Abstract
The light and scanning electron microscopic observations were carried out for anatomical features of leaf, pollens and powder.Microscopic studies provide useful information for identification and authentication of adulteration in A. maritima. Nutritional analysis of A. maritima revealed that life fundamental macromolecules such as carbohydrates (49.63 %) crude proteins (13.17 %) and crude fibers (21.06 %) were present in sufficient quantity while crude fats (4.11 %) reported in low quantity. The life essential elements such as Mg (9.472 ± 0.011), Ca (4.152 ± 0.135) and Fe (4.112 ± 0.002) were found in high concentration while heavy metals reported under the safety threshold of WHO. These observations favored A. maritima an alternative of food.Appreciable quantity of phenolics (17.64 ± 0.574) and flavonoids (7.67 ± 0.069) were found while qualitatively active phytochemicals were reported.
The FTIR characterization of A. maritima crude powder revealed chromatogram in 3328.61 to 408.68 frequency range and 24 characteristic peaks on the basis of which different compounds of biological importance were classified. HPLC-UV technique quantifiedand identified six phenolic compounds morin,epigallocatechin gallate, catechin hydrate,ellagic acid, pyrogallol andrutin. Identification of compounds through GC–MS chromatogram revealed the presence of 46 compounds in methanolic fraction however 17 compounds of biological importance were selected.
In-vitro biological evaluation of A. maritima for antioxidant, antimicrobial, antidiabetic (12.61 ± 0.113 %) and cytotoxic activities (LC50 = 20 μg/ml) suggested that methanolic fractions exhibited the highest activity as compared to chloroform and ethyl acetate fractions. The MIC values of 10 or 15 mg/ml were recorded for most of the fungal pathogens. Antibacterial activity revealed 3.75 mg/ml of MIC values against B. subtilis and 1.87 mg/ml against S. aureus, E. coli and P. aeruginosa. In-vivo biological evaluation revealed thatmaximum inhibition was observed for crude extract at 250 mg/kg body weight. The mechanism underlined in-vivo analgesic responses was carried out which revealed that naloxone (morphine and tramadol antagonist) showed no prominent effect while Glibenclamide pretreatment minutely modified the analgesic action. These observations clearly indicted the absence of opiod receptors and involvement of ATP sensitive potassium channels.
Keywords: Biological drug evaluation, Microscopic identification, Phytochemical characterization
1. Introduction
Among the angiosperm family Asteraceae is a largest family which includes over 20,000 species which are cosmopolitan in nature. Economically important genus Artemisia includes in family Asteraceae distributed along Europe, North America and Asia (Shehata et al., 2015). Artemisia is represented by well known 25 species in Pakistan (Zeb et al., 2019). Artemisia belongs to Anthemideae tribe (Nigam et al., 2019). The genus Artemisia of Anthemideae tribe has diverse biological and chemical contents and contains essential oil due to which current evaluations focused on phyto-constituent of this genus (Abad et al., 2012). Focused studies are required on Artemisia due to ecological, economic and species diversity (Vallès et al., 2003).
Decoction of A. maritima is important to cure intermittent fever (Ahmad et al., 2016). Leaf extraction of A. maritima is also used in Pakistan for skin diseases (Fahad and Bano, 2012). Artemisia maritima traditionally called “Zoon, Rooner and Tarkha” in the Northeastern area of Pakistan is used as anti-inflammatory, antimalarial and also antiseptic (Hayat et al., 2009). Artemisia campestris subsp. maritima have been evaluated for its antimicrobial, anti-inflammatory and anti-rheumatic activities (Behmanesh et al., 2007).
In the Sub-Continent of Indo-Pak the native communities and herbal industries generally face the difficulties in proper identification. They are made misguided and deal with completely different taxa (Khan et al., 2011).The raw materials used by the pharmaceutical industry and people are usually obtained from market, which may be contaminated, substituted or adulterated accidently or deliberately (Handa, 2004).The drug identification involves physical, chemical, biochemical and biological features (Alamgir, 2017). Present study designed to carry out various pharmacognostic features of A. maritima todifferentiate and authenticate it on the basis of various physical, chemical, biochemicals, and biological pharmacognostic features.
2. Materials and methods
2.1. Plant materials
Plant material shade dried after collected and was grounded using blender. An identified specimen was deposited to the herbarium of Islamia College Peshawar with voucher number ICP090618. Plant material of 2 kg was macerated in 10 L of methanol with occasional shaking was kept for one week. The extract was then filtered using Whatman filter paper No. 45. The filtrate obtained was evaporated in a rotary evaporator at 40 °C and about 50 g of methanolic crude extract was obtained. These processes were repeated twice. Crudemethanolic extract of (5 g) was stored in refrigerator at 2–4 °C for further use. The remaining extract was suspended with various organic solvents by their increasing polarity i.e. chloroform and ethyl acetate. All the fractions were dried using rotary evaporator. The dried fractions were stored at 4 °C in the refrigerator for future use (Jamil et al., 2012).
2.2. Chemicals and reagents
The chemical reagentsused inproximate analyses were NaOH, H2SO4, HNO3, C2SO and HClO4. Reagents used in Phytochemical analysis and characterization were FeCl3, NH3, CHCL3, Na2CO3, NaNO3, C₆H₂(OH)3CO₂H, C27H30O16, KBr, column gradients system consists of solvent B (deionized water: methanol: acetic acid in the ratio of 180: 100: 20; v/v) and solvent C (water: methanol: acetic acid in the ratio of 80: 900: 20; v/v). Chemicals and reagents for in-vitro biological activities were (CH3)2SO, (DMSO), C18H12N5O6 (DPPH), C6H8O6 (Ascorbic acid), C6N6FeK3 (Potassium ferric cyanide), sabouraud dextrose agar (SDA), Turbinafine, Cefixime-USP, commercial sea salt, starch, Iodine, α-amylase, KI (potassium iodide), C25H43NO18 (Acarbose). Chemicals and reagents for in-vivo biological activities were CH3COOH (Acetic acid), CH2O (Formalin), C16H25NO2 (Tramadol), C19H21NO4 (Naloxone).
2.3. Microscopic characterization
Microscopic characterization such as light microscopy and scaning electron microscopy was carried out using standard procedure as adapted by Singh et al., 2018, Khan and Khan, 2020 using light microscope (Meiji MX5200H) and scanning electron microscope (JEOL-JSM 5910).
2.4. Proximate analysis (nutritional and elemental analysis)
Nutritional contents such as crude fat, crude protein, crude fiber and carbohydrate percentage were calculated by using method of Charles et al. (2005) as:
| (1) |
| (2) |
| (3) |
| (4) |
Determination of total ash (%), dry matter, moisture and gross food energy were calculated by Sadia et al. (2014) using the relation as given:
| (5) |
| (6) |
| (7) |
| (8) |
Elemental analysis was performed according to the standard procedure of Allen et al. (1974) with the help of Shimadzu AA-670 atomic absorption spectrophotometer using the relation:
| (9) |
2.5. Phytochemical screening (qualitative and quantitative)
Qualitative screening of tannins, saponin, flavonoid, terpenoid and alkaloid were carried using Khan et al. (2010). Quantitatively total phenolic contents (TPC) and total flavonoid contents (TFC) Sakanaka et al. (2005).
2.6. Phytochemical characterization
The Fourier Transform Infrared (FTIR) spectroscopy analysis was performed following Deshmukh and Ghanawat (2020). HPLC-UV characterization and quantification were carried out according to Zeb (2015). The chemical investigations through gas chromatography mass spectrometry (GC/MS) carried out according to Yaşar et al. (2005).
2.7. In-vitro biological evaluation
The DPPH free radical scavenging activity, total antioxidant capacity (TAQ) and total reducing power (TRP) was calculated according to Phull et al. (2017). In antifungal activity-seven fungal pathogenic strains e.g. Aspergillus niger, Fusarium solani, Aspergillus fumigatus, Mucor specie, Helminthosporium solani, Candida albicans and Aspergillus flavus were used. In antibacterial activity-four pathogenic strains were used including gram positive bacteria (Staphylococcus aureus and Bacillus subtilis) and gram negative (Pseudomonas aeruginosa and Escherichia coli) according to Jamil et al. (2012). Cytotoxic potential was carried out according to Meyer-Alber et al. (1992) by using the relation as:
| (10) |
Determination of α-amylase inhibition by colorimetric assay as adapted by Funke & Melzig (2006) was used as:
| (11) |
Whereas: ODx, ODy and ODz are absorbance of test samples, negative control and blank.
2.8. In-vivo biological evaluation
In-vivo biological analgesic activities such as writhing test by acetic acid and licking response by formalin according to Shoaib et al. (2019). Hot plate test was carried out according to Eidi et al. (2016).While mechanistic approach for the modulation of pain among all fractions and extracts carried out according to Muhammad et al. (2012).
3. Results
3.1. Microscopic characterization
-
a.
Foliar and pollen characterization
The leaf and pollen anatomical features were studied out using light microscope and scanning electron microscope given in Table 1. Resultswere obtained in micro meter (µm) with ±SEM. The observations recorded are given in Fig. 1 and Fig. S1.
-
b.
Powder characterization
Table 1.
LM and SEM of leaf and pollen characters of A. maritima.
| Foliar Characters (µm) ± SEM | Adaxial surface | Abaxial surface | Pollen Character (µm) ± SEM | Observations |
|---|---|---|---|---|
| Length of epidermal cell | 11.4 ± 0.84 | 11.09 ± 1.74 | Polar Diameter | 3.3 ± 1.342 |
| Width of epidermal cell | 7.2 ± 0.72 | 5.98 ± 1.65 | ||
| No. of epidermal cells | 53.4 ± 4.72 | 59.6 ± 7.98 | Equatorial Diameter | 5 ± 0.997 |
| Length of guard cells | 3.2 ± 0.38 | 2.58 ± 0.41 | ||
| Width of guard cells | 2.1 ± 0.41 | 1.7 ± 0.44 | P/E Ratio | 0.67 ± 2.24 |
| No. of stomata | 13.4 ± 0.50 | 30.4 ± 8.11 | Colpi Length | 3.314 ± 1.4 |
| Length of stomata | 11.3 ± 0.63 | 9.7 ± 0.80 | ||
| Width of stomata | 8.5 ± 0.84 | 7.98 ± 0.63 | Colpi Width | 2.56 ± 1.65 |
| Length of subsidiary cell | 12.8 ± 1.56 | 10.86 ± 1.91 | ||
| Width of subsidiary cells | 6.6 ± 1.90 | 6.3 ± 1.35 | Exine Thickness | 1.17 ± 2.07 |
| Length of stomatal pore | 3.1 ± 0.36 | 2.68 ± 0.54 | ||
| Width of stomatal pore | 2.1 ± 0.25 | 1.88 ± 0.43 | No of fertile pollen | 12.71 ± 5.50 |
| Length of Trichome | N/A | 11.38 ± 3.22 | ||
| Width of Trichome | N/A | 3.24 ± 0.29 | No of sterile pollen | 5.143 ± 2.56 |
| Stomatal Index | 25.1 ± 5.8 | 51.1 ± 16.6 |
Mean ± SEM (n = 7 number), LM = Light microscope and SEM = Scanning electron microscope.
Fig. 1.
Scanning electron microscopy (SEM) of pollens of A. maritima (A = pollen pore, B = group of pollen with equatorial view, C = polar view and D pollen colpi).
Powder drug of A. maritima were evaluated for different characters (Table S1). Organoleptic evaluation revealed yellowish brown colour, aromatic odour and slightly salty taste. The observations under light and scanning electron are given in Fig. S2.
3.2. Proximate analysis
-
a.
Nutritional analysis
The evaluation of A. maritima for nutritional analysis (in grams per 100 g dry weight) revealed good information for various nutritional components such ascrude fats, crude proteins, crude fibers, ash contents, moisture contents and carbohydrates (Fig. S3).
-
b.
Elemental analysis
Elemental analysis of different elements (Fig. S4) such as calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), iron (Fe), copper (Cu), zinc (Zn), manganese (Mn), Lead (Pb), cadmium (Cd) and chromium (Cr) were evaluated. The results revealed high concentration of Mg contents (9.47 ± 0.01) followed by Ca (4.15 ± 0.13) and Fe contents (4.11 ± 0.002). The current study showed that none of the heavy metals contents (Pb, Cd and Cr) reported in A. maritima exceeded the safety threshold.
3.3. Phytochemical analysis
Qualitatively and quantitatively various phytochemical were determined and results have been shown in Table S2.
3.4. Phytochemical characterization
3.4.1. The Fourier Transform Infrared (FTIR) spectroscopy
TheFTIR chromatogramfrom the crude powder of A. maritima (Fig.S5) revealed 24 characteristic peaks and confirmed the presence of medicinally important functional groups (Table S3 identified from the literature) in the frequency range (3328.61 to 408.68).
3.4.2. HPLC-UV characterization
The HPLC-UV chromatogram (Fig. S6) of methanolic extracts of A. maritima revealed identification and quantificationof six compounds (Table 2). Results revealed high concentration of Pyrogallol (1215.147 µg/ml) followed by morin (38.546 µg/ml).
Table 2.
Identification and quantification of phenolic compounds in A. maritima extracts.
| No. of Peak | Retention time (min) | Phenolic compounds Identity | HPLC-UV λmax (nm) | Peak Area of sample | Peak Area of standard | Concentration (µg/ml) | Identification Reference |
|---|---|---|---|---|---|---|---|
| 1 | 8 | Epigallocatechin gallate | 320 | 61.796 | 7261.47 | 0.085 | Reference Standard |
| 2 | 12 | Morin | 320 | 77.093 | 20 | 38.546 | Reference Standard |
| 3 | 16 | Ellagic acid | 320 | 271.117 | 319.24 | 8.492 | Reference Standard |
| 4 | 20 | Catechin hydrate | 320 | 22.426 | 78 | 2.875 | Reference Standard |
| 5 | 22 | Rutin | 320 | 48.514 | 2241.2 | 0.216 | Reference Standard |
| 6 | 28 | Pyrogallol | 320 | 123.216 | 1.014 | 1215.147 | Reference Standard |
3.4.3. Gas chromatography mass spectrometry (GC/MS)
GC–MS chromatogram (Fig. S7) of A. maritima identified the presence of 46 compounds however 15 compounds of biological importance were selected (Table S4).
3.5. In-vitro biological evaluation
3.5.1. Antioxidant activities
DPPH free radical scavenging activity, total reducing power assay (TRP) and total antioxidant capacity (TAC) were performed for different fractions of A. maritima.Antioxidant activities revealed that methanolic fractions exhibited the highest antioxidant activity (Table3).
Table 3.
Ascorbic acid equivalent (% TRP, TAC and DPPH) antioxidant activity.
| Fractions | Antioxidant assay |
||
|---|---|---|---|
| TRP | TAC | DPPH | |
| Methanolic | 66.7 ± 0.311a | 44.02 ± 0.21a | 80.7 ± 0.097a |
| Chloroform | 54.4 ± 0.415b | 37.35 ± 0.368b | 79.087 ± 0.0489b |
| Ethyl acetate | 39.24 ± 0.144c | 16.43 ± 0.276c | 73.1 ± 0.036c |
Key: Values shown in the table are mean ± SE (n = 3). The means which share different superscript (a-c) letters in the columns are significantly (p < 0.05) different from each other.
3.5.2. Antifungal activities
Antifungal activities of A. maritima crude methanolic extracts and their subsequent fractions (ethyl acetate and chloroform) were performed using agar tube dilution method at a concentration of 15 mg/ml (Table 4). The statistically variable results (at P < 0.05)revealedthat methanol and chloroform fractions were found effective.The most active fractions were evaluated for MIC value using disc diffusion method which revealed that most of the fungal strains were inhibited with MIC values of 10 or 15 mg/ml (Table S5).
Table 4.
Antifungal activities of A. maritima (using agar tube dilution method).
| Fractions | Fungal strains |
|||||||
|---|---|---|---|---|---|---|---|---|
| A. niger | A. flavus | A. fumigatus | Mucor sp. | H. solani | C. albicans | F. solani | ||
| %Inh ± SE | %Inh ± SE | %Inh ± SE | %Inh ± SE | %Inh ± SE | %Inh ± SE | %Inh ± SE | ||
| Agar tube dilution method | Meth | 44 ± 1.41ab | 32.5 ± 2.12a | 48 ± 1.41c | 60 ± 1.41a | 63.5 ± 3.53c | 68 ± 1.41bc | 59 ± 1.41a |
| Chl | 55.5 ± 2.12c | 44 ± 2.82b | 25 ± 4.24a | 62 ± 2.82a | 54.5 ± 6.36ab | 71 ± 9.89bc | 63.5 ± 0.70 a | |
| E.a | 45.5 ± 4.94ab | 57 ± 1.41c | 39.5 ± 3.53b | 58.5 ± 2.12a | 54 ± 1.41ab | 53 ± 11.31a | 63.5 ± 2.12 a | |
| PC(+) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
Key: % Inh (percentage inhibition in mm), Meth (methanol), Chl (Chloroform), E.a (ethyl acetate), PC (positive control i.e. turbinafine).The means which share different superscript (a-c) letters in the columns are significantly (p < 0.05) different from each other.
3.5.3. Antibacterial activities
Antibacterial assay was performed against the selected bacterial strains and six concentrations viz. 0.937 mg/ml, 1.875 mg/ml, 3.75 mg/ml, 7.5 mg/ml, 15 mg/ml and 30 mg/ml of each samples were used.The results (Table. 5) revealed that all the fractions were found to inhibit the growth of selected bacterial strains however methanolic fractions showed excellent activities at P < 0.05.Moreover, the active fractions were selected for MICs calculation the results are given in Table S6.
Table 5.
Results of antibacterial activities (zone of inhibitionin mm) of different fractions of A. maritima.
| Bacterial Strains | Gram positive bacteria |
Gram negative bacteria |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Bacillus subtilis |
Staphylococcus aureus |
Escherichia coli |
Pseudomonas aeruginosa |
||||||||||
| Fractions | Conc. a | Conc. b | Conc. c | Conc. a | Conc. b | Conc. c | Conc. a | Conc. b | Conc. c | Conc. a | Conc. b | Conc. c | |
| Disc diffusion method | Meth | 19 ± 1.41a | 17 ± 1.41a | 15 ± 1.41a | 18.5 ± 2.12a | 15 ± 1.41a | 10.5 ± 2.12a | 18.5 ± 2.12a | 15 ± 1.41a | 10.5 ± 2.12a | 18.5 ± 2.12ab | 15 ± 1.41a | 10.5 ± 2.12a |
| CHl | 14.5 ± 0.70bc | 13.5 ± 0.70b | 11 ± 0b | 6.5 ± 0.70c | 5.5 ± 0.70c | 3.5 ± 2.12c | 14.5 ± 0.70bc | 10.5 ± 0.70bc | 7 ± 1.41bc | 19 ± 2.82ab | 12 ± 1.41bc | 6 ± 1.41bc | |
| E.a | 13.5 ± 0.70bc | 9.5 ± 0.70c | 6.5 ± 0.70c | 12 ± 1.41b | 9.5 ± 0.70b | 8.5 ± 0.70b | 12.5 ± 0.70bc | 9.5 ± 2.12bc | 7 ± 2.82bc | 15 ± 2.82c | 10.8 ± 0.28bc | 9 ± 1.41bc | |
| PC1 + ve | 28.5 ± 0.70 | 26.5 ± 0.70 | 25.5 ± 0.70 | 28.5 ± 0.70 | 27 ± 0.70 | 28.4 ± 0.56 | 28.5 ± 0.70 | 27.35 ± 0.49 | 28.25 ± 0.35 | 27.95 ± 0.07 | 27.1 ± 0.14 | 27.87 ± 0.18 | |
| PC2 + ve | 19.5 ± 0.70 | 17.5 ± 0.70 | 18.5 ± 0.70 | 19.5 ± 0.70 | 18 ± 0.35 | 20.3 ± 0.42 | 19.2 ± 0.28 | 18.45 ± 0.63 | 19.35 ± 0.49 | 19.2 ± 0.28 | 18.36 ± 0.51 | 19.17 ± 0.24 | |
Key: PC1 and PC2 (positive control i.e.Cefixime & Roxithromycin) Conc (concentration). Valuesshown in the table are mean ± SE (n = 3). The means which share different superscript letters (a-c) in the columns are significantly (p < 0.05) different from each other.
3.5.4. Cytotoxic activity
The methanolic extracts of A. maritimashowed significant cytotoxic activity at all concentration with LC50 value of 20 μg/ml (Table S7).
3.5.5. Alpha-amylase anti-diabetic activity
In-vitro alpha-amylase enzyme inhibition activity of the extracts (Table S8) revealed highest enzyme inhibition (12.61 ± 0.113 %) for methanolic fractions.
3.6. In-vivo biological evaluation
Results from crude extracts and fractions revealed no mortality even at a maximum dose up to 1500 mg/kg (b.w) when orally administered. Hence, 125and 250 mg/kg dose for crude extracts and 75 mg/kg for subsequent fractions were chosen to evaluate analgesic activities.
3.6.1. Writhing test by acetic acid
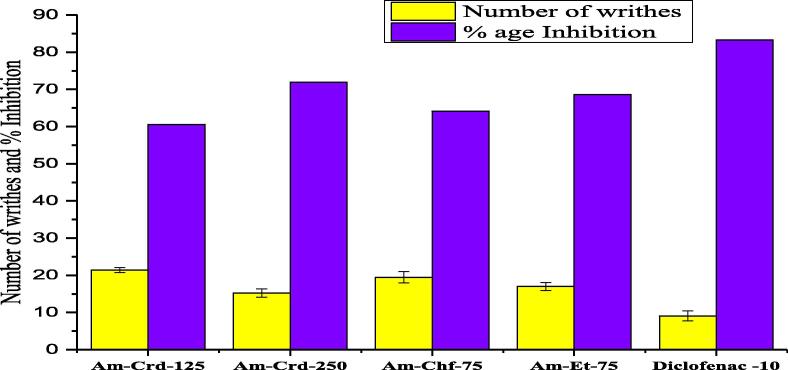
The results revealed a dose dependent pain relief and maximum inhibition (77.17 %, P < 0.001, n = 8) for the crude extract of A. maritima (Am- Crd) at 250 mg/kg as compared to control (Diclofenac sodium) at a dose of 10 mg/kg (88.42 %) (Fig. 2). Formalin test results of Phase-I and phase-IIrevealed dose-dependent inhibitory effectin mice with formalin induced nociception. The crude extracts (Am-Crd) at 250 mg/kg and ethyl acetate fractions at 70 mg/kgrevealed significantly higher inhibition of the neurogenic and inflammatory phases when compared with control (morphine 5 mg/kg and indomethacin 10 mg/kg) (Fig. 3).
Fig. 2.
A. acid induced writhing model analgesic activity of A. maritime. Key: Am = A. maritima, Crd = Crude, Chf = Chloroform, Et = Ethyl acetate,
Fig. 3.
Formalin induced paw licking model analgesic activity of A. maritima of phase-I and Phase-II.
3.6.2. Hot plate test
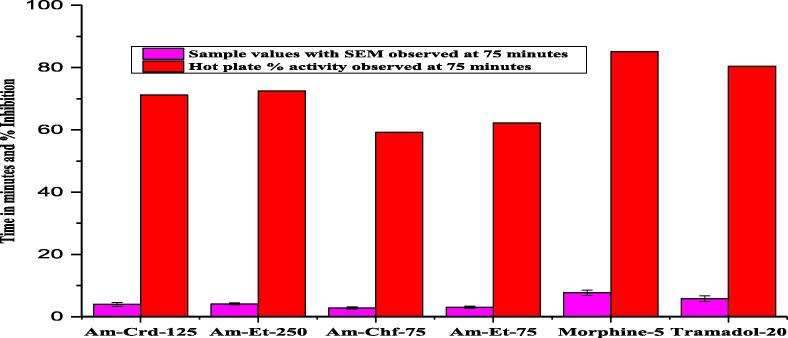
The central analgesic effect of the samples is commonly assessed by Hot plate test. A significant inhibition was shown by crude extract (Am-Crd) at a dose of 250 and 125 mg/kg followed by fractions of ethyl acetate (Am-Et-75) and chloroform (Chf-75)as compared to the control group (tramadol and morphine)with the result of 80.45 % (P < 0.001, n = 8) and 85.12 % (P < 0.001, n = 8) (Fig. 4).
Fig. 4.
Hot plate tail flicking model analgesic activity of A. maritima.
The crude extracts and fractions of chloroform and ethyl acetate exhibited an activity resembling more to that of morphine and tramadol, giving an idea towards the involvement of central mechanism. Therefore, in next step, a non-selective opioid antagonist (naloxone) was used in response of agonistic effects of morphine to find out the possible involvement of opioid receptor.
3.6.3. Involvement of opioid receptor
The possible involvement of opioid receptor was determined using hot plate test. A reversal of inhibitory potential was observed in animals pre-treated with naloxone. The results show that naloxone (morphine and tramadol antagonist) showed no prominent effect. This agonistic and antagonistic effect of morphine/tramadol and naloxone indicates the absence of possible involvement of opioid receptors (Table S9).
3.6.4. Involvement of ATP-sensitive K+ channel pathway
A possible role for ATP sensitive potassium channel in the analgesic effect of the plant extract and fractions was investigated. Glibenclamide pretreatment minutely modified the analgesic action of the tested samples. This observation suggests that ATP sensitive potassium channels are very partly involved in the analgesic action of the tested samples (Table S10).
4. Discussion
Medicinal plants faced issues related to adulteration of morphologically similar species (Ahmed et al., 2019). Genus Artemisia consist closely similar but morphologically different species which make Artemisia extremely difficult to identify correctly (Ghafoor 2002). The role of microscopy is highly important in the validation and identification of novel medicinal plants (Fatima et al., 2018). Pollen grains studies are extensively used in taxonomy for identification of different closely related flowering plants (Bahadur et al., 2019). The foliar epidermal featuresare used as an excellent diagnostic tool for the determination of taxonomic interaction in plants (Hussain et al., 2019). Microscopy of powder drugs helps in the authentication, identification and adulteration detection in the herbal drugs (Soni et al., 2011).The present microscopic studies of different parameters of A. maritima supported by previous studies such as;Hameed et al., 2020, Hussain et al., 2019, Ivashchenko and Ivanenko, 2017, Hayat et al., 2010. Working on the bark of different plants Singh et al., 2018, Xavier et al., 2015, Khan and Khan, 2020 reported similar organoleptic properties, tissues and cells. The current scheme of study is an easy step to distinguish unadulterated from adulterated ones.
From nutritional point of view value of fats fall <2 % because it is a macronutrient of less abundance. The protein level is usually 5 % or above and varies in different fruits (Demir and Özcan, 2001).In proximate analysis ash contents reveals the direct estimation of total quantity of minerals in a specific sample (Younis et al., 2016). The current study revealed that A. maritima contain sufficient nutrient which are in agreement with (Younis et al., 2016), Penuel et al., 2013, Sadia et al., 2014. The proper maintenance of physiological and biochemical functions of life need proper amount of minerals (Pontieri et al., 2014). Human health faces serious challenges and affecting globally due to the deficiency of minerals (Subhani et al., 2015).The current study revealed that A. maritima was found to contain higher concentration of Ca, Na, K and Zn. Heavy metals quantified in the current study did not exceededpermissible limits of WHO safety threshold.
According to Stanojević et al., 2009, Mustafa et al., 2010 methanolic fractions were found rich with total phenolic and total flavonoid contents similar results found in the current study.The quantity of phenolic contentsreported in A. maritimain the current study parallel to those reported by Pereira et al., 2018, Dib et al., 2017, Megdiche-Ksouri et al., 2015, Ghlissi et al., 2016, Al Jahid et al., 2016 with minor variations. Plants (herbs, vegetables, fruits) have a broad range of free radical scavenging constituents such as flavonoids, phenolic acids, tannins, alkaloids, terpenoids and other metabolites with high antioxidant activity (Krishnaiah et al., 2011). Different reports such as Pandey et al., 2017, Abiri et al., 2018, and Sharopov et al. (2020) confirmed the antioxidant status genus Artemisa. The antibacterial assays revealed that methanolic extracts and fractions of chloroform and ethyl acetate were found effective. The study such as Khan et al., 2014, Stappen et al., 2014 reported greater antimicrobial activity.The findings of cytotoxic effects of A. maritima supported byprevious work done of Pereira et al., 2018, Abiri et al., 2018. The highest antidiabetic activity supported by findings of Chen et al., 2019, Fatima et al., 2017. The in-vitro studies of the current studies provide details of different strains extracts and fractions which are different from the previous work done. Moreover they studied different species of Artemisia.These activities may be attributed to the active constituents present in A. maritima.
The abdominal constriction assay induced with acetic acid is helpful for finding out peripheral analgesic response (Neto et al., 2005). Formalin induced nociception is used to quantify the potentials of a substance to relieve continuous moderate pain produced (Oliveira et al., 2009). To find out the spinal pathways in the regulation of pain response the tail immersion test is used (Khatun et al., 2015).The opioid receptors are widely distributed in the peripheral and central nervous systems. In response to noxious stimuli the endogenous opiods activate the opioid receptors (Koneru et al., 2009). The cellular membrane glycoprotein are opioid receptors which are responsible to change the conduction of potassium (K+) and calcium (Ca+) ions (Du Pen et al., 2004). The study outcomes for possible mechanism of painare in agreement with the previous findings of Habib and Waheed (2013), (Afsar et al., 2013) and (Qing-Hu et al., 2015).Previous work done on different Artemisia species for analgesic response also reported dose dependent and solvent based activities such as; Hadi et al., 2014, Habib and Waheed, 2013, Ashok and Upadhyaya, 2013. The analgesic activities in the current study confirmed the traditional uses of genus Artemisia administered as pain with scientific basisand confirmed the existence of antinociceptive potentials of A.maritima.
According to Singh et al. (2010), spectroscopic techniques can be used in quality control of herbal medicines.The findings of current study for FTIR are supported by previous studies such as Kim et al., (2009). The compounds quantified and reported in GC–MS and HPLC analysis (pyrogallol and ellagic acid in high quantity) confirmed its active biological natureas antioxidant, antimicrobial, cytotoxic, antidiabetic and analgesicfrom the literature such as Cynthia et al., 2018, Sampath et al., 2021, Mondal et al., 2020, Zheng et al., 2018, Savic et al., 2019, Gupta et al., 2019, Fatima et al., 2017, Chen et al., 2018. Therefore, it is concluded that the presence of these compounds are responsible for the potentmedicinal nature of the A. maritimaand can be attributed to biological activities revealed by A.maritima in the current study.
5. Conclusion
Genus Artemisia is highly medicinal and well known for its medicinal and pharmacological value. The species of genus Artemisia are closely related therefore proper authentication and identification is highly important. This study was therefore focused on the microscopic characterization which revealed useful information for identification of the selected plant. Biological evaluation (In-vitro and In-vivo) revealed good results which concluded that A. maritima has an excellent potential to treat the diseases related to our performed experiments. These results also confirmed the ethno pharmacological uses of this species for different ailments. The phytochemical characterization provided useful information about the active constituents and biomolecules found in A. maritima which may be attributed to the observed biological potential. However, further experiments in this connection are needed to confirm the observed biological potential and also to isolate responsible compounds in pure state.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful the Deanship of Scientific Research program at Princess Nourahbint Abdulrahman University through the Fast-track Research Funding Program. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R33), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2022.103419.
Contributor Information
Shah Zaman, Email: shahzaman@uom.edu.pk.
Barkatulllah, Email: barkatullah@icp.edu.pk.
Muhammad Zahoor, Email: zahoor@uom.edu.pk.
Syed Wadood Ali Shah, Email: wadodalishah@uom.edu.pk.
Zahid Ullah, Email: zahidtaxon@uswat.edu.pk.
Riaz Ullah, Email: rullah@ksu.edu.sa.
Amal Alotaibi, Email: amaalotaibi@pnu.edu.sa.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abad M.J., Bedoya L.M., Apaza L., Bermejo P. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 2012;17(3):2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiri R., Silva A.L.M., de Mesquita L.S.S., de Mesquita J.W.C., Atabaki N., de Almeida E.B., Shaharuddin N.A., Malik S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018;109:403–415. doi: 10.1016/j.foodres.2018.03.072. [DOI] [PubMed] [Google Scholar]
- Afsar S.K., Kumar K.R., Gopal J.V., Raveesha P. Assessment of anti-inflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wistar albino rats. J. Pharm. Res. 2013;7(6):463–467. [Google Scholar]
- Ahmad M., Khan M.P.Z., Mukhtar A., Zafar M., Sultana S., Jahan S. Ethnopharmacological survey on medicinal plants used in herbal drinks among the traditional communities of Pakistan. J. Ethnopharmacol. 2016;184:154–186. doi: 10.1016/j.jep.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Ahmed S.N., Ahmad M., Zafar M., Rashid S., Yaseen G., Sultana S., Siddiq Z., Kilic O., Ozdemir F.A., Kayani S. Comparative light and scanning electron microscopy in authentication of adulterated traded medicinal plants. Microsc. Res. Tech. 2019;82(7):1174–1183. doi: 10.1002/jemt.23266. [DOI] [PubMed] [Google Scholar]
- Al Jahid A., Essabaq S., Elamrani A., Blaghen M., Jamal Eddine J. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the hydro-alcoholic extract of Artemisia campestris L. leaves from south eastern Morocco. J. Biol. Act. Prod. Nat. 2016;6(5–6):393–405. [Google Scholar]
- Alamgir A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts. Springer; Cham: 2017. Origin, definition, scope and area, subject matter, importance, and history of development of pharmacognosy; pp. 19–60. [Google Scholar]
- Allen S.E., Grimshaw H.M., Parkinson J.A., Quarmby C. Blackwell Scientific Publications; 1974. Chemical Analysis of Ecological Materials. [Google Scholar]
- Ashok P.K., Upadhyaya K. Evaluation of analgesic and anti-inflammatory activities of aerial parts of Artemisia vulgaris L. in experimental animal models. J. Biol. Act. Prod. Nat. 2013;3(1):101–105. [Google Scholar]
- Bahadur S., Ahmad M., Zafar M., Sultana S., Begum N., Ashfaq S., Gul S., Khan M.S., Shah S.N., Ullah F., Saqib S., Ayaz A. Palyno-anatomical studies of monocot taxa and its taxonomic implications using light and scanning electron microscopy. Microsc. Res. Tech. 2019;82(4):373–393. doi: 10.1002/jemt.23179. [DOI] [PubMed] [Google Scholar]
- Behmanesh B., Heshmati G.A., Mazandaran M., Rezaei M.B., Ahmadi A.R., Ghaemi E.O., Nosrat S.B. Chemical composition and antibacterial activity from essential oil of Artemisia sieberi Besser subsp. Sieberi in North of Iran. Asian J. Plant Sci. 2007;6(3):562–564. [Google Scholar]
- Charles A.L., Sriroth K., Huang T.C. Proximate composition, mineral contents, hydrogen cyanide and phytic acid of 5 cassava genotypes. Food Chem. 2005;92(4):615–620. [Google Scholar]
- Chen L., Teng H., Cao H. Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food Chem. Toxicol. 2019;127:182–187. doi: 10.1016/j.fct.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Chen H., Yang X., Yu Z., Cheng Z., Yuan H.u., Zhao Z., Wu G., Xie N., Yuan X., Sun Q., Zhang W. Synthesis and biological evaluation of α-santonin derivatives as anti-hepatoma agents. Eur. J. Med. Chem. 2018;149:90–97. doi: 10.1016/j.ejmech.2018.02.073. [DOI] [PubMed] [Google Scholar]
- Cynthia I.F., Hery S., Akhmad D. Antibacterial and antioxidant activities of pyrogallol and synthetic pyrogallol dimer. Res. J. Chem. Environ. 2018;22:39–48. [Google Scholar]
- Demir F., Özcan M. Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J. Food Eng. 2001;47(4):333–336. [Google Scholar]
- Deshmukh S.V., Ghanawat N.A. Quantification of mineral elements in Hardwickiabinataroxb.-an endemic plant. Asian J. Pharm. Clin. Res. 2020;13(2):44–46. [Google Scholar]
- Dib I., Angenot L., Mihamou A., Ziyyat A., Tits M. Artemisia campestris L.: Ethnomedicinal, phytochemical and pharmacological review. J. Herb. Med. 2017;7:1–10. [Google Scholar]
- Du Pen S.L., Du Pen A.R., Warfield C.A., Bajwa Z.H. Principles and Practice of Pain Medicine. McGraw-Hill; New York: 2004. Neuraxial drug delivery; p. 733. [Google Scholar]
- Eidi A., Oryan S., Zaringhalam J., Rad M. Antinociceptive and anti-inflammatory effects of the aerial parts of Artemisia dracunculus in mice. Pharm. Biol. 2016;54(3):549–554. doi: 10.3109/13880209.2015.1056312. [DOI] [PubMed] [Google Scholar]
- Fahad S., Bano A. Ethnobotanical and physiological studies of some endangered plant species collected from two different altitudes in Gilgit Baltistan. Pak. J. Bot. 2012;44:165–170. [Google Scholar]
- Fatima N., Hafizur R.M., Hameed A., Ahmed S., Nisar M., Kabir N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017;56(2):591–601. doi: 10.1007/s00394-015-1103-y. [DOI] [PubMed] [Google Scholar]
- Fatima A., Zafar M., Ahmad M., Yaseen G., Sultana S., Gulfraz M., Khan A.M. Scanning electron microscopy as a tool for authentication of oil yielding seed. Microsc. Res. Tech. 2018;81(6):624–629. doi: 10.1002/jemt.23017. [DOI] [PubMed] [Google Scholar]
- Funke I., Melzig M.F. Traditionally used plants in diabetes therapy: phytotherapeutics as inhibitors of alpha-amylase activity. Rev. Bras. Farmacogn. 2006;16(1):1–5. [Google Scholar]
- Ghafoor A. In: Flora of Pakistan. Ali S.I., Qaiser M., editors. Missouri Botanical Garden; St. Louis, Missouri: 2002. Asteraceae (I)-Anthemideae; pp. 93–161. [Google Scholar]
- Ghlissi Z., Sayari N., Kallel R., Bougatef A., Sahnoun Z. Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed. Pharmacother. 2016;84:115–122. doi: 10.1016/j.biopha.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Gupta P., Mohammad T., Khan P., Alajmi M.F., Hussain A., Rehman M.T., Hassan M.I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: A targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019;118:109245. doi: 10.1016/j.biopha.2019.109245. [DOI] [PubMed] [Google Scholar]
- Habib M., Waheed I. Evaluation of anti-nociceptive, anti-inflammatory and antipyretic activities of Artemisia scopariahydromethanolic extract. J. Ethnopharmacol. 2013;145(1):18–24. doi: 10.1016/j.jep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Hadi A., Hossein N., Shirin P., Najmeh N., Abolfazl M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int. J. Pharm. Sci. Res. 2014;38:237–244. [Google Scholar]
- Hameed A., Zafar M., Ullah R., Shahat A.A., Ahmad M., Cheema S.I., Majeed S. Systematic significance of pollen morphology and foliar epidermal anatomy of medicinal plants using SEM and LM techniques. Microsc. Res. Tech. 2020;83(8):1007–1022. [Google Scholar]
- Handa S.S. ProceedIngs of the 1st JoInt Workshop on Quality Control and Standardization of Traditional MedicIne Indo-ChIna Experience. 2004. Indian efforts for quality control and standardization of herbal drugs/products; pp. 8–10. [Google Scholar]
- Hayat M.Q., Ashraf M., Khan M.A., Yasmin G., Shaheen N., Jabeen S. Phylogenetic relationships in Artemisia spp. (Asteraceae) based on distribution of foliar trichomes. Int. J. Agric. Biol. 2009;11:553–558. [Google Scholar]
- Hayat M.Q., Ashraf M., Khan M.A., Yasmin G., Shaheen N., Jabeen S. Palynological study of the Genus Artemisia (Asteraceae) and its systematic implications. Pak. J. Bot. 2010;42(2):751–763. [Google Scholar]
- Hussain A., Hayat M.Q., Sahreen S., Bokhari S.A.I. Unveiling the foliar epidermal anatomical characteristics of genus Artemisia (Asteraceae) from Northeast (Gilgit-Baltistan), Pakistan. Int. J. Agric. Biol. 2019;21(3):630–638. [Google Scholar]
- Ivashchenko I.V., Ivanenko G.F. Morphological and anatomical structure of leaves of Artemisia abrotanum (Asteraceae) introduced in Zhytomyr Polissya. Mod. Phytomorphol. 2017;11:35–42. [Google Scholar]
- Jamil M., Mirza B., Qayyum M. Isolation of antibacterial compounds from Quercus dilatata L. through bioassay guided fractionation. Ann. Clin. Microbiol. 2012;11(1):1–11. doi: 10.1186/1476-0711-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Abbasi A.M., Dastagir G., Nazir A., Shah G.M., Shah M.M., Shah M.H. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as a potential source to cure infectious diseases. BMC Compl. Altern. Med. 2014;14(1):1–10. doi: 10.1186/1472-6882-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Ahmad M., Zafar M., Sultana S., Marwat S.K., Shaheen S., Nazir A. Medico-botanical and chemical standardization of pharmaceutically important plant of Tricholepischaetolepis (Boiss) Rech. f.J. Med. Plant Res. 2011;5(8):1471–1477. [Google Scholar]
- Khan A.S., Ihsan I., Shah A.A., Razia B. Preliminary phytochemical screening of some plants of ethnobotanical importance from district Gilgit of Northern Areas, Pakistan. Hamdard Med. 2010;53(4):102–105. [Google Scholar]
- Khan S.A., Khan B. Anatomy, micromorphology, and physiochemical analysis of Rhus succedanea var. himalaica root. Microsc. Res. Tech. 2020;83(4):424–435. doi: 10.1002/jemt.23430. [DOI] [PubMed] [Google Scholar]
- Khatun A., Imam M.Z., Rana M.S. Antinociceptive effect of methanol extract of leaves of Persicaria hydropiper in mice. BMC Compl. Altern. Med. 2015;15(1):1–8. doi: 10.1186/s12906-015-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Min S.R., Kim J., Park S.K., Kim T.I., Liu J.R. Rapid discrimination of commercial strawberry cultivars using Fourier transform infrared spectroscopy data combined by multivariate analysis. Plant Biotechnol. Rep. 2009;3(1):87–93. [Google Scholar]
- Koneru A., Satyanarayana S., Rizwan S. Endogenous opioids: their physiological role and receptors. Glob. J. Pharmacol. 2009;3(3):149–153. [Google Scholar]
- Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89(3):217–233. [Google Scholar]
- Megdiche-Ksouri W., Trabelsi N., Mkadmini K., Bourgou S., Noumi A., Snoussi M., Ksouri R. Artemisia campestris phenolic compounds have antioxidant and antimicrobial activity. Ind. Crops Prod. 2015;63:104–113. [Google Scholar]
- Meyer-Alber A., Hartmann H., Stümpel F., Creutzfeldt W. Mechanism of insulin resistance in CCl4-induced cirrhosis of rats. Gastroenterology. 1992;102(1):223–229. doi: 10.1016/0016-5085(92)91804-d. [DOI] [PubMed] [Google Scholar]
- Mondal M., Saha S., Hossain M., Al Foyjul I., Sarkar C., Hossain S., Kundu S.K. Phytochemical profiling and evaluation of bioactivities of methanolic and ethyl acetate extracts of Marsdenia tenacissima leaves. J. Herbs Spices Med. Plants. 2020;26(4):405–422. [Google Scholar]
- Muhammad N., Saeed M., Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Compl. Altern. Med. 2012;12(1):59. doi: 10.1186/1472-6882-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa R.A., Hamid A.A., Mohamed S., Bakar F.A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010;75(1):C28–C35. doi: 10.1111/j.1750-3841.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- Neto A.G., Costa J.M.L.C., Belati C.C., Vinholis A.H.C., Possebom L.S., Da Silva Filho A.A., e Silva M.L.A. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J. Ethnopharmacol. 2005;96(1–2):87–91. doi: 10.1016/j.jep.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Nigam M., Atanassova M., Mishra A.P., Pezzani R., Devkota H.P., Plygun S., Sharifi-Rad J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019;14(7) [Google Scholar]
- Oliveira R.R., Góis R.M., Siqueira R.S., Almeida J.R., Lima J.T., Nunes X.P., Quintans-Júnior L.J. Antinociceptive effect of the ethanolic extract of Amburana cearensis (Allemão) AC Sm., Fabaceae, in rodents. Rev. Bras. Farmacogn. 2009;19(3):672–676. [Google Scholar]
- Pandey B.P., Thapa R., Upreti A. Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgaris and Gaultheria fragrantissima collected from Nepal. Asian Pac. J. Trop. Med. 2017;10(10):952–959. doi: 10.1016/j.apjtm.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Penuel B.L., Khan E.M., Maitera M.O. Properties of proximate composition and elemental analysis of Citrullus Vulgaris (Guna) seed. BEPLS. 2013;2(2):39–46. [Google Scholar]
- Pereira C.G., Barreira L., Bijttebier S., Pieters L., Marques C., Santos T.F., Custódio L. Health promoting potential of herbal teas and tinctures from Artemisia campestris subsp. maritima: from traditional remedies to prospective products. Sci. Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-23038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phull A.R., Majid M., Haq I.U., Khan M.R., Kim S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017;97:468–480. doi: 10.1016/j.ijbiomac.2017.01.051. [DOI] [PubMed] [Google Scholar]
- Pontieri P., Troisi J., Di Fiore R., Di Maro A., Bean S.R., Tuinstra M.R., Roemer E., Boffa A., Del Giudice A., Pizzolante G., Alifano P., Giudice L.D. Mineral contents in grains of seven food-grade sorghum hybrids grown in a Mediterranean environment. Aust. J. Crop. Sci. 2014;8(11):1550–1559. [Google Scholar]
- Qing-Hu W.A.N.G., Na-Yin-Tai D.A.I., Rong-Jun W.U., Jie-Si W.U. Analgesic effects and structural elucidation of two new flavone C-glycosides from Artemisasacrorum. CJNM. 2015;13(10):786–790. doi: 10.1016/S1875-5364(15)30080-7. [DOI] [PubMed] [Google Scholar]
- Sadia H., Ahmad M., Sultana S., Abdullah A.Z., Teong L., Zafar M., Bano A. Nutrient and mineral assessment of edible wild fig and mulberry fruits. Fruits. 2014;69(2):159–166. [Google Scholar]
- Sakanaka S., Tachibana Y., Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89(4):569–575. [Google Scholar]
- Sampath G., Shyu D.J., Rameshkumar N., Krishnan M., Sivasankar P., Kayalvizhi N. Synthesis and characterization of pyrogallol capped silver nanoparticles and evaluation of their in vitro anti-bacterial, anti-cancer profile against AGS cells. J. Clust. Sci. 2021;32:549–557. [Google Scholar]
- Savic I.M., Jocic E., Nikolic V.D., Popsavin M.M., Rakic S.J., Savic-Gajic I.M. The effect of complexation with cyclodextrins on the antioxidant and antimicrobial activity of ellagic acid. Pharm. Dev. Technol. 2019;24(4):410–418. doi: 10.1080/10837450.2018.1502318. [DOI] [PubMed] [Google Scholar]
- Sharopov F.S., Salimov A., Numonov S., Safomuddin A., Bakri M., Salimov T., Habasi M. Chemical composition, antioxidant, and antimicrobial activities of the essential oils from Artemisia annua L. growing wild in Tajikistan. Nat. Prod. Commun. 2020;15(5) [Google Scholar]
- Shehata E., Grigorakis S., Loupassaki S., Makris D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015;149:462–469. [Google Scholar]
- Shoaib M., Shah A., Wadood S., Ali N., Umar N., Shah I., Tahir M.N. A possible mechanistic approach of synthetic flavonoids in the management of pain. Pak. J. Pharm. Sci. 2019;32(3) [PubMed] [Google Scholar]
- Singh D., Aeri V., Ananthanarayana D.B. Development of standard operating protocol for slide preparation of powdered bark samples with varying grinding techniques. Pharmacogn. 2018;J10(2) [Google Scholar]
- Singh S.K., Jha S.K., Chaudhary A., Yadava R.D.S., Rai S.B. Quality control of herbal medicines by using spectroscopic techniques and multivariate statistical analysis. Pharm. Biol. 2010;48(2):134–141. doi: 10.3109/13880200903059388. [DOI] [PubMed] [Google Scholar]
- Soni N., Lal V.K., Agrawal S., Verma H. Pharmacognostical and phytophysico-chemical profile of Curculigoorchioides (Gaertn) Adv. Res. Pharm. Biol. 2011;1:130–138. [Google Scholar]
- Stanojević L., Stanković M., Nikolić V., Nikolić L., Ristić D., Čanadanovic-Brunet J., Tumbas V. Antioxidant activity and total phenolic and flavonoid contents of Hieracium pilosella L. extracts. Sensors. 2009;9(7):5702–5714. doi: 10.3390/s90705702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappen I., Wanner J., Tabanca N., Wedge D.E., Ali A., Khan I.A., Jirovetz L. Chemical composition and biological effects of Artemisia maritima and Artemisia nilagirica essential oils from wild plants of western Himalaya. Planta Med. 2014;80(13):1079–1087. doi: 10.1055/s-0034-1382957. [DOI] [PubMed] [Google Scholar]
- Subhani G.M., Ahmad J., Subhani A., Hussain M., Mahmood A. Nutritional diversity in spring wheat with chronological perspective and its association with grain yield. Aust. J. Agric. Res. 2015;9(5):363–371. [Google Scholar]
- Vallès J., Torrell M., Garnatje T., Garcia Jacas N., Vilatersana R., Susanna A. The genus Artemisia and its allies: phylogeny of the subtribe Artemisiinae (Asteraceae, Anthemideae) based on nucleotide sequences of nuclear ribosomal DNA internal transcribed spacers (ITS) Plant Biol. 2003;5(3):274–284. [Google Scholar]
- Xavier S.K., Devkar R.A., Chaudhary S., Shreedhara C.S., Setty M.M. Pharmacognostical standardisation and HPTLC quantification of Gallic acid in Homonoia riparia Lour. Pharmacogn. J. 2015;7(6):383–388. [Google Scholar]
- Yaşar A., Üçüncü O., Güleç C., İnceer H., Ayaz S., Yayl N. GC-MS analysis of chloroform extracts in flowers, stems, and roots of Tripleurospermum callosum. Pharm. Biol. 2005;43(2):108–112. [Google Scholar]
- Younis S., Khan Z.I., Ahmad K., Sher M., Batool A.I., Arshad F., Ahmad M.S. A comparative study on nutritional composition of some selected wild plants of semi-arid environment in Punjab, Pakistan. Fresenius Environ. Bull. 2016:5960–5966. [Google Scholar]
- Zeb A. A reversed phase HPLC-DAD method for the determination of phenolic compounds in plant leaves. Anal. Methods. 2015;7(18):7753–7757. [Google Scholar]
- Zeb S., Ali A., Zaman W., Zeb S., Ali S., Ullah F., Shakoor A. Pharmacology, taxonomy and phytochemistry of the genus Artemisia specifically from Pakistan: a comprehensive review. PBR. 2019 [Google Scholar]
- Zheng L., Lee J., Yue L.M., Lim G.T., Yang J.M., Ye Z.M., Park Y.D. Inhibitory effect of pyrogallol on α-glucosidase: Integrating docking simulations with inhibition kinetics. Int. J. Biol. Macromol. 2018;112:686–693. doi: 10.1016/j.ijbiomac.2018.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.