Abstract
Objectives
Fatigue is a disabling symptom in people with RA. This study aims to describe the prevalence, risk factors and longitudinal course of fatigue in early RA.
Methods
Demographic, clinical, quality of life (QoL), comorbidities and laboratory data were from the Early RA Network (ERAN), a UK multicentre inception cohort of people with RA. Fatigue was measured using the vitality subscale of the 36-item Short Form Health Survey, where higher values represent better QoL. Baseline prevalences of fatigue classifications were age and sex standardized. Linear regression, hierarchical growth curve modelling and group-based trajectory modelling (GBTM) were utilized.
Results
At baseline (n = 1236, 67% female, mean age 57 years), the mean vitality was 41 (s.d. 11) and disease duration was 11 months (interquartile range 7–18). Age- and sex-standardized prevalence rates of fatigue and severe fatigue were 44% (95% CI 39, 50) and 19% (95% CI 15, 23), respectively. Fatigue changed little over 3 years and five measurement occasions β = −0.13 (95% CI −0.23, −0.02). GBTM identified two subgroups, which we named ‘Fatigue’ (53%) and ‘No-fatigue’ (47%). Female sex, worse pain, mental health and functional ability were associated with greater fatigue and predicted Fatigue group membership (area under the receiver operating characteristics curve = 0.81). Objective measures of inflammation—swollen joint count and ESR—were not significantly associated with fatigue.
Conclusions
Fatigue is prevalent and persistent in early RA. Diverse characteristics indicative of central mechanisms are associated with persistent fatigue. Management of fatigue might require interventions targeted at central mechanisms in addition to inflammatory disease modification. People who require such interventions might be identified at presentation with early RA.
Keywords: fatigue, rheumatoid arthritis, trajectories, inflammation, central mechanisms
Rheumatology key messages.
Fatigue is prevalent and remains stable regardless of improvements in inflammatory disease activity.
People with persistent fatigue could be identified early, representing targets for fatigue-lowering interventions.
Traits indicative of central mechanisms are associated with persistent fatigue in early RA.
Introduction
Fatigue is a common debilitating symptom in many musculoskeletal diseases. In RA, the prevalence of fatigue has been reported to be between 40 and 70% [1–3]. This large variation stems from a heterogeneous RA population, at various levels of disease activity, and from the use of different fatigue measurement tools. Fatigue is associated with greater healthcare utilization and worse outcomes. Fatigue is also associated with huge economic consequences, being responsible for sickness absence and loss of employment, culminating in an overall poorer quality of life (QoL) in these individuals [4].
Fatigue was recommended as a core outcome measure in RA clinical trials and a key symptom whose absence is required to indicate RA remission from the patient’s perspective more than a decade ago. However, in spite of the resulting increase in RA fatigue research, the exact causal mechanisms remain elusive [5]. Fatigue was traditionally thought to be a consequence of an inflammatory process, but despite the innovations in anti-inflammatory therapeutics, fatigue remains a problem for many people with RA [6].
Several conceptual causal models have been proposed, incorporating biopsychosocial and environmental factors as direct and cumulative causal effects. Supporting evidence is largely based on cross-sectional studies and studies involving individuals with long-standing disease [6, 7]. Few longitudinal studies describe the progression of fatigue over time, and a large proportion of these studies include people with long-standing disease and disease refractory to first-line anti-rheumatic therapy [8]. Fatigue in RA has been characterized as persistent and stable over time or to improve in some participant subgroups but persist in others. Generalization from these study findings is limited by small sample sizes and heterogeneity between study populations and contexts [8–10].
Several studies have shown the benefit of early treatment of RA on disease activity. Clinical trials have demonstrated a small reduction in fatigue levels with the use of biologic DMARDs (bDMARDs), although the extent to which these findings can be generalized to other DMARDs or to early RA is unknown [11]. This study aims to characterize the prevalence and course of fatigue and to identify characteristics associated or predictive of fatigue in people with early RA. We hypothesized that groups of individuals may exist who exhibit varying fatigue progression and that identification of these discrete trajectory groups might already be possible in individuals with early disease, based on demographic or clinical characteristics.
Methods
Data sources
Data from the Early Rheumatoid Arthritis Network (ERAN) were analysed. The ERAN has been described in detail elsewhere [12]. In brief, people with a symptom duration of <2 years were recruited on clinician diagnosis of RA from outpatient clinics in the UK and Ireland between 2002 and 2012. Participants were excluded from the cohort if they were found to have an alternative diagnosis at follow-up and thus were not analysed in this study. If participants did not attend study visits at their centre for any reason, they became lost to follow-up. Demographic, clinical and laboratory data were collected at baseline, 6 months and annually thereafter. The ERAN contains a wealth of information on early RA and has been used in several published articles on the epidemiology of early RA and in the development of National Institute of Health and Care Excellence decision rules [13]. Data from the first five assessment visits, equivalent to 3 years of follow-up, were used for longitudinal analyses in this study. Ethical approval was from the UK National Health Service (Trent Research Ethics Committee reference 01/4/047). All participants gave written informed consent and the study was conducted in accordance with the Declaration of Helsinki. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies (Supplementary Table S1, available at Rheumatology online).
Measures
Outcome
Fatigue was measured using the vitality subscale of the Medical Outcomes Study 36-item Short Form Health Survey (SF-36) [14]. In accordance with the developer’s scoring guidelines, UK population normalized values were utilized, resulting in a 0–100 scale, with higher scores signifying a better QoL. Vitality has good psychometric properties and is often used to assess fatigue in musculoskeletal research [14].
A binary (yes/no) fatigue variable was derived from the vitality subscale to estimate fatigue prevalence. The crude prevalence of fatigue and severe fatigue was estimated as the proportion of participants with vitality ≤1 s.d. and 2 s.d. of the UK population mean score of 50, respectively [15]. The minimum clinically important difference (MCID) of SF-36 vitality was 1 s.d. of UK normalized values [16]. Prevalence values standardized to the European standard population (ESP) 2013 were estimated using the R statistical software ‘Epitools’ package (R Foundation for Statistical Computing, Vienna, Austria) [17]. ESP is an artificial population structure based on the population structures of European countries. It is used to estimate age- and sex-standardized rates.
Exposures
Demographic characteristics self-reported age at the onset of disease (in years), sex (male/female), ethnicity (white/non-white), BMI (kg/m2) and smoking status (never, current and ex) were included in the analysis.
Inflammation/disease activity was measured using ESR (in mm/h), tender (TJC) and swollen joint counts (SJC) in 28 joints, each scaled 0–28, and the Patient’s Global Assessment of Disease Activity (PGA) on a 0–100 mm visual analogue scale [18]. In addition, the 28-joint DAS (DAS28), a composite index of disease activity comprising TJC, SJC, PGA and ESR, was assessed [19]. Seropositivity was classified according to the presence of RF and/or positive or borderline anti-CCP antibodies [20]. The presence or absence of nodules on clinical examination was also assessed.
Comorbidities were assessed using the Rheumatic Disease Comorbidity Index (RDCI) [21].
Pain and mental health were measured with the SF-36 bodily pain (SF36BP) and mental health (SF36MH) subscales, respectively. Like vitality, these subscales were normalized to the UK population on a 0–100 scale, with higher values representing a better QoL. The SF36MH assesses mental symptoms and psychological well-being [22, 23].
Disability/functional limitation was measured using the HAQ Disability Index (HAQ-DI). This questionnaire assesses the level of functional ability by examining functional activities such as walking, eating, reach, grip and usual activities. It is scored on a scale of 0–3, with higher scores indicating greater disability [24].
Medication use was represented by a binary (yes/no) variable, with the affirmative comprising participants prescribed DMARDs.
Disease duration was measured by the self-reported time since that onset of symptoms (in months).
Time was assessed as the study measurement occasion. The first measurement occasion was defined as that at baseline and the second was at 3–6 months. Subsequent measurements were conducted annually from the baseline. Data were used up to the fifth measurement occasion (3 years from baseline).
Statistical analysis
The overall study sample comprised data from the first five measurement occasions. Data for subsequent measurement occasions were excluded due to high attrition rates. Sample data were summarized using descriptive statistics at baseline and at each measurement occasion.
Factors associated with baseline vitality scores were examined using Spearman’s correlation coefficients whose magnitudes were interpreted according to published guidelines [25]. Baseline vitality scores were also examined using univariate and multiple linear regression, with robust standard errors. Multicollinearity was tested using the variance inflation factor (VIF). Residuals were examined graphically to assess linear regression assumptions.
Individuals with three or more vitality measurements were included in the longitudinal analysis using hierarchical growth curve analysis. Models with random intercepts for person and a random slope for person and time were used to capture between-person variability over time [26]. Linear, quadratic and linear spline trajectories were investigated. Following trajectory selection, the unconditional (model without covariates) and conditional (with covariates) models were examined. The model that best suited the data based on the Bayesian information criterion (BIC), log-likelihood (LL) and Akaike information criterion (AIC) was selected [27]. Missing data were considered missing at random (MAR) and handled using multiple imputation using chained equations, accounting for the longitudinal structure of the data. A total of 100 imputations using 10 burn-in iterations were conducted using the ‘miceadds’ R package [28, 29].
Fatigue trajectories were investigated using group-based trajectory modelling (GBTM). GBTM is an application of finite mixture models that gathers individuals into subgroups with similar growth trajectories based on the estimated probability of group membership. Model fit was assessed using AIC, BIC, LL and entropy. Entropy indicates how well groups are separated and how well individuals fit into their respective groups [30]. The resultant group assignment was assessed using posterior probabilities and odds of correct classification. Posterior probabilities are the probabilities of group membership derived from parameter estimates of the model. An average posterior probability of >0.7 and odds of correct classification >5 for each group were deemed acceptable [31]. GBTM was conducted using the Stata ‘Traj’ plugin [31].
Baseline risk factors for fatigue group membership were investigated using univariate and multivariable logistic regression analysis. Model discrimination was examined using the area under the receiver operating characteristics (ROC) curve.
Sensitivity analyses were conducted by restricting the study population to participants who had a self-reported disease duration of <6 months and restricting longitudinal analysis to participants with five complete vitality measurements.
Analyses were performed using Stata 16 (StataCorp, College Station, TX, USA) and R. P-values ≤0.05 were considered statistically significant unless stated otherwise.
Results
The ERAN sample at baseline comprised 1236 participants, 992 of whom had vitality scores recorded at baseline. Three or more vitality scores were recorded by the fifth measurement occasion for 792 participants, with vitality scores for 493 individuals in year 3 (Supplementary Fig. S1, available at Rheumatology online).
Table 1 describes the study sample at baseline. The sample was predominantly female (67%), of white ethnicity (96%), with a mean age of 57 years (s.d. 14) and a mean vitality score of 41 (s.d. 11). A total of 61% of participants were seropositive. The mean pain and mental health scores at baseline were 33 (s.d. 10) and 42 (s.d. 11), respectively, and the median disease duration was 11 months [interquartile range (IQR) 7–18]. The mean baseline DAS 28 with ESR (DAS28-ESR) was 4.7 (s.d. 1.6). Descriptive characteristic of the ERAN population at each measurement occasion showed an improvement in disease activity/inflammatory characteristics (TJC, SJC, PGA, DAS28-ESR) and HAQ (Supplementary Table S2, available at Rheumatology online).
Table 1.
Overview of the ERAN population at baseline
| Characteristics | Values |
|---|---|
| SF-36 vitality, mean (s.d.) | 41.82 (11.14) |
| Demographics | |
| Age at onset, years, mean (s.d.) | 57.01 (14.03) |
| Female, n (%) | 839 (67.88) |
| White ethnicity, n (%)* | 1196 (97.00) |
| BMI, kg/m2, median (IQR) | 26.84 (16.80–29.76) |
| Never smoker, n (%) | 477 (40.02) |
| Ex-smoker, n (%) | 404 (33.89) |
| Current smoker, n (%) | 311 (26.09) |
| Disease activity/inflammation | |
| DAS28-ESR, mean (s.d.) | 4.68 (1.56) |
| TJC, median (IQR) | 5.00 (2–11) |
| SJC, median (IQR) | 4.00 (1–9) |
| Seropositive, n (%) | 655 (61.04) |
| Erosions present, n (%) | 331(29.4) |
| Nodule present, n (%) | 100 (9.52) |
| Haemoglobin, mg/dl, mean (s.d.) | 13.10 (1.44) |
| ESR, mm/h, median (IQR) | 24.00 (12–41) |
| PROMs, mean (s.d.) | |
| SF36BP | 33.77 (10.63) |
| SF36MH | 46.34 (11.15) |
| HAQ | 1.08 (0.76) |
| PGA | 43.50 (25.6) |
| Medication/comorbidity | |
| Medication use, n (%) | 935 (78.31) |
| RDCI, median (IQR) | 1.00 (0–2) |
| Disease duration, months, median (IQR) | 12.00 (7–18) |
PROMs: patient-reported outcome measures.
Prevalence and associations of fatigue at baseline
More than 75% of the study population reported lower vitality scores than the mean UK general population value of 50. The age- and sex-standardized prevalence of fatigue and severe fatigue at baseline was 44% (95% CI 39, 50) and 19% (95% CI 15, 23), respectively. Similar prevalence values were seen in participants with a disease duration of <6 months (Supplementary Table S3, available at Rheumatology online).
Pain and mental health scores showed moderate positive correlation with vitality scores at baseline (r = 0.54 and 0.61, respectively; P ≤ 0.05). The HAQ was negatively correlated with vitality (r = −0.50, P ≤ 0.05). Associations between vitality scores and other variables at baseline were weak (Supplementary Table S4, available at Rheumatology online).
Female sex, worse pain, mental health, TJC, SJC, PGA, haemoglobin, SJC, RDCI, ESR, DAS28-ESR and higher BMI were significantly associated with vitality at baseline in the bivariate regression analysis (Supplementary Table S5, available at Rheumatology online). Table 2 describes the multivariable linear regression analysis; female sex, worse mental health, pain, HAQ and TJC were significantly associated with vitality. Collectively these characteristics explained about half of the vitality variability at baseline (R2 = 0.49). Mental health and pain showed the largest contribution to vitality (standardized β = 0.42 and 0.24, respectively). Multicollinearity was not detected (mean VIF = 1.47, none ≥ 2) and linear regression assumptions were not violated (Supplementary Fig. S2A and B, available at Rheumatology online).
Table 2.
Multivariable predictors of vitality at baseline
| Multivariable linear regression (n = 973, R2 = 0.49) | |||
|---|---|---|---|
| Characteristic | Unit | Coefficient (95% CI) | Standardized values |
| Age at onset | Years | 0.01 (−0.03, 0.05) | 0.01 |
| Sex | Female | −2.00 (−3.11, −0.86)* | −0.08 |
| TJC | 0–28 | −0.10 (−0.19, −0.02)* | −0.06 |
| Pain | 0–100 | 0.21 (0.15, 0.28)* | 0.24 |
| Mental health | 0–100 | 0.42 (0.36, 0.47)* | 0.42 |
| HAQ | 0–3 | −2.43 (−3.37, −1.50)* | −0.17 |
P ≤ 0.05. Values are linear regression coefficients.
Course of fatigue over time
A total of 729 participants were included in the longitudinal analysis. These participants appeared similar to the total ERAN population in terms of measured covariates (Supplementary Table S6, available at Rheumatology online). Missing data proportions are provided in Supplementary Fig. S3, available at Rheumatology online.
Mean vitality remained relatively stable throughout the period of the study with little variability between measurement occasions (Fig. 1 and Supplementary Fig. S4, available at Rheumatology online). The linear growth model was selected as the best fit to the data based on fit statistics (Supplementary Table S7A and B, available at Rheumatology online). From the unconditional linear growth model (model without covariates), the mean vitality score at baseline was 42.32 (95% CI 41.58, 43.06), with an increase in vitality over the study duration of β = 0.19 (95% CI 0.05, 0.33) at each measurement occasion. The fully conditional model showed little change over time [β = −0.13 (95% CI −0.23, −0.02)] and the slope of the vitality trajectory was not affected by the presence of covariates (Supplementary Table S8, available at Rheumatology online).
Fig. 1.
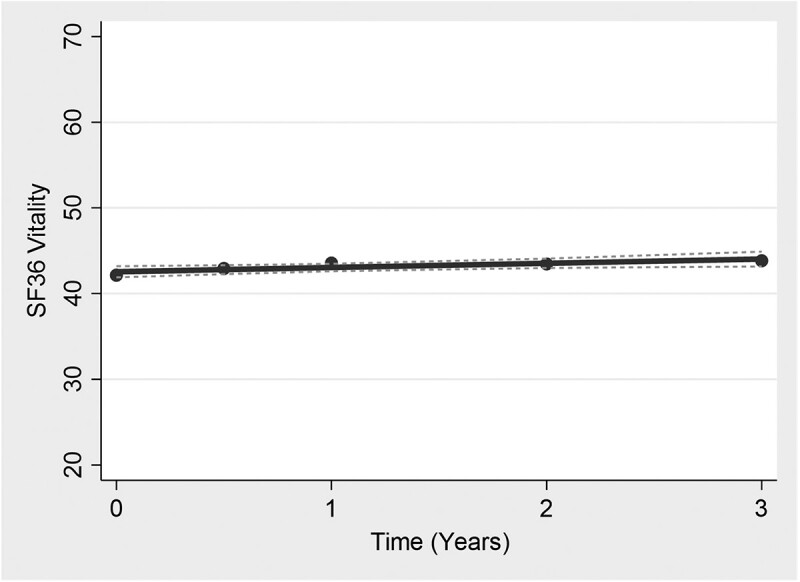
Vitality trajectories over measurement occasions
GBTM
The GBTM analysis of vitality scores revealed two trajectory groups, which we named the ‘Fatigue’ (47%) and ‘No-fatigue’ (53%) groups (Fig. 2). The two-trajectory group estimates had a lower AIC, BIC and LL and a higher entropy than a three-trajectory group model (Supplementary Table S9, available at Rheumatology online). The average posterior probabilities and odds of correct classification were 94% and 13.7 for the Fatigue group and 95% and 21.19 for the No-fatigue group, respectively (Supplementary Table S10, available at Rheumatology online). Participants in the No-fatigue group had a mean vitality score of 49.67 (95% CI 48.70, 50.64), while in the Fatigue group the mean vitality score was >1 s.d. less than the average population levels, 35.23 (95% CI 34.45, 36.00) (Supplementary Table S11, available at Rheumatology online).
Fig. 2.
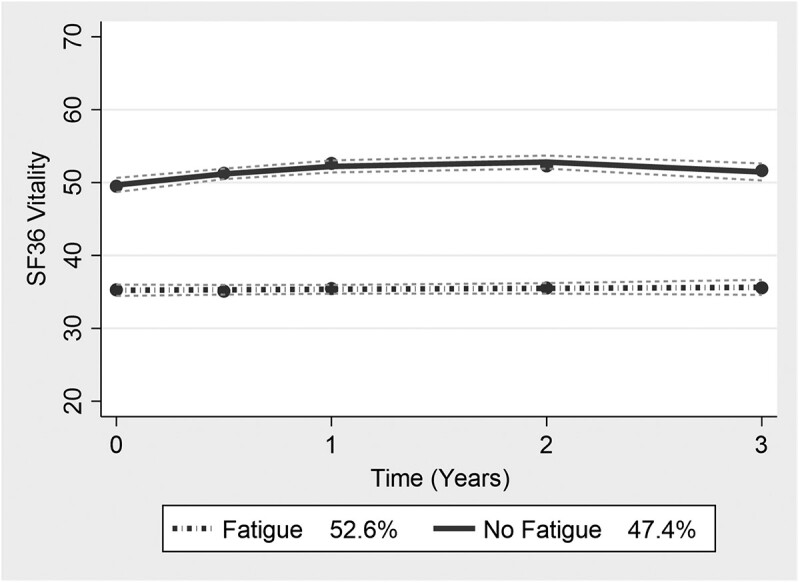
Vitality trajectory groups based on GBTM analysis
Table 3 describes the baseline characteristics of the vitality trajectory groups. Individuals in the Fatigue group were predominantly female, had a higher DAS28, worse pain and mental health and worse HAQ and PGA than those in the No-fatigue group. Table 4 shows the results of the univariate and multivariable regression analysis estimating the association between baseline characteristics and group membership. Female sex, higher BMI, higher HAQ, worse mental health, worse pain, higher DAS28 and higher RDCI at baseline were all associated with the Fatigue group. The area under the receiver operating characteristics curve was 0.81 (Supplementary Fig. S5, available at Rheumatology online).
Table 3.
Baseline characteristics based on trajectory groups
| Characteristic | Unit | Baseline |
|
|---|---|---|---|
| Group 1 (Fatigue; n = 391) | Group 2 (No-fatigue; n = 338) | ||
| Demographics | |||
| Age at onset, mean (s.d.) | Years | 56.44 (13.06) | 56.67 (14.00) |
| Sex, n (%) | Female | 292 (74.68) | 201 (59.47) |
| White ethnicity, n (%) | Yes | 384 (98.21) | 325 (96.15) |
| BMI, median (IQR) | kg/m2 | 27.91 (24.75–31.63) | 26.29 (23.72–29.36) |
| Smoking status, n (%) | |||
| Never | 137 (36.05) | 143 (43.73) | |
| Current | 109 (28.68) | 78 (23.85) | |
| Ex-smoker | 134 (35.26) | 106 (32.42) | |
| Disease activity/inflammation | |||
| DAS28-ESR, mean (s.d.) | 5.14 (1.47) | 4.39 (1.53) | |
| TJC, median (IQR) | 0–28 | 8 (3–14) | 4 (1–9) |
| SJC, median (IQR) | 0–28 | 5 (2–10) | 4 (1–9) |
| Seropositive, n (%) | Yes | 211 (63.94) | 178 (61.38) |
| Erosions, n (%) | Yes | 113 (30.87) | 100 (32.36) |
| Nodules, n (%) | Yes | 36 (10.11) | 28 (9.21) |
| Haemoglobin, mean (s.d.) | mg/dl | 13.08 (1.40) | 13.15 (1.46) |
| ESR, median (IQR) | mm/h | 27 (13–47) | 20 (12–36) |
| PROMs, mean (s.d.) | |||
| SF36BP | 0–100 | 29.21 (9.14) | 37.90 (9.14) |
| SF36VT | 0–100 | 35.20 (8.51) | 49.09 (8.39) |
| SF36MH | 0–100 | 42.33(10.84) | 52.02 (9.15) |
| HAQ | 0–3 | 1.29 (0.72) | 0.79 (0.67) |
| PGA | 0–100 | 50.74 (23.60) | 35.92 (24.09) |
| Comorbidity/medication | |||
| RDCI, median (IQR) | 0–9 | 1 (0–2) | 0 (0–1) |
| Medication, n (%) | Yes | 318 (81.33) | 291 (86.09) |
| Disease duration, median (IQR) | Months | 12 (7–23) | 13 (8–21) |
SF36VT: SF-36 vitality.
Table 4.
Baseline predictors of vitality group membership
| Variable | Univariate logistic regression |
Multiple logistic regression |
|
|---|---|---|---|
| Unit | OR (95% CI) | OR | |
| Age | Years | 1.01 (0.99, 1.02) | 1.01 (0.99–1.02) |
| BMI | kg/m2 | 1.07* (1.02, 1.11) | 1.06* (1.02–1.10) |
| HAQ | 0–3 | 1.57* (1.09, 2.27) | 1.69* (1.22–2.33) |
| Mental health | 0–100 | 0.94* (0.92, 0.96 | 0.94* (0.92–0.96) |
| Bodily pain | 0–100 | 0.95* (0.93, 0.98) | 0.96* (0.93–0.98) |
| Female | Yes | 1.71* (1.08, 2.71) | 1.94* (1.28–2.95) |
| Comorbidities (RDCI) | 0–9 | 1.56* (1.20, 1.54) | 1.32* (1.12–1.56) |
| PGA | 0–100 | 1.00 (0.99, 1.01) | |
| TJC | 0–28 | 1.02 (0.99, 1.06) | |
| SJC | 0–28 | 1.02 (0.99, 1.04) | |
| ESR | mm/h | 0.99 (0.99, 1.00) | |
| Medication | Yes | 0.83 (0.48, 1.46) | |
| DAS28-ESR | 1.40* (1.25, 1.56) | ||
P ≤ 0.05.
Univariate and multiple logistic regressions with odds ratios (ORs) with 95% CI. Multiple logistic regression model included age, sex and additional variables significantly predictive of vitality group membership in univariate analysis, except that DAS28-ESR was excluded due to substantial collinearity with component indices.
Sensitivity analysis showed similar results when data from participants who had a disease duration of <6 months at baseline or from participants with five complete SF-36 vitality measurements were analysed (Supplementary Tables S12A to 13B, available at Rheumatology online). Analyses using unimputed data also provided similar results.
Discussion
Fatigue is prevalent in early RA and associated with worse patient-reported outcomes even in early disease. Our study found that in early RA, more than half of the RA population experienced clinically significant fatigue. Fatigue remained persistent and did not undergo a clinically significant change over time. Females with worse mental health, pain and functional ability at presentation with RA were more likely to experience fatigue throughout the course of disease.
Our study aligns with previous studies that reported similar high prevalence rates in established RA in both UK and non-UK populations [1, 2], highlighting the problem across the disease course and in different populations.
This study assessed the course of fatigue over the first 3 years of RA and overall the mean vitality scores were remarkably stable over the duration, with values less than the MCID of vitality [16], meaning that people with fatigue at baseline continued to report fatigue longitudinally. A deeper examination of the data using trajectory analyses found two distinct groups, with and without fatigue. This result reflects our findings on the prevalence of fatigue, as not all people experience clinically important fatigue in RA; however, those who experience fatigue at baseline continued to report fatigue at follow-up.
We identified that female sex, pain, mental health and functional ability showed a consistent association with fatigue when examined cross-sectionally and predicted people more likely to belong to the Fatigue group. This concurs with findings from another study [32] and is mirrored in people with inflammatory and non-inflammatory diseases, including post-viral fatigue and post-chemotherapy fatigue [33, 34]. Interestingly, many RA-related characteristics such as inflammation and duration of disease were not consistently significantly associated with fatigue cross-sectionally and longitudinally in our analyses [35]. These results corroborate findings from other cross-sectional studies that found little association between traditional measures of inflammation (ESR and CRP) and fatigue [36, 37]. Although some previous studies reported an association between inflammation and fatigue, these studies used either univariate analysis or a composite measure of disease activity (e.g. DAS28) [32, 38]. In addition, fatigue has been demonstrated to persist in the presence of well-controlled inflammatory disease and in conditions with no strong evidence of a systemic inflammatory component (e.g. fibromyalgia) [39].
Female sex and worse mental health, pain and functional capacity were consistently associated with fatigue in our study. These factors, and indeed fatigue, are components of a cluster of characteristics described as fibromyalgianess. Fibromyalgianess is associated with central sensitization, involving hyperexcitement of the central neurons characterized by amplification of noxious stimulus and increased sensitivity to environmental stimuli such as heat or light [40]. Central sensitization is an established pain mechanism in musculoskeletal diseases and evidence of an association with fatigue has been reported [41]. Central sensitization could explain fatigue persistence and association with pain, metal health and lower functional capacity and overall worse outcomes even in the presence of well-controlled inflammatory disease [42, 43].
It is noteworthy that randomized controlled trials (RCTs) showed a small improvement in fatigue levels after 6 months of follow-up after treatment with bDMARD therapies, although these studies did not examine the long-term changes in fatigue [44]. It remains possible therefore that fatigue may originate from an inflammatory process, whereby pro-inflammatory cytokines fundamental to the development of an immune response trigger long-term changes in brain architecture, neural pathways and sensitization, culminating in central sensitization and fibromyalgianess [45].
RCTs have also demonstrated a therapeutic ‘window of opportunity’ in RA management associated with better outcomes in terms of radiological damage and disability [11]. There is some debate about the duration of this therapeutic window, however, up to 2 years post-diagnosis is supported by the literature. It is proposed that intervening while the disease process is less mature and more reversible would facilitate modulation of the disease. This non-linear progression has been demonstrated in studies describing disease activity, pain and psychological distress in RA [12]. Our study, although not designed to examine the effect of treatment, revealed that fatigue was present at the start of RA and underscores another consideration that the causes of fatigue may occur much earlier in the disease course, possibly even in preclinical stages of the disease. More studies in populations at risk of developing RA may provide greater insights into the mechanisms of fatigue [46].
Previous studies on the progression of fatigue in established RA identified additional groups with improving fatigue and worsening fatigue. In one of the studies, individuals were assigned to groups a priori based on values of the fatigue measure on a population starting bDMARD therapy [8]. The improving fatigue group could result from higher baseline fatigue in the study population and/or better control of inflammation with the initiation of bDMARD therapy. Another study found two persistent fatigue groups in women only; in contrast, our study did not elicit the effect of sex on trajectory groups, although females had lower starting fatigue than males in our study population. Overall, the pattern of persistent fatigue in certain groups of individuals with RA was consistent across all studies [8, 47].
Current RA treatment guidelines include therapeutic management with DMARDs and access to a multidisciplinary team to manage other symptoms that affect the QoL of these individuals, including fatigue. There is little published data on the uptake of these services and a paucity of information on specific interventions geared towards the management of symptoms in people with RA, suggesting that they are not used very frequently. This is probably due to the hope that improving control of inflammatory disease might resolve other problematic symptoms, and maybe also the challenges of identifying people with definite central sensitization. Additional tools to identify people with central mechanism traits in clinical practice are being developed and would provide a means to identify individuals who may require additional treatments beyond peripheral pain and inflammation [48, 49].
This study is subject to some limitations. High attrition rates precluded the inclusion of additional time points to the longitudinal analysis. However, GBTM produces unbiased results under the MAR assumption [30] and we did not detect an effect from attrition on our findings. Data were assumed to be missing at random. Results may be subject to bias if data were missing not at random, although sensitivity analyses using unimputed data provided similar findings. Cases were eligible for participation in the ERAN if they had a physician diagnosis of RA, and were subsequently excluded if the clinical diagnosis was revised. However, it remains possible that some participants may have been inadvertently included with diagnoses other than RA.
RA treatment strategies have evolved since the inception of this cohort and it is not clear if these new treatment strategies would present a different picture. This study did not address some notable factors associated with fatigue (e.g. sleep quality). Univariate GBTM was used, but perhaps modelling joint trajectories of other significant fatigue risk factors would provide more insight into the nature of heterogeneity observed in this analysis.
Conclusion
Fatigue is a prevalent symptom in RA, even in early disease. Those with fatigue at baseline were likely to continue to report fatigue at follow-up. Diverse baseline characteristics indicative of central mechanisms are associated with persistent fatigue. Management of fatigue might require complex interventions targeted at central mechanisms in addition to disease modification, and people who require such interventions might be identified at presentation with early RA.
Funding: This work is supported by the Vs Arthritis Pain Centre (grant 20777) and Onosi Ifesemen’s PhD is partially funded by the ERAN.
Disclosure statement: D.F.M. received grant support from Pfizer and Eli Lilly. D.A.W. received grant support from Pfizer, Eli Lilly and UCB and provided consultancy for Pfizer, GlaxoSmithKline and AbbVie.
Supplementary Material
Contributor Information
Onosi Sylvia Ifesemen, Academic Rheumatology; Pain Centre Versus Arthritis.
Daniel Frederick McWilliams, Academic Rheumatology; Pain Centre Versus Arthritis; NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham.
Sam Norton, Institute of Psychiatry Psychology & Neuroscience Centre for Rheumatic Diseases, Faculty of Life Sciences & Medicine, Kings College.
Patrick D W Kiely, Department of Rheumatology, St Georges Hospital London.
Adam Young, Centre for Health Services and Clinical Research Basic and Clinical Science Unit Department of Clinical, Pharmaceutical and Biological Science School of Life and Medical Sciences, University of Hertfordshire, London.
David Andrew Walsh, Academic Rheumatology; Pain Centre Versus Arthritis; NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham; Department of Rheumatology, Sherwood Forest NHS Foundation Trust, Nottinghamshire, UK.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Wolfe F, Hawley DJ, Wilson K.. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 1996;23:1407–17. [PubMed] [Google Scholar]
- 2. Overman CL, Kool MB, Da Silva JA, Geenen R.. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin Rheumatol 2016;35:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nikolaus S, Bode C, Taal E, van de Laar MA.. Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 2013;65:1128–46. [DOI] [PubMed] [Google Scholar]
- 4. Katz P. Fatigue in rheumatoid arthritis. Curr Rheumatol Rep 2017;19:25. [DOI] [PubMed] [Google Scholar]
- 5. Kirwan J, Heiberg T, Hewlett S. et al. Outcomes from the Patient Perspective Workshop at OMERACT 6. J Rheumatol 2003;30:868–72. [PubMed] [Google Scholar]
- 6. Hewlett S, Chalder T, Choy E. et al. Fatigue in rheumatoid arthritis: time for a conceptual model. Rheumatology 2011;50:1004–6. [DOI] [PubMed] [Google Scholar]
- 7. Geenen R, Dures E.. A biopsychosocial network model of fatigue in rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2019;58(Suppl 5):v10–v21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Provan SA, Michelsen B, Sexton J, Uhlig T, Hammer HB.. Trajectories of fatigue in actively treated patients with established rheumatoid arthritis starting biologic DMARD therapy. RMD Open 2020;6:e001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Repping-Wuts H, Fransen J, van Achterberg T, Bleijenberg G, van Riel P.. Persistent severe fatigue in patients with rheumatoid arthritis. J Clin Nurs 2007;16:377–83. [DOI] [PubMed] [Google Scholar]
- 10. van Steenbergen HW, Tsonaka R, Huizinga TWJ, Boonen A, van der Helm-van Mil AHM.. Fatigue in rheumatoid arthritis; a persistent problem: a large longitudinal study. RMD Open 2015;1:e000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chauffier K, Salliot C, Berenbaum F, Sellam J.. Effect of biotherapies on fatigue in rheumatoid arthritis: a systematic review of the literature and meta-analysis. Rheumatology (Oxford) 2012;51:60–8. [DOI] [PubMed] [Google Scholar]
- 12. McWilliams DF, Dawson O, Young A. et al. Discrete trajectories of resolving and persistent pain in people with rheumatoid arthritis despite undergoing treatment for inflammation: results from three UK cohorts. J Pain 2019;20:716–27. [DOI] [PubMed] [Google Scholar]
- 13. Gibson L, Hernández Alava M, Wailoo A. Progression of disease in people with rheumatoid arthritis treated with non biologic therapies. Report by the decision support unit. School of Health and Related Research, University of Sheffield, 2015.
- 14. Hewlett S, Hehir M, Kirwan JR.. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum 2007;57:429–39. [DOI] [PubMed] [Google Scholar]
- 15. van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P.. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:1294–302. [DOI] [PubMed] [Google Scholar]
- 16. Bjorner JB, Wallenstein GV, Martin MC. et al. Interpreting score differences in the SF-36 vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin 2007;23:731–9. [DOI] [PubMed] [Google Scholar]
- 17. Aragon TJ, Fay MP, Wollschlaeger D, Omidpanah A. epitools: Epidemiology Tools. version 0.5-10.1. Tools for training and practicing epidemiologists including methods for two-way and multi-way contingency tables. https://cran.r-project.org/web/packages/epitools/
- 18. Nikiphorou E, Radner H, Chatzidionysiou K. et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther 2016;18:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells G, Becker JC, Teng J. et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee AN, Beck CE, Hall M.. Rheumatoid factor and anti-CCP autoantibodies in rheumatoid arthritis: a review. Clin Lab Sci 2008;21:15. [PubMed] [Google Scholar]
- 21. England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K.. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken) 2015;67:865–72. [DOI] [PubMed] [Google Scholar]
- 22. Hawker GA, Mian S, Kendzerska T, French M.. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011;63(Suppl):S240–52. [DOI] [PubMed] [Google Scholar]
- 23. Bech P, Olsen LR, Kjoller M, Rasmussen NK.. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int J Methods Psychiatr Res 2003;12:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bruce B, Fries JF.. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schober P, Boer C, Schwarte LA.. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763–8. [DOI] [PubMed] [Google Scholar]
- 26. Curran PJ, Obeidat K, Losardo D.. Twelve frequently asked questions about growth curve modeling. J Cogn Dev 2010;11:121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burnham KP, Anderson DR.. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 2004;33:261–304. [Google Scholar]
- 28. Robitzsch A, Grund S, Henke T, Robitzsch MA. Package ‘miceadds’. Vienna: R Foundation for Statistical Computing, 2017.
- 29. Carpenter JR, Smuk M.. Missing data: a statistical framework for practice. Biom J 2021;63:915–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagin DS, Odgers CL.. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 31. Daniel SN. Group-based trajectory modeling: an overview. Ann Nutr Metab 2014;65:205–10. [DOI] [PubMed] [Google Scholar]
- 32. Rat A-C, Pouchot J, Fautrel B. et al. Factors associated with fatigue in early arthritis: results from a multicenter national French cohort study. Arthritis Care Res 2012;64:1061–9. [DOI] [PubMed] [Google Scholar]
- 33. Esbensen BA, Stallknecht SE, Madsen ME, Hagelund L, Pilgaard T.. Correlations of fatigue in Danish patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis. PLoS One 2020;15:e0237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bearne LM, Bieles J, Georgopoulou S. et al. Fatigue in adults with primary antiphospholipid syndrome: findings from a mixed-methods study. Lupus 2020;29:924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Minnock P, Veale DJ, Bresnihan B, FitzGerald O, McKee G.. Factors that influence fatigue status in patients with severe rheumatoid arthritis (RA) and good disease outcome following 6 months of TNF inhibitor therapy: a comparative analysis. Clin Rheumatol 2015;34:1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stebbings S, Herbison P, Doyle TC, Treharne GJ, Highton J.. A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: disparity in associations with disability, anxiety and sleep disturbance. Rheumatology (Oxford) 2010;49:361–7. [DOI] [PubMed] [Google Scholar]
- 37. Bergman MJ, Shahouri SH, Shaver TS. et al. Is fatigue an inflammatory variable in rheumatoid arthritis (RA)? Analyses of fatigue in RA, osteoarthritis, and fibromyalgia. J Rheumatol 2009;36:2788–94. [DOI] [PubMed] [Google Scholar]
- 38. Huyser BA, Parker JC, Thoreson R. et al. Predictors of subjective fatigue among individuals with rheumatoid arthritis. Arthritis Rheum 1998;41:2230–7. [DOI] [PubMed] [Google Scholar]
- 39. Druce KL, Bhattacharya Y, Jones GT, Macfarlane GJ, Basu N.. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2016;55:1786–90. [DOI] [PubMed] [Google Scholar]
- 40. Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 2007;36:339–56. [DOI] [PubMed] [Google Scholar]
- 41. Druce KL, McBeth J.. Central sensitization predicts greater fatigue independently of musculoskeletal pain. Rheumatology 2019;58:1923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee YC, Napadow V, Loggia ML.. Editorial: functional connectivity: dissecting the relationship between the brain and “pain centralization” in rheumatoid arthritis. Arthritis Rheumatol 2018;70:977–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolfe F. Fibromyalgianess. Arthritis Care Res 2009;61:715–6. [DOI] [PubMed] [Google Scholar]
- 44. Almeida C, Choy EH, Hewlett S. et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2016; 6:CD008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Korte SM, Straub RH.. Fatigue in inflammatory rheumatic disorders: pathophysiological mechanisms. Rheumatology (Oxford) 2019;58:v35–v50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deane KD, Norris JM, Holers VM.. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010;36:213–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Druce KL, Jones GT, Macfarlane GJ, Verstappen SM, Basu N.. The longitudinal course of fatigue in rheumatoid arthritis: results from the Norfolk Arthritis Register. J Rheumatol 2015;42:2059–65. [DOI] [PubMed] [Google Scholar]
- 48. Akin-Akinyosoye K, Frowd N, Marshall L. et al. Traits associated with central pain augmentation in the Knee Pain In the Community (KPIC) cohort. Pain 2018;159:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ifesemen OS, McWilliams DF, Ferguson E. et al. Central Aspects of Pain in Rheumatoid Arthritis (CAP-RA): protocol for a prospective observational study. BMC Rheumatol 2021;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.


