Abstract
To examine long-term effects of early intervention services (EIS) for first-episode psychosis, we compared Heinrichs-Carpenter Quality of Life (QLS) and Positive and Negative Syndrome Scale (PANSS) scores and inpatient hospitalization days over 5 years with data from the site-randomized RAISE-ETP trial that compared the EIS NAVIGATE (17 sites; 223 participants) and community care (CC) (17 sites; 181 participants). Inclusion criteria were: age 15–40 years; DSM-IV diagnoses of schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, or psychotic disorder not otherwise specified; first psychotic episode; antipsychotic medication taken for ≤6 months. NAVIGATE-randomized participants could receive NAVIGATE from their study entry date until NAVIGATE ended when the last-enrolled NAVIGATE participant completed 2 years of treatment. Assessments occurred every 6 months. 61% of participants had assessments conducted ≥2 years; 31% at 5 years. Median follow-up length was CC 30 months and NAVIGATE 38 months. Primary analyses assumed data were not-missing-at-random (NMAR); sensitivity analyses assumed data were missing-at-random (MAR). MAR analyses found no significant treatment-by-time interactions for QLS or PANSS. NMAR analyses revealed that NAVIGATE was associated with a 13.14 (95%CI:6.92,19.37) unit QLS and 7.73 (95%CI:2.98,12.47) unit PANSS better improvement and 2.53 (95%CI:0.59,4.47) fewer inpatient days than CC (all comparisons significant). QLS and PANSS effect sizes were 0.856 and 0.70. NAVIGATE opportunity length (mean 33.8 (SD = 5.1) months) was not associated (P = .72) with QLS outcome; duration of untreated psychosis did not moderate (P = .32) differential QLS outcome. While conclusions are limited by the low rate of five-year follow-up, the data support long-term benefit of NAVIGATE compared to community care.
Keywords: coordinated specialty care, schizophrenia, follow-up, early intervention services, first episode psychosis
Introduction
Early intervention services (EIS)1 for first-episode psychosis (FEP) provide enriched treatment for defined periods and produce better outcomes than standard care during EIS participation.2 However, the British Lambeth Early Onset (LEO) study,3 the Danish OPUS I trial,4,5 and the Norwegian Optimal Treatment Project (OTP)6 which provided EIS from 1.5 to 2 years did not find better symptom outcomes after EIS discontinuation4–6 and only OPUS I found fewer hospitalization days with EIS (solely during the first 3 years).
The RAISE-Early Treatment Program (RAISE-ETP) (Clinicaltrials.gov registration NCT01321177) was the first US-based, multi-center randomized trial comparing an EIS (NAVIGATE) to standard care. NAVIGATE was associated with better treatment retention and improvement in symptoms and quality of life but no difference in inpatient hospitalization over the first 2 years of participation.7 This report extends outcome comparisons to trajectories over 5 years, a period encompassing EIS, and subsequent non-EIS treatment.
Methods
RAISE-ETP’s design has been described.8 Participants were aged 15–40 years, had Structured Clinical Interview for DSM-IV (SCID)9-verified diagnoses of schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, or psychotic disorder not otherwise specified, had experienced only one psychotic episode and had taken ≤6 months of lifetime antipsychotic medications.
Details of NAVIGATE have been reported10,11 (see Supplemental Materials). NAVIGATE includes four coordinated interventions: personalized medication management (PMM) with a computer decision-support system; family psychoeducation (FE); resilience-focused individual therapy (IRT); and supported education/employment (SEE). Manuals are available at https://navigateconsultants.org/manuals.html. FE and IRT teach information and skills; once acquired, participation tapers. SEE is provided as needed. PMM continues for the full duration of NAVIGATE treatment. RAISE-ETP fidelity assessments12 found relatively strong IRT, FE, PPM, and NAVIGATE team structure implementation and adequate SEE implementation.
The control condition, “Community Care” (CC), was treatment determined by clinician choice without restrictions. CC sites received training on recruitment, research data collection, and enhancing retention in research assessments but no training about treatment.
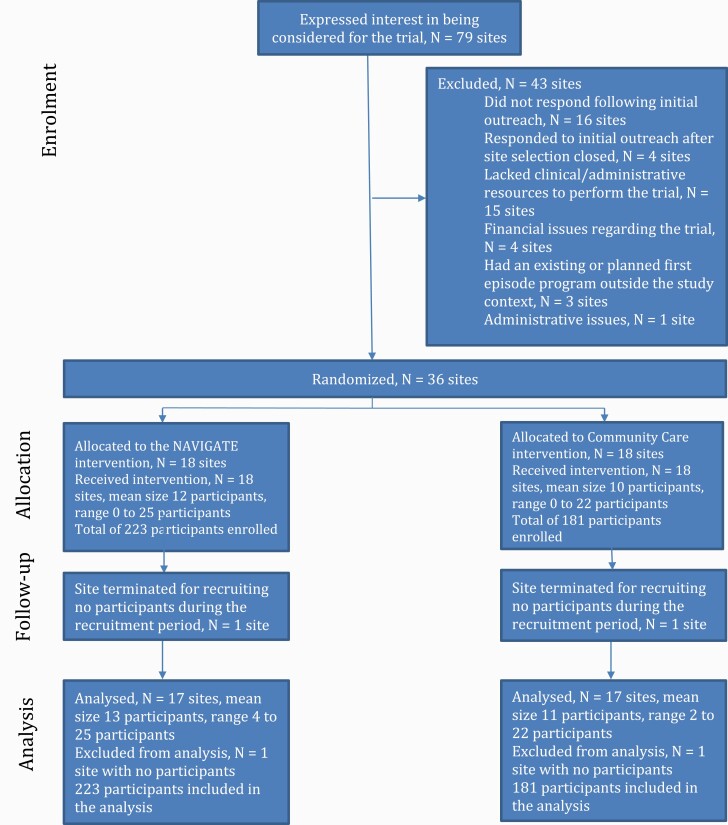
RAISE-ETP utilized site (cluster) randomization because providing NAVIGATE and separate CC treatment as required by patient randomization was not feasible at community facilities. Outcomes were participant level. Sites were required to be community treatment facilities, have no preexisting first-episode program, and have sufficient resources to provide NAVIGATE if so randomized. Thirty-five centers were selected via national search.8 To minimize the potential for sites randomized to the two treatment conditions to differ, sites were assigned before randomization to groups with sites within each group having similar racial/ethnic patient distribution and location. Study statisticians then randomly assigned sites within each group 1:1 to provide NAVIGATE or CC to all their eligible participants. Thus, treatment assignment of sites was random but constrained so that sites with similar characteristics were equally assigned to the treatment conditions. One site in each condition recruited no participants and were terminated. An additional site was subsequently recruited and randomized by the statisticians. These procedures resulted in 17 NAVIGATE and 17 CC sites (figure 1).
Fig. 1.
Participant flow.
To examine whether length of NAVIGATE treatment influenced outcomes, the protocol specified a varying length by participant of opportunity for NAVIGATE. All NAVIGATE-randomized participants had a minimum length of 2 years of opportunity for NAVIGATE but by design most participants had longer opportunity. All NAVIGATE-randomized participants started NAVIGATE at the time of enrollment. Enrollment occurred between July 2010 and July 2012. NAVIGATE was available until July 2014. By then, the last enrolled participant (enrolled in July 2012) had had the opportunity to receive 2 years of NAVIGATE. Those enrolled before July 2012 had >2 years of opportunity (individual enrollment date to July 2014) for NAVIGATE. After July 2014, NAVIGATE ended (eg the computer decision-support system was deactivated) and participants received care as determined by the sites (ie remain with current providers, transfer to other agency providers, or transfer to another agency).
At study entry, adult participants and guardians of those under 18 provided written consent and those under 18 written assent. A second written consent was obtained for procedures after July 2014. The study was approved by the coordinating center’s and participating sites’ institutional review boards.
Assessment occurred every 6 months for 5 years. Participants were encouraged to continue assessments even if they discontinued treatment. The primary outcome measure was the Heinrichs-Carpenter Quality of Life Scale (QLS)13 covering sense of purpose, motivation, emotional and social interaction, role functioning, and engagement in regular activities. QLS total scores range from 0 (worst) to 126 (best). The assessment protocol used until July 2014 has been reported.8 Afterwards the battery of every 6-month assessments consisted of the QLS, the Positive and Negative Syndrome Scale (PANSS)14 to measure symptoms, and the Service Use and Resource Form (SURF).15,16 The SURF provided participant report of days of hospitalization, confirmed when possible by records. All QLS and PANSS interviews were obtained via videoconferencing by remote raters masked to treatment assignment and study site; SURF interviews included masked and unmasked assessments depending upon study phase and site. The last assessment occurred in October 2017.
Sample size: RAISE-ETP was a 3-level cluster-randomized RCT with level 1 representing the measurement occasion nested within patients and centers, level 2 representing the patient nested within center, and level 3 the center. A sample of 400 was estimated to provide power of ≥0.80 to detect a difference of at least 7 (ie 0.35 SD units with SD = 20) QLS units at month 24 between the conditions assuming a Level 2 intra-class correlation (ICC) of 0.30 and a Level 3 ICC of 0.10.17
Statistical Analyses
Because complete long-term assessment of young people with psychosis is difficult to obtain (eg4,18) and dropout by young people with psychosis can be driven by positive (eg moving for school opportunity) negative (eg lack of insight) or neutral (eg moving with parents to a new location) clinical events, we first examined missing data patterns. The three assumptions about missing data employed with usual longitudinal trial data are missing completely at random (MCAR), missing at random (MAR), and not missing at random (NMAR). NMAR (ie the factors associated with dropout are not fully captured in the observed data) is the more general assumption compared with MAR (ie the factors associated with dropout are captured by the observed data) and the generally unrealistic MCAR assumption of covariate independent missingness. The a-priori analytic plan required that the assumptions of a MAR model be examined and specified a NMAR model if the MAR assumption was not justified. We compared characteristics of participants with a post-baseline assessment who (1) participated only during the first 2 years (the minimum treatment duration for NAVIGATE-randomized participants) or (2) participated after the first 2 years. Measures examined were those at baseline and at the last evaluation during the first 2 years. As presented in the Results and Supplemental Material, the comparisons revealed complex treatment-related missing data patterns which led to our decision to model the data using NMAR approaches.
Two NMAR data analysis approaches are selection models19 and pattern mixture models.20 Selection models assume that outcomes are subject to selection effects and include a model for dropout and for outcome which are linked through a shared parameter such as one or more random-effects.21 Pattern mixture models stratify the sample into mutually exclusive missing data patterns and evaluate the joint likelihood conditional on each missing data pattern. Selection models were the primary approach to analysis of QLS and PANSS data; pattern mixture models were used for hospitalization data.
Analyses followed the intention-to-treat principle. The a-priori analysis plan specified that variables that differed between the two treatment groups at baseline and were correlated with outcome be included as covariates in the analyses.7 Based upon these criteria, baseline characteristics of sex, race, and current student status were included in all models.
Estimation of QLS and PANSS Outcomes
Modeling for the NMAR shared parameter model used the approach of Hedeker and Gibbons22 (pp 295–302). Analysis of QLS and PANSS total and subscale scores used a 3-level mixed effects linear regression model:
with a random intercept and a random slope for level 2 (patient) and a random intercept for level 3 (site). Time is the square root of month/6 to accommodate time trend nonlinearity. Graphical depictions are in the original metric of month and therefore curvilinear. The drop-out model included the main effect of treatment, two shared random effects from the outcome model, and the treatment by random effect interactions.
The treatment effect is measured by slope differences between treatment groups. The shared parameters between the longitudinal and time-to-dropout parts of the model were the main effect of treatment, patient-level random intercepts and slopes, and the interactions between treatment and the two random effects.
Sensitivity analyses of QLS and PANSS outcomes are described in Supplemental Materials. These included outcome analyses (MAR and completer) that take less account of dropout effects than the more general NMAR models.
Estimation of Other Outcomes
NAVIGATE-randomized participants had different lengths of opportunity for NAVIGATE. To explore opportunity effects, opportunity was added as a time-varying covariate (CC participant opportunity was set as 0) to a model of QLS total score with treatment and the treatment-by-time interaction.
To examine duration of untreated psychosis (DUP), the length of time between psychotic symptom onset and initiation of antipsychotic treatment, as a moderator of treatment effects, main-effects, two-way and three-way interactions with treatment and time were added to the models. The DUP-by-treatment-by-time interaction tests whether treatment-related effects are independent of DUP.
Poisson mixed-effects model with site as random intercept was used to analyze number of hospital days. Least square means obtained from SAS GLIMMIX procedure were exponentiated to obtain average hospitalization days for each treatment by pattern mixture category. Two years was the minimum duration of NAVIGATE treatment offered to participants randomized to NAVIGATE and follow-up lasted 5 years. The three pattern mixture categories to analyze were chosen as (1) last assessment before 2 years, (2) last assessment ≥2 years but <5 years, and (3) last assessment at 5 years. Pooled estimate for each treatment was obtained following Hedeker and Gibbons22 and the corresponding standard error obtained using the Delta method.23
Effect Sizes (ES)
Effect sizes for the QLS and PANSS were calculated as the estimated mean difference of an outcome of interest divided by the estimated pooled standard deviation at 5 years.
Results
Figure 1 presents participant flow. NAVIGATE sites enrolled 223 individuals and CC 181. Baseline participant characteristics have been published7 and are summarized in Supplemental Table 1. Participants had a mean age of 23 and 72.5% were men. Fifty-three percent met DSM-IV criteria for schizophrenia; the next most frequent diagnoses were schizoaffective disorder (21%) and schizophreniform disorder (13%).
NAVIGATE Services
The RAISE-ETP SURF included questions about NAVIGATE-type services. Rates of these services (table 1) were much higher at NAVIGATE compared with CC sites before the end of NAVIGATE treatment in July 2014. Rates after July 2014 were substantially lower than before July 2014 and similar between CC and NAVIGATE sites. During the provision of NAVIGATE before July 2014, NAVIGATE psychosocial interventions rates were higher during the first 2 years of participation than afterwards (Supplemental Table 2).
Table 1.
Service Use and Resource Form (SURF) Data on NAVIGATE-type Services Provided Before and After July 2014
| SURF Question [Corresponding NAVIGATE Service] | SURFs Covering Period to July 2014 | SURFs Covering Period After July 2014 | ||
|---|---|---|---|---|
| % of Yes Responses on Community Care SURFs | % of Yes Responses on NAVIGATE SURFs | % of Yes Responses on Community Care SURFs | % of Yes Responses on NAVIGATE SURFs | |
| Have you met with a person who is helping you get a job in the community or furthering your education (for example a supported employment specialist)? [Supported education and employment] | 10.4% | 33.0% | 7.5% | 10.0% |
| Has your family met with a mental health provider to help them understand and address your situation? [Family psychoeducation] | 5.4% | 26.2% | 3.0% | 1.9% |
| Have you had individual sessions with a mental health provider that help you work on your goals and look positively towards the future? [Individual resiliency training] | 29.5% | 53.7% | 4.1% | 7.5% |
| Were you asked to record your symptoms and side effects before you met with your psychiatrist or nurse practitioner? [NAVIGATE COMPASS medication and health visit] | 10.9% | 50.9% | 5.6% | 8.2% |
Data Patterns
One hundred fifty-eight participants had a last observation before 2 years of follow-up, 126 had their last observation at ≥2 years but <5 years and 120 had an observation at 5 years. Median time for last assessment for CC was 30 months and 38 months for NAVIGATE (comparison Log-rank P-value = .547) (Supplemental Figure 1). Our design encouraged participants to continue assessment even if they discontinued treatment. Examination (Supplemental Materials) of the SURF assessments showed that some participants provided assessments when not in treatment.
The only baseline characteristic with detectable differences between participants with a post-baseline assessment who participated only during the first 2 years and those who participated longer was treatment assignment (Supplemental Table 4). In contrast, several differences were detectable for last observation within the first 2 years (Supplemental Table 5). Those who participated longer had better scores on the PANSS and QLS and reported that they were more likely to complete the study.
Overall, the most common causes of dropout recorded by sites were lost to follow-up (34%) followed by declined assessments (10%) and moving out of area (10%) (Supplemental Table 6).
The NMAR dropout models (table 2) revealed that CC participants who discontinued had lower quality of life and more severe psychotic symptomatology. The reverse was found for NAVIGATE participants where discontinuation was associated with increased quality of life and lower psychotic symptom severity. Specifically, CC dropouts had decreased QLS scores over time (marginal maximum likelihood estimate [MMLE] = −1.248, SE = 0.327, P < .001) with an overall difference of 3.16*–1.248 = −3.94 units whereas NAVIGATE dropouts had increased QLS scores of (−1.248 + 1.555)*3.16 = 0.97 units over time (treatment-by-random-time trend interaction MMLE = 1.555, SE = 0.436, P < .001). Over the 5-year period, scores for CC and NAVIGATE dropouts differed by 1.555*3.16 = 4.91 units. CC dropouts had increased PANSS scores over time (MMLE = 1.281, SE = 0.277, P < .001), but those in the NAVIGATE group had decreased PANSS scores over time (treatment by trend interaction MMLE = −1.287, SE = 0.328, P < .001). Over the 5-year period, there is a difference of 9.97 units between CC dropout PANSS scores and NAVIGATE group dropout PANSS scores. These complex treatment-related missing data patterns further supported the appropriateness of our NMAR approach. Outcomes for participants who dropout at different study phases are presented in Supplemental Figure 2.
Table 2.
Quality of Life Total Score and Positive and Negative Syndrome Scale Total Score Analyses Based Upon Shared Parameter Model
| Estimate | SE | P-value | |
|---|---|---|---|
| Quality of Life Total Score | |||
| Outcome model | |||
| Treatment | −2.812 | 1.829 | .125 |
| Month | 2.452 | 0.707 | .001 |
| Treatment by month | 4.162 | 1.002 | <.001 |
| Dropout model | |||
| Treatment | −0.150 | 0.169 | .376 |
| Random intercept | 0.053 | 0.168 | .753 |
| Random slope | −1.248 | 0.327 | <.001 |
| Treatment by random intercept | −0.385 | 0.209 | .067 |
| Treatment by random slope | 1.555 | 0.436 | <.001 |
| Positive and Negative Syndrome Scale Total Score | |||
| Outcome model | |||
| Treatment | 2.580 | 1.464 | .079 |
| Month | −3.895 | 0.562 | <.001 |
| Treatment by month | −2.445 | 0.764 | .002 |
| Dropout model | |||
| Treatment | −0.141 | 0.166 | .397 |
| Random intercept | −0.134 | 0.181 | .459 |
| Random slope | 1.281 | 0.277 | <.001 |
| Treatment by random intercept | 0.351 | 0.209 | .094 |
| Treatment by random slope | −1.287 | 0.328 | <.001 |
The table presents marginal maximum likelihood estimates (MMLE), standard errors, and P-values for main effects of treatment, time, and the treatment by time interaction. Time is measured in months and to linearize the time effect a square root transformation22 is used. The dropout model relates treatment, person-specific deviations in the intercept and slope of the time trends, and the treatment by time trend interactions to the probability of dropout, which is jointly modeled with the treatment-related effects on the two outcome measures. To obtain the estimated treatment difference for a specific month, the square root of month is multiplied by the treatment by month interaction. The main effect of treatment is the treatment difference at baseline. The main effect of month is the month effect in the Community Care group.
Primary Outcome
QLS Total Score ( table 2 and figure 2 ).
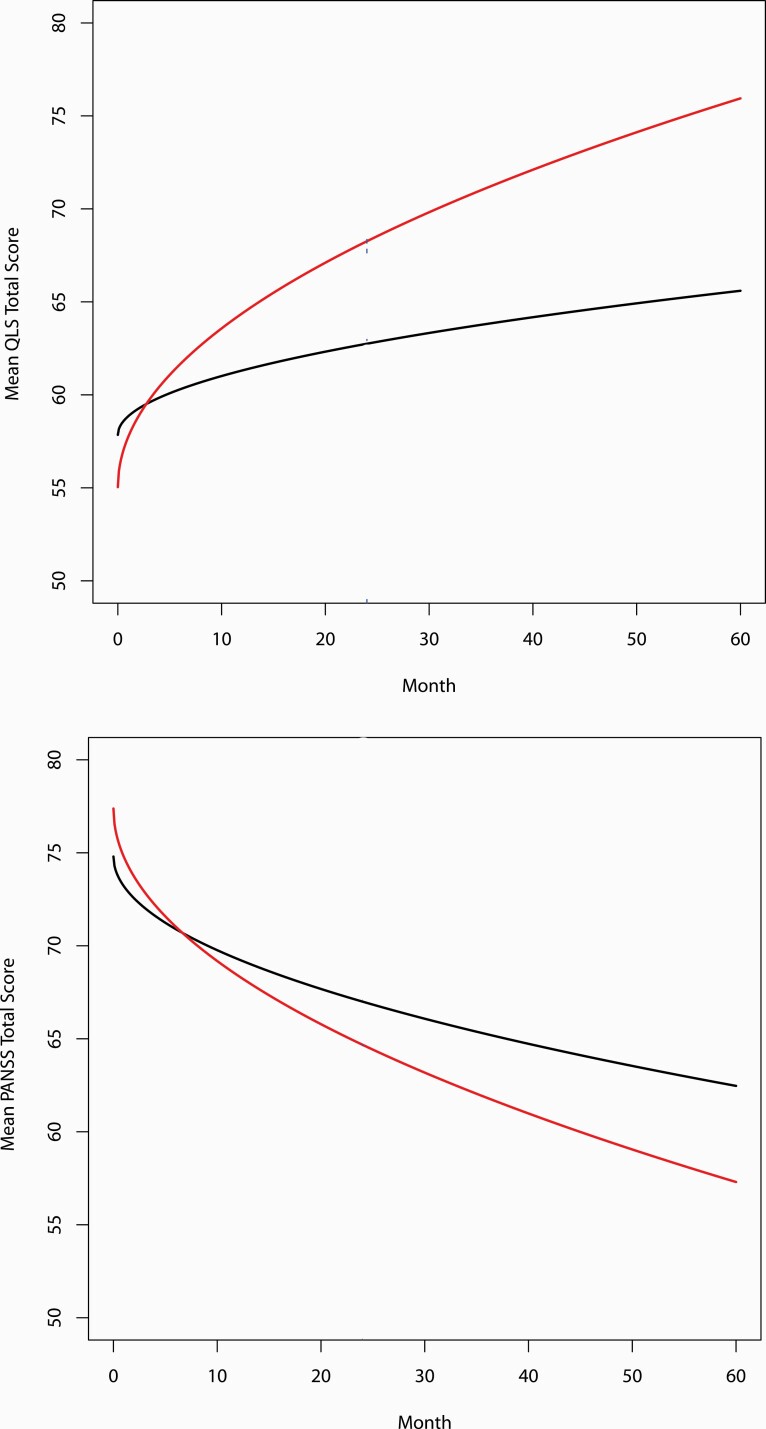
Fig. 2.
Shared parameter model of Heinrichs Carpenter Quality of Life Scale (QLS) total scores and Positive and Negative Syndrome Scale (PANSS) total scores.Dark line = Community Care estimated from model; Red line = NAVIGATE estimated from model.
The ICC for the baseline total score was 0.087. The shared parameter outcome model revealed a significant overall treatment-by-time interaction (MMLE = 4.162, SE = 1.002, P < .001). This estimate is based on the square root of month/6; the unit difference over the five year trajectory is computed as 3.16*4.16 = 13.14 units favoring NAVIGATE, with the multiplier 3.16 being the sqrt(60/6). Effect size was 0.856.
Sensitivity analyses (Supplemental Materials) included analyses that account less for dropout effects than the NMAR models. If dropout effects are important, analyses that account less for dropout effects should detect less differences between CC and NAVIGATE if CC dropouts had worse clinical trajectories than NAVIGATE dropouts as revealed in the dropout models. This was the pattern observed. The MAR analysis that takes into account dropout effects based upon observed baseline and covariate outcomes (but not unobserved outcomes as NMAR does) showed an estimated QLS treatment difference favoring NAVIGATE, as the NMAR analyses did, but the P-value was at a trend instead of a significant level.
Secondary Outcomes
PANSS Total Score ( table 2 and figure 2 ).
The shared parameter outcome model identified a significant overall treatment-by-time interaction (MMLE = −2.445, SE = 0.764, P < .002), with a −7.73 unit score difference over the five-year study favoring NAVIGATE. Effect size was 0.70.
QLS and PANSS Subscale Scores ( table 3 ).
Table 3.
Shared Parameter Model for Quality of Life Subscale and Positive and Negative Syndrome Factor Scores. Treatment-By-Time Interactions
| Years 1–5 | |||
|---|---|---|---|
| Treatment-by-time Interactiona | |||
| Estimateb | SE | P-value | |
| Heinrichs-Carpenter Quality of Life Scale Subscales | |||
| Interpersonal relations | 1.88 | 0.44 | <.0001 |
| Instrumental role | 1.22 | 0.41 | .003 |
| Intrapsychic foundation | 0.94 | 0.35 | .007 |
| Common objects and activities | 0.20 | 0.10 | .044 |
| Positive and Negative Syndrome Scale Factor Scores 24 | |||
| Positive | −0.59 | 0.21 | .005 |
| Negative | −0.39 | 0.26 | .130 |
| Disorganized/concrete | −0.38 | 0.12 | .001 |
| Excited | −0.42 | 0.13 | .001 |
| Depressed | −0.34 | 0.15 | .023 |
Baseline characteristics of sex, race, and current student status were included in all models.
a‘time’ is defined as: SQRT(month/6) where month ranges from 0 to 60. Month is the month of the assessment.
bEstimate is interpreted as the difference of outcome slopes with respect to ‘time’ between NAVIGATE and Community Care treatment.
Treatment-by-time interactions for all the QLS subscales (interpersonal relations, instrumental role, intrapsychic foundation, and common objects and activities) were statistically significant. For the PANSS, the positive, disorganized/concrete, excited, and depressed subscales were also statistically significant. Estimated treatment effects were in the same direction but of smaller magnitude than their corresponding total score. Only the PANSS negative symptoms subscale treatment-by-time effect was not significant.
Length of Opportunity for NAVIGATE and Its Effect on QLS Total Scores.
Mean opportunity to receive NAVIGATE for NAVIGATE-randomized participants was 33.8 (SD = 5.1) months (range 24.8–44.4, median = 33.9 months). NAVIGATE opportunity length was not a significant effect on QLS total score outcomes (MMLE = 0.0403, SE = 0.1103, P = .72).
DUP and QLS Total Scores ( Supplemental Figure 2 ).
The test for the moderating effect of DUP on differential treatment related effects over time was not significant (MMLE = 0.080, SE = 0.081, P = .32).
Days of Hospitalization.
The overall estimate of days hospitalized, pooled across drop-out categories, was 5.97 (SD = 16.9) for CC and 3.46 (SD = 10.5) for NAVIGATE. Z-score for the pooled difference was 2.53 and the corresponding P-value = .02. The least-square means for hospitalized days by pattern mixture category for CC vs NAVIGATE were: 0.91 vs 1.41 for <2 years follow-up (N = 158), 9.01 vs 5.14 for ≥2 years but <5 years follow-up (N = 127) and 11.28 vs 3.58 for 5 years of follow-up (N = 119). Means differed significantly only for the 5-year category (P < .01).
Serious Adverse Events.
One NAVIGATE participant died and one CC participant committed a homicide. Suicide attempts without hospitalization occurred in 2 NAVIGATE and 5 CC participants. Seventy-two NAVIGATE and 65 CC participants had ≥ one psychiatric hospitalization; 15 in both conditions had a medical hospitalization. One participant in each condition became pregnant.
Discussion
The shared parameter analyses revealed substantial benefits over a 5-year timeframe from NAVIGATE compared to CC treatment with effect size of 0.856 on QLS and 0.70 on PANSS total scores. The differential improvement across 5 years for NAVIGATE-treated participants was 7.73 points for the PANSS and 13.14 points for the QLS total scores. For context, the minimal clinically-important difference for QLS total scores is 5.3 points25 and RAISE-ETP baseline QLS scores estimated from the shared parameter analyses were only 55.04 for NAVIGATE and 57.85 for CC. CC treatment was associated with QLS and PANSS score improvements. Thus, the NAVIGATE differential gains over CC treatment were additional gains beyond the improvements found with an effective CC treatment. The QLS and PANSS subscale analyses showed that the NAVIGATE differential improvement was found across almost all subscales suggesting a broad effect.
NAVIGATE treatment was associated with fewer days of inpatient hospitalization over 5 years. Other EIS studies have found reduced hospitalization compared with usual care during active treatment,2 but not enduring effects.3–6 Our 2.53 hospitalization days difference between NAVIGATE and CC may be small from an individual perspective but substantial from a policy perspective when considered across many individuals in large scale initiatives.
Among studies examining EIS vs usual care long-term effects, RAISE-ETP’s sample size was similar to OPUS I4 (404 vs 547) and the minimum RAISE-ETP EIS treatment duration (24.8 months) was also similar to the fixed treatment duration of 18 months with LEO3 and 24 months with OPUS4 and OTP.6 Despite these similarities, there are important study differences. The EIS studied and the health care delivery environments differed and there are notable methodologic differences. Our outcomes were outcome trajectories over 5 years versus the mostly cross-sectional outcomes in other studies. Further, we used NMAR analysis models versus the MAR models in other work. Future research is needed to evaluate the influence of these factors.
Our design included variable lengths of opportunity for NAVIGATE services by participant with a minimum opportunity of 2 years. NAVIGATE opportunity length (range 24.8–44.4 months) was not associated with 5-year QLS trajectories. One possible factor is that NAVIGATE psychosocial services are manual-based and the treatment model is for participants to no longer attend sessions once they complete the appropriate manual sections (participants can return for additional sessions if needed). Previously published data on services during the first 2 years of treatment showed decreased service use over time7 and the currently presented data (Supplemental Table 2) on services provided after the first two years show further decreases in NAVIGATE services; both consistent with the NAVIGATE treatment model. We evaluated outcome for all participants entering treatment; in contrast three trials26–28have examined continuing EIS after completing 2 years of EIS. Our dropout analyses show that the characteristics of patients who start treatment and those who have finished 2 years of treatment may differ. Albert and colleagues26 compared 3 additional years of OPUS treatment (N = 197) to regular care (N = 203) and at 5 years found no difference in negative symptoms (primary outcome) nor in psychotic dimension remission, suicidal ideation, substance abuse, medication compliance, months employed, and number of bed days. Chang and colleagues27 compared one additional year of EIS (N = 82) to regular care (N = 78) and assessed participants yearly for 3 years. At year 1, Social and Occupational Functioning Assessment Scale (SOFAS)29 scores, the primary outcome, favored the intervention group but not at 2- or 3-year follow-up. No differences in PANSS positive, negative, and general psychopathology scores were found at the 2- and 3-year follow-up. Malla and colleagues28 compared 3 years of additional EIS (N = 110) and regular care (N = 110). Over the follow-up period, intervention participants had greater length of time in remission of positive and negative symptoms. Given the divergence of study results, additional research is needed about which patients may benefit from extended EIS.
We did not find a significant moderating effect of DUP on QLS outcomes between treatment conditions over 5 years whereas we did in a prior analysis covering the first 2 years with the same sample.7 Comparison with other work is limited by the use of different study outcome measures. Findings in other work have been suggestive of an effect on negative symptoms but not other outcomes and only for participants with short DUP. Albert and colleagues30 examined outcomes of the extended OPUS treatment comparison described above with three different classifications of DUP length: DUP ≤1 month vs >1 month; DUP ≤3 months vs >3 months and DUP ≤6 months vs >6 months. No differences between extended OPUS and regular treatment were found with the DUP ≤1 month and ≤6 month comparisons. In the DUP ≤3 month comparison, trend level differences favoring extended OPUS treatment were found for disorganization symptoms and negative symptoms but not for psychotic symptoms, remission, work/school, or days in hospital. Dama and colleagues31 reported a significant DUP-by extended EIS vs return to usual care interaction effect on negative symptoms over 3 years using a DUP ≤3 month cutoff but not with other cutoffs and no effect on positive symptoms. Further research is needed to clarify whether DUP moderates the long-term effects of EIS given that EIS clinics often exclude individuals with long DUP (see32).
Generalizability
Our data are from clinics with no preexisting EIS programs located in 21 states, bolstering generalizability. Our sites had to have an interest in treatment research and be capable of providing NAVIGATE. They may have had interests and resources that differed from typical community clinics. If this occurred, it may have minimized differences between NAVIGATE and typical care, making our estimates of long-term benefit conservative.
Limitations
Our primary analyses used a NMAR approach, the most general assumption about missing data effects, but may not have accounted fully for the effects of missing data given the substantial dropout rate. Sensitivity MAR analyses found effects in the same direction as the MNAR analyses but differences between NAVIGATE and CC were not significant.
The SURF data about NAVIGATE-type services provided before and after NAVIGATE formally ended in July 2014 show a substantial decrease after July 2014. However, our protocol did not require that NAVIGATE participants be transferred to other agencies or clinicians once NAVIGATE ended. NAVIGATE participants who continued treatment at their agency may have received some degree of enhanced services over standard care if the skills acquired during the active phase of NAVIGATE provision were continued by clinicians trained in NAVIGATE or generalized within the agency.
Summary
While conclusions are limited by the low rate of five-year follow-up, the data support long-term benefit of NAVIGATE compared to community care. These benefits are important for making individual decisions about treatment and for policy decisions about program development, implementation, and support.
Supplementary Material
Acknowledgments
We are very grateful for the participation of the hundreds of patients and families who made the study possible with their time and commitment. We thank and acknowledge the work of the many clinicians, research assistants and administrators at the participating sites. Dr Robinson has been a consultant to Advocates for Human Potential, American Psychiatric Association, C4 Innovations, Costello Medical Consulting, Health Analytics, Innovative Science Solutions, Janssen, Lundbeck, Neurocrine, Neuronix, Otsuka, Teva and US WorldMeds and has received grant support from Otsuka. Dr Schooler has consulted or served on Advisory Boards for Alkermes, Allergan, GWPharmaceuticals, Intracellular Therapies, Lundbeck, Otsuka and Teva. She has received grant support form Otsuka. Ms. Marcy is executive director for the Vanguard Research Group. She reports grants from Alkermes, Boehringer-Ingelheim, Janssen, Lundbeck, NeuroRX, Otsuka, Roche and Takeda, and personal fees from Otsuka. Dr. Gibbons is a founder of Adaptive Testing Technologies. He has served as an expert witness for Merck, Pfizer and GlaxoSmithKline. Dr. Rosenheck serves on a Data Monitoring Committee sponsored by Boehringer Ingelheim Pharmaceuticals. Dr. Correll has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Relmada, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Relmada, Rovi, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of LB Pharma. Dr. Miller has received honoraria from INC Research for service on Data Monitoring Committees. Dr. Kane has been a consultant to or received honoraria for lectures from Alkermes, Allergan, Dainippon Sumitomo, Intracellular Therapies, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, and Teva. He has received grant support from Janssen, Lundbeck and Otsuka. He is a shareholder of The Vanguard Research Group and LB Pharma. Drs. Robinson, Meyer-Kalos, Gottlieb and Glynn and Mr. Lynde and Ms. Gingerich provide training and consultation about implementing NAVIGATE treatment that can include compensation. Dr. Mayer-Kalos, Dr. Gottlieb, Dr. Glynn, Ms. Gingerich and Mr. Lynde report no other conflicts. Drs. Brown, John, Mueser, Penn, Addington, Brunette, Estroff, Mr. Pipes and Ms. Severe report no conflicts.
Contributor Information
Delbert G Robinson, Departments of Psychiatry and of Molecular Medicine, The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; The Feinstein Institutes for Medical Research, Institute of Behavioral Science, Manhasset, NY, USA; The Zucker Hillside Hospital, Psychiatry Research, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA.
Nina R Schooler, Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, NY, USA.
Patricia Marcy, Vanguard Research Group, Glen Oaks, NY, USA.
Robert D Gibbons, Center for Health Statistics, University of Chicago, Chicago, IL, USA.
C Hendricks Brown, Departments of Psychiatry and Behavioral Sciences, Preventive Medicine and Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Majnu John, The Feinstein Institutes for Medical Research, Institute of Behavioral Science, Manhasset, NY, USA; Department of Mathematics, Hofstra University, Hempstead, NY, USA.
Kim T Mueser, Center for Psychiatric Rehabilitation, Boston University, Boston, MA, USA.
David L Penn, Department of Psychology, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA; School of Behavioural and Health Sciences, Australian Catholic University, Melbourne, Victoria, Australia.
Robert A Rosenheck, Department of Psychiatry, Yale Medical School, New Haven, CT, USA.
Jean Addington, Department of Psychiatry, University of Calgary Cumming School of Medicine, Calgary, AB, Canada.
Mary F Brunette, Department of Psychiatry, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Christoph U Correll, Departments of Psychiatry and of Molecular Medicine, The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; The Feinstein Institutes for Medical Research, Institute of Behavioral Science, Manhasset, NY, USA; The Zucker Hillside Hospital, Psychiatry Research, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA; Department of Child and Adolescent Psychiatry, Charite Universitatsmedizin, Berlin, Germany.
Sue E Estroff, Department of Social Medicine, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA.
Piper S Mayer-Kalos, Department of Psychiatry & Behavioral Sciences, University of Minnesota Medical School, Minneapolis, MN, USA.
Jennifer D Gottlieb, Division of Population Behavioral Health Innovation and Harvard Medical School, Department of Psychiatry, Cambridge Health Alliance, Cambridge, MA, USA.
Shirley M Glynn, Semel Institute of Neuroscience and Human Behavior, University of California, Los Angeles, CA, USA.
David W Lynde, Departments of Psychiatry and of Molecular Medicine, The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA.
Susan Gingerich, Departments of Psychiatry and of Molecular Medicine, The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA.
Ronny Pipes, Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Alexander L Miller, Department of Psychiatry and Behavioral Sciences San Antonio, UT Health San Antonio, TX, USA .
Joanne B Severe, Departments of Psychiatry and of Molecular Medicine, The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA .
John M Kane, Departments of Psychiatry and of Molecular Medicine, The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; The Feinstein Institutes for Medical Research, Institute of Behavioral Science, Manhasset, NY, USA; The Zucker Hillside Hospital, Psychiatry Research, North Shore - Long Island Jewish Health System, Glen Oaks, NY, USA.
Funding
Funding for the RAISE-ETP study was provided by the National Institute of Mental Health (NIMH), Bethesda, MD (HHSN-271-2009-00019C; PI: Dr. Kane). As a NIMH contract, NIMH had input into the design and conduct of the trial. NIMH staff did not participate in the analyses presented in this report. The contents of this report are solely the responsibility of the authors and do not necessarily represent the views of NIMH or the U.S. Department of Health and Human Services.
References
- 1. NIMH. Evidence-based treatments for first episode psychosis: components of coordinated specialty care. https://www.nimh.nih.gov/health/topics/schizophrenia/raise/evidence-based-treatments-for-first-episode-psychosis-components-of-coordinated-specialty-care.shtml. Accessed June 4, 2019.
- 2. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gafoor R, Nitsch D, McCrone P, et al. Effect of early intervention on 5-year outcome in non-affective psychosis. Br J Psychiatry. 2010;196(5):372–376. [DOI] [PubMed] [Google Scholar]
- 4. Bertelsen M, Jeppesen P, Petersen L, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry. 2008;65(7):762–771. [DOI] [PubMed] [Google Scholar]
- 5. Secher RG, Hjorthøj CR, Austin SF, et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull. 2014;41(3):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sigrúnarson V, Gråwe RW, Morken G. Integrated treatment vs. treatment-as-usual for recent onset schizophrenia; 12 year follow-up on a randomized controlled trial. BMC Psychiatry. 2013;13:200. doi: 10.1186/1471-244X-13-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2015;173(4):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. First M, Spitzer R, Gibbon M, Williams J.. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Reseach, New York State Psychiatric Institute; 2002. [Google Scholar]
- 10. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680–690. doi: 10.1176/appi.ps.201400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson DG, Schooler NR, Correll CU, et al. Psychopharmacological treatment in the RAISE-ETP Study: outcomes of a manual and computer decision support system based intervention. Am J Psychiatry. 2018;175(2):169–179. doi: 10.1176/appi.ajp.2017.16080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueser KT, Meyer-Kalos PS, Glynn SM, et al. Implementation and fidelity assessment of the NAVIGATE treatment program for first episode psychosis in a multi-site study. Schizophr Res. 2019;204:271–281. doi: 10.1016/j.schres.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 14. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 15. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. AJP. 2006;163(12):2080–2089. doi: 10.1176/ajp.2006.163.12.2080. [DOI] [PubMed] [Google Scholar]
- 16. Rosenheck R, Kasprow W, Frisman L, Liu-Mares W. Cost-effectiveness of supported housing for homeless persons with mental illness. Arch Gen Psychiatry. 2003;60(9):940–951. doi: 10.1001/archpsyc.60.9.940. [DOI] [PubMed] [Google Scholar]
- 17. Roy A, Bhaumik DK, Aryal S, Gibbons RD. Sample size determination for hierarchical longitudinal designs with differential attrition rates. Biometrics. 2007;63(3):699–707. [DOI] [PubMed] [Google Scholar]
- 18. Addington J, Addington D. Outcome after discharge from an early psychosis program. Schizophr Res. 2008;106(2–3):363–366. doi: 10.1016/j.schres.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 19. Heckman JJ. Sample selection bias as a specification error. Econometrica: J Econom Soc. 1979;15:3–161. [Google Scholar]
- 20. Little RJ. Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc. 1993;88(421):125–134. [Google Scholar]
- 21. Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Stat Med. 2006;25(1):143–163. [DOI] [PubMed] [Google Scholar]
- 22. Hedeker D, Gibbons RD.. Longitudinal Data Analysis. Vol 451. Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- 23. van der Vaart AW. Asymptotic Statistics (Cambridge Series in Statistical and Probabilistic Mathematics). Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 24. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137(1):246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falissard B, Sapin C, Loze JY, Landsberg W, Hansen K. Defining the minimal clinically important difference (MCID) of the Heinrichs–carpenter quality of life scale (QLS). Int J Methods Psychiatr Res. 2016;25(2):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albert N, Melau M, Jensen H, et al. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ. 2017;356:i6681. doi: 10.1136/bmj.i6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang WC, Kwong VWY, Lau ESK, et al. Sustainability of treatment effect of a 3-year early intervention programme for first-episode psychosis. Br J Psychiatry. 2017;211(1):37–44. doi: 10.1192/bjp.bp.117.198929. [DOI] [PubMed] [Google Scholar]
- 28. Malla A, Joober R, Iyer S, et al. Comparing three-year extension of early intervention service to regular care following two years of early intervention service in first-episode psychosis: a randomized single blind clinical trial. World Psychiatry. 2017;16(3):278–286. doi: 10.1002/wps.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:9. [DOI] [PubMed] [Google Scholar]
- 30. Albert N, Melau M, Jensen H, Hastrup LH, Hjorthøj C, Nordentoft M. The effect of duration of untreated psychosis and treatment delay on the outcomes of prolonged early intervention in psychotic disorders. npj Schizophr. 2017;3(1):34. doi: 10.1038/s41537-017-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dama M, Shah J, Norman R, et al. Short duration of untreated psychosis enhances negative symptom remission in extended early intervention service for psychosis. Acta Psychiatr Scand. 2019;140(1):65–76. doi: 10.1111/acps.13033. [DOI] [PubMed] [Google Scholar]
- 32. Snapshot of state plans for using the Community Mental Health Block Grant 10 percent set-aside to address first episode psychosis. https://www.nasmhpd.org/sites/default/files/Snapshot_of_State_Plans.pdf. Published 2018. Accessed June 4, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.