Abstract
Background and Hypothesis
Facial Emotion Recognition is a key domain of social cognition associated with psychotic disorders as a candidate intermediate phenotype. In this study, we set out to investigate global and specific facial emotion recognition deficits in first-episode psychosis, and whether polygenic liability to psychotic disorders is associated with facial emotion recognition.
Study Design
828 First Episode Psychosis (FEP) patients and 1308 population-based controls completed assessments of the Degraded Facial Affect Recognition Task (DFAR) and a subsample of 524 FEP and 899 controls provided blood or saliva samples from which we extracted DNA, performed genotyping and computed polygenic risk scores for schizophrenia (SZ), bipolar disorder (BD), and major depressive disorder (MD).
Study Results
A worse ability to globally recognize facial emotion expressions was found in patients compared with controls [B= −1.5 (0.6), 95% CI −2.7 to −0.3], with evidence for stronger effects on negative emotions (fear [B = −3.3 (1.1), 95% CI −5.3 to −1.2] and anger [B = −2.3 (1.1), 95% CI −4.6 to −0.1]) than on happiness [B = 0.3 (0.7), 95% CI −1 to 1.7]. Pooling all participants, and controlling for confounds including case/control status, facial anger recognition was associated significantly with Schizophrenia Polygenic Risk Score (SZ PRS) [B = −3.5 (1.7), 95% CI −6.9 to −0.2].
Conclusions
Psychosis is associated with impaired recognition of fear and anger, and higher SZ PRS is associated with worse facial anger recognition. Our findings provide evidence that facial emotion recognition of anger might play a role as an intermediate phenotype for psychosis.
Keywords: facial affect recognition, genetic liability, first episode psychosis
Introduction
Psychotic disorders are polygenic syndromes, with many common genetic variants contributing to the risk of illness onset. The Psychiatric Genomic Consortium (PGC) identified 108 specific genetic loci reaching genome-wide significance for schizophrenia. These are common genetic variants that can be summarized into an individual polygenic risk score (PRS).1-3 Before the GWAS era, studies on twins and first-degree relatives of patients suggested that the genes implicated in the risk of schizophrenia and related disorders affect some heritable traits on the causal pathway to the illness.4 Those traits, known as intermediate phenotypes, are “simpler clues to genetic underpinnings than the disease syndrome itself,” 5 and putatively more directly influenced by risk genes.6 Therefore, they might represent useful research targets to help unravel the biological mechanisms contributing to these disorders.
Patients with psychosis tend to show impairments in social cognition which refers to the set of psychological processes involved in the perception, encoding, storage, retrieval, and regulation of information about other people and self. Facial emotion recognition is a key domain of social cognition that has been extensively studied in schizophrenia and other psychotic disorders. Indeed, deficits in facial emotion recognition represent a well-replicated finding in schizophrenia,7,8 detected at psychosis onset with the same severity as at more advanced stages of illness, especially for negative emotions.9-11 Whether the deficits in psychosis concern recognition of all emotions, or whether there is differential recognition ability across emotions, still requires further investigation, but increasing evidence, from meta-analysis9 and from the largest studies to date,11,12 suggests the most prominent deficits are in fear and/or anger.
Facial emotion recognition was reported to remain stable in psychosis over a 3-year follow-up period.13,14 Furthermore, unaffected relatives of psychotic patients show intermediate performance compared with their affected relatives and controls,15-18 supporting the hypothesis that emotion recognition might be a marker of liability for psychotic disorders. Emotion recognition has therefore been suggested as an intermediate phenotype which, as with other cognitive domains, was found to be polygenic.19,20
Studies testing the genetic association between psychotic disorders and facial emotion recognition ability have had mixed results. Germine et al.21 found an association between PRS for schizophrenia and social cognition—in particular, facial emotion identification efficiency – in two different samples, spanning from childhood to young adulthood; the findings suggest a potential role of emotion recognition in the genetic risk for schizophrenia. The study focused on emotion recognition in general and did not assess recognition of specific emotions. However, Xavier22 found no association with general facial emotion recognition in ~700 patients with chronic schizophrenia testing SZ PRS, and Coleman et al20 found that, after correction for 33 statistical tests, there were no significant associations between polygenic risk scores for mental disorders and facial emotion recognition ability (neither general nor specific) in a large population cohort of ~4000 children aged 8 years old. Although Coleman et al’s negative study is the largest study to date (to our knowledge) to examine this topic, it is possible that genetic risk for schizophrenia only manifests in emotion recognition deficits after age 8. We, therefore, reasoned that examining, in a large sample, associations between PRS for schizophrenia and emotion recognition, could help resolve previous partly contradictory results. We also wanted to examine any relationship between genetic risk for schizophrenia and recognition of specific emotions. While our primary focus is on risk for schizophrenia, we also wanted to examine risk for other mental disorders (Bipolar disorder and depression) to help put any associations into context, acknowledging recent findings on shared genetic components among psychiatric disorders.23
In the current study, we report data concerning recognition of angry, fearful, happy, and neutral faces from the large multi-country European Network of national schizophrenia networks studying Gene-Environment Interactions (EUGEI) case-control study of first-episode psychosis. We aimed to confirm previous findings that FEP patients’ exhibit lower facial recognition of fear and anger compared with controls, and we wished to take advantage of this well-powered study to investigate the extent to which facial recognition deficits in psychosis extend to other emotions. We hypothesized that polygenic risk score for schizophrenia (SZ PRS), would be associated with lower emotion recognition ability, particularly as regards to fear and anger. In secondary analyses, we examined whether PRS for Bipolar Disorder and for Major Depression would be associated with emotion recognition deficits.
Materials and Methods
Design and Procedure
The EU-GEI study Work-Package 2 (WP2) employed a case-control design collecting data with an extensive battery of demographic, clinical, social, and biological measures (Core assessment); psychological measures, and cognitive tasks. EU-GEI WP2 participants with complete Degraded Facial Affect Recognition (DFAR) task and Benton Face Recognition Test (BFRT) data were included in the current study. All the researchers involved in administering the assessments undertook a training organized by a technical working committee of the overall EU-GEI study at the beginning and throughout the study. Inter-rater reliability (0.75) was assessed annually to warrant comparability of procedures and methods across sites.
Participants
Participants were recruited and assessed as part of the incidence and first episode case-control study, conducted as part of the EU-GEI programme.24-26 The study was designed to investigate risk factors for psychotic disorders between May 1, 2010, and April 1, 2015, in 17 catchment areas in England, France, the Netherlands, Italy, Spain, and Brazil.
Patients were included if they met the following criteria during the recruitment period: (a) aged between 18 and 64 years; (b) presentation with a clinical diagnosis for an untreated FEP, even if longstanding (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes F20-F33); (c) resident within the catchment area at first presentation. Exclusion criteria were: (a) previous contact with psychiatric services for psychosis; (b) psychotic symptoms with any evidence of organic causation; and (c) transient psychotic symptoms resulting from acute intoxication (ICD-10: F1x.5).
Inclusion criteria for controls were: (a) aged between 18 and 64 years; (b) resident within a clearly defined catchment area corresponding to that of cases at the time of consent into the study; (c) sufficient command of the primary language at each site to complete assessments; and (d) no current or past psychotic disorder. To select a population-based sample of controls broadly representative of local populations in relation to age, sex, and ethnicity, a mixture of random and quota sampling was adopted. Quotas for control recruitment were based on the most accurate local demographic data available, and then filled using a variety of recruitment methods, including through (1) random sampling from lists of all postal addresses (e.g., in London); (2) stratified random sampling via General Practitioner (GP) lists (e.g., in London and Cambridge) from randomly selected surgeries; and (3) ad hoc approaches (e.g., internet and newspaper adverts, leaflets at local stations, shops, and job centers). All participants provided informed, written consent. Ethical approval was provided by relevant research ethics committees in each of the study sites. All data were stored anonymously.
Measures
Information about age, sex, and self-reported ethnicity was collected from cases and controls using the Medical Research Council (MRC) Sociodemographic Schedule.27 Psychopathology was assessed using the OPerational CRITera system (OPCRIT).28 Item response modeling was previously used to develop a bi-factor model composed of general and specific dimensions of psychotic symptoms (positive, negative, disorganization, mania, and depression).29 The Community Assessment of Psychic Experience (CAPE) was used as a self-report measurement of lifetime psychotic experiences in controls with good reliability for all the languages spoken in the EUGEI catchment areas (http://www.cape42.homestead.com/). Previous factor analyses on the CAPE showed a three-factor structure of positive, negative, and depressive dimensions.30 The short form of the Wechsler Adult Intelligence Scale (WAIS) III31,32 was administered as an indicator of general cognitive ability (IQ). We used the Degraded Facial Affect Recognition (DFAR) task,33 which has been used in numerous previous psychiatric research studies; 12-15,34-38 it assesses emotional face recognition in degraded photographs of four different actors (two females, and two males) representing four emotions: anger, fearful, happy, and neutral. Subjects were presented with 64 trials, and 16 presentations in each condition on a computer screen and asked to indicate the expression of each face by a button press (1 for angry, 2 for happy, 3 for fearful, and 4 for neutral). Variables generated by DFAR performance were the percentage of correctly recognized total facial expressions (DFAR total), neutral (DFAR neutral), happy (DFAR happy), fearful (DFAR fearful), and angry facial expressions (DFAR angry). The type of misinterpretation for each emotion was also computed. To account for general facial recognition ability, the short form (16 items) of the Benton Facial Recognition test (BFRT)39 was administered to measure the ability to match non-emotional unfamiliar faces. We excluded the poorest performers by excluding participants who scored equal or below chance level (≤25%) on DFAR total (FEP N = 14, controls N = 17), as they may not have engaged with the task, and we covaried for BFRT to account for general facial recognition performance.
Polygenic Risk Scores
The case–control genotyped WP2 EUGEI sample (N = 2169; cases’ samples N = 920, controls’ samples N = 1248) included DNA extracted from blood (N = 1857) or saliva (N = 312). The samples were genotyped at the MRC Centre for Neuropsychiatric Genetics and Genomics in Cardiff (UK) using a custom Illumina HumanCoreExome-24 BeadChip genotyping array covering 570 038 genetic variants. For genotype Quality Control, we excluded SNPs with minor allele frequency <0.5%, Hardy Weinberg Equilibrium P < 10− 6, missingness >2%. For sample Quality Control, we excluded samples with >2% missing genotype, heterozygosity Fhet >0.14 or <−0.11, and those who presented genotype–phenotype sex mismatch or clustered with African ancestry in Principal Components Analysis (PCA) (N = 170). PCA was applied to genotype data to detect and correct our analysis for population stratification.40 The final sample of 1720 individuals (1112 of European ancestry, 608 of any other ancestries but not black African) comprised 1041 controls and 679 patients. Imputation was performed through the Michigan Imputation Server, using the Haplotype Reference Consortium reference panel with the Eagle software for inferring haplotype phase, and Minimac3 for genotype imputation.41-43 The imputed variants with r2 <0.6, MAF <0.1% or missingness >1% were excluded.
The polygenic risk scores for schizophrenia, bipolar disorder, and major depression disorder were built using, as training data sets, the results from the last available mega-analyses from the Psychiatric Genomics Consortium (PGC).3,44-47 In PRSice, individuals’ number of risk alleles in the target sample was weighted by the log odds ratio from the discovery sample and summed into the PRSs at 0.05 SNPs Pt-thresholds (a priori selected). We excluded people of homogeneous African ancestry since in this population the SZ PRS from the PGC2 we calculated, as reported by other studies,1,48 failed to explain a significant proportion of the variance (R2 = 1.1%, P = .004).
Statistical Analysis
Analyses were conducted in STATA 15.49 Preliminary descriptive analyses were performed using chi-square and t-tests to examine the differences in age, sex, ethnicity, IQ, BFRT, and DFAR scores between cases and controls. Linear mixed-effects models were built to estimate the relationship between overall and emotion-specific DFAR scores with case/control status, adjusted for age, sex, ethnicity, BFRT, and IQ as covariates, and country as a random effect. Those analyses were repeated with errors patterns for each condition as outcome variables (see supplement). Regression coefficients represent the strength of association between emotion recognition and case/control status; we inspected 83% confidence intervals50 on these coefficients from separate regressions to examine whether the strength of association between emotion recognition and case-control status differed significantly for different emotions (reported in supplement). We also performed repeated measures mixed model analysis, examining emotion by case-control status interaction terms (see supplement). Associations between emotion recognition and symptom dimensions in cases and PLE in controls were examined using Pearson’s correlation coefficient (reported in supplement). To investigate whether lower emotion recognition ability was associated with the liability for schizophrenia, bipolar, and major depression disorders, we considered SZ, BP, and MDD PRSs as predictors adjusting for case/control status, age, sex, BFRT, IQ, and 20 principal components (PCs) to control for population stratification in linear mixed-effects models with country as a random effect. Our primary interest in the PRS analysis was in examining relations between schizophrenia PRS and negative facial emotion recognition (fear and anger). Other PRS analyses were to provide context and are of secondary interest. Therefore, in consonance with Rothman,51 corrections for multiple testing were not applied.
Results
Sample Characteristics
FEP patients and controls were included in the current study if data on both DFAR and BFRT were available. This led to a sample of 828 FEP patients and 1308 controls for the analysis.
Patients were younger (mean age = 30.9 ± 10.6 vs. 36.2 ± 13; t = 9.9, P < .001), with more men [61.8% (512) vs. 47.4% (620); χ2(1) = 42.4, P < .001], and more frequently from minority ethnic backgrounds (χ2(5) = 49.3, P < .001) compared with controls (table 1). The aforementioned differences are those expected when comparing psychotic patients with the general population.
Table 1.
Demographic and cognitive characteristics of the sample included in the analysis
| Controls N=1308 | FEP N=828 | Df | Test Statistics | P value | |
|---|---|---|---|---|---|
| Age (mean; sd) | 36.2 (13) | 30.9 (10.6) | 2134 | t = 9.9 | <.001 |
| Sex (male %; N) | 47.4 (620) | 61.8 (512) | 1 | χ 2 = 42.4 | <.001 |
| Ethnicity (%; N) | |||||
| White | 77.4 (1012) | 64.4 (533) | 5 | χ 2 = 49.3 | <.001 |
| Black | 8.4 (110) | 14.9 (123) | |||
| Mixed | 8.6 (112) | 11.5 (95) | |||
| Asian | 2.3 (30) | 2.9 (24) | |||
| North African | 1.7 (22) | 4.2 (35) | |||
| Other | 1.7 (22) | 2.2 (18) | |||
| IQ (mean; SD) | 102.7 (17.7) | 85.6 (18.1) | 2041 | t = 20.9 | <.001 |
IQ, intelligence quotient.
Table 1 also shows that IQ scores were lower in cases compared with controls.
Facial Emotion Recognition and Psychosis
All DFAR scores and BFRT score were lower in patients compared with controls (tables 2 and 3). After adjusting for age, sex, ethnicity, BFRT score, and IQ, case-control status was still associated with worse ability to globally recognize facial emotion expressions [B = −1.5 (0.6), 95% CI −2.7 to −0.3; P = .013]. The specific emotions with the largest case control regression coefficients were fear [B = −3.3 (1.1), 95% CI −5.3 to −1.2; P = .002] and anger [B = −2.3 (1.1), 95% CI −4.6 to −0.1; P = .041]. Regression coefficients for case control status were significantly stronger for fear and anger expression recognition than for the happy facial expression recognition analysis (see for inspection of not overlapping 83% confidence interval, table S4 in supplement). Emotion by case–control status interaction analysis also indicated more prominent deficits in anger and fear recognition in psychosis (table S8a,b in supplement). The analysis on misattribution patterns (table S2 in supplement) highlighted that fearful expressions were mostly mistaken for neutral [B = 2.2, 95% CI 0.2 to 4.2; P = .033] and happy emotions [B = 1.2, 95% CI 0.5 to 1.8; P = .001] by patients, whereas angry faces were incorrectly recognized as neutral [B = 1.8, 95% CI −0.03 to 3.5; P = .053].
Table 2.
DFAR and BFRT scores in FEP and controls
| Controls N = 1308 | FEP N = 828 | Df | Test statistics | P value | |
|---|---|---|---|---|---|
| DFAR total | 72.4 (12.1) | 68.5 (13.6) | 2134 | t = 6.9 | <.001 |
| DFAR neutral | 79.9 (17) | 75.9 (21.9) | 2134 | t = 4.7 | <.001 |
| DFAR happy | 87.8 (13.3) | 86.2 (15.1) | 2134 | t = 2.6 | .0095 |
| DFAR fearful | 55.2 (20.9) | 50 (21.7) | 2134 | t = 5.5 | <.001 |
| DFAR angry | 66.8 (22.3) | 61.9 (23.1) | 2134 | t = 4.8 | <.001 |
| BFRT score | 22.1 (2.2) | 21.2(2.8) | 2134 | t = 8.2 | <.001 |
Table 3.
DFAR scores’ prediction by case/control status
| Model | Case/control status | |||
|---|---|---|---|---|
| B | SE | 95% CI | P value | |
| DFAR totala | −3.2 | 0.6 | −4.3 to −2.1 | <.001 |
| DFAR totalb | −1.5 | 0.6 | −2.7 to −0.3 | .013 |
| DFAR neutrala | −2.9 | 0.9 | −4.6 to −1.2 | .001 |
| DFAR neutralb | −0.6 | 0.9 | −2.4 to 1.2 | .506 |
| DFAR happya | −0.9 | 0.6 | −2.2 to 0.4 | .160 |
| DFAR happyb | 0.3 | 0.7 | −1 to 1.7 | .639 |
| DFAR feara | −5.1 | 0.9 | −6.9 to −3.1 | <.001 |
| DFAR fearb | −3.3 | 1.1 | −5.3 to −1.2 | .002 |
| DFAR angera | −3.9 | 1 | −5.9 to −1.9 | <.001 |
| DFAR angerb | −2.3 | 1.1 | −4.6 to −0.1 | .041 |
Note: DFAR, degraded facial affect recognition.
aAdjusted for age, sex, ethnicity, BFRT score.
bAdjusted for age, sex, ethnicity, BFRT score, IQ. Models were random-intercept models that included one random effect to allow DFAR scores to vary across countries.
Facial Emotion Recognition and PRSs for Schizophrenia, Bipolar Disorder, and Major Depression
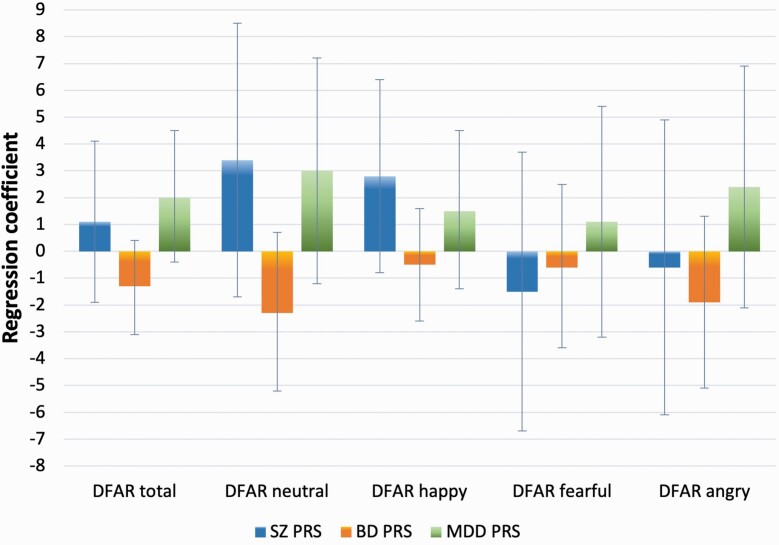
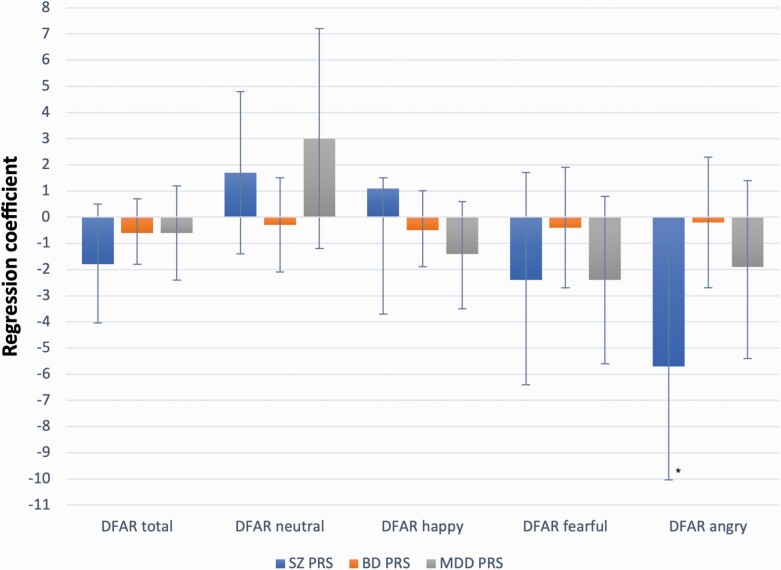
The analysis on PRSs was performed in a subsample of 524 FEP and 899 population controls, controlling for case/control status and other covariates (see methods). Results are summarized in table 4 and illustrated in figure 1 for cases and figure 2 for controls separately.
Table 4.
DFAR scores’ prediction by SZ PRS, BD PRS, and MDD PRS
| Model | SZ PRS | |||
|---|---|---|---|---|
| B | SE | 95% CI | P value | |
| DFAR total | −0.7 | 0.9 | −2.5 to 1.1 | .466 |
| DFAR neutral | 2.6 | 1.4 | −0.1 to 5.4 | .060 |
| DFAR happy | 0.3 | 1.1 | −1.8 to 2.4 | .784 |
| DFAR fear | −2.4 | 1.6 | −5.6 to 0.8 | .136 |
| DFAR anger | −3.5 | 1.7 | −6.9 to −0.2 | .040 |
| BD PRS | ||||
| DFAR total | −0.6 | 0.5 | −1.7 to 0.4 | .220 |
| DFAR neutral | −0.8 | 0.8 | −2.4 to 0.7 | .297 |
| DFAR happy | −0.5 | 0.6 | −1.8 to 0.7 | .383 |
| DFAR fear | −0.6 | 0.9 | −2.4 to 1.2 | .525 |
| DFAR anger | −0.7 | 0.9 | −2.7 to 1.2 | .459 |
| MDD PRS | ||||
| DFAR total | 0.2 | 0.7 | −1.3 to 1.6 | .793 |
| DFAR neutral | 2.7 | 1.1 | 0.5 to 4.9 | .017 |
| DFAR happy | −0.3 | 0.9 | −1.9 to 1.4 | .743 |
| DFAR fear | −1.2 | 1.3 | −3.8 to 1.3 | .347 |
Note: Linear regression models adjusted for case/control status, age, sex, BFRT, IQ, and 20 PCs.
SZ, schizophrenia; BD, bipolar disorder; MDD, major depression disorder; PRS, polygenic risk score; DFAR, degraded facial affect recognition.
Fig. 1.
Associations between DFAR scores and SZ, BD, and MDD PRSs in FEP. Error bars indicate 95% CI.
Fig. 2.
Associations between DFAR scores and SZ, BD, and MDD PRSs in controls. Error bars indicate 95% CI. *P = .009.
SZ PRS was negatively associated with DFAR anger [B = −3.5 (1.7), 95% CI −6.9 to −0.2; P = .040], and the strength of the effect for anger was notably stronger than the strength of the effect for happiness (supplement table S5). PRS for Major Depression and Bipolar PRS was not significantly associated with facial emotion recognition (table 4; figures 1 and 2). When testing the association separately for cases and controls, SZ PRS prediction of DFAR angry held statistical significance in controls only [B = −5.7 (2.2), 95% CI −10 to −1.4; P = .009] (figure 2).
Exploratory Analyses by Country
Due to suggestions that emerged during the review process, we conducted exploratory analyses to probe differences between countries. Case-control deficits appeared (on inspection) especially prominent in Italy and Brazil (figure S3). We, therefore, repeated analyses, but now including country by case-control status as interaction terms. Results (supplementary table S9), indicated there were interactions between case-control status and country (driven by Italy and Brazil) for global DFAR and for anger recognition, but not fear recognition. This provides evidence that fear recognition deficit conclusions can be drawn from analyses without interaction terms, and so can be generalized across countries, but anger recognition deficits in psychosis appear to differ across countries. We also conducted a sensitivity analysis of repeating some analyses having excluding participants from Italy and Brazil (table S10).
Discussion
The present study was conducted to investigate impairments in an important social cognition domain, facial emotion recognition, at first presentation for a psychotic disorder. For this purpose, we used the largest to date incidence sample of FEP patients and population-based controls. Moreover, we tested the association between global and specific emotion recognition and genetic susceptibility to schizophrenia and affective disorders.
As we expected, facial emotions were poorly recognized by patients compared with controls. This is in line with previous literature on patients at the first episode as well as with multi-episode and long-standing schizophrenia which reported a generalized deficit in emotion recognition.9,10,12,13,52 In the same sample, we recently demonstrated that the patient group had deficits in probabilistic reasoning, yet these were no longer present after adjusting for IQ, and indeed were fully mediated by deficits in IQ.53 Associations between emotion recognition and general cognitive abilities were previously reported in both childhood54 and adulthood.15 In our sample adjusting for IQ leads to a partial, but not complete, attenuation of the effect size (table 3), though the picture is more complex when probing differences between countries (see supplementary material). Facial emotion recognition difficulty in psychosis may be partially, but not entirely, related to general intellectual deficit and may in some circumstances be present even when general cognitive ability is preserved. We found that FEP patients had the most difficulty in recognizing fearful and angry faces, and were indeed statistically more impaired on these emotions than on happiness. This is broadly consistent with previous literature on early psychosis, but we go beyond prior studies as the current large sample size allows us to examine relative effect sizes with a degree of precision using confidence intervals. Barkl9 examining specific emotions’ identification accuracy in their meta-analysis, found that recognition of fear was the most consistent deficit across six studies. Catalan13 and Caldiroli12 reported a more prominent deficit of anger identification, in 64 FEP patients and 110 actively unwell FEP patients respectively, compared to controls. Fett,38 employed a very large sample size (n = 1032 patients with nonaffective psychosis and n = 579 controls) and found patient deficits in anger and fear recognition but not in recognition of happy and neutral faces; however, they did not formally compare the effect sizes of group differences between emotions. Together, our data and prior studies indicate that fear and anger are the facial emotions with the most prominent deficits in psychosis. Moreover, the examination of error patterns made by our participants revealed that negative emotions were more mistaken for either neutral or happy by patients; this is in line with previous studies taking into account error patterns in FEP.11,13 We did not find significant difficulties in recognizing neutral faces (after adjustment for IQ and other covariates), in contrast to some prior studies13,55 nor was there any tendency to misattribute neutral and happy facial expressions for emotions with negative valence. Those results, along with either weak or no associations with any symptom dimensions (correlation coefficients ranging from –0.03 to 0.1, see supplement) or with psychotic-like experiences (correlation coefficients ranging from –0.01 to 0.1, see supplement), are consistent with the hypothesis of a specific impairment of recognizing negative emotions not strongly related to levels of symptomatology or salience misattribution,15 but to social-emotional processing disturbances possibly preceding the onset of the disorder.56
The evidence from our case-control analysis for a specific emotion identification deficit in psychosis was partly corroborated in our study by the PRS analysis. Genetic liability to schizophrenia was associated with greater impairment in identifying angry emotional faces. We note that a previous study testing the association between schizophrenia polygenic risk score and facial emotion recognition by Xavier22 on a sample of ~700 patients with chronic schizophrenia did not detect any association between SZ PRS and facial emotion identification, perhaps because of insufficient power or because there may be other factors in chronic patients that cloud the association such as current illness state; 12 similarly, in our study no association between SZ PRS and emotion identification reached conventional statistical significance when we tested SZ PRS in patients only (N = 524). Whereas, the association between genetic risk for schizophrenia and worse anger recognition ability held in controls when analyzed separately, probably due to increased power (N = 899).
A PRS analysis is in some ways analogous to traditional familial risk studies in relatives of patients. Research conducted on siblings and first-degree relatives of patients suffering from psychotic disorders found deficits in recognizing negative emotions in facial expressions compared to controls15,16,57-59 although to a lesser degree than patients, though we acknowledge that not all familial studies have shown significant sibling differences.37 To help put the schizophrenia PRS result into context, we went on to examine the genetics underpinning bipolar and major depression disorders and the ability to identify facial emotional expressions, but there were no significant associations. There is strong evidence for deficits in facial emotion recognition in affective disorder.10,18,60 However, our primary genomic interest was in the schizophrenia PRS analysis given that (1) the FEP sample has more nonaffective, schizophrenia spectrum psychosis than bipolar psychosis or depressive psychosis, and (2) prior findings indicate that schizophrenia PRS explains more variance in schizophrenia caseness than the other psychiatric PRSs do for their respective disorders.61 We note that the affective disorder PRSs are less well developed compared to the schizophrenia PRS (because of factors relating to genetic architecture in the case of depression and smaller discovery GWAS sample size for bipolar disorder). Further work in larger sample sizes, using improved PRS in future, will be required to examine the specificity of the PRS associations across different disorders with different emotions.
Limitations and Strengths
There are limitations to our study. We assessed facial emotion recognition ability using a static task that does not provide temporally transient signals in stimuli as real-world perception.62 As different tests will probe slightly different aspects of emotion recognition, it will be important to examine the consistency of results using different measures. Nonetheless, studies using more ecological dynamic task found no differences in terms of quality of impairment.63,64 We acknowledge the possibility that the high accuracy rate for neutral and happy faces in both cases and controls might be more related to the psychometric property of the DFAR task rather than the absence of impairment. Future research may employ social cognition measures recommended by international research groups to improve replicability. While our sample size is very large for a case-control study of cognition, it is modest for genetic analyses. In addition, the multi-ethnic origin of our participants required further reduction of the sample size. Furthermore, in the PRS analyses, we controlled for population stratification by adjusting for 20 PCs.65 In accordance with Rothman,52 we did not correct for multiple testing; p values and confidence intervals of all tests are fully reported in tables, as recommended, and should be cautiously interpreted.
Our study has several strengths. This is the largest study to date of social cognition in patients at the onset of their psychosis illness and population controls, and the large size permits us to examine relative emotional specificity. As a moderate involvement of general cognitive ability in emotion recognition was previously detected,15,66 our study is strengthened by taking into account IQ and general face recognition ability. The multi-site sample from different centers across Europe and Brazil increases the generalizability of our results,9,11 and provides some ability for a preliminary exploration of cultural differences. Cultural and country differences could be investigated further in future, ideally in larger samples, with a more fine-grained analysis of why emotion recognition abilities may differ between countries and how this interacts with illness.
Conclusions
Our results indicate a predominantly negative emotion facial recognition impairment in early psychosis, mainly involving fear and anger. Additionally, our findings provide further evidence to consider angry emotion recognition as an intermediate phenotype for psychosis, shedding light on specific emotion identification ability associated with common genetic risk variants for schizophrenia.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Acknowledgments
The authors would like to acknowledge the EU-GEI WP2 nonauthor members for their cooperation in the study (see supplement for a full list).
Contributor Information
Giada Tripoli, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy; Department of Psychosis Studies, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, UK.
Diego Quattrone, Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre, South London and Maudsley NHS Foundation Trust, King’s College London, London, UK; Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Mannheim, Germany and National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, UK.
Laura Ferraro, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy.
Charlotte Gayer-Anderson, Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, London, UK.
Caterina La Cascia, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy.
Daniele La Barbera, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy.
Crocettarachele Sartorio, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy.
Fabio Seminerio, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy.
Victoria Rodriguez, Department of Psychosis Studies, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, UK.
Ilaria Tarricone, Department of Medical and Surgical Science, Psychiatry Unit, Alma Mater Studiorum Università di Bologna , Bologna, Italy.
Domenico Berardi, Department of Biomedical and NeuroMotor Sciences, Psychiatry Unit, Alma Mater Studiorum Università di Bologna , Bologna, Italy.
Stéphane Jamain, Institut National de la Santé et de la Recherche Médicale, Faculté de Médecine, Université Paris-Est, Creteil, France.
Celso Arango, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health. Hospital General Universitario Gregorio Marañón, School of Medicine, Universidad Complutense, IiSGM, CIBERSAM, Madrid, Spain.
Andrea Tortelli, Etablissement Public de Santé Maison Blanche, Paris, France.
Pierre-Michel Llorca, Université Clermont Auvergne, Clermont-Ferrand, France.
Lieuwe de Haan, Department of Psychiatry, Early Psychosis Section, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Eva Velthorst, Department of Psychiatry, Early Psychosis Section, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Department of Psychiatry, Icahn School of Medicine at Mount Sinai, NY.
Julio Bobes, Department of Medicine, Psychiatry Area, School of Medicine, Universidad de Oviedo, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Oviedo, Spain.
Miquel Bernardo, Barcelona Clinic Schizophrenia Unit, Neuroscience Institute, Hospital clinic, Department of Medicine, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Spain.
Julio Sanjuán, Department of Psychiatry, School of Medicine, Universidad de Valencia, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Valencia, Spain.
Jose Luis Santos, Department of Psychiatry, Servicio de Psiquiatría Hospital “Virgen de la Luz”, Cuenca, Spain.
Manuel Arrojo, Department of Psychiatry, Psychiatric Genetic Group, Instituto de Investigación Sanitaria de Santiago de Compostela, Complejo Hospitalario Universitario de Santiago de Compostela, Spain.
Cristina Marta Del-Ben, Division of Psychiatry, Department of Neuroscience and Behaviour, Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil.
Paulo Rossi Menezes, Department of Preventive Medicine, Faculdade de Medicina, Universidade of São Paulo, São Paulo, Brazil.
Els van der Ven, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, Maastricht, The Netherlands; Vrije Universiteit Amsterdam, Department of Clinical, Neuro- and Developmental Psychology.
Peter B Jones, Department of Psychiatry, University of Cambridge, Cambridge, UK; CAMEO Early Intervention Service, Cambridgeshire & Peterborough NHS Foundation Trust, Cambridge, UK.
Hannah E Jongsma, Psylife Group, Division of Psychiatry, University College London, London, UK.
James B Kirkbride, Psylife Group, Division of Psychiatry, University College London, London, UK.
Sarah Tosato, Section of Psychiatry, Department of Neuroscience, Biomedicine and Movement, University of Verona, Verona, Italy.
Antonio Lasalvia, Section of Psychiatry, Azienda Ospedaliera Universitaria Integrata di Verona , Verona, Italy.
Alex Richards, Division of Psychological Medicine and Clinical Neurosciences, MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, Cardiff, UK.
Michael O’Donovan, Division of Psychological Medicine and Clinical Neurosciences, MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, Cardiff, UK.
Bart P F Rutten, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, Maastricht, The Netherlands.
Jim van Os, Department of Biomedicine, Neuroscience, and Advanced Diagnostics, University of Palermo, Palermo, Italy; Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, Maastricht, The Netherlands; Department Psychiatry, Brain Centre Rudolf Magnus, Utrecht University Medical Centre, Utrecht, The Netherlands.
Craig Morgan, Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, London, UK.
Pak C Sham, Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; Centre for Genomic Sciences, Li KaShing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Marta Di Forti, Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre, South London and Maudsley NHS Foundation Trust, King’s College London, London, UK.
Robin M Murray, Department of Psychosis Studies, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, UK.
Graham K Murray, Department of Psychiatry, University of Cambridge, Cambridge, UK; CAMEO Early Intervention Service, Cambridgeshire & Peterborough NHS Foundation Trust, Cambridge, UK; Institute for Molecular Bioscience, University of Queensland, Australia.
Funding
The EU-GEI Project was funded by the European Community’s Seventh Framework Programme under grant agreement No. HEALTH-F2-2010-241909 (Project EU-GEI). The Brazilian study was funded by the Säo Paulo Research Foundation under grant number 2012/0417-0. Funders were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript, and decision to submit the manuscript for publication.
Disclosure
M. Di Forti reports personal fees from Janssen, outside the submitted work. R.M. Murray reports personal fees from Janssen, Lundbeck, Sunovion, and Otsuka, outside of the submitted work. M. Bernardo reports grants and personal fees from Adamed, Janssen-Cilag, Otsuka, and Abbiotics; personal fees from Angelini and Casen Recordati; and grants from Lundbeck and Takeda, outside of the submitted work. P.B. Jones reports personal fees from being a member of the scientific advisory boards for Janssen and Ricordati, outside of the submitted work. C. Arango reports personal fees from Acadia, Ambrosseti, Gedeon Richter, Janssen Cilag, Lundbeck, Merck, Otsuka, Roche, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda; and grants from CIBERSAM, Familia Alonso, Fundacion Alicia Koplowitz, the European Commission, the Spanish Ministry of Science and Universities, and the Comunidad de Madrid, during the conduct of the study. J. Bobes has received research grants and served as consultant, advisor, or speaker for AB-Biotics, Acadia Pharmaceuticals, Ambrosseti-Angelini, Casen Recordati, D&A Pharma, Exeltis, Gilead, Indivior, Janssen-Cilag, Lundbeck, Mundipharma, Otsuka, Pfizer, Roche, Sage Therapeutics, Servier, Schwabe Farma Ibérica, Shire, Takeda, research funding from the Spanish Ministry of Economy and Competitiveness – Centro de Investigación Biomedica en Red area de Salud Mental (CIBERSAM) and Instituto de Salud Carlos III-, Spanish Ministry of Health, Social Services and Equality - Plan Nacional Sobre Drogas outside of the submitted work. The other authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1. International Schizophrenia C, Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iyegbe C, Campbell D, Butler A, Ajnakina O, Sham PC. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for GxE research. Soc Psychiatry Psychiatr Epidemiol. 2014;49:169–182. [DOI] [PubMed] [Google Scholar]
- 4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 5. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. [DOI] [PubMed] [Google Scholar]
- 6. Wickham H, Murray RM. Can biological markers identify endophenotypes predisposing to schizophrenia? Int Rev Psychiatry. 1997;9(4):355–364. [Google Scholar]
- 7. Chan RCK, Li H, Cheung EFC, Gong Q. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178(2):381–390. [DOI] [PubMed] [Google Scholar]
- 8. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36(5):1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barkl SJ, Lah S, Starling J, Hainsworth C, Harris AWF, Williams LM. Facial emotion identification in early-onset psychosis. Schizophr Res. 2014;160(1-3):150–156. [DOI] [PubMed] [Google Scholar]
- 10. Daros AR, Ruocco AC, Reilly JL, Harris MSH, Sweeney JA. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr Res. 2014;153(1–3):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosnjak Kuharic D, Makaric P, Kekin I, et al. Differences in facial emotional recognition between patients with the first-episode psychosis, multi-episode schizophrenia, and healthy controls. J Int Neuropsychol Soc. 2018;25(2):165–173. [DOI] [PubMed] [Google Scholar]
- 12. Caldiroli A, Buoli M, Serati M, Cahn W, Altamura AC. General and social cognition in remitted first-episode schizophrenia patients: a comparative study. Eur Arch Psychiatr Clin Neurosci. 2016;266(7):639–647. [DOI] [PubMed] [Google Scholar]
- 13. Catalan A, Gonzalez de Artaza M, Bustamante S, et al. Differences in facial emotion recognition between first episode psychosis, borderline personality disorder and healthy controls. PLoS One. 2016;11(7):e0160056. doi: 10.1371/journal.pone.0160056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maat A, van Montfort SJT, de Nijs J, et al. Emotion processing in schizophrenia is state and trait dependent. Schizophr Res. 2015;161(2-3):392–398. [DOI] [PubMed] [Google Scholar]
- 15. Andric S, Maric NP, Mihaljevic M, Mirjanic T, van Os J. Familial covariation of facial emotion recognition and IQ in schizophrenia. Psychiatry Res. 2016;246:52–57. [DOI] [PubMed] [Google Scholar]
- 16. Bediou B, Asri F, Brunelin J, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br J Psychiatry. 2018;191(2):126–130. [DOI] [PubMed] [Google Scholar]
- 17. Kohler CG, Richard JA, Brensinger CM, et al. Facial emotion perception differs in young persons at genetic and clinical high-risk for psychosis. Psychiatry Res. 2014;216(2):206–212. [DOI] [PubMed] [Google Scholar]
- 18. Ruocco AC, Reilly JL, Rubin LH, et al. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr Res. 2014;158(1-3):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson EB, Kirby A, Ruparel K, et al. The genetic architecture of pediatric cognitive abilities in the Philadelphia Neurodevelopmental Cohort. Mol Psychiatry. 2015;20(4):454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coleman JRI, Lester KJ, Keers R, Munafo MR, Breen G, Eley TC. Genome-wide association study of facial emotion recognition in children and association with polygenic risk for mental health disorders.. Am J Med Genet B Neuropsychiatr Genet. 2017;9999:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Germine L, Robinson EB, Smoller JW, et al. Association between polygenic risk for schizophrenia, neurocognition and social cognition across development. Transl Psychiatry. 2016;6(10):e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xavier RM, Dungan JR, Keefe RSE, Vorderstrasse A. polygenic signal for symptom dimensions and cognitive performance in patients with chronic schizophrenia. Schizophrenia Res. Cogn. 2018;12:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Donovan MC, Owen MJ. The implications of the shared genetics of psychiatric disorders. Nat Med. 2016;22:1214–1219. [DOI] [PubMed] [Google Scholar]
- 24. Gayer-Anderson C, Jongsma HE, Di Forti M, et al. The EUropean Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI) – incidence and first-episode case-control programme. Soc Psychiatry Psychiatr Epidemiol. 2020;55:645–657. [DOI] [PubMed] [Google Scholar]
- 25. Jongsma HE, Gayer-Anderson C, Lasalvia A, et al. Treated incidence of psychotic disorders in the multinational eu-gei study. JAMA Psychiatry. 2018;75(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mallett R. Sociodemographic Schedule. London: Section of Social Psychiatry, Institute of Psychiatry; 1997. [Google Scholar]
- 28. McGuffin P, Farmer A, Harvey IA. Polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–770. [DOI] [PubMed] [Google Scholar]
- 29. Quattrone D, Di Forti M, Gayer-Anderson C, et al. Transdiagnostic dimensions of psychopathology at first episode psychosis: findings from the multinational EU-GEI study. Psychol Med. 2019;49(8):1378–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. [DOI] [PubMed] [Google Scholar]
- 31. Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46(2-3):209–215. [DOI] [PubMed] [Google Scholar]
- 32. Velthorst E, Levine SZ, Henquet C, et al. To cut a short test even shorter: Reliability and validity of a brief assessment of intellectual ability in Schizophrenia—a control–case family study. Cognit Neuropsychiatry. 2013;18(6):574–593. [DOI] [PubMed] [Google Scholar]
- 33. van ‘t Wout M, Aleman A, Kessels RPC, Larøi F, Kahn RS. Emotional processing in a non-clinical psychosis-prone sample. Schizophr Res. 2004;68(2):271–281. [DOI] [PubMed] [Google Scholar]
- 34. van Ommen MM, van Beilen M, Cornelissen FW, et al. The prevalence of visual hallucinations in non-affective psychosis, and the role of perception and attention. Psychol Med. 2016;46:1735–1747. [DOI] [PubMed] [Google Scholar]
- 35. de Nijs J, Meijer JH, de Haan L, et al. Associations between olfactory identification and (social) cognitive functioning: a cross-sectional study in schizophrenia patients and healthy controls. . Psychiatric Res. 2018;266:147–151. [DOI] [PubMed] [Google Scholar]
- 36. Meijer J, Simons CJP, Quee PJ, Verweij K, Group I. Cognitive alterations in patients with non-affective psychotic disorder and their unaffected siblings and parents. Acta Psychiatr Scand. 2012;125:66–76. [DOI] [PubMed] [Google Scholar]
- 37. Fett AKJ, Maat A; GROUP Investigators . Social cognitive impairments and psychotic symptoms: what is the nature of their association? Schizophr Bull. 2013;39(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tognin S, Catalan A, Modinos G, et al. Emotion recognition and adverse childhood experiences in individuals at clinical high risk of psychosis. Schizophr Bull. 2020;46(4):823–833. 10.1093/schbul/sbz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benton AL, Van Allen MW. Impairment in facial recognition in patients with cerebral disease. Cortex. 1968;4:344–IN1. [PubMed] [Google Scholar]
- 40. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. [DOI] [PubMed] [Google Scholar]
- 41. Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Loh P-R, Danecek P, Palamara PF, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48(11):1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCarthy S, Das S, Kretzschmar W, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173(7):1705–1715.e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stahl EA, Breen G, Forstner AJ, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ripke S, Wray NR, Lewis CM, et al. ; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium . A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howard DM, Adams MJ, Clarke T-K, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vassos E, Di Forti M, Coleman J, et al. An examination of polygenic score risk prediction in individuals with first-episode psychosis. Biol Psychiatry. 2017;81(6):470–477. [DOI] [PubMed] [Google Scholar]
- 49. StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- 50. Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. 2003;3(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 52. Romero-Ferreiro MV, Aguado L, Rodriguez-Torresano J, Palomo T, Rodriguez-Jimenez R, Pedreira-Massa JL. Facial affect recognition in early and late-stage schizophrenia patients. Schizophr Res. 2016;172(1–3):177–183. [DOI] [PubMed] [Google Scholar]
- 53. Tripoli G, Quattrone D, Ferraro L, et al. Jumping to conclusions, general intelligence, and psychosis liability: findings from the Multicentric EU-GEI Case-Control Study. Psychol Med. 2021;51(4):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lawrence K, Campbell R, Skuse D. Age, gender, and puberty influence the development of facial emotion recognition. Front Psychol. 2015;6:761. 10.3389/fpsyg.2015.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160(10):1768–1774. [DOI] [PubMed] [Google Scholar]
- 56. van Dijke A, van ‘t Wout M, Ford JD, Aleman A. Deficits in degraded facial affect labeling in schizophrenia and borderline personality disorder. PLoS One. 2016;11(6):e0154145. 10.1371/journal.pone.0154145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leppänen JM, Niehaus DJH, Koen L, Du Toit E, Schoeman R, Emsley R. Deficits in facial affect recognition in unaffected siblings of Xhosa schizophrenia patients: evidence for a neurocognitive endophenotype. Schizophr Res. 2008;99(1):270–273. [DOI] [PubMed] [Google Scholar]
- 58. Allott KA, Rice S, Bartholomeusz CF, et al. Emotion recognition in unaffected first-degree relatives of individuals with first-episode schizophrenia. Schizophr Res. 2015;161(2):322–328. [DOI] [PubMed] [Google Scholar]
- 59. Martin D, Croft J, Pitt A, Strelchuk D, Sullivan S, Zammit S. Systematic review and meta-analysis of the relationship between genetic risk for schizophrenia and facial emotion recognition. Schizophr Res. 2020;218:7–13. doi: 10.1016/j.schres.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 60. Dalili MN, Penton-Voak IS, Harmer CJ, Munafò MR. Meta-analysis of emotion recognition deficits in major depressive disorder. Psychol Med. 2014;45(6):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martin AR, Daly MJ, Robinson EB, Hyman SE, Neale BM. Predicting polygenic risk of psychiatric disorders. Biol Psychiatry. 2019;86(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav Cogn Neurosci Rev. 2002;1(1):21–62. [DOI] [PubMed] [Google Scholar]
- 63. Johnston PJ, Enticott PG, Mayes AK, Hoy KE, Herring SE, Fitzgerald PB. Symptom correlates of static and dynamic facial affect processing in schizophrenia: evidence of a double dissociation? Schizophr Bull. 2008;36(4):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hargreaves A, Mothersill O, Anderson M, Lawless S, Corvin A, Donohoe G. Detecting facial emotion recognition deficits in schizophrenia using dynamic stimuli of varying intensities. Neurosci Lett. 2016;633:47–54. [DOI] [PubMed] [Google Scholar]
- 65. Peterson RE, Kuchenbaecker K, Walters RK, et al. Genome-wide association studies in ancestrally diverse populations: opportunities. Methods Pitfalls Recomm. Cell. 2019;179(3):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ventura J, Wood RC, Jimenez AM, Hellemann GS. Neurocognition and symptoms identify links between facial recognition and emotion processing in schizophrenia: meta-analytic findings. Schizophr Res. 2013;151(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.