultured in minimal medium at pH 4.5 (thin lines) or minimal medium at pH 4.5 plus 0.5 M NaCl (thick lines). The histograms show GFP expression from the indicated strains subcultured in either low (thin lines)- or high (thick lines)-osmolarity media. Peak fluorescences are indicated for each population. Bacteria subcultured in LB broth did not express significant levels of GFP (data not shown).
DISCUSSION
We have established that OmpR regulates transcription of the two-component regulatory system SsrA-SsrB in serovar Typhimurium. While an ompR mutant has been shown to be avirulent in mice, no known OmpR-regulated genes have been assigned a clear role in virulence during Salmonella infection (reviewed in reference 33). Our study suggests that the avirulent phenotype of the ompR strain is at least partially a result of OmpR regulating ssrA and thereby the genes of the type III secretion system encoded on SPI-2, which are essential for virulence in mice.
The DNase I protection assays show that the OmpR protein binds to the ssrA promoter region (Fig. 4). This is only the second example of biochemical data showing a response regulator protein directly binding to the promoter region of a different two-component regulatory system. In Bacillus subtilis, the PhoP protein of the PhoP-PhoR two-component system directly binds to the promoter region of the resD gene of the ResD-ResE sensor-kinase system, which regulates cytochrome c biogenesis (7). There is also genetic evidence of other two-component regulatory system cascades. For example, the PhoP-PhoQ system in Salmonella controls the expression of the two-component system PmrA-PmrB, which in turn controls expression of a subset of PhoP-activated genes (75). Furthermore, Soncini and Groisman reported that all PhoP-activated genes are induced by Mg2+ limitation, but those that are PmrA-PmrB dependent also respond to acid, independent of PhoP-PhoQ. Thus, multiple signals can affect different steps in two-component regulatory cascades.
What is Salmonella sensing before and after host cell invasion? In vitro, the invasion genes encoded on SPI-1 are induced at pH 8 (the approximate pH of the gut lumen) and repressed by low pH and low osmolarity (conditions of the phagosome) (4, 15, 28). After Salmonella enters the macrophage, the Salmonella-containing vacuole acidifies to pH 4.0 to 5.0 within 30 min and ssrA and ssaH transcription increases (70) (Fig. 2). Phagosome acidification is required for Spi2 gene induction, and low-pH minimal medium induces SPI-2 genes in vitro (6, 12) (Fig. 6). Thus, SPI-1 and SPI-2 appear to be reciprocally regulated. High pH and high osmolarity induce SPI-1 and repress SPI-2, whereas low pH and low osmolarity repress SPI-1 and induce SPI-2.
In addition to OmpR-dependent positive regulation, repression of ssrA gene expression by unknown factors is evident. Successive deletions of the region upstream of the ssrA ATG in plasmids pANT30.1 and pANT30.2 resulted in extracellular bacteria expressing increasing amounts of GFP, indicating that we may have deleted regions necessary for repression of ssrA transcription (Fig. 5). Many different things could repress ssrA expression. For example, environmental changes might affect DNA conformation and result in altered ssrA expression. Changes in DNA topology have been shown to affect expression of a variety of genes (17, 18, 27, 39). OmpR protein itself might be an activator as well as a repressor of ssrA transcription. In E. coli, OmpR acts as an activator of ompF transcription in low-osmolarity conditions but acts as a repressor of ompF transcription in high-osmolarity conditions (reviewed in reference 68). Another possibility is that SsrB or another protein binds to the ssrAB promoter region, with or without OmpR, to activate or repress transcription. Further experiments are needed to confirm or reject these hypotheses.
We showed that timely induction of SPI-2 inside macrophages is dependent upon OmpR (Fig. 2). However, if the SsrA-SsrB two-component regulatory system can eventually detect the vacuolar environment in the absence of OmpR and turn on the SPI-2 type III secretion system, then why is an ompR mutant still deficient for survival inside macrophages upon complement-mediated entry (Fig. 3)? We speculate that the timing of environmental sensing and response is critical for Salmonella. Salmonella must respond immediately to the host intracellular environment in order to turn on the appropriate genes to avoid host defenses. The replication assay, which measures bacterial growth within macrophages at 23 h postinfection, cannot determine when after invasion SPI-2 genes function, but at least one SPI-2 gene, SsaB (SpiC), is known to act within hours of invasion (78). In the absence of OmpR, low levels of SsrA accumulate enough to allow ssaH expression eventually but not enough to allow Salmonella to survive and replicate inside the host cell (Fig. 3). This indicates either that low levels of Spi2 are not sufficient to support replication or that OmpR regulates other unidentified genes necessary for survival and replication inside macrophages.
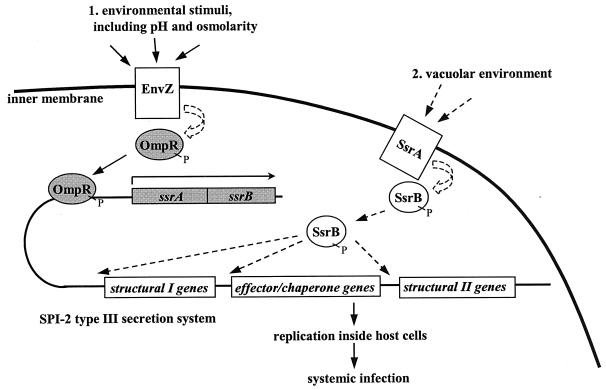
OmpR was originally identified as the response regulator for the osmolarity-sensing gene EnvZ in E. coli (2, 68). By analogy with E. coli, we presumed that EnvZ is the sensor through which OmpR is responding in order to regulate ssrAB in Salmonella. Our model predicts that there are at least two tiers of ssrA regulation: EnvZ senses the low pH and low osmolarity of the vacuole, and SsrA is a second sensor, perhaps for later signals in the vacuolar compartment. We do not know what the environmental stimulus for SsrA is at this point. It has been suggested that phosphate starvation may be another signal Salmonella is sensing inside the vacuole, though we have not tested this (16). Collaboration between the OmpR-EnvZ and SsrA-SsrB systems would yield efficient detection of and response to the intracellular environment (Fig. 8).
FIG. 8.
Model for Salmonella SPI-2 regulation inside host macrophages. The OmpR-EnvZ system responds to the intracellular environment, possibly stimulated by the acidic pH and low osmolarity of the phagosome. OmpR binds to the ssrA promoter region to activate transcription of the ssrAB genes. Later, SsrB detects a different environmental stimulus in the vacuole. SsrB activates expression of the type III secretion system encoded within SPI-2, which then allows for replication inside cells and systemic infection in mice. The solid arrows indicate supporting data from this study and others (12, 35, 36, 64, 74). OmpR and SsrB are hypothesized to be phosphorylated (as indicated by “P”) in this model. The shaded proteins and genes represent those studied here. The dashed arrows indicate speculative function based on protein homology, not experimental evidence.
The survival of Salmonella demands that the bacterium respond immediately to its surroundings, especially the host cell vacuole. It must turn on the appropriate genes to counter the potentially fatal intracellular host environment. We established here that the OmpR protein of the global two-component regulatory system OmpR-EnvZ regulates the SPI-2 two-component regulatory system, SsrA-SsrB, which in turn activates the type III secretion system in SPI-2. As more information on coordinate gene regulation in Salmonella is discovered, we will gain a greater understanding of how pathogens use complex networks of genetic regulation both to exert control and to maintain flexibility in response to the host milieu.
ACKNOWLEDGMENTS
We wholeheartedly thank M. Igo and K. Huang for providing purified OmpR and MBP-EnvZ proteins, as well as interesting discussions. We thank Tom Silhavy for useful discussion as well as Raphael Valdivia for his constructs and insights throughout the project. We are grateful to Joan Mecsas, Denise Monack, Lalita Ramakrishnan, and Sara Fisher for critical readings of the manuscript.
A. K. Lee was supported by training grant 5T32 AI07328-11 from the Dept. of Microbiology and Immunology, Stanford University, and by a contract with Protein Design Labs, Inc., Fremont, Calif. C.S.D. was supported by postdoctoral fellowship no. 99-146-01-MBC from the American Cancer Society.
REFERENCES
- 1.Abshire K Z, Neidhardt F C. Analysis of proteins synthesized by Salmonella typhimurium during growth within a host macrophage. J Bacteriol. 1993;175:3734–3743. doi: 10.1128/jb.175.12.3734-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alphen W V, Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977;131:623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Ausubel, F. M., et al. (ed.). Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 5.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuzon C R, Banks G, Deiwick J, Hensel M, Holden D W. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol Microbiol. 1999;33:806–816. doi: 10.1046/j.1365-2958.1999.01527.x. [DOI] [PubMed] [Google Scholar]
- 7.Birkey S M, Liu W, Zhang X, Duggan M F, Hulett F M. Pho signal transduction network reveals direct transcriptional regulation of one two-component system by another two-component regulator: Bacillus subtilis PhoP directly regulates production of ResD. Mol Microbiol. 1998;30:943–953. doi: 10.1046/j.1365-2958.1998.01122.x. [DOI] [PubMed] [Google Scholar]
- 8.Bogdanove A J, Beer S V, Bonas U, Boucher C A, Collmer A, Coplin D L, et al. Unified nomenclature for broadly conserved hrp genes of phytopathogenic bacteria. Mol Microbiol. 1996;20:681–683. doi: 10.1046/j.1365-2958.1996.5731077.x. [DOI] [PubMed] [Google Scholar]
- 9.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 10.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatfield S N, Dorman C J, Hayward C, Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect Immun. 1991;59:449–452. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 13.Collazo C M, Galan J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 14.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 15.Daefler S. Type III secretion by Salmonella typhimurium does not require contact with a eukaryotic host. Mol Microbiol. 1999;31:45–51. doi: 10.1046/j.1365-2958.1999.01141.x. [DOI] [PubMed] [Google Scholar]
- 16.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1774. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 17.Dorman C J. Flexible response: DNA supercoiling, transcription and bacterial adaptation to environmental stress. Trends Microbiol. 1996;4:214–216. doi: 10.1016/0966-842X(96)30015-2. [DOI] [PubMed] [Google Scholar]
- 18.Dorman C J, Barr G C, Bhriain N N, Higgins C F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988;170:2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichelberg K, Galan J E. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect Immun. 1999;67:4099–4105. doi: 10.1128/iai.67.8.4099-4105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finlay B B. Molecular and cellular mechanisms of Salmonella pathogenesis. Curr Top Microbiol Immunol. 1994;192:163–185. doi: 10.1007/978-3-642-78624-2_8. [DOI] [PubMed] [Google Scholar]
- 24.Forst S, Kalve I, Durski W. Molecular analysis of OmpR binding sequences involved in the regulation of ompF in Escherichia coli. FEMS Microbiol Lett. 1995;131:147–151. doi: 10.1111/j.1574-6968.1995.tb07769.x. [DOI] [PubMed] [Google Scholar]
- 25.Galan J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 26.Galan J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galan J E, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-del Portillo F, Foster J W, Maguire M E, Finlay B B. Characterization of the micro-environment of Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 29.Garcia Vescovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 30.Garcia Vescovi E, Soncini F C, Groisman E A. The role of the PhoP/PhoQ regulon in Salmonella virulence. Res Microbiol. 1994;145:473–480. doi: 10.1016/0923-2508(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 31.Gibson M M, Ellis E M, Graeme-Cook K A, Higgins C F. OmpR and EnvZ are pleiotropic regulatory proteins: positive regulation of the tripeptide permease (tppB) of Salmonella typhimurium. Mol Gen Genet. 1987;207:120–129. doi: 10.1007/BF00331499. [DOI] [PubMed] [Google Scholar]
- 32.Groisman E A, Chiao E, Lipps C J, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groisman E A, Heffron F. Regulation of Salmonella virulence by two-component regulatory systems. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 319–332. [Google Scholar]
- 34.Heithoff D M, Conner C P, Hentschel U, Govantes F, Hanna P C, Mahan M J. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181:799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 36.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang F C, Holden D W. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 37.Heran Darwin K, Miller V L. InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J Bacteriol. 1999;181:4949–4954. doi: 10.1128/jb.181.16.4949-4954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heyde M, Portalier R. Regulation of major outer membrane porin proteins of Escherichia coli K 12 by pH. Mol Gen Genet. 1987;208:511–517. doi: 10.1007/BF00328148. [DOI] [PubMed] [Google Scholar]
- 39.Higgins C F, Dorman C J, Stirling D A, Waddell L, Booth I R, May G, Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 40.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 41.Huang K J, Igo M M. Identification of the bases in the ompF regulatory region, which interact with the transcription factor OmpR. J Mol Biol. 1996;262:615–628. doi: 10.1006/jmbi.1996.0540. [DOI] [PubMed] [Google Scholar]
- 42.Huang K J, Lan C Y, Igo M M. Phosphorylation stimulates the cooperative DNA-binding properties of the transcription factor OmpR. Proc Natl Acad Sci USA. 1997;94:2828–2832. doi: 10.1073/pnas.94.7.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston C, Pegues D A, Hueck C J, Lee A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 45.Jones B, Pascopella L, Falkow S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr Opin Immunol. 1995;7:474–478. doi: 10.1016/0952-7915(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 46.Jones B D, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–3752. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowarz L, Coynault C, Robbe-Saule V, Norel F. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J Bacteriol. 1994;176:6852–6860. doi: 10.1128/jb.176.22.6852-6860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung K Y, Finlay B B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liljestrom P, Laamanen I, Palva E T. Structure and expression of the ompB operon, the regulatory locus for the outer membrane porin regulon in Salmonella typhimurium LT-2. J Mol Biol. 1988;201:663–673. doi: 10.1016/0022-2836(88)90465-2. [DOI] [PubMed] [Google Scholar]
- 51.Liljestrom P, Maattanen P L, Palva E T. Cloning of the regulatory locus ompB of Salmonella typhimurium LT-2. II. Identification of the envZ gene product, a protein involved in the expression of the porin proteins. Mol Gen Genet. 1982;188:190–194. doi: 10.1007/BF00332674. [DOI] [PubMed] [Google Scholar]
- 52.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loewen P C, Hengge-Aronis R. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 54.Mecsas J, Strauss E J. Molecular mechanisms of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller S I. PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence? Mol Microbiol. 1991;5:2073–2078. doi: 10.1111/j.1365-2958.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 57.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller S I, Mekalanos J J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills D M, Bajaj V, Lee C A. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol. 1995;15:749–759. doi: 10.1111/j.1365-2958.1995.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 60.Mills S D, Ruschkowski S R, Stein M A, Finlay B B. Trafficking of porin-deficient Salmonella typhimurium mutants inside HeLa cells: ompR and envZ mutants are defective for the formation of Salmonella-induced filaments. Infect Immun. 1998;66:1806–1811. doi: 10.1128/iai.66.4.1806-1811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norel F, Robbe-Saule V, Popoff M Y, Coynault C. The putative sigma factor KatF (RpoS) is required for the transcription of the Salmonella typhimurium virulence gene spvB in Escherichia coli. FEMS Microbiol Lett. 1992;78:271–276. doi: 10.1016/0378-1097(92)90039-q. [DOI] [PubMed] [Google Scholar]
- 64.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Neal C R, Gabriel W M, Turk A K, Libby S J, Fang F C, Spector M P. RpoS is necessary for both the positive and negative regulation of starvation survival genes during phosphate, carbon, and nitrogen starvation in Salmonella typhimurium. J Bacteriol. 1994;176:4610–4616. doi: 10.1128/jb.176.15.4610-4616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 67.Pratt L A, Silhavy T J. Identification of base pairs important for OmpR-DNA interaction. Mol Microbiol. 1995;17:565–573. doi: 10.1111/j.1365-2958.1995.mmi_17030565.x. [DOI] [PubMed] [Google Scholar]
- 68.Pratt L A, Silhavy T J. Porin regulation of Escherichia coli. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 105–127. [Google Scholar]
- 69.Rathman M, Barker L P, Falkow S. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect Immun. 1997;65:1475–1485. doi: 10.1128/iai.65.4.1475-1485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rathman M, Sjaastad M D, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schechter L M, Damrauer S M, Lee C A. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol. 1999;32:629–642. doi: 10.1046/j.1365-2958.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 73.Shea J E, Beuzon C R, Gleeson C, Mundy R, Holden D W. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67:213–219. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soncini F C, Groisman E A. Two-component regulatory systems can interact to process multiple environmental signals. J Bacteriol. 1996;178:6796–6801. doi: 10.1128/jb.178.23.6796-6801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stein M A, Leung K Y, Zwick M, Garcia-del Portillo F, Finlay B B. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol Microbiol. 1996;20:151–164. doi: 10.1111/j.1365-2958.1996.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 77.Thomas A D, Booth I R. The regulation of expression of the porin gene ompC by acid pH. J Gen Microbiol. 1992;138:1829–1835. doi: 10.1099/00221287-138-9-1829. [DOI] [PubMed] [Google Scholar]
- 78.Uchiya K, Barbieri M A, Funato K, Shah A H, Stahl P D, Groisman E A. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uhl M A, Miller J F. Bordetella pertussis BvgAS virulence control system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 80.Valdivia R H, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol. 1996;22:367–378. doi: 10.1046/j.1365-2958.1996.00120.x. [DOI] [PubMed] [Google Scholar]
- 81.Valdivia R H, Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277:2007–2011. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]