Abstract
Background
In general, plant protein intake was inversely associated with mortality in studies in middle-aged adults. Our aim was to evaluate the long-term associations of animal and plant protein intake with mortality in older adults.
Methods
A prospective cohort study including 1 139 community-dwelling older adults (mean age 75 years, 56% women) living in Tuscany, Italy, followed for 20 years (InCHIANTI study) was analyzed. Dietary intake by food frequency questionnaires and clinical information were assessed 5 times during the follow-up. Protein intakes were expressed as percentages of total energy. Time-dependent Cox regression models adjusted for confounders were used to assess the association between plant and animal protein intake, and mortality.
Results
During the 20 years of follow-up (mean: 12 years), 811 deaths occurred (292 of cardiovascular- and 151 of cancer-related causes). Animal protein intake was inversely associated with all-cause (hazard ratio [HR] per 1% of total energy from protein increase, 95% confidence interval [CI]: 0.96, 0.93–0.99) and cardiovascular mortality (HR per 1% of total energy from protein increase, 95% CI: 0.93, 0.87–0.98). Plant protein intake showed no association with any of the mortality outcomes, but an interaction with baseline hypertension was found for all-cause and cardiovascular mortality (p < .05).
Conclusions
Animal protein was inversely associated with all-cause and cardiovascular mortality in older adults. Further studies are needed to provide recommendations on dietary protein intake for older adults.
Keywords: Cohort study, Diet, Longevity, Nutrition, Protein
Total protein requirements in older adults are higher than in middle-aged adults (1), and inadequately low protein intake is frequent among older adults (2). In previous studies, the relationship between protein intake and mortality was different depending on the source of dietary protein (whether from animal or plant sources) (3–6). In middle-aged adults, higher animal protein intake was associated with an increased cardiovascular mortality, while plant protein exhibited an inverse relationship (3–7). Studies carried out in older adults (≥65 years) observed an inverse association between total protein intake and mortality (8,9). However, only one study evaluated the different associations between protein sources and mortality in older adults. Chan et al. (10) observed an inverse association between plant protein intake and all-cause mortality among women but not in men. In men, animal protein was inversely associated with mortality.
Previous studies carried out in older adults showed that higher plant protein intake was associated with reduced physical decline and a composite index of unhealthy aging (11,12). However, intrinsic characteristics of animal protein (ie, amino acid profile, protein digestibility) have been highlighted in a recent review on healthy aging and sarcopenia prevention in older adults, suggesting to prioritize the consumption of high-quality, nutrient-dense sources of protein (eg, eggs, milk, fish, and some specific plant sources) (13). Therefore, the adequate combination of source, quality, and quantity of dietary protein is still under discussion (13).The present study aimed to evaluate the association of animal and plant protein intake with all-cause, cardiovascular, and cancer mortality after 20 years of follow-up in an Italian cohort study (InCHIANTI study). Our hypothesis is that plant, but not animal, protein would be inversely associated with mortality.
Participants
Study Design
The InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study included community-dwelling older adults living in the Chianti geographic area (Tuscany, Italy). Study details have been previously reported (14).
The Italian National Institute of Research and Care of Aging Institutional Review and MedStar Research Institute (Baltimore, MD) approved the study protocol, and all participants signed an informed consent.
The current report followed the Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-NUT) guidelines (Supplementary Table 1) (15).
Study Population
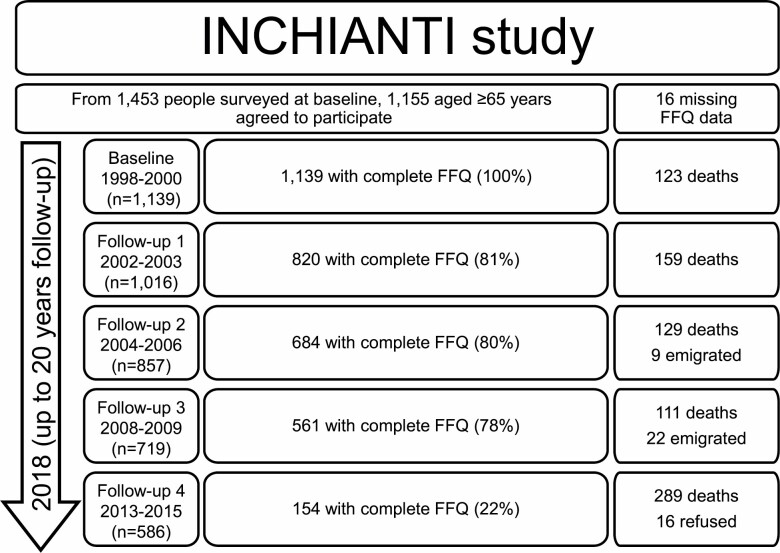
About 1 453 community-dwelling adults were randomly selected from the population registries of 2 Italian communities: Greve in Chianti and Bagno a Ripoli, using a multistage, stratified sampling method. Baseline participation rate was 91.7%, and 1 155 participants aged 65 years or older were evaluated. Sixteen participants had missing data on dietary questionnaires and the studied population included 1 139 participants (Figure 1). Clinical data were available in more than 95% of the cases during all the follow-up evaluations, except for the Follow-up 4 in which 80% had available data. In total, 4 317 observations from 1 139 participants were included in the analysis.
Figure 1.
InCHIANTI study flowchart of participants across follow-up assessments. FFQ = food frequency questionnaire.
Method
Dietary Intake
Habitual dietary intake was assessed by trained interviewers using the validated Italian version of the food frequency questionnaire (FFQ) developed and validated in the European Prospective Investigation into Cancer and Nutrition—Italy study (16). This FFQ asked the frequency of consumption during the previous year of 240 items (Supplementary Table 2), which were then summarized into 60 foods. Nutrient data for these specific foods were obtained from the Food Composition Database for Epidemiological Studies in Italy (17). For this analysis, dietary data at baseline and during the follow-up were considered (Figure 1). In the 15 years follow-up, the dietary assessment was only conducted in one site, Bagno a Ripoli.
Mediterranean Diet Adherence Score
Adherence to the Mediterranean diet was computed using a 9-point linear scale as described by Trichopoulou et al. (18). The resulting Mediterranean diet adherence score ranged between 0 (no adherence) and 9 (high adherence).
Covariates
Covariates were selected on the basis of existent literature and previous associations with mortality in the InCHIANTI study (3,7,19). Age, sex, years of education, economic situation, disability in activities of daily life (disabled ADL ≥1 or not), and physical activity (categorized into 3 levels) were assessed through questionnaires. Economic situation was evaluated using the following question: “How do you feel about your economic situation?” Possible answers were “cannot say,” “bad,” “satisfactory,” or “good.” Smoking habits were self-reported, and participants were classified into never smokers, former smokers, or current smokers. Height and weight were measured, and body mass index (BMI) was calculated in kg/m2. Global cognitive performance was assessed with the Mini-Mental State Examination (MMSE). Comorbidities considered were hypertension, diabetes, impaired renal function (3 categories, normal: glomerular filtration rate [GFR] >60 ml/min, mild: GFR <60 ml/min but ≥30 ml/min, and severe: GFR <30 ml/min), chronic obstructive pulmonary disease (COPD), ischemic heart disease (IHD, angina pectoris or myocardial infarction), cerebrovascular disease (stroke or transient ischemic attack), peripheral artery disease, congestive heart failure (CHF), Parkinson’s disease, dementia, cognitive impairment (MMSE <24 points), and cancer. They were defined using standard clinical definitions by algorithms combining information from self-reported physician diagnoses, pharmacological treatments, medical history, clinical examinations, and blood tests. The presence of comorbidities was updated in every follow-up evaluation. In addition, cognitive decline was defined as ≥3 points decline in MMSE between follow-up evaluations (20).
Outcome Assessment
Mortality data on 20 years were collected using the Mortality General Registry from the Tuscany Region, as well as death certificates delivered after the decease of participants to the municipal registry office. Mortality records were coded by the 9th and 10th revision of the International Classification of Diseases (ICD-9 or -10).
Statistical Analysis
Data preparation
To express macronutrients intakes as a percentage of total energy, we used the following energy equivalents: 9 kcal/g for fat and fat subtypes, 4 kcal/g for carbohydrates and protein, and 7 kcal/g for alcohol intake. Total, animal, and plant protein intake as a percentage of total energy was categorized into quintiles for descriptive statistics. In 166 participants (15%), BMI and renal function at baseline were missing and values were imputed by the median. During the follow-up, missing values in clinical information (n = 206 observations, 4.7%) and in dietary data (n = 959 observations, 22.2%, Figure 1) were imputed by the Last Observation Carried Forward. Dummy variables were created to identify participants with imputed values at baseline and during follow-up.
Descriptive analysis
Continuous variables are presented as means (SD) or medians (interquartile range). Categorical variables are expressed as percentages. Differences in general characteristics across quintiles of total, animal, or plant protein intake and in participants with and without hypertension were assessed using generalized linear models adjusted for age and sex. To evaluate differences in dietary data across the follow-up assessments, linear mixed models using site- and individual-specific random effects were used. Intraclass correlation coefficients were calculated.
Survival analysis
A multivariable nutrient density model was used for Cox regression models with adjustment for total energy and percentage of energy from subtypes of fats, total or animal and plant protein, and alcohol as described previously (3,7). For the main analyses, we used 2 time-dependent Cox models stratified by site: Model 1 included total or animal and plant protein (continuous) adjusted for age, sex, total energy, and percentage of energy from saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), and alcohol. Model 2 was further adjusted for BMI, years of education, economic situation, smoking, ADL disability, physical activity, impaired renal function, diabetes, COPD, hypertension, IHD, cerebrovascular disease, peripheral artery disease, CHF, Parkinson’s disease, dementia, cognitive impairment, cognitive decline, cancer, and Mediterranean diet score (continuous). Visual inspection of scaled Schoenfeld residuals was used to check for the proportional hazard assumption and they were mostly satisfied except for hypertension. In consequence, Cox regression models also included hypertension as a stratification variable.
We compared the models with and without restricted cubic splines with 3 knots by the likelihood ratio test to assess for nonlinear relationships between exposures and outcomes.
Interactions between total, animal, or plant protein intake and age, sex, physical activity, BMI categories, diabetes, smoking, ADL disability, hypertension, IHD, cerebrovascular disease, CHF, cognitive impairment, and cancer were tested using likelihood ratio tests. Additionally, animal and plant protein intakes were categorized by tertiles, and an interaction term was modeled in the fully adjusted Cox regression model.
Sensitivity analyses were done after the exclusion of participants with imputed values, participants who died within the first 2 years of follow-up, and participants with baseline cancer, severely impaired kidney function, or cognitive impairment. Last, we created a dummy variable to identify the different periods between follow-up evaluations (from 0 to 4) and included interaction terms between it and total or animal and plant protein in Cox regression models. The same approach was used to test for the difference of effect between study sites in participants with and without imputations, considering that FFQs of the last follow-up were only available in one site of the study.
For the statistical analyses, SPSS version 25.0 (IBM, Armonk, NY, USA) and R 4.0.5 (R Foundation, Vienna, Austria) were used.
Results
Baseline Characteristics
The studied population consisted of 1 139 participants (56% women) with a mean age of 75 ± 8 years at baseline. Mean (SD) intake of total protein was 74 (21) g/day, and the normalized value by weight was 1.1 (0.3) g/kg of body weight per day. Overall, 63 (1) % of total protein intake was animal protein. Sources of animal protein were 26% for dairy products, 26% for processed meat products, 20% for red meat, 7.7% for fish and seafood, 6.3% for chicken, 2.7% for eggs, and the rest for other meats. Sources of plant protein were 73% for cereals, 11.4% for vegetables, 9.0% for fruits and nuts, and 5.3% for legumes.
According to quintiles of total protein intake (as % of total energy from protein), participants in the highest compared to the lowest quintile were more likely to be women, less educated, and to present diabetes at baseline (Table 1). There were no differences in plant protein as a percentage of energy across the quintiles of total protein intake. Participants in the highest quintile of total protein intake tended to consume more meat and dairy products, fish, and seafood, as well as less fruits, cereals, and alcohol, and showed a lower Mediterranean diet score compared to those in the lowest quintile (Supplementary Table 3).
Table 1.
Baseline Characteristics of the Population According to Quintiles of Total, Animal, or Plant Protein Intake as % of Energy From Protein in the InCHIANTI Study
| Total Protein | Animal Protein | Plant Protein | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 1 139) | Q1 (227) | Q3 (228) | Q5 (227) | Q1 (228) | Q3 (229) | Q5 (228) | Q1 (227) | Q3 (227) | Q5 (227) |
| Clinical characteristics | |||||||||
| Age (years) | 75 ± 7 | 75 ± 8 | 76 ± 7 | 74 ± 7 | 75 ± 8 | 76 ± 8† | 77 ± 8 | 76 ± 8 | 75 ± 7 |
| Women (%) | 42 | 52 | 70* | 38 | 55 | 72* | 64 | 59 | 51* |
| BMI (kg/m²)* | 27 ± 4 | 27 ± 4 | 27 ± 4 | 27 ± 4 | 27 ± 4 | 27 ± 4 | 27 ± 4 | 27 ± 4 | 27 ± 4 |
| Education (years) | 6 ± 3 | 5 ± 3 | 5 ± 3† | 6 ± 3 | 5 ± 3 | 5 ± 3 | 6 ± 4 | 5 ± 3 | 5 ± 3* |
| Economic situation (%) | |||||||||
| Cannot say | 2 | 4 | 2 | 3 | 2 | 3 | 3 | 5 | 4 |
| Bad | 34 | 31 | 27 | 30 | 34 | 31 | 39 | 28 | 23 |
| Satisfactory | 54 | 55 | 59 | 57 | 54 | 54 | 48 | 57 | 59 |
| Good | 9 | 10 | 11 | 10 | 9 | 11 | 10 | 9 | 14† |
| Current smoking (%) | 33 | 27 | 21 | 35 | 25 | 24 | 31 | 24 | 31 |
| ADL disability (%) | 6 | 11 | 12 | 3 | 11 | 15† | 13 | 12 | 6‡ |
| Physical activity (%) | |||||||||
| Sedentary | 21 | 23 | 30 | 20 | 20 | 31 | 27 | 29 | 20 |
| Light | 37 | 42 | 44 | 37 | 45 | 43 | 41 | 40 | 45 |
| Mod–high | 41 | 35 | 26 | 43 | 35 | 25 | 30 | 31 | 35 |
| Hypertension (%) | 55 | 56 | 56 | 56 | 59 | 56 | 52 | 56 | 57‡ |
| Diabetes (%) | 7 | 15 | 22* | 6 | 13 | 18* | 8 | 16 | 16† |
| IRF (%)* | |||||||||
| Mild | 27 | 32 | 33 | 25 | 33 | 33 | 34 | 33 | 27 |
| Severe | 3 | 1 | 2 | 3 | 3 | 0 | 2 | 1 | 3 |
| IHD (%) | 6 | 7 | 11‡ | 5 | 7 | 9 | 5 | 8 | 8 |
| Cerebrovascular disease (%) | 5 | 8 | 6 | 6 | 8 | 7 | 5 | 7 | 8 |
| PAD (%) | 11 | 11 | 8 | 10 | 10 | 9 | 10 | 12 | 12 |
| CHF (%) | 4 | 4 | 7‡ | 4 | 6 | 6 | 2 | 8 | 6 |
| COPD (%) | 11 | 7 | 7 | 11 | 7 | 7 | 7 | 11 | 6 |
| Cancer (%) | 5 | 3 | 7 | 4 | 7 | 8 | 5 | 5 | 3 |
| Dementia (%) | 5 | 7 | 7 | 4 | 4 | 10 | 7 | 9 | 5 |
| Cognitive impairment (%) | 23 | 31 | 38† | 21 | 31 | 40† | 35 | 32 | 31 |
| Parkinson’s disease (%) | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 |
| Dietary characteristics | |||||||||
| Energy (103 kcal/day) | 2.1 ± 0.6 | 1.9 ± 0.5 | 1.6 ± 0.4* | 2.1 ± 0.7 | 1.9 ± 0.5 | 1.7 ± 0.5* | 1.8 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.6 |
| Total protein (% E) | 13 ± 1 | 16 ± 1 | 19 ± 1* | 13 ± 1 | 16 ± 1 | 19 ± 1* | 16 ± 2 | 16 ± 2 | 16 ± 2 |
| Animal protein (% E) | 7 ± 1 | 10 ± 1 | 13 ± 2* | 7 ± 1 | 10 ± 1 | 14 ± 1* | 12 ± 2 | 10 ± 2 | 8 ± 2* |
| Plant protein (% E) | 5.6 ± 1.0 | 5.9 ± 1.0 | 5.7 ± 1.0 | 6.4 ± 1.0 | 5.8 ± 0.8 | 5.1 ± 0.9* | 4.3 ± 0.5 | 5.8 ± 0.1 | 7.2 ± 0.5* |
| SFA (% E) | 10 ± 2 | 10 ± 2 | 11 ± 2* | 9 ± 2 | 10 ± 2 | 12 ± 2* | 12 ± 2 | 11 ± 2 | 8 ± 2* |
| MUFA (% E) | 14 ± 3 | 15 ± 3 | 16 ± 3* | 14 ± 3 | 15 ± 3 | 17 ± 3* | 16 ± 3 | 15 ± 3 | 14 ± 3* |
| PUFA (% E) | 3.1 ± 0.7 | 3.3 ± 0.7 | 3.6 ± 0.6* | 3.0 ± 0.7 | 3.3 ± 0.6 | 3.7 ± 0.6* | 3.4 ± 0.6 | 3.4 ± 0.7 | 3.3 ± 0.7 |
| Carbohydrates (% E) | 52 ± 7 | 52 ± 6 | 47 ± 6* | 54 ± 7 | 51 ± 5 | 46 ± 6* | 46 ± 7 | 50 ± 5 | 56 ± 5* |
| Alcohol (% E) | 6 (10) | 3 (5) | 0 (3)* | 5 (10) | 3 (7) | 0 (5)* | 5 (10) | 2 (7) | 1 (4)* |
Notes: BMI = body mass index; ADL = activities of daily life; PA = physical activity; IHD = ischemic heart disease; PAD = peripheral artery disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; SFA = saturated fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid. Data for continuous variables are shown as mean ± SD or median (interquartile range). Cutoff points of intake category (percentage of total energy) for total protein were Quintile 1, <14.1%; Quintile 2, 14.1% to <15.2%; Quintile 3, 15.2% to <16.3%; Quintile 4, 16.3% to <17.6%; and Quintile 5, ≥17.6%. For animal protein were Quintile 1, <8.1%; Quintile 2, 8.1% to <9.3%; Quintile 3, 9.3% to <10.5%; Quintile 4, 10.5% to <12.0%; and Quintile 5, ≥12.0%. For plant protein were Quintile 1, <4.9%; Quintile 2, 4.9% to <5.5%; Quintile 3, 5.5% to <6.0%; Quintile 4, 6.0% to <6.6%; and Quintile 5, ≥6.6%.
*Data available in 973 subjects.
*p for trend <.001, †p for trend <.01, ‡p for trend <.05 using age- and sex-adjusted generalized linear models.
Across quintiles of animal protein intake (as % of total energy from protein), differences in sociodemographic, clinical characteristics, and dietary intake data were similar to the results across total protein intake quintiles (Table 1 and Supplementary Table 3).
Participants in the highest quintile of plant protein intake (as % of total energy from protein) were more likely to be men, less educated, with a better self-reported economic situation, and to present a higher prevalence of hypertension and diabetes than those in the lowest quintile (Table 1). Total energy and total protein intakes (as % of total energy from protein) were not different across plant protein quintiles.
The prevalence of chronic diseases during the follow-up is shown in Supplementary Figure 1. Throughout the follow-up, small differences were observed in macronutrients intake data as % of total energy (variations within the ±12% of baseline values across follow-up evaluations). Intraclass correlation coefficients of macronutrients intake data were within the range of 0.23–0.50.
Association Between Dietary Protein Sources and Mortality
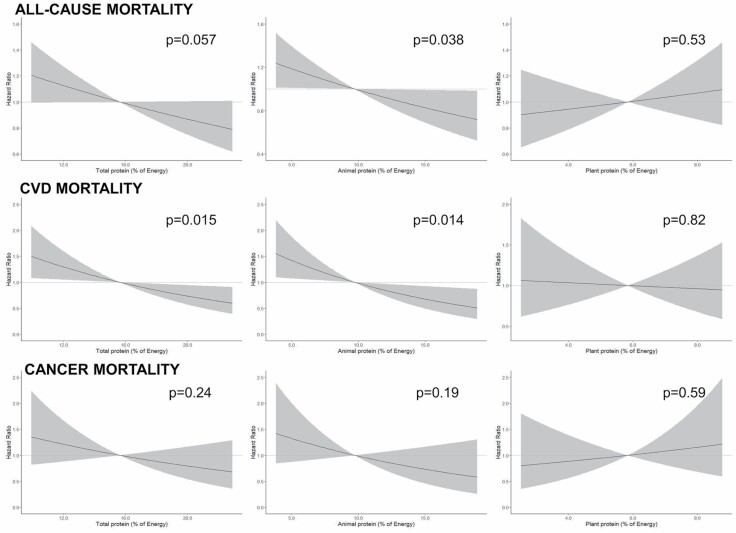
During the 20 years of follow-up (mean: 12 ± 6 years), 811 deaths occurred (292 of cardiovascular diseases (CVDs) and 151 of cancer-related causes). Associations between total, animal, and plant protein intake and mortality outcomes are shown in Figure 2 and Supplementary Table 4. In the fully adjusted model, a statistically significant inverse association was observed between total protein and cardiovascular mortality. Animal protein was inversely associated with both all-cause and cardiovascular mortality. Neither total, nor animal, nor plant protein intakes were associated with cancer mortality (Figure 2), or mortality from other causes (p > .05). Similar results were obtained according to quintiles of total, animal, and plant protein intake (Supplementary Table 5). Statistically significant associations were linear in all cases, and nonlinear terms did not improve the models’ fit.
Figure 2.
Association between total, animal, and plant protein intake and all-cause, cardiovascular, and cancer mortality. Cox regression stratified by site and baseline hypertension adjusted for age, sex, total energy, percentage of energy from saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, alcohol, total or plant and animal protein, body mass index, years of education, economic situation, smoking status (current vs former and nonsmoker), activities of daily living disability, physical activity, impaired renal function, diabetes, ischemic heart disease, cerebrovascular disease, peripheral artery disease, congestive heart failure, chronic obstructive pulmonary disease, cancer, dementia, cognitive impairment, cognitive decline, Parkinson’s disease, and Mediterranean diet adherence score. Number of participants: 1 139. Number of observations = 4 317. Shaded areas are the 95% confidence intervals. CVD = cardiovascular disease.
Statistically significant interactions are shown in Supplementary Figure 2. For all-cause mortality, there was a statistically significant interaction between plant protein and hypertension (lower risk for all-cause mortality per 1% of total energy from plant protein in participants without baseline hypertension). For cardiovascular mortality, an interaction was observed between animal protein and IHD (lower risk for cardiovascular mortality per 1% of total energy from animal protein in participants with IHD), and between plant protein and hypertension (lower risk for cardiovascular mortality per 1% of total energy from plant protein in participants without baseline hypertension), and IHD (lower risk for cardiovascular mortality per 1% of total energy from plant protein in participants without IHD). For cancer mortality, statistically significant interactions were between plant protein and ADL disability (lower risk for cancer mortality per 1% of total energy from plant protein in participants without ADL disability), and cognitive impairment (lower risk for cancer mortality per 1% of total energy from plant protein in participants without cognitive impairment). No other interaction was statistically significant for any of the mortality outcomes. In addition, no interaction was observed between animal protein and plant protein intake for any of the mortality outcomes (Supplementary Figure 3).
Hypertension had a major effect on the association between plant protein and all-cause and cardiovascular mortality. Indeed, plant protein displayed an inverse, marginally significant association with cardiovascular mortality among participants without baseline hypertension (hazard ratio, 95% confidence interval: 0.80, 0.63–1.02, Supplementary Figure 2). Participants with hypertension were more likely to be women and showed higher age and sex-adjusted prevalence of obesity, impaired renal function, IHD, peripheral artery disease, and CHF (Supplementary Table 6).
In sensitivity analyses, results were similar after the exclusion of participants with imputed values, participants who died within the first 2 years of the follow-up, participants with severe impaired renal function at baseline, baseline cancer, or with cognitive impairment, who may be inaccurate in reporting food intake (Supplementary Table 4).
Across the follow-up period, the association between plant protein and cardiovascular mortality was significantly reduced during the last follow-up periods of the study (p = .032). No other difference of effects was observed during the follow-up assessments for any mortality outcome. Likewise, there was no difference of effect between sites whether using the data with or without imputations (p > .05).
Discussion
Higher intakes of animal protein were associated with lower risk for all-cause and cardiovascular mortality in this study of community-dwelling older adults after 20 years of follow-up.
The present study is the first showing an inverse association between animal protein and mortality in older women and men from a Mediterranean country. Chan et al. (10) reported a similar association in older men, but not in women. The borderline statistically significant inverse association between total protein intake and overall mortality is also in agreement with other studies carried out in older adults (8–10). Overall, total and animal protein intake displayed similar relationships with all-cause and cardiovascular mortality.
Increased animal protein intake may be inversely associated with mortality in older adults through its protective effects on muscle strength, frailty, sarcopenia, or immune responses, all of which merit further studies (21). Increased intake of total or animal protein was positively associated with muscle strength (22), which in turn was inversely associated with all-cause mortality in a recent meta-analysis of studies in older adults (23). Moreover, chronic or acute inflammatory conditions may impair the direct relationship between protein intake and muscle strength in older adults, increasing dietary protein requirements (1).
In this study, plant protein was mostly coming from cereals, and this fact could be related to the null association between plant protein and mortality. Indeed, in Asian studies where an inverse association between plant protein and all-cause mortality was observed, legumes and pulses contributed to approximately 25% of total plant protein (7,10). Recently, Huang et al. (6) reported an inverse association between plant protein and mortality in a US cohort. However, they observed that this association decreased in participants aged 65 years or older and with a follow-up of more than 10 years. Thus, our results are not in disagreement, but are complimentary by studying an older age population. Because animal protein intake doubled the one of plant protein, our results must be interpreted considering this different contribution to the amount of total protein. If intrinsic characteristics of animal protein (ie, its amino acid profile) and/or the overall levels of total protein intake were responsible for its inverse association with mortality in older adults requires further investigations. Meeting total protein intake requirements in older adults is challenging (1,2), and further studies designed to compare the health effects of animal and plant protein in older adults are required.
Hernandez-Alonso et al. (24) observed a positive association between higher animal protein intake and all-cause and CVD mortality in the PREDIMED study. However, major differences in the baseline prevalence of hypertension (83%) and diabetes (49%) prevent a direct comparison between their study and our study. Recently, an analysis from the Rotterdam study showed a positive association between animal protein and all-cause mortality (4). They analyzed 7 786 participants with a mean (SD) age of 64 (9) years without CVD, diabetes, or cancer at baseline during a median follow-up of 13 years. There was a significant interaction between sex and animal protein intake, and the results were confirmed only in men but not in women. Compared to our population, besides the differences in age and of comorbid conditions, there were major differences in dietary intake. For instance, total fat energy was lower in our study (27% vs 35% of total energy) and qualitatively different with a higher MUFA intake (14% vs 12% of total energy) and lower SFA and PUFA intakes (10% vs 14% and 3.2% vs 7.0% of total energy, respectively). In line with the effect of fatty acid composition of the consumed foods, they found that substitution of dairy or meat protein (correlated with SFA intake) for carbohydrates was the largest contributor to the association between animal protein and all-cause mortality (4). These dietary peculiarities may have further contributed to the differences in our results. Notably, our results were adjusted by overall diet quality (using the Mediterranean diet score), which has major implications shaping the relationship between intake of protein from different sources and mortality.
The strengths of the current study include a long follow-up in a well-established cohort of older adults, and the inclusion of repeated dietary assessments to reduce bias from measurement errors in dietary questionnaires. Our study also has limitations. The relatively small sample size and low incidence of cancer-related deaths could have compromised the statistical power. Medical advice could have affected the dietary choices. Indeed, higher plant protein intakes are encouraged within a DASH diet compared to a Western diet (25). Residual confounding may remain, even though we adjusted the analyses by a Mediterranean diet adherence score. Last, our results may be affected by survivor bias, the mean age at baseline was 75 years, and require validation in other studies with a longer follow-up and in non-Mediterranean populations.
In conclusion, refuting our initial hypothesis, animal protein intake was inversely associated with all-cause and cardiovascular mortality in older adults. Further studies are needed to deliver recommendations on dietary protein intake for older adults.
Supplementary Material
Acknowledgments
We would like to thank all the participants of the study and the staff involved in the InCHIANTI study.
Contributor Information
Tomás Meroño, Biomarkers and Nutrimetabolomics Laboratory, Department of Nutrition, Food Sciences and Gastronomy, Faculty of Pharmacy and Food Sciences, Food Innovation Net (XIA), Nutrition and Food Safety Research Institute (INSA), University of Barcelona, Barcelona, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Instituto de Salud Carlos III, Madrid, Spain.
Raúl Zamora-Ros, Biomarkers and Nutrimetabolomics Laboratory, Department of Nutrition, Food Sciences and Gastronomy, Faculty of Pharmacy and Food Sciences, Food Innovation Net (XIA), Nutrition and Food Safety Research Institute (INSA), University of Barcelona, Barcelona, Spain; Unit of Nutrition and Cancer, Catalan Institute of Oncology, Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain.
Nicole Hidalgo-Liberona, Biomarkers and Nutrimetabolomics Laboratory, Department of Nutrition, Food Sciences and Gastronomy, Faculty of Pharmacy and Food Sciences, Food Innovation Net (XIA), Nutrition and Food Safety Research Institute (INSA), University of Barcelona, Barcelona, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Instituto de Salud Carlos III, Madrid, Spain.
Montserrat Rabassa, Biomarkers and Nutrimetabolomics Laboratory, Department of Nutrition, Food Sciences and Gastronomy, Faculty of Pharmacy and Food Sciences, Food Innovation Net (XIA), Nutrition and Food Safety Research Institute (INSA), University of Barcelona, Barcelona, Spain.
Stefania Bandinelli, Geriatric Unit, ASL Toscana Centro, Firenze, Italy.
Luigi Ferrucci, Clinical Research Branch, National Institute on Aging, NIH, Baltimore, Maryland, USA.
Massimiliano Fedecostante, Geriatria, Accettazione geriatrica e Centro di ricerca per l’invecchiamento, IRCCS INRCA, Ancona, Italy.
Antonio Cherubini, Geriatria, Accettazione geriatrica e Centro di ricerca per l’invecchiamento, IRCCS INRCA, Ancona, Italy.
Cristina Andres-Lacueva, Biomarkers and Nutrimetabolomics Laboratory, Department of Nutrition, Food Sciences and Gastronomy, Faculty of Pharmacy and Food Sciences, Food Innovation Net (XIA), Nutrition and Food Safety Research Institute (INSA), University of Barcelona, Barcelona, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Instituto de Salud Carlos III, Madrid, Spain.
Funding
The InCHIANTI study was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health (PE-2011-02350413) and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164, 263 MD 821336, N.1-AG-1-1, N.1-AG-1-2111, and N01-AG-5-0002). This study was further supported by Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), AC19/00096, funded by Instituto de Salud Carlos III and co-funded by European Regional Development Fund “A way to make Europe” and the award of the Generalitat de Catalunya’s Agency AGAUR (2017SGR1546) and ICREA Academia 2018. IDIBELL is a member of the CERCA Programme, Generalitat de Catalunya.
M.R. and R.Z.-R. would like to thank the “Sara Borrell” (CD16/00157) and “Miguel Servet” (CP15/00100) research contracts, respectively, from the Carlos III Institute of Health and the European Social Fund (ESF). T.M. would like to thank the Ayuda para contratos “Juan de la Cierva Incorporación” Ayuda IJCI 2017-32534 financiada por Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación (MCIN/AEI)/10.13039/501100011033.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author Contributions
T.M., R.Z.-R., and C.A.-L. designed the research; T.M., N.H.-L., M.R., S.B., L.F., A.C., and C.A.-L. conducted the research; T.M., R.Z.-R., N.H.-L., and M.R. performed statistical analysis; T.M., N.H.-L., and M.R. wrote the first draft of the manuscript; R.Z.-R., S.B., L.F., M.F., A.C., and C.A.-L. provided critical revision; and C.A.-L. had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1. Bauer J, Biolo G, Cederholm T, et al. . Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 2. Gaytán-González A, Ocampo-Alfaro MdeJ, Torres-Naranjo F, et al. . Dietary protein intake patterns and inadequate protein intake in older adults from four countries. Nutrients. 2020;12(10):1–17. doi: 10.3390/nu12103156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song M, Fung TT, Hu FB, et al. . Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453–1463. doi: 10.1001/jamainternmed.2016.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Z, Glisic M, Song M, et al. . Dietary protein intake and all-cause and cause-specific mortality: results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35(5):411–429. doi: 10.1007/s10654-020-00607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naghshi S, Sadeghi O, Willett WC, Esmaillzadeh A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2020;370:m2412. doi: 10.1136/bmj.m2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang J, Liao LM, Weinstein SJ, Sinha R, Graubard BI, Albanes D. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern Med. 2020;180(9):1173–1184. doi: 10.1001/jamainternmed.2020.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Budhathoki S, Sawada N, Iwasaki M, et al. ; Japan Public Health Center–based Prospective Study Group . Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med. 2019;179(11):1509–1518. doi: 10.1001/jamainternmed.2019.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bates CJ, Mansoor MA, Pentieva KD, Hamer M, Mishra GD. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr. 2010;104(6):893–899. doi: 10.1017/S0007114510001236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaslavsky O, Zelber-Sagi S, Hebert JR, et al. . Biomarker-calibrated nutrient intake and healthy diet index associations with mortality risks among older and frail women from the Women’s Health Initiative. Am J Clin Nutr. 2017;105(6):1399–1407. doi: 10.3945/ajcn.116.151530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan R, Leung J, Woo J. High protein intake is associated with lower risk of all-cause mortality in community-dwelling Chinese older men and women. J Nutr Health Aging. 2019;23(10):987–996. doi: 10.1007/s12603-019-1263-1 [DOI] [PubMed] [Google Scholar]
- 11. Ortolá R, Struijk EA, García-Esquinas E, Rodríguez-Artalejo F, Lopez-Garcia E. Changes in dietary intake of animal and vegetable protein and unhealthy aging. Am J Med. 2020;133(2):231–239.e7. doi: 10.1016/j.amjmed.2019.06.051 [DOI] [PubMed] [Google Scholar]
- 12. Coelho-Junior HJ, Calvani R, Gonçalves IO, et al. . High relative consumption of vegetable protein is associated with faster walking speed in well-functioning older adults. Aging Clin Exp Res. 2019;31(6):837–844. doi: 10.1007/s40520-019-01216-4 [DOI] [PubMed] [Google Scholar]
- 13. Nunes EA, Currier BS, Lim C, Phillips SM. Nutrient-dense protein as a primary dietary strategy in healthy ageing: please sir, may we have more? Proc Nutr Soc. 2021;80(2):264–277. doi: 10.1017/S0029665120007892 [DOI] [PubMed] [Google Scholar]
- 14. Ferrucci L, Bandinelli S, Benvenuti E, et al. . Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 15. Lachat C, Hawwash D, Ocké MC, et al. . Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-nut): an extension of the STROBE statement. PLoS Med. 2016;13(6):e1002036. doi: 10.1371/journal.pmed.1002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152 [DOI] [PubMed] [Google Scholar]
- 17. Bartali B, Turrini A, Salvini S, et al. . Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr. 2004;38(1):51–60. doi: 10.1016/s0167-4943(03)00084-0 [DOI] [PubMed] [Google Scholar]
- 18. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 19. Zamora-Ros R, Rabassa M, Cherubini A, et al. . High concentrations of a urinary biomarker of polyphenol intake are associated with decreased mortality in older adults. J Nutr. 2013;143(9):1445–1450. doi: 10.3945/jn.113.177121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabassa M, Cherubini A, Zamora-Ros R, et al. . Low levels of a urinary biomarker of dietary polyphenol are associated with substantial cognitive decline over a 3-year period in older adults: the invecchiare in Chianti study. J Am Geriatr Soc. 2015;63(5):938–946. doi: 10.1111/jgs.13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8(6):359. doi: 10.3390/nu8060359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci. 2016;71(3):356–361. doi: 10.1093/gerona/glv184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J. Associations between handgrip strength and disease-specific mortality including cancer, cardiovascular, and respiratory diseases in older adults: a meta-analysis. J Aging Phys Act. 2020;28(2):320–331. doi: 10.1123/japa.2018-0348 [DOI] [PubMed] [Google Scholar]
- 24. Hernández-Alonso P, Salas-Salvadó J, Ruiz-Canela M, et al. . High dietary protein intake is associated with an increased body weight and total death risk. Clin Nutr. 2016;35(2):496–506. doi: 10.1016/j.clnu.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 25. Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr. 2015;6(6):712–728. doi: 10.3945/an.115.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.