Abstract
Objectives
Evidence-based treatment protocols are currently lacking for immune-mediated necrotizing myopathy (IMNM). In this multicentre retrospective study, we examined baseline clinical characteristics and treatment variables that may predict short-term outcomes of patients with IMNM.
Methods
Muscle biopsies from the John Hunter Hospital and the Royal Adelaide Hospital obtained between 2012 and 2019 were reviewed at a single laboratory at South Australia Pathology. All biopsies with histological features of IMNM were identified. Demographics of study subjects, clinical information and myositis-specific antibody status were recorded along with muscle strength, serum creatine kinase (CK) and treatment regimens at baseline and 3 and 6 months. Primary outcome measures were muscle strength and serum CK at 3 and 6 months. Mixed-effects regression models in a Bayesian framework were performed using the R statistical package.
Results
Female sex, older age, initial prednisone dose and i.v. methylprednisolone were associated with greater improvement in serum CK. In patients with moderate–severe disease at baseline, early IVIG was associated with greater improvement in hip flexor strength at 6 months.
Conclusion
Early IVIG was associated with clinical improvement in the short-term follow-up in IMNM. Female sex, older age, initial oral prednisone dose and initial use of i.v. methylprednisolone were associated with better biochemical improvement.
Keywords: immune-mediated necrotizing myopathy, intravenous immunoglobulin, DMARD, predictors of outcome
Rheumatology key messages.
Early IVIG use was associated with greater gains in hip flexor strength for moderate–severe IMNM.
Older age, female sex and initial use of i.v. methylprednisolone were associated with a greater biochemical response during follow-up.
Conventional synthetic DMARDs were not associated with greater improvement in serum CK or muscle strength.
Introduction
Immune-mediated necrotizing myopathy (IMNM) is a recently recognized inflammatory muscle disorder characterized by subacute proximal muscle weakness, elevated serum creatine kinase (CK) and myofibre necrosis with a paucity of inflammation on histopathology [1, 2]. It is subtyped according to myositis-specific antibodies, specifically antibodies against 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), signal recognition particle (SRP) and those negative for both antibodies (seronegative). Due to its recent recognition, it was not distinguished from polymyositis in the 2017 EULAR/ACR Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies [3].
The optimal treatment strategy for IMNM is unknown. Conventional treatment approaches for IMNM have been similar to those for other inflammatory myopathies, including corticosteroids, agents such as methotrexate and azathioprine, IVIG and rituximab. However, IMNM is considered more refractory to immunosuppression, with poor muscle strength recovery [1, 2, 4, 5]. The current evidence supports the use of IVIG, especially for anti-HMGCR-associated IMNM [4, 6]. Herein we examine clinical and treatment variables that may affect short-term outcomes.
Patients and methods
Study design and ethics
This was a retrospective study performed at John Hunter Hospital (New Lambton Heights, NSW, Australia) and Royal Adelaide Hospital (Adelaide, SA, Australia). The protocol was approved by the human research ethics committees at the John Hunter Hospital and Royal Adelaide Hospital.
Patient selection
The Anatomical Pathology Laboratory at South Australia Pathology diagnoses necrotizing myopathy when the dominant histological changes are that of myofibre necrosis, with a relative paucity of lymphocytic infiltrates [6]. All biopsies from both hospitals between 2012 and 2019 were recorded in the laboratory database. This database was searched to identify cases with this diagnosis and each biopsy report was reviewed by the authors for verification. The medical records of subjects with necrotizing myopathy were reviewed. At both centres, subjects were initially reviewed monthly and, when stabilized, 3 and 6 month assessments were performed. Data recorded at each visit as part of standard clinical care included muscle strength assessments, serum CK levels and treatment regimen.
The diagnosis of IMNM was made based on clinician final diagnosis, consistent with the published international consensus [6]. Only subjects with appropriate clinical, biochemical and serological features were included. Demographics, clinical information including symptom duration, exposure to statin therapy and myositis-specific antibody (MSA) status were recorded, along with muscle strength, serum CK levels and treatment regimens at baseline and follow-up.
Myositis-specific antibodies were tested using a line immunoassay (Euroimmun, Lübeck, Germany) that detects antibodies against Mi-2, TIF1-γ, MDA5, NXP2, SAE1, Ku, PM/Scl100, PM/Scl75, Jo-1, SRP, PL-7, PL-12, OJ, EJ and Ro52. HMGCR antibody testing was performed at PathWest [7].
Information on initial treatment, defined as treatment given upon clinical and histological diagnosis, was extracted from the medical records. Baseline characteristics were collected at the time of initial treatment, hence all subjects were treatment naïve.
Outcome measures
Primary outcomes were serum CK levels and muscle strength at 3 and 6 months.
In determining muscle strength, at presentation, clinicians recorded detailed muscle strength according to the Medical Research Council (MRC) scale. During follow-up, the most consistently examined muscle group was the hip flexors. Furthermore, a previous study demonstrated that hip flexors were the most severely affected in IMNM [8]. Hence we chose hip flexor strength as an outcome measure. Due to the small number of subjects, to enable meaningful statistical analyses, we limited our grading of hip flexor strength to four levels instead of using the full MRC scale. Specifically, grade 3 in our analysis corresponds to MRC grade 5, grade 2 to MRC grade 4, grade 1 to moderate weakness or MRC grade 3 and grade 0 to severe weakness or MRC grades 0–2. MRC grades 0–2 were combined, as they correspond to strengths weaker than anti-gravity and hence significant functional impairment. We considered a score change of 1 to be clinically relevant. Although our scale has not been formally validated, it is likely to correspond to a functional and therefore clinically meaningful change.
The secondary outcome was glucocorticoid dose during follow-up.
Statistical analyses
Statistical analyses were performed with the R statistical programme [version 4.0.1; R Foundation for Statistical Computing, Vienna, Austria (https://www.r-project.org)]. Ordinal mixed-effects regression models were used to assess the predictive ability of patients’ clinical and treatment characteristics on outcomes. Given the variability of baseline CK, we performed two different univariate analyses. First, for each variable, we fitted a mixed-effects model including a fixed categorical effects for time since diagnosis, the log of initial CK and the variable of interest. Mean CK levels were estimated from the posterior distributions, with 95% highest posterior density intervals. This model allows estimation of the effects of each variable on serum CK, adjusted to the baseline CK value. We also included a model on the percentage reduction in CK, which included fixed categorical effects for time and the variable of interest. Multivariate regression models for CK were also performed. Multiple imputations were used for missing values. The probability of direction (PD) in the Bayesian convention is expressed as the following: >0.95, possible; >0.97, likely; >0.99, probable; >0.999, certain.
For muscle strength, we examined the effect of early IVIG (within 1 month of diagnosis) and i.v. pulse methylprednisolone (IVMP) on hip flexor strength during follow-up. Since clinicians may be more likely to use IVIG early in subjects with moderate–severe disease, we also included a subgroup analysis.
Results
Patient demographics and baseline characteristics
Forty-six patients with histological findings of IMNM were identified during the study period. Twelve patients were excluded. Six were excluded due to unavailability of clinical records through patients being managed at other institutions or by private rheumatologists and six because of alternative clinical diagnosis (anti-synthetase syndrome, n = 3; dermatomyositis, n = 1; overlap myositis, n = 1; statin-induced rhabdomyolysis, n = 1). The demographics and baseline characteristics of the 34 included subjects are shown in Table 1. The median age at diagnosis was 65 years and there was an equal sex distribution. There was significant variability in the serum CK at baseline [median 6546 U/l (range 334–211 066 U/l)]. Of the MSAs, 15/34 (44.1%) had HMGCR antibodies, 5/34 (17.6%) had SRP antibodies and 13/34 (35.3%) were seronegative. The conventional synthetic DMARDS (cs-DMARDs) in this cohort included mycophenolate, methotrexate and azathioprine. IVMP was administered to 16/34 (47.1%) subjects as part of initial treatment. Ten (29.4%) received early IVIG.
Table 1.
Demographics and baseline characteristics (N = 34)
| Characteristics | Values |
|---|---|
| Sex, n (%) | |
| Male | 18 (52.9) |
| Female | 16 (47.1) |
| Age at diagnosis (years), n (%) | |
| <60 | 9 (26.5) |
| >60 | 25 (73.5) |
| Duration of symptoms, n (%) | |
| <5 weeks | 7 (20.6) |
| 5–24 weeks | 13 (38.2) |
| 25–52 weeks | 5 (14.7) |
| >1 year | 9 (26.5) |
| Antibody status, n (%) | |
| HMGCR | 15 (44.1) |
| SRP | 6 (17.6) |
| Negative | 12 (35.3) |
| Statin use, n (%) | |
| Previous | 5 (14.7) |
| Current | 20 (58.8) |
| Never | 7 (20.6) |
| Initial CK | |
| Median | 6546 U/l |
| Range | 344–211 066 U/l |
| Initial hip flexor strengtha (grade 0–3), n (%) | |
| Grade 3 | 0 (0.0) |
| Grade 2 | 8 (23.5) |
| Grade 1 | 17 (50.0) |
| Grade 0 | 9 (26.5) |
| Initial treatment, n (%) | |
| GC alone | 14 (41.2) |
| GC + IVIG | 9 (26.5) |
| GC + cs-DMARDs | 10 (29.4) |
| Initial IVMP, n (%) | |
| Yes | 16 (47.1) |
| No | 18 (52.9) |
| Initial use of cs-DMARDs, n (%) | |
| Yes | 13 (38.2) |
| No | 21 (61.8) |
| Early IVIG, n (%) | |
| Yes | 10 (29.4) |
| No | 24 (70.6) |
Due to the small number of subjects in the study, we limited the number of grades of the hip flexors in our cohort by combining MRC grades 0–2 into one group.
Predictors of serum CK
In our cohort, CK nearly normalized over the follow-up period. The average percentage reduction in CK was 83% and 92% at 3 months and 6 months, respectively.
In the univariate analyses, adjusted for baseline CK, female sex, older age, higher initial oral prednisone dose and IVMP use were associated with lower CK at follow-up. Similarly, female sex, higher initial oral prednisone dose and IVMP were associated with a higher percentage reduction in CK (PD >0.95 for all variables). In contrast, cs-DMARDs appeared to be associated with only a more minor reduction in CK (Supplementary Fig. S1, available at Rheumatology online).
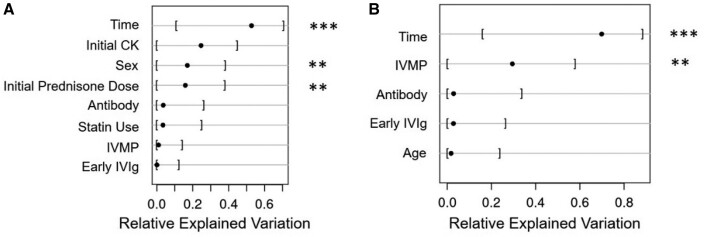
Two multivariate models were performed. The first included time since diagnosis, log of baseline CK and the clinical variables of interest. The second model, on percentage CK reduction, included variables of interest and time since diagnosis. In the first model, initial prednisone dose was more important than early IVIG or IVMP in predicting serum CK. In the second model, IVMP was the only treatment-related variable on the percentage reduction of CK. This suggests that during short-term follow-up, IVMP rather than IVIG was associated with more rapid improvement in CK (Fig. 1). Antibody status did not appear to be significant.
Fig. 1.
Bayesian proportional odds ordinal logistic model on CK
Models show fractions of change in CK attributable to each predictor variable. A higher relative explained variation suggests the particular predictor is more relevant to the change in CK at follow-up. (A) Multivariate analysis for serum CK at follow-up including a categorical variable time, log of baseline CK and variables of interest. This model shows the fraction of change in CK values attributable to each predictor variable. Initial treatment, duration of symptoms and age were dropped from the full model due to redundancy. (B) Multivariate analysis for percentage CK reduction. Asterisks (**) denote a PD >0.97.
Response of hip flexor strength to early treatment with IVIG
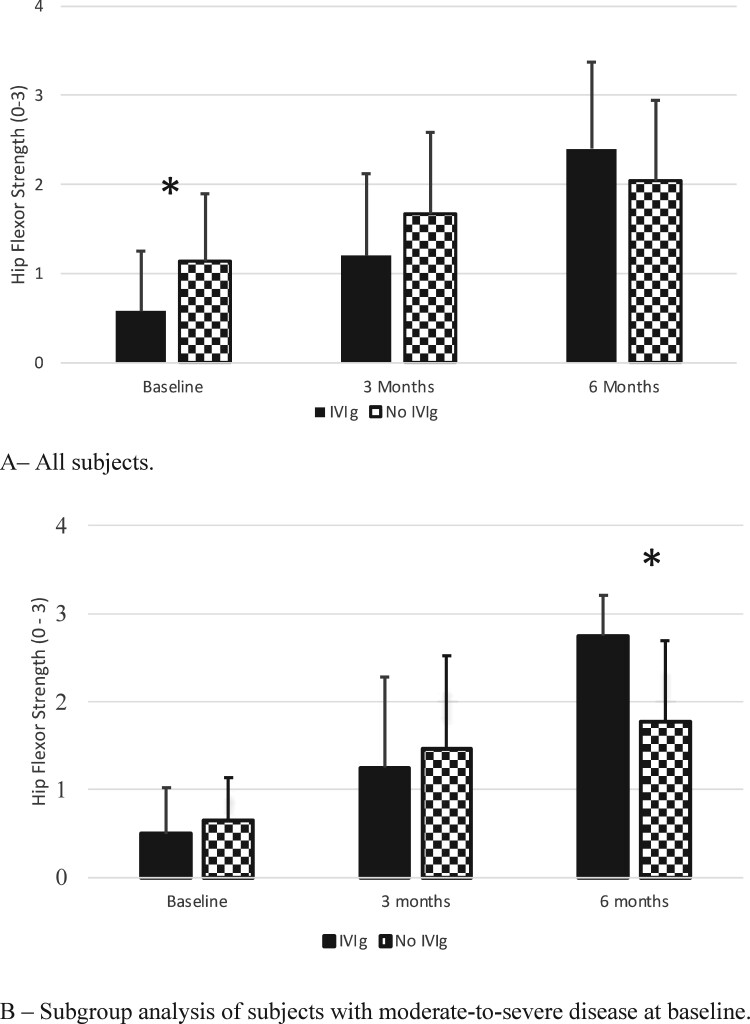
We examined the effects of early IVIG on hip flexor strength. There was no baseline difference between the early IVIG and no early IVIG groups in sex, age, serum CK, antibody status, IVMP use or initial prednisone dose (Supplementary Table S1, available at Rheumatology online). However, the early IVIG group had significantly worse hip flexor strength compared with the no early IVIG group (mean 0.58 vs 1.14, respectively; P = 0.03), suggesting clinicians’ preference to use IVIG early for more severe disease. This difference between the two groups disappeared during follow-up (Fig. 2A), suggesting that the early IVIG group had greater improvement than the no early IVIG group. Numerically, at 3 months, the improvement between the groups was similar, 0.50 in both groups (P = 1.000). However, at 6 months the mean increase in the early IVIG group was 1.70, compared with 0.87 in the no early IVIG group (P = 0.02).
Fig. 2.
Effect of early IVIG on hip flexor strength
(A) All subjects. At baseline, hip flexor strength was significantly lower in the early IVIG group compared with the no early IVIG group. At 3 and 6 months follow-up, this difference was no longer observed. (B) Subgroup analysis of subjects with moderate–severe disease at baseline. At baseline, there was no difference in hip flexor strength between the groups. At 6 months, the group that received early IVIG had significantly better hip flexor strength compared with the no early IVIG group. y-axis: Grading of hip flexor strength. Grade 0 correspond to MRC grades 0–2, grade 1 corresponds to MRC grade 3, grade 2 corresponds to MRC grade 4 and grade 3 correspond to MRC grade 5.
To explore whether the observed response was primarily dependent on the effect of IVIG rather than initial disease severity, we performed a subgroup analysis of the 26 subjects with moderate–severe weakness at baseline (9/26 early IVIG and 17/26 no early IVIG). There was no difference in baseline CK levels, mean hip flexor strength, sex, age, antibody status, initial prednisone dose or IVMP use between the early IVIG and no early IVIG groups (Supplementary Table S2, available at Rheumatology online). At 6 months, the early IVIG group had a higher mean hip flexor strength compared with the no early IVIG group (2.75 vs 1.77, respectively; P = 0.012; Fig. 2B). Hence early IVIG treatment was associated with a greater gain in hip flexor strength, with the benefit seen mainly in patients with moderate–severe disease.
We examined effects of IVMP use on hip flexor strength. Baseline hip flexor strength was significantly worse in the IVMP group (mean 0.56 vs 1.32 for the no IVMP group; P = 0.001). However, there was no difference in the mean increase in hip flexor strength between the two groups at 3 or 6 months (Supplementary Table S3, available at Rheumatology online).
Effects of IVIG on glucocorticoid dose
We next examined whether early IVIG was associated with a reduction in the prednisone dose. There was no difference in the prednisone dose between the early IVIG and no early IVIG groups at baseline or follow-up. However, there was a slightly higher mean percentage reduction (66% vs 52%) in prednisone use in the early IVIG compared with the no early IVIG group (Supplementary Table S4, available at Rheumatology online).
Discussion
In the present study, early introduction of IVIG appeared to be beneficial in IMNM; the gains are especially demonstrable in those with moderate–severe weakness.
We show that older age and female sex were associated with a better biochemical response, consistent with results from previously published cohorts [6, 8–10].
The treatment of IMNM remains empirical, based on data from retrospective studies and expert opinions [11]. The benefit of IVIG has been demonstrated in other inflammatory myopathies, such as dermatomyositis [5, 10]. Previous studies support the use of IVIG in the treatment of IMNM, especially in the subset with anti-HMGCR antibodies [12–14]. In our cohort, we found that in subjects with moderate–severe muscle weakness at baseline, early IVIG was associated with better outcomes as measured by gains in muscle strength during short-term follow-up. This is consistent with previous studies in which early treatment was associated with improved outcomes [15].
Glucocorticoids constitute an important therapeutic component for IMNM, but when used alone are insufficient [16, 17]. Therefore cs-DMARDs are used for immunomodulatory and steroid-sparing effects [11]. However, we were unable to assess the effects of cs-DMARDs in our cohort because no single cs-DMARD was used consistently and the sample size was small. The effect of early IVIG on prednisone dose as a secondary outcome did not reach statistical significance. This reflects the previous findings that IMNM is more resistant to therapy, further underscoring the importance of improving treatment protocols.
Consistent with prior reports, the initial use of IVMP was associated with a rapid reduction in CK [17]. Although IVMP had been suggested to have the potential to induce remission, especially in dermatomyositis, such a benefit on muscle strength was not seen here.
The limitations inherent to retrospective studies need to be considered. Importantly, use of IVIG and IVMP was based on clinician choice, making our analyses prone to confounding. Nonetheless, the strength of this multicentre study lies in the homogeneously defined population identified in a single laboratory with expertise in interpretation of muscle pathology. Our findings should prompt larger prospective studies to verify our findings and to further assess the response to IVIG in distinct serological subsets of IMNM.
Supplementary Material
Acknowledgements
J.W., V.L. and G.M. conceived the research questions and project. J.W. and M.W. collected clinical data. C.O. performed the statistical analyses. J.W. wrote the manuscript. All authors read and approve the final manuscript.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Jeremy X Wang, Department of Rheumatology, John Hunter Hospital, New Lambton Heights, NSW.
Michael Wilkinson, Department of Rheumatology, Royal Adelaide Hospital, Adelaide, SA.
Christopher Oldmeadow, Hunter Medical Research Institute, New Lambton Heights, NSW.
Vidya Limaye, Department of Rheumatology, Royal Adelaide Hospital, Adelaide, SA; Discipline of Medicine, University of Adelaide, Adelaide, SA.
Gabor Major, Department of Rheumatology, John Hunter Hospital, New Lambton Heights, NSW; Faculty of Medicine, University of Newcastle, Newcastle, NSW, Australia.
Data availability statement
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Basharat P, Christopher-Stine L.. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep 2015;17:72. [DOI] [PubMed] [Google Scholar]
- 2. Day JA, Limaye V.. Immune-mediated necrotising myopathy: a critical review of current concepts. Semin Arthritis Rheum 2019;49:420–9. [DOI] [PubMed] [Google Scholar]
- 3. Lundberg IE, Tjärnlund A, Bottai M. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tiniakou E, Pinal-Fernandez I, Lloyd TE. et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford) 2017;56:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bronner IM, Hoogendijk JE, Wintzen AR. et al. Necrotising myopathy, an unusual presentation of a steroid-responsive myopathy. J Neurol 2003;250:480–5. [DOI] [PubMed] [Google Scholar]
- 6. Allenbach Y, Mammen AL, Benveniste O, Stenzel W, Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord 2018;28:87–99. [DOI] [PubMed] [Google Scholar]
- 7. Limaye V, Bundell C, Hollingsworth P. et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 2015;52:196–203. [DOI] [PubMed] [Google Scholar]
- 8. Pinal-Fernandez I, Parks C, Werner JL. et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res (Hoboken) 2017;69:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon PA, Winer JB, Hoogendijk JE, Choy EH.. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2012;3:CD003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landon-Cardinal O, Koumako C, Hardouin G. et al. Severe axial and pelvifemoral muscle damage in immune-mediated necrotizing myopathy evaluated by whole-body MRI. Semin Arthritis Rheum 2020;50:1437–40. [DOI] [PubMed] [Google Scholar]
- 11. Kusumoto T, Okamori S, Masuzawa K. et al. Development of necrotizing myopathy following interstitial lung disease with anti-signal recognition particle antibody. Intern Med 2018;57:2045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giudizi MG, Cammelli D, Vivarelli E. et al. Anti-HMGCR antibody-associated necrotizing myopathy: diagnosis and treatment illustrated using a case report. Scand J Rheumatol 2016;45:427–9. [DOI] [PubMed] [Google Scholar]
- 13. Allenbach Y, Benveniste O, Stenzel W, Boyer O.. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol 2020;16:689–701. [DOI] [PubMed] [Google Scholar]
- 14. Meyer A, Troyanov Y, Drouin J. et al. Statin-induced anti-HMGCR myopathy: successful therapeutic strategies for corticosteroid-free remission in 55 patients. Arthritis Res Ther 2020;22:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim J, Rietveld A, De Bleecker JL. et al. Seronegative patients form a distinctive subgroup of immune-mediated necrotizing myopathy. Neurol Neuroimmunol Neuroinflamm 2019;6:e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M.. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015;72:996–1003. [DOI] [PubMed] [Google Scholar]
- 17. de Souza JM, Hoff LS, Shinjo SK.. Intravenous human immunoglobulin and/or methylprednisolone pulse therapies as a possible treat-to-target strategy in immune-mediated necrotizing myopathies. Rheumatol Int 2019;39:1201–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.