Abstract
The known 41 flagellar, chemotaxis, and motility genes of Sinorhizobium (Rhizobium) meliloti contained in the “flagellar regulon” are organized as seven operons and six transcription units that map to a contiguous 45-kb chromosomal region. By probing gene expression on Western blots and with lacZ fusions, we have identified two master regulatory genes, visN and visR, contained in one operon. The gene products probably form a heterodimer, VisNR, acting as a global transcription activator of other flagellar genes. The related 27-kDa VisN and VisR proteins are LuxR-type proteins with typical ligand- and DNA-binding domains. The vis operon itself is constitutively transcribed; however, to activate flagellar genes, VisNR seemingly requires the binding of a yet-unknown effector. Gene expression in tester strains with known deficiencies revealed a hierarchy of three classes of flagellar genes: class I comprises visN and visR; class II, controlled by VisNR, comprises flagellar assembly (class IIA) and motor (class IIB) genes; and class III comprises flagellin and chemotaxis genes that require functional class I and class IIA genes for expression. In contrast to their enterobacterial counterparts, mot genes belong to class II without exerting control over class III genes. While the general hierarchy of gene expression resembles the enterobacterial scheme, the assignment of mot genes to class IIB and the global control by a LuxR-type VisNR activator are new features distinguishing the S. meliloti flagellar gene system.
Bacterial motility and chemotaxis are essential qualities for optimum adaptation to different environments. Flagellar synthesis and motility are maximal under nutritional stress and require 2 to 3% of the energy of a cell. Therefore, the expression of some 50 genes involved in this process is strictly regulated by a hierarchy of controls (19). The current paradigm of flagellar gene regulation was derived from studies of Escherichia coli and Salmonella enterica serovar Typhimurium (13, 15, 18, 20, 29). The E. coli chemotaxis, flagellar, and motility genes map in four separate clusters often referred to as the flagellar regulon. The genes of this regulon are organized in three classes that are expressed in hierarchial order, although their map positions do not necessarily reflect the assignments to these classes. Class I is represented by a master operon that encodes the transcription activators, FlhC and FlhD, which in turn regulate the expression of class II (18). This includes genes that determine the flagellar basal body and the flagellin-specific export apparatus and fliA, which encodes a ς28 (ςF) transcription factor for class III.
Sinorhizobium (Rhizobium) meliloti, a member of the alpha subgroup of proteobacteria, exhibits significant deviations from the enterobacterial (gamma subgroup) paradigm of chemotaxis in its flagellar structure and mode of rotation (23). The complex, rigid flagellar filaments of S. meliloti consist of four closely related flagellin subunits, FlaA, FlaB, FlaC, and FlaD, encoded by four linked but independently transcribed genes (24, 33). The right-handed flagellar helices rotate exclusively in the clockwise mode, and swimming cells respond to tactic stimuli by modulating their flagellar rotary speed (31, 32). Two novel motor proteins, MotC and MotD, are essential players in the control of flagellar rotary speed (23). The organization of the S. meliloti chemotaxis (che), flagellar (fla, flg, flh, and fli), and motility (mot) genes is distinctly different from that in enterobacteria, since all known 41 genes are clustered in one contiguous 45-kb chromosomal region (33). Among these are 10 genes of a che operon, four mot genes (23), 15 flg, flh, and fli genes encoding components of the basal body and the flagellar export apparatus, 4 flagellin (fla) genes, and 5 genes of hitherto unknown function. Notably, MotA is encoded in the same operon as FliM, FliN, and FliG, and a new gene, orf38, necessary for flagellum formation, is part of the motB-motC-motD operon (33). In keeping with established nomenclature (20), we refer to the entirety of these genes as the flagellar regulon.
We have identified in S. meliloti two related members of the LuxR family, VisN and VisR (for “vital for swimming”), formerly named Orf12 and Orf13, respectively (33), that function as global activators of the flagellar regulon. Transcriptional activators of the LuxR family have been found in various bacterial species, where they regulate such different processes as conjugation, cell division, and antibiotic production (6, 34). Typical members of this family are TraR, which regulates plant-pathogenic genes of Agrobacterium tumefaciens (5, 22), and RhiR and RaiR, which regulate the nodulation genes of Rhizobium leguminosarum and Rhizobium etli (3, 8, 26). LuxR is best known for its role in quorum sensing, i.e., the ability to monitor population density by sensing the external concentration of autoinducer molecules, notably homoserine lactones. All these transcription factors have similar structures with a ligand-binding domain and DNA-binding domains of the helix-turn-helix type, which place them in the LuxR-FixJ-UhpA-NarL superfamily of transcription factors (6, 9). The LuxR-like regulators, VisN and VisR, of S. meliloti described here act as master controls of a gene cascade that encodes flagellar, motor, and chemotaxis proteins.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Derivates of E. coli K-12 and S. meliloti MVII-1 (12) and the plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Markers | Source or reference |
|---|---|---|
| E. coli | ||
| S17-1 | recA endA thi hsdR RP4-2 Tc::Mu::Tn7 Tpr Smr | 30 |
| S. meliloti | ||
| RU10/406 | Wild-type strain | 7, 14 |
| RU11/001 | Smr; spontaneous streptomycin-resistant derivative of RU10/406 (wild type) | 25 |
| RU11/011a | Δ(flaA-flaC)::Smr Nmr; nonmotile | 25 |
| RU11/300A | Smr Tcr; RU11/001(pRU1770) | This study |
| RU11/300B | Smr Tcr; RU11/317(pRU1770) | This study |
| RU11/300C | Smr Tcr; RU11/318(pRU1770) | This study |
| RU11/317 | Smr ΔvisR | This study |
| RU11/318 | Smr ΔvisN | This study |
| RU11/391 | Smr Nmr Gmr; RU11/318(pRU1755) | This study |
| RU11/397 | Smr Nmr Gmr; RU11/317(pRU1757) | This study |
| RU11/513 | Smr ΔmotBC | 23 |
| RU11/800 | Smr ΔfliM | This study |
| RU11/801 | Smr Δorf38 | This study |
| RU11/802 | Smr ΔmotA | This study |
| Plasmids | ||
| pML122 | Kmr Gmr | 16 |
| pPHU234b | Tcr; promoterless lacZ | 11 |
| pRU1755 | Kmr Gmr; recombinant of pML122 and 969-bp HindIII-XbaI fragment containing visN | This study |
| pRU1756 | Kmr Gmr; recombinant of pML122 and 1.7-kb XbaI-XhoI fragment containing visN and visR | This study |
| pRU1757 | Kmr Gmr; recombinant of pML122 and 930-bp SacI-XhoI fragment containing visR | This study |
| pRU1770 | Tcr; visN-lacZ fusion | This study |
| pRU2250 | Tcr; tlpA-lacZ fusion | This study |
| pRU2269 | Tcr; motA-lacZ fusion | This study |
| pRU2274 | Tcr; flaA-lacZ fusion | This study |
| pRU2278 | Tcr; orf38-lacZ fusion | This study |
Media and growth conditions.
E. coli strains were grown in Luria broth (19) at 37°C. S. meliloti strains were grown in TYC at 30°C (23). Motile cells prepared for immunoblots and β-galactosidase assays (see below) were grown overnight in TYC, diluted in 15 ml of RB (7) to an optical density at 600 nm (OD600) = 0.05, layered on Bromfield plates (31), and grown on a slowly rotating platform at 30°C for 17 h to an OD600 of ca. 0.1 to 0.5. Swarm plates containing Bromfield medium and 0.3% Bacto Agar were inoculated with 3-μl droplets of the test culture and incubated at 30°C for 2 days. Antibiotics were used at the following final concentrations: for E. coli, kanamycin, 50 mg/liter; gentamicin, 10 mg/liter; and tetracycline, 10 mg/liter; for S. meliloti, neomycin, 100 mg/liter; streptomycin, 600 mg/liter; gentamicin, 20 mg/liter; and tetracycline, 10 mg/liter.
DNA methods.
S. meliloti chromosomal DNA was isolated and purified as previously described (31). Plasmid DNA was purified with Qiagen spin columns or Qiagen 100 tips, as described by the manufacturer (Qiagen, Hilden, Germany). DNA fragments or PCR products were purified from agarose gels by use of the QiaEX DNA purification kit (Qiagen). PCR amplification of chromosomal DNA (33) and Southern analyses followed previously published protocols (23, 33). DNA was sequenced by the method of Sanger et al. (27) with a Quick Denature Sequenase kit (Amersham Buchler, Braunschweig, Germany) or with an ABI 310 automatic sequencer (Applied Biosystems, Weiterstadt, Germany). Sequences were aligned and compared by using GCG sequence analysis software (4); similarities were calculated by the method of Henikoff and Henikoff (9).
Gene replacement and complementation.
Deletions were generated in vitro by using two PCR steps as described by Higuchi (10). PCR products containing the desired deletions were cloned into the mobilizable suicide vector pK18 mob sacB (28) and used to transform E. coli S17-1. Clones were sequenced to ascertain the accuracy of PCR-generated portions. Filter crosses of E. coli S17-1 and S. meliloti and sequential selections on neomycin and on 10% sucrose were performed as previously described (31). Complementation with pML122-based recombinant genes (Table 1) followed established protocols (16).
Immunoblots.
Aliquots (1 ml) of cell culture of wild-type S. meliloti RU11/001 and various deletion strains at an OD600 of 0.3 were harvested by centrifugation at 20,000 × g for 6 min, resuspended in 20 μl of sodium dodecyl sulfate sample buffer, and heated to 100°C for 8 min. Samples were stored at −20°C. Fla, FliM, and MotC proteins were separated electrophoretically in a 10 to 15% linear acrylamide gradient and CheY1 was separated in a 12.5 to 20% linear gradient by the method of Laemmli (17). Electrophoretic transfer of proteins from gels to 0.45-μm-pore-size nitrocellulose (Hybond ECL; Amersham) was performed in a tank blot device (Biological Laboratories, Harvard University) for 1.5 h at 500 mA. The nitrocellulose blots were blocked overnight at room temperature in 80 mM Na2HPO4–20 mM NaH2PO4–100 mM NaCl–0.1% (vol/vol) Tween 20 (pH 7.5) (Bio-Rad)–5% instant nonfat dry milk on a shaking platform. The blots were probed with anti-FliM-MalE, anti-Fla, and anti-CheY1 polyclonal antibodies at a 1:1,000 dilution for 2 h. Anti-MotC polyclonal antibody was purified, as described by Platzer et al. (23), at a 1:250 dilution for 3 h. The blots were washed three times (10 min each), incubated with donkey anti-rabbit horseradish peroxidase-linked whole immunoglobulin antibody (Amersham) diluted 1:2,500, and washed four times (10 min each). Detection of bands by enhanced chemiluminescence (Amersham) was performed as specified by the manufacturer.
Construction of lacZ fusions and β-galactosidase assay.
The broad-host range vector pPHU234 and two derivates, pPHU235 and pPHU236 (Table 1), served as vehicles for translational fusions to a promoterless lacZ (11). Five lacZ fusions were constructed to test gene expression in S. meliloti: (i) pRU1770, a recombinant of a 217-bp XbaI-HindIII visN (9-bp) fragment and pPHU234; (ii) pPHU2250, a recombinant of a 1,969-bp EcoRI-PstI tlpA (165-bp) fragment and pPHU235; (iii) pRU2269, a recombinant of a 786-bp EcoRI-HindIII motA (15-bp) fragment and pPHU235; (iv) pRU2274, a recombinant of a 550-bp EcoRI-PstI flaA (62-bp) fragment and pPHU236; and (v) pRU2278, a recombinant of a 561-bp orf38 (23-bp) fragment and pPHU 234 (lengths of coding sequences are given in parentheses). The resulting lacZ fusion plasmids were used to transform E. coli S17-1 and then conjugally transferred to wild-type and mutant S. meliloti strains by a streptomycin-tetracycline double selection, as described by Labes et al. (16). Cultures of S. meliloti cells containing lacZ fusions were sampled, diluted in Z-buffer to an OD600 of 0.2, permeabilized with 1 drop of toluene, and assayed for β-galactosidase activity by the method of Miller (21).
RESULTS
VisN and VisR are LuxR-type regulatory proteins.
The visN and visR genes form an operon located centrally in the S. meliloti flagellar regulon between fliF and two operons (starting with flhB and motA, respectively) with opposite transcription polarity relative to all other genes (33). The derived polypeptide sequences of the structurally related 27-kDa VisN and VisR proteins revealed distinct similarities to the transcription activator LuxR (Fig. 1). LuxR-like factors typically consist of a receptor module with ligand-binding and oligomerization domains (located in the N-terminal half) and an activator module with the DNA-binding site featuring a helix-turn-helix motif and the transcription activation domain (located in the C-terminal half) (5). The entire LuxR with its autoinducer binding, oligomerization, and DNA-binding domains exhibits some 40% similarity (9) to the VisN and VisR polypeptides. The similarity to LuxR is more prominent among the DNA-binding domains with 39% identical and 60% similar residues for VisN and 42% identical and 60% similar residues for VisR, respectively. Both VisN and VisR contain the characteristic helix-turn-helix motifs, which have 36% identity and 60% similarity to each other. With respect to LuxR, the lowest sequence conservation prevails in the ligand-binding domains of VisN and VisR, suggesting a new specificity for a yet-unknown ligand.
FIG. 1.
Alignment of the LuxR (6) and VisN and VisR (33) polypeptide sequences. Comparisons of VisN and VisR each to LuxR with regard to identical (one-letter amino acid symbols) or similar residues (+; assigned according to the system of Henikoff and Henikoff [9]) are depicted above (VisN and LuxR) and below (VisR and LuxR) the sequences, respectively. LuxR-type functional domains are numbered as follows: 1, autoregulation; 2, ligand binding; 3, oligomerization; 4, transcription activation. The three components of the conserved helix-turn-helix DNA-binding motif (1, 9) are marked by black bars.
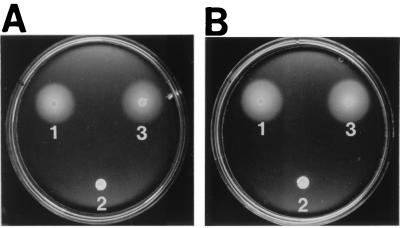
visN and visR knockout mutants were used to study the presumptive regulatory role of these genes. In-frame deletions of visN and visR constructed to avoid polar effects were used for allelic exchange of the wild-type genes by homologous recombination (28, 31). The resulting mutants, RU11/318 (ΔvisN) and RU11/317 (ΔvisR), were nonmotile under phase-contrast microscopy and on swarm plates (Fig. 2, spot 2). Electron microscopy revealed that both mutants were nonflagellate, pointing to impaired flagellar synthesis. Complementation by separate expression of the homologous wild-type alleles introduced on plasmids pRU1755 (visN) and pRU1757 (visR) completely restored swimming and swarming proficiency (Fig. 2, spot 3). The data clearly suggest that the gene products of visN and visR are acting in trans and are both required for flagellar synthesis.
FIG. 2.
Effects on swarming of in-frame deletions of visR (A) and visN (B) and complementation by the wild-type alleles. (A) Swarm 1, RU11/001 (wild type); swarm 2, RU11/317 (ΔvisR); swarm 3, RU11/397 (ΔvisR/visR). (B) Swarm 1 RU11/001 (wild type); swarm 2, RU11/318 (ΔvisN); swarm 3 RU11/391 (ΔvisN/visN). Strains to be tested were transferred by micropipette (3 μl) onto Bromfield swarm plates and incubated at 30°C for 2 days. The diameter of a swarm ring reflects the motile proficiency of a given strain.
VisN and VisR are master controls of the flagellar regulon.
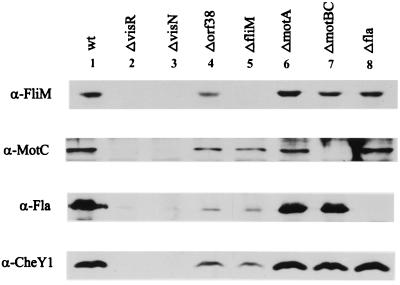
Features of the ΔvisN and ΔvisR deletion mutants, notably the total absence of flagella, led us to ask whether VisN and VisR are global regulators of the entire cluster of chemotaxis, flagellar, and motility genes (flagellar regulon). We therefore tested the expression of representative basal-body, motor, flagellin, and chemotaxis genes by Western analysis (Fig. 3) and by lacZ fusions (Table 2) in wild-type S. meliloti and in defined tester mutant background. Polyclonal antibodies against recombinant FliM, MotC, CheY1, and purified flagellar filaments (Fla) were used to probe gene expression of the four “indicator” genes, i.e., fliM, encoding a constituent of the basal flagellar body; motC, encoding a flagellar motor protein; flaA to flaC, encoding the flagellin subunits; and cheY1, encoding a chemotaxis response regulator (23, 24, 31, 32, 33). The results, shown in Fig. 3 (lanes 2 and 3), confirm the presumed role of VisN and VisR as global regulators, since none of the three genes representing major operons and the fla genes were expressed, neither in the ΔvisN (RU11/318) nor in the ΔvisR (RU11/317) mutant background. In a control experiment with wild-type S. meliloti, each indicator gene produced a strong signal (lane 1). Since visN and visR mutants each failed to express the fli, mot, fla, and che genes, we postulate that VisN and VisR may form a functional heterodimer, VisNR, that acts as transcription activator on target promoters. This is an intriguing new function for a LuxR-like regulatory protein.
FIG. 3.
Western blot analysis of gene expression in wild-type and mutant S. meliloti by using polyclonal anti-FliM (α-FliM), anti-MotC (α-MotC), anti-flagellin (α-Fla), and anti-CheY1 (α-CheY1) antibodies. Equal amounts of total cell protein from strains 1 (RU11/001 [wild type]), 2 (RU11/317 [ΔvisR]), 3 (RU11/318 [ΔvisN]), 4 (RU11/801 [Δorf38]), 5 (RU11/800 [ΔfliM]), 6 (RU11/802 [ΔmotA]), 7 (RU11/513 [ΔmotBC]), and 8 (RU11/011 [ΔflaA–flaC]) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted on nitrocellulose, and detected with specific antibody as described in Materials and Methods. Note the lack of a signal where the indicator gene was deleted.
TABLE 2.
Expression of five promoters fused to lacZ in seven different mutant tester strains
| Plasmid (lacZ fusion) | Relative promoter activity in following mutant backgrounda:
|
||||||
|---|---|---|---|---|---|---|---|
| RU11/317 (ΔvisR) (class I) | RU11/318 (ΔvisN) (class I) | RU11/801 (Δorf38) (class IIA) | RU11/800 (ΔfliM) (class IIA) | RU11/802 (ΔmotA) (class IIB) | RU11/513 (ΔmotBC) (class IIB) | RU11/011 (ΔflaA-C) (class III) | |
| pRU1770 (vis operon)b | 0.79 | 1.24 | 0.92 | 1.05 | 1.16 | 1.03 | 1.12 |
| pRU2269 (mofli operon)b | 0.01 | 0.03 | 1.42 | 1.43 | 1.31 | 1.02 | 1.53 |
| pRU2278 (mot operon)b | 0 | 0.04 | 1.23 | 1.31 | 0.65 | 1.26 | 1.20 |
| pRU2274 (flaA) | 0.04 | 0.02 | 0.34 | 0.22 | 0.97 | 0.96 | 1.52 |
| pRU2250 (che operon)b | 0 | 0 | 0.07 | 0.05 | 0.60 | 0.68 | 1.05 |
Relative activities are expressed as the ratio of β-galactosidase activities in a given mutant background to the wild-type background (not shown). Transcription from five promoters (left column) was determined via plasmid-borne lacZ fusions (see Materials and Methods). Mean values of four Miller assays (21) were averaged, with standard deviations ranging between 0.01 and 0.15.
Operons controlled by the specified promoters contain the following genes (33): vis, visN-R; mofli, motA fliMNG; mot, orf38 motBCD; che, tlpA orf2 cheY1AWRB Y2D orf10.
Immunoblots of the four indicator genes have been extended to extracts of six additional strains bearing in-frame deletions in orf38, fliM, motA, motBC, and flaA to flaC (Fig. 3, lanes 4 to 8). By including these knockout mutants, a hierarchial order of gene expression within the flagellar regulon was derived. The results in Fig. 3 reveal differential expression of the four indicator genes depending on the mutant allele tested. The data facilitate a subdivision of the flagellar regulon into three classes of expression, with visNR as the master operon (class I); orf38, fliM, motA, and motBC as class II; and the che and fla genes as class III.
This scheme has been refined and confirmed by probing transcription from the promoters that correspond to the four indicator genes plus the vis operon itself. Table 2 lists the β-galactosidase activities of different lacZ fusions measured in seven tester strains and expressed in proportion to activities determined in wild-type S. meliloti RU11/001 (not shown). Ideally, the ratio of mutant to wild-type activity is 1 if the mutant allele exerts no control over the promoter tested and is close to 0 if the native allele is needed for gene expression. Accordingly, visN and visR (class I) are required for transcription of basal-body (fli), motor (mot), flagellin (flaA), and chemotaxis (che) genes but not for vis transcription itself (except for moderate repression exerted by VisN on vis promoter activity, as seen in strain RU11/317). fliM and orf38 (class IIA) exert control over flagellin (flaA) and chemotaxis (che) gene transcription but not over basal-body and motor genes. The mot genes (class IIB) have no control over the other classes, except for a slight stimulation of chemotaxis gene expression, which was, however, not seen on immunoblots (Fig. 3, lanes 6 and 7). The flaA gene (class III) has no control over other indicator genes. Increased expression levels listed under ΔflaA (Table 2, right-hand column) may reflect the titration of sigma factor that is normally engaged by strong fla promoters (24). We conclude that the vis operon lies at the top of the hierarchy as the sole class I operon, with both its gene products being absolutely required for the expression of the other genes in the flagellar regulon, i.e., those belonging to class II and class III. Class II genes have been subdivided into classes IIA (orf38 and fliM) and IIB (motA and motBC) depending on whether they control class III genes (fla and che) or not. Expression of the latter depends on class I and class IIA but not class IIB genes (Fig. 3 and Table 2).
Genetic control of the vis operon.
The combination of a lacZ fusion (pRU1770) and in-frame deletions of visN and visR used to probe transcription control of the vis genes (Table 2) has been extended to testing their expression at two extremes of bacterial growth. Exponentially growing motile cells and starved nonmotile cells (Table 3) with and without VisN or VisR were compared for expression of the vis operon. High β-galactosidase activities observed under these two conditions indicate constitutive expression (again, with some 20% repression by VisN alone [Table 2]) of visN and visR throughout all stages of growth with a 25% up-regulation in response to starvation (low energy). On the other hand, immunoblots and observations of swimming cells (data not shown) tell us that flagellar and motility genes are preferentially expressed in a “window” between early and mid-exponential growth but not in stationary or low-energy phases. Therefore, an additional regulatory element that controls the onset and close of VisNR activity (controlling motility) during growth must exist. We propose that it is the binding of a hitherto unknown effector to VisN, VisR, or both that triggers the transcription of class II genes.
TABLE 3.
In vivo vis promoter activity in exponentially growing and starved wild-type and mutant strains of S. meliloti
DISCUSSION
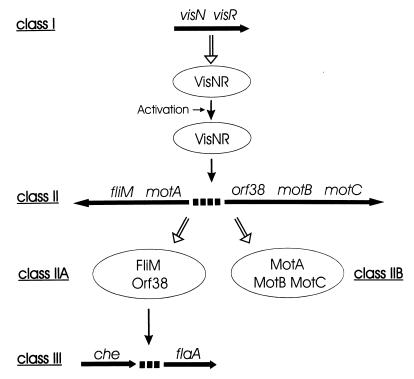
The stepwise assembly of enterobacterial flagella is reflected by a regulatory cascade of three classes of genes securing the sequential biosynthesis of flagellar and motor components as needed (20). Similarly, the present study of gene expression within the S. meliloti flagellar regulon yielded three major classes of operons and genes that are expressed in hierarchical order. However, the molecular nature of two LuxR-type global activators and the different class assignment of mot genes are distinguishing new features of the S. meliloti system. The interdependence of gene expression deduced from Western blots and reporter gene assays (Fig. 3 and Table 2) is diagrammed in Fig. 4. Class I, represented by the vis operon, encodes two LuxR-type master regulatory proteins, VisN and VisR, both required for the expression of all flagellar, motility, and chemotaxis genes tested. The vis operon itself is constitutively expressed, with possible modulation by VisN and low metabolic energy; it does not require other gene products for transcription. However, quasiconstitutive transcription of vis (Table 3), on one hand, and the fact that motility is limited mostly to exponential growth, on the other hand, are contradictory unless one assumes posttranslational activation of VisNR by the binding of an effector (see below). Class II includes genes encoding basal-body components and motor proteins. Their expression requires the function of visN and visR but of no other genes of the flagellar regulon. Class III comprises cheY1, representing the chemotaxis operon (33), and flaA, a principal flagellin gene. Their expression requires class I and class IIA genes. The mot genes are also contained in class II operons, but they do not control chemotaxis and flagellation and have thus been assigned to class IIB.
FIG. 4.
Regulation scheme of the S. meliloti flagellar, motility, and chemotaxis gene system falling into a hierarchy of three expression classes. The transcription polarities of operons and flaA are indicated by horizontal arrows drawn below the gene symbols (33). Translation to gene products (ellipsoids) is indicated by open white arrows, and positive regulatory controls are indicated by solid arrows. The postulated heterodimeric structure of VisNR, posttranslational activation by an unknown effector, and subclassification of basal-body (IIA) and motor (IIB) genes are included.
Although a similar hierarchy of gene expression exists within the enterobacterial flagellar regulon (20), there are new features in the S. meliloti regulatory cascade. The global regulators, VisN and VisR, are of the LuxR type without structural resemblance to the enterobacterial master controls, FlhC and FlhD (18). Since both VisN and VisR are needed for gene activation, it is plausible to assume that they function as a heterodimer, VisNR. Multimerization is similarly reported of LuxR, the transcription activator of Vibrio fischeri luminescence (2). Like LuxR (6), VisNR supposedly requires the binding of a yet unknown effector for function. Unlike in quorum sensing (6), such ligand molecules are not present among those excreted into the medium by motile or densely grown bacteria (34), since S. meliloti cells showed no response when concentrated culture medium from motile or from densely grown cells was added (data not shown). We are currently testing endogenous metabolites and chemotactic attractants as candidate effectors of VisNR. Another complication is introduced by dissimilar polypeptide structures of the ligand-binding sites of VisN and VisR (Fig. 1) that may reflect different ligand-binding specificities. Given the heterodimeric molecular structure of functional VisNR, two different receptor domains conceivably require two different ligands for activation. Obviously, the correct mixture of two ligand molecules may not be readily available in the cell. Alternatively, the heterodimeric receptor domains may cooperate in binding a single effector molecule at their common interface.
The Mot proteins (notably MotA and MotB) of S. meliloti are encoded by different class II operons (33), unlike in E. coli and S. enterica serovar Typhimurium, where they map in one operon assigned to class III (20). The S. meliloti motA gene (class IIB) is part of an operon that also contains the C-ring component genes, fliMNG (class IIA). The motBCD genes (class IIB), on the other hand, belong to a separate operon together with orf38 (class IIA), which may encode a structural component of the basal body. Does this genetic (and regulatory) separation of the motA and motBCD genes and their linkage to basal-body genes reflect known differences in the S. meliloti mode of flagellar rotation requiring a more complex motor structure (23)? It needs to be elucidated whether the basal-body (rotor) and the four motor (stator) genes require coassembly as a way of securing their correct positioning relative to each other inside and outside the cytoplasmic membrane.
While VisN and VisR directly control class II genes, their control over class III genes (flaA and the che operon) is most likely to be an indirect one. The dependence of class III gene expression on the completion of basal-body structure and flagellar export (class IIA genes) implies a similar mode of control to that which operates in enterobacteria (20). In E. coli and S. enterica serovar Typhimurium, ς28-mediated transcription of class III genes is inhibited by an anti-sigma factor, FlgM, which is expelled into the medium upon completion of the flagellar export apparatus, thus releasing ς28 for class III gene transcription. We expect that ongoing efforts toward completing the genetic map of the S. meliloti flagellar regulon (33) will, among other genes, also reveal class II genes that control class III transcription.
ACKNOWLEDGMENTS
We thank Andrea Brinnich for excellent technical assistance and Iris Kobl for expert artwork.
This study was supported by grant Schm68/24-3 from the Deutsche Forschungsgemeinschaft.
Footnotes
Dedicated to Professor Wolfram Heumann on the occasion of his 85th birthday.
REFERENCES
- 1.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus R P, Dickerson R E. Structure of the Escherichia coli response regulator NarL. Biochemistry. 1996;35:11053–11061. doi: 10.1021/bi960919o. [DOI] [PubMed] [Google Scholar]
- 2.Choi S H, Greenberg E P. Genetic evidence for multimerization of LuxR, the transcription activator of Vibrio fischeri luminescence. Mol Mar Biol Biotechnol. 1992;1:408–413. [Google Scholar]
- 3.Cubo M T, Economou A, Murphy G, Johnston A W B, Downie J A. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J Bacteriol. 1992;174:4026–4035. doi: 10.1128/jb.174.12.4026-4035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuqua W C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuqua W C, Winans S C, Greenberg P. Quorum sensing in bacteria: the LuxR/LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Götz R, Limmer N, Ober K, Schmitt R. Motility and chemotaxis in two strains of Rhizobium with complex flagella. J Gen Microbiol. 1982;128:789–798. [Google Scholar]
- 8.Gray K M, Pearson J P, Downie J A, Boboye B E A, Greenberg E P. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere-expressed genes. J Bacteriol. 1996;178:372–376. doi: 10.1128/jb.178.2.372-376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henikoff S, Henikoff J G. Amino acid substitution matrices from protein blocks. Prof Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi R. Using PCR to engineer DNA. In: Erlich H A, editor. PCR technology. Principles and applications for DNA amplification. New York, N.Y: Stockton Press; 1989. pp. 61–70. [Google Scholar]
- 11.Hübner P, Willison J C, Vignais P M, Bickle T A. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991;173:2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamberger W. An Ouchterlony double diffusion study on the interaction between legume lectins and rhizobial cell surface antigens. Arch Microbiol. 1979;121:83–90. [Google Scholar]
- 13.Komeda Y, Suzuki H, Ishidsu J-I, Iino T. The role of cAMP in flagellation of Salmonella typhimurium. Mol Gen Genet. 1975;142:289–298. doi: 10.1007/BF00271253. [DOI] [PubMed] [Google Scholar]
- 14.Krupski G, Götz R, Ober K, Pleier E, Schmitt R. Structure of complex flagellar filaments in Rhizobium meliloti. J Bacteriol. 1985;162:361–366. doi: 10.1128/jb.162.1.361-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutsukake K, Ohya Y, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labes M, Pühler A, Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990;89:37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Matsumura P. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J Bacteriol. 1994;176:7345–7351. doi: 10.1128/jb.176.23.7345-7351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luria S E, Adams F N, Ting R C. Transduction of lactose utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- 20.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 22.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 23.Platzer J, Sterr W, Hausmann M, Schmitt R. Three genes of a motility operon and their role in flagellar rotary speed variation in Rhizobium meliloti. J Bacteriol. 1997;179:6391–6399. doi: 10.1128/jb.179.20.6391-6399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pleier E, Schmitt R. Identification and sequence analysis of two related flagellin genes in Rhizobium meliloti. J Bacteriol. 1989;171:1467–1475. doi: 10.1128/jb.171.3.1467-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pleier E, Schmitt R. Expression of two Rhizobium meliloti flagellin genes and their contribution to the complex filament structure. J Bacteriol. 1991;173:2077–2085. doi: 10.1128/jb.173.6.2077-2085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosemeyer V, Michiels J, Verreth C, Vanderleyden J. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J Bacteriol. 1998;180:815–821. doi: 10.1128/jb.180.4.815-821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 29.Silverman M, Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974;120:1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon R, O'Connell M, Labes M, Pühler A. Plasmid vectors for the genetic analysis and manipulation of Rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;18:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 31.Sourjik V, Schmitt R. Different roles of CheY1 and CheY2 in the chemotaxis of Rhizobium meliloti. Mol Microbiol. 1996;22:427–436. doi: 10.1046/j.1365-2958.1996.1291489.x. [DOI] [PubMed] [Google Scholar]
- 32.Sourjik V, Schmitt R. Phosphotransfer between CheA, CheY1, and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry. 1998;37:2327–2335. doi: 10.1021/bi972330a. [DOI] [PubMed] [Google Scholar]
- 33.Sourjik V, Sterr W, Platzer J, Bos I, Haslbeck M, Schmitt R. Mapping of 41 chemotaxis, flagellar and motility genes to a single region of the Sinorhizobium meliloti chromosome. Gene. 1998;223:283–290. doi: 10.1016/s0378-1119(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 34.Swift S, Throup J P, Williams P, Salmond G P C, Stewart G S A B. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]