Abstract
Introduction: The prevalence of cardiovascular disease (CVD) is rising in Sub-Saharan Africa, but it is not known whether current risk assessment tools predict coronary atherosclerosis in the region. Furthermore, sex-specific performance and interaction with HIV serostatus has not been well studied. Methods: This cross-sectional study compared ASCVD risk scores and detectable coronary artery calcium (CAC>0) by sex in Kampala, Uganda (n = 200). The cohort was enriched for persons living with HIV, and all participants had at least one CVD risk factor. We fit log binomial regression models and constructed ROC curves to assess the correlation between ASCVD scores and CAC>0. Results: The mean age was 56. 62% were female and 50% of both men and women were living with HIV. The median 10-year ASCVD risk score was significantly higher in men (11.0%, IQR 7.6–19.4%) than in women (5.1%, IQR 3.2–8.7%), although the prevalence of CAC>0 was similar (8.1 vs 10.5%, p = 0.63). Each 10% increase in ASCVD risk was associated with increased risk of CAC>0 in men (PR 1.59, 95% CI 1.00–2.55, p = 0.05) but not women (PR 1.15, 95% CI 0.44–3.00, p = 0.77). ROC curves demonstrated an AUC of 0.57 for women vs 0.70 for men. Adjustment for HIV serostatus improved the predictive value of ASCVD in women only (AUC 0.78, p = 0.02). Conclusions: ASCVD risk score did not correlate with the presence of CAC in women. When HIV status was added to the ASCVD risk score, correlation with CAC was improved in women but not in men.
Keywords: Coronary artery disease, Coronary artery calcium, ASCVD pooled Cohort equations (PCE), Human immunodeficiency virus (HIV), Sex, Uganda
1. Introduction
The prevalence of cardiovascular disease is rising in sub-Saharan Africa as countries in the region undergo both demographic and epidemiologic transitions [[1], [2], [3], [4]]. The trend is largely driven by a growing burden of traditional risk factors for atherosclerosis, including hypertension, diabetes, and obesity [[5], [6], [7]]. Accurate risk-prediction tools are an essential component of the prevention strategy for atherosclerotic cardiovascular disease (ASCVD). However, widely-used risk estimators such as the Pooled Cohort Equations (PCE) [8] have not been adequately validated in sub-Saharan Africa and may not accurately predict risk in this heterogeneous population [[9], [10], [11]].
Previous work has identified specific patterns of ASCVD risk across sub-Saharan Africa that suggest the PCE may not apply to this context. First, historic data established male sex as an independent cardiovascular risk factor [[12], [13], [14]]. However, studies in sub-Saharan Africa have identified higher rates of age-standardized cardiovascular mortality in women [2] which may be partially explained by a higher burden of traditional risk factors, particularly obesity and the metabolic syndrome [[15], [16], [17]]. Second, the interaction between traditional and non-traditional risk factors such as HIV may moderate risk profiles in significant ways [18]. In the global north, there is a well-established association between HIV infection, chronic immune activation and an increased risk of coronary heart disease [[19], [20], [21], [22], [23], [24]]. HIV is estimated to increase the risk of cardiovascular disease 2-fold. In sub-Saharan Africa, where HIV is more endemic, this translates to 10–15% of the population attributable risk for ASCVD [21].
Ascertainment of clinical ASCVD outcomes can be challenging in sub-Saharan contexts due to constrained healthcare resources, making direct validation of risk estimators challenging. Coronary artery calcium (CAC) scoring via computed tomography has been associated with HIV serostatus elsewhere, and is therefore proposed as an important marker of subclinical ASCVD in this setting [25]. We undertook the current study to investigate the correlation of the PCE 10-year risk score with CAC among adults living in Kampala, Uganda, with particular attention to sex and HIV serostatus.
2. Material and methods
2.1. Participants
For this cross-sectional study, 100 persons living with HIV (PLWH) were enrolled from a population of patients in ambulatory care currently receiving antiretroviral therapy (ART) at the Joint Clinical Research Centre near Kampala, Uganda. The Joint Clinical Research Centre was established in 1991 to provide high-quality HIV care and has since grown to support a wide variety of clinical services and academic research initiatives. Enrollment for this study began in April 2015 and extended through May 2017. We prospectively identified 100 age- and sex-matched HIV-negative controls in a 1:1 ratio, recruited from the community or hospital-based internal medicine clinics within the catchment area of the Joint Clinical Research Centre. All participants were 45 years of age or older. To enrich the study population for cardiovascular risk, enrollment criteria required all participants to have at least one traditional cardiovascular risk factor (hypertension, diabetes mellitus, smoking, or high cholesterol) as assessed by self-report and correlation with the enrolling clinic's medical record.
2.2. Study procedures
Self-reported demographics, smoking status, and medical history were obtained using standardized questionnaires and clinical chart review. Socioeconomic status (SES) was defined using a previously validated survey of household asset ownership [26]. Diabetes and hypertension were defined as having a self-reported history of the condition or being on a medication for the condition. Smoking was defined as a self-report of current use of cigarettes. Among PLWH, current and nadir CD4+ count, time since HIV diagnosis, current ART and total duration of ART were abstracted from the medical record. A physical examination was conducted, including height, weight, blood pressure, waist and hip measurement. All control subjects were confirmed negative with a rapid HIV test. After a 12-h fast, blood was drawn for clinical labs including a lipoprotein panel and inflammatory markers.
All subjects underwent non-contrast, electrocardiogram-gated cardiac computed tomography (CT) for calcium scoring. Scans were performed on a 128-slice multidetector CT scanner (Siemens; Munich, Germany). Coronary artery calcium (CAC) score was measured offline by a radiologist (GE) using a Siemens Syngo workstation. Calcified lesions were defined as having ≥6 pixels with density >130 Hounsfield units, and total CAC score was calculated using the Agatston method [27].
Biomarkers of inflammation and immune activation were measured in batch from cryopreserved plasma samples. Interleukin-6 (IL-6) was measured by electrochemiluminescence (Meso Scale Diagnostics, Rockville, MD, USA). Soluble CD14 (sCD14; R&D Systems) and soluble CD163 (sCD163; R&D Systems) were measured by ELISA. High sensitivity C-reactive protein (hsCRP) was measured by nephelometry (Siemens, Munich, Germany).
2.3. Statistical analysis
We first described the demographic and cardiometabolic risk profile among study participants stratified by sex. Statistical comparisons between groups (female vs male) were made using t-tests and Wilcoxon rank-sum tests for continuous variables, and chi-squared or Fisher exact tests for categorical variables as appropriate. Household asset ownership data was used to generate an index of socioeconomic status using a principal-components analysis. Biomarkers were analyzed after log transformation and categorized by tertile to allow relative comparisons with the cohort, due to the lack of standardized thresholds for clinical disease prediction with the markers studied. Missingness was <3% for all variables. The American College of Cardiology (ACC) Pooled Cohort Equations Risk Estimator was used to assess the 10-year risk of an ASCVD event among all study participants. ASCVD is defined as coronary heart disease death, non-fatal MI, fatal stroke and non-fatal stroke. Race was categorized as “other” for all participants due to a lack of data supporting the applicability of US-defined racial constructs to this context.
In the primary analysis, we first categorized the distribution of predicted ASCVD risk and detectable calcified coronary plaque (defined as CAC score >0) for the entire cohort and among the cohort stratified by sex. We then used log binomial regression to assess the relationship between ASCVD risk score and CAC>0 both in a univariable model and in multivariable models adjusted for 1) HIV status and 2) individual inflammatory markers by sex. We assessed the predictive value of ASCVD risk score for CAC>0 by generating ROC curves for 1) ASCVD alone, 2) ASCVD and HIV and 3) ASCVD and inflammatory markers. STATA 14.0 was used for analysis; p < 0.05 was considered statistically significant.
2.4. Ethical review
All study procedures conducted in Uganda were reviewed and approved by the Institutional Review Board of University Hospitals Cleveland Medical Center, the Joint Clinical Research Centre (JCRC; Kampala, Uganda) and the Uganda National Council for Science and Technology. All participants signed written informed consent.
3. Results
Characteristics of study participants are displayed by gender in Table 1 and by HIV serostatus in Supplemental Table 1. Women were more likely than men to have hypertension (91% vs 75%, p < 0.01), obesity (60% vs 16%, p < 0.01) and to fall in the lowest wealth tertile (41% vs 21%. P = 0.02). Women had both higher mean LDL levels (144 mg/dL, SD 46 vs 128 mg/dL, SD 38, p < 0.01) and mean HDL levels (57 mg/dL, SD 14 vs 52 mg/dL, SD 14, p = 0.02).
Table 1.
Demographic indicators.
| Women (N = 124) | Men (N = 76) | p-value | |
|---|---|---|---|
| Age (mean, SD) | 55.7 (6.2) | 55.5 (7.0) | 0.79 |
| Systolic BP (mean, SD) | 157.1 (25.9) | 154.1 (27.7) | 0.44 |
| Diastolic BP (mean, SD) | 93.0 (12.5) | 94.9 (16.9) | 0.37 |
| Hypertensiona (n, %) | 113 (91.1) | 57 (75.0) | <0.01 |
| Total cholesterol level (mg/dl, mean, SD) | 219.3 (55.4) | 201.3 (49.7) | 0.02 |
| LDL level (mg/dl, mean, SD) | 144.4 (46.2) | 127.6 (38.1) | <0.01 |
| HDL level (mg/dl, mean, SD) | 57.4 (13.9) | 52.4 (14.2) | 0.02 |
| BMI (n, %) | <0.01 | ||
| Underweight <18.5 (n,%) | 0 (0.0) | 3 (4.0) | – |
| Normal 18.5–24.9 (n, %) | 16 (13.0) | 29 (38.2) | – |
| Overweight 25–29.9 (n, %) | 34 (27.6) | 32 (42.1) | |
| Obese≥30 (n, %) | 73 (59.4) | 12 (15.8) | – |
| Fasting glucose (mg/dl, mean, SD) | 119.3 (57.5) | 124.6 (61.0) | 0.54 |
| Diabetesa (n, %) | 39 (31.5) | 32 (42.1) | 0.13 |
| SES index (n, %) | 0.02 | ||
| First tertile | 49 (41.2) | 16 (21.3) | – |
| Second tertile | 35 (29.4) | 30 (40.0) | – |
| Third tertile (wealthiest) | 35 (29.4) | 29 (38.7) | – |
| Current smoker (n, %) | 3 (2.4) | 5 (6.6) | 0.26 |
| hsCRP (μg/ml, median, IQR) | 2.8 (0.8–5.1) | 1.2 (0.5–4.1) | 0.02c |
| hsCRP (μg/ml, n, prop) | 0.02 | ||
| hsCRP <1 | 37 (29.8) | 37 (48.7) | – |
| hsCRP 1–3 | 30 (24.2) | 16 (21.1) | – |
| hsCRP >3 | 57 (46.0) | 23 (30.3) | – |
| sCD14 (pg/ml, median, IQR) | 1,534,371 (1,328,543–1,826,348) | 1,433,311 (1,128,021–1,737,784) | 0.04c |
| sCD14 (pg/ml, n, %) | 0.24 | ||
| First tertile | 36 (29.0) | 31 (40.8) | – |
| Second tertile | 45 (36.3) | 22 (29.0) | – |
| Third tertile | 43 (34.7) | 23 (30.3) | – |
| sCD163 (ng/ml, median, IQR) | 666.5 (530.5–910.5) | 583.0 (427.5–805.5) | 0.02c |
| sCD163 (ng/ml, n, %) | 0.05 | ||
| First tertile | 34 (27.4) | 33 (43.4) | – |
| Second tertile | 43 (34.7) | 24 (31.6) | – |
| Third tertile | 47 (37.9) | 19 (25.0) | – |
| IL-6 (pg/ml, median, IQR) | 0.7 (0.5–1.1) | 0.6 (0.4–0.9) | 0.74c |
| HIV (n, %) | 62 (50.0) | 38 (50.0) | 1.0 |
| HIV-Specific Indicators | |||
| Women (n = 62) | Men (n = 38) | p-value | |
| Nadir CD4 (median, IQR) | 141 (75–199) | 148 (65–216) | 0.99b |
| Current protease inhibitor use (n, %) | 16 (25.8) | 3 (8.1) | 0.04 |
| Current abacavir use (n, %) | 2 (3.2) | 4 (10.5) | 0.20 |
Hypertension and diabetes self-reported and confirmed w/clinic records.
p-value via Wilcoxon rank sum.
p-value via natural log transformation.
Rates of smoking were low in both groups. Markers of systemic inflammation were overall higher in women than men. Women were more likely to have a CRP >3 μg/ml (46% of women vs 30% of men, p = 0.02), and more likely to fall into the highest tertile for sCD14 level (35% vs 30%p = 0.04), and sCD163 level (38% vs 25%, p = 0.02). There was no difference in IL-6 level by sex.
The median calculated 10-year ASCVD risk score was significantly higher in men (11.0%, IQR 7.6–19.4%) than in women (5.1%, IQR 3.2–8.7%) and men were more likely to fall into a higher ASCVD risk category (p < 0.01, Table 2). However, there was no difference in the prevalence of detectable coronary artery calcium (CAC>0) by sex (PR 0.77, 95% CI 0.32–1.86, p = 0.56, Table 3).
Table 2.
Comparison of ASCVD 10-year risk categories by sex (N, %) Race = other.
| ASCVD Risk | Female (N = 123) | Male (N = 75) | P-value |
|---|---|---|---|
| <5% | 54 (43.9) | 8 (10.7) | |
| 5–7.5% | 28 (22.8) | 10 (13.3) | |
| 7.5–10% | 18 (14.6) | 15 (20.0) | |
| ≥10% | 23 (18.7) | 42 (56.0) | |
| Total | 123 (100) | 75 (100) | <0.01 |
Table 3.
Adjusted prevalence ratio for coronary artery calcium score > 0 and AUROC for adjusted models.
| Variables | 4A. All |
4B. Women |
4C. Men |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APR | P-value | AUROC | Pseudo-R2 | APR | P-value | AUROC | Pseudo-R2 | APR | P-value | AUROC | Pseudo-R2 | |
| ASCVD only (each 10 pt increase in 10-year risk) | 1.46 | 0.05 | 0.62 | 0.03 | 1.15 | 0.77 | 0.57 | <0.01 | 1.59 | 0.05 | 0.70 | 0.06 |
| ASCVD + HIV | 1.56 | 0.02 | 0.67 | 0.05 | 2.16 | 0.17 | 0.78 | 0.13 | 1.55 | 0.07 | 0.71 | 0.07 |
| ASCVD + scd163 | 1.50 | 0.04 | 0.71 | 0.08 | 1.16 | 0.77 | 0.75 | 0.12 | 1.63 | 0.05 | 0.68 | 0.07 |
In univariable models, 10-year ASCVD risk score was correlated with CAC>0 in men (PR 1.59 for each 10% increase in ASCVD risk, 95% CI 1.00–2.55, p = 0.05), but not in women (PR 1.14, 95% CI 0.44–3.01, p = 0.78, Supplemental Table 2, Fig. 1). HIV was associated with CAC>0 in women (PR 9.0, 95% CI 1.18–68.92, p = 0.03), but not in men (PR 0.60, 95% CI 0.15–2.34, p = 0.46). The AUROC for ASCVD risk score as a predictor of CAC>0 was 0.57 among women and 0.70 among men (Table 3). When the model was adjusted to include HIV, the AUROC improved from 0.57 to 0.78 for women (p = 0.02 for difference between the models, Fig. 2A). There was no significant change in the AUROC for men (adjusted AUROC 0.71, p = 0.71, Fig. 2B). Finally, when the ASCVD risk model was adjusted for sCD163 (a general marker of macrophage activation) rather than HIV, the AUROC improved to 0.75 for women (p = 0.04) but remained similar at 0.68 for men (p = 0.52).
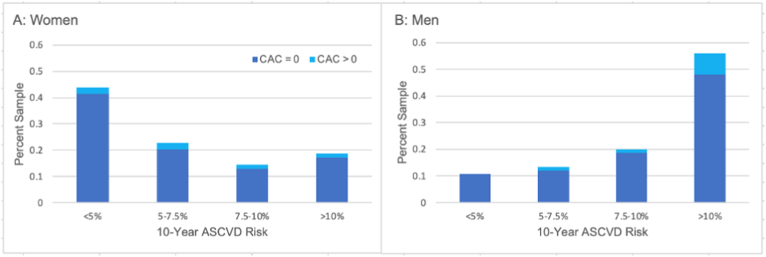
Fig. 1.
Distribution of Coronary Artery Calcium Scores by ASCVD Risk Group. The percentage of the sample with and without detectable coronary artery calcium is displayed by 10-year predicted risk of atherosclerotic cardiovascular disease. Prevalence of detectable coronary artery calcium is not significantly associated with 10-year ASCVD risk in women (1A) but increases with increasing ASCVD risk in men (1B).
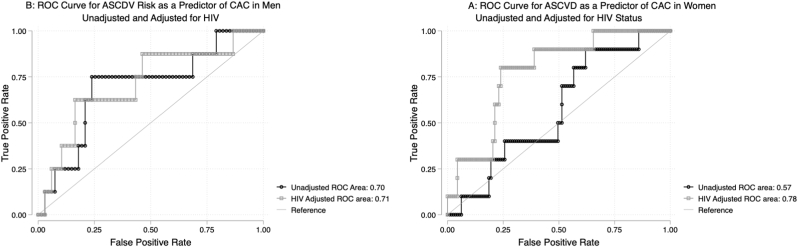
Fig. 2.
ROC Curves for the Predictive Value of ASCVD Risk for CAC>0. Receiver operating characteristic curves are demonstrated for the association between 10-year ASCVD risk as predicted by the pooled cohort equations and the prevalence of coronary artery calcium in women (2A) and men (2B) with and without adjustment for HIV. In women, the AUROC for 10-year ASCVD risk score as a predictor of CAC was only 0.58, indicating a poor correlation, but improved to 0.78 after adjustment for HIV. In men, the unadjusted AUROC was 0.70, indicating moderate correlation and did not change with addition of HIV to the model.
4. Discussion
Among this population of middle-aged adults in Uganda, there was no statistically significant difference in prevalence of CAC by sex. Men had higher predicted ASCVD risk scores using the PCE. Women had a higher prevalence of hypertension, obesity, and elevated LDL cholesterol. Higher ASCVD risk scores were associated with a higher likelihood of detectable CAC among men but not women, and HIV was associated with detectable CAC among women but not men. Including HIV status into regression models improved model performance at predicting CAC for women. Our results suggest that prevalence of subclinical coronary atherosclerosis, as defined by CAC, is reasonably well balanced by sex in this population, but that risk estimation via the PCE does not adequately discriminate risk among women.
4.1. ASCVD risk estimation and CAC
The goal of ASCVD primary prevention is to match the intensity of intervention to an individual's risk of disease [28]. While we are unable to link ASCVD risk to the prospective capture of overt clinical events in this study, a strength of our data is the ability to correlate risk estimation using the PCE with the presence of subclinical disease using CAC.
We found similar predicted levels of ASCVD risk using the PCE as the large US-based CAC Consortium study. In both cohorts, the predicted risk among women was about half the risk of men [29]. In that study, Shaw et al. found that 40% of women and 63% of men had CAC >0. These results were nearly identical to the US-based Multi-Ethnic Study of Atherosclerosis (MESA) results published a decade earlier [30]. However, our cohort demonstrated a much lower prevalence of CAC (∼10%) among both men and women despite similar age and selection for the presence of risk factors [31]. Our findings lend support to those by Wu et al. that risk estimators developed in the United States or Europe may significantly overestimate ASCVD risk in other settings [32]. Additionally, our findings suggest that differential risk for CAC between men and women may not be consistent in all contexts.
4.2. The role of HIV and sex
Due to the recognition of HIV as a significant risk-modifier for ASCVD, an HIV-specific ASCVD risk estimator has been developed [33], but lacks validation in HIV endemic countries [24]. Consequently, it is not known how risk estimators perform among HIV-positive patients in HIV-endemic settings, or how sex interacts with HIV in such populations.
Our study sought to respond to this gap in the literature by including a high proportion of women and PLWH in Uganda. In this study, risk estimation using the PCE demonstrated poor discrimination for CAC among women despite a significant burden of traditional risk factors. Adding either HIV status or soluble CD163 (a marker of inflammation) improved the performance of the risk prediction model for women only, though we are not able to determine how this may translate into clinical cardiovascular events over time.
There are several proposed mechanisms for sex-based differences in the development of cardiovascular disease among PLWH. HIV infection has been associated with early reproductive aging, which may reduce the protective effects of naturally occurring female sex hormones over time and result in relatively higher risk of atherosclerosis [34]. Interestingly, residence in sub-Saharan Africa was associated with a more advanced reproductive aging among women with HIV enrolled in the REPREIVE trial in comparison to other world regions [35]. HIV-associated macrophage activation may additionally drive atherosclerosis among women to a greater degree than men, and this risk may be modified by unmeasured, nontraditional risk factors In Sub-Saharan Africa such as indoor air pollution that increase chronic inflammation [36].
A US-based study previously demonstrated that women with HIV have lower rates of coronary plaque as assessed by coronary CT angiography in compared to men [37]. While these results seem to contrast with our findings, such discrepancies highlight the importance of context-specific risk assessments for sub-Saharan populations. Additional social, cultural and dietary factors may play a significant role, and these associations should be further investigated in subsequent research.
4.3. CAC and clinical outcomes
While this study evaluates CAC as marker of subclinical atherosclerosis, CAC also has a role in future prediction of ASCVD events. Previous work has demonstrated that CAC provides additional information predictive of future adverse events beyond risk estimators alone, particularly for those with intermediate or uncertain risk profiles [38]. While some work has shown that CAC may be more useful for reclassifying ASCVD risk in men [39], Shaw et al. demonstrated that any detectable CAC was associated with a higher risk of cardiovascular mortality among women compared to men, and that this differential risk was magnified in subgroups with multivessel CAC or large lesions [29]. Overall, incorporation of CAC into a risk-prediction model has been shown to mitigate the relative contributions of other demographic factors such as age and sex [40]. CAC therefore remains compelling as a technique to refine risk prediction tools, particularly in regions where the role of traditional demographic risk factors remains uncertain and the ascertainment of primary outcome events, such as stroke and myocardial infarction, poses a clinical challenge.
4.4. Limitations
By nature of the cross-sectional study design and small sample size, we are not able to draw conclusions about the directionality of effects of HIV or CVD risk factors on CAC or assess for interaction with specific HIV parameters such as type of antiretroviral therapy. We are also unable to assess the relationships between HIV, CVD risk factors and the ultimate presentation with hard CVD events such as MI and stroke. Our recruitment of patients through clinical sites may limit generalizability to patients in contact with medical care. Finally, this work was not designed to calibrate the PCE risk-assessment tool for specific risk percentages in this population, either globally or in reference to US-defined race categories.
5. Conclusions
We found a substantive relative difference in 10-year ASCVD risk estimation via the pooled cohort equations by sex among a study population in Uganda enriched for people living with HIV. However, there were no sex-based differences in the presence of coronary artery calcification as detected by CT. Inclusion of HIV improved model performance among women but not men. Additional research is needed to further explore the contributions of sex and HIV status to risk estimation for this population, establish accurate risk prediction tools, and to build mitigation strategies that respond to unique risk profiles.
Author contributions
KJK and CTL conceived the study. CK, GE, IS, BG, MSB performed data collection and interpretation. KJK, MJS and CTL planned the analysis, which was performed by KJK. KJK and MSD prepared the first draft of the manuscript, and all authors contributed to its development and the interpretation of the analysis. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding
This work was supported in part by the National Institutes of Health (K23 HL123341 to CTL and R01 HL141053 to MJS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
CTL has received a research grant from Gilead Sciences and has served on an advisory board for Esperion Therapeutics. MSB has received a research grant from Sanofi, consulting fees from Bayer and speaker fees from Novartis, EMS, NovoNordisk and GE Healthcare. No other authors have financial disclosures.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2022.200136.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gouda H.N., Charlson F., Sorsdahl K., Ahmadzada S., Ferrari A.J., Erskine H., Leung J., Santamauro D., Lund C., Aminde L.N., Mayosi B.M., Kengne A.P., Harris M., Achoki T., Wiysonge C.S., Stein D.J., Whiteford H. Burden of non-communicable diseases in sub-saharan Africa, 1990–2017: results from the global burden of disease study 2017. Lancet Global Health. 2019;7 doi: 10.1016/S2214-109X(19)30374-2. e1375–e1387. [DOI] [PubMed] [Google Scholar]
- 2.Mensah G., Roth G., Sampson U., Moran A., Feigin V., Forouzanfar M., Naghavi M., Murray C. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013: cardiovascular topic. Cardiovasc. J. Afr. 2015;26 doi: 10.5830/CVJA-2015-036. S6–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran A., Forouzanfar M., Sampson U., Chugh S., Feigin V., Mensah G. The epidemiology of cardiovascular diseases in sub-saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog. Cardiovasc. Dis. 2013;56:234–239. doi: 10.1016/j.pcad.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onen C.L. Epidemiology of ischaemic heart disease in sub-Saharan Africa : review article, Cardiovasc. J. Afr. 2013;24:34–42. doi: 10.5830/CVJA-2012-071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gersh B.J., Sliwa K., Mayosi B.M., Yusuf S. Novel therapeutic concepts * the epidemic of cardiovascular disease in the developing world: global implications. Eur. Heart J. 2010;31:642–648. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 6.Keates A.K., Mocumbi A.O., Ntsekhe M., Sliwa K., Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat. Rev. Cardiol. 2017;14:273–293. doi: 10.1038/nrcardio.2017.19. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S., Hawken S., Ôunpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Goff D.C., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R., Greenland P., Lackland D.T., Levy D., O'Donnell C.J., Robinson J.G., Schwartz J.S., Shero S.T., Smith S.C., Sorlie P., Stone N.J., Wilson P.W.F. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American heart association task force on practice guidelines. Circulation. 2013;129 doi: 10.1161/01.cir.0000437741.48606.98. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosepele M., Hemphill L.C., Palai T., Nkele I., Bennett K., Lockman S., Triant V.A. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American heart association (AHA) atherosclerotic cardiovascular disease (ASCVD) risk score among HIV-infected patients in sub-saharan Africa. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muiru A.N., Bibangambah P., Hemphill L., Sentongo R., Kim J.-H., Triant V.A., Bangsberg D.R., Tsai A.C., Martin J.N., Haberer J.E., Boum Y., Plutzky J., Hunt P.W., Okello S., Siedner M.J. Distribution and performance of cardiovascular risk scores in a mixed population of HIV-infected and community-based HIV-uninfected individuals in Uganda. JAIDS J. Acquir. Immune Defic. Syndr. 2018;78:458–464. doi: 10.1097/QAI.0000000000001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaziano T.A., Abrahams-Gessel S., Alam S., Alam D., Ali M., Bloomfield G., Carrillo-Larco R.M., Prabhakaran D., Gutierrez L., Irazola V., Levitt N.S., Miranda J.J., Bernabe-Ortiz A., Pandya A., Rubinstein A., Steyn K., Xavier D., Yan L.L. Comparison of nonblood-based and blood-based total CV risk scores in global populations. Glob. Heart. 2016;11:37. doi: 10.1016/j.gheart.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Kalin M.F., Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids. 1990;55:330–352. doi: 10.1016/0039-128X(90)90058-J. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Connor E. Sex differences in coronary heart disease: why are women so superior? The 1995 ancel keys lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.CIR.95.1.252. [DOI] [PubMed] [Google Scholar]
- 14.D'Agostino R.B., Grundy S., Sullivan L.M., Wilson P. For the CHD risk prediction group, validation of the framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 15.Njelekela M.A., Mpembeni R., Muhihi A., Mligiliche N.L., Spiegelman D., Hertzmark E., Liu E., Finkelstein J.L., Fawzi W.W., Willett W.C., Mtabaji J. Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc. Disord. 2009;9:30. doi: 10.1186/1471-2261-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magodoro I.M., Feng M., North C.M., Vořechovská D., Kraemer J.D., Kakuhikire B., Bangsberg D., Tsai A.C., Siedner M.J. Female sex and cardiovascular disease risk in rural Uganda: a cross-sectional, population-based study. BMC Cardiovasc. Disord. 2019;19:96. doi: 10.1186/s12872-019-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaspers Faijer-Westerink H., Kengne A.P., Meeks K.A.C., Agyemang C. Prevalence of metabolic syndrome in sub-Saharan Africa: a systematic review and meta-analysis. Nutr. Metabol. Cardiovasc. Dis. 2020;30:547–565. doi: 10.1016/j.numecd.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein M.J., Bogorodskaya M., Bloomfield G.S., Vedanthan R., Siedner M.J., Kwan G.F., Longenecker C.T. Cardiovascular complications of HIV in endemic countries. Curr. Cardiol. Rep. 2016;18:113. doi: 10.1007/s11886-016-0794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currier J.S., Taylor A., Boyd F., Dezii C.M., Kawabata H., Burtcel B., Maa J.-F., Hodder S. Coronary heart disease in HIV-infected individuals:, JAIDS. J. Acquir. Immune defic. Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Freiberg M.S., Chang C.-C.H., Kuller L.H., Skanderson M., Lowy E., Kraemer K.L., Butt A.A., Bidwell Goetz M., Leaf D., Oursler K.A., Rimland D., Rodriguez Barradas M., Brown S., Gibert C., McGinnis K., Crothers K., Sico J., Crane H., Warner A., Gottlieb S., Gottdiener J., Tracy R.P., Budoff M., Watson C., Armah K.A., Doebler D., Bryant K., Justice A.C. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah A.S.V., Stelzle D., Lee K.K., Beck E.J., Alam S., Clifford S., Longenecker C.T., Strachan F., Bagchi S., Whiteley W., Rajagopalan S., Kottilil S., Nair H., Newby D.E., McAllister D.A., Mills N.L. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha A., Ma Y., Scherzer R., Hur S., Li D., Ganz P., Deeks S.G., Hsue P.Y. Role of T-cell dysfunction, inflammation, and coagulation in microvascular disease in HIV. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsue P.Y., Waters D.D. HIV infection and coronary heart disease: mechanisms and management. Nat. Rev. Cardiol. 2019;16:745–759. doi: 10.1038/s41569-019-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feinstein M.J., Hsue P.Y., Benjamin L.A., Bloomfield G.S., Currier J.S., Freiberg M.S., Grinspoon S.K., Levin J., Longenecker C.T., Post W.S. Null null, characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American heart association. Circulation. 2019;140 doi: 10.1161/CIR.0000000000000695. e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Post W.S., Budoff M., Kingsley L., Palella F.J., Witt M.D., Li X., George R.T., Brown T.T., Jacobson L.P. Associations between HIV infection and subclinical coronary atherosclerosis. Ann. Intern. Med. 2014;160:458. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filmer D., Pritchett L.H. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 27.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 28.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., Michos E.D., Miedema M.D., Muñoz D., Smith S.C., Virani S.S., Williams K.A., Yeboah J., Ziaeian B. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140 doi: 10.1161/CIR.0000000000000678. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw L.J., Min J.K., Nasir K., Xie J.X., Berman D.S., Miedema M.D., Whelton S.P., Dardari Z.A., Rozanski A., Rumberger J., Bairey Merz C.N., Al-Mallah M.H., Budoff M.J., Blaha M.J. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur. Heart J. 2018;39:3727–3735. doi: 10.1093/eurheartj/ehy534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClelland R.L., Chung H., Detrano R., Post W., Kronmal R.A. Distribution of coronary artery calcium by race, gender, and age: results from the multi-ethnic study of atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 31.Alencherry B., Erem G., Mirembe G., Ssinabulya I., Yun C.-H., Hung C.-L., Siedner M.J., Bittencourt M., Kityo C., McComsey G.A., Longenecker C.T. Coronary artery calcium, HIV and inflammation in Uganda compared with the USA. Open Heart. 2019;6 doi: 10.1136/openhrt-2019-001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y., Liu X., Li X., Li Y., Zhao L., Chen Z., Li Y., Rao X., Zhou B., Detrano R., Liu K. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114:2217–2225. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- 33.Krikke M., Hoogeveen R., Hoepelman A., Visseren F., Arends J. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for The Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-: CVD risk prediction in HIV-infected patients. HIV Med. 2016;17:289–297. doi: 10.1111/hiv.12300. [DOI] [PubMed] [Google Scholar]
- 34.Stone L., Looby S.E., Zanni M.V. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr. Opin. HIV AIDS. 2017;12:585–593. doi: 10.1097/COH.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanni M.V., Currier J.S., Kantor A., Smeaton L., Rivard C., Taron J., Burdo T.H., Badal-Faesen S., Lalloo U.G., Pinto J.A., Samaneka W., Valencia J., Klingman K., Allston-Smith B., Cooper-Arnold K., Desvigne-Nickens P., Lu M.T., Fitch K.V., Hoffman U., Grinspoon S.K., Douglas P.S., Looby S.E. Correlates and timing of reproductive aging transitions in a global cohort of midlife women with human immunodeficiency virus: insights from the REPRIEVE trial. J. Infect. Dis. 2020;222 doi: 10.1093/infdis/jiaa214. S20–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mocumbi A.O., Stewart S., Patel S., Al-Delaimy W.K. Cardiovascular effects of indoor air pollution from solid fuel: relevance to sub-saharan Africa. Curr. Environ. Health Rep. 2019;6:116–126. doi: 10.1007/s40572-019-00234-8. [DOI] [PubMed] [Google Scholar]
- 37.Foldyna B., Fourman L.T., Lu M.T., Mueller M.E., Szilveszter B., Neilan T.G., Ho J.E., Burdo T.H., Lau E.S., Stone L.A., Toribio M., Srinivasa S., Looby S.E., Lo J., Fitch K.V., Zanni M.V. Sex differences in subclinical coronary atherosclerotic plaque among individuals with HIV on antiretroviral therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2018;78:421–428. doi: 10.1097/QAI.0000000000001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Rourke R.A., Brundage B.H., Froelicher V.F., Greenland P., Grundy S.M., Hachamovitch R., Pohost G.M., Shaw L.J., Weintraub W.S., Winters W.L., Forrester J.S., Douglas P.S., Faxon D.P., Fisher J.D., Gregoratos G., Hochman J.S., Hutter A.M., Kaul S., O'Rourke R.A., Weintraub W.S., Winters W.L., Wolk M.J. American College of Cardiology/American heart association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease: committee members. Circulation. 2000;102:126–140. doi: 10.1161/01.CIR.102.1.126. [DOI] [PubMed] [Google Scholar]
- 39.Nakao Y.M., Miyamoto Y., Higashi M., Noguchi T., Ohishi M., Kubota I., Tsutsui H., Kawasaki T., Furukawa Y., Yoshimura M., Morita H., Nishimura K., Kada A., Goto Y., Okamura T., Tei C., Tomoike H., Naito H., Yasuda S. Sex differences in impact of coronary artery calcification to predict coronary artery disease. Heart. 2018;104:1118–1124. doi: 10.1136/heartjnl-2017-312151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClelland R.L., Jorgensen N.W., Budoff M., Blaha M.J., Post W.S., Kronmal R.A., Bild D.E., Shea S., Liu K., Watson K.E., Folsom A.R., Khera A., Ayers C., Mahabadi A.-A., Lehmann N., Jöckel K.-H., Moebus S., Carr J.J., Erbel R., Burke G.L. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors. J. Am. Coll. Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.