Abstract
Background
Mature progression-free survival (PFS) data from the phase III J-ALEX study showed superiority for alectinib versus crizotinib [hazard ratio (HR) 0.37, 95% confidence interval (CI) 0.26-0.52; median PFS 34.1 versus 10.2 months, respectively] in advanced ALK (anaplastic lymphoma kinase)-positive non-small-cell lung cancer (NSCLC). Overall survival (OS) data were immature (HR 0.80, 99.8799% CI 0.35-1.82) at the time of data cut-off (30 June 2018). We report final OS data after ≥5 years of follow-up.
Patients and methods
ALK inhibitor naive Japanese patients who were chemotherapy naive or had received one prior chemotherapy regimen were enrolled. Patients were randomized to receive alectinib 300 mg (n = 103) or crizotinib 250 mg (n = 104) twice daily until progressive disease, unacceptable toxicity, death, or withdrawal. The primary endpoint was independent review facility-assessed PFS, with OS (not fully powered) as a secondary endpoint.
Results
Median duration of OS follow-up was 68.6 months with alectinib and 68.0 months with crizotinib. Treatment with alectinib did not prolong OS relative to crizotinib (HR 1.03, 95.0405% CI 0.67-1.58; P = 0.9105). Five-year OS rates were 60.9% (95% CI 51.4-70.3) with alectinib and 64.1% (95% CI 54.9-73.4) with crizotinib. In total, 91.3% (n = 95/104) of crizotinib-treated patients and 46.6% (n = 48/103) of alectinib-treated patients received at least one subsequent anticancer therapy. After study drug discontinuation, 78.8% of patients in the crizotinib arm switched to alectinib, while 10.7% of patients in the alectinib arm switched to crizotinib as a first subsequent anticancer therapy. Patients randomized to crizotinib tended to switch treatment earlier than those randomized to alectinib.
Conclusion
Final OS analysis from J-ALEX did not show superiority of alectinib to crizotinib; this result was most likely confounded by treatment crossover. Alectinib remains a standard of care for the treatment of patients with advanced ALK-positive NSCLC.
Key words: alectinib, ALK-positive NSCLC, crizotinib, J-ALEX, overall survival
Highlights
-
•
Final OS analysis from J-ALEX after ≥5 years’ follow-up; not fully powered.
-
•
Alectinib treatment did not prolong OS relative to crizotinib (HR 1.03, 95.0405% CI 0.67-1.58; P = 0.9105).
-
•
This was possibly due to cross-over of more crizotinib-treated patients to alectinib as a first subsequent therapy (78.8%).
-
•
5-year OS rates were 60.9% (95% CI 51.4-70.3) with alectinib and 64.1% (95% CI 54.9-73.4) with crizotinib.
-
•
Alectinib remains a preferred standard of care for the treatment of patients with advanced ALK-positive NSCLC.
Introduction
Alectinib, a highly selective anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (TKI), is a preferred first-line therapy for patients with advanced ALK-positive non-small-cell lung cancer (NSCLC).1,2 The randomized, multicenter, open-label, phase III J-ALEX study compared the efficacy and safety of alectinib, at the approved Japanese dose of 300 mg twice daily (b.i.d.), with crizotinib in Japanese patients with ALK inhibitor-naïve advanced ALK-positive NSCLC.3 The approved Japanese dose for alectinib, 300 mg twice daily (b.i.d.), was used in J-ALEX based on data from the Japanese AF-001JP study, while the approved dose for alectinib outside of Japan is 600 mg b.i.d.4,5 At the time of the primary endpoint analysis (data cut-off: 3 December 2015), alectinib demonstrated superior progression-free survival (PFS) relative to crizotinib according to an independent review facility [IRF; hazard ratio (HR) 0.34, 99.7% confidence interval (CI) 0.17-0.71, stratified log-rank P < 0.0001].3 Analysis of the central nervous system (CNS) efficacy of alectinib in J-ALEX showed that alectinib prolonged the time to progression of CNS metastases and prevented the development of new CNS lesions in patients without baseline CNS disease.6
Mature PFS data from J-ALEX (data cut-off: 30 June 2018) continued to demonstrate superiority in IRF-assessed PFS for alectinib versus crizotinib (HR 0.37, 95% CI 0.26-0.52; median PFS 34.1 versus 10.2 months with crizotinib).7 Overall survival (OS) data remained immature with 30.1% of events recorded in the alectinib arm and 31.7% of events in the crizotinib arm (HR 0.80, 99.8799% CI 0.35-1.82).7 The J-ALEX study was closed at the clinical data cut-off date of 30 June 2018, and OS follow-up and data collection of subsequent treatment were continued after the close of the study. Here, we report the final OS analysis of J-ALEX after at least 5 years of follow-up from the last patient.
Methods
Study design and patients
The design of the J-ALEX study (JapicCTI-132316; JO28928) has been described previously.3 ALK inhibitor naive Japanese patients with advanced ALK-positive NSCLC, who were chemotherapy naive or had received one prior chemotherapy regimen were enrolled. Key eligibility criteria included age ≥20 years; stage IIIB, IV, or recurrent ALK-positive NSCLC; Eastern Cooperative Oncology Group (ECOG) performance status 0-2; and at least one investigator-assessed measurable lesion according to RECIST version 1.1. Patients with treated or asymptomatic brain metastases were also eligible. ALK positivity was confirmed centrally by immunohistochemistry and FISH, or by RT-PCR, using tissue or cell samples.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice in Japan. The study protocol was reviewed by the institutional review board from the perspective of ethical, scientific, and medical validity. All patients provided written informed consent prior to any study-related procedures.
Treatment
Patients were randomized 1:1 via an interactive web response system to receive oral alectinib 300 mg or oral crizotinib 250 mg b.i.d. until progressive disease (PD), unacceptable toxicity, death, or withdrawal. Randomization was stratified according to ECOG performance status (0/1 versus 2); treatment line (first versus second); and clinical stage (IIIB/IV versus recurrent). Treatment crossover between arms after study drug discontinuation was permitted. Patients initially randomized to the crizotinib arm who withdrew due to PD, prior to the approval of alectinib in Japan, were permitted to receive alectinib during the study.
Endpoints
The primary study endpoint was PFS as assessed by an IRF. Secondary endpoints included OS, objective response rate, duration of response, time to response, time to progression of brain metastases in patients who presented with them at baseline, time to onset of brain metastases in patients who did not present with them at baseline, health-related quality of life, safety, and pharmacokinetics. IRF-assessed PFS was defined as the time from randomization to confirmation of PD assessed by the IRF or death, whichever occurred first. OS was defined as the time from randomization to death from any cause.
Statistical analyses
A sample size of 200 patients was planned primarily to detect a clinically meaningful difference in IRF-assessed PFS, assuming an enrollment period of 44 months. The study was not fully powered primarily for the endpoint of OS, but it was estimated that 150 OS events would provide 70% statistical power for a superiority hypothesis, under a targeted HR of 0.667.3 The final analysis of OS was planned to be conducted when 150 events have accrued or when the sponsor decides to terminate the trial, whichever occurs first. In this study, superiority in OS could be tested in a hierarchical fashion only if the null hypothesis for the superiority in PFS was rejected. Multiplicity by multiple analysis, including two formal interim analyses of OS, was adjusted by O’Brien and Fleming-type alpha spending function. The Kaplan-Meier methodology was used to estimate time to event endpoints. A stratified Cox regression model using the stratification factors for randomization was used to estimate the HR and its CI of OS between treatment arms. Efficacy analyses were carried out on the intent-to-treat population, which comprised all randomized patients.
Results
Baseline characteristics
Patients were enrolled between November 2013 and August 2015. In total, 207 patients were randomized to receive alectinib (n = 103) or crizotinib (n = 104).3 Baseline patient characteristics were generally balanced, but there was a greater proportion of patients who presented with brain metastases at baseline in the crizotinib arm (n = 29/104, 27.9%) compared with the alectinib arm (n = 14/103, 13.6%; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100527). However, this imbalance did not impact the conclusion from the primary analysis.3
Efficacy
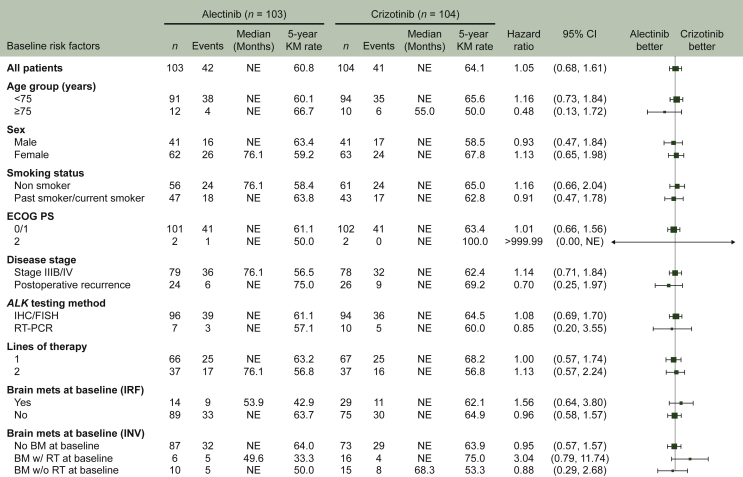
At the time of the final OS analysis in late 2020, when a minimal follow-up duration of 5 years for each patient was reached, the median duration of follow-up was 68.6 months (range 6-81) with alectinib and 68.0 months (range 2-79) with crizotinib. In total, 42 death events (40.8%) were recorded in the alectinib arm and 41 (39.4%) in the crizotinib arm. Superiority in OS of alectinib to crizotinib was not demonstrated (HR 1.03, 95.0405% CI 0.67-1.58, P = 0.9105); median OS was not reached in either treatment arm (Figure 1). The 5-year OS rate was 60.9% (95% CI 51.4-70.3) with alectinib and 64.1% (95% CI 54.9-73.4) with crizotinib. Subgroup analyses for OS were generally consistent with the results for all patients (Figure 2).
Figure 1.
Final OS in the ITT population.
CI, confidence interval; ITT, intent to treat; NE, not estimable; OS, overall survival. aThe O’Brien–Fleming rejection boundary was P < 0.049595 for the final OS analysis.
Figure 2.
OS subgroup analysis.
BM, brain metastases; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; IRF, independent review facility; INV, investigator-assessed; KM, Kaplan-Meier; Mets, metastases; NE, not estimable; OS, overall survival; RT, radiotherapy; w/, with; w/o, without.
Subsequent therapies
Overall, 91.3% (n = 95/104) and 46.6% (n = 48/103) of patients received one or more subsequent anticancer therapies in the crizotinib and alectinib arms, respectively, most commonly an ALK TKI. Access to other next-generation ALK TKIs occurred in 25.2% of patients in the alectinib arm and 82.7% of patients in the crizotinib arm (Table 1). After study drug discontinuation, 78.8% (n = 82/104) of patients originally randomized to receive crizotinib switched over to alectinib, while 10.7% (n = 11/103) of patients randomized to receive alectinib switched over to crizotinib. Subsequent anticancer therapies in patients that discontinued first treatment due to lack of efficacy are summarized in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100527. A further 56.7% (n = 59/104) of patients in the crizotinib arm and 37.9% (n = 39/103) of patients in the alectinib arm received a second subsequent anticancer therapy (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100527).
Table 1.
First subsequent anticancer therapies after alectinib or crizotinib
| Patients, n (%) | Alectinib (n = 103) | Crizotinib (n = 104) |
|---|---|---|
| Receiving at least one treatment | 48 (46.6) | 95 (91.3) |
| ALK TKI | 26 (25.2) | 86 (82.7) |
| Alectinib | 0 (0) | 82 (78.8) |
| Crizotinib | 11 (10.7) | 0 (0) |
| Brigatinib | 6 (5.8) | 1 (1.0) |
| Lorlatinib | 4 (3.9) | 3 (2.9) |
| Ceritinib | 5 (4.9) | 0 (0) |
| Chemotherapy | 18 (17.5) | 7 (6.7) |
| Pemetrexed | 13 (12.6) | 5 (4.8) |
| Carboplatin | 8 (7.8) | 2 (1.9) |
| Cisplatin | 5 (4.9) | 3 (2.9) |
| Paclitaxel | 1 (1.0) | 2 (1.9) |
| Tegafur | 2 (1.9) | 0 (0) |
| Docetaxel | 1 (1.0) | 0 (0) |
| Zoledronic acid | 1 (1.0) | 0 (0) |
| VEGF inhibitor (bevacizumab) | 4 (3.9) | 1 (1.0) |
| Cancer immunotherapy (nivolumab) | 2 (1.9) | 0 (0) |
| RANKL inhibitor (denosumab)a | 2 (1.9) | 2 (1.9) |
ALK, anaplastic lymphoma kinase; RANKL, receptor activator of nuclear factor kappa-B ligand; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Administered as part of supportive care.
Patients were permitted to change from their initial treatment to a subsequent treatment for various reasons, including PD (i.e. after study drug discontinuation, termed ‘treatment switching’). Patients in the crizotinib arm tended to have their treatment switched earlier than those in the alectinib arm (Figure 3). In a non-randomized subset of patients who had their treatment switched, OS following the first treatment switch was more favorable in the crizotinib arm than in the alectinib arm (Figure 4).
Figure 3.
Time from randomization to first treatment switch.
CI, confidence interval; NE, not estimable.
Figure 4.
OS from initiation of first treatment switch in patients receiving subsequent therapies.
CI, confidence interval; NE, not estimable; OS, overall survival.
Discussion
In this final OS analysis of the J-ALEX study, prolongation of OS in the alectinib arm (at the approved Japanese dose of 300 mg b.i.d.) was not observed relative to the crizotinib arm. However, OS at this final analysis was not fully powered because the number of events was 83, which is approximately half of the planned number of 150 events. The final OS analysis was planned to be conducted when 150 events had accrued or when the sponsor decided to terminate the trial, whichever occurred first. Although the OS data had not reached 150 events, the sponsor decided to conduct the final OS analysis after at least 5 years of follow-up from the last patient was completed, as it was assumed that it would take a long period until 150 events accrued as the survival rate of each TKI arm was greater than was estimated. A 5-year follow-up from the last patient enrollment was considered as a relevant and substantial follow-up period for this analysis in advanced NSCLC. In addition, a greater proportion of patients in the crizotinib arm had their initial treatment switched compared with the alectinib arm. The majority (82.7%) of patients in the crizotinib arm received an ALK TKI, including 78.8% of patients who received alectinib as a first subsequent treatment. Patients initially randomized to receive crizotinib tended to have their treatment switched earlier than those randomized to receive alectinib mainly due to earlier determination of PD with crizotinib. In addition, the proportion of patients receiving more than one subsequent anticancer therapy was higher in the crizotinib arm relative to the alectinib arm.
Although patient characteristics at change of treatment may vary from those at baseline, patients in the crizotinib arm who had their treatment switched demonstrated a numerically longer OS from the initial treatment switch than those in the alectinib arm who switched treatment. These findings suggest that the OS results from J-ALEX may have been confounded by the crossover of a large proportion of patients (78.8% in the crizotinib arm received alectinib as first subsequent anticancer therapy). This was also observed in a phase II prospective analysis in patients with ALK-positive NSCLC treated with alectinib following PD on crizotinib.8 Despite OS in J-ALEX being similar in the alectinib and crizotinib arms, superior PFS for alectinib in J-ALEX has been previously reported and the frequency of adverse events (AEs), such as gastrointestinal toxicity, was lower with alectinib than with crizotinib.3
In the phase III, randomized, global ALEX study, the globally approved alectinib dose of 600 mg b.i.d. demonstrated a clinically meaningful improvement in OS versus crizotinib in patients with treatment-naïve, advanced ALK-positive NSCLC; 5-year OS rates were 62.5% with alectinib and 45.5% with crizotinib.9 OS data remain immature with 37% of required events recorded. Median OS in ALEX was not reached with alectinib and was 57.4 months with crizotinib (stratified HR 0.67, 95% CI 0.46-0.98).9 At data cut-off, 55.3% patients in the alectinib arm and 75.5% patients in the crizotinib arm experienced PD. Access to other next-generation ALK TKIs occurred in 38.1% patients who progressed in the alectinib arm of ALEX, and in 53.5% patients who progressed in the crizotinib arm.9 In this study, 82.7% (n = 86/104) of patients in the crizotinib arm and 25.2% (n = 26/103) of patients in the alectinib arm received other ALK TKIs as the first subsequent anticancer therapy after study drug discontinuation. It is interesting to note that 38.8% (n = 40/103) of patients in the alectinib arm and 76.9% (n = 80/104) patients in the crizotinib arm experienced treatment discontinuation due to the lack of efficacy, and access to other ALK TKIs occurred in 35.0% (n = 14/40) and 91.3% (n = 73/80) of patients who progressed in the alectinib arm and crizotinib arm, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100527). It should also be noted that a lower dose of alectinib than the globally approved dose was used in J-ALEX. However, a population pharmacokinetic and exposure-response analysis demonstrated that alectinib 300 mg b.i.d. dose can be bridged to the 600 mg b.i.d. dose.10 Taken together, this suggests that the difference in the frequency of other next-generation ALK TKIs, including alectinib in the crizotinib arm, being used as subsequent therapies may have had a greater impact on the OS result than the difference in dose between the ALEX and J-ALEX studies.
The next-generation ALK TKIs, brigatinib and lorlatinib, are more effective than crizotinib in the treatment of advanced ALK-positive NSCLC, with suggested activity against broader resistant mutations, indicating that they may also be effective against alectinib-resistant populations.11 Indeed, lorlatinib (median PFS 5.5 months12) or brigatinib (median PFS 7.3 months13) have demonstrated efficacy in patients who have progressed on alectinib. Both agents are approved in the first-line treatment setting, but AEs such as increased blood creatine phosphokinase with brigatinib and neurocognitive effects with lorlatinib have been widely reported.11 These findings could support the use of alectinib as initial therapy, followed by newer ALK TKIs once alectinib resistance develops. Therefore, alectinib may be considered a preferred first-line treatment option from the perspective of AE management and acquired resistance follow-up.
Results of the J-ALEX study suggest that alectinib contributes to the long-term survival of patients with advanced ALK-positive NSCLC, with median OS not reached in either treatment arm, and 5-year OS rates of approximately 60%. Published 5-year OS rates in patients with advanced NSCLC receiving other agents are considerably lower than in J-ALEX.
The superior efficacy of alectinib relative to crizotinib in advanced ALK-positive NSCLC can be partially explained by its greater CNS activity. For reference, CNS activity data from J-ALEX for the clinical cut-off date of 30 June 2018 are provided in Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100527. Previous results from J-ALEX showed that alectinib prolonged the time to progression of CNS metastases in patients who presented with them at baseline and delayed the onset of new CNS lesions in patients who did not present with them at baseline.6 Similar findings were reported in the global ALEX study.14 The high CNS protection activity of alectinib was also reported in a retrospective analysis in select patients with advanced ALK-positive NSCLC failing crizotinib treatment; patients receiving alectinib experienced a lower incidence of CNS progression than those receiving ceritinib (cause-specific HR 0.10, 95% CI 0.01-0.78).15
Conclusion
In the J-ALEX study, alectinib resulted in long-term survival in patients with advanced ALK-positive NSCLC, although prolongation of OS was not observed relative to the crizotinib arm, possibly due to treatment crossover. Median OS was not reached in either treatment arm, with 5-year OS rates of approximately 60%, and the study was not powered primarily for OS. These findings suggest that alectinib remains a preferred standard of care for the treatment of patients with advanced ALK-positive NSCLC.
Acknowledgements
The authors gratefully acknowledge the investigators, staff, and patients involved in this study. Third-party medical writing assistance, under the direction of the authors, was provided by Joanne Bowes, PhD, of Ashfield MedComms, an Ashfield Health company, and was funded by Chugai Pharmaceutical Co. Ltd. T. Kumagai is deceased. This paper is dedicated to his memory.
Funding
This work was supported by Chugai Pharmaceutical Co. Ltd (no grant number).
Disclosure
KH declares personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb (BMS), Chugai, Lilly, MSD, Ono, Pfizer, Taiho, and Takeda; and grants from AstraZeneca, BMS, Chugai, Lilly, AbbVie, and MSD. TH declares grants and personal fees from AstraZeneca, BMS, Chugai, Kissei, MSD, Nippon Boehringer Ingelheim, Novartis, Ono, Pfizer, Taiho, and Takeda; and grants from AbbVie, Astellas, Daiichi-Sankyo, Eisai, Ignyta, Janssen, and Merck Serono. HN declares honoraria for lectures from AstraZeneca, Chugai, Nippon Boehringer Ingelheim, Pfizer, and Eli Lilly Japan; institutional grants from AstraZeneca, Chugai, and Pfizer. MM declares grants and personal fees from AstraZeneca, BMS, Chugai, Eli Lilly, Kissei, Merck Serono, Nippon Boehringer Ingelheim, Novartis, Ono, Pfizer, and Taiho. YHK declares lecture fees from Chugai, AstraZeneca, Eli Lilly, Boehringer Ingelheim, MSD, Taiho, and Nippon Kayaku. KA declares honoraria from AstraZeneca, BMS, Chugai, MSD, and Ono. TS declares grants from AbbVie, Chugai, Daiichi-Sankyo, Eli Lilly Japan, Kissei Pharmaceutical, Merck Biopharma, MSD, Novartis, Pfizer, and Takeda; personal fees from AstraZeneca, BMS, Chugai, Covidien Japan, Daiichi-Sankyo, Eli Lilly Japan, Kyowa Hakko Kirin, MSD, Mochida Pharmaceutical, Nippon, Novartis, Ono, Pfizer, Taiho, Takeda, and Thermo Fisher Scientific; and is an employee of Precision Medicine Asia. YT declares grants and lecture fees from Chugai, Pfizer, Boehringer Ingelheim, Takeda, Novartis, Ono, Eli Lilly, AstraZeneca, MSD, Taiho, and Towa; grants from Chugai, Boehringer Ingelheim, Takeda, Taiho, Daiichi-Sankyo, Nippon Kayaku, and MSD, Eli Lilly, and Kyowa Hakko Kirin; and study support from BMS, MSD, AstraZeneca, and AbbVie. MN declares grants and personal fees from Ono, BMS, Pfizer, Chugai, Eli Lilly, Taiho, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Merck Biopharma, Daiichi-Sankyo, and Takeda; and personal fees from Boehringer Ingelheim, Teijin, and AbbVie. HY declares honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Delta-Fly Pharma, Lilly, Kyowa Kirin Co. Ltd, MSD, Novartis, Nippon Kayaku, Ono, Pfizer, and Taiho. TK declares grants from AstraZeneca, Chugai, Eli Lilly, Merck Biopharma, MSD, Nippon Boehringer Ingelheim, Ono, Pfizer, Taiho, Takeda, and The Osaka Foundation for the Prevention of Cancer and Lifestyle related Diseases; personal fees from BMS, Nippon Boehringer Ingelheim, Ono, Pfizer, and Taiho; and consulting fees from Takeda. SW declares grants and personal fees from Boehringer Ingelheim and personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Lilly, Nippon Kayaku, Novartis, Ono, Pfizer, and Taiho. KG declares honoraria or personal fees from Amgen Astellas BioPharma, Amgen, Amoy Diagnostics, Boehringer Ingelheim Japan, BMS, Bayer Yakuhin, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly Japan, Guardant Health, Janssen, Kyowa Kirin, Life Technologies Japan, MSD, Novartis, Ono, Otsuka, Pfizer Japan, Taiho, and Takeda; institutional funding from Amgen Astellas BioPharma, Amen, Boehringer Ingelheim Japan, Bristol-Myers Squibb, Bayer Yakuhin, Daiichi-Sankyo, Eisai, Eli Lilly Japan, Ignyta, Janssen, Kissei Pharmaceutical, Kyowa Kirin, Loxo Oncology, Medical & Biological Laboratories, Merck Biopharma, Merus, MSD, NEC, Novartis, Ono, Sumitomo Dainippon Pharma, Spectrum Pharmaceuticals, Sysmex, Haihe Biopharma, Taiho, Takeda, and Turning Point Therapeutics. MS declares honoraria from Chugai, Pfizer, Takeda, AstraZeneca, MSD, Novartis, Eli Lilly, Bristol-Myers Squibb, Ono, Taiho, and Merck; grants from Chugai, MSD, IQVIA, EPS, Janssen, Amgen, Taiho, Ono, Pfizer, AbbVie, Daiichi-Sankyo, and Eisai. TK declares grants and personal fees from Chugai, AstraZeneca, Eli Lilly Japan, Taiho, BMS, MSD, Kyowa Hakko Kirin, Daiichi-Sankyo, and AbbVie; grants from Merck Biopharma, Amgen, and Sanofi; and personal fees from Ono, Pfizer Japan, Nippon Boehringer Ingelheim, Nippon Kayaku, Novartis, Takeda, and Bayer. TS declares honoraria or personal fees from AstraZeneca, Chugai, Boehringer Ingelheim, Novartis, MSD, Taiho, Daiichi-Sankyo, Ono, BMS, Nippon Kayaku, and Pfizer; and grants from AstraZeneca, Chugai, Boehringer Ingelheim, Novartis, and MSD. KN declares honoraria from Astellas, AstraZeneca, MSD, Ono, Daiichi-Sankyo, Taiho, BMS, Medical Review, Thermo Fisher Scientific, KYORIN, Nikkei Business Publications, Takeda, Chugai, Eli Lilly Japan, Merck Biopharma, Novartis, Pfizer Japan, CareNet, YODOSHA, Hisamitsu, MEDICUS SHUPPAN Publishers, Yomiuri Telecasting Corporation, Nanzando, Roche Diagnostics, Nippon Kayaku, Bayer Yakuhin, Kyowa Kirin, AbbVie, Amgen, 3H Clinical Trial, Nichi-Iko, Nippon Boehringer Ingelheim, and Medical Mobile Communications; research funding from MSD, ICON Japan, Takeda, Eli Lilly Japan, BMS, Taiho, PARAXEL International, Ono, Sysmex Corporation, A2 Healthcare, AbbVie, Chugai, Nippon Boehringer Ingelheim, SymBio Pharmaceuticals, AstraZeneca, Astellas, Novartis, EPS International, CMIC Shift Zero, Eisai, Mochida, GlaxoSmithKline, Kyowa Hakko Kirin, EPS Corporation, Daiichi-Sankyo, IQVIA Services Japan/Quintiles, Pfizer Japan, Bayer Yakuhin, Otsuka, PRA Health Sciences, Merck Biopharma, Covance Japan, Medical Research Support, Sanofi, Syneos Health, Pfizer R&D Japan, PPD-SNBL, and Japan Clinical Research Operations; consulting or advisor role for Astellas, Pfizer Japan, Takeda, KYORIN, Eli Lilly Japan, and Ono. TM declares honoraria from AstraZeneca, Bristol-Myers Squibb, Chugai, MSD, Nippon Boehringer Ingelheim, Novartis, Ono, Pfizer, Taiho, Takeda, Amgen, Invitae, Merck Biopharma, Thermo Fisher Scientific, and Eli Lilly; research grants from Daiichi-Sankyo, MSD, Nippon Boehringer Ingelheim, Chugai, Ono, Taiho, Ethicon, and Bridge Biotherapeutics. NY declares consulting fees from Chugai, honoraria or personal fees from MSD, AstraZeneca, Ono, Thermo Fisher Scientific, Daiichi-Sankyo, Taiho, Takeda, Chugai, Eli Lilly Japan, Boehringer Ingelheim, Novartis, Pfizer, BMS, Nippon Kayaku, GlaxoSmithKline, Sanofi, Hisamitsu, and Merck Biopharma; data safety monitoring board or advisory board participation for MSD, AstraZeneca, Ono, Taiho, Takeda, Chugai, Eli Lilly Japan, Boehringer Ingelheim, Novartis, Pfizer, BMS, Life Technologies Japan, Nippon Kayaku, Amgen, Guardant Health Japan, and Janssen; leadership or fiduciary role in The Japan Lung Cancer Society, Japanese Association of Supportive Care in Cancer, and West Japan Oncology Group. TA, TY, and ST are employees of Chugai. TT declares honoraria, personal fees, and grants from Chugai, Taiho, Eli Lilly, Ono, Nippon Boehringer Ingelheim, BMS, Daiichi-Sankyo, Eisai, Yakult Honsha, Novartis, Astellas, MSD, Nippon Kayaku, Kyowa Kirin, and CMIC ShiftZero.
Data sharing
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). For further details on Chugai’s Data Sharing Policy and how to request access to related clinical study documents, see here (www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html).
Supplementary data
References
- 1.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N.H., Robinson A.G., Temin S., et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39:1040–1091. doi: 10.1200/JCO.20.03570. [DOI] [PubMed] [Google Scholar]
- 3.Hida T., Nokihara H., Kondo M., et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 4.Gadgeel S.M., Gandhi L., Riely G.J., et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 5.Seto T., Kiura K., Nishio M., et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 6.Nishio M., Nakagawa K., Mitsudomi T., et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2018;121:37–40. doi: 10.1016/j.lungcan.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa K., Hida T., Nokihara H., et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2020;139:195–199. doi: 10.1016/j.lungcan.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Shaw A.T., Gandhi L., Gadgeel S., et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok T., Camidge D.R., Gadgeel S.M., et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 10.Hsu J.C., Jaminion F., Guerini E., et al. Population pharmacokinetics (popPK) and exposure-response (ER) analyses bridge J-ALEX to the global population with an alectinib (ALC) 600mg bid dosing regimen. J Clin Oncol. 2017;35 [Google Scholar]
- 11.Naito T., Shiraishi H., Fujiwara Y. Brigatinib and lorlatinib: their effect on ALK inhibitors in NSCLC focusing on resistant mutations and central nervous system metastases. Jpn J Clin Oncol. 2021;51:37–44. doi: 10.1093/jjco/hyaa192. [DOI] [PubMed] [Google Scholar]
- 12.Borghaei H., Gettinger S., Vokes E., et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishio M., Yoshida T., Kumagai T., et al. Brigatinib in Japanese patients with ALK-positive NSCLC previously treated with alectinib and other tyrosine kinase inhibitors: Outcomes of the phase 2 J-ALTA trial. J Thorac Oncol. 2021;16:452–463. doi: 10.1016/j.jtho.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Gadgeel S., Peters S., Mok T., et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29:2214–2222. doi: 10.1093/annonc/mdy405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo C.-H.S., Tung P.-H., Huang A.C.-C., et al. A retrospective study of alectinib versus ceritinib in patients with advanced non-small-cell lung cancer of anaplastic lymphoma kinase fusion in whom crizotinib treatment failed. BMC Cancer. 2021;21:309. doi: 10.1186/s12885-021-08005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.