Abstract
BACKGROUND
The beneficial effects of hormonal therapy in stimulating spermatogenesis in patients with non-obstructive azoospermia (NOA) and either normal gonadotrophins or hypergonadotropic hypogonadism prior to surgical sperm retrieval (SSR) is controversial. Although the European Association of Urology guidelines state that hormone stimulation is not recommended in routine clinical practice, a significant number of patients undergo empiric therapy prior to SSR. The success rate for SSR from microdissection testicular sperm extraction is only 40–60%, thus hormonal therapy could prove to be an effective adjunctive therapy to increase SSR rates.
OBJECTIVE AND RATIONALE
The primary aim of this systematic review and meta-analysis was to compare the SSR rates in men with NOA (excluding those with hypogonadotropic hypogonadism) receiving hormone therapy compared to placebo or no treatment. The secondary objective was to compare the effects of hormonal therapy in normogonadotropic and hypergonadotropic NOA men.
SEARCH METHODS
A literature search was performed using the Medline, Embase, Web of Science and Clinicaltrials.gov databases from 01 January 1946 to 17 September 2020. We included all studies where hormone status was confirmed. We excluded non-English language and animal studies. Heterogeneity was calculated using I2 statistics and risk of bias was assessed using Cochrane tools. We performed a meta-analysis on all the eligible controlled trials to determine whether hormone stimulation (irrespective of class) improved SSR rates and also whether this was affected by baseline hormone status (hypergonadotropic versus normogonadotropic NOA men). Sensitivity analyses were performed when indicated.
OUTCOMES
A total of 3846 studies were screened and 22 studies were included with 1706 participants. A higher SSR rate in subjects pre-treated with hormonal therapy was observed (odds ratio (OR) 1.96, 95% CI: 1.08–3.56, P = 0.03) and this trend persisted when excluding a study containing only men with Klinefelter syndrome (OR 1.90, 95% CI: 1.03–3.51, P = 0.04). However, the subgroup analysis of baseline hormone status demonstrated a significant improvement only in normogonadotropic men (OR 2.13, 95% CI: 1.10–4.14, P = 0.02) and not in hypergonadotropic patients (OR 1.73, 95% CI: 0.44–6.77, P = 0.43). The literature was at moderate or severe risk of bias.
WIDER IMPLICATIONS
This meta-analysis demonstrates that hormone therapy is not associated with improved SSR rates in hypergonadotropic hypogonadism. While hormone therapy improved SSR rates in eugonadal men with NOA, the quality of evidence was low with a moderate to high risk of bias. Therefore, hormone therapy should not be routinely used in men with NOA prior to SSR and large scale, prospective randomized controlled trials are needed to validate the meta-analysis findings.
Keywords: non-obstructive azoospermia, testicular extraction sperm surgery, hypergonadotropic hypogonadism, selective oestrogen receptor modulators, aromatase inhibitors, gonadotrophins
Introduction
Non-obstructive azoospermia (NOA) is the absence of sperm in the ejaculate secondary to impaired spermatogenesis (Schlegel, 2004) and represents the most severe form of male infertility. NOA is estimated to affect 1% of the male population and 10–20% of patients presenting with infertility (Jarow et al., 1989). Biochemical hypogonadism is present in almost half of all patients with NOA (Bobjer et al., 2012; Reifsnyder et al., 2012).
The use of hormone therapy in men with NOA and hypergonadotropic hypogonadism (i.e. primary hypogonadism) or eugonadism is controversial (Kim and Schlegel, 2008; Reifsnyder et al., 2012; Kumar, 2013; Shiraishi, 2015) with mixed outcomes reported in the literature although it is widely practiced.
Intratesticular testosterone (ITT) is required for spermiogenesis and serum testosterone has been shown to be an inaccurate surrogate for ITT level with differences ranging from 40- to 181-fold (Jarow et al., 2001; McLachlan, 2002; Coviello et al., 2004; Roth et al., 2010).
In hypergonadotropic hypogonadism, both human and animal data suggest a pathological desensitization of the FSH receptor (FSHR) caused by high circulating levels of gonadotrophins (Gnanaprakasam et al., 1979; Namiki et al., 1985, 1987; Themmen et al., 1991; Foresta et al., 2004). It has been postulated that hormone therapy may benefit patients with hypergonadotropic hypogonadism by using GnRH to suppress gonadotrophin levels and thereby overcoming Sertoli cell receptor desensitization caused by chronically raised FSH levels (Foresta et al., 2004, 2009). Foresta et al. (2009) conducted a randomized controlled trial (RCT) in hypergonadotropic men in which treatment with GnRH to induce hypogonadotropism followed by recombinant LH and FSH improved semen parameters and pregnancy rates.
The existence of a testosterone independent pathway for spermatogenesis, through supraphysiological FSH stimulation, provides a rationale for hormone stimulation therapy in both eugonadal and hypergonadotropic hypogonadism patients (Huhtaniemi, 2018; Oduwole et al., 2018a,b). Oduwole et al. (2018b) observed that constitutively activating FSHR mutations in mice were able to maintain spermatogenesis even in the absence of androgen signalling including treatment with the anti-androgen Flutamide. Furthermore, a case report (Gromoll et al., 1996) of a male with an FSHR-D567G mutation who exhibited normal spermatogenesis after hypophysectomy suggests that a strong constitutive FSH stimulation can compensate for a deficiency in LH and testosterone.
The current European Association of Urology (EAU) guidelines on Male Sexual and Reproductive Health do not advocate hormone stimulation therapy in idiopathic NOA (Salonia et al., 2021). However, a survey reported that 64.9% of urologists prescribe empiric hormone therapy to treat idiopathic male infertility, with clomiphene citrate the most commonly prescribed drug for both general and fertility-trained urologists (Ko et al., 2012). This may be attributable to the fact that surgical sperm retrieval (SSR) rates in patients with NOA have remained static (40–60%) over the last 10 years (Shiraishi et al., 2012; Corona et al., 2019). Therefore, hormone therapy has been proposed as an adjunctive therapy to improve fertility outcomes (i.e. SSR rates and production of sperm into the ejaculate) in men with NOA.
This is the first systematic review and meta-analysis to investigate the effects of hormone therapy on SSR rate. The primary outcome of the meta-analysis was the SSR rate in men with NOA who were treated with hormone therapy. The secondary outcome was comparison of SSR rates according to baseline hormone status (hypergonadotropic versus normogonadotropic NOA men).
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines and was registered in the international prospective register of systematic reviews (PROSPERO, ID CRD42019145226).
Literature search
A literature search was performed using the Medline, Embase, Web of Science and Clinicaltrials.gov databases from 01 January 1946 to 17 September 2020. Search terms included: azoospermia, selective oestrogen receptor modulators, tamoxifen, clomiphene, gonadotropins, gonadotropin releasing hormone, aromatase inhibitors, anastrozole, letrozole, testolactone, chorionic gonadotropin, human chorionic gonadotropin, menotropins, human menopausal gonadotropin, sperm retrieval, testicular sperm extraction, microdissection testicular sperm extraction, testicular sperm aspiration and the corresponding abbreviations.
Inclusion and exclusion criteria
For the systematic review, we included prospective and retrospective case series, case-control studies and RCTs. Studies for possible inclusion needed to confirm subjects with NOA and the hormone status of the participants and the type(s) and duration of hormone treatment. Non-English language and animal studies were excluded. We included abstracts and full-text studies. There were no age restrictions, and we included all patients with NOA irrespective of genetics status. In the case of multiple publications with overlapping cohorts, we included only the most recent study unless specified otherwise. For the meta-analysis, we only included controlled studies. We included multiple cohort studies when one arm fulfilled the aforementioned criteria.
Study selection
Screening of the study abstracts was performed by two independent reviewers (T.T. and D.F.). Any discrepancy was discussed, and consensus achieved by a third reviewer (C.N.J.). Full-text articles were retrieved and underwent further utility assessment by two independent reviewers (T.T. and D.F.) with any differences being adjudicated by a third reviewer (S.M.). In cases where outcome measures were absent from the full-text article, the authors of the study were contacted to provide the raw data.
Outcomes and quality assessment of included studies
There is no reference gonadotrophin or testosterone level to achieve optimal spermatogenesis in men with either eugonadism or with hypergonadotropic hypogonadism. We therefore compared the differences in serum testosterone, FSH and LH among each type of hormone treatment where applicable. For the purpose of the systematic review, we accepted mean or median cohort testosterone values as a representation of overall cohort hormone status. A successful sperm retrieval was defined as the presence of a single spermatozoon or more. Conventional testicular sperm extraction (TESE) was defined as single or multiple random biopsies of the testicular tissue while microdissection TESE was defined as TESE under magnification utilizing the technique previously described by Schlegel (1999).
Where indicated, hormone status was defined according to the reference ranges utilized in each individual study or authors descriptions of hormone status (e.g. normal hormone profiles). In cases of ambiguity, the authors were contacted for clarification and in the absence of a response, an FSH level of ≥12 mUI/ml and an LH ≥10 mUI/ml was used to define hypergonadotropic hypogonadism as these were the most common (mode) upper limit thresholds utilized in all the included studies. Similarly, hypogonadism was defined as a serum testosterone level <8.8 nmol/l as this was the average (mean) lowest reference threshold for hypogonadism in the included studies. If a single gonadotrophin was raised (FSH or LH) than this was categorized as hypergonadotropic. In addition to this, men with a raised FSH or LH and a normal testosterone were classified as compensated hypergonadotropic hypogonadism.
Full-text articles were studied, and the outcome measures recorded included baseline hormone parameters, type and duration of hormone agent, type of surgery, SSR rates, sperm production in the ejaculate and adverse events.
The risk of bias was evaluated using the ROBINS-1 tool (Sterne et al., 2016) for non-RCTs (Aydos et al., 2003; Hussein et al., 2013; Gul, 2016; Cocci et al., 2018; Hu et al., 2018) included in the meta-analysis. Two reviewers (T.T. and D.F.) performed independent assessments of risk of bias with discrepancies being resolved by a third reviewer (S.M.).
Meta-analysis and statistical analysis
Only controlled studies were included for the meta-analysis. We pooled data and performed a meta-analysis of all controlled trials to determine whether hormone stimulation (irrespective of class) improved SSR rates in hypergonadotropic men with NOA and eugonadal men with NOA. We also studied whether hormone therapy improved the SSR rate overall (irrespective of hormone status). Sensitivity analyses were performed when indicated.
Heterogeneity in SSR was assessed using I2 statistics. Even when low heterogeneity was detected, a random-effect model was applied because the validity of tests of heterogeneity can be limited with a small number of component studies. We used funnel plots and the Begg adjusted rank correlation test to estimate possible publication or disclosure bias (Begg and Mazumdar, 1994); however, undetected bias may still be present, because these tests have low statistical power when the number of trials is small. Overall SSR is expressed as a mean percentage (95% CI). All data were calculated using the Comprehensive Meta-analysis Version 2, Biostat, and (Englewood, NJ, USA). a value of P < 0.05 was considered significant.
Results
Evidence synthesis
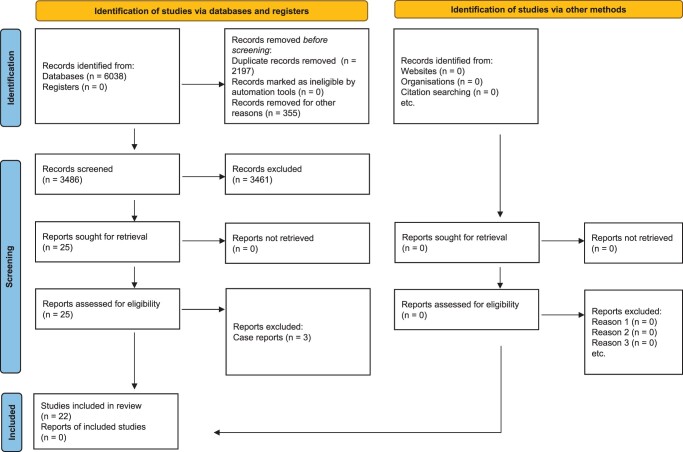
Figure 1 shows the PRISMA flow-chart of the studies. We screened 3846 studies and included 22 studies of which 10 were case-control studies, 11 were case series and 1 was an RCT.
Figure 1.
PRISMA flow chart for the selection of studies on hormone therapy and sperm retrieval rates in men with non-obstructive azoospermia. PRISMA, Preferred Reporting Items For Systematic Reviews and Meta-analysis.
For the purposes of the systematic review, we subdivided the cohorts of NOA into hypergonadotropic hypogonadism (Table I) and eugonadism (Table II). Any study which included a mixture of eugonadal and hypergonadotropic hypogonadism patients were analysed separately (Table III).
Table I.
Studies assessed in the systematic review that evaluated the use of hormone stimulation therapy in men with non-obstructive azoospermia and hypergonadotropic hypogonadism.
| Study (year) | Design | Population | Genetics | Mean age (SD) (*range) in years **=median | Intervention regime | Type of surgery | Hormone changes after hormone therapy | Rates of sperm returning to the ejaculate/surgical sperm retrieval (patients with NOA only) | Pregnancy Live birth rates | Adverse events | Strengths | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shiraishi et al. (2012) | Case control |
|
Chromosomal abnormalities excluded | 34 (5.7) |
|
Secondary mTESE | hCG only cohort:
|
|
NR |
|
• Control included |
|
hCG and FSH cohort:
| ||||||||||||
|
| ||||||||||||
| Shiraishi et al. (2016) | Case series | cHH NOA (n = 21) | Chromosomal abnormalities excluded | 32.2 (3.1) (*29–36) |
|
Secondary mTESE |
|
SSR via mTESE:
|
|
Acne: 3/21 (14.3%) | • Pregnancy/live birth rates measured |
|
|
| ||||||||||||
| Hu et al. (2018) | Case control |
|
Chromosomal abnormalities excluded |
|
Control group: no treatment |
Secondary mTESE | Intervention group:
|
|
NR | Symptoms of androgen deprivation (e.g erectile dysfunction) on Goserelin: 10/25 (40%)
|
• Control included |
|
|
| ||||||||||||
| Pavlovich et al. (2001) | Case series |
|
Chromosomal abnormalities included | 37 (*31–43) |
|
Not applicable |
|
Rate of sperm in the ejaculate: 0/12 |
NR | Asymptomatic deranged Liver function tests 8/43 (18.6%)
|
|
|
|
| ||||||||||||
| Saylam et al. (2011) | Case series |
|
NR | 34.92 (6.66) (*26–49) |
|
Not applicable |
|
Rate of sperm in the ejaculate:
|
NR | Mild headaches:
|
|
|
|
| ||||||||||||
| Cavallini et al. (2013) | RCT |
|
Chromosomal abnormalities excluded | Intervention group:
|
|
Not applicable | Intervention group:
|
Rate of sperm in the ejaculate:
|
PR: 0/46 (0%) | Loss of libido, loss of hair, + cutaneous rash: 4/22 (18.2%)
|
|
|
| Shoshany et al. (2017) | Case series |
|
Chromosomal abnormalities excluded | **37 (*32–41) | Anastrazole 1 mg once daily for 4 months | Primary mTESE |
|
Rate of sperm in the ejaculate: 0/28
|
NR | Joint pain, lower limb swelling, low libido, ocular pruritus/pain, depression, mastalgia, + dry mouth: 8/86 (9.3%)
|
|
|
|
| ||||||||||||
| Reifsnyder et al. (2012) | Case control |
|
|
35 |
|
Primary mTESE | Decreased post-treatment FSH in intervention group compared to control (P = 0.02) |
|
No significant difference in, PR and LBR | NR |
|
|
|
| ||||||||||||
| Majzoub et al. (2016) | Case control |
|
|
32.9 |
|
Primary mTESE | Statistically significant increase in testosterone in intervention group compared to controls (P = 0.01), but no difference in FSH and LH | SSR via mTESE
|
|
NR |
|
|
|
| ||||||||||||
| Amer et al. (2020) | Case control |
|
NR |
|
|
Secondary mTESE | NR | SSR via mTESE:
|
NR | NR |
|
|
|
| ||||||||||||
| Sujenthiran et al. (2019) | Case series |
|
All subjects: Klinefelter syndrome | **33 (IQR 30–34) |
|
NR | NR | SSR via mTESE:
|
Intervention group:
|
NR | • Pregnancy/live birth rates measured |
|
CC, clomiphene citrate; cHH, compensated hypergonadotropic hypogonadism; E2, serum oestrogen; HH, hypergonadotropic hypogonadism; IQR, interquartile range; LBR, live birth rate; mTESE, microtesticular sperm extraction; NOA, non-obstructive azoospermia; NR, not reported; PR, pregnancy rate; puFSH, purified urinary FSH; RCT, randomized control trial; SSR, successful surgical sperm retrieval; T:E, testosterone oestrogen ratio; tT, serum total testosterone.
Table II.
Studies assessed in the systematic review that evaluated the use of hormone stimulation therapy in eugonadal men and non-obstructive azoospermia.
| Study (year) | Design | Population | Genetics | Mean age (SD) (*range) in years **=median | Intervention regime | Type of surgery | Hormone changes after hormone therapy | Rates of sperm returning to the ejaculate/surgical sperm retrieval (patients with NOA only) | Pregnancy Live birth rate | Adverse events | Strengths | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aydos et al. (2003) | Case control |
|
Chromosomal abnormalities included | 29 (*21–39) |
|
Primary cTESE | FSH increase in intervention group vs controls (P < 0.001) | SSR via cTESE:
|
NR | No adverse effects observed |
|
|
|
| ||||||||||||
| Selman et al. (2006) | Case series | NG NOA (n = 49) | Chromosomal abnormalities excluded | (*32–41) |
|
Secondary cTESE | NR | Rate of sperm in the ejaculate:
|
|
NR | • Pregnancy/live birth rates measured |
|
|
| ||||||||||||
| Efesoy et al.(2009) | Case series | NG NOA (n = 11) | NR | 31.1 (4.52) |
|
Primary mTESE | Increase in FSH (P = 0.004) | Rate of sperm in the ejaculate:
|
NR | No adverse events observed | • Prospective |
|
|
| ||||||||||||
| Gul (2016) | Case control |
|
Chromosomal abnormalities excluded | 34 (5.7) |
|
Primary cTESE (and if this failed then mTESE) | NR | SSR via cTESE and mTESE:
|
No significant difference in FR, PR and LBR | No adverse events observed |
|
|
|
| ||||||||||||
| Cocci et al. (2018) | Case control |
|
NR | 35.5 (4.3) |
|
Primary cTESE | NR | Rate of sperm in the ejaculate:
|
Increased FR and PR in treated group vs controls (P < 0.05) | NR |
|
|
SSR via cTESE:
| ||||||||||||
|
| ||||||||||||
| Cavallini et al. (2011) | Case series | NG NOA (N = 4) | Chromosomal abnormalities excluded | 37.3 (*29–44) | Letrozole 2.5 mg, orally, once daily for 6 months | Not applicable |
|
Rate of sperm in the ejaculate: 4/4 (100%) | NR | Loss of libido, Cutaneous rash, and anxiety |
|
|
|
| ||||||||||||
| Hussein et al. (2013) | Case control |
|
NR | 26.7 (4.9) | Intervention groups:
|
Primary mTESE |
|
Rate of sperm in the ejaculate:
|
NR | Paradoxical decrease in serum tT level on CC: 16/496 (3.2%) |
|
|
SSR via mTESE:
| ||||||||||||
|
| ||||||||||||
| Song and Qian (2012) | Case series |
|
Chromosomal abnormalities excluded | (*25–39) | Testosterone undecanoate 40 mg twice daily and TC 10 mg twice daily for 4 months | Not applicable | Increase in FSH and LH (P < 0.01) | Rate of sperm in the ejaculate: NOA patients: 4/4 (100%)
|
NR | NR |
|
|
|
| ||||||||||||
| Sen et al. (2020) | Case control |
|
NR |
|
|
Primary mTESE | Intervention group serum tT increased from 8.03 (±0.97) to 15.66 (±2.20) | Rate of sperm in the ejaculate:
|
NR | NR | • Control included |
|
SSR via mTESE:
| ||||||||||||
CC, clomiphene citrate; cTESE, conventional testicular sperm extraction; FR, fertilization rate; HH, hypergonadotropic hypogonadism; I.M., intramuscular injection; LBR, live birth rate; mTESE, microtesticular sperm extraction; NG, normogonadotropic eugonadism; NGH, normogonadotropic hypogonadism; NOA, non-obstructive azoospermia; NR, not reported; PR, pregnancy rate; rFSH, recombinant FSH; S.C., subcutaneous injection; SSR, successful surgical sperm retrieval; TC, tamoxifen citrate; tT, serum total testosterone.
Table III.
Studies assessed in the systematic review that evaluated the use of hormone stimulation therapy in a mixed cohort of both eugonadal and hypergonadotrophic hypogonadism non-obstructive azoospermia men.
| Study (year) | Design | Population | Genetics | Mean age (SD) (*range) in years **=median | Intervention regime | Type of surgery | Hormone changes | Rates of sperm returning to the ejaculate/surgical sperm retrieval (NOA patients only) | Pregnancy Live birth rate | Adverse events | Strengths | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kumar et al. (1990) | Case series | NG and cHH NOA (n = 50) andOligospermia (n = 29) | Chromosomal abnormalities excluded | 31 (4.7) | 2000 units hCG, twice a week for 6 months OrCC (50 mg once a day, 25 days per month for 6 months) | Not applicable | NR | Rate of sperm in the ejaculate: 0/50 (0%) | NA | NR |
|
|
|
| ||||||||||||
| Kobori et al. (2015) | Case series | HH, cHH and NG NOA (n = 26) | Chromosomal abnormalities excluded | 34.6 (*29–38) | 75 IU FSH twice a week for the first 3 months, then 150 IU twice a week subsequently | Not applicable | NR | Rate of sperm in the ejaculate: 5/26 (19.2%)
|
PR: 2/26 (7.7%) LBR: 1/26 (3.9%) |
NR | −Pregnancy/live birth rates measured |
|
CC, clomiphene citrate; cHH, compensated hypergonadotropic hypogonadism; HH, hypergonadotropic hypogonadism; LBR, live birth rate; NG, normogonadotropic eugonadism; NOA, non-obstructive azoospermia; NR, not reported; PR, pregnancy rate; SSR, successful surgical sperm retrieval.
Men with non-obstructive azoospermia and hypergonadotropic hypogonadism
There have been 11 studies (Pavlovich et al., 2001; Saylam et al., 2011; Reifsnyder et al., 2012; Shiraishi et al., 2012, 2016; Cavallini et al., 2013; Majzoub et al., 2016; Shoshany et al., 2017; Hu et al., 2018; Sujenthiran et al., 2019; Amer et al., 2020) investigating the use of hormone therapy in men with NOA and primary hypogonadism. The literature predominantly consisted of case series (n = 5) (Pavlovich et al., 2001; Saylam et al., 2011; Shiraishi et al., 2016; Shoshany et al., 2017; Sujenthiran et al., 2019) and case-control studies (n = 5) (Reifsnyder et al., 2012; Shiraishi et al., 2012; Majzoub et al., 2016; Hu et al., 2018; Amer et al., 2020) with only one RCT (Cavallini et al., 2013). There were four studies solely utilizing aromatase inhibitors (Pavlovich et al., 2001; Saylam et al., 2011; Cavallini et al., 2013; Shoshany et al., 2017), two studies investigating gonadotrophin therapy (Shiraishi et al., 2012, 2016) and three studies investigating multiple hormone agents (aromatase inhibitors, gonadotrophins, selective oestrogen receptor modulators (SERM’s) and combinations e.g. aromatase inhibitors and hCG) (Reifsnyder et al., 2012; Majzoub et al., 2016; Sujenthiran et al., 2019). Two studies investigated the use of gonadotrophins with an anti-gonadotrophin agent (either in the form of goserelin or exogenous testosterone) (Hu et al., 2018; Amer et al., 2020). The literature included three studies analysing patients undergoing primary TESE (Reifsnyder et al., 2012; Majzoub et al., 2016; Shoshany et al., 2017), four studies investigated patients undergoing secondary TESE (Shiraishi et al., 2012, 2016; Hu et al., 2018; Amer et al., 2020) and one study did not report the operation status (Sujenthiran et al., 2019). There were three studies investigating only the effect of hormone therapy on NOA men producing sperm in their ejaculate (Pavlovich et al., 2001; Saylam et al., 2011; Cavallini et al., 2013). There were five studies that excluded chromosomal abnormalities (Shiraishi et al., 2012, 2016; Cavallini et al., 2013; Shoshany et al., 2017; Hu et al., 2018), four studies included patients with these abnormalities (Pavlovich et al., 2001; Reifsnyder et al., 2012; Majzoub et al., 2016; Sujenthiran et al., 2019) and two studies did not report on genetic findings (Saylam et al., 2011; Amer et al., 2020). The treatment duration ranged from 2 to 6.5 months.
Of the case-control studies, the outcomes were variable; one study (Shiraishi et al., 2012) investigating hCG and FSH showed a statistically significant improvement in SSR in those receiving hormone therapy compared to no treatment (21.4% versus 0%, respectively P < 0.05) while two studies (Reifsnyder et al., 2012; Amer et al., 2020) reported no significant differences in SSR between the treatment and control cohorts. Two studies (Majzoub et al., 2016; Hu et al., 2018) observed improved SSR outcomes with hormone stimulation compared to no treatment but no statistical significance analysis was performed. The single RCT observed that the use of aromatase inhibitors resulted in all NOA patients (n = 6) producing sperm in the ejaculate compared to zero in the control group who did not receive any hormone therapy (n = 6) but it is unclear whether this was statistically significant. The cause for these differences in outcomes is unclear but may be related to study heterogenicity with regards to the patient cohorts, operation status (primary versus secondary TESE) and treatment protocol.
Overall, the following adverse effects were reported with the use of hormone therapy: acne, gynaecomastia, deranged liver function tests, headache, loss of libido, hair loss, joint pain, cutaneous rash, lower limb swelling, ocular pruritus, depression, mastalgia and dry mouth. In three studies, the dropout rates owing to treatment side effects were 9.3% (Shoshany et al., 2017), 18.2% (Cavallini et al., 2013) and 40% (Hu et al., 2018). The main limitation to the current literature is the lack of standardization in terms of treatment regimens and patient cohorts, few studies report pregnancy or live birth rates, and a large proportion of the data is retrospective, case series. Furthermore, there is no clear trend regarding whether hormone therapy improves SSR outcomes compared to no treatment or placebo.
Men with non-obstructive azoospermia and eugonadism
There have been eight studies (Aydos et al., 2003; Selman et al., 2006; Efesoy et al., 2009; Cavallini et al., 2011; Song and Qian, 2012; Hussein et al., 2013; Gul, 2016; Cocci et al., 2018) investigating the use of hormone therapy in men with NOA and eugonadism. The literature consisted of case series (n = 4) (Selman et al., 2006; Efesoy et al., 2009; Cavallini et al., 2011; Song and Qian, 2012) and case-control studies (n = 4) (Aydos et al., 2003; Hussein et al., 2013; Gul, 2016; Cocci et al., 2018) with no RCTs. One study solely utilized aromatase inhibitors (Cavallini et al., 2011), five studies investigated gonadotrophin therapy (Aydos et al., 2003; Selman et al., 2006; Efesoy et al., 2009; Gul, 2016; Cocci et al., 2018) and one study investigated multiple hormone agents (SERMs, gonadotrophins) (Hussein et al., 2013). One study investigated the use of SERMs with exogenous testosterone (Song and Qian, 2012). The data included four studies analysing patients undergoing primary TESE (Aydos et al., 2003; Efesoy et al., 2009; Hussein et al., 2013; Gul, 2016; Cocci et al., 2018) and one study investigated patients undergoing secondary TESE (Selman et al., 2006). There were two studies investigating only the effect of hormone therapy in men with NOA producing sperm in their ejaculate (Cavallini et al., 2011; Song and Qian, 2012), and the treatment duration ranged from 3 to 7 months. There were four studies that excluded chromosomal abnormalities (Selman et al., 2006; Cavallini et al., 2011; Song and Qian, 2012; Gul, 2016), one study that included chromosomal abnormalities (Aydos et al., 2003) and three studies that did not report on the genetic status of the participants (Efesoy et al., 2009; Hussein et al., 2013; Cocci et al., 2018).
Of the case-control studies, the outcomes were inconsistent; two studies (employing gonadotrophins) did not show any statistically significant difference in SSR between those receiving hormone therapy and those proceeding straight to TESE (Aydos et al., 2003; Gul, 2016). However, Cocci et al. (2018) observed that the use of gonadotrophins increased both SSR rate (P < 0.05) and production of sperm into the ejaculate (P < 0.05) compared to no hormone therapy. Similarly, Hussein et al. (2013) studied multiple hormone therapy agents (SERMs, gonadotrophins and a combination of SERMs and gonadotrophins) and reported that hormone therapy increased both SSR rate (P < 0.05) and production of sperm into the ejaculate (P < 0.05) compared to the control group not receiving any treatment. The cause for the differences in outcomes reported in the literature is unclear but may be related to differences in patient cohorts and treatment regimens and durations.
The following adverse effects were reported with the use of hormone therapy: loss of libido, cutaneous rash, anxiety and a paradoxical decline in testosterone levels.
The main limitation to the current evidence is the lack of standardization in terms of patient cohorts, treatment regimens and outcome reporting, with few studies report pregnancy or live birth rates and a large proportion of the data being retrospective, case series.
Studies including men with eugonadal and hypergonadotropic hypogonadal non-obstructive azoospermia
Two case series (Kumar et al., 1990; Kobori et al., 2015) have investigated the use of hormone therapy in a mixed cohort of NOA men with hypergonadotropic hypogonadism and eugonadism. One study solely utilized gonadotrophin therapy (Kobori et al., 2015) and one study investigated the use of either gonadotrophin or clomiphene citrate use (Kumar et al., 1990). Both studies reported the rate of sperm production in the ejaculate and excluded chromosomal abnormalities. No adverse effects were reported in either of the studies. The effects of hormone therapy on the production of sperm in the ejaculate were inconsistent between studies and both studies were limited because the data was retrospective and lacked control cohorts.
Meta-analysis
For the meta-analysis, we only included controlled studies and, owing to the limited number of studies, we pooled data for all hormone classes. Hence, no analysis was performed on the individual drug classes.
Of the retrieved texts, we analysed 10 studies (Tables I and II). Among them, five studies (Reifsnyder et al., 2012; Shiraishi et al., 2012; Majzoub et al., 2016; Hu et al., 2018; Amer et al., 2020) included hypergonadotropic subjects whereas five (Aydos et al., 2003; Hussein et al., 2013; Gul, 2016; Cocci et al., 2018; Sen et al., 2020) included normogonadotropic men. The characteristics of the retrieved studies are reported in Tables I and II. The retrieved studies included 985 patients with a mean (±SD) age of 31.9 ± 4.2 years and a mean follow-up of 17.2 ± 9.4 weeks. The modality of treatment and the drug dosages differed among studies (Tables I and II).
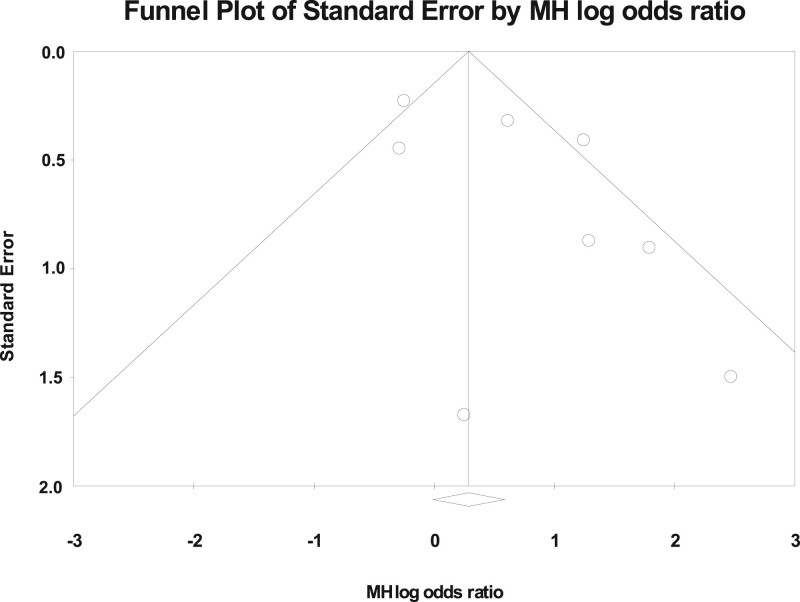
The I2 in trials assessing overall SSR was 58.2 (P < 0.01). A funnel plot and Begg adjusted rank correlation test (Kendall’s τ: 0.00 P = 1.00) was non-significant suggesting publication bias was not present. Figure 2 demonstrates the standard error of sperm retrieval rate by Mantel–Haenszel log odds ratio.
Figure 2.
A funnel plot of standard error of sperm retrieval rate by Mantel–Haenszel log odds ratio. MH, Mantel–Haenszel.
Overall, a higher SSR in subjects pre-treated with hormone therapy was observed (odds ratio (OR) 1.96, 95% CI: 1.08–3.56, P = 0.03) (Fig. 3).
Figure 3.
Effect of hormone therapy on surgical sperm retrieval rate in men with non-obstructive azoospermia. A Forest plot demonstrating the individual and cumulative odds ratios for surgical sperm retrieval.
Sensitivity analysis, excluding one study enrolling only patients with Klinefelter syndrome (Majzoub et al., 2016), confirmed the previous observation that hormone therapy was associated with a higher SSR (OR 1.90, 95% CI: 1.03–3.51, P = 0.04) (Fig. 4).
Figure 4.
Effect of hormone therapy on surgical sperm retrieval rate, including only patients with Klinefelter syndrome. A Forest plot demonstrating the individual and cumulative odds ratios for surgical sperm retrieval. This analysis excluded the study by Majzoub et al. (2016). We excluded this study, as it only included Klienfelter syndrome patients and we wanted to see if this disproportionately affected the results and thus whether are results would be applicable to a non-Klienfelter population.
Further subgroup analysis of baseline hormone status demonstrated only a significant improvement in normogonadotropic men (OR 2.13, 95% CI: 1.10–4.14, P = 0.02) (Fig. 5) but not in hypergonadotropic subjects (OR 1.73, 95% CI: 0.44–6.77, P = 0.43) (Fig. 6).
Figure 5.
Effect of hormone therapy on surgical sperm retrieval rate in normogonadotropic men with non-obstructive azoospermia. A Forest plot demonstrating the individual and cumulative odds ratios for surgical sperm retrieval.
Figure 6.
Effect of hormone therapy on surgical sperm retrieval rate in hypergonadotropic men with non-obstructive azoospermia. A Forest plot demonstrating the individual and cumulative odds ratios for surgical sperm retrieval.
Finally, when the only study not published as a full text (Sen et al., 2020) was excluded, there was a non-statistically significant trend towards a higher SSR in the normogonadotropic group compared to the hypergonadotropic cohort (OR 1.9, 95% CI: 0.95–3.78, P = 0.07).
Risk of bias
The risk of bias analysis is demonstrated in Tables IV and V. A limitation to the data was that none of the studies were randomized and most of the evidence was at risk of confounding bias. The main merits of the literature were that there was a low risk of bias from missing data. Overall, six of the studies were categorized as being of serious risk of bias and four studies of moderate risk of bias.
Table IV.
Risk of bias for studies included in the meta-analysis that investigated eugonadal men with non-obstructive azoospermia.
| Risk of bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study name (year) | Study design | Confounding | Patient selection | Interventions classification | Deviation form intended interventions | Missing data | Measurement outcomes | Selection of reported result | Outcome |
| Aydos et al. (2003) | Case control | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Cocci et al. (2018) | Case control | Serious | Low | Moderate | Low | Low | Serious | Low | Serious |
| Gul (2016) | Case control | Moderate | Moderate | Serious | Low | Low | Serious | Low | Serious |
| Hussein et al (2013) | Case control | Serious | Serious | Serious | Moderate | Low | Serious | Moderate | Serious |
Table V.
Risk of bias for studies included in the meta-analysis that investigated men with non-obstructive azoospermia and hypergonadotropic hypogonadism.
| Risk of bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study name (year) | Study design | Confounding | Patient selection | Interventions classification | Deviation form intended interventions | Missing data | Measurement outcomes | Selection of reported result | Outcome |
| Hu et al. (2018) | Case control | Low | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Shiraishi et al (2012) | Case control | Moderate | Low | Low | Moderate | Low | Low | Low | Moderate |
| Reifsnyder et al. (2012) | Case control | Serious | Serious | Moderate | Moderate | Low | Low | Moderate | Serious |
| Majzoub et al (2016) | Case control | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Sen et al. (2020) | Case control | Serious | Low | Low | Low | Low | Moderate | Low | Serious |
| Amer et al (2020) | Case control | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
Discussion
This is the first systematic review and meta-analysis investigating hormone stimulation therapy in men with NOA and either primary hypogonadism or normal hormone status.
Currently, there are no established pharmacological therapies to treat NOA in men with primary hypogonadism, while rates of successful SSR have been reported to be only 47% (Corona et al., 2019). Within this context, hormone therapies have been used empirically by reproductive clinicians to improve the chances of sperm retrieval, although there are limited large-scale RCTs supporting this in clinical practice. There is a theoretical rationale (Tharakan et al., 2020) to the use of hormone therapy prior to a TESE, as ITT is required for spermiogenesis and human studies have observed that hormone therapy can increase ITT (Shinjo et al., 2013). A study comparing men with hypergonadotropic hypogonadism NOA to those with obstructive azoospermia observed that the former group had more testicular interstitial fibrosis than the latter and the use of hCG was associated with a reduction in fibrotic areas (Oka et al., 2017). However, it remains unclear as to the optimal level of ITT to facilitate spermatogenesis and improve SSR. Moreover, the measurement of ITT requires testicular aspiration, which is an invasive procedure and there is a poor correlation between serum testosterone and ITT levels (Tharakan et al., 2020). A transgenic murine study suggested that an increase of FSH may also contribute to stimulation of spermatogenesis despite a low ITT (Oduwole et al., 2018b); however, this needs to be validated by further data, and the optimal level of FSH elucidated, especially given that another transgenic mice study (Allan et al., 2004) reported that FSH stimulation alone was unable to produce complete spermatogenesis. Therefore, many clinicians have utilized hormone therapy empirically given the theoretical plausibility and lack of alternative treatments. However, the available literature is of low-quality evidence with an abundance of retrospective case series, with only one RCT and a small number of case-controlled studies. Furthermore, we observed moderate heterogeneity (I2 = 58.2, P < 0.01) in the meta-analysis data. The current literature is inconsistent in terms of therapies, duration of treatment, patient cohorts (genetic status, mixed cohorts of oligospermic men and men with NOA) and surgical techniques (primary versus secondary TESE). Moreover, several studies had missing data, with particular reference to post-treatment hormone levels and adverse events outcomes. Furthermore, because a wide range of treatment regimens were utilized, the optimal hormone therapy or duration of treatment to optimize SSR rates remains unclear.
Within these limitations, our meta-analysis demonstrated that, overall, hormone therapy significantly improved SSR (OR 1.96, P = 0.03). Given the paucity of controlled studies, we were unable to perform a sub-analysis on the individual hormone therapy classes. However, when stratifying by baseline hormone status, the effect of hormone therapy on SSR was only seen in men with normal gonadotrophin levels and not in those who were hypergonadotropic. The underlying mechanisms for this are unclear but could be related to the fact that hypergonadotropic hypogonadism may reflect a more severe form of disease with irreversible damage to spermatogenesis and hence is a condition refractory to hormone therapy. Furthermore, in this subset of patients FSH levels are already increased and therefore further hyperstimulation is likely to have less pronounced effects on spermatogenesis. However, there are currently no animal or human studies in the literature to validate this theory.
Murine studies have demonstrated differential endocrinological and reproductive outcomes from the disruption of the androgen receptor in different cell types of the testes. Wang et al. (2009) reported that cell-specific androgen receptor knockout in germ cells resulted in normal gonadotrophin and testosterone levels, testicular size, sperm count and fertility. However, cell-specific androgen receptor knockout in Leydig cells was associated with hypergonadotropic hypogonadism, decreased testicular size and azoospermia. Extrapolating this data to our study, these findings suggest that androgen receptor polymorphisms could also be responsible for the different endocrinological and reproductive characteristics of NOA and may also affect the response to hormone therapy. Moreover, there is data showing that polymorphisms in the FSHR may affect hormone profiles (Lindgren et al., 2012), sperm parameters (Lindgren et al., 2012) and contribute to different responses to hormone therapy (Selice et al., 2011). Lindgren et al. (2016) reported that men homozygous for the Thr307Thr/Asn680Asn single-nucleotide polymorphism combination had a significantly lower FSH (P = 0.009) and total testosterone level (P < 0.0001) but a higher sperm concentration (P = 0.040) and testicular volume (P = 0.002) compared with carriers of other FSHR variants. Selice et al. (2011) observed that the use of FSH therapy only conferred to a statistically significant improvement of sperm parameters in oligospermic men who were homozygote Ala307-Ser680/Ala307-Ser680 or had heterozygote Thr307-Asn680/Ala307-Ser680 common allelic variants. These studies suggest that the effects of hormone therapy may be dependent on genetic alterations in the androgen receptor or FSHR but further studies specifically investigating non-azoospermic men and the effects on SSR rates are needed.
We observed that all identified controlled studies had moderate to serious risk of bias (Tables IV and V). Therefore, although our findings have suggested that hormone therapy may be beneficial in eugonadal NOA men, it is based on low-quality evidence with a significant risk of bias. The current literature is also deficient with regards to information pertaining to the costs of different hormone treatments. Furthermore, no study reported on the prevalence of hypogonadal symptoms in their study cohorts. This would be a useful parameter to assess, as it could potentially justify the use of hormonal manipulation for the dual benefits of infertility and symptomatic male hypogonadism. Moreover, few studies have included data on pregnancy and live birth rates, which is needed to understand how hormone therapy may ultimately influence the quality of sperm and ART outcomes. Therefore, we would not recommend hormone stimulation therapy outside of clinical trials.
There were several limitations to this study. Most of the studies were not randomized or prospective and do not report study participation rate. Thus, the findings of the meta-analysis should be treated with caution given the high risk of selection bias. Furthermore, different hormone assays were utilized presenting a further source of bias. In addition to this, SSR outcomes are influenced by both surgical and embryological factors, including the type of surgery (Bernie et al., 2015), experience of the surgeon (Ishikawa et al., 2010), and the methods used to process the sperm from testicular tissue (Crabbé et al., 1998). Furthermore, many of these studies are not consistent in standardized reported outcomes such as surgical technique used and quantity and quality of sperm retrieved. Available data did not allow us to correct for any of these confounding factors. Moreover, another prognostic factor to sperm retrieval surgery is histopathological subtype (Flannigan et al., 2017), although most studies did not report data pertaining to this confounding variable. However, it must be noted that it is common for NOA patients to have a mixed histopathological pattern (McLachlan et al., 2007). We were unable to provide any analysis regarding aetiology and its effects on SSR, which represents a further limitation (e.g. some genetic or acquired conditions, such as azoospermia factor microdeletions, confer a worse prognosis for SSR outcomes (Kamp et al., 2001)). In most studies, there were no comparison of markers of testicular function (such as testicular size, and Leydig and Sertoli cellular secretory function parameters: insulin like three peptide, inhibin B and anti-Müllerian hormone) and therefore this study was unable to exclude these confounding factors.
Conclusion
This systematic review and meta-analysis observed that the current literature pertaining to hormone stimulation in men with NOA provides low-quality evidence and is at moderate or severe risk of bias. Within these limitations, hormone therapy overall appears to increase SSR rate but only in men with NOA and normal gonadotrophin status. However, there is a paucity of controlled trials to provide any evidence-based recommendations, and no firm inferences can be provided given the poor quality of the data. Moreover, many studies do not report adverse events. Therefore, based upon the current literature we cannot advocate the use of hormone therapy in men with NOA until further high powered, RCTs are performed.
Contributor Information
Tharu Tharakan, Department of Urology, Imperial Healthcare NHS Trust, Charing Cross Hospital, London, UK; Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK.
Giovanni Corona, Endocrinology Unit, Medical Department, Azienda Usl Bologna Maggiore-Bellaria Hospital, Bologna, Italy.
Daniel Foran, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK.
Andrea Salonia, Department of Experimental Oncology/Unit of Urology, URI, IRCCS Ospedale San Raffaele, Milan, Italy; Department of Urology, University Vita-Salute San Raffaele, Milan, Italy.
Nikolaos Sofikitis, Department of Urology, Ioannina University School of Medicine, Ioannina, Greece.
Aleksander Giwercman, Department of Translational Medicine, Lund University, Lund, Sweden.
Csilla Krausz, Department of Experimental and Clinical Biomedical Sciences, University Hospital of Careggi (AOUC), University of Florence, Florence, Italy.
Tet Yap, Department of Urology, Guy’s and St Thomas’ Hospital, London, UK.
Channa N Jayasena, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK.
Suks Minhas, Department of Urology, Imperial Healthcare NHS Trust, Charing Cross Hospital, London, UK.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
T.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: T.T., C.N.J. and S.M. Acquisition of data: T.T., G.C., D.F. and T.Y. Analysis and interpretation of data: T.T., N.S., A.G, C.K., C.N.J., S.M., G.C., T.Y., A.S., N.S., A.G., C.K. and C.N.J. Drafting of the manuscript: T.T. and D.F. Critical revision of the manuscript for important intellectual content: G.C., S.M., C.N.J., A.S., N.S., A.G., C.K., T.Y. and A.S. Statistical analysis: T.T., T.Y. and G.C. Obtaining funding: none. Administrative, technical or material support: None. Supervision: C.N.J. and S.M. Other: none. Financial disclosures: T.T. certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received or pending), are the following: none.
Funding
C.N.J.’s research was funded by the NIHR Biomedical Research Centre at Imperial College Healthcare NHS Trust and National Institute for Health Research (NIHR) post doctorial fellowship. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. T.T. is supported by Imperial Private Services Fellowship.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Allan CM, Garcia A, Spaliviero J, Zhang FP, Jimenez M, Huhtaniemi I, Handelsman DJ.. Complete sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology 2004;145:1587–1593. [DOI] [PubMed] [Google Scholar]
- Amer MK, Ahmed HEH, GamalEl Din SF, Fawzy Megawer A, Ahmed AR.. Evaluation of neoadjuvant gonadotropin administration with downregulation by testosterone prior to second time microsurgical testicular sperm extraction: a prospective case–control study. Urologia 2020;87:185–190. [DOI] [PubMed] [Google Scholar]
- Aydos K, Ünlü C, Demirel LC, Evirgen O, Tolunay Ö.. The effect of pure FSH administration in non-obstructive azoospermic men on testicular sperm retrieval. Eur J Obstet Gynecol Reprod Biol 2003;108:54–58. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088. [PubMed] [Google Scholar]
- Bernie AM, Mata DA, Ramasamy R, Schlegel PN.. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril 2015;104:1099–1103. [DOI] [PubMed] [Google Scholar]
- Bobjer J, Naumovska M, Giwercman YL, Giwercman A.. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl 2012;35:688–694. [DOI] [PubMed] [Google Scholar]
- Cavallini G, Beretta G, Biagiotti G.. Preliminary study of letrozole use for improving spermatogenesis in non-obstructive azoospermia patients with normal serum FSH. Asian J Androl 2011;13:895–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini G, Biagiotti G, Bolzon E.. Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian J Androl 2013;15:806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocci A, Cito G, Russo GI, Falcone M, Capece M, Timpano M, Della Camera PA, Morselli S, Tasso G, Morelli G. et al. Effectiveness of highly purified urofollitropin treatment in patients with idiopathic azoospermia before testicular sperm extraction. Urologia 2018;85:19–21. [DOI] [PubMed] [Google Scholar]
- Corona G, Minhas S, Giwercman A, Bettocchi C, Dinkelman-Smit M, Dohle G, Fusco F, Kadioglou A, Kliesch S, Kopa Z. et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: a systematic review and meta-analysis. Hum Reprod Update 2019;25:733–757. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, Yan X, Brown TR, Wright WW, Zirkin BR. et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl 2004;25:931–938. [DOI] [PubMed] [Google Scholar]
- Crabbé E, Verheyen G, Silber S, Tournaye H, Van de Velde H, Goossens A, Van Steirteghem A.. Enzymatic digestion of testicular tissue may rescue the intracytoplasmic sperm injection cycle in some patients with non-obstructive azoospermia. Hum Reprod 1998;13:2791–2796. [DOI] [PubMed] [Google Scholar]
- Efesoy O, Cayan S, Akbay E.. The efficacy of recombinant human follicle-stimulating hormone in the treatment of various types of male-factor infertility at a Single University Hospital. J Androl 2009;30:679–684. [DOI] [PubMed] [Google Scholar]
- Flannigan R, Bach PV, Schlegel PN.. Microdissection testicular sperm extraction. Transl Androl Urol 2017;6:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C, Bettella A, Spolaore D, Merico M, Rossato M, Ferlin A.. Suppression of the high endogenous levels of plasma FSH in infertile men are associated with improved Sertoli cell function as reflected by elevated levels of plasma inhibin B. Hum Reprod 2004;19:1431–1437. [DOI] [PubMed] [Google Scholar]
- Foresta C, Selice R, Moretti A, Pati MA, Carraro M, Engl B, Garolla A.. Gonadotropin administration after gonadotropin-releasing-hormone agonist: a therapeutic option in severe testiculopathies. Fertil Steril 2009;92:1326–1332. [DOI] [PubMed] [Google Scholar]
- Gnanaprakasam MS, Chen CJH, Sutherland JG, Bhalla VK.. Receptor depletion and replenishment processes: in vivo regulation of gonadotropin receptors by luteinizing hormone, follicle stimulating hormone and ethanol in rat testis. Biol Reprod 1979;20:991–1000. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Simoni M, Nieschlag E.. An activating mutation of the follicle-stimulating hormone receptor autonomously sustains spermatogenesis in a hypophysectomized man. J Clin Endocrinol Metab 1996;81:1367–1370. [DOI] [PubMed] [Google Scholar]
- Gul U. The effect of human chorionic gonadotropin treatment before testicular sperm extraction in non-obstructive azoospermia. J Clin Anal Med 2016;7:55–59. [Google Scholar]
- Hu X, Ding Z, Hong Z, Zou Z, Feng Y, Zhu R, Ma J, Ge X, Li C, Yao B.. Spermatogenesis improved by suppressing the high level of endogenous gonadotropins in idiopathic non-obstructive azoospermia: a case control pilot study. Reprod Biol Endocrinol 2018;16:91–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I. Mechanisms in endocrinology: hormonal regulation of spermatogenesis: mutant mice challenging old paradigms. Eur J Endocrinol 2018;179:R143–R150. [DOI] [PubMed] [Google Scholar]
- Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C.. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int 2013;111:E110–E114. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nose R, Yamaguchi K, Chiba K, Fujisawa M.. Learning curves of microdissection testicular sperm extraction for nonobstructive azoospermia. Fertil Steril 2010;94:1008–1011. [DOI] [PubMed] [Google Scholar]
- Jarow JP, Chen H, Rosner W, Trentacoste S, Zirkin BR.. Assessment of the androgen environment within the human testis: Minimally invasive method to obtain intratesticular fluid. J Androl 2001;22:640–645. [PubMed] [Google Scholar]
- Jarow JP, Espeland MA, Lipshultz LI.. Evaluation of the azoospermic patient. J Urol 1989;142:62–65. [DOI] [PubMed] [Google Scholar]
- Kamp C, Huellen K, Fernandes S, Sousa M, Schlegel PN, Mielnik A, Kleiman S, Yavetz H, Krause W, Küpker W. et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod 2001;7:987–994. [DOI] [PubMed] [Google Scholar]
- Kim HH, Schlegel PN.. Endocrine manipulation in male infertility. Urol Clin North Am 2008;35:303–318. [DOI] [PubMed] [Google Scholar]
- Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES.. Empirical medical therapy for idiopathic male infertility: a survey of the American urological association. J Urol 2012;187:973–978. [DOI] [PubMed] [Google Scholar]
- Kobori Y, Suzuki K, Iwahata T, Shin T, Sato R, Nishio K, Yagi H, Arai G, Soh S, Okada H.. Induction of spermatogenesis by rhFSH for azoospermia due to spermatogenic dysfunction with maturation arrest: five case series. Syst Biol Reprod Med 2015;61:168–170. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jayakumar B, Khurana ML, Prakash V, Yaraghavan MV, Shukla NK, Amini AC, Karmarkar MG, Ahuja MMS.. Testicular histology and gonadotropin levels in infertile men with non-obstructive oligo-/azoospermia. Natl Med J India 1990;3:212–216. [PubMed] [Google Scholar]
- Kumar R. Medical management of non-obstructive azoospermia. Clinics 2013;68:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren I, Giwercman A, Axelsson J, Lundberg Giwercman Y.. Association between follicle-stimulating hormone receptor polymorphisms and reproductive parameters in young men from the general population. Pharmacogenet Genomics 2012;22:667–672. [DOI] [PubMed] [Google Scholar]
- Lindgren K, Nordqvist S, Karehed K, Sundstrom-Poromaa I, Akerud H.. The effect of a specific histidine-rich glycoprotein polymorphism on male infertility and semen parameters. Reprod Biomed Online 2016;33:180–188. [DOI] [PubMed] [Google Scholar]
- Majzoub A, Arafa M, Al Said S, Agarwal A, Seif A, Al Naimi A, El Bardisi H.. Outcome of testicular sperm extraction in nonmosaic Klinefelter syndrome patients: what is the best approach? Andrologia 2016;48:171–176. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, Robertson DM.. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab 2002;87:546–556. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE.. Histological evaluation of the human testis—approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod 2007;22:2–16. [DOI] [PubMed] [Google Scholar]
- Namiki M, Nakamura M, Okuyama A, Sonoda T, Itatani H, Sugao H, Sakurai T, Nishimune Y, Matsumoto K.. Reduction of human and rat testicular follicle stimulating hormone receptors by human menopausal gonadotrophin in vivo and in vitro. Clin Endocrinol (Oxf) 1987;26:675–684. [DOI] [PubMed] [Google Scholar]
- Namiki M, Okuyama A, Sonoda T, Miyake A, Aono T, Matsumoto K.. Down-regulation of testicular follicle-stimulating hormone receptors by human menopausal gonadotropin in infertile men. Fertil Steril 1985;44:710–712. [DOI] [PubMed] [Google Scholar]
- Oduwole OO, Peltoketo H, Huhtaniemi IT.. Role of follicle-stimulating hormone in spermatogenesis. Front Endocrinol (Lausanne) 2018a;9:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduwole OO, Peltoketo H, Poliandri A, Vengadabady L, Chrusciel M, Doroszko M, Samanta L, Owen L, Keevil B, Rahman NA. et al. Constitutively active follicle-stimulating hormone receptor enables androgen-independent spermatogenesis. J Clin Invest 2018b;128:1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Shiraishi K, Matsuyama H.. Effects of human chorionic gonadotropin on testicular interstitial tissues in men with non-obstructive azoospermia. Andrology 2017;5:232–239. [DOI] [PubMed] [Google Scholar]
- Pavlovich CP, King P, Goldstein M, Schlegel PN.. Evidence of a treatable endocrinopathy in infertile men. J Urol 2001;165:837–841. [PubMed] [Google Scholar]
- Reifsnyder JE, Ramasamy R, Husseini J, Schlegel PN.. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2012;188:532–536. [DOI] [PubMed] [Google Scholar]
- Roth MY, Lin K, Amory JK, Matsumoto AM, Anawalt BD, Snyder CN, Kalhorn TF, Bremner WJ, Page ST.. Serum LH correlates highly with intratesticular steroid levels in normal men. J Androl 2010;31:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cocci A, Corona G, Dimitropoulos K, Gül M, Hatzichristodoulou G. et al. EAU Guidelines on Sexual and Reproductive Health. 2021. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Sexual-and-Reproductive-Health-2021.pdf (15 April 2022, date last accessed).
- Salonia A, Rastrelli G, Hackett G, Seminara SB, Huhtaniemi IT, Rey RA, Hellstrom WJG, Palmert MR, Corona G, Dohle GR, Khera M. et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Primers 2019;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylam B, Efesoy O, Ayan S.. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril 2011;95:809–811. [DOI] [PubMed] [Google Scholar]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14:131–135. [DOI] [PubMed] [Google Scholar]
- Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev 2004;16:561–572. [DOI] [PubMed] [Google Scholar]
- Selice R, Garolla A, Pengo M, Caretta N, Ferlin A, Foresta C.. The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int J Androl 2011;34:306–312. [DOI] [PubMed] [Google Scholar]
- Selman H, De Santo M, Sterzik K, Cipollone G, Aragona C, El-Danasouri I.. Rescue of spermatogenesis arrest in azoospermic men after long-term gonadotropin treatment. Fertil Steril 2006;86:466–468. [DOI] [PubMed] [Google Scholar]
- Sen S, Chaw TE, Ling YS, Xuemei Z, Rajesh H.. Effect of recombinant HCG pretreatment on surgical sperm retrieval rates in patients with non-obstructive azoospermia—an audit of our practice. Fertil Steril 2020;113:E21–E22. [Google Scholar]
- Shinjo E, Shiraishi K, Matsuyama H.. The effect of human chorionic gonadotropin-based hormonal therapy on intratesticular testosterone levels and spermatogonial DNA synthesis in men with non-obstructive azoospermia. Andrology 2013;1:929–935. [DOI] [PubMed] [Google Scholar]
- Shiraishi K. Hormonal therapy for non-obstructive azoospermia: basic and clinical perspectives. Reprod Med Biol 2015;14:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K, Ishikawa T, Watanabe N, Iwamoto T, Matsuyama H.. Salvage hormonal therapy after failed microdissection testicular sperm extraction: a multi-institutional prospective study. Int J Urol 2016;23:496–500. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Ohmi C, Shimabukuro T, Matsuyama H.. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod 2012;27:331–339. [DOI] [PubMed] [Google Scholar]
- Shoshany O, Abhyankar N, Mufarreh N, Daniel G, Niederberger C.. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertil Steril 2017;107:589–594. [DOI] [PubMed] [Google Scholar]
- Song B, Qian WP.. Effectiveness of combined testosterone undecanoate with tamoxifen citrate treatment in men with idiopathic azoospermia or serious oligozoospermia. J Reprod Contracept 2012;23:254–258. [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujenthiran A, Tracey J, Broussil P, Homa S, Dajani Y, Kopeika Y, Shabbir M, Yap T.. MP46-05 Hormone stimulation prior to micro-TESE improves success rates in Klinefelter syndrome patients. J Urol 2019;201:e679. [Google Scholar]
- Tharakan T, Salonia A, Corona G, Dhillo W, Minhas S, Jayasena C.. The role of hormone stimulation in men with nonobstructive azoospermia undergoing surgical sperm retrieval. J Clin Endocrinol Metab 2020;105:dgaa556. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Blok LJ, Post M, Baarends WM, Hoogerbrugge JW, Parmentier M, Vassart G, Grootegoed JA.. Follitropin receptor down-regulation involves a cAMP-dependent post-transcriptional decrease of receptor mRNA expression. Mol Cell Endocrinol 1991;78:R7–R13. [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Tzeng CR, Chang C.. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 2009;30:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.