Abstract
Crassulacean acid metabolism (CAM) is a mode of photosynthesis that evolved in response to decreasing CO2 levels in the atmosphere some 20 million years ago. An elevated ratio of O2 relative to CO2 caused many plants to face increasing stress from photorespiration, a process exacerbated for plants living under high temperatures or in water-limited environments. Today, our climate is again rapidly changing and plants’ ability to cope with and adapt to these novel environments is critical for their success. This review focuses on CAM plant responses to abiotic stressors likely to dominate in our changing climate: increasing CO2 levels, increasing temperatures, and greater variability in drought. Empirical studies that have assessed CAM responses are reviewed, though notably these are concentrated in relatively few CAM lineages. Other aspects of CAM biology, including the effects of abiotic stress on the light reactions and the role of leaf succulence, are also considered in the context of climate change. Finally, more recent studies using genomic techniques are discussed to link physiological changes in CAM plants with the underlying molecular mechanism. Together, the body of work reviewed suggests that CAM plants will continue to thrive in certain environments under elevated CO2. However, how CO2 interacts with other environmental factors, how those interactions affect CAM plants, and whether all CAM plants will be equally affected remain outstanding questions regarding the evolution of CAM on a changing planet.
The evolutionary history, physiology, and molecular function of CAM photosynthesis provides clues as to how CAM plants will fare under future climate change scenarios.
Background
Whereas photosynthesis is arguably the most central component of a plant’s metabolism, aspects of the photosynthetic machinery have evolved in response to environmental stressors. In particular, the carbon reactions (or “dark” reactions) are susceptible to conditions that promote photorespiration (Box 1) in plants, such as high temperatures or a lack of water. RuBisCO—the enzyme that catalyzes the fixation of atmospheric CO2 in C3 plants—can fix both CO2 and O2. When RuBisCO interacts with O2, plants undergo photorespiration, a process that expends energy with no net carbon gain. High temperatures favor O2 fixation over CO2 due to the enzymatic kinetics of RuBisCO and a lack of water promotes stomatal closure, limiting the amount of CO2 available to RuBisCO. To combat this photorespiratory stress, some plants use carbon concentrating mechanisms (CCMs) to actively increase CO2 concentrations around RuBisCO. C4 photosynthesis and Crassulacean acid metabolism (CAM) are the two major CCMs in angiosperms, and each has evolved many times independently across diverse plant lineages (Keeley and Rundel, 2003; Edwards and Ogburn, 2012; Edwards, 2019).
Box 1.
Photorespiration and the evolution of CCMs
RuBisCO is both a carboxylase and an oxygenase, and under certain conditions, RuBisCO’s oxygenation function increases, leading to photorespiration (Bauwe et al., 2010; Eisenhut et al., 2019). Environmental conditions can cause an increase in oxygenation and therefore photorespiration: high temperatures alter RuBisCO’s enzyme kinetics to increase its specificity for O2, whereas drought conditions force the closure of stomata, leading to a draw down of available CO2 for RuBisCO. Photorespiration refers specifically to the process by which plants remove O2 from the substrate (ribulose bisphosphate, or RuBP) so that it can be available again for CO2. Photorespiration can be a costly process, accounting for greater than 20% yield losses in agricultural crops (Walker et al., 2016). Although photorespiration can have other benefits, including aiding in nitrogen and serine metabolism (Eisenhut et al., 2019), high rates of photorespiration can be detrimental to plant growth. Plants have evolved CCMs to cope with photorespiratory stress (Sage et al., 2012; Mallmann et al., 2014); CCMs concentrate CO2 around the site of RuBisCO carboxylation, preventing RuBisCO from interacting with O2 in any meaningful way (Keeley and Rundel, 2003; Raven et al., 2008).
CCMs function to increase the levels of CO2 around RuBisCO but accomplish it in different ways (Sage et al., 2012; Edwards, 2019). C4 plants spatially separate the initial atmospheric carbon capture from the subsequent RuBisCO-catalyzed conversion of carbon into sugars (Björkman and Gauhl, 1969; Berry et al., 1970; Edwards et al., 1970). C4 plants use phosphoenolpyruvate carboxylase (PPC) expressed in the mesophyll cells to initially convert CO2 (in the form of bicarbonate) into an organic acid, typically malic acid (Kortschak et al., 1965; Hatch and Slack, 1966, 1968). This acid is shuttled from the mesophyll to the adjacent bundle sheath cells, where it is decarboxylated, creating high concentrations of CO2 around RuBisCO. CAM plants use a nearly identical biochemical pathway (Thomas, 1949), but the separation of atmospheric CO2 capture and RuBisCO function happens temporally, rather than spatially. CAM plants open stomata for gas exchange predominantly during the night, convert incoming CO2 into malic acid, and store that acid over the night period in the vacuole (Osmond, 1978). During the day, malic acid is moved out of the vacuole for decarboxylation, providing high CO2 concentrations around RuBisCO. CAM plants, by limiting the bulk of gas exchange to the night period, reduce evapotranspiration and are considered to be considerably more water-use efficient than either C3 or C4 plants (Nobel, 1991; Borland et al., 2014).
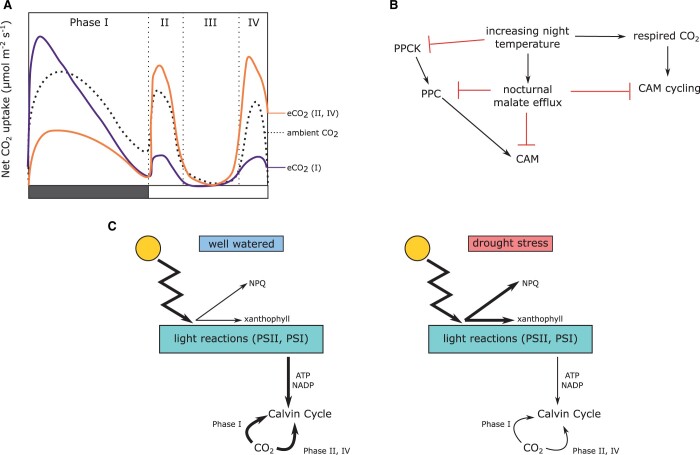
CAM is found in over 33 plant families (Winter and Smith, 1996; Cushman, 2001), including desert species, tropical epiphytes, and even aquatic plants, resulting in an impressive amount of phenotypic variation (Dodd et al., 2002). CAM is typically divided into four distinct phases: phase I includes nocturnal CO2 uptake and carboxylation into malic acid; phase II is distinguished by an early morning stomatal opening and carboxylation via RuBisCO, before malate is decarboxylated from the vacuole; phase III is a period of stomatal closure during the majority of the day period, while malate is decarboxylated; and phase IV is characterized by stomatal opening and RuBisCO carboxylation driven by a draw down of malate concentrations (Figure 1A). The degree to which a plant obtains its carbon via these phases delineates different variants of CAM (Box 2). For example, constitutive CAM plants are those that take up the majority of their carbon via phase I; they are often treated as immune to environmental conditions; however, numerous studies have shown constitutive CAM species can modulate the proportion of CAM used depending on their environmental conditions (Nobel, 1988; Heyduk et al., 2016). C3+CAM species can obtain carbon through both C3 and CAM pathways, including during phase III (Figure 1B), whereas facultative CAM species can upregulate phase I in response to abiotic stressors, such as drought and salt stress (Figure 1C).
Figure 1.
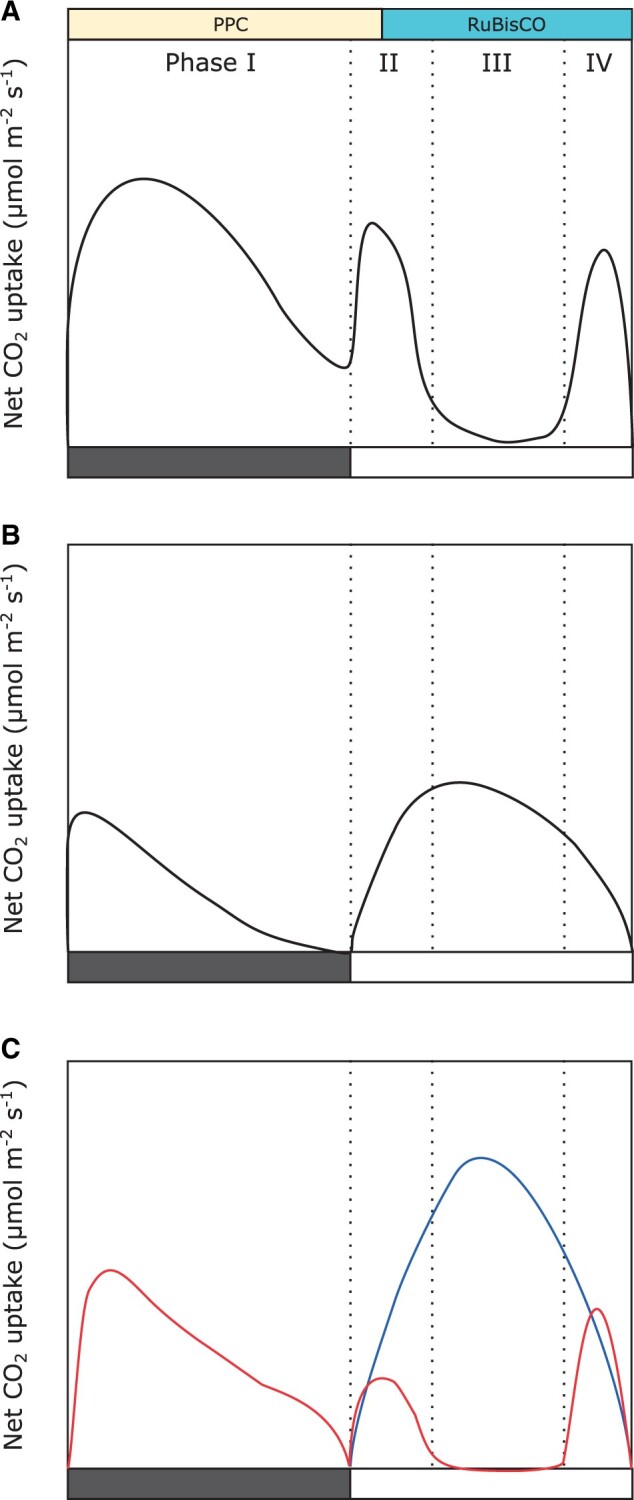
Daily gas exchange in the different types of CAM. General gas exchange plots for (A) constitutive CAM species, (B) C3+CAM species, and (C) facultative CAM species (well watered = blue, drought = red) across the four typical phases of CAM. Filled and unfilled boxes at the bottom of each graph represent night and day, respectively. After Winter and Smith (1996).
Box 2.
Types of CAM photosynthesis
Constitutive CAM: when the majority of CO2 is derived from nocturnal carboxylation via PPC. Constitutive CAM will still have phases II and IV stomatal opening and some carboxylation via RuBisCO.
C3+CAM: the use of a mix of both pathways, though a substantial amount of CO2 is obtained via the C3 pathway. C3+CAM plants can also be facultative (see below). The typical threshold for defining this category of photosynthesis is that more than half of CO2 is obtained via C3.
Facultative CAM: a CAM cycle that can be up-regulated in response to abiotic stress, including drought and salt stress. Typically found in plants that use C3+CAM.
Like C4, CAM is thought to have evolved in response to decreasing CO2 levels in the atmosphere some 20–30 million years ago (Edwards and Ogburn, 2012). The drop in atmospheric CO2 relative to O2 in the Miocene would have led to higher rates of photorespiration in C3 plants, particularly in high-light and warm environments (Ehleringer et al., 1991). Phylogenetic studies show C4 grasses diversified during the Miocene (Grass Phylogeny Working Group II, 2012), and similar ages are estimated for a number of eudicot C4 lineages (Christin et al., 2011). CAM lineages likewise originated and diversified in the Miocene (Good-Avila et al., 2006; Arakaki et al., 2011; Hernández-Hernández et al., 2014). Whereas past environmental conditions may have promoted the evolution of C4 and CAM, less is known about how future climate scenarios will affect plants using CCMs, particularly CAM. Increasing atmospheric CO2 concentrations on their own should minimize the benefit of CCMs over C3 photosynthesis, but climate change is not univariate: in addition to increasing CO2, rising temperatures, and increasingly variable precipitation will interact to create novel environments. Herein described is what is known about how CAM species respond to environmental perturbations, including what has been learned from recent genomics studies in CAM stress tolerance, to hypothesize how CAM species will fare in a changing global climate.
CAM under stress
The intrinsic stress-adapted properties of CAM plants have garnered attention for the potential use of CAM species as climate-proofed food and fuel crops; a recent review of their leaf properties discussed both adaptations to potential future climate scenarios, as well as the implications of those adaptations (e.g. tissue succulence) to biofuel production (Pereira et al., 2021). Others have reviewed the predicted responses of CCMs more generally, particularly focusing on C4 species, which have a larger body of prior research (Sage and Stata, 2021). Here, three specific environmental changes are considered—elevated CO2, higher temperatures, and drought stress—and their effects on CAM.
CO2
CCMs elevate CO2 concentrations inside photosynthetic cells, theoretically saturating RuBisCO with CO2 so that photorespiration is minimized. However, CAM plants still have direct carboxylation of atmospheric CO2 by RuBisCO, particularly late in the day during phase IV. It is worth noting that photorespiratory genes are still present and active in CAM plants (Whitehouse et al., 1991; Lüttge, 2010; Heyduk et al., 2019), photorespiration provides benefits to plants through nitrogen and serine metabolism (Eisenhut et al., 2019), and generally the degree to which photorespiration is reduced in CAM species is unclear. The reliance on both PPC and RuBisCO in CAM plants has resulted in varying empirical responses to elevated CO2 levels, though in general CAM plants respond to elevated CO2 with increased biomass (Nobel and Hartsock, 1986; Drennan and Nobel, 2000; Ceusters and Borland, 2011). However, the mechanism of increased biomass varies; some species have increased nocturnal CO2 uptake under elevated CO2, whereas in others daytime CO2 uptake increases at the expense of nocturnal carboxylation (Drennan and Nobel, 2000; Figure 2A). An increase in nocturnal PPC carboxylation is unexpected: PPC is CO2 specific, unlike RuBisCO, and therefore CO2 does not need to compete with O2. In other words, PPC is saturated at current atmospheric CO2 levels (Drennan and Nobel, 2000). However, PPC is thought to require carbonic anhydrase (CA), an enzyme that converts atmospheric CO2 into bicarbonate (HCO3−), which greatly speeds up the process relative to passive CO2 → HCO3−. Recent functional genetics and genomic studies have shown limited activity of CA in both C4 and CAM species (Studer et al., 2014; Brilhaus et al., 2016; Heyduk et al., 2019; Wai et al., 2019). If CA is indeed low-functioning or non-functional and plants are relying on passive conversion of CO2 to bicarbonate, increases in atmospheric CO2 could increase the rate of CO2 to bicarbonate conversion in the absence of CA, and thus increase overall nocturnal carboxylation rates. Additionally, Agave deserti grown in elevated CO2 levels had nocturnal CO2 assimilation rates that peaked early in the night and dropped off (Nobel and Hartsock, 1979), perhaps indicating a limitation on storage of malate. In other words, the anatomy of the leaves and the function of the vacuole in CAM species are important when interpreting CAM responses to increased CO2 (Töpfer et al., 2020) (see the “Anatomical considerations” and “Temperature”).
Figure 2.
Possible effects of environmental perturbations on CAM. A, Effects of elevated CO2 concentrations on the four phases of CAM (based on data summarized in Drennan and Nobel, 2000). Filled and unfilled boxes at the bottom represent night and day, respectively. Dashed line showed standard net CO2 uptake curve at ambient CO2 levels, whereas purple and orange lines indicate two possible CAM responses: increase in phase I CO2 uptake (purple) or increase in phases II and IV uptake (orange). B, Schematic showing possible effects of increasing night temperatures on enzymes, malate efflux from the vacuole, and alterations to the carbon cycle in CAM plants. C, Changes to energy demands and dissipation under well-watered and drought-stress conditions for a CAM plant, where CO2 can be supplied either by phase I or phases II and IV. Arrow thickness indicates the amount of flow through that part of the pathway. Drought stress can reduce CO2 uptake and result in an imbalance of energy entering the system versus energy required; excess energy can be dissipated via NPQ and xanthophyll. ATP, adenosine triphosphate; NADP, nicotinamide adenine dinucleotide phosphate.
The effects of elevated CO2 on facultative or C3+CAM species are even less studied than the responses of constitutive CAM species. Unlike constitutive CAM, C3+CAM plants are presumably not saturating RuBisCO via the CAM carboxylation pathway, and so could benefit from increased CO2 levels in the atmosphere. Indeed, in a meta-analysis of C3, C4, and CAM plants, overall biomass increases in CAM species are intermediate to that of C3 (large gains) and C4 (small gains), likely because many of the species surveyed in the study have facultative CAM ability (Poorter and Navas, 2003). Even so, there were only nine CAM species available for the meta-analysis, precluding generalizations to all CAM species. This analysis also did not analyze carbon uptake patterns, so little is known about whether C3+CAM or facultative CAM plants will up- or downregulate across the four phases. The large phenotypic diversity and flexibility of CAM—both within species and across lineages—likely means there is a high variability in CAM species’ responses to elevated CO2. Much of this variation will be driven by elevated CO2 interactions with other environmental aspects, such as temperature and water availability.
Temperature
The effects of temperature on any physiological process are often integrated across biological levels; in the case of CAM, aspects of stomatal behavior, water relations, and enzymatic activity are all involved. CAM plants have the highest levels of nocturnal CO2 fixation when nighttime temperatures are sufficiently lower than during the day—typically 5°C–10°C (Nobel, 1988). One potential explanation for this characteristic day–night difference in optimal temperatures lies in the properties of the tonoplast or the vacuolar membrane (Kluge and Schomburg, 1996). Tonoplast membrane properties change in response to temperatures: at high temperatures, they become more permeable, allowing greater rates of passive efflux of malate from the vacuole (Friemert et al., 1988; Kliemchen et al., 1993); cooler temperatures solidify the lipid membrane and prevent efflux. Despite these intrinsic properties, the tonoplast was found to play a role in high temperature acclimation in CAM species via increased rigidity of the membrane, but this acclimation came at the cost of daytime efflux of malate from the vacuole (Kliemchen et al., 1993). More recent work showed that although the tonoplast appears to be acclimating to warmer temperatures via an increase in the proportion of proteins:lipids in the membrane, the lipids were increasingly composed of unsaturated fatty acids, allowing the tonoplast to maintain fluidity even while overall rigidity increased (Lin et al., 2008). Lüttge (2000) postulated that the tonoplast serves as a central regulator of CAM, with more control over the entire pathway than even the expression of PPC; additional research in this area is warranted to understand the role of the tonoplast membrane in the regulation of CAM across a range of temperatures and species (Kluge and Schomburg, 1996).
Whereas movement of malic acid from the vacuole may occur partially through a passive mechanism, influx into the vacuole is thought to happen due to an electrochemical gradient generated by the active pumping of H+ into the vacuole (Lüttge and Ball, 1979; Cheffings et al., 1997; Holtum et al., 2005). There is also evidence for efflux from the vacuole via a malate transporter in the tonoplast (Emmerlich et al., 2003; Wai et al., 2017), but additional studies are needed to verify this transporter’s role in CAM malate movement. The rate of efflux from the vacuole and subsequent decarboxylation of malate can impact the duration of daytime phases of CAM (Grams et al., 1997; Dever et al., 2015; Ceusters et al., 2021). Temperature changes affecting malate movement across the tonoplast are therefore important considerations for understanding CAM plants under climate change.
Enzymes involved in CAM biochemistry have temperature optima and can be adversely affected by higher or lower temperatures. PPC, the main carboxylating enzyme in the CAM pathway, is a critical component of nocturnal carbon fixation. Comparisons between CAM Crassula argentea and C4Zea mays showed that whereas the C4 enzyme had a detrimental conformational change at temperatures above 27°C, the CAM enzyme continued to have increased activity at higher temperatures (Wu and Wedding, 1987). Similar experiments in Kalanchoe species show PPC enzyme activity is higher under warmer temperatures (Lee et al., 2007), but negative regulation via malate sensitivity also increases (Carter et al., 1995). This negative regulation by malate is avoided in CAM plants at night by the phosphorylation of PPC by a dedicated kinase, PPC kinase (PPCK). PPCK mRNA expression increases with increasing temperatures (Hartwell et al., 1996; Borland et al., 1999), though this has not been explored at temperatures higher than 30°C. Whereas enzyme activities appear to increase at warmer temperatures, future research should experimentally test temperatures >30°C and effects on additional CAM enzymes. Moreover, broadening these studies to include transcription and translation in addition to enzyme activity will help to better characterize the complex response of CAM biochemistry to increasing temperatures (Figure 2B).
CAM plants will also be differentially affected by temperature extremes depending on the extent to which they rely on the CAM pathway for carbon gain. Whereas higher nocturnal temperatures decreased transpiration and overall photosynthesis in CAM species (Neales, 1973; Neales et al., 1980), higher daytime temperatures did not affect daytime conductance as greatly (Nobel and Hartsock, 1979; Nobel, 1988). In other words, CAM plants that have appreciable C3 photosynthesis during the day may not be as affected by higher temperatures in terms of overall total carbon gain. High daytime temperatures will also affect other processes in CAM leaves, including light reactions and general cell stress, but those are unlikely to be unique to CAM plants. Increased nocturnal temperatures might limit atmospheric CO2 fixation, but will also increase respiration; many CAM plants can take respired CO2 and convert it to malic acid for subsequent daytime fixation. Studies have shown that whereas some CAM species can have negligible CO2 fixation at night under high temperatures, malic acid accumulation remains high, suggesting a recycling of respired CO2 that can act as a buffer against photosynthetic loss under warmer night temperatures (Medina and Osmond, 1981; Nobel, 1988). However, negative feedback loops described above (e.g. malate efflux and decreased PPC activity) might hinder even CAM cycling under extreme temperatures (Figure 2B).
The diversity of CAM species both across the green plant phylogeny, as well as across habitat types, means any prediction of how CAM plants will fare under changing temperatures will need to be conditioned on their current temperature preferences. For example, there is no single temperature optimum for all CAM plants experimentally examined to date. In fact, work in Agave showed that optimal day/night temperature regimes for different species unsurprisingly mirrored temperatures found in their natural habitats; in another study, temperature had relatively little effect on biomass of Agave angustifolia (Holtum and Winter, 2014). In Kalanchoe species, plants acclimated to lower day/night temperature regimes did not have an endogenous CAM cycle at elevated temperatures, whereas plants acclimated to higher temperatures for 4 weeks were able to maintain CAM function at high temperatures (Grams et al., 1995; Yamori et al., 2014).
Drought
CAM is often discussed in the context of water limitation and whereas that is undoubtedly a major factor in the evolution and success of CAM plants, responses to drought in CAM plants are variable. Much of the drought-response research has understandably focused on C3+CAM species, particularly those that can facultatively upregulate CAM in response to drought (Winter et al., 2008; Fleta-Soriano et al., 2015; Winter, 2019; Heyduk et al., 2020). Indeed, how facultative CAM plants sense drought stress and induce CAM photosynthesis remains unanswered. Less research has focused on how constitutive CAM plants respond to drought stress, even though abiotic factors can affect nocturnal CO2 carboxylation rates (Winter, 2019). In seedlings of constitutive Clusia and Kalanchoe species, drought induced elevated nocturnal CO2 fixation rates (Winter et al., 2008) and in CAM Yucca aloifolia, drought stress reduced nocturnal CO2 fixation (Heyduk et al., 2016). The cause of the different drought responses may be differences across lineages or the ages of the plants studied (i.e. seedlings versus mature), but additional studies are required for generalizable conclusions.
Water limitation varies in strength and duration across the year for many plants and CAM plants are no different. Comparisons of CAM and C3 tank bromeliads during the dry season in the Campo Rupestre of Brazil showed that whereas CAM plants could maintain metabolism under drought via nocturnal photosynthesis, it came at a cost: CAM bromeliads lost larger amounts of leaf water whereas the C3 species conserved water by severely limiting stomatal opening (Marques et al., 2021). The constitutive CAM saguaro cactus (Carnegiea gigantea) exhibits seasonal variation in the levels of CAM and C3 photosynthesis employed (Bronson et al., 2011). Extreme drought over 6 months shifted constitutive CAM Phalaenopsis “Edessa” plants to CAM idling, whereby atmospheric CO2 assimilation is abolished but respired CO2 is refixed (Ceusters et al., 2019). Clearly, CAM species are also negatively affected by drought conditions, but can persist under those conditions for far longer than C3 or C4 species (Nobel, 1991). Additional studies on CAM species responses to drought stress are needed to better understand the diversity of mechanisms underlying their tolerance, as well as the variation in this trait across CAM phenotypes and lineages (though see “CAM under stress in the genomics era” for examples of drought studies on facultative CAM species).
In addition to empirical evidence, models of CAM photosynthesis have been developed to estimate productivity of CAM species on marginal lands (Hartzell et al., 2021), to assess limitations to and benefits of the CAM pathway (Töpfer et al., 2020), and to compare overall potential productivity of CAM species to those of C3 and C4 (Hartzell et al., 2018; Shameer et al., 2018). These models parameterize water relations and thus can make predictions of how plants fare under simulated drought conditions. Specifically, CAM had higher carbon gain compared with C3 and C4 under a prolonged drought and had overall lower transpiration rates, suggesting their ability to maintain a water supply near the roots for a longer period of time (Hartzell et al., 2018). A separate simulation study found that although CO2 uptake was maintained under drought in CAM, CO2 uptake rates decreased relative to control, particularly in phases II and IV, and eventually affecting even phase I (Bartlett et al., 2014). The existence of models that have parameterized the complexity of CAM systems—from circadian rhythms and water limitation to carbon assimilation and irradiance—holds great promise for simulations to understand how environmental perturbations like drought, temperature, and CO2 levels will affect CAM species.
Beyond carbon: abiotic stress effects on photosystems
Environmental perturbations of water availability, temperature, and CO2 concentration will impact carbon capture efficiency and overall growth of CAM plants. But like any organism, CAM plants are complex systems and focusing only on CO2 uptake is narrow in scope. In particular, the carbon reactions of photosynthesis are directly tied to the light reactions; energy generated by photosystems II and I (PSII and PSI) is used in the Calvin cycle in all plants, regardless of any CCM. In CAM plants, both the light reactions and the Calvin cycle are temporally separated from the nocturnal CO2 fixation via PPC. The amount of CO2 decarboxylated during the day during phase III, and the amount of CO2 obtained from the atmosphere in phases II and IV, impacts the amount of energy from light reactions demanded by the Calvin cycle (Figure 2C). Therefore, environmental stressors like heat and drought can affect the photosystems of CAM plants directly and indirectly, the latter through their effects on carbon fixation.
In most plants, excess light will cause damage to the photosystems responsible for transferring energy through the light reactions; irreversible damage is known as photoinhibition and plants have multiple mechanisms to transfer extra energy either as heat (non-photochemical quenching, NPQ) or through other molecules (e.g. xanthophyll) (Demmig-Adams and Adams, 1992). Excess energy can also result if there is an imbalance between light availability and sink demand (i.e. the Calvin cycle). CAM plants employ the same NPQ strategies as C3 species and appear to have evolved protection mechanisms that prolong the amount of time CAM plants can survive in environmental conditions that might lead to photoinhibition. For example, 6 weeks of drought in the CAM orchid Phalaenopsis “Edessa” induced CAM idling, whereby plants had constantly closed stomata and relied on recycled, respired CO2 to maintain metabolism (Ceusters et al., 2019). Measures of photosystem performance showed reductions in photochemical activity in favor of energy dissipation, which matched the flux demands for carbon while CAM idling limited CO2 availability (Ceusters et al., 2019). Four months of drought stress in Agave salmiana resulted in a decrease in chlorophyll b content, increased NPQ, and decreased PSII function; upon re-watering, all measures of photosystem function recovered to control levels, again suggesting that the integrity of photosystems was maintained over a long duration drought (Campos et al., 2014).
A number of other studies on desert and tropical CAM plants have suggested the same idea: CAM species, under drought, have enhanced abilities to reduce photoinhibition and can resume normal function rapidly post-drought (de Mattos et al., 1999; Cela et al., 2009; Masrahi et al., 2015). The ability of CAM plants to maintain low-level metabolic function (e.g. CAM idling) under drought allows maintenance of photosystems in a way that enables rapid recovery. In this respect, climate change-induced drought stress is likely to affect carbon reactions directly in CAM plants and indirectly affect light reaction efficiency and recovery. Light reactions are of course affected by other environmental cues, including higher temperatures. The effect of high temperatures on photoinhibition processes in CAM plants has not been extensively explored.
Anatomical considerations
Aside from a shared biochemical pathway, CAM plants often (though not always) have succulent leaves or stems that can store water for use during periods of drought (Borland et al., 2018; Grace, 2019). Succulent leaf morphology additionally benefits CAM by providing large cells for malate storage, but can limit CO2 diffusion by reducing air spaces between cells (Nelson and Sage, 2008; Zambrano et al., 2014; Males, 2018). The ability of CO2 to move through the tissue is known as conductance; low conductance of CO2 during the daytime when carboxylation occurs via RuBisCO increases the proportion of oxygenation to carboxylation, and likely leads to increased photorespiratory stress in plants. CAM species Kalanchoe daigremontiana has one of the lowest internal CO2 conductance values measured in plants (Maxwell et al., 1997); such low conductance values could impede phases I, II, and IV carboxylation in CAM plants. Succulent leaves tend to also have a higher leaf mass per unit area (LMA); in general, high LMA leaves are often associated with resource-limited environments and tend to have lower nutrient concentrations than leaves with lower LMA (Reich et al., 1997; Poorter et al., 2009). A comparison of thick leaves (high LMA, both succulent and non-succulent) to thin leaves (low LMA) showed that thicker leaves will maintain lower nutrient status even under high-nutrient conditions. This leads to limitations in photosynthetic capacity, decreases the sink strength of RuBisCO, and therefore potentially limits conductance of CO2 through the leaf (Nielsen et al., 1997).
Succulent tissues store large amounts of water, in addition to being reservoirs for malate. Both the water content and the overall thickness of succulent tissues can reduce heat stress in leaves by increasing the overall thermal mass—in other words, increasing how long it takes for temperatures within the tissue to rise. For example, thicker leaves (high LMA) were shown to reduce heat stress via models comparing arid plant species (Curtis et al., 2012; Leigh et al., 2012). This was especially true under environmental conditions that limited effective evaporative cooling, such as low wind speeds (Leigh et al., 2012). The water stored in succulent leaves is, of course, best used in times of drought; succulent plants can quickly mobilize water reserves to preserve metabolic function and growth (Ogburn and Edwards, 2010), even under prolonged drought (Goldstein et al., 1991; Pimienta-Barrios et al., 2002; Nobel, 2006). The succulent nature of many arid CAM plants means they are likely to have the additional benefit of stored water under future drought regimes over C3 and C4 species.
CAM under stress in the genomics era
In the last decade, a number of studies have begun to explore the genetic components of CAM photosynthesis, with reference genomes available (Cai et al., 2015; Ming et al., 2015; Yang et al., 2017; Wai et al., 2019; Wickell et al., 2021), additional transcriptomic studies (Abraham et al., 2016; Brilhaus et al., 2016; Heyduk et al., 2018a, 2018b, 2019; Gilman et al., 2022), and exciting functional genetics and genomics research ongoing (Boxall et al., 2017, 2020; Lim et al., 2019). Most studies that have examined abiotic stressors on CAM in a genomics context have focused on drought (though see Shakeel et al., 2013), limiting our understanding of how abiotic stress response is regulated in CAM plants more generally, and providing an opportunity for further research. Nearly all studies that assess -omic changes in CAM species in response to abiotic stress focus on transcriptomic responses, with a few exceptions. Brilhaus et al. (2016) and Heyduk et al. (2019) also assessed metabolite changes and Abraham et al. (2016) assessed transcript, protein, and metabolite turnover across the diel cycle in Agave americana, but did not do so under abiotic-stress conditions. Additional studies exploring transcription to translation and the regulation of both are needed to understand the full response of CAM plants to abiotic factors.
Surprisingly, only a handful of the genomic studies in CAM have explored the effects of environmental factors on constitutive CAM species (the majority focus on facultative CAM). In the constitutive CAM Agave sisalana, over 3,000 genes had differential expression in response to drought stress, including genes involved in purine and thiamine metabolism and carbohydrate processing (Sarwar et al., 2019). This study only sampled at a single time point, however, and therefore did not capture the temporal dynamics of CAM and how they change under drought stress. In a comparison between constitutive CAM and C3+CAM species in the Agavoideae (Asparagaceae), C3+CAM species had an increase in the use of CAM photosynthesis under drought, whereas constitutive CAM species were only slightly negatively affected physiologically, evidenced by decreased leaf acid accumulation and a reduction in daytime CO2 uptake (Heyduk et al., 2018b, 2022). The same study explored differences in expression of sugar metabolism genes and stomatal regulators, as CAM plants are thought to have dampened stomatal responses to daytime opening cues (e.g. blue light) and are likely affected by drought signals differently than C3 stomata (Males and Griffiths, 2017). Sugar metabolism genes had differential expression under drought in CAM and C3+CAM species, though analysis of stomatal regulatory genes was inconclusive. A drought experiment on constitutive CAM Y.aloifolia showed overall dampening of CAM physiology after 7 days drought and an almost total restriction of daytime CO2 uptake (Heyduk et al., 2016). Gene expression of core CAM pathway genes also decreased under drought, corroborating measured physiological responses (Heyduk et al., 2019).
In facultative CAM plants, CAM is upregulated directly in response to an environmental stress. As a result, in-depth transcriptomic studies have been conducted on facultative CAM species with particular attention to drought response. Unsurprisingly, multiple studies find up-regulation of core CAM pathway transcripts in independent facultative lineages (Brilhaus et al., 2016; Heyduk et al., 2019; Wai et al., 2019; Gilman et al., 2022). Whereas the photosynthetic response to drought in these species is predictable, several other pathways are affected by drought. Expression of ABA signaling transcripts was strongly induced during drought stress in facultative CAM Talinum triangulare (Brilhaus et al., 2016). Starch and sugar metabolic genes had differential expression as well, though it’s unclear if those are direct responses to drought or related to the induction of CAM. Talinum triangulare also showed induction of light stress-response genes, increase in gene expression of catabolic processes for fatty acids, and reduced expression of genes involved in the cell cycle and DNA replication. Together the transcriptomic responses in T. triangulare suggest that although CAM induction reduces water loss, multiple aspects of the plant are still dealing with stress incurred from drought. In general, describing the discrete pathways involved in CAM upregulation and a more general stress response in facultative CAM species is difficult (Wai et al., 2019), and would require the presence of a closely related outgroup without CAM induction. Whereas closely related C3+CAM and C3 species of the genus Yucca were drought stressed (Heyduk et al., 2019), analysis of the subsequent gene expression data focused on CAM induction but not drought responses directly.
Conclusions and future directions
CAM photosynthesis is a complex trait in that it requires the careful integration of multiple aspects of plant biology, including photosynthetic physiology and light reactions, metabolite movement, and anatomy. Whereas CAM plants are an evolutionary response to decreasing CO2 concentrations, they are almost assuredly not doomed to nonexistence under our higher CO2 future. CAM plants will likely continue to maintain an advantage of C3 and C4 in areas where water is limiting, though the extent to which that advantage continues under various CO2 concentrations and drought extremes remains unknown. Importantly, the current understanding of CAM responses to climate-change factors is based on a very small number of species, concentrated in the Agavoideae, Cactaceae, and Crassulaceae. These represent a small fraction of the total diversity of CAM plants; future research should aim to increase the breadth of species considered, particularly tropical CAM plants. On the other hand, detailed functional genetics could be expanded in model CAM systems like Kalanchoe to further understand the mechanisms by which CAM plants respond to stressors associated with climate change. Distinctions in plant responses to climate should also be explored between constitutive CAM species and those that are C3+CAM or facultatively CAM. Finally, the largest area lacking in our understanding of CAM plants under climate-change scenarios is how they will fare under the interactive effects of abiotic stress. Increasing CO2 levels also will increase temperatures and can affect long-term weather patterns. A few notable studies have examined the effect of multiple variables on CAM: Cylindropuntia imbricata (CAM) had an advantage over Bouteloua eriopoda (C3) under elevated CO2 conditions and drought stress; that advantage disappeared in well-watered conditions (Yu et al., 2019). On the other hand, CAM and C4 plants fared worse under elevated CO2 when temperature was also elevated (Wang et al., 2008). Additional studies in diverse lineages are required to fully understand how CAM plants respond to the interactions of abiotic stress.
CAM plants represent a sizable fraction of the flora on the planet and are important species in both tropical and arid environments. The current body of empirical evidence has barely scratched the surface of understanding how CAM plants respond to abiotic stress and the implications for climate change (see “Outstanding Questions”). The effects of climate change are already being seen. Our ability to predict threats to species and ecosystems requires further interrogation into how CAM species will fare under a changing climate.
ADVANCES.
Climate change will impact plant growth and productivity not only through increasing CO2 concentrations, but also via the interactive effects of CO2, increasing temperatures, and increasing rainfall variability.
The response of CAM plants to environmental perturbation that mirrors projected climate-change extremes—including elevated CO2, higher temperatures, and drought stress—is highly variable across lineages.
Physiological and genomic analyses of CAM plant responses to drought have shown alterations to photosynthesis, carbohydrate metabolism, stomatal regulation, light reactions, and the core CAM biochemical pathway.
OUTSTANDING QUESTIONS.
What are the interactive effects of drought, elevated CO2 concentration, and high temperature on CAM photosynthesis?
How does the response to these interactions vary across lineages? For example, will tropical and arid CAM plants have fundamentally different shifts in photosynthetic physiology and fitness in response to climate change?
How will tropical species in the Orchidaceae and Bromeliaceae, which represent the largest diversity of CAM species but are relatively understudied in terms of physiological responses to environmental perturbation, respond to climate change?
Acknowledgments
Thanks to Dr. Edward McAssey, Dr. Cody Howard, Ian Gilman, and two anonymous reviewers for thoughtful discussions and comments on the manuscript.
Funding
This work was not supported by any particular funding source.
Conflict of interest statement. None declared
K.H. conducted the research and wrote the paper.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/General-Instructions) is: Karolina Heyduk (heyduk@hawaii.edu).
References
- Abraham PE, Yin H, Borland AM, Weighill D, Lim SD, De Paoli HC, Engle N, Jones PC, Agh R, Weston DJ, et al. (2016) Transcript, protein and metabolite temporal dynamics in the CAM plant Agave. Nat Plants 2: 16178. [DOI] [PubMed] [Google Scholar]
- Arakaki M, Christin P-A, Nyffeler R, Lendel A, Eggli U, Ogburn RM, Spriggs E, Moore MJ, Edwards EJ (2011) Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proc Natl Acad Sci USA 108: 8379–8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett MS, Vico G, Porporato A (2014) Coupled carbon and water fluxes in CAM photosynthesis: modeling quantification of water use efficiency and productivity. Plant Soil 383: 111–138 [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses of plants to high light stress. Annu Rev 43: 599–626 10.1146/annurev.pp.43.060192.003123 [DOI] [Google Scholar]
- Berry JA, Downton WJS, Tregunna EB (1970) The photosynthetic carbon metabolism of Zea mays and Gomphrena globosa: the location of the CO2 fixation and the carboxyl transfer reactions. Can J Bot 48: 777–786 [Google Scholar]
- Björkman O, Gauhl E (1969) Carboxydismutase activity in plants with and without β-carboxylation photosynthesis. Planta 88: 197–203 [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Jenkins GI, Wilkins MB, Nimmo HG (1999) Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in Crassulacean acid metabolism. Plant Physiol 121: 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Weston DJ, Schlauch KA, Tschaplinski TJ, Tuskan GA, Yang X, Cushman JC (2014) Engineering Crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci 19: 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Leverett A, Hurtado-Castano N, Hu R, Yang X (2018) Functional anatomical traits of the photosynthetic organs of plants with Crassulacean acid metabolism. InAdams WW III, Terashima I, eds, The Leaf: A Platform for Performing Photosynthesis. Springer International Publishing, Cham, pp 281–305 [Google Scholar]
- Boxall SF, Dever LV, Kneřová J, Gould PD, Hartwell J (2017) Phosphorylation of phosphoenol pyruvate carboxylase is essential for maximal and sustained dark CO2 fixation and core circadian clock operation in the obligate Crassulacean acid metabolism species Kalanchoë fedtschenkoi. Plant Cell 29: 2519–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall SF, Kadu N, Dever LV, Kneřová J, Waller JL, Gould PJD, Hartwell J (2020) Kalanchoë PPC1 is essential for Crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. Plant Cell 32: 1136–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhaus D, Bräutigam A, Mettler-Altmann T, Winter K, Weber APM (2016) Reversible burst of transcriptional changes during induction of Crassulacean acid metabolism in Talinum triangulare. Plant Physiol 170: 102–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson DR, English NB, Dettman DL, Williams DG (2011) Seasonal photosynthetic gas exchange and water-use efficiency in a constitutive CAM plant, the giant saguaro cactus (Carnegiea gigantea). Oecologia 167: 861–871 [DOI] [PubMed] [Google Scholar]
- Cai J, Liu X, Vanneste K, Proost S, Tsai W-C, Liu K-W, Chen L-J, He Y, Xu Q, Bian C, et al. (2015) The genome sequence of the orchid Phalaenopsis equestris. Nat Genet 47: 65–72 [DOI] [PubMed] [Google Scholar]
- Campos H, Trejo C, Peña-Valdivia CB, García-Nava R, Conde-Martínez FV, del Cruz-Ortega MR (2014) Photosynthetic acclimation to drought stress in Agave salmiana Otto ex Salm–Dyck seedlings is largely dependent on thermal dissipation and enhanced electron flux to photosystem I. Photosynth Res 122: 23–39 [DOI] [PubMed] [Google Scholar]
- Carter P, Wilkins M, Nimmo H, Fewson C (1995) Effects of temperature on the activity of phosphoenolpyruvate carboxylase and on the control of CO2 fixation in Bryophyllum fedtschenkoi. Planta 196: 375–380 [Google Scholar]
- Cela J, Arrom L, Munné-Bosch S (2009) Diurnal changes in photosystem II photochemistry, photoprotective compounds and stress-related phytohormones in the CAM plant, Aptenia cordifolia. Plant Sci 177: 404–410 [Google Scholar]
- Ceusters J, Borland AM (2011) Impacts of elevated CO2 on the growth and physiology of plants with Crassulacean acid metabolism. InLüttge UE, Beyschlag W, Büdel B, Francis D, eds, Progress in Botany Vol 72. Springer, Berlin, Heidelberg, pp 163–181 [Google Scholar]
- Ceusters N, Borland AM, Ceusters J (2021) How to resolve the enigma of diurnal malate remobilisation from the vacuole in plants with Crassulacean acid metabolism? New Phytol 229: 3116–3124 [DOI] [PubMed] [Google Scholar]
- Ceusters N, Valcke R, Frans M, Claes JE, Van den Ende W, Ceusters J (2019) Performance index and PSII connectivity under drought and contrasting light regimes in the CAM Orchid Phalaenopsis. Front Plant Sci 10: 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheffings CM, Pantoja O, Ashcroft FM, Smith JA (1997) Malate transport and vacuolar ion channels in CAM plants. J Exp Bot 48: 623–631 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Sage RF, Arakaki M, Edwards EJ (2011) C(4) eudicots are not younger than C(4) monocots. J Exp Bot 62: 3171–3181 [DOI] [PubMed] [Google Scholar]
- Curtis EM, Leigh A, Rayburg S (2012) Relationships among leaf traits of Australian arid zone plants: alternative modes of thermal protection. Aust J Bot 60: 471–483 [Google Scholar]
- Cushman JC (2001) Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol 127: 1439–1448 [PMC free article] [PubMed] [Google Scholar]
- Dever LV, Boxall SF, Kneřová J, Hartwell J (2015) Transgenic perturbation of the decarboxylation phase of Crassulacean acid metabolism alters physiology and metabolism but has only a small effect on growth. Plant Physiol 167: 44–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (2002) Crassulacean acid metabolism: plastic, fantastic. J Exp Bot 53: 569–580 [DOI] [PubMed] [Google Scholar]
- Drennan PM, Nobel PS (2000) Responses of CAM species to increasing atmospheric CO2 concentrations. Plant Cell Environ 23: 767–781 [Google Scholar]
- Edwards EJ (2019) Evolutionary trajectories, accessibility, and other metaphors: the case of C4 and CAM photosynthesis. New Phytol 223: 1742–1755 [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM (2012) Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. Int J Plant Sci 173: 724–733 [Google Scholar]
- Edwards GE, Lee SS, Chen TM, Black CC (1970) Carboxylation reactions and photosynthesis of carbon compounds in isolated mesophyll and bundle sheath cells of Digitariasanguinalis (L.) Scop. Biochem Biophys Res Commun 39: 389–395 [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW (1991) Climate change and the evolution of C4 photosynthesis. Trends Ecol Evol 6: 95–99 [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Roell M-S, Weber APM (2019) Mechanistic understanding of photorespiration paves the way to a new green revolution. New Phytol 223: 1762–1769 [DOI] [PubMed] [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleta-Soriano E, Pintó-Marijuan M, Munné-Bosch S (2015) Evidence of drought stress memory in the facultative CAM, Aptenia cordifolia: Possible role of phytohormones. PLoS ONE 10: e0135391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friemert V, Heininger D, Kluge M, Ziegler H (1988) Temperature effects on malic-acid efflux from the vacuoles and on the carboxylation pathways in Crassulacean-acid-metabolism plants. Planta 174: 453–461 [DOI] [PubMed] [Google Scholar]
- Gilman IS, Moreno-Villena JJ, Lewis ZR, Goolsby EW, Edwards EJ (2022) Gene co-expression reveals the modularity and integration of C4 and CAM in Portulaca. Plant Physiol 189: 735–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G, Andrade JL, Nobel PS (1991) Differences in water relations parameters for the Chlorenchyma and the Parenchyma of Opuntia ficus-indica under wet versus dry conditions. Funct Plant Biol 18: 95–107 [Google Scholar]
- Good-Avila SV, Souza V, Gaut BS, Eguiarte LE (2006) Timing and rate of speciation in Agave (Agavaceae). Proc Natl Acad Sci USA 103: 9124–9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace OM (2019) Succulent plant diversity as natural capital. Plants People Planet 1: 336–345 [Google Scholar]
- Grams T, Borland AM, Roberts A, Griffiths H, Beck F, Luttge U (1997) On the mechanism of reinitiation of endogenous Crassulacean acid metabolism rhythm by temperature changes. Plant Physiol 113: 1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grams TEE, Kluge M, Lüttge U (1995) High temperature-adapted plants of Kalanchoë daigremontiana show changes in temperature dependence of the endogenous CAM rhythm. J Exp Bot 46: 1927–1929 [Google Scholar]
- Grass Phylogeny Working Group II (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193: 304–312 [DOI] [PubMed] [Google Scholar]
- Hartwell J, Smith LH, Wilkins MB, Jenkins GI, Nimmo HG (1996) Higher plant phosphoenolpyruvate carboxylase kinase is regulated at the level of translatable mRNA in response to light or a circadian rhythm. Plant J 10: 1071–1078 [Google Scholar]
- Hartzell S, Bartlett MS, Inglese P, Consoli S, Yin J, Porporato A (2021) Modelling nonlinear dynamics of Crassulacean acid metabolism productivity and water use for global predictions. Plant Cell Environ 44: 34–48 [DOI] [PubMed] [Google Scholar]
- Hartzell S, Bartlett MS, Porporato A (2018) Unified representation of the C3, C4, and CAM photosynthetic pathways with the Photo3 model. Ecol Modell 384: 173–187 [Google Scholar]
- Hatch MD, Slack CR (1966) Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J 101: 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD, Slack CR (1968) A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J 106: 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández T, Brown JW, Schlumpberger BO, Eguiarte LE, Magallón S (2014) Beyond aridification: multiple explanations for the elevated diversification of cacti in the New World Succulent Biome. New Phytol 202: 1382–1397 [DOI] [PubMed] [Google Scholar]
- Heyduk K, Burrell N, Lalani F, Leebens-Mack J (2016) Gas exchange and leaf anatomy of a C3-CAM hybrid, Yucca gloriosa (Asparagaceae). J Exp Bot 67: 1369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, Hwang M, Albert V, Silvera K, Lan T, Farr K, Chang T-H, Chan M-T, Winter K, Leebens-Mack J (2018a) Altered gene regulatory networks are associated with the transition from C3 to Crassulacean acid metabolism in Erycina (Oncidiinae: Orchidaceae). Front Plant Sci 9: 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, McAssey EV, Leebens-Mack J (2022) Differential timing of gene expression and recruitment in independent origins of CAM in the Agavoideae (Asparagaceae). New Phytol doi:10.1111/nph.18267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, Ray JN, Ayyampalayam S, Leebens-Mack J (2018b) Shifts in gene expression profiles are associated with weak and strong Crassulacean acid metabolism. Am J Bot 105: 587–601 [DOI] [PubMed] [Google Scholar]
- Heyduk K, Ray JN, Ayyampalayam S, Moledina N, Borland A, Harding SA, Tsai C-J, Leebens-Mack J (2019) Shared expression of Crassulacean acid metabolism (CAM) genes predates the origin of CAM in the genus Yucca. J Exp Bot 70: 6597–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, Ray JN, Leebens-Mack J (2020) Leaf anatomy is not correlated to CAM function in a C3+CAM hybrid species, Yucca gloriosa. Ann Bot 127: 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, Smith JAC, Neuhaus HE (2005) Intracellular transport and pathways of carbon flow in plants with Crassulacean acid metabolism. Funct Plant Biol 32: 429–449 [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Winter K (2014) Limited photosynthetic plasticity in the leaf-succulent CAM plant Agave angustifolia grown at different temperatures. Funct Plant Biol 41: 843–849 [DOI] [PubMed] [Google Scholar]
- Keeley JE, Rundel PW (2003) Evolution of CAM and C4 carbon‐concentrating mechanisms. Int J Plant Sci 164: S55–S77 [Google Scholar]
- Kliemchen A, Schomburg M, Galla HJ, Lüttge U, Kluge M (1993) Phenotypic changes in the fluidity of the tonoplast membrane of Crassulacean-acid-metabolism plants in response to temperature and salinity stress. Planta 189: 403–409 [DOI] [PubMed] [Google Scholar]
- Kluge M, Schomburg M (1996) The tonoplast as a target of temperature effects in Crassulacean acid metabolism. InWinter K, Smith JAC, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer, Berlin, Heidelberg, pp 72–77 [Google Scholar]
- Kortschak HP, Hartt CE, Burr GO (1965) Carbon dioxide fixation in sugarcane leaves. Plant Physiol 40: 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W-Z, Kim B-G, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci USA 104: 15959–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh A, Sevanto S, Ball MC, Close JD, Ellsworth DS, Knight CA, Nicotra AB, Vogel S (2012) Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol 194: 477–487 [DOI] [PubMed] [Google Scholar]
- Lim SD, Lee S, Choi W-G, Yim WC, Cushman JC (2019) Laying the foundation for Crassulacean acid metabolism (CAM) biodesign: Expression of the C4 metabolism cycle genes of CAM in Arabidopsis. Front Plant Sci 10: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Wang YM, Nose A, Hong HTK, Agarie S (2008) Effects of high night temperature on lipid and protein compositions in tonoplasts isolated from Ananas comosus and Kalanchoë pinnata leaves. Biol Plant 52: 59 [Google Scholar]
- Lüttge U (2000) The tonoplast functioning as the master switch for circadian regulation of Crassulacean acid metabolism. Planta 211: 761–769 [DOI] [PubMed] [Google Scholar]
- Lüttge U (2010) Photorespiration in Phase III of Crassulacean Acid Metabolism: Evolutionary and Ecophysiological Implications. Springer, Berlin, Heidelberg, pp 371–384 [Google Scholar]
- Lüttge U, Ball E (1979) Electrochemical investigation of active malic acid transport at the tonoplast into the vacuoles of the CAM plant Kalanchoë daigremontiana. J Membr Biol 47: 401–422 [Google Scholar]
- Males J (2018) Concerted anatomical change associated with Crassulacean acid metabolism in the Bromeliaceae. Funct Plant Biol 45: 681–695 [DOI] [PubMed] [Google Scholar]
- Males J, Griffiths H (2017) Stomatal biology of CAM plants. Plant Physiol 174: 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Bräutigam A, Lercher MJ, Weber APM, Westhoff P, Gowik U (2014) The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. Elife 3: e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AR, Duarte AA, de Souza FA, de Lemos-Filho JP (2021) Does seasonal drought affect C3 and CAM tank-bromeliads from campo rupestre differently? Flora 282: 151886 [Google Scholar]
- Masrahi YS, Al-Turki TA, Sayed OH (2015) Photosynthetic adaptation and survival strategy of Duvalia velutina in an extremely arid environment. Photosynthetica 53: 555–561 [Google Scholar]
- de Mattos EA, Herzog B, Lüttge U (1999) Chlorophyll fluorescence during CAM-phases in Clusia minor L. under drought stress. J Exp Bot 50: 253–561 [Google Scholar]
- Maxwell K, von Caemmerer S, Evans JR (1997) Is a low internal conductance to CO2 diffusion a consequence of succulence in plants with Crassulacean acid metabolism? Aust J Plant Physiol 24: 777 [Google Scholar]
- Medina E, Osmond CB (1981) Temperature dependence of dark CO2 fixation and acid accumulation in Kalanchoe daigremontiana. Funct Plant Biol 8: 641–649 [Google Scholar]
- Ming R, VanBuren R, Wai CM, Tang H, Schatz MC, Bowers JE, Lyons E, Wang M-L, Chen J, Biggers E, et al. (2015) The pineapple genome and the evolution of CAM photosynthesis. Nat Genet 47: 1435–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neales TF (1973) The effect of night temperature on CO2 assimilation, transpiration, and water use efficiency in Agave americana L. Aust J Bio Sci 26: 705–714 [Google Scholar]
- Neales TF, Sale PJM, Meyer CP (1980) Carbon dioxide assimilation by pineapple plants, Ananas comosus (L.) Merr. II. Effects of variation of the day/night temperature regime. Funct Plant Biol 7: 375–385 [Google Scholar]
- Nelson EA, Sage RF (2008) Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. J Exp Bot 59: 1841–1850 [DOI] [PubMed] [Google Scholar]
- Nielsen SL, Enríquez S, Duarte CM (1997) Control of PAR-saturated CO2 exchange rate in some C3 and CAM plants. Biol Plant 40: 91–101 [Google Scholar]
- Nobel PS (1991) Achievable productivities of certain CAM plants: basis for high values compared with C3 and C4 plants. New Phytol 119: 183–205 [DOI] [PubMed] [Google Scholar]
- Nobel PS (1988) Environmental Biology of Agaves and Cacti. Cambridge University Press [Google Scholar]
- Nobel PS (2006) Parenchyma–chlorenchyma water movement during drought for the hemiepiphytic cactus Hylocereus undatus. Ann Bot 97: 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS, Hartsock TL (1986) Short-term and long-term responses of Crassulacean acid metabolism plants to elevated CO(2). Plant Physiol 82: 604–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS, Hartsock TL (1979) Environmental influences on open stomates of a Crassulacean acid metabolism plant, Agave deserti. Plant Physiol 63: 63–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn RM, Edwards EJ (2010) Chapter 4—The ecological water-use strategies of succulent plants. InKader J-C, Delseny M, eds, Advances in Botanical Research. Academic Press, pp 179–225 [Google Scholar]
- Osmond CB (1978) Crassulacean acid metabolism: A curiosity in context. Annu Rev Plant Physiol 29: 379–414 [Google Scholar]
- Pereira PN, Niechayev NA, Blair BB, Cushman JC (2021) Chapter 10—Climate change responses and adaptations in crassulacean acid metabolism (CAM) plants. In KM Becklin, JK Ward, DA Way, eds, Advances in Photosynthesis and Respiration. Springer International Publishing, Cham, pp 283–329 [Google Scholar]
- Pimienta-Barrios E, González del Castillo-Aranda ME, Nobel PS (2002) Ecophysiology of a wild platyopuntia exposed to prolonged drought. Environ Exp Bot 47: 77–86 [Google Scholar]
- Poorter H, Navas M-L (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157: 175–198 [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182: 565–588 [DOI] [PubMed] [Google Scholar]
- Raven JA, Cockell CS, De La Rocha CL (2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Phil Trans R Soc Lond B Biol Sci 363: 2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94: 13730–13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Sage RF, Stata M (2021) Chapter 8—Terrestrial CO2-concentrating mechanisms in a high CO2 world. InBecklin KM, Ward JK, Way DA, eds, Photosynthesis, Respiration, and Climate Change. Springer International Publishing, Cham, pp 193–250 [Google Scholar]
- Sarwar MB, Ahmad Z, Rashid B, Hassan S, Gregersen PL, Leyva MD la O, Nagy I, Asp T, Husnain T (2019) De novo assembly of Agave sisalana transcriptome in response to drought stress provides insight into the tolerance mechanisms. Sci Rep 9: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel SN, Aman S, Haq NU, Heckathorn SA, Luthe D (2013) Proteomic and Transcriptomic analyses of Agave americana in response to heat stress. Plant Mol Biol Rep 31: 840–851 [Google Scholar]
- Shameer S, Baghalian K, Cheung CYM, Ratcliffe RG, Sweetlove LJ (2018) Computational analysis of the productivity potential of CAM. Nat Plants 4: 165–171 [DOI] [PubMed] [Google Scholar]
- Studer AJ, Gandin A, Kolbe AR, Wang L, Cousins AB, Brutnell TP (2014) A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a CA1CA2 double mutant in maize. Plant Physiol 165: 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M (1949) Physiological studies on acid metabolism in green plants. I. CO2 fixation and CO2 liberation in Crassulacean acid metabolism. New Phytol 48: 390–420 [Google Scholar]
- Töpfer N, Braam T, Shameer S, Ratcliffe RG, Sweetlove LJ (2020) Alternative Crassulacean acid metabolism modes provide environment-specific water-saving benefits in a leaf metabolic model. Plant Cell 32: 3689–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai CM, VanBuren R, Zhang J, Huang L, Miao W, Edger PP, Yim WC, Priest HD, Meyers BC, Mockler T, et al. (2017) Temporal and spatial transcriptomic and microRNA dynamics of CAM photosynthesis in pineapple. Plant J 92: 19–30 [DOI] [PubMed] [Google Scholar]
- Wai CM, Weise SE, Ozersky P, Mockler TC, Michael TP, VanBuren R (2019) Time of day and network reprogramming during drought induced CAM photosynthesis in Sedum album. PLoS Genet 15: e1008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, VanLoocke A, Bernacchi CJ, Ort DR (2016) The costs of photorespiration to food production now and in the future. Annu Rev Plant Biol 67: 107–129 [DOI] [PubMed] [Google Scholar]
- Wang D, Heckathorn SA, Barua D, Joshi P, Hamilton EW, Lacroix JJ (2008) Effects of elevated CO2 on the tolerance of photosynthesis to acute heat stress in C3, C4, and CAM species. Am J Bot 95: 165–176 [DOI] [PubMed] [Google Scholar]
- Whitehouse DG, Rogerrs WJ, Tobin AK (1991) Photorespiratory enzyme activities in C3 and CAM forms of the facultative CAM plant, Mesembryanthemum crystallimum L. J Exp Bot 42: 485–492 [Google Scholar]
- Wickell D, Kuo L-Y, Yang H-P, Dhabalia Ashok A, Irisarri I, Dadras A, de Vries S, de Vries J, Huang Y-M, Li Z, et al. (2021) Underwater CAM photosynthesis elucidated by Isoetes genome. Nat Commun 12: 6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K (2019) Ecophysiology of constitutive and facultative CAM photosynthesis. J Exp Bot. doi:10.1093/jxb/erz002 [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JAM (2008) On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. J Exp Bot 59: 1829–1840 [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC (1996) An introduction to Crassulacean acid metabolism. InWinter K, Smith JAC, eds, Crassulacean Acid Metabolism. Springer-Verlag, pp 1–10 [Google Scholar]
- Wu MX, Wedding RT (1987) Temperature effects on phosphoenolpyruvate carboxylase from a CAM and a C(4) Plant: A comparative study. Plant Physiol 85: 497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119: 101–117 [DOI] [PubMed] [Google Scholar]
- Yang X, Hu R, Yin H, Jenkins J, Shu S, Tang H, Liu D, Weighill DA, Cheol Yim W, Ha J, et al. (2017) The Kalanchoë genome provides insights into convergent evolution and building blocks of Crassulacean acid metabolism. Nat Commun 8: 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, D’Odorico P, Collins SL, Carr D, Porporato A, Anderegg WRL, Gilhooly WP III, Wang L, Bhattachan A, Bartlett M, et al. (2019) The competitive advantage of a constitutive CAM species over a C4 grass species under drought and CO2 enrichment. Ecosphere 10: e02721 [Google Scholar]
- Zambrano VAB, Lawson T, Olmos E, Fernández-García N, Borland AM (2014) Leaf anatomical traits which accommodate the facultative engagement of Crassulacean acid metabolism in tropical trees of the genus Clusia. J Exp Bot 65: 3513–3523 [DOI] [PubMed] [Google Scholar]