Abstract
Background
Activation of leukemia inhibitory factor (LIF) is linked to an immunosuppressive tumor microenvironment (TME), with a strong association between LIF expression and tumor-associated macrophages (TAMs). MSC-1 (AZD0171) is a humanized monoclonal antibody that binds with high affinity to LIF, promoting antitumor inflammation through TAM modulation and cancer stem cell inhibition, slowing tumor growth. In this phase I, first-in-human, open-label, dose-escalation study, MSC-1 monotherapy was assessed in patients with advanced, unresectable solid tumors.
Materials and methods
Using accelerated-titration dose escalation followed by a 3 + 3 design, MSC-1 doses of 75-1500 mg were administered intravenously every 3 weeks (Q3W) until progression or unmanageable toxicity. Additional patients were enrolled in selected cohorts to further evaluate safety, pharmacokinetics (PK), and pharmacodynamics after escalation to the next dose had been approved. The primary objective was characterizing safety and determining the recommended phase II dose (RP2D). Evaluating antitumor activity and progression-free survival (PFS) by RECIST v1.1, PK and immunogenicity were secondary objectives. Exploratory objectives included pharmacodynamic effects on circulating LIF and TME immune markers.
Results
Forty-one patients received treatment. MSC-1 monotherapy was safe and well tolerated at all doses, with no dose-limiting toxicities. The maximum tolerated dose was not reached and the RP2D was determined to be 1500 mg Q3W. Almost half of the patients had treatment-related adverse events (TRAEs), with no apparent trends across doses; no patients withdrew due to TRAEs. There were no objective responses; 23.7% had stable disease for ≥2 consecutive tumor assessments. Median PFS was 5.9 weeks; 23.7% had PFS >16 weeks. On-treatment changes in circulating LIF and TME signal transducers and activators of transcription 3 signaling, M1:M2 macrophage populations, and CD8+ T-cell infiltration were consistent with the hypothesized mechanism of action.
Conclusions
MSC-1 was very well tolerated across doses, with prolonged PFS in some patients. Biomarker and preclinical data suggest potential synergy with checkpoint inhibitors.
Key words: leukemia inhibitory factor, monoclonal antibody, solid tumors, STAT3, safety
Highlights
-
•
The anti-LIF antibody MSC-1 was safe and well tolerated in the first-in-human trial in patients with advanced solid tumors.
-
•
No patient had an objective response but 23.7% of patients had PFS exceeding 16 weeks.
-
•
Tumor biomarker changes reflect the hypothesized mechanism of action and suggest a potential role in combination regimens.
Introduction
Leukemia inhibitory factor (LIF), a multifunctional member of the interleukin-6 (IL-6) family of cytokines, is a novel target in oncology. LIF is involved in many physiological and pathological processes,1, 2, 3, 4, 5, 6, 7, 8, 9 and LIF expression correlates with poor prognosis in multiple tumor types, including pancreatic cancer,10, 11, 12 glioblastoma multiforme,13,14 colorectal cancer,15 breast cancer,16 lung cancer,14,17 head and neck cancer,17 and nasopharyngeal carcinoma.18 However, the specific role of LIF in tumors has not yet been fully elucidated.
LIF signals through the heterodimeric glycoprotein 130 (gp130)/LIF receptor (LIFR) complex at the cell surface.19, 20, 21 Binding and activation of LIFR triggers the recruitment of gp130, leading to phosphorylation of signal transducers and activators of transcription 3 (pSTAT3) by Janus kinase (JAK) and induction of downstream signaling pathways that regulate proliferation and survival, i.e. JAK/STAT3, phosphoinositide 3-kinase/protein kinase B, and mitogen-activated protein kinase.19,22 LIF is hypothesized to drive disease progression by acting on multiple aspects of cancer biology involved in tumor growth (through activation of tumor stemness and epithelial-to-mesenchymal transition), metastasis, and resistance to anticancer therapy (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.10053011, 12, 13, 14). Evidence supports the concept that LIF promotes immunosuppressive polarization of human macrophages, which blunts the infiltration and functionality of CD8+ T-effector cells and impairs the effect of programmed cell death-1 (PD-1) inhibitors.14 In preclinical studies, activating LIF has been linked to an immunosuppressive tumor microenvironment (TME), with a strong association between LIF expression and M2 tumor-associated macrophages (TAMs), which comprise a major fraction of tumor-infiltrating myeloid cells within the TME.14 In contrast, LIF was significantly less correlated with M1 macrophages, which are immunostimulatory.23
MSC-1 (AZD0171) is a first-in-class, humanized immunoglobulin G subclass 1 monoclonal antibody that binds with high affinity to LIF.24 It is a potent and specific LIF antagonist, which inhibits LIF signaling by binding to an epitope that overlaps with the gp130 receptor binding site on LIF.25,26 MSC-1 potently inhibits LIF-induced pSTAT3 signaling in vitro and in vivo.24 Preclinical evidence suggests that it promotes antitumor inflammation through TAM modulation, which induces CD8+ T-cell activity and improves response to PD-1 inhibitors.14,24,26
The activity of MSC-1 monotherapy and in combination with a PD-L1 inhibitor has been demonstrated in preclinical studies, using the CT26 and MC38 syngeneic mouse tumor models.24,26 MSC-1 monotherapy decreased tumor growth and drove reprogramming of the TME from an immunosuppressive M2-like state to an immunostimulatory M1-like state, while decreasing regulatory T cells and increasing T-effector cells and natural killer cells.24, 25, 26 Combination therapy with MSC-1 and a PD-1 inhibitor induced durable slowing of tumor growth more than PD-1 inhibitor monotherapy in these tumor models, suggesting a synergistic mechanism of action.26 It is hypothesized that in patients with advanced solid tumors, MSC-1 could effectively block LIF signaling, activate immune-mediated antitumor effects, and inhibit cancer stem cells.
Here, we report the results of the dose-escalation part of a first-in-human, phase I study (NCT03490669), which aimed to determine the recommended phase II dose (RP2D), safety and tolerability, pharmacokinetics and pharmacodynamics (PK/PD), and clinical activity of MSC-1 as monotherapy in patients with advanced solid tumors.
Materials and methods
Study design and participants
This open-label study enrolled patients aged 18 years or older with histologically or cytologically proven advanced, unresectable solid tumors for which there was no curative therapy and who had progressed on standard-of-care (SoC) treatment, or were intolerant to or had no available SoC, or found SoC unacceptable. Patients had to have documented progression on or following the last line of therapy; measurable disease as per Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST v1.1), documented by computed tomography (CT) and/or magnetic resonance imaging (MRI); an archival tumor sample for LIF expression analysis; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; weight ≥37.5 kg; adequate organ function; life expectancy ≥12 weeks; and resolution of all acute, reversible toxic effects of prior therapy of surgical procedure to grade ≤1 (except alopecia and peripheral neuropathy, to grade ≤2).
The key exclusion criteria were symptomatic or unstable central nervous system primary tumor or metastases and/or carcinomatous meningitis; previous or concurrent malignancy that could affect compliance with protocol or interpretation of results; prior systemic therapy within 4 weeks of study entry or 5 half-lives before study entry, whichever was shorter; radiation therapy or significant surgery within 21 days of study entry; ascites or pleural effusion requiring large-volume para- or pleurocentesis within 4 weeks of study entry; history of congenital or acquired immunodeficiency syndrome, or current use of immunosuppressive therapy; grade 3/4 peripheral neuropathy [as per Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v4.03)]; and therapeutic anticoagulation for a thromboembolic event (prophylactic anticoagulation was allowed).
The study was initiated with an accelerated-titration dose-escalation scheme, which was completed after enrollment of three patients (two at the 75-mg dose level and one at the 225-mg dose level). The study then proceeded according to a classic 3 + 3 design, with enrollment of three patients per cohort and expansion to six patients in the event of a dose-limiting toxicity (DLT). If ≥2 out of 6 patients experienced a DLT, the previous dose was to be expanded to further evaluate safety (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100530). In cohorts 3-5 [750-1500 mg once every 3 weeks (Q3W)], additional patients were enrolled to further evaluate safety, PK, and PD after the initial patients had completed the 21-day DLT period and the Data Review Committee (DRC) had decided to escalate to the next cohort level. Additional enrollment in each of these cohorts could not exceed 12 patients.
Dosage and administration of study treatment
MSC-1 was administered as a 60-min intravenous (i.v.) infusion. The five planned dose levels were 75, 225, 750, 1125, and 1500 mg Q3W. The starting dose and frequency of administration were based on preclinical data, including cynomolgus monkey PK data, which were allometrically scaled to calculate human PK parameters. Flat dosing was chosen based on recent evidence showing no major difference in PK variability between weight-based and flat dosing and the relative safety of the molecule in preclinical research. Intrapatient dose escalation to successively higher dose levels was allowed, upon consultation with the medical monitor and only to dose levels cleared by the DRC for safety and tolerability. Treatment continued until disease progression or unacceptable adverse events (AEs).
Study objectives
The primary objectives were to evaluate the safety and tolerability of MSC-1 in patients with advanced solid tumors, and determine the RP2D for MSC-1 monotherapy. The key secondary objectives were to characterize the PK and immunogenicity of MSC-1, and assess its efficacy, including objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS) by RECIST v1.1. The key exploratory objectives were to assess the relationships of PK, PD, and MSC-1 exposure to patient safety and antitumor activity, and characterize the PD effects of MSC-1 in blood as well as in the TME using analysis of pre-treatment and on-treatment biopsies.
Study assessments
AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA) 20.1. A DLT was defined as a grade 3 AE as per CTCAE v4.03 within 21 days of starting treatment that was considered at least possibly related to MSC-1 treatment, based upon the determination of the investigator (and agreed upon at the subsequent safety/dose-escalation meeting with the DRC). Self-limited grade 3 AEs could be deemed non-DLTs in the case of fatigue, nausea, vomiting, or diarrhea that resolved to grade ≤2 within 72 h with appropriate medical therapy; transient (lasting <72 h) grade 3 biochemical abnormalities that were considered clinically insignificant; grade 3 neutropenia that lasted <72 h; or grade 3 thrombocytopenia without clinically significant bleeding. Any treatment-related grade 4 or 5 toxicity was considered a DLT, and MSC-1 treatment was permanently discontinued. A treatment-related AE (TRAE) of any grade that delayed the start of cycle 2 day 1 dosing by >14 days may also have been considered a DLT, upon agreement with the DRC.
The maximum tolerated dose (MTD) was defined as the dose level below the level associated with DLTs in two out of three patients (or two out of six patients). The RP2D was the dose chosen by the DRC for use in the expansion cohorts and could be identical to the MTD or a lower dose than the MTD. The planned expansion phase of the study was cancelled due to the robustness of the escalation phase results.
Clinical activity was assessed by RECIST v1.1, with tumor measurements carried out at prespecified time points. CT/MRI scans of the chest, abdomen, and pelvis were carried out to assess disease status at screening, every 6 weeks from cycle 1 day 1 independent of cycle length, whenever disease progression was suspected, and at the end of treatment. For patients continuing on study after 6 months, scans were carried out every 12 weeks. Tumor imaging was continued on this calendar schedule regardless of any delays in dosing. Confirmatory assessments were required for complete response (CR) and partial response (PR).
Blood samples were collected at multiple time points to determine PK parameters, incidence, and titer of antidrug antibodies (ADAs) and circulating total and free LIF levels. Additional details are provided in the Supplementary Methods, available at https://doi.org/10.1016/j.esmoop.2022.100530.
Statistical analysis
The safety population consisted of all patients who received at least one dose of MSC-1. The PK population consisted of all patients who received at least one dose of MSC-1 and had evaluable PK data. The efficacy population consisted of all patients who had an evaluable screening or baseline assessment and at least one post-treatment tumor assessment.
The number of patients enrolled depended on the observed safety and PK profile and the number of dose escalations required to define the RP2D. Approximately 14-48 patients were anticipated (five dose levels). Additional patients could replace patients who withdrew early for progressive disease or other reasons unrelated to toxicity. Given the exploratory nature of the dose-escalation phase, the sample size was not based on power calculations.
For the efficacy analysis, ORR was defined as the percentage of patients with a confirmed CR or PR as defined by RECIST v1.1, and calculated along with its 95% confidence interval (CI), determined by the Clopper–Pearson method. The best overall response (BOR) used overall response at each time point and established the best one for each patient. DCR was defined as the percentage of patients with a confirmed CR, PR, or stable disease for ≥2 consecutive tumor assessments, and calculated along with its 95% CI, determined by the Clopper–Pearson method. PFS was defined as the time from the first dose of MSC-1 to the first documentation of objective tumor progression or death due to any cause. The median and range of PFS were estimated from the Kaplan-Meier survival curve and summarized by cohort.
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethical considerations
All patients provided written, informed consent to their participation in the study. The study protocol was approved by the institutional review board for each participating center, and the study was run in accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines on Good Clinical Practice, as well as any applicable local laws and requirements.
Results
Demographic and baseline characteristics
The study was conducted from 21 May 2018 to 23 September 2019. The data extract date was 16 October 2019. Forty-one patients were enrolled and treated. Demographic and baseline characteristics are summarized in Table 1. Median age was 64 years (range, 36-78 years). The majority of patients were white (85.4%) and had an ECOG PS of 1 (80.5%).
Table 1.
Demographic and baseline characteristics in the safety population
| Characteristic | Overall population (n = 41) |
|---|---|
| Age, years | |
| Median (min, max) | 64.0 (36, 78) |
| Sex, n (%) | |
| Female | 21 (51.2) |
| Male | 20 (48.8) |
| Race, n (%) | |
| Asian | 2 (4.9) |
| Black or African-American | 4 (9.8) |
| White | 35 (85.4) |
| ECOG performance status, n (%) | |
| 0 | 8 (19.5) |
| 1 | 33 (80.5) |
| Primary cancer diagnosis, n (%)a | |
| Colorectal cancer | 5 (12.2) |
| Head and neck cancer | 4 (9.8) |
| Melanoma | 1 (2.4) |
| Non-small-cell lung cancer | 2 (4.9) |
| Ovarian cancer | 4 (9.8) |
| Pancreatic adenocarcinoma | 13 (31.7) |
| Prostate cancer | 3 (7.3) |
| Othera | 9 (22.0) |
| Stage IV at study entry, n (%) | 41 (100) |
| Time from diagnosis to first dose of study treatment, months | |
| Median (min, max) | 30.6 (6.0, 179.4) |
| Prior anticancer treatments received, n (%) | |
| Prior surgery | 34 (82.9) |
| Prior radiotherapy | 22 (53.7) |
| Prior lines of anticancer therapy received, n (%) | |
| 1-2 | 9 (22.0) |
| 3-4 | 20 (48.8) |
| ≥5 | 12 (29.3) |
| Setting of prior anticancer therapy, n (%)b | |
| Neoadjuvant | 7 (17.1) |
| Adjuvant | 15 (36.6) |
| Palliative | 10 (24.4) |
| Metastatic | 32 (78.0) |
| Maintenancec | 3 (7.3) |
| Time from last systemic anticancer therapy, months | |
| Median (min, max) | 1.4 (0.7, 37.4) |
ECOG, Eastern Cooperative Oncology Group.
Other includes appendiceal adenocarcinoma (n = 2), cholangiocarcinoma (n = 2), fallopian tube carcinoma (n = 1), myxoid liposarcoma (n = 1), retroperitoneal paraganglioma (n = 1), squamous cell carcinoma of the anus (n = 1), and uterine sarcoma (n = 1).
Multiple responses could be selected for therapy setting.
Maintenance of stable disease.
The most common tumor types were pancreatic adenocarcinoma (31.7%), colorectal carcinoma (12.2%), head and neck cancer (9.8%), ovarian cancer (9.8%), and prostate cancer (7.3%). Information on microsatellite stability was not collected for patients with colorectal carcinoma. At the time of entry into the study, all patients had stage IV disease, as defined by the tumor–node–metastasis (TNM) classification.
Patients were heavily pre-treated: 48.8% had received three to four prior lines of anticancer therapy in the metastatic/palliative setting and 29.3% had received five or more prior lines of therapy. In total, 17.1% had previously received nivolumab, 7.3% had received ipilimumab, 4.9% each had received atezolizumab, durvalumab, or pembrolizumab, and 2.4% had received tremelimumab. The proportion of patients with primary refractoriness versus acquired resistance to immunotherapy was not analyzed.
Patient disposition
Treatment was discontinued for all patients due to radiographic disease progression (35 patients), clinical disease progression (4 patients), withdrawal of consent (1 patient), or death (1 patient; not treatment-related).
Safety
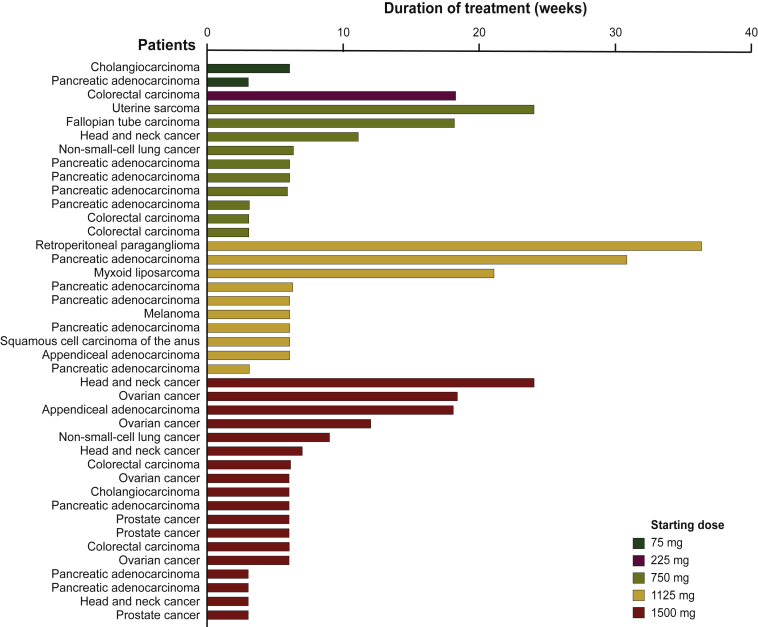
Median duration of exposure was 6.0 weeks (range, 3.0-36.4 weeks), with a median number of treatment cycles of 2 (range, 1-12) (Figure 1). No patients had a dose modification due to a TRAE. Six patients had dose escalations, as permitted by protocol. All six patients were escalated into the next cohort, including one originally assigned to cohort 2 (225 mg), two originally assigned to cohort 3 (750 mg), and three originally assigned to cohort 4 (1125 mg).
Figure 1.
Duration of exposure in the safety population. Duration of exposure (weeks) of MSC-1 is defined as: [(date of the last infusion - date of the first infusion) + 21 days]/7.
MSC-1 was safe and well tolerated. All patients experienced all-cause AEs and 56.1% of patients experienced grade ≥3 AEs (Table 2). Fatigue, decreased appetite, and back pain were the most common all-cause AEs (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100530). Overall, 46.3% of patients had TRAEs; all were grade 1/2 except for one grade 3 TRAE in the 75 mg cohort (aspartate aminotransferase increase). There was no trend across doses in the number of patients experiencing TRAEs. The most frequently reported TRAEs were fatigue (19.5%) and nausea (9.8%), which were all grade 1/2 (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100530). No patients withdrew from the study due to a TRAE, and there were no treatment-related deaths.
Table 2.
Summary of adverse events in the safety population
| Adverse events, n (%) | MSC-1 Q3W |
Overall (n = 41) | ||||
|---|---|---|---|---|---|---|
| 75 mg (n = 2) | 225 mg (n = 1) | 750 mg (n = 10) | 1125 mg (n = 10) | 1500 mg (n = 18) | ||
| Overall (all-cause) Grade ≥3 |
2 (100) 2 (100) |
1 (100) — |
10 (100) 7 (70.0) |
10 (100) 4 (40.0) |
18 (100) 10 (55.6) |
41 (100) 23 (56.1) |
| TRAEs Grade ≥3 |
1 (50.0) 1 (50.0) |
1 (100) — |
4 (40.0) — |
3 (30.0) — |
10 (55.6) — |
19 (46.3) 1 (2.4) |
| Serious AEs | 2 (100) | – | 7 (70.0) | 1 (10.0) | 9 (50.0) | 19 (46.3) |
| Serious TRAEs | — | — | — | — | 1 (5.6) | 1 (2.4) |
| Fatal AEsa Fatal TRAEs |
1 (50.0) — |
— — |
1 (10.0) — |
— — |
3 (16.7) — |
5 (12.2) — |
| DLTs | — | — | — | — | — | — |
| Infusion-related AEs | — | 1 (100) | 1 (10.0) | — | — | 2 (4.9) |
| Patients whose MSC-1 treatment was: | ||||||
| Delayed due to AE | — | — | 1 (10.0) | — | — | 1 (2.4) |
| Interrupted due to AE | — | — | 1 (10.0) | — | 1 (5.6) | 2 (4.9) |
| Withdrawn due to AE | — | — | — | — | — | — |
AE, adverse event; Q3W, once every 3 weeks; TRAE, treatment-related adverse event.
Four fatal AEs were due to disease progression: one multi-organ failure in a patient with metastatic pancreatic cancer; one cardiac arrest in a patient with metastatic non-small-cell lung cancer; one case of hepatic insufficiency due to progressive pancreatic cancer; and one case of metastatic non-small-cell lung cancer with respiratory failure. Additionally, there was one sudden death not otherwise specified, considered unrelated to study treatment.
In total, 46.3% of patients experienced at least one serious AE; five of these were fatal (Table 2). One serious AE (osteonecrosis of the mandible) in a patient with head and neck cancer (adenoid cystic carcinoma) was considered to be possibly treatment-related. The lesion was observed at the site of prior high-dose external beam radiotherapy and the patient had a history of prior receptor activator of nuclear factor κ-Β ligand (RANKL) inhibitor therapy and periodontal disease.
No patient experienced a DLT, and the MTD was not reached. The RP2D was determined to be 1500 mg Q3W.
Efficacy
Clinical activity was evaluable in 38 patients. None had a CR or PR. The BOR was stable disease for ≥2 consecutive tumor assessments for 9 (23.7%) patients, and stable disease followed by progressive disease in 4 (10.5%) patients; 25 (65.8%) patients had progressive disease. The DCR was therefore 23.7% (95% CI 11.4% to 40.2%). The overall median PFS was 5.9 weeks (95% CI 2.1 to 28.3 weeks), with nine patients having a PFS of >16 weeks.
One patient with advanced pancreatic cancer and four prior lines of therapy (all with shorter duration than time on MSC-1) remained on treatment for 28 weeks, with a decrease of ∼40% in the sum of the longest diameter of target lesions from baseline to last scan, but progression in a non-target abdominal lesion (meeting the definition of progressive disease). A reduction in carbohydrate antigen 19-9 levels (from 1752 U/ml at baseline to 1069 U/ml at cycle 3, and 1530 U/ml at the end of treatment) was also seen (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100530), with improvement in symptom control.
Pharmacokinetics
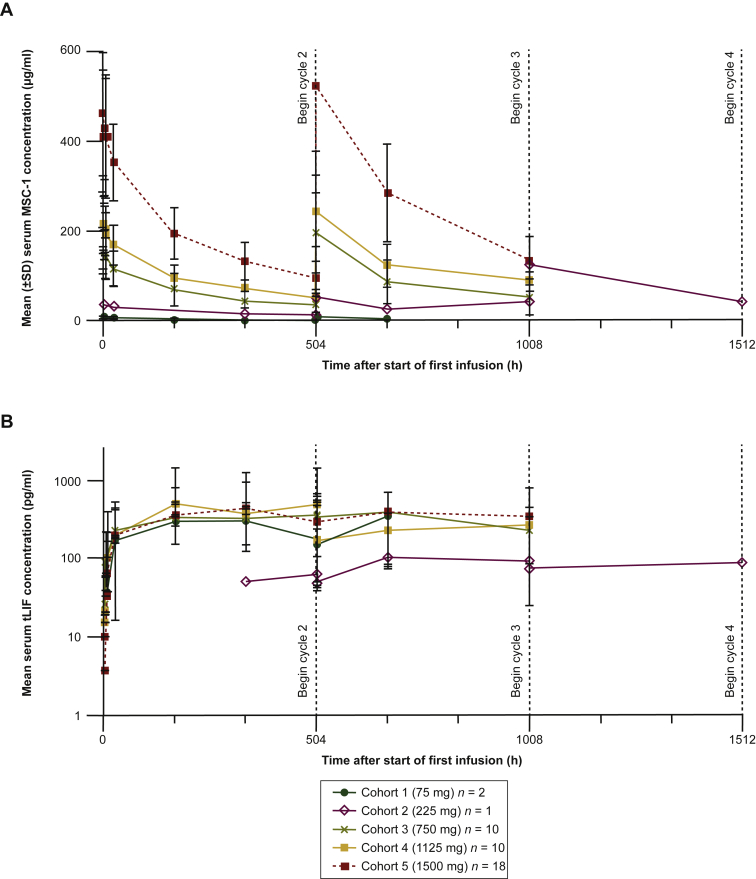
All 41 patients received at least one infusion of MSC-1 and were included in the PK evaluation. For nearly all patients, maximum MSC-1 concentration (Cmax) was reached within 4 h of the start of infusion (3 h after the end of the infusion). Afterward, serum concentrations declined in a generally biphasic manner. Non-compartmental PK parameters were calculated with the PK data from the first treatment cycle. At the lowest dose (75 mg Q3W), the geometric mean of estimated terminal half-life (t½) was 168 h, or 7.0 days. At higher doses, t½ values were relatively consistent, at ∼13 days. MSC-1 exposure parameters were near dose-proportional at doses from 225 mg to 1500 mg Q3W (Figure 2A). While total clearance (CL) was highest at the lowest dose, it was relatively stable at 11.9-16.6 ml/h (0.29-0.40 l/day) at higher doses. Along with near dose-proportional PK, this CL range is consistent with linear elimination characteristics for a monoclonal antibody, suggesting that at doses higher than 75 mg Q3W, MSC-1 is either not cleared by target-mediated drug disposition (TMDD) or the TMDD CL mechanism has been saturated. The volume of distribution at steady state (Vss) at doses above 75 mg Q3W was 5-7 l, which is typical of a monoclonal antibody. Serum trough (pre-dose) concentrations increased through the first three treatment cycles. Although data were limited after these cycles, visual inspection of trough concentrations suggested that MSC-1 steady state was attained by cycle 4, and that there was an ∼1.6-fold increase in MSC-1 concentrations at steady state compared with the first administration of MSC-1.
Figure 2.
Summary of PK and total LIF concentrations by cohort. (A) Geometric mean ± SD of serum MSC-1 concentration–time profiles and (B) mean ± SD of serum total LIF concentration–time profiles following Q3W i.v. infusion of MSC-1 in patients with advanced solid tumors.
i.v., intravenous; LIF, leukemia inhibitory factor; PK, pharmacokinetics; Q3W, once every 3 weeks; SD, standard deviation; tLIF, total LIF.
Pharmacodynamics
Circulating total LIF concentrations were evaluated as a measure of target engagement (i.e. LIF stabilization), based on the observation that for many cytokines, the half-life in circulation is increased once the cytokine is bound to the antibody.27 Free LIF and total LIF were measured in all patients. Free LIF concentrations were only measured in pre-dose samples and could only be detected in 4 out of 41 patients (2 patients dosed at 750 mg, 1 dosed at 1125 mg, and 1 dosed at 1500 mg), and the concentrations were all below 9 pg/ml. Total LIF concentrations (which measured both free LIF and antibody-bound LIF) could be quantitated in all patients post-dose. As expected for an antibody-bound cytokine,27 total LIF concentrations generally increased to a relatively stable level for each patient by the end of the second treatment cycle (Figure 2B), although cycle-to-cycle total LIF measurements were highly variable for most patients. Interpatient variability in total LIF levels was also high, as expected based on the heterogeneity in LIF expression previously observed across and within tumor types.14 Despite the increase in total LIF concentration, free LIF concentration is expected to decrease with treatment. The increase in total LIF concentration was probably due mostly to antibody-bound LIF, which is inactive. In this study, however, free LIF concentrations were non-detectable pre-treatment, so the assay would not have detected further decrease and therefore, post-treatment samples were not measured. Of note, free MSC-1 concentrations (in the PK assay) exceeded total LIF concentrations (PD assay) by 5-6 log.
Immunogenicity
MSC-1 had a benign immunogenicity profile. Three patients had pre-existing ADAs in the 1125 mg cohort, with titers ≤2. In one patient in that cohort, the pre-existing ADAs were treatment-boosted; the titer for the end-of-treatment sample had a low value of 4. One patient dosed with 1500 mg Q3W had treatment-induced ADAs, with a titer of <2.
Tumor biomarker analysis
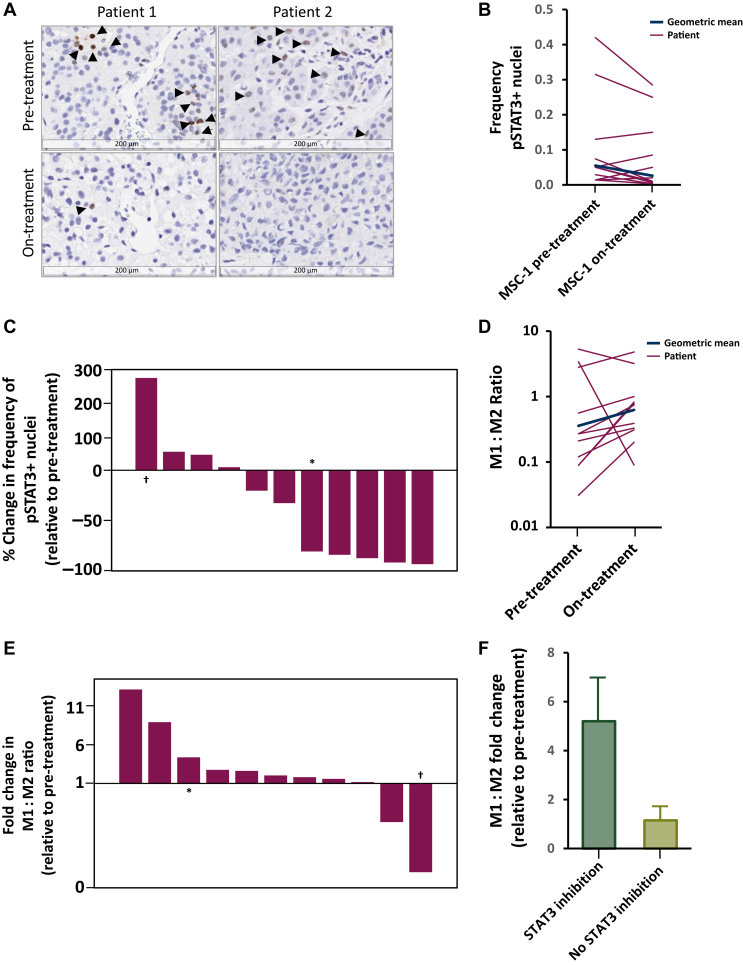
Paired biopsies were analyzed for 11 patients who had received MSC-1 doses of 750-1500 mg (Figure 3A). The pairs were examined for changes in STAT3 signaling, the M1:M2 macrophage ratio, and CD8+ T-cell infiltration into tumors. As expected, highly variable levels of pSTAT3 positivity were observed at baseline. Overall, 7/11 patients showed inhibition of STAT3 signaling relative to the pre-treatment biopsy (Figure 3B), with 5/11 patients showing >75% inhibition (Figure 3C). Individual patient data are presented in Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100530.
Figure 3.
STAT3 and macrophage polarization biomarker analysis of paired pre-/on-treatment biopsies from matched metastatic lesions of 11 patients. (A) Representative images of biopsies with IHC staining for phosphorylated STAT3 (black arrows: pSTAT3+ nuclei). (B) pSTAT3+ nuclei frequencies in pre-/on-treatment samples for each patient (log-scale). (C) Percentage change in the frequency of pSTAT3+ nuclei normalized to the pre-treatment frequency for all patients, ordered by change. (D) Measured M1(MHCII+/CD68+):M2(CD163 or CD206) ratio in pre-/on-treatment samples for each patient (log-scale). (E) Fold change in the M1:M2 ratio between pre- and on-treatment biopsies for all patients. (F) M1:M2 ratio fold change between pre- and on-treatment samples for samples stratified on the basis of an observed pSTAT3+ nuclei decrease.
∗Archival/on-treatment pair.
†Pre-/on-treatment pair from non-matched metastatic lesions.
The relative frequencies of immunostimulatory macrophage phenotypes (M1, CD68+/MHCII+) were increased compared to immunosuppressive types (M2, CD163+ or CD206+) following treatment in the majority of patients (Figure 3D). The M1:M2 ratio increased in 9/11 patients in the on-treatment samples relative to baseline (Figure 3E). Samples showing decreased levels of STAT3 phosphorylation also showed greater M1:M2 ratio increases relative to baseline (Figure 3F). The dose relationships of M1:M2 skewing and STAT3 inhibition were not analyzed, as on-treatment biopsies were collected only at higher doses. Individual patient data are presented in Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100530.
Levels of CD8+ T-cell infiltration were highly variable at baseline, with the mean cellular frequency being <2%. Increased infiltration was observed in a subset of patients, with the largest change being from 12% at baseline to 46% in the on-treatment sample. Data are presented in Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2022.100530. The small number of patients with stable disease precluded any analysis of relationships to these biomarkers.
Discussion
In the dose-escalation phase of this first-in-human, phase I study, the LIF antagonist MSC-1 as monotherapy was safe and very well tolerated at doses of 75, 225, 750, 1125, and 1500 mg in patients with advanced solid tumors. No DLTs were detected. The MTD was not reached and the RP2D was determined to be 1500 mg Q3W, based on safety, PK, stabilization and accumulation of total LIF, and evidence of STAT3 pathway inhibition and M1:M2 macrophage skewing. MSC-1 showed a dose-proportional exposure increase at doses between 225 and 1500 mg, with a half-life of around 13 days, and relatively stable CL between 11.9 and 16.6 ml/h; Vss at this dose range was 5-7 l. Both CL and Vss values suggest that MSC-1 had a typical PK profile for a monoclonal antibody.
Almost half of the patients had TRAEs (mostly grade 1/2), with no apparent trends across doses. The most frequently reported TRAEs were fatigue and nausea, and no patients withdrew from the study due to a TRAE. No patients had liver function tests that met Hy’s Law criteria, despite increases in hepatic liver enzyme and bilirubin levels. One patient had a serious AE, osteonecrosis of the jaw, that was possibly treatment-related. The patient had adenoid cystic carcinoma that had been previously treated with a RANKL inhibitor, and a history of periodontal disease. The osteonecrosis lesion was observed at the site of prior high-dose external beam radiotherapy. None of the fatal serious AEs were considered to be treatment-related. MSC-1 had a benign immunogenicity profile, with only two patients showing positive and very low ADA titers over the course of treatment.
Some evidence of clinical activity was observed, with 23.7% of patients having stable disease for ≥2 consecutive tumor assessments. The proportion of patients whose PFS exceeded 16 weeks was also 23.7%. Although antitumor activity was limited, biomarker data from paired biopsies showed on-treatment changes in the TME that were consistent with the hypothesized mechanism of action of MSC-1. High levels of peripheral LIF target engagement after MSC-1 treatment were detected in all patients by the end of the second treatment cycle. Mechanistic changes were observed in tumor PD biomarkers, including inhibition of LIF signaling (reduction in pSTAT3), increased CD8+ T-cell infiltration, and M1:M2 macrophage skewing in several patients, favoring immunostimulatory over immunosuppressive TAM populations. Overall, these data support further development of MSC-1 in solid tumors, particularly in combination with chemotherapy and immune checkpoint inhibitors. MSC-1 has been shown to decrease tumor growth,25,26 which could provide synergy if it were used following chemotherapy/radiotherapy or surgery, to prevent regrowth. Furthermore, as LIF expression in human cancer samples has been associated with poor patient outcomes and resistance to immune checkpoint therapy,24,28,29 combining MSC-1 with immune checkpoint inhibitors is hypothesized to enable antitumor inflammation by modulating TAMs to promote CD8+ T-cell activity.14 This is supported by preclinical studies in mouse tumor models.14,26
Conclusions
In this phase I study, MSC-1 was shown to be safe and well tolerated in patients with advanced solid tumors. Although antitumor activity was limited, stabilization of total circulating LIF concentrations and biomarker evidence of immunological reprogramming in the TME support the therapeutic hypothesis for MSC-1. Based on the promising activity and corresponding TME changes in patients with previously treated pancreatic ductal adenocarcinoma (PDAC), a phase II trial of first-line MSC-1 (AZD0171) plus the programmed death-ligand 1 inhibitor durvalumab and chemotherapy has been initiated in patients with metastatic PDAC and CD8+ T-cell infiltration (NCT04999969).29
Acknowledgements
We thank Dr Sergio Santillana for his support and stimulating discussion during study and for critically reviewing the manuscript, and AstraZeneca personnel for reviewing the manuscript.
Funding
This work was supported by Northern Biologics (no grant number). Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Carole Mongin-Bulewski, PhD, of Ashfield MedComms (Manchester, UK), an Ashfield Health company, and was funded by AstraZeneca (no grant number).
Disclosure
EB has provided advisory work for BioNTech SE and Imaging Endpoints LLC, and has participated in a speaker’s bureau for Ipsen. AMS has received fees and/or research grants from AstraZeneca, Northern Biologics, Merus, Kura Oncology, Surface Oncology, Lilly Oncology, Pfizer, Black Diamond Therapeutics, BeiGene, and Relay Therapeutics. EG has received personal fees for a consultant/advisory role from Janssen, Seattle Genetics, TFS HealthScience, Alkermes, Thermo Fisher Scientific, Bristol Myers Squibb, and MSD; and research grants from Menarini Diagnostics and Glycotope Biotechnology GmbH. IB has received research funding from Northern Biologics, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, MSD, Novartis, OrionPharma, Regeneron, Seattle Genetics, Shattuck Labs, and VCN Biosciences SL; institutional grants from Cellex Foundation, La Caixa Foundation, and Banco Bilbao Vizcaya Argentaria Foundation (BBVA Foundation); educational grants from Bristol Myers Squibb; consulting fees from Achilles Therapeutics PLC, Bristol Myers Squibb, Cancer Expert Now, Etherna Immunotherapies Nv, Merck Serono, MSD, and Rakuten; payment of honoraria from Bristol Myers Squibb, Merck Serono, and MSD; support from attending meetings and/or travel from Merck Serono and MSD; and has a leadership or fiduciary role in another board, society, committee, or advocacy group, paid or unpaid, with ESMO Head and Neck track, EORTC Head and Neck group, and Cancer Core Europe Clinical Taskforce. MVV has received personal fees from Roche outside the work submitted. AS has received research funding from AstraZeneca, Northern Biologics, Alkermes, Array BioPharma/Pfizer, Bayer, Bristol Myers Squibb, GlaxoSmithKline, Janssen Oncology/Johnson & Johnson, Merck, Novartis, Regeneron Pharmaceuticals, Roche, Surface Oncology, Symphogen, Treadwell Therapeutics, and Oncorus; and personal fees from Bristol Myers Squibb, Merck, and Oncorus. MO has received personal fees from Bristol Myers Squibb, Merck, and MSD; non-financial support from Bristol Myers Squibb and MSD; and research grants from the Spanish Society of Medical Oncology, CRIS Contra el Cancer Foundation, and ASO Conquer Cancer Foundation, all outside of the work submitted. NJL has received honoraria for advisory boards from Innovent Biologics; and research funding from AstraZeneca, Northern Biologics, Alpine Immune Sciences, ALX Oncology, Apexian Pharmaceuticals, Ascentage Pharma, Alexion Pharmaceuticals, Asana, BeiGene, CytomX, Constellation Pharmaceutical, Cerulean Pharma, Formation Biologics (Forbius), Forty Seven, Ikena Oncology, Incyte Corporation, Inhibrx, Innovent Biologics, Jounce Therapeutics, Merck, Mersana Therapeutics, Pfizer, Regeneron Pharmaceuticals, Symphogen, and TaiRx. KH is a former employee and holds shares of Northern Biologics. RMH is a former employee and holds shares of Northern Biologics. DM is a former employee and holds shares of Northern Biologics. FH has received funding from Northern Biologics for the present work as part of Applied BioMath. JH has received funding from Northern Biologics for the present work as part of Applied BioMath; and owns shares of Pfizer. PG is a former employee and holds shares of Northern Biologics. JA is a former employee and holds shares of Northern Biologics. AK was a consultant to Northern Biologics at the time the study was conducted. PJV is a former employee and holds shares of Northern Biologics. RW is a former employee and holds shares of Northern Biologics. JS is a co-founder of Mosaic Biomedicals and has ownership interests from Mosaic Biomedicals and Northern Biologics; and had received grant/research support from Mosaic Biomedicals, Northern Biologics, Roche Glycart AG, and F. Hoffmann-La Roche Ltd. LLS has received financial compensation for consulting/advisory work from Merck, Pfizer, Celgene, AstraZeneca, Morphosys, Roche, GeneSeeq, Loxo Oncology, Oncorus, Symphogen, Seattle Genetics, GlaxoSmithKline, Voronoi, Treadwell Therapeutics, Arvinas, Tessa Therapeutics, Navire Pharma, Relay Therapeutics, and Rubius Therapeutics; and research grants/support from Novartis, Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca, Merck, Celgene, Astellas, AbbVie, Amgen, Symphogen, Intensity Therapeutics, Mirati Therapeutics, Shattuck Labs, and Avid Radiopharmaceuticals. DMH is an employee of Loxo Oncology at Lilly, a wholly owned subsidiary of Eli Lilly (salary and equity). DVH is an employee of McKesson Corporation; he has stock or other ownership interests in Medtronic, CerRx, SynDevRx, UnitedHealthcare, Anthem Inc, Stromatis Pharma, Systems Oncology, StingRay Therapeutics, Forma Therapeutics, and Orpheus Bioscience; he has received financial compensation for consulting/advisory work from DNAtrix, Esperance Pharmaceuticals, Five Prime Therapeutics, Imaging Endpoints, Medical Prognosis Institute, Senhwa Biosciences, Tolero Pharmaceuticals, Alpha Cancer Technologies, Arvinas, Bellicum Pharmaceuticals, CanBas, Horizon Discovery, Lixte Biotechnology, Oncolyze, Translational Drug Development (TD2), Aadi Bioscience, Aptose Biosciences, BiolineRx, CytomX Therapeutics, EMD Serono, Evelo Biosciences, Fujifilm, Kura Oncology, Phosplatin Therapeutics, Sotio, Strategia Therapeutics, Synergene Therapeutics, 7 Hills Pharma, Actinium Pharmaceuticals, Cancer Prevention Pharmaceuticals, Geistlich Pharma, Huya Bioscience International, Immunophotonics, Genzada Pharmaceuticals, L.E.A.F. Pharmaceuticals, Oncology Venture, Turning Point Therapeutics, Verily Life Sciences, Athenex, Samus Therapeutics, Aeglea Biotherapeutics, Novita Pharmaceuticals, NuCana, Vicus Therapeutics, Codiak Biosciences, Agenus, Kelun, Radimmune Therapeutics, Samumed, Sobi, BioXcel therapeutics, Bryologyx, Celgene, BioPharma Services, Sirnaomics, AIMed, Boston Scientific, Corcept Therapeutics, Erimos Pharmaceuticals, Gimbal Bio, Amunix Pharmaceuticals, Pfizer, Apeiron Biologics, GiraFpharma, Axis Therapeutics, DrugCendR, ImmuneOncia, Orphagen Pharmaceuticals, Array BioPharma, MaveriX Oncology, Northern Biologics, Viracta Therapeutics, Varian Biopharma, Xerient Pharma, AlaMab Therapeutics, Avesta76 Therapeutics, Bessor Pharma, NeoTx, Decoy Biosystems, Noxxon Pharma, RefleXion Medical, and Reglagene; and has received research grants/support from Eli Lilly, Genentech, Celgene, Incyte, Merrimack Pharmaceuticals, Plexxikon, Minneamrita Therapeutics, AbbVie, Aduro Biotech, Cleave Biosciences, CytRx Corporation, Daiichi Sankyo, Deciphera Pharmaceuticals, Endocyte, Exelixis, Five Prime Therapeutics, Gilead Sciences, Merck, Pfizer, Pharmacyclics, Phoenix Biotechnology, Samumed, Strategia Therapeutics, and Halozyme. JT reports personal financial interests in the form of scientific consultancy role for Array BioPharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech, Inc., HalioDx SAS, Hutchison MediPharma, Ikena Oncology, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati Therapeutics, Inc., NeoPhore, Novartis, Orion Biotechnology, Peptomyc SL, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Servier, Taiho Pharmaceutical, Tessa Therapeutics, and TheraMyc Limited.
Supplementary data
References
- 1.Stewart C.L., Kaspar P., Brunet L.J., et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 2.Williams R.L., Hilton D.J., Pease S., et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf D., Gearing D.P. Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc Natl Acad Sci U S A. 1989;86:5948–5952. doi: 10.1073/pnas.86.15.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesnokova V., Auernhammer C.J., Melmed S. Murine leukemia inhibitory factor gene disruption attenuates the hypothalamo-pituitary-adrenal axis stress response. Endocrinology. 1998;139:2209–2216. doi: 10.1210/endo.139.5.6016. [DOI] [PubMed] [Google Scholar]
- 5.Rao M.S., Sun Y., Escary J.L., et al. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- 6.Cheng J.G., Chen J.R., Hernandez L., Alvord W.G., Stewart C.L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci U S A. 2001;98:8680–8685. doi: 10.1073/pnas.151180898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J.R., Cheng J.G., Shatzer T., Sewell L., Hernandez L., Stewart C.L. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141:4365–4372. doi: 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- 8.Rosario G.X., Hondo E., Jeong J.W., et al. The LIF-mediated molecular signature regulating murine embryo implantation. Bio Reprod. 2014;91:66. doi: 10.1095/biolreprod.114.118513. [DOI] [PubMed] [Google Scholar]
- 9.Schofield G., Kimber S.J. Leukocyte subpopulations in the uteri of leukemia inhibitory factor knockout mice during early pregnancy. Biol Reprod. 2005;72:872–878. doi: 10.1095/biolreprod.104.034876. [DOI] [PubMed] [Google Scholar]
- 10.Kamahora H., Ogawa M., Ishiko T., Sakamoto K., Baba H. Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells: involvement of regulation of LIF and its receptor expression. Int J Oncol. 2007;30:977–983. [PubMed] [Google Scholar]
- 11.Shi Y., Gao W., Lytle N.K., et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569:131–135. doi: 10.1038/s41586-019-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M.T., Fer N., Galeas J., et al. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat Commun. 2019;10:3055. doi: 10.1038/s41467-019-11044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peñuelas S., Anido J., Prieto-Sánchez R.M., et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Garcia M., Bonfill-Teixidor E., Planas-Rigol E., et al. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8(+) T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun. 2019;10:2416. doi: 10.1038/s41467-019-10369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H., Yue X., Zhao Y., et al. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat Commun. 2014;5:5218. doi: 10.1038/ncomms6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Yang Q., Yu H., et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget. 2014;5:788–801. doi: 10.18632/oncotarget.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrengues J., Bourget I., Pons C., et al. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014;7:1664–1678. doi: 10.1016/j.celrep.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Liu S.C., Tsang N.M., Chiang W.C., et al. Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Invest. 2013;123:5269–5283. doi: 10.1172/JCI63428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicola N.A., Babon J.J. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26:533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue X., Wu L., Hu W. The regulation of leukemia inhibitor factor. Cancer Cell Microenviron. 2015;2:e877. doi: 10.14800/ccm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taupin J.L., Pitard V., Dechanet J., Miossec V., Gualde N., Moreau J.F. Leukemia inhibitory factor; part of a large ingathering family. Int Rev Immunol. 1998;16:397–426. doi: 10.3109/08830189809043003. [DOI] [PubMed] [Google Scholar]
- 22.Onishi K., Zandstra P.W. LIF signaling in stem cells and development. Development. 2015;142:2230–2236. doi: 10.1242/dev.117598. [DOI] [PubMed] [Google Scholar]
- 23.Liu R., Liao Y-Z., Zhang W., Zhou H-H. Relevance of immune infiltration and clinical outcomes in pancreatic ductal adenocarcinoma subtypes. Frontiers Oncol. 2021;10 doi: 10.3389/fonc.2020.575264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borazanci E, Schram A, Braña I, et al. Phase 1 dose escalation of MSC-1, a humanized anti-LIF monoclonal antibody, in patients (pts) with advanced solid tumors: Updated Results. In: Abstract Book of the 44th ESMO Congress (ESMO 2019); 2019 September 27 – October 1; Barcelona Spain. Ann Oncol 2019;30(5 Suppl):Abstract nr 1196P.
- 25.Sinclair A, Hallett R, Giblin P, et al. MSC-1 is a first-in-class humanized monoclonal antibody that modulates the tumor microenvironment by inhibiting a novel cancer immunotherapy target, LIF. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 April 14–18; Chicago, IL. Philadelphia (PA): AACR; Cancer Res 2018;78(13 Suppl):Abstract nr 1751.
- 26.Candido J, Kar G, Zhang B, et al. AZD0171 (anti-LIF) combines productively with chemotherapy and anti-PD-L1 in mouse models of cancer. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022; 2022 April 8–13. Philadelphia (PA): AACR; Cancer Res 2022;82(12 Suppl):Abstract nr 1293.
- 27.Roskos L.K., Schneider A., Vainshtein I., et al. PK-PD modeling of protein drugs: implications in assay development. Bioanalysis. 2011;3:659–675. doi: 10.4155/bio.11.28. [DOI] [PubMed] [Google Scholar]
- 28.Loriot Y., Marabelle A., Guégan J.P., et al. Plasma proteomics identifies leukemia inhibitory factor (LIF) as a novel predictive biomarker of immune-checkpoint blockade resistance. Ann Oncol. 2021;32:1381–1390. doi: 10.1016/j.annonc.2021.08.1748. [DOI] [PubMed] [Google Scholar]
- 29.O’Kane P, Macarulla T, Balogun F, et al. A phase 2 trial of first-line AZD0171 + durvalumab and chemotherapy in patients with metastatic pancreatic ductal adenocarcinoma and CD8+ T cell infiltration. In: Proceedings of the 113th Annual Meeting of the American Association for Cancer Research; 2021 April 8-13; New Orleans, LA. Philadelphia, PA: AACR; 2022. Abstract nr CT126.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.