Abstract
Sodium (Na+) and potassium (K+) homeostasis is essential for plant survival in saline soils. A member of the High-Affinity K+ Transporter (HKT) family in rice (Oryza sativa), OsHKT1;1, is a vital regulator of Na+ exclusion from shoots and is bound by a MYB transcription factor (OsMYBc). Here, we generated transgenic rice lines in the oshkt1;1 mutant background for genetic complementation using genomic OsHKT1;1 containing a native (Com) or mutated (mCom) promoter that cannot be bound by OsMYBc. In contrast to wild-type (WT) or Com lines, the mCom lines were not able to recover the salt-sensitive phenotype of oshkt1;1. The OsMYBc-overexpressing plants were more tolerant to salt stress than WT plants. A yeast two-hybrid screen using the OsMYBc N-terminus as bait identified a rice MYBc stress-related RING finger protein (OsMSRFP). OsMSRFP is an active E3 ligase that ubiquitinated OsMYBc in vitro and mediated 26S proteasome-mediated degradation of OsMYBc under semi-in vitro and in vivo conditions. OsMSRFP attenuated OsMYBc-mediated OsHKT1;1 expression, and knockout of OsMSRFP led to rice salt tolerance. These findings uncover a regulatory mechanism of salt response that fine-tunes OsHKT1;1 transcription by ubiquitination of OsMYBc.
Salt stress attenuates MYBC STRESS-RELATED RING FINGER PROTEIN (OsMSRFP) expression, preventing degradation of the OsMYBc transcription factor and inducing the high-affinity K+ transporter OsHKT1;1.
Introduction
Saline soil is a major inhibiting factor of plant growth and crop production (Munns and Tester, 2008; Ismail and Horie, 2017). Plants have evolved several strategies to cope with salt stress, such as signals that activate stress response genes through signal transduction pathways, and altering plant metabolism and ion channel permeability (Zhu, 2002). Plants have multiple Sodium (Na+) transport systems to circumvent Na+ toxicity. The High-affinity Potassium (K+) Transporter (HKT1s) and Na+/H+ Exchanger (NHXs) transporters are crucial determinants of cellular Na+ homeostasis, with HKT1s and NHX7 (another name Salt Overly Sensitive 1 [SOS1]) controlling net flux across the plasma membrane, and NHX1/NHX2 controlling movement across the tonoplast membrane into the vacuole (Mickelbart et al., 2015; van Zelm et al., 2020). Besides, NHX1 and NHX2 at the tonoplast also contribute to salinity tolerance by favorably adjusting cellular K+ homeostasis (Sze and Chanroj, 2018; Bassil et al., 2019).
Plant HKTs are allocated into two subfamilies. Electrophysiology investigations have shown that subfamily 1 is Na+-selective, and subfamily 2 is permeable to Na+ and K+, depending on external Na+(K+) concentrations (Jabnoune et al., 2009). In rice (Oryza sativa), quantitative trait loci analysis has identified the OsHKT1;5 gene as a salt-tolerance determinant. Electrophysiological and physiological analyses showed that this protein was a Na+-selective transporter that controls Na+ unloading from the xylem vessels (Ren et al., 2005). Using T-DNA mutants, Kobayashi et al. (2017) further demonstrated the involvement of OsHKT1;5 in xylem Na+ unloading in leaf sheaths, and Na+ exclusion in the phloem to prevent Na+ transfer to young leaf blades. Genetic and physiological evidence has shown that other subfamily 1 members in rice, namely, OsHKT1;1 and OsHKT1;4, are also involved in mechanisms of Na+ exclusion from leaf blades during salt stress (Cotsaftis et al., 2012; Takagi et al., 2015; Wang et al., 2015; Suzuki et al., 2016; Campbell et al; 2017). More recently, Imran et al. (2021) reported that there are one full-length OsHKT1;3 and five variants in japonica rice. These six OsHKT1;3 showed smaller Na+ currents when expressed in Xenopus laevis oocytes, but their functions in plants have not been fully elucidated.
In contrast to the functions of HKT transporters, little is known about their promoter structure roles and transcriptional regulation (Agarwal et al., 2018). In Arabidopsis (Arabidopsis thaliana), an ABA responsive ABEs motif (GCGGCTTT) and ABRE motif (ACGTGT) in the promoter of HKT1;1 have been identified as crucial elements in response to salt and to ABA. They are found to be regulated by ABSCISIC ACID-INSENSITIVE4 (ABI4) and CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR 6 (CAMTA6), respectively (Shkolnik-Inbar et al., 2013, 2019). Using a yeast one-hybrid approach, a MYB coiled-coil type transcription factor, OsMYBc, was found to bind to the OsHKT1;1 promoter at AAANATNC (C/T) fragments (Wang et al., 2015). The MYB family of proteins is a group of functionally diverse transcription factors which regulate various biological processes, including stress responses (Dubos et al., 2010). Mutation of the OsMYBc-binding nucleotides resulted in a decrease in OsHKT1;1 promoter activity, suggesting that OsMYBc positively regulated OsHKT1;1 transcription (Wang et al., 2015). In this study, we confirmed the functions of OsMYBc-OsHKT1;1 promoter binding in salt tolerance at the genetic level and identified an E3 ligase that ubiquitinates OsMYBc and regulates its degradation.
Ubiquitin-mediated protein proteolysis plays a vital role in controlling the abundance of key regulatory proteins and enzymes and acts as a central modifier of signaling in plants (Vierstra, 2009). Once a protein target is ubiquitinated via ATP-dependent reaction cascades, which are sequentially conducted by the E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligase, the ubiquitylated protein is usually broken down by the ubiquitin/26S proteasome system (UPS; Sadanandom et al., 2012). As a typical example, when auxin levels rise in plant cells, the Aux/IAA proteins are degraded by the 26S proteasome, resulting in the depression of auxin response factor and activation of transcriptional responses (Chapman and Estelle, 2009). Recent findings have also established the role of the UPS in abiotic and biotic threats (Sadanandom et al., 2012; Pan et al., 2020). In rice, the DROUGHT-HYPERSENSITIVE (DHS) gene encodes a Really Interesting New Gene (RING)-type protein that negatively regulates Rice Outermost Cell-specific 4 (ROC4) protein levels by promoting its UPS-mediated degradation. ROC4 directly binds to and activates the expression of BODYGUARD, which positively regulates wax biosynthesis, thereby affecting drought tolerance responses (Wang et al., 2018). Therefore, the E3 ligase DHS cooperates with its ubiquitination substrate ROC4 to fine-tune wax biosynthesis and the drought stress response in rice (Wang et al., 2018). In Arabidopsis, the endoplasmic reticulum-localized E3 ligase—Salt-and Drought-Induced RING1 (SDIR1)—plays a key role in regulating abscisic acid (ABA)-related seed germination and salt stress response, by ubiquitinating its substrate SDIR1-Interacting Protein1 (SDIRIP1). SDIRIP1 selectively regulates the expression of the downstream basic region/leucine zipper motif transcription factor gene ABI5 (Zhang et al., 2015).
More recently, a Salt-Induced RING Finger Protein 2 (OsSIRP2) gene was identified. The OsSIRP2 protein exhibited protein degradation activity toward transketolase 1 (OsTKL1) in vitro. Moreover, overexpression of OsSIRP2 conferred salinity and osmotic stress tolerance in plants (Chapagain et al., 2018).
Although diverse signaling roles of ubiquitin E3 ligase are found in plants, the targets for ubiquitination, their interactors, and their molecular functions within the regulatory context have yet to be defined (Sadanandom et al., 2012). In this study, we identified MYBc Stress-Related RING Finger Protein (MSRFP) as an active E3 ligase that ubiquitinates the OsMYBc transcription factor. We further identified the physiological roles of the interactions of OsMSRFP and OsMYBc in regulating the expression of OsHKT1;1, which regulates Na+ transport and salt tolerance in rice.
Results
Binding of the transcription factor OsMYBc to the OsHKT1;1 promoter is essential for salt tolerance in rice
Previously, we reported that OsHKT1;1 controls Na+ concentrations and prevents Na+ toxicity in leaf blades, and is regulated by the OsMYBc transcription factor. The OsMYBc binding fragment (AAANATNCC/T) was identified within the OsHKT1;1 promoter (Wang et al., 2015). To determine the functions of the OsHKT1;1 and OsMYBc interaction, base mutations were simultaneously performed in three OsMYBc binding elements within the 2,103-bp promoter region of OsHKT1;1, as indicated in Figure 1A. The mutated OsHKT1;1 promoter was fused to the genomic region of the OsHKT1;1 gene and transformed into the oshkt1;1 mutant, which was a retrotransposon (Tos17) insertion mutant of OsHKT1;1 isolated from the Tos17 Insertion Mutant Database (https://tos.nias.affrc.go.jp/index.html.en; Miyao et al., 2003; Wang et al., 2015). This approach generated the complemented mOsHKT1;1-COM lines (mCom1 and mCom2). The OsHKT1;1-COM (Com1 and Com2) lines that were previously obtained through transformation with the native promoter and the genomic region of OsHKT1;1 into the oshkt1;1 mutant (Wang et al., 2015) were used as a control. Another control was “TosWT,” which was isolated from the same seed populations as oshkt1;1 by screening for individuals that show no insertion of the Tos17 in the OsHKT1;1 gene (Miyao et al., 2003; Horie et al., 2007; Wang et al., 2015). Reverse transcription–quantitative polymerase chain reaction (RT–qPCR) analysis revealed that the mutant promoter (mCom1 and mCom2) was not able to complement OsHKT1;1 expression, but the native promoter could complement OsHKT1;1 expression, suggesting that OsMYBc binding is essential to promote OsHKT1;1 expression (Figure 1B).
Figure 1.
Binding of transcription factor OsMYBc to OsHKT1;1 promoter is essential to salt tolerance of rice. A, The schematic illustration of the mutation in three positions of OsMYBc binding-element in the OsHKT1;1 promoter region. The base mutations and positions of OsMYBc binding elements AAATATNCC/T are indicated. Two mutations (−76 to −68 and −275 to −267 bp) for OsMYBc binding on sense strand, and one mutation (−1,476 to −1,468 bp) for the OsMYBc binding on antisense strand. B, RT-qPCR analysis of OsHKT1;1 expression in the WT, TosWT, oshkt1;1 mutant, oshkt1;1 background complemented with genomic OsHKT1;1 containing a native (Com1 and Com2) or mutated promoter that cannot be bound by OsMYBc (mCom1 and mCom2). Rice UBQ5 gene was used as the internal reference to normalize OsHKT1;1 expression. Data represent means ± standard deviation (SD) (n = 3 independent pools of seedlings, three plants per pool). C, Phenotypes of WT, TosWT, and different mutant plants under salt stress. Two-week-old hydroponically grown seedlings were treated with 120 mM NaCl for 7 days and then allowed to recover for 7 days. D–F, Fresh weight (D), dry weight (E), and survival rate (F) of plants under salt stress. Data (D–F) represent means ± SD (n = 3 independent pools of seedlings, 30 plants per pool). The experiments were repeated 3 times with similar results. Significant differences from the WT in each group are indicated by asterisk (*P ≤ 0.05, **P ≤ 0.01, Students t test; (B), (D–F)).
To determine whether OsMYBc binding to OsHKT1;1 regulates salt tolerance in rice, we compared the growth of wild-type (WT), TosWT, oshkt1;1, Com, and mCom plants under salt-stress conditions. The 14-day-old plants were hydroponically grown in the presence of 120-mM NaCl for 7 days and then grown for the following 7 days in the absence of NaCl. Consistent with the previous studies (Wang et al., 2015), oshkt1;1 exhibited a salt-sensitive phenotype, including reduced fresh and dry weight and higher seedling death, as compared with WT or TosWT (Figure 1, C–F). The Com lines exhibited a similar phenotype to WT and TosWT plants. In contrast, the mCom lines displayed a similar phenotype to the oshkt1;1 mutant (Figure 1, C–F). These results indicate that OsMYBc binding to the OsHKT1;1 promoter is essential for salt tolerance in rice.
As OsHKT1;1 regulates salt tolerance through the control of Na+ concentration in shoots (Wang et al., 2015), we speculated whether the inhibition of OsMYBc binding to the OsHKT1;1 promoter would affect Na+ accumulation. As shown in Supplemental Figure S1A, both the oshkt1;1 mutant and mCom1 lines accumulated more Na+ in the shoots than the WT, TosWT, and Com1 lines. The Na+ concentration in xylem sap extracted from the oshkt1;1 and mCom1 lines was higher than those in the xylem sap of the WT, TosWT, and Com1 lines, whereas that in phloem sap from the oshkt1;1 and mCom1 was lower than those in the phloem of the WT, TosWT, and Com1 lines (Supplemental Figure S1, B and C). The results suggest that OsMYBc binding to the OsHKT1;1 promoter is important for the control of Na+ net accumulation in shoots.
Overexpression of OsMYBc increases OsHKT1;1 expression and salt tolerance
Next, we determined whether OsHKT1;1 and OsMYBc were expressed in identical tissues. First, the OsMYBc transcript level was examined using RT–qPCR. At the vegetative stage, OsMYBc was expressed mainly in the leaf blade (6-week-old, fourth leaves), moderately in the leaf sheath and basal stem, and the lowest in roots (Figure 2A). The pattern was similar to that of OsHKT1;1 (Wang et al., 2015). At the reproductive stage, OsMYBc expression in roots was comparable to that in stems, nodes, internodes, blades, and sheaths. However, less OsMYBc was expressed in the spikelets (Supplemental Figure S2). To investigate the spatial expression of the OsMYBc gene, transverse cross-sections of the transgenic rice lines containing the GUS reporter controlled by the OsMYBc promoter were examined. In roots, GUS expression was found mainly in the stele, especially in phloem cells (Figure 2, B and C). These results agreed with a previous observation for OsHKT1;1 (Wang et al., 2015). In leaf blades, GUS activities were detected in the vascular tissue as well as in the mesophyll cells (Figure 2D).
Figure 2.
Expression pattern of OsMYBc in rice plants and overexpression of OsMYBc enhances salt tolerance of seedlings. A, Relative expression levels of OsMYBc in various organs in the vegetative stage analyzed by RT-qPCR. Six-week-old rice seedlings were used. The UBQ5 gene was used as an internal reference for normalizing data. Data represent means ± SD from three tissue pools with three plants per pool. B–D, GUS staining of ProMYBc::GUS in transgenic plants. B, Root cross-section (Bar = 500 μm). C, Partial enlargements of (B) (Bar = 500 μm). D, Cross section of leaf blade (Bar = 20 μm). c, cortex; ep, epidermis; ex, exodermis; sc, sclerenchyma; ph, phloem; xy, xylem; px, protoxylem; mx, metaxylem; m, mesophyll. E and F, Relative expression of OsMYBc (E) and OsHKT1;1 (F) in 2-week-old plants analyzed by RT-qPCR. Rice seedlings were treated with 120 mM NaCl for 3 h (F, NaCl treatment) or without NaCl (E and “control” in F), the whole plants were sampled to RT-qPCR analysis. Data were normalized to those of the WT (E, the level of WT was set to 1; F, the level of WT control was 1). The UBQ5 gene was used as an internal reference. Values are means ± SD (n = 3 independent pools, five plants per pool). Significant difference compared with WT (*P ≤ 0.05, **P ≤ 0.01; Student’s t test). G, Survival rate. The 2-week-old seedlings were treated with 120 mM NaCl for 7 days and then allowed to recover for 7 days. Data represent means ± SD from three pools with 30 plants per pool for each line. Significant difference compared with WT (*P ≤ 0.05, **P ≤ 0.01, Student’s t test).
OsMYBc expression was induced by NaCl, mannitol, ABA, and H2O2 treatments (Supplemental Figure S3, A–C), which was similar to the pattern for OsHKT1;1 (Wang et al., 2015). Furthermore, overexpression of OsMYBc led to an increase in OsHKT1;1 expression under salt stress (Figure 2, E and F). This genetic manipulation resulted in decreased Na+ and increased K+ in shoots and roots (Supplemental Figure S4, A–D), and improved salt tolerance in rice (Figure 2G; Supplemental Figure S4, E–G). These results are identical to previous findings that showed that knockout of OsMYBc led to salt sensitivity (Wang et al., 2015). Taken together, these results indicate that OsMYBc positively regulates salt tolerance by binding to the OsHKT1;1 promoter.
Identification of OsMSRFP as an OsMYBc-interacting protein
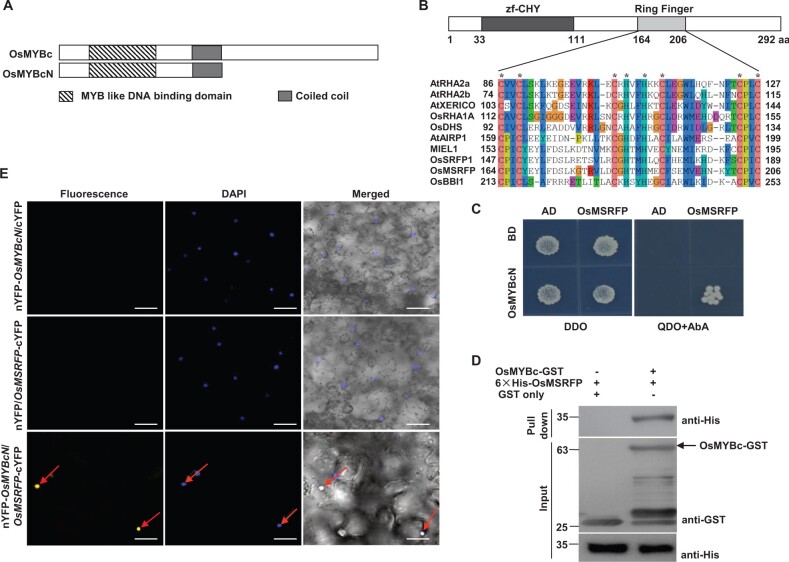
To further understand the regulation and mode of action of OsMYBc, we performed a yeast two-hybrid (Y2H) screen to search for the proteins that interacted with OsMYBc. An OsMYBc version that contained a deletion at its C-terminal transcriptional activation domain (OsMYBcN) was used as bait (Figure 3A) to screen a Y2H cDNA library generated from mRNAs isolated from rice seedlings treated with 100-mM NaCl. Among 98 positive clones, 7 were characterized as containing the same protein (encoded by Os12g35320) (Supplemental Table S1). The deduced protein of this gene belongs to RING finger proteins, featuring eight conserved Cys and His residues (C3H2C3; Marino et al., 2013; Zhang et al., 2015; Figure 3B). RING finger proteins play key roles in developmental processes and stimuli responses (Kim and Kim, 2013; Zhang et al., 2015). However, the function of the identified protein here was unknown, and the protein was named the O.sativa MYBc-Stress-related RING Finger Protein (OsMSRFP).
Figure 3.
OsMSRFP interacts with OsMYBc. A, Schematic illustration of the structures of OsMYBc and OsMYBcN. B, Schematic illustration of the structure of OsMSRFP and alignment of amino acid sequences in the Ring finger domain of OsMSRFP with representative RING-H2 type finger proteins using the CLUSTALX2 program. Zf-CHY represents CHY zinc finger domain. Asterisks indicate conserved Cys and His residues. C, Interaction between OsMYBcN and OsMSRFP verified by Y2H assay. BD, bait vector pGBKT7; AD, prey vector pGADT7; OsMYBcN and OsMSRFP were cloned into pGBKT7 and pGADT7 respectively. Yeast cells were grown on SD/-Leu/-Trp (DDO) and SD/-Ade/-His/-Leu/-Trp + 125 ng/mL AbA (QDO + AbA) synthetic dropout medium respectively. D, Pull-down assays for interaction between OsMSRFP and OsMYBc. OsMYBc-GST was used as the bait to pull down His-OsMSRFP. The GST alone was used as a control. An equal amount of His-OsMSRFP was used in pull-down assays and as the input control. E, BiFC visualization of the interaction between OsMYBcN and OsMSRFP in N. benthamiana leaves. The images were taken under a confocal microscope after transfection for 60 h. Nucleus of leaf epidermal cells were stained with 4′,6-diamidino-2-phenylindole. The red arrows indicate the reconstituted YFP fluorescence that detected in the combination of nYFP-OsMYBcN with OsMSRFP-cYFP samples. The combinations of empty vector cYFP with nYFP-OsMYBcN and empty vector nYFP with OsMSRFP-cYFP, respectively, served as negative controls. Bars = 20 μm.
Phylogenetic tree analysis showed that OsMSRFP-like protein was widely present in monocotyledonous and dicotyledonous plants. OsMSRFP exhibited 58% and 61% amino-acid identity with MYB30-INTERACTING E3 LIGASE 1 (MIEL1; At5g18650; Marino et al., 2013; Lee and Seo, 2016) and O.sativa Stress-Related RING Finger Protein 1(OsSRFP1, LOC_Os03g22680; Fang et al., 2015), respectively. OsMSRFP even exhibited greater similarity with the potential E3 ligase from Brachypodium distachyum (Bradi.4g05690), sorghum (Sorghum bicolor) (SORBI.008g117200), and maize (Zea mays) (GRMZM.2g056270) with identity values of 79.8%, 84.3%, and 84.3%, respectively, although their functions have not been reported (Supplemental Figure S5).
A Y2H screen was used to confirm the potential physical interaction between OsMYBc and OsMSRFP. The bait (pGBKT7-OsMYBcN, N-terminus of OsMYBc) and the prey (pGADT7-OsMSRFP) plasmids were co-transformed into yeast Y2H Gold cells. The co-transformed yeast cells were grown on synthetic dropout medium lacking Leu and Trp (shortly SD/–Leu/–Trp), and the medium lacking Ade, His, Leu, and Trp supplemented with 125-ng mL−1 AbA (shortly SD/–Ade/–His/–Leu/–Trp + AbA), respectively. These yeast cells could survive on SD/−Leu/−Trp and SD/−Ade/−His/−Leu/−Trp + AbA medium plates (Figure 3C). However, other yeast cells harboring the vectors pGBKT7 and pGADT7, vector pGBKT7 and pGADT7-OsMSRFP, or vector pGADT7 and pGBKT7-OsMYBcN could only grow on SD/-Leu/-Trp medium plates (Figure 3C). The results suggest that OsMYBcN and OsMSRFP interact in yeast cells.
Next, we determined the interaction between OsMSRFP and full-length OsMYBc using a pull-down assay. GST-OsMYBc and His-OsMSRFP were expressed in Escherichia coli. GST-OsMYBc (but not GST only) was able to pull-down His-OsMSRFP, further supporting the interaction between OsMYBc and OsMSRFP (Figure 3D).
We next determined whether the expression of OsMSRFP is co-localized with OsMYBc in the same cell type/tissue. GUS reporter revealed that the OsMSRFP expression in roots was found mainly in the stele, especially in phloem cells (Supplemental Figure S6, A and B). In leaf blades, the GUS activities were detected in the vascular tissues as well as in the mesophyll cells (Supplemental Figure S6C). These results agreed with the observation for OsMYBc (Figure 2, B and C), as well as OsHKT1;1 (Wang et al., 2015).
The tissue-specific expression patterns of OsMSRFP were determined using RT–qPCR. At the vegetative stage, OsMSRFP was more highly expressed in leaf blades and sheaths than in roots (Supplemental Figure S7A), which was similar with the pattern of OsMYBc (Figure 2A). At the reproductive stage, OsMSRFP was expressed in the tested tissues, especially highly in flag leaf blade and sheath (Supplemental Figure S7B). Together, these results indicate that OsMSRFP expression localizes with OsMYBc, which provides the spatial possibility for their interaction.
OsMYBc and OsMSRFP physically interact in the nucleus
To examine the OsMYBc–OsMSRFP interaction in cells, we performed bimolecular fluorescence complementation (BiFC) assays, which enable detection of protein–protein interactions with subcellular localization information (Waadt et al., 2008). The N-terminal sequence of OsMYBc (OsMYBcN) and full-length OsMSRFP were fused to the N- and C-termini of Venus to produce nYFP-OsMYBcN and OsMSRFP-cYFP, respectively. These were transiently expressed in Nicotiana benthamiana leaves. Confocal observation confirmed the OsMYBcN–OsMSRFP interaction in the nucleus, which was visualized by staining with 4′,6-diamidino-2-phenylindole, a nuclear marker (Figure 3E). In contrast, the negative controls consisting of nYFP with OsMSRFP-cYFP or cYFP with nYFP-OsMYBcN, failed to produce any YFP signal (Figure 3E). OsMSRFP-eGFP was localized in the nucleus (Supplemental Figure S8). Taken together, these results indicate that OsMYBc can interact with OsMSRFP in vivo.
OsMSRFP is an active E3 ubiquitin ligase able to ubiquitinate OsMYBc in vitro
The phylogenetic analysis revealed that OsMSRFP exhibited 58% similarity with MIEL1, which is an Arabidopsis RING-type E3 ubiquitin ligase that interacts with and ubiquitinates MYB30, leading to MYB30 proteasomal degradation and downregulation of its transcriptional activity (Supplemental Figure S5; Marino et al., 2013). We investigated whether OsMSRFP displayed ligase activity. Total protein was extracted from N. benthamiana leaves infiltrated with 35S::OsMSRFP-eGFP constructs. The ubiquitinated form of OsMSRFP-eGFP was detectable in the N. benthamiana leaves, indicating that OsMSRFP is an active E3 ligase (Figure 4A).
Figure 4.
OsMSRFP shows E3 Ub ligase activity and ubiquitinates OsMYBc in vitro. A, OsMSRFP shows E3 Ub ligase activity in transiently expressed N. benthamiana leaves. The GFP vector was used as the negative control. B, OsMSRFP is an active E3 Ub ligase and ubiquitinates OsMYBc in vitro. His-OsMSRFP fusion protein could ubiquitinate OsMYBc in the presence of 6× His-WE1 (wheat E1), 6×His-AtUBC8 (Arabidopsis E2), His-tagged ubiquitin (6× His-AtUBQ14) and GST tagged OsMYBc (lane 4). The 6× His itself was used as a negative control (lane 1). Mutation in integral RING catalytic domain (OsMSRFPm) resulted in no OsMSRFP-mediated ubiquitination observed (lane 2). The specificity of the interaction between OsMSRFP and OsMYBc was compared with the OsMSRFP paralog, OsSRFP1 (lane 3). The protein gel blot was analyzed using anti-GST antibody. The vertical solid line indicates a ubiquitinated smear ladder.
Next, we tested whether OsMYBc is a substrate for OsMSRFP E3 ubiquitin ligase activity. Incubation of GST-tagged OsMYBc with recombinant wheat (Triticum aestivum) E1 (WE1, His-tagged), Ubiquitin-Conjugating E2 from Arabidopsis (AtUBC8), and OsMSRFP enabled the detection of a stronger and higher protein ladder than that absent of OsMSRFP (Figure 4B, lane 4 versus 1). OsMSRFP contains a C3H2C3-type RING domain in which Cys and His residues are highly conserved, and are important for E3 ligase activity (Marino et al., 2013; Fang et al., 2015; Figure 3B). We did triple mutation (C183, H185, and H188) to Tyr, which led to the abolishment in E3 ubiquitin ligase activity of OsMSRFP (OsMSRFPm; Figure 4B, lane 2). Finally, the previously described active RING-type E3 ligase protein, OsSRFP1 (Fang et al., 2015; LOC_Os03g22680, Supplementary Figure S5) was not able to ubiquitinate OsMYBc (Figure 4B, lane 3). Taken together, the results demonstrate that OsMSRFP specifically ubiquitinates OsMYBc in vitro.
OsMSRFP mediates OsMYBc protein degradation
We next performed semi-in vitro ubiquitination degradation experiments (Oh et al., 2017) to investigate whether OsMSRFP-mediated ubiquitination of OsMYBc occurs in a 26S proteasome-dependent manner and whether it is involved in the salt response. To this end, OsMYBc-GST protein was incubated with crude protein extracted from WT rice leaves with or without NaCl (120 mM) treatment, and the degradation pattern was monitored. As shown in Figure 5A, OsMYBc-GST protein was degraded by the extracts from WT leaves in a time-dependent manner, and the degradation was diminished by salt treatment. An OsMYBc-like protein (Os08g25820) displayed about 70% similarity with OsMYBc (Supplemental Figure S9), but was not immunoprecipitated by OsMSRFP (Supplemental Figure S10). This Os08g25820 protein also showed a time-dependent degradation that was not affected by salt treatment. The tag protein GST did not change during a 4-h incubation regardless of the presence or absence of salt treatment (Figure 5A). Thus the OsMYBc degradation caused by rice extracts is inhibited by salt signaling, suggesting a feedback regulation mechanism to enhance OsMYBc activity (Figure 5A).
Figure 5.
Cell free assay of OsMYBc degradation regulated by salt stress or OsMSRFP protein. A, Bacterially expressed OsMYBc-GST, Os08g25820-GST, and GST were incubated with leaf crude extracts from 14-day-old WT (Dongjin) seedlings treated with or without 120 mM NaCl for the indicated time. About 50 μM MG132 was used as 26S proteasome inhibitor. B, The recombinant OsMYBc-GST, Os08g25820-GST, and GST were incubated with leaf crude extracts from 14-day-old WT (Nipponbare) and cr-osmsrfp1 mutant seedlings for indicated time. About 50 μM MG132 was used as 26S proteasome inhibitor. The data were obtained from three independent experiments, and relative band intensities were quantified and normalized relative to the control (or WT) at time 0. C, The recombinant OsMYBc-GST was incubated for 1 h with leaf crude extracts prepared from N. benthamiana leaves transiently expressed GFP, OsMSRFP-GFP, and OsMSRFPm-GFP, respectively. Rubisco large subunits were used in (A–C) as loading controls.
To explore whether OsMSRFP in the rice extracts is involved in the regulation of OsMYBc degradation, osmsrfp mutants (cr-osmsrfp1 and cr-osmsrfp2) were generated using the CRISPR/Cas9 editing system (Supplemental Figure S11). The results revealed that the OsMYBc-GST level decreased more slowly upon exposure to the crude extracts from the cr-osmsrfp lines than to those from WT (cr-osmsrfp1 in Figure 5B, cr-osmsrfp2 in Supplemental Figure S12). In contrast, the OsMYBc-GST level decreased more rapidly upon exposure to the crude extracts of the OsMSRFP-OE transgenic lines than to those from WT (Supplemental Figure S13, A–C). In addition, the Os08g25820-GST level decreased upon exposure to the crude extracts of the cr-osmsrfp lines to a similar degree to the extracts from WT, suggesting that OsMSRFP did not affect Os08g25820 protein degradation (Figure 5B). Furthermore, the decrease in OsMYBc-GST and Os08g25820-GST level was effectively inhibited by the 26S proteasome inhibitor MG132 (50 μM) in extracts of the WT and cr-osmsrfp mutants (or OsMSRFP-OE) (Figure 5, A and B; Supplemental Figures S12 and S13). Taken together, these results suggest that OsMSRFP can accelerate the degradation of OsMYBc, which is subject to regulation by the UPS.
The OsMSRFP-dependent turnover of OsMYBc was further investigated using a transient expression system in N.benthamiana. The addition of OsMSRFP-GFP protein crude extracts transiently expressed in N. benthamiana leaves to an OsMYBc-GST protein solution resulted in decreased OsMYBc content (Figure 5C, lane 2). Treatment with MG132 protected OsMYBc from degradation, demonstrating that OsMSRFP-mediated degradation of OsMYBc was proteasome-dependent (Figure 5C, lane 3). Importantly, the OsMSRFP catalytic mutant OsMSRFPm (Marino et al., 2013; Fang et al., 2015; more information in “Materials and methods”), which is not able to ubiquitinate OsMYBc in vitro, did not affect OsMYBc-GST protein levels, further demonstrating that the catalytic activity of OsMSRFP is required to negatively regulate OsMYBc protein levels (Figure 5C, lane 4).
OsMSRFP attenuates OsMYBc-mediated OsHKT1;1 expression
Next, we determined the effects of OsMSRFP and OsMYBc interactions on OsHKT1;1 transcription. For this purpose, we constructed a GUS reporter fused with the OsHKT1;1 promoter, as well as effector vectors harboring OsMYBc-Flag, OsMSRFP-GFP, or OsMSRFPm-GFP under the control of a 35S promoter (Figure 6A). The OsHKT1;1 promoter-driven GUS (ProOsHKT1;1::GUS) reporter was co-expressed with different effectors in N. benthamiana leaves for 60 h. The results showed that ProOsHKT1;1::GUS activity was stimulated by the expression of OsMYBc-Flag as compared with by the Flag vector control (Figure 6B). The OsMYBc-mediated activation of ProOsHKT1;1::GUS was significantly reduced when they were co-expressed with OsMSRFP-GFP, but not with OsMSRFPm-GFP (Figure 6B).
Figure 6.
OsMSRFP reduces OsMYBc-mediated OsHKT1;1 expression. A, Schematic representation of reporter and effector constructs. B, Fluorimetric GUS assays in N. benthamiana leaves. The proOsHKT1;1::GUS reporter was co-expressed with (1) GFP vector; (2) Flag vector; (3) OsMYBc-Flag (Effector 1); (4) OsMYBc-Flag + OsMSRFP-GFP (Effector 2) and (5) OsMYBc-Flag + OsMSRFPm-GFP (Effector 3), respectively. The leaves were gathered to measure enzymatic GUS activities using MUG as substrate. Results are presented as means ± SD (**P ≤ 0.01, student’s t test) from five independent biological replicates. Three repeated experiments were performed with similar results. MU, methylumbelliferone. C, OsMSRFP reduces OsMYBc accumulation in leaves. The N. benthamiana leaves expressing the vectors indicated in (B) were used to perform Western blot. The antibodies against Flag and GFP were used to detect OsMYBc and OsMSRFP protein, respectively. Rubisco large subunits were used as loading controls.
Western blotting further revealed that OsMSRFP-GFP suppressed OsMYBc-Flag accumulation, and OsMSRFPm-GFP did not affect OsMYBc-Flag levels (Figure 6C). Taken together, the in vivo data demonstrate that OsMSRFP negatively regulates OsMYBc-mediated OsHKT1;1 expression via the 26S proteasome-mediated degradation pathway.
OsMSRFP is involved in regulation of salt tolerance in rice
To investigate the functions of OsMSRFP in rice in response to salt stress, the growth of WT and cr-osmsrfp lines was compared after they were hydroponically grown in a culture solution containing 120-mM NaCl. Phenotypic analysis showed that two CRISPR lines (cr-osmsrfp1 and cr-osmsrfp2) were more tolerant to salt stress than WT as exemplified by an increased seedling survival rate (Figure 7, A and B). Furthermore, mutations of OsMSRFP enhanced the salt-induced OsHKT1;1 expression in rice seedlings (Figure 7C). To quantify the physiological functions of OsMSRFP, we further determined Na+ and K+ concentrations in roots and shoots. Compared with the WT, the two CRISPR lines accumulated obviously less Na+ and more K+ in both roots and shoots (Figure 7, D–G), whereas, OsMSRFP-overexpressing plants accumulated more Na+ and less K+ in shoots and roots than in the WT (Supplementary Figure S14). The results suggest that OsMSRFP is involved in rice salt tolerance via regulation of Na+ and K+ accumulation.
Figure 7.
Mutation of OsMSRFP results in salt tolerance in rice seedlings. Two-week-old seedlings were hydroponically grown with or without 120 mM NaCl. A, Representative photographs of WT and CRISPR lines (cr-osmsrfp1 and cr-osmsrfp2) treated with NaCl for 7 days and then recovered for 7 days. B, Survival rate of rice plants treated as (A). C, Relative expression levels of OsHKT1;1 in seedlings of WT and cr-osmsrfp lines under 120 mM NaCl treatments for 3 h. The transcript levels of OsHKT1;1 were normalized to those of rice UBQ5 gene that was used as the internal reference. D and E, Na+ contents in roots (D) and shoots (E). F and G, K+ contents in roots (F) and shoots (G). Data in (B) to (G) represent means ± SD from three independent biological replicates. Significant differences between WT and cr-osmsrfp lines are indicated (B–G) by asterisk (*P ≤ 0.05, **P ≤ 0.01, student’s t test).
Discussion
Up to date, a series of members of the HKT family have been functionally characterized in various plants (Wang et al., 2015; Suzuki et al., 2016; Kobayashi et al., 2017; Huang et al., 2020). Evidence that OsHKT1;1 is involved in limiting Na+ accumulation in leaves and salt tolerance in rice has been reported previously (Wang et al., 2015). This HKT transporter, together with other OsHKT1 members such as OsHKT1;4 and OsHKT1;5, contribute to Na+ exclusion from leaf blades in rice exposed to salt stress (Cotsaftis et al., 2012; Takagi et al., 2015; Wang et al., 2015; Suzuki et al., 2016; Kobayashi et al., 2017). In this study, we determined the transcriptional regulation of OsHKT1;1 by a MYB coiled-coil transcription factor. Importantly, an E3 ligase OsMSRFP was identified, which ubiquitinates OsMYBc and regulates its degradation. Our work elucidated the fine-tuned transcriptional regulation of the Na+ transporter, through which a balance is established between Na+ detoxification and energy-saving for survival and growth when rice plants are challenged by salt-stress environments.
OsMYBc positively regulates salt tolerance by binding to the OsHKT1;1 promoter
The Na+ transporters HKT1s and NHXs are important for controlling cellular Na+ homeostasis (Mickelbart et al., 2015). The mechanisms of posttranslation modification for the NHX7 (SOS1) have been investigated previously; namely, SOS1 is phosphorylated by SOS2 kinase, which is controlled by a SOS3 calcium sensor (Yang and Guo, 2018). In the case of HKT transporters, a comparison of alleles of rice HKT1;5 (previously called SKC1) associated with sensitivity (Koshihikari; KSKC1 allele) and tolerance (Nona Bokra; NSKC1 allele) to salt revealed that Nona Bokra HKT1;5 displayed greater selective Na+ transport compared to Koshihikari HKT1;5, owing to four amino-acid substitutions in the loop domains (Ren et al., 2005; Mickelbart et al., 2015). In wheat, allelic variation in HKT1;5s within Triticum species also influences transporter structure and activity, leading to pronounced variations in salt tolerance (Munns et al., 2012; Platten et al., 2013; Mickelbart et al., 2015). Chen et al. (2017) reported that Mg2+ transported by the OsMGT1 protein is essential for OsHKT1;5 activity, which is responsible for salt tolerance. Based on sequence comparisons of HKT1 between Arabidopsis and its halophytic relative Thellungiella salsuginea (Ts), a single amino acid in the pore-loop domain, Asp (D207 in Ts) versus Asn (N211 in At), determines the differential selectivity for Na+ and K+ (Ali et al., 2016).
Previously, OsHKT1;1 transcription was found to be regulated by OsMYBc at the promoter sequences AAATATGCC and AAATATGCT (Wang et al., 2015). Genetic evidence in present work confirmed that OsMYBc binding to the AAATATGCC/T motifs is essential for both OsHKT1;1 transcription and salt tolerance. This conclusion was supported by the following observations: (1) disruption of OsMYBc binding to the OsHKT1;1 promoter resulted in salt sensitivity (Figure 1, A and C) and (2) overexpression of OsMYBc not only increased OsHKT1;1 expression but also increased salt tolerance (Figure 2F; Supplemental Figure S4). The spatial expression of OsMYBc in the phloem and xylem parenchyma cells of roots and in the phloem parenchyma cells of leaf blades overlapped with that of OsHKT1;1 (Figure 2; Wang et al., 2015). Therefore, OsMYBc activated OsHKT1;1 expression may contribute to both Na+ unloading from the xylem and Na+ loading into the phloem in leaves for Na+ recirculation. The phloem-mediated Na+ recirculation from shoots to roots or from younger leaves to older leaves is possibly another approach mediated by OsMYBc-OsHKT1;1 combination to control the level of Na+ in young leaves (Wang et al., 2015; Ismail and Horie, 2017).
Transcriptional regulation of HKT transporters was also observed in other reports. OsbZIP72 was recently found as a transcriptional modulator to with the ability to induce the expression of OsHKT1;1 in response to environmental stresses through an ABA-dependent regulatory pathway (Wang et al., 2021). In contrast to OsMYBc and OsbZIP72, ABI4 in Arabidopsis is a repressor of the transcription of AtHKT1;1 (Shkolnik-Inbar et al., 2013). Higher AtHKT1;1 expression in xylem parenchyma cells was observed in abi4 mutant plants and lower levels of expression in ABI4-overexpressing plants. Consequently, the abi4 mutants show increased salt tolerance, which is related to increased AtHKT1;1 expression that leads to increased Na+ unloading from the xylem vessels thus lower Na+ accumulation in shoots (Shkolnik-Inbar et al., 2013). Results from both rice and Arabidopsis appear to indicate that cytokinin signaling participates in regulation of OsHKT1;1 (AtHKT1;1) expression through the type-B response regulators O.sativa (Arabidopsis) RESPONSE REGULATOR 1, OsRRs (ARRs) (Ismail and Horie, 2017). In addition, a CaM-binding transcription activator 6 (CAMTA6) has been found to be indispensable for NaCl and ABA-regulated expression of AtHKT1;1 (Shkolnik et al., 2019). Therefore, transcription of HKT1;1 is controlled precisely by complex mechanisms in different plants.
OsMSRFP is a RING-type ligase that ubiquitinates OsMYBc and negatively regulates salt tolerance
Another finding in this work is identification of OsMSRFP as a RING-type E3 ligase that ubiquitinates OsMYBc. It has been widely reported that RING proteins play critical roles in the drought stress response and ABA signaling (Bu et al., 2009; Li et al., 2011; Wang et al., 2018; Pan et al., 2020). For example, SDIR1, a RING finger E3 ligase, acts upstream of ABI5, ABF3, and ABF4 and participates in the ABA-, salt-, and drought-stress pathways (Zhang et al., 2007). UBC27 interacts with the RING E3 ligase AIRP3 and forms an E2–E3 pair to promote ABI1 degradation and transduction of ABA signals (Pan et al., 2020).
In plants, the stability of some transcriptional regulators is controlled by the ubiquitin-mediated proteasome (Skelly et al., 2016). Typically, the hormones auxin and jasmonate promote the recruitment of transcriptional repressors to the Skp1/Cullin/F-box (SCF) E3 ligases, SCFTIR1, and SCFCOI1, respectively, leading to their proteasome-mediated degradation and the activation of hormone-responsive gene expression. Here, we describe OsMSRFP, a previously uncharacterized rice E3 ubiquitin ligase that contributes to the fine-tuning of plant salt stress responses through ubiquitination and proteasomal degradation of OsMYBc. OsMSRFP was identified by Y2H screening of a cDNA library prepared from rice seedlings exposed to salt stress using the N-terminal containing the MYB-like DNA binding domain of OsMYBc as bait (Figure 3A). In agreement with this finding, we have shown that OsMSRFP and OsMYBc colocalized and interacted in the N. benthamiana nucleus (Figure 3E). OsMSRFP was able to ubiquitinate OsMYBc in vitro, providing further confirmation of this protein interaction. Functional characterization of OsMSRFP revealed that this protein is an active E3 ligase that is able to ubiquitinate OsMYBc (Figure 4B). Under normal (unstressed) condition, the co-localization of the OsMYBc and OsMSRFP E3 ligase in the same cell type (tissue) in the plant provides spatial possibility of their interaction in situ (Figure 2; Supplemental Figures S6 and S7). After salt stress was initiated, up-expression of OsMYBc was temporally in accordance with down-expression of OsMSRFP, suggesting that OsMSRFP might regulate OsMYBc levels during the salt response (Supplemental Figure S15). In addition, OsMSRFP might regulate OsMYBc specifically, as OsMYBc-like protein Os08g25820 that was not co-immunoprecipitated by OsMSRFP (Supplemental Figure S10) was not regulated by the extracts containing OsMSRFP (Figure 5). Interestingly, the Os08g25820 is not the candidate within positive OsHKT1;1 interactions from the yeast one hybrid screen (Wang et al., 2015). Together, these results provide a link between OsMSRFP-mediated proteasomal degradation of OsMYBc and the suppression of rice tolerance to salt stress.
A question is then raised, what is potential cellular and physiological importance of OsMSRFP regulation of OsMYBc dynamics? In Arabidopsis, E3 ligase-mediated proteasomal degradation of the MYB transcriptional factor and negative regulation of the hypersensitive response have been reported (Marino et al., 2013). Such negative regulatory mechanisms are necessary and essential for plants to temporally and spatially regulate the activation of programmed cell death in response to pathogen attack, and to precisely control developmental processes, because the establishment of defense is a costly response (Marino et al., 2013). Similarly, salt responses and tolerance are also costly processes, in which the bulk of transcriptional changes occur after the onset of salt stress (Deinlein et al., 2014). As OsHKT1;1 activity is specific to Na+ flux (Jabnoune et al., 2009), the repression of its expression and stimulation of expression of other solute transporters for mineral uptake and transport is believed to be beneficial for plant growth under normal conditions. In transient expression systems of N. benthamiana, we did find that OsMSRFP attenuated OsHKT1;1 expression by promoting OsMYBc degradation (Figure 6). More importantly, salt-induced OsHKT1;1 expression was impaired in OsMSRFP-overexpressed rice (Supplementary Figure S14), further suggesting that OsMSRFP negatively regulates OsHKT1;1 transcription in planta. Whereas, when rice plants were exposed to salt stress, OsMYBc expression was induced and OsMSRFP expression was inhibited (Supplemental Figure S15), indicating that inter-repression occurred between OsMSRFP and OsMYBc. Indeed, we found that knockout of OsMSRFP gene improved rice salt tolerance (Figure 7). Therefore, we propose that during the salt response, unknown signals may inhibit OsMSRFP activity, which releases OsMYBc and finally induces transcriptional regulation of stress-inducible genes such as OsHKT1;1 (Figure 8).
Figure 8.
Model for OsMYBc and OsMSRFP coordinative regulation of OsHKT1;1 transcription and salt tolerance. In the absence of salt stress, OsMSRFP interacts with OsMYBc and leads to its proteasomal degradation, thus regulates the turnover of OsMYBc, avoiding superfluous OsMYBc-mediated activation of salt stress-induced gene expression. Under salt stress, signal(s) is supposed to block the activity of OsMSRFP, which leads to the increase of “non-ubiquitinated” OsMYBc (active form). As a result, OsMYBc binds to OsHKT1;1 promoter to activate its expression and trigger the positive regulation of salt stress response.
In conclusion, our results provide genetic evidence that OsMYBc binding is essential for the promoter activity of OsHKT1;1 and its roles in controlling Na+ accumulation in rice shoots. Furthermore, OsMSRFP, a RING-type E3 ubiquitin ligase that targets OsMYBc for proteasomal degradation, participates in negative regulatory mechanisms that involve posttranslational modification, thus providing an efficient means to temporally and spatially regulate the activation of salt-induced genes. This regulatory mechanism illustrates the sophisticated fine-tuning of rice responses to salt tolerance.
Materials and methods
Plant materials, cultivation, and stress treatment
The oshkt1;1 mutant (in the Nipponbare background), TosWT, complementation lines OsHKT1;1-COM (Com1 and Com2) and the osmybc mutant (in the Kitaake background) were prepared as described previously (Wang et al., 2015).
Rice (O.sativa) seedlings were grown hydroponically in Yoshida’s culture solution as previously described (Zhou et al., 2013). Plants were cultured in a growth chamber at 28°C/25°C (day/night) under a 14-h light/10-h dark photoperiod (∼500 μmol m−2 s−1).
To evaluate the salt tolerance of rice plants, 14-day-old seedlings were treated with 120-mM NaCl for 7 days, then the seedlings were transferred to a culture solution without NaCl for recovery. After an additional 7 days, the seedlings with completely yellow aerial parts were scored as dead, and the survival rate of the seedlings was analyzed according to the method described by Zhang et al. (2009).
Nicotiana benthamiana seeds were germinated and grown in sterilized nutrient-enriched soil in a growth chamber with a 14-h light/10-h dark photoperiod and a 25°C/22°C day/night temperature regime. The N. benthamiana seedlings were used for transient expression of proteins.
Determination of Na+ and K+ concentrations
The shoots and roots of rice seedlings were harvested separately, and Na+ and K+ concentrations were determined according to the methods described by Wang et al. (2015). Briefly, after drying at 80°C for 2 days until constant weight was achieved, each sample was digested in 5 mL of nitric acid at 90°C for 8 h. The mixture was diluted to 25 mL with distilled water and analyzed using an inductively coupled plasma-optical emission spectrometry instrument (ICP-OES; Perkin Elmer, Waltham, MA, USA).
Na+ concentration in the phloem sap was determined according to the method of Ren et al. (2005). Rice plants were hydroponically cultured for 30 days. The phloem sap was collected after treatment with 25-mM NaCl for 2 days. For each replicate, four plants were detached using a blade, and the cut ends were kept in a solution of 20-mM K2EDTA with pH 7.5 for at least 1 min. Then, the shoots were transferred to 1 mL of solution containing 15-mM K2EDTA with pH 7.5 for 4 h and left in an illumination incubator under 90% relative humidity to reduce transpiration. The glutamine (Gln) released in the ethylene diamine tetraacetic acid (EDTA) solution was used as an internal standard and was analyzed using an amino-acid analyzer (L8900; Hitachi). The Na+ concentration in the phloem sap is expressed as the ratio of Na+ to Gln.
The Na+ concentration in xylem sap was measured according to the method described by Horie et al. (2007) with minor modifications. Rice plants were grown for the collection of phloem sap. The shoots (2 cm above the roots) were severed using a blade. Except for the first drops following the initial incisions of shoots, the xylem sap was collected for 4 h after incision by covering the cut stem with a microcentrifuge tube containing a piece of absorbent cotton.
Concurrent with the phloem and xylem sap collection, the shoots of rice seedlings grown under the same conditions were collected for the measurement of Na+ concentrations.
The Na+ levels in shoots, and phloem and xylem sap were measured using ICP-OES.
Vector constructs and rice plant transformation
Three OsMYBc-binding elements (AAANATNCC/T) in the 2,103-bp region upstream of the initial codon of OsHKT1;1 were mutated (−76 to −68: AAATATGCC mutated to AAAGGGGCC; −275 to −267: AAATATGCT mutated to AAAAAAGGT; −1,476 to −1,468: GGTATATTT mutated to GGTGGACCT) using the Quick Change site-directed mutagenesis kit (Stratagene). The mutated OsHKT1;1 promoter was fused to the 2,248-bp genomic region of OsHKT1;1 and cloned into the modified vector pSuper1300 to obtain the Prom::OsHKT1;1 construct (Cao et al., 2013).
To construct the OsMYBc and OsMSRFP overexpression vectors, the full-length coding sequences (CDS) of OsMYBc and OsMSRFP were, respectively, cloned into the pSuper1300 and modified pCAMBIA1301 vectors to obtain the pSuper::OsMYBc (under the control of the Super promoter) and proZmUBQ1::OsMSRFP (under the control of the maize ubiquitin promoter) constructs, respectively.
OsMSRFP knockout mutants were generated using the CRISPR/Cas9 editing system according to previous methods with some modifications (Tian et al., 2021). The coding sequence of OsMSRFP with the locus number Os12g35320 was obtained from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). The specific guide RNA (gRNA) sequences listed in Supplemental Table S2 were designed using CRISPR-PLANT database (https://www.genome.arizona.edu/crispr) to reduce potential off-targets. The gRNA spacer was cloned into the binary vector pRGEB32, followed by introduction into WT (cv. Nipponbare) plants via Agrobacterium tumefaciens-mediated transformation. Mutation detection was performed using primer pairs flanking the gRNA-targeted sites.
To construct the proOsMYBc::GUS and proOsMSRFP::GUS vector, a 2,195-bp and 2,010-bp promoter region upstream of the 5′-terminal sites in the genome sequences of OsMYBc and OsMSRFP (according to the Promoter 2.0 Prediction Server [http://www.cbs.dtu.dk/services/Promoter/]) were, respectively, cloned into pCAMBIA1301 to replace the 35S promoter region (Li et al., 2017).
The sequence of the OsMYBc full-length CDS was cloned into the frame in front of the Flag coding region in the pCAMBIA1307 vector to create the 35S::OsMYBc-Flag fusion protein construct under the control of the 35S promoter (Dong et al., 2016). The full-length CDS of OsMSRFP and OsMSRFPm (mutations in the catalytic RING domain in which the key amino acids C183, H185, and H188 were mutated to Tyr) were fused to modified pCAMBIA1307 at the N-terminal of eGFP to produce the 35S::OsMSRFP-eGFP and 35S::OsMSRFPm-eGFP constructs, respectively (Dong et al., 2016).
The Prom::OsHKT1;1, pSuper::OsMYBc, proZmUBQ1::OsMSRFP, proUbi::OsMSRFP-Crispr, proOsMYBc::GUS, and proOsMSRFP::GUS constructs were introduced into A.tumefaciens strain EHA105. The constructs were then transformed into rice calluses of the oshkt1;1 mutant (Prom::OsHKT1;1), Kitaake (pSuper::OsMYBc), Dongjin (proZmUBQ1::OsMSRFP), and Nipponbare (proUbi::OsMSRFP-Crispr, proOsMYBc::GUS, and proOsMSRFP::GUS) cultivars following the protocol reported by Nishimura et al. (2006).
The primers used in this paper are listed in Supplemental Table S2, and the vectors and restriction sites are listed in Supplemental Table S3.
RNA isolation and RT–qPCR
Total RNA was extracted from rice tissues using Trizol (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. From l μg of total RNA, cDNA was synthesized using a Primer Script RT Reagent kit with gDNA Eraser (TaKaRa) following the manufacturer’s procedures. The reaction products were diluted two-fold and used as templates. Quantitative PCR analysis was performed as described previously (Bartley et al., 2013; Suzuki et al., 2016). UBQ5 gene was used as the internal reference to normalize gene expression data. The primers used for OsHKT1;1, OsMYBc, OsMSRFP, and UBQ5 are listed in Supplemental Table S2.
GUS staining and fluorimetric GUS assays
Histochemical analysis of GUS-stained tissues of rice plants was performed as described previously (Ai et al., 2009). Fluorimetric GUS assays were carried out according to the method described by Marino et al. (2013). The vectors were transformed into N. benthamiana leaves via an Agrobacterium-mediated transfer system. After expression for 60 h, the leaves were ground in liquid nitrogen, extracted in GUS buffer (50 mM Na2HPO4–NaH2PO4, pH 7.0, 10-mM β-mercaptoethanol, 10-mM Na2EDTA, 0.1% [w/v] sarcosyl, and 0.1% [v/v] Triton X-100), and centrifuged for 5 min at 10,000 g. One milligram of total protein in the supernatants was used to fluorimetrically measure enzymatic GUS activities using a microplate reader (Infinite M200 PRO; Switzerland), using 4-methylumbelliferyl-β-d-glucuronide (Sigma-Aldrich, St Louis, MO, USA) as a substrate. Standard curves were prepared with a range of increasing concentrations of 4-methylumbelliferone (Sigma-Aldrich).
Y2H assays
Rice RNA, which was used to create a cDNA library, was extracted from seedlings treated with 100-mM NaCl for 0, 1, 2, 4, 8, 24, and 48 h. A total of 5 μg of combined RNA comprising equal ratios was used to prepare the cDNA library (Wang et al., 2015). The N-terminal fragment of OsMYBc (133 amino acids) was fused to the GAL4-binding domain in pGBKT7 (Clontech, Mountain View, CA, USA) as the bait construct. Screening of interaction clones was carried out via mating according to the manufacturer’s instructions (Clontech Y2H assay). For the Y2H assays, prey proteins were fused to the GAL4 activation domain in pGADT7 (Clontech). Bait and prey constructs were co-transformed into yeast Y2H Gold cells. Positive clones were identified by the ability to grow on SD medium minus adenine/histidine/leucine/tryptophan and containing 125 ng mL−1 Aureobasidin A. The primers and the restriction sites used for the pGBKT7-OsMYBcN and pGADT7-OsMSRFP constructs are listed in Supplemental Tables S2 and S3, respectively.
In vitro pull-down assays
In vitro pull-down assays were conducted as described previously (Dong et al., 2014). Briefly, 10 μL of GST beads and 1 μg of purified GST, GST-OsMYBc, or GST-08g25820 fusion proteins were mixed in GST binding buffer (20-mM Tris–HCl, pH 7.5, 250-mM NaCl, 10% [v/v]glycerol, and 1-mM PMSF) and rotated for 2 h at 4°C. Next, 1 μg of His-OsMSRFP was added to each mixture with an additional 1 h of rotation. The GST beads were then washed with binding buffer 4 times and boiled with 5× protein loading sample buffer (258-mM Tris–HCl, pH 6.8, 8% [w/v] Na+ dodecyl sulfate [SDS], 40% [v/v] glycerol, 0.4% [w/v] Coomassie Brilliant Blue, and 0.4 M β-mercaptoethanol). The protein samples were subjected to 12% [w/v]SDS/PAGE. Input and pull-down prey proteins were detected by immunoblotting using anti-His and anti-GST monoclonal antibodies (Sigma-Aldrich). The primers and the restriction sites used for the pGEX-4T-GST-OsMYBc, pGEX-4T-GST-Os08g25820, and pET-28a-His-OsMSRFP constructs are listed in Supplemental Tables S2 and S3, respectively.
BiFC assays and subcellular localization of OsMSRFP
For the BiFC assays, OsMYBcN and OsMSRFP were fused to the vectors of pSPYNE (R) 155 (VN) and pSPYCE (M) (VC) (Waadt et al., 2008) to produce nYFP-OsMYBcN and OsMSRFP-cYFP, respectively. Combinations of VN with OsMSRFP-cYFP and nYFP-OsMYBcN with VC were used as negative controls. Then, nYFP-OsMYBcN/VC, VN/OsMSRFP-VC, and nYFP-OsMYBcN/OsMSRFP-cYFP were transiently expressed in N. benthamiana leaves. Leaves were collected after transfection for 60 h. For subcellular localization of OsMSRFP, the 35S::OsMSRFP-eGFP construct was transiently expressed in N. benthamiana leaves using the Agrobacterium-infection method (Yang et al., 2000). Fluorescence was detected using a confocal laser scanning microscope (Zeiss LSM780, Germany). The GFP fluorophore was excited with a 488-nm laser at 20% intensity and the emission was detected at 490–542 nm. The YFP fluorophore was excited with a 514-nm laser at 20% intensity and the emission was detected at 530–550 nm. The confocal gains were set to 700 mm.
The primers and the restriction sites used for the nYFP-OsMYBcN, OsMSRFP-cYFP, and 35S::OsMSRFP-eGFP constructs are listed in Supplemental Tables S2 and S3 respectively.
In vitro ubiquitination assay
For the in vitro ubiquitination assay, crude extract containing recombinant wheat (T.aestivum) E1 (GI: 136632), purified OsMYBc (1 μg) fused with the GST tag, purified AtUBC8 (E2, At5g41700; 40 ng), Arabidopsis ubiquitin (AtUBQ14; At4g02890; 2 μg), OsMSRFP (2 μg), OsMSRFPm (2 μg), and OsSRFP1 (2 μg) fused with His tags were used. His was used as the negative control for His-OsMSRFP. Reactions were performed following a previously described protocol (Xie et al., 2002). The reaction was stopped by the addition of 5× protein loading sample buffer and boiling for 10 min. The protein was resolved via SDS–PAGE, and the ubiquitinated proteins were visualized by immunoblotting with anti-GST antibody (Sigma-Aldrich). C183, H185, and H188 in the catalytic RING domain of OsMSRFP were mutated to Tyr using a Quick Change site-directed mutagenesis kit (Stratagene). The primers and the restriction sites used for the pET-28a-His-OsSRFP1 and pET-28a-His-OsMSRFPm (183, 185, and 188) constructs are listed in Supplemental Tables S2 and S3, respectively.
In vitro degradation assays
Soluble crude extracts (including 50 mM Tris—MES, pH 8.0; 0.5-M sucrose, 1-mM MgCl2, 10-mM EDTA, 5-mM DTT, and protease inhibitor cocktail Complete Mini tablets [Sigma-Aldrich]) were prepared from WT (with or without exposure to 120-mM NaCl for 3 h), cr-osmsrfp, or proZmUBQ1::OsMSRFP rice seedlings. The crude extracts (10 µg of total protein) were incubated with GST-OsMYBc or GST-Os08g25820 (500 ng) recombinant proteins for 1, 2, and 4 h in the presence or absence of 50-μM MG132 (Tocris). The reaction was stopped by adding 5× protein loading sample buffer and examined by immunoblot analysis using anti-GST (Sigma-Aldrich) antibodies.
In the transient expression system, the crude extracts were prepared from N. benthamiana leaves transiently expressed 35S::OsMSRFP-eGFP and 35S::OsMSRFPm-eGFP, respectively. The recombinant OsMYBc-GST was incubated with the above extracts respectively.
Bioinformatic analysis
Multiple amino acid sequence alignments of MYB proteins were made by Clustal Omega program and visualized by Jalview version 2.10. The phylogenetic trees of MYB and MSRFP were generated using MEGA version 7.0 software.
Statistical analyses
Data were analyzed via Student’s t test using the SPSS version 10 program.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: OsHKT1;1, LOC_Os04g51820; OsMYBc, LOC_Os09g12770; OsMSRFP, LOC_Os12g35320, MIEL1, At5g18650; OsSRFP1, LOC_Os03g22680; OsUBQ5, LOC_Os01g22490; AtUBC8, At5g41700; AtUBQ14, At4g02890.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Binding of OsMYBc to the OsHKT1;1 promoter is essential for controlling Na+ contents in shoots, xylem and phloem sap of rice plants under salt stress.
Supplemental Figure S2. Relative expression levels of OsMYBc in the reproductive stage analyzed by RT-qPCR.
Supplemental Figure S3. RT-qPCR analysis of the expression of OsMYBc in response to abiotic stresses.
Supplemental Figure S4. Overexpression of OsMYBc enhances salt tolerance of transgenic rice seedlings.
Supplemental Figure S5. The phylogenetic tree from OsMSRFP and its putative paralogs constructed using MEGA version 7.0 software.
Supplemental Figure S6. GUS staining of ProMSRFP::GUS in transgenic plants.
Supplemental Figure S7. Relative expression levels of OsMSRFP in the vegetative and reproductive stage analyzed by RT-qPCR.
Supplemental Figure S8. Sub-cellular localization of OsMSRFP in leaf epidermal cells of N. benthamiana.
Supplemental Figure S9. Sequence analysis of OsMYBs transcription factors.
Supplemental Figure S10. Pull-down assays for the interaction between OsMSRFP and OsMYBc (or Os08g25820).
Supplemental Figure S11. Generation of osmsrfp mutants using CRISPR/Cas9 gene editing.
Supplemental Figure S12. Cell free assay of OsMYBc degradation regulated by the extracts from cr-osmsrfp2 rice plants.
Supplemental Figure S13. Cell free assay of OsMYBc degradation regulated by OsMSRFP protein from OsMSRFP-OE rice plants.
Supplemental Figure S14. Overexpression of OsMSRFP results in salt sensitivity.
Supplemental Figure S15. RT-qPCR analysis of the expression of OsMYBc and OsMSRFP in response to salt stress.
Supplemental Table S1. Positive interactions from yeast two-hybrid screen.
Supplemental Table S2. The primers used in this study.
Supplemental Table S3. The restriction sites used to construct the vectors.
Supplementary Material
Acknowledgments
We thank Dr. Qi Xie (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, China) for providing the plasmids used for ubiquitination assay. We also thank Lingling Wu and Yang Bai (Nanjing Agricultural University, China) for their assistance in the experiments.
Funding
This work was supported by grants from the Ministry of Science and Technology in China (grants no. 2022YFE0198100), from the National Natural Science Foundation of China (grants no. 32171956), and from the Fundamental Research Funds for the Central University to W. Zhang.
Conflict of interest statement. None declared.
Contributor Information
Longyun Xiao, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China; College of Biotechnology, Jiangsu University of Science and Technology, Zhenjiang 212100, China.
Yiyuan Shi, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Rong Wang, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Yu Feng, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Lesheng Wang, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Hongsheng Zhang, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Xingyu Shi, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Guangqin Jing, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Ping Deng, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Tengzhao Song, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China.
Wen Jing, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China; Key Laboratory of Crop Physiology Ecology and Production Management, Ministry of Agriculture, Nanjing Agricultural University, Nanjing 210095, China.
Wenhua Zhang, State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China; Key Laboratory of Crop Physiology Ecology and Production Management, Ministry of Agriculture, Nanjing Agricultural University, Nanjing 210095, China.
L.X., W.J., and W.Z. conceived and designed the research. L.X. performed experiments with Y.S., Y.F., L.W., H.Z., X.S., G.J., P.D., and T.S. R.W. gave the materials and suggestions. L.X., W.J., and W.Z. analyzed the data and wrote the article. W.Z. and W.J. agree to serve as the authors responsible for contact and ensure communication.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) are Wenhua Zhang (whzhang@njau.edu.cn) and Wen Jing (jingwen@njau.edu.cn).
References
- Agarwal PK, Dave A, Agarwal P (2018) Transcriptional regulation of salinity stress: role and spatio-temporal expressions of ion-transporter gene promoters. Biol Plant 62: 641–646 [Google Scholar]
- Ai PH, Sun SB, Zhao JN, Fan XR, Xin WJ, Guo Q, Yu L, Shen QR, Wu P, Miller AJ, et al. (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809 [DOI] [PubMed] [Google Scholar]
- Ali A, Raddatz N, Aman R, Kim S, Park HC, Jan M, Baek D, Khan IU, Oh DH, Lee SY, et al. (2016) A single amino-acid substitution in the sodium transporter HKT1 associated with plant salt tolerance. Plant Physiol 171: 2112–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley LE, Peck ML, Kim SR, Ebert B, Manisseri C, Chiniquy D, Sykes R, Gao LF, Rautengarten C, Vega-Sánchez ME, et al. (2013) Overexpression of a BAHD acyltransferase, OsAt10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol 161: 1615–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Zhang SQ, Gong HJ, Tajima H, Blumwald E (2019) Cation specificity of vacuolar NHX-type cation/H+ antiporters. Plant Physiol 179: 616–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu QY, Li HM, Zhao QZ, Jiang HL, Zhai QZ, Zhang J, Wu XY, Sun JQ, Xie Q, Wang DW, et al. (2009) The Arabidopsis RING finger E3 ligase RHA2a is a novel positive regulator of abscisic acid signaling during seed germination and early seedling development. Plant Physiol 150: 463–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MT, Bandillo N, Al Shiblawi FRA, Sharma S, Liu K, Du Q, Schmitz AJ, Zhang C, Véry AA, Lorenz AJ, et al. (2017) Allelic variants of OsHKT1;1 underlie the divergence between indica and japonica subspecies of rice (Oryza sativa) for root sodium content. PLoS Genet 13: e1006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LY, Wang LH, Zheng M, Cao H, Ding L, Zhang XL, Fu Y (2013) Arabidopsis AUGMIN subunit8 is a microtubule plus-end binding protein that promotes microtubule reorientation in hypocotyls. Plant Cell 25: 2187–2201 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chapagain S, Park YC, Kim JH, Jang CS (2018) Oryza sativa salt-induced RING E3 ligase 2 (OsSIRP2) acts as a positive regulator of transketolase in plant response to salinity and osmotic stress. Planta 247: 925–939 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M (2009) Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 43: 265–285 [DOI] [PubMed] [Google Scholar]
- Chen ZC, Yamaji N, Horie T, Che J, Li J, An G, Ma JF (2017) A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiol 174: 1837–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsaftis O, Plett D, Shirley N, Tester M, Hrmova M (2012) A two-staged model of Na+ exclusion in rice explained by 3D modeling of HKT transporters and alternative splicing. PLoS One 7: e39865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinlein U, Stephan AB, Horie T, Luo W, Xu GH, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19: 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Tang DF, Gao ZX, Yu RB, Li KL, He H, Terzaghi W, Deng XW, Chen HD (2014) Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell 26: 3630–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZJ, Yu YW, Li SH, Wang J, Tang SJ, Huang RF (2016) Abscisic Acid antagonizes ethylene production through the ABI4-mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis. Mol Plant 9: 126–135 [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (. 2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15: 573–581 [DOI] [PubMed] [Google Scholar]
- Fang HM, Meng QL, Xu JW, Tang HJ, Tang SY, Zhang HS, Huang J (2015) Knock down of stress inducible OsSRFP1 encoding an E3 ubiquitin ligase with transcriptional activation activity confers abiotic stress tolerance through enhancing antioxidant protection in rice. Plant Mol Biol 87: 441–458 [DOI] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26: 3003–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kuang L, Wu L, Shen Q, Han Y, Jiang L, Wu D, Zhang G (2020) The HKT transporter HvHKT1;5 negatively regulates salt tolerance. Plant Physiol 182: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran S, Tsuchiya Y, Tran STH, Katsuhara M (2021) Identification and characterization of rice OsHKT1;3 variants. Plants 10: 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Horie T (2017) Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu Rev Plant Biol 68: 405–34 [DOI] [PubMed] [Google Scholar]
- Jabnoune M, Espeout S, Mieulet D, Fizames C, Verdeil JL, Conéjéro G, Rodríguez-Navarro A, Sentenac H, Guiderdoni E, Abdelly C, et al. (2009) Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol 150: 1955–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim WT (2013) Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett 587: 2584–2590 [DOI] [PubMed] [Google Scholar]
- Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, et al. (2017) OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J 91: 657–670 [DOI] [PubMed] [Google Scholar]
- Lee HG, Seo PJ (2016) The Arabidopsis MIEL1 E3 ligase negatively regulates ABA signalling by promoting protein turnover of MYB96. Nature Commu 7: 12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Jiang HL, Bu QY, Zhao QZ, Sun JQ, Xie Q, Li CY (2011) The Arabidopsis RING finger E3 ligase RHA2b acts additively with RHA2a in regulating abscisic acid signaling and drought response. Plant Physiol 156: 550–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang FW, Yan PW, Jing W, Zhang CX, Kudla J, Zhang WH (2017) A phosphoinositide-specific phospholipase C pathway elicits stress-induced Ca2+ signals and confers salt tolerance to rice. New Phytol 214: 1172–1187 [DOI] [PubMed] [Google Scholar]
- Marino D, Froidure S, Canonne J, Khaled SB, Khafif M, Pouzet C, Jauneau A, Roby D, Rivas S (2013) Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defense .Nat Commun 4: 1476–1485 [DOI] [PubMed] [Google Scholar]
- Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet 16: 237–251 [DOI] [PubMed] [Google Scholar]
- Miyao A, Tanaka K, Murata K, Sawaki H, Takeda S, Abe K, Shinozuka Y, Onosato K, Hirochika H (2003) Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. Plant Cell 15: 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, et al. (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat Biotechnol 30: 360–364 [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Nishimura A, Aichi I, Matsuoka M (2006) A protocol for Agrobacterium mediated transformation in rice. Nat Protoc 1: 2796–2802 [DOI] [PubMed] [Google Scholar]
- Oh TR, Kim JH, Cho SK, Ryu MY, Yang SW, Kim WT (2017) Atairp2 E3 ligase affects ABA and high-salinity responses by stimulating its ATP1/SDIRIP1 substrate turnover. Plant Physiol 174: 2515–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Lin B, Yang X, Liu L, Xia R, Li J, Wu Y, Xie Q (2020) The UBC-AIRP3 ubiquitination complex modulates ABA signaling by promoting the degradation of ABI1 in Arabidopsis .Proc Natl Acad Sci USA 117: 27694–27702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Egdane JA, Ismail AM (2013) Salinity tolerance, Na+ exclusion and allele mining of HKT1;5 in Oryza sativa and O. glaberrima: many sources, many genes, one mechanism? BMC Plant Biol 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37: 1141–1146 [DOI] [PubMed] [Google Scholar]
- Sadanandom A, Bailey M, Ewan R, Lee J, Nelis S (2012) The ubiquitin–proteasome system: central modifier of plant signalling. New Phytol 196: 13–28 [DOI] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Adler G, Bar-Zvi D (2013) ABI4 downregulates expression of the sodium transporter HKT1;1 in Arabidopsis roots and affects salt tolerance. Plant J 73: 993–1005 [DOI] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Finkler A, Pasmanik-Chor M, Fromma H (2019) CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR 6: a key regulator of Na+ homeostasis during germination. Plant Physiol 180: 1101–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Frungillo L, Spoel SH (2016) Transcriptional regulation by complex interplay between post-translational modifications. Curr Opin Plant Biol 33: 126–132 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yamaji N, Costa A, Okuma E, Kobayashi NI, Kashiwagi T, Katsuhara M, Wang C, Tanoi K, Murata Y, et al. (2016) OsHKT1;4-mediated Na+ transport in stems contributes to Na+ exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biol 16: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Chanroj S (2018) Plant endomembrane dynamics: studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol 177: 875–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Tamiru M, Abe A, Yoshida K, Uemura A, Yaegashi H, Obara T, Oikawa K, Utsushi H, Kanzaki E, et al. (2015) MutMap accelerates breeding of salt-tolerant rice cultivar. Nat Biotechnol 33: 445–449 [DOI] [PubMed] [Google Scholar]
- Tian Q, Shen L, Luan J, Zhou Z, Guo D, Shen Y, Jing W, Zhang B, Zhang Q, Zhang W (2021) Rice Shaker potassium channel OsAKT2 positively regulates salt tolerance and grain yield by mediating K+ redistribution. Plant Cell Environ 44: 2951–2965 [DOI] [PubMed] [Google Scholar]
- van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol. 71: 403–433 [DOI] [PubMed] [Google Scholar]
- Vierstra RD (2009) The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56: 505–516 [DOI] [PubMed] [Google Scholar]
- Wang BX, Liu Y, Wang YF, Jingfang L, Ming C, Zhiguang S, Bo X, Bo Y, Tingmu C, Baiguan L, et al. (2021) OsbZIP72 is involved in transcriptional gene-regulation pathway of abscisic acid signal transduction by activating rice high-affinity potassium transporter OsHKT1;1. Rice Sci 28: 257–267 [Google Scholar]
- Wang R, Jing W, Xiao LY, Jin YK, Shen LK, Zhang WH (2015) The Rice high-affinity potassium transporter1;1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiol 168: 1076–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Tian XJ, Zhao QZ, Liu ZQ, Li XF, Ren YK, Tang JQ, Fang J, Xu QJ, Bu QY (2018) The E3 ligase DROUGHT HYPERSENSITIVE negatively regulates cuticular wax biosynthesis by promoting the degradation of transcription factor ROC4 in rice. Plant Cell 30: 228–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Guo HS, Dallman G, Fang S, Weissman AM, Chua NH (2002) SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 419: 167–170 [DOI] [PubMed] [Google Scholar]
- Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217: 523–539 [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22: 543–551 [DOI] [PubMed] [Google Scholar]
- Zhang HW, Cui F, Wu YR, Lou LJ, Liu LJ, Tian MM, Ning YS, Shu K, Tang SY, Xie Q (2015) The RING finger ubiquitin E3 ligase SDIR1 targets SDIR1-INTERACTING PROTEIN1 for degradation to modulate the salt stress response and ABA signaling in Arabidopsis. Plant Cell 27: 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tian LH, Zhao JF, Song Y, Zhang CJ, Guo Y (2009) Identification of an apoplastic protein involved in the initial phase of salt stress response in rice root by two-dimensional electrophoresis. Plant Physiol 149: 916–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang C, Li Y, Zheng N, Chen H, Zhao Q, Gao T, Guo H, Xie Q (2007) SDIR1 is a RING finger E3 ligase that positively regulates stress responsive abscisic acid signaling in Arabidopsis. Plant Cell 19: 1912–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang F, Deng P, Jing W, Zhang W (2013) Characterization and mapping of a salt-sensitive mutant in rice (Oryza sativa L.). J Integr Plant Biol 55: 504–513 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.